Skuteczność inhibitorów reniny w obniżaniu ciśnienia krwi u osób z pierwotnym nadciśnieniu tętniczym.
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomized, double‐blind, placebo‐controlled, parallel group study. | |
| Participants | 1342 patients were randomized to 6 different combination treatment groups. Inclusion criteria: Not reported Exclusion criteria: Not reported. Baseline characteristics: Mean age was 55.1 + 10 years; 19% patients were > 65 years old; 73% participants were male; race not reported, mean sitting SBP and DBP, pulse pressure and heart rate was not reported | |
| Interventions | Placebo (N = 153) and aliskiren 150 mg (N = 157) monotherapy (Other combination therapy groups and amlodipine 2.5 mg and 5 mg monotherapy groups are not described) Treatment duration = 8 weeks | |
| Outcomes | Primary: Change From Baseline in MSDBP to End of Study (Week 8); Secondary: Change From Baseline in MSDBP to End of Study (Week 8); Percentage of patients achieving goal BP; serious adverse events; total adverse events | |
| Notes | Study CSPA100A1301 is not published in a journal. It is registered on clinicalTrials.gov. Identifier: NCT01237223 (results are posted) It is registered on Novartis Clinical Trial Results database (results are not posted) EMA does not possess CSR for this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomized ; no description is available. |
| Allocation concealment (selection bias) | Unclear risk | No description is available. |
| Blinding (performance bias and detection bias) | Low risk | Clinical study synopsis reported study as double‐blind (Subject, Outcomes Assessor). In order to adequately blind the study, patients were required to take a total of 3 tablets and 2 capsules of study medication throughout the study. |
| Incomplete outcome data (attrition bias) | High risk | 33 (21.6%) patients in placebo group and 15 (9.6%) patients in aliskiren 150 mg group did not complete the study. Reasons for withdrawal differed (adverse events 12 in placebo and 4 in A 150 mg; unsatisfactory therapeutic effect, 20 in placebo and 6 in A 150 mg). It is not known how missing data were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available to assess selective reporting bias. Clinical study synopsis does not provide details. |
| Other bias | High risk | Study sponsored by Novartis. Study is not published in a journal. NCT01237223 ‐ short clinical synopsis is posted on clinical Trial.gov Novartis Clinical Trial Results database (results are not posted) |
| Methods | A double‐blind, multicentre, randomized, placebo‐controlled, multifactorial study was conducted at 208 centres across 18 countries (Argentina, Australia, Canada, Colombia, Denmark, Finland, Greece, Italy, Mexico, Panama, Peru, Romania, Russia, South Africa, Spain, Sweden, Taiwan and the USA). | |
| Participants | 1688 patients were randomized to 9 different monotherapy as well as combination treatment groups. 596 were randomized to aliskiren 150 mg, 300 mg and placebo groups. Inclusion criteria: Men and women aged >18 years with primary hypertension; MSDBP > 95 mmHg and <110 mmHg at randomization; an absolute difference of ≤ 10 mmHg in their MSDBP during the last 2 visits of the single‐blind run‐in period. Exclusion criteria: grade III hypertension (MSDBP >110 mmHg or MSSBP > 180 mmHg); secondary hypertension; a history of severe cardiovascular or cerebrovascular disease; type 1 or type 2 diabetes mellitus that was not well‐controlled (glycosylated haemoglobin [HbA1c] 48.0%; severe renal impairment; a history of dialysis; or a history of nephrotic syndrome; or hepatic disease; a history of hepatic encephalopathy; oesophageal varices; or portocaval shunt; pregnant or lactating women. Women of childbearing potential had to be using effective contraceptive methods for inclusion in the study; Baseline characteristics: Mean age 54.1 + 10.7 years; 17.2% of participants aged 65 years or older; Caucasian (62.1%) Black (19.9%), Asian patients (6.6%), others 11.4%; Mean body mass index 30.3 + 5.4 (Kg/m2); 11.0% of participants had diabetes; mean sitting SBP/DBP at baseline was 157 + 11.7/99.5 + 3.8 mmHg; mean sitting pulse pressure or heart rate was not reported. | |
| Interventions | Aliskiren 150 mg (N = 195), aliskiren 300 mg (N = 203), or placebo (N = 198). (Two amlodipine monotherapy and four aliskiren/amlodipine combination groups) Treatment was administered once daily at 800 hours, except on clinic visit days, when patients were instructed to delay the treatment until all assessments had been completed. Treatment duration = 8 weeks. | |
| Outcomes | Primary: antihypertensive efficacy of the combination of aliskiren/amlodipine was superior to each of the component monotherapies, as assessed by change in MSDBP from baseline to week‐8 endpoint across doses. Secondary: change in MSSBP from baseline to week‐8 endpoint; proportion of patients achieving BP control (< 140/90mmHg) at week 8; Change in mean 24 h ambulatory BP from baseline to week‐8 endpoint in a subgroup of patients; Changes in PRA from baseline to week‐8 endpoint in a subset of patients; All adverse events (AEs) and serious AEs. | |
| Notes | Study CSPA100A2305 is published in a journal as Littlejohn 2013 It is registered on clinicalTrials.gov. Identifier: NCT00739973 (results are posted) It is registered on Novartis Clinical Trial Results database (results are posted) EMA does not possess CSR for this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned in an equal ratio, using a validated interactive voice response system, to one of the nine treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Described as double‐blind. In order to adequately blind the study, patients were required to take a total of 3 tablets and 2 capsule of study medication throughout the study. There is no mention of how outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | High risk | 30 (15.2%) patients discontinued in placebo group; 19 (9.7%) each in aliskiren 150 mg and 300 mg groups. The incidence of withdrawal due to unsatisfactory therapeutic effect was lower in the combination groups (0.5% to 1.1%) than in the placebo (8.6%) and monotherapy (1.1% to 4.1%) groups. Other common reasons for discontinuation included AEs and withdrawal of consent, with the incidence generally similar across treatment groups. Three patients were mis‐randomized, as they were discontinued from the single‐blind period and were not treated in the double‐blind period. For endpoint analyses, the last observation was carried forward for patients who did not have a measurement at week 8. |
| Selective reporting (reporting bias) | Unclear risk | All primary and secondary outcomes were reported. Pulse pressure and heart rate were not reported at baseline or end of treatment. |
| Other bias | High risk | J Zhang, H Hsu and DL Keefe are employees of Novartis Pharmaceuticals Corporation and are therefore eligible for Novartis stock and stock options. The remaining authors declare no conflict of interest. EMA does not possess CSR for this study. |
| Methods | Multicentre, randomized, placebo‐controlled, double‐blind, parallel group study. | |
| Participants | 615 were recruited from 29 clinical centres in Japan and 455 were randomized. 160 withdrew during the single‐blind period (138 not meeting protocol requirements,15 due to adverse events and 7 withdrawal of consent). Inclusion criteria: Japanese men and women with essential hypertension between the ages of 20 and 80 years; MSDBP > 90 mm Hg and < 110 mm Hg and MSSBP < 180 mmHg Exclusion criteria: Severe hypertension (MSDBP >110 mmHg and/or mean sitting systolic BP (MSSBP) >180 mmHg); secondary hypertension, suspected malignant hypertension; a history of severe cardiovascular or cerebrovascular disease; type 1 or type 2 diabetes mellitus receiving insulin or with poor glucose control (glycosylated haemoglobin [HbA1c] > 8%); serious hepatic or renal disease; history of pancreatitis; malignant tumours in the last 5 years; autoimmune disease; anaemia; gout or hyperthyroidism; apparent dehydration; pregnancy; Patients receiving treatment for gastric or duodenal ulcer or had taken any investigational medications within the last 12 weeks; patients with autoimmune disease, symptomatic anaemia, hypothyroidism, gout, or unable to comply with the protocol. Baseline characteristics: Mean age 53 + 11years; Males: 73%; MSSBP 156 + 11.8 mmHg and MSDBP 99.6 + 4.4 mmHg; Mean sitting pulse: 75 + 9.8 beats per minute; Pulse pressure was not reported. | |
| Interventions | 455 patients were randomized to Placebo: N = 115; aliskiren 75 mg: N = 115; aliskiren 150 mg: N = 112; aliskiren 300 mg: N = 113 Duration of treatment = 13 weeks | |
| Outcomes | Primary: Change in trough MSDBP from baseline at endpoint. | |
| Notes | Study CSPP100A1201 is published in a journal as Kushiro 2006 It is not registered on clinicalTrials.gov It is registered on Novartis Clinical Trial Results database (results are not posted) CSR without appendices was received from EMA on March 11th 2016 BP data were reported at the end of the double‐blind period at 8 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Drug allocation tables were prepared by computer‐generated random numbers and patients were assigned to treatment groups via central allocation." |
| Allocation concealment (selection bias) | Low risk | "The allocation schedule was then concealed until the key code was broken, so all patients, investigators, collaborators and the sponsor were unaware of the treatment assignments throughout the study." |
| Blinding (performance bias and detection bias) | Low risk | "The study drugs and placebo were indistinguishable in terms of appearance, shape, packaging form labelling etc". "To maintain blinding, all patients took three tablets a day (two aliskiren tablets plus one placebo, two placebo tablets plus one aliskiren, or three placebo tablets)." "The key code was broken after all CRF were completed and after data for analysis was locked". |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons for withdrawal during washout and single‐blind period: 138 (22.4%) ‐not meeting requirement specified in the protocol; 15 (2.4%) due to adverse events and 7(1.1%) due to withdrawal of consent. Of 455 patients randomized, 21 (4.4%) were withdrawn due to major protocol deviations. Most withdrawals 11(9.6%) occurred in the placebo group; 5 (4.3%) withdrew in 75 mg group; 3 (2.7%) in 150 mg group and 2 (1.8%) in the 300 mg group. (page 67 of CSR). Last observation was carried forward in patients with missing data during DB period at week 8. |
| Selective reporting (reporting bias) | Unclear risk | All primary and secondary outcomes were reported as stated in the protocol. Pulse pressure was not reported at baseline or endpoint. Heart rate was reported at baseline but no data are reported on change in heart rate at endpoint. These are available in appendices that were not provided by EMA. |
| Other bias | High risk | Manufacturer sponsored. Conflict of interest of authors has not been provided. Patients could have been selected based on their response to other drugs acting on the renin‐angiotensin system. Patients with adverse events were excluded during washout and single‐blind period. Study was not registered with clinicalTrials.gov. |
| Methods | A randomized , double‐blind, placebo‐ and active‐controlled, parallel‐group, multicentre, comparative study. | |
| Participants | 1206 patients were enrolled from 53 Japanese centres and 761 were randomized. Inclusion criteria: Adult (20 to 75 years old) Asian outpatients with essential hypertension defined as MSDBP > 90 mmHg and < 110 mmHg at visit 2, and > 95 mmHg and < 110 mmHg at visit 3; difference in MSDBP measured at visit 2 and visit 3 needed to be < 10mmHg. Exclusion criteria: MSSBP measured > 180 mmHg and/or if the MSDBP > 110mmHg at visit 1, 2, or 3; Suspected or confirmed secondary hypertension; malignant hypertension; severe signs of: cardiac disease, renal disease, hepatic disease, cerebrovascular disorder; pancreatitis, or history of pancreatitis; treatment of duodenal or gastric ulcer; over dehydration or electrolyte abnormalities of clinical concern; type I or type II diabetes mellitus (HbA1c greater than 8% at start of run‐in period (visit 1); history of malignant tumours including leukaemia and lymphoma within the past 5 years; history of autoimmune diseases; anaemia; fecal occult blood at start of run‐in period; hypothyroidism; exposure to aliskiren or placebo within 12 weeks of start of run‐in period; history of hypersensitivity to ARBs or drugs with similar chemical structures to aliskiren; alcoholic patients, or those with history of drug abuse within 52 weeks of study start date; patients considered unlikely to comply with the requirements specified in the protocol by the investigator. Baseline characteristics: Mean age 52 + 10.2 years, with 12.6% of participants aged 65 years or older; Oriental (100%); Male 73.2%; Mean BMI 25.6 + 3.8; Diabetes 8.7%; MSSBP/MSDBP 152.0 + 11.5/99.0 + 4.0 mmHg; Mean sitting pulse was 73.0 + 10.4 bpm; mean sitting pulse pressure was not reported. | |
| Interventions | 761 patients were randomized Placebo (N=156), aliskiren 150 mg (N = 302), losartan 50 mg (N = 303) Duration of treatment = 8 weeks | |
| Outcomes | Primary: change from baseline in MSDBP Secondary: change from baseline in MSSBP; responder rate (percentage of patients a MSDBP < 90 mmHg and/or at least 10 mmHg reduction from baseline), blood pressure control rate (percentage of patients with a MSDBP < 90 mmHg and MSSBP < 140 mmHg, changes from baseline of the standing and supine systolic and diastolic blood pressures; adverse events, vital signs, laboratory tests, ECG and fecal occult blood. | |
| Notes | Study CSPP100A1301 is not published in a journal. It is registered on clinicalTrials.gov. Identifier: NCT003344110 (results are not posted) It is registered on Novartis Clinical Trial Results database (results are posted) CSR without appendices was received from EMA on May 23rd 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment groups by central randomization. Participants were provided with drug numbers by SPP 100 subject registration Centre and drug numbers were recorded in the eCRF by the investigators for use as randomization numbers and study drug was allocated according to the numbers. |
| Allocation concealment (selection bias) | Low risk | The allocation table was prepared using computer‐generated random numbers. The table was sealed by the person responsible for drug allocation and kept in strict confidence through unblinding. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind. Study drugs were identical in packaging, labelling, administration schedule, appearance, and odour. Patients were required to take a placebo during the placebo run‐in period (4 weeks) and were given 2 tablets according to the patient's assigned group. Participants, investigators, sub investigators, site staff, evaluators and data analysts remained blind to the identity of the treatment from randomization to database lock. |
| Incomplete outcome data (attrition bias) | Unclear risk | Discontinuations from the study ‐ placebo: N = 15 (9.6%) and aliskiren 150 mg: N = 14 (4.6%). Last observation was carried forward in patients with missing data during DB period at week 8. Missing data at week 8 had very little effect on analysis of results for MSSBP and MSDBP. |
| Selective reporting (reporting bias) | Unclear risk | Most primary and secondary outcomes were reported. Baseline and endpoint BP data was reported. Pulse rate was reported at baseline but not reported at endpoint. Pulse pressure was not reported. These may be available in appendices that were not provided by EMA. |
| Other bias | High risk | Manufacturer sponsored. Conflicts of interests unknown. Study is not published in a journal. Of the 1206 patients enrolled, 445 were not randomized (286 did not meet protocol requirements; 89 had abnormal laboratory value; 39 had adverse event; 22 withdrew consent and 9 had protocol violation). |
| Methods | Multicentre randomized, double‐blind, placebo‐controlled parallel‐group study. | |
| Participants | 793 patients were enrolled from 56 centres in USA, Germany and Belgium of which 652 were randomized. 141 patients were excluded (69 due to failure to meet BP criteria; 19 due to withdrawal of consent and 15 due to abnormal test procedure results). Inclusion criteria: Men and women aged 18 years or older, with mild‐to‐moderate essential hypertension (MSDBP > 95 mmHg and < 110 mmHg). Exclusion criteria: Severe hypertension (MSDBP > 110mmHg and/or MSSBP > 180mmHg); secondary hypertension; a history of severe cardiovascular or cerebrovascular disease; type 1 or type 2 diabetes mellitus receiving insulin or with poor glucose control (glycosylated haemoglobin [HbA1c] > 8%); serious hepatic or renal disease; history of malignancy; history of severe or life‐threatening diseases; history of drug or alcohol abuse; apparent dehydration; and pregnancy or nursing mothers. Baseline characteristics: Mean age 56 + 11.5 years; 22.7% patients were > 65 years old; Male 50.2% ; Caucasians 76.8%; Black 17.2%; Other 6%; mean BMI 30.8 + 6.3; MSSBP 152.2 + 11.2 mmHg and MSDBP 99.0 + 3.6 mmHg; Mean sitting pulse: 72.8 + 8.7 bpm; and mean pulse pressure was not reported. | |
| Interventions | 652 patients randomized Placebo: N = 131; aliskiren 150 mg: N = 127; aliskiren 300 mg: N = 130; aliskiren 600 mg: N = 130; Irbesartan 150 mg: N = 134 Duration of treatment = 8 weeks | |
| Outcomes | Primary: change from baseline in trough MSDBP | |
| Notes | Study CSPP100A2201 is published in a journal as Gradman 2005. It is not registered on clinicalTrials.gov It is registered on Novartis Clinical Trial Results database (results are not posted) CSR without appendices was received from EMA on January 25th 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization with a block size of 5 and stratified by region was performed by the interactive voice response system provider using a validated system that automates the random assignment of treatment groups to randomization numbers." |
| Allocation concealment (selection bias) | Low risk | "Randomization data were kept strictly confidential until completion of the study and blinded data cleaning process. Access during the study was available only to authorized persons who maintained the randomization database and were not involved in the conduct of the study. The database lock procedure was followed to merge clinical data and treatment codes for analyses after the completion of the study and data cleaning." |
| Blinding (performance bias and detection bias) | Low risk | All medications were identical in appearance. "All study personnel and participants remained blinded to the treatment assignment for the duration of the study." |
| Incomplete outcome data (attrition bias) | High risk | Withdrawal rate was 22 (16.8%) in the placebo group; 12 (9.4%) in aliskiren 150 mg; 11 (8.5%) in aliskiren 300 mg and 10 (7.7%) in aliskiren 600 mg. Reason for withdrawal differed ‐ 7.6% in placebo group withdrew due to lack of therapeutic effect and 0.8% to 1.6% withdrawal in aliskiren treatment groups. How missing data were analyzed has not been reported. |
| Selective reporting (reporting bias) | Unclear risk | Most primary and secondary outcomes were reported. Baseline and endpoint BP data was reported. Pulse rate was reported at baseline but not reported at endpoint. Change in pulse pressure was reported without standard deviation on page 53 of the CSR. |
| Other bias | High risk | Manufacturer sponsored. Authors are employees of Novartis Pharmaceuticals Corporation and are therefore eligible for Novartis stock and stock options. Patients could have been selected based on their response to other drugs acting on the renin‐angiotensin system. Study is not registered on www.ClinicalTrials.gov |
| Methods | Multicentre, randomized, double‐blind placebo‐controlled, multifactorial, parallel‐group study | |
| Participants | 1441 patients were enrolled from 94 centres in the USA, Germany, France, Denmark, and Poland, of which 1123 were randomized to 11 treatment groups. 318 patients discontinued single‐blind period (13.4% due to abnormal test procedure; 3.3% due to abnormal laboratory values; 2.8% due to withdrawal of consent; 1.5% due to adverse events; 0.5% due to protocol violation; 0.1% due to unsatisfactory therapeutic effect; 0.3% due to condition no longer required study drug; 0.1% due to administrative problems and 0.1% due to lost to follow‐up). Inclusion criteria: Adult men and women 18 years or older with mild‐to‐moderate essential hypertension (MSDBP 95 mmHg to < 110 mmHg). Exclusion criteria: Severe hypertension (MSSBP 180 mmHg or more or MSDBP of 110 mmHg or more); secondary hypertension; type I or uncontrolled diabetes mellitus; type 2 diabetes mellitus; history of severe cardiovascular or cerebrovascular disease or other life‐threatening medical conditions. Baseline characteristics: Mean age 56 + 12.2 years; 24.9% were > 65 years of age; Male 55.9%; Caucasians 92.1%; Black 6.28; other 1.2%; mean BMI 29.5 + 5.0; MSSBP 153.3 + 12.0 mmHg and MSDBP 99.0 + 3.5 mmHg; mean sitting pulse 72 + 9.3 bpm; mean pulse pressure is not reported. | |
| Interventions | 1123 patients were randomized Placebo: N = 177; aliskiren 75 mg: N = 179; aliskiren 150 mg: N = 178; aliskiren 300 mg: N = 175; several valsartan monotherapy groups and combination therapy groups. Duration of treatment = 8 weeks | |
| Outcomes | Primary: change from baseline to endpoint in MSDBP of monotherapy with aliskiren at all doses versus placebo. Secondary: change from baseline in MSSBP of aliskiren monotherapy as well as in combination therapy groups; safety and tolerability of aliskiren; of aliskiren combination therapy; impact of treatment on selected biomarker. | |
| Notes | Study CSPP100A2203 is published in a journal as Pool 2007. It is not registered on clinicalTrials.gov It is not registered on Novartis Clinical Trial Results database. CSR without appendices was received from EMA on April 12th 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization by region was performed by the interactive voice response system provider using a validated system that automates the random assignment of treatment groups to randomization numbers." |
| Allocation concealment (selection bias) | Low risk | Each centre received a supply of medication packs each labelled with a unique number. "Randomization codes were kept strictly confidential until the database was locked." |
| Blinding (performance bias and detection bias) | Unclear risk | Aliskiren 75 mg, aliskiren 150 mg were supplied as capsule and aliskiren 300 mg as tablet. Placebo was supplied both as a capsule and in tablet form. To adequately blind the study, patients had to take 3 tablets/capsules of study medication. Insufficient information to determine if blinding was successful. Blinding of outcome assessor is not reported. Appendices were not provided by EMA. |
| Incomplete outcome data (attrition bias) | Unclear risk | 15 (8.5%) participants withdrew from placebo group; 21 (11.7%) from aliskiren 75 mg group; 13 (7.3%) from aliskiren 150 mg group and 9 (5.1%) from aliskiren 300 mg group. Reason for withdrawal differed between groups ‐ lack of therapeutic effect was noted in 2.8% in placebo group; 5.6% in aliskiren 75 mg; 2.2% in aliskiren 150 mg and 2.3% in aliskiren 300 mg group. How missing data will be analyzed is not reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was available and primary and secondary outcomes were reported. Pulse rate was reported at baseline but no detail provided at endpoint. Pulse pressure is not reported at baseline or endpoint. This information may be available in the appendices that were not provided with the CSR by the EMA. |
| Other bias | High risk | Manufacturer sponsored. Conflict of interest of authors has not been provided. Patients could have been selected based on their response to other drugs acting on the renin‐angiotensin system. Study is not registered with clinicalTrials.gov or Novartis Clinical Trial Results database. |
| Methods | Multicentre, randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled. multifactorial, parallel‐group study of aliskiren monotherapy compared to placebo and combination therapy of aliskiren with HCTZ compared to component monotherapies. | |
| Participants | 3190 patients were enrolled from 19 countries in Argentina, Brazil, Canada, Columbia, Finland, France, Germany, Guatemala, Italy, the Netherlands, Norway, Peru, Poland, Russia, Slovakia, Spain, Sweden, Taiwan and USA in the single‐blind placebo run‐in period. 2776 were randomized, of which 2558 (92.1%) completed the study and 204 (7.3%) discontinued. Discontinuation rate was highest in the placebo group most often due to unsatisfactory therapeutic effect (2%) and those due to adverse events (2.3%). Inclusion criteria: Women and men aged 18 years or older with mild‐to‐moderate essential hypertension (MSDBP from > 95 mmHg to < 110 mmHg) at visit 3. Female patients were either postmenopausal, surgically sterile or using effective contraceptive methods. Exclusion criteria: Pregnant or nursing women; patients with severe hypertension (DBP > 110 mmHg and /or SBP > 180 mmHg) ; those with secondary hypertension; cerebrovascular accident, MI; coronary bypass surgery; TIA within last 12 months; Keith ‐ Wagener grade III to IV hypertensive retinopathy; malignancy including leukaemia and lymphoma within the past five years but excluding basal cell carcinoma; drug or alcohol abuse; gouty arthritis; current diagnosis of heart failure; angina pectoris requiring pharmacotherapy other than sublingual nitroglycerin; second or third degree heart block without a pace maker; any potential life‐threatening arrhythmia or clinically significant valvular disease; surgical or medical conditions which might significantly alter the absorption, distribution, metabolism, or excretion of study medication; known or suspected contraindications to aliskiren, including history of allergy to study drug and previous participation in an investigation clinical study within 1 month of visit 1 of the study; Participants with type I or 2 diabetes mellitus with poor glycaemic control; laboratory serum sodium or potassium values ≥ 5.5 mEq/L; hepatic disease; or renal impairment; history of major gastrointestinal tract surgery or current or previously active inflammatory bowel disease were excluded. Baseline characteristics: Mean age 54.6 + 11.6 years; 21.1% patients were > 65 years of age; Male 54.8%; Caucasians 85.4%; Black 4.6%; Asian 2.5%; Native American 1.8% and others 5.7%; MSSBP 153.6 + 12.2 mmHg and MSDBP 99.2 + 3.6 mmHg; mean sitting pulse pressure was 72.2 beats/minute | |
| Interventions | Placebo: N = 195; aliskiren 75 mg: N = 184; aliskiren 150 mg: N = 185; aliskiren 300 mg: N = 183; HCTZ monotherapy groups and aliskiren combination therapy groups. Duration of treatment = 8 weeks | |
| Outcomes | Primary: Change from baseline in MSDBP. Secondary: change from baseline in MSSBP, assessment of dose‐response efficacy, proportion of patients showing a successful response MSDBP < 90 mmHg or 10 mmHg or greater reduction from baseline, proportion of patients achieving BP control (<140 mmHg/90 mmHg), safety and tolerability, effects of treatment on plasma renin activity and renin concentration. | |
| Notes | Study CSPP100A2204 is published in a journal as Villamil 2007. It is registered on clinicalTrials.gov. Identifier: NCT00219024 (results are posted). It is registered on Novartis Clinical Trial Results database (results are not posted). CSR without appendices was received from EMA on April 6th 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization was performed by Covance Inc. using validated system that automates random assignment. The detail of the method used is not reported in the CSR. The randomization method and list is provided in appendix 5.2, which was not available. 14 patients received randomization numbers in error, were not treated with DB treatment and did not provide post baseline data. One patient completed the single‐blind phase and was assigned DB treatment without randomization and medication assignment by IVRS. Two patients received placebo instead of the assigned medication for 9 or 10 days and were subsequently discontinued due to protocol violation. |
| Allocation concealment (selection bias) | Unclear risk | Randomization number was provided along with a unique medication number for the packages of study drug to be dispensed. No further detail is provided. |
| Blinding (performance bias and detection bias) | Low risk | The identity of the treatment was concealed by the use of drugs that were identical in packaging, labelling, appearance, odour and schedule of administration. A double‐dummy design was used because the identity of the doses of aliskiren and HCTZ could not be disguised due to their different forms. |
| Incomplete outcome data (attrition bias) | Unclear risk | Total withdrawals were 22 (11.3%) in placebo group 15 (8.2%) in aliskiren 75 mg group; 16 (8.6%) in aliskiren 150 mg group and 17 (9.3%) in aliskiren 300 mg group. (Page 49 of CSR). The reasons for withdrawal in the placebo group were higher due to unsatisfactory therapeutic effect as compared to aliskiren groups. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was available and primary and secondary outcomes were reported. Pulse rate was reported at baseline but no detail provided at endpoint. Pulse pressure is not reported at baseline or endpoint. This information may be available in the appendices that were not provided with the CSR by the EMA. |
| Other bias | High risk | Manufacturer sponsored. Conflict of interest of authors has not been reported. Patients could have been selected based on their response to other drugs acting on the renin‐angiotensin system. |
| Methods | Randomized double‐blind placebo‐controlled, parallel‐group, multicentre study. | |
| Participants | Patients were recruited at 68 centres internationally (Canada, Guatemala, Korea, the Netherlands and USA). Of the 833 patients who entered single‐blind period, 672 were randomized to 4 double‐blind treatment groups. 63 (9.4%) discontinued treatment (15.1% due to abnormal test procedure; 0.4% due to abnormal laboratory values; 2.5% due to withdrawal of consent; 1.5% due to adverse events; 0.4% due to protocol violation; 0.1% due to unsatisfactory therapeutic effect; 0.1% due to condition no longer required study drug; 0.1% due to administrative problems and 0.5% due to lost to follow‐up). Inclusion criteria: Men and women aged 18 years or over with mild‐to‐moderate essential hypertension (MSDBP > 95 mmHg and <110 mmHg. Exclusion criteria: Patients who previously entered an aliskiren study; severe hypertension (MSDBP > 110 mmHg and/or MSSBP > 180 mmHg); secondary hypertension; known Keith‐Waegner grade III or IV hypertensive retinopathy; history of hypertensive encephalopathy or cerebrovascular accident; TIA during 12 months prior to visit 1; current diagnosis of heart failure (NYHA class II ‐ Iv); history of MI, coronary bypass surgery or any percutaneous intervention during 6 months prior to visit 1; current angina pectoris requiring pharmacotherapy; second or third degree heart block without a pacemaker; potentially life‐threatening arrhythmia; clinically significant valvular disease; type I or II diabetes mellitus with poor glycaemic control HbA1C > 9%; serum sodium less than lower limit of normal and serum potassium < 3.5 mEq/L or > 5.5 mEq/L or dehydration at visit 1; any surgical or medical condition that might significantly alter absorption, distribution, metabolism or excretion of the drug; history of malignancy including leukaemia or lymphoma; history of drug or alcohol abuse within last 12 months; pregnant or nursing women; and history of non compliance to medical regimens or unwillingness to comply with study protocol. Baseline characteristics: Mean age 53 + 10.5 years; 13.1% were > 65 years; Male 61.6%; Caucasians 61.3%; Black 12.4%; Asian 18%; Others 8.3%; Mean BMI 29 + 5.9 kg/m2; MSDBP 99.6 + 3.7 mmHg and MSSBP 152.1 + 12.4 mmHg. | |
| Interventions | Placebo: N = 165; aliskiren 150 mg: N = 172; aliskiren 300 mg: N = 169; aliskiren 600 mg: N = 166 Duration of treatment = 8 weeks | |
| Outcomes | Primary: change in MSDBP from baseline | |
| Notes | Study CSPP100A2308 is published in a journal as Oh 2007 It is registered on clinicalTrials.gov. Identifier: NCT00219128 (results are not posted) It is registered on Novartis Clinical Trial Results database (results are not posted) CSR without appendices was received from EMA on February 20th 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization list is produced using a validated system that automates the random assignment of treatment groups to randomized numbers in the specified ratios to insure treatment assignment is unbiased and concealed from patients and investigator staff. |
| Allocation concealment (selection bias) | Low risk | Randomization list is produced using a validated system that automates the random assignment of treatment groups to randomized numbers in the specified ratios to insure treatment assignment is unbiased and concealed from patients and investigator staff. |
| Blinding (performance bias and detection bias) | Low risk | Patients, investigator and staff performing the assessment and data analysis will remain blind to the identity of the treatment from the time of randomization until database lock and kept strictly confidential. The identity of the treatments will be concealed by the use of study drugs that are identical in packaging, labelling, schedule of administration, appearance, taste and odour. Patients were instructed to take 3 tablets of study medication per dose throughout the study at approximately 8.00am. Unblinding will occur in the case of patient emergencies and at the end of the conclusion of the study. |
| Incomplete outcome data (attrition bias) | Unclear risk | of the 833 patients enrolled in SB period, 671 (80.6%) completed SB period and were then randomized to DB treatment and 162 (19.4%) discontinued. 30 (18.2%) patients withdrew in placebo group; 10 (5.8%) in aliskiren 150 mg group; 9 (5.3%) in aliskiren 300 mg group and 14 (8.4%) in aliskiren 600 mg group. Reasons for withdrawal differed between treatment groups ‐ unsatisfactory therapeutic effect in 17 (10.3%) in placebo; 3 (1.7%) in aliskirenA 150 mg; 1 (0.6%) in aliskiren 300 mg and 4 (2.4%) in aliskiren 600 mg. For each patient, the last post baseline measurement during double blind‐treatment period was carried forward as endpoint measurement for the variable analyzed. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was available and primary and secondary outcomes were reported. Pulse rate and pulse pressure were not reported at baseline or endpoint. This information may be available in the appendices that were not provided with the CSR by the EMA. |
| Other bias | High risk | Manufacturer sponsored. Author Keefe D is employee of Novartis. Conflict of interest of other authors has not been provided in the published article. Patients could have been selected based on their response to other drugs acting on the renin‐angiotensin system. |
| Methods | Randomized, double‐blind, parallel‐group, placebo‐ and active‐controlled multicentre dose titration study. After 3 weeks, randomized patients receiving aliskiren 150 mg or HCTZ 12.5 mg underwent forced titration to doubled doses of their respective treatments. After an additional 3 weeks, patients in the placebo group were reassigned to aliskiren 300 mg or hydrochlorothiazide 25 mg for 20 weeks. | |
| Participants | 1440 patients were screened of which 1275 were enrolled in the single‐blind run‐in period. During this period 151 (11.8%) patients discontinued (most common reasons were abnormal laboratory test 7.1%, which included patients who did not meet the blood pressure criteria for randomization; withdrawal of consent in 2.6% and adverse events 0.9%). 1124 patients were randomized to receive once‐daily treatment with aliskiren 150 mg, hydrochlorothiazide 12.5 mg, or placebo. 978 (87%) completed the double‐blind period. Inclusion criteria: Patients aged ≥18 years with MSDBP > 90 mmHg and < 110 mmHg at the single‐blind placebo run‐in visit. At randomization, patients had to have a MSDBP > 95 mmHg and < 110 mmHg and show a absolute difference of < 10 mmHg in MSDBP from their previous study visit. Exclusion criteria: Patients with were severe hypertension (MSDBP ≥110 mmHg and/or MSSBP ≥180 mmHg); suspected secondary or malignant hypertension; Known Keith‐Waegner grade III or IV hypertensive retinopathy; malignancy excluding basal cell carcinoma within the last 5 years; drug or alcohol abuse in the last 12 months; current diagnosis of heart failure (NYHA class II ‐ IV); angina pectoris requiring pharmacological treatment; second or third degree heart block without a pace maker; clinically significant valvular disease; potentially life‐threatening or symptomatic arrhythmia; TIA; coronary bypass surgery or PCI during 12 months prior to visit 1; type 1 diabetes mellitus (DM); type 2 DM, poorly controlled (glycosylated haemoglobin > 9.0% or microalbuminuria at visit 1); serious hepatic, pancreatic, or renal disease; any surgical or medical condition that might significantly alter absorption, distribution, metabolism or excretion of the drug; history of major gastrointestinal tract surgery such as gastrectomy, gastroenterostomy, or bowel resection; history of active inflammatory bowel disease; currently active gastritis, duodenal or gastric ulcer; gastrointestinal bleeding in the past 3months; any history of pancreatitis, pancreatic injury, or evidence of impaired pancreatic function or abnormal lipase or amylase during past 12 months of visit 1; evidence of hepatic disease, history of hepatic encephalopathy; history of oesophageal varices; history of portocaval shunt; evidence of renal impairment or dialysis or nephrotic syndrome; current treatment with cholesterol absorption inhibitors; proteinuria or serum sodium less than lower limit of normal and/or serum potassium < 3.5 mEq/L or dehydration at visit 1; clinically significant allergy; pregnant and breast feeding women were excluded. Baseline characteristics: Mean age 56 + 11 years; 22.8% were > 65 years of age; male 56%; Caucasians 99%; Asian (0.7%) Black 0.2% and other 0.2%; Mean BMI 29.1 + 4.8 kg/m2;and diabetes in 10.9% patients; MSSBP 154.2 + 11.2 mmHg and MSDBP 99 + 3.4 mmHg | |
| Interventions | Placebo: N = 221; aliskiren 150 mg N = 459; HCTZ 12.5 mg N = 444; After 3 weeks on therapy, patients underwent forced titration to aliskiren 300 mg and HCTZ 25 mg. After 6 weeks, patients receiving placebo were reassigned to aliskiren 300 mg (N = 108) or HCTZ 25 mg (N = 113). Duration of treatment = 26 weeks (monotherapy data useful at week 3 for 150 mg dose and at week 6 for 300 mg dose) | |
| Outcomes | Primary: Change from baseline to endpoint in MSDBP. Secondary: changes in MSSBP at week 26 endpoint and MSDBP and MSSBP at week 52 endpoint; comparison of the BP‐lowering efficacy of aliskiren 300 mg monotherapy and hydrochlorothiazide 25 mg monotherapy at week 12 endpoint; evaluation of the proportion of patients with response to treatment and BP control at the week 26 and week 52 endpoints; and comparison of the long‐term safety and tolerability of an aliskiren regimen with a HCTZ regimen. | |
| Notes | Study CSPP100A323 is published in a journal as Schmieder 2009 It is registered on clinicalTrials.gov. Identifier: NCT00219154 (results are posted) It is registered on Novartis Clinical Trial Results database (results are not posted) CSR without appendices was received on May 27th 2016. BP data at end of the aliskiren monotherapy period is analyzed at the end of 3 weeks for 150 mg and 300 mg dose. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization by centre was performed by the interactive voice response system provider with the use of a validated system that automates the random assignment of patients to randomization numbers." The IVRS assigned a 2=part patient number. The first part was the centre number and the second part was one of the series of numbers allocated to the centre. |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed using the procedure to ensure that treatment assignment was unbiased and concealed from the patient and investigator staff. Data were kept strictly confidential until the time of unblinding. Novartis Pharmaceuticals Corporation was responsible for managing the database and conducting audits" |
| Blinding (performance bias and detection bias) | Low risk | Aliskiren 150 mg and 300 mg were provided as film ‐coated tablets each of a different size, shape and colour. The placebos to aliskiren 150 mg and 300 mg were matched in respective size, shape and colour to the active tablets. HCTZ 12.5 and 25 mg and placebo to HCTZ were provided as identically appearing capsules. |
| Incomplete outcome data (attrition bias) | Unclear risk | During the 6‐week double‐blind, placebo‐controlled treatment period, 67 patients (6.0%) discontinued study treatment. Discontinuations were significantly higher (P = 0.05) in the placebo group 21 (9.5%) than in the aliskiren group 20 (4.4%) but were not significantly greater than in the HTCZ group 26 (5.9%). The most common reason for discontinuation in placebo group was adverse events, withdrawal of consent and unsatisfactory therapeutic response each occurring in 2.7% patients. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was available and primary and secondary outcomes were reported at 26 weeks. Pulse rate and pulse pressure are not reported at baseline or at week 3, week 6 and study endpoint. This information may be available in the appendices that were not provided with the CSR by the EMA. Most data in the CSR are reported at 26 weeks. Data that can be used at 3 and 6 weeks for this review have been partially reported. MSDBP data with SEM are reported at week 6 in the CSR. Other MSSBP and MSDBP data have been calculated from the graph. SAE data detail at week 3 and 6 have not been reported. |
| Other bias | High risk | Study supported by Novartis. Authors received research grants, consulting and speaker fees from Novartis. |
| Methods | Randomized, double‐blind, parallel‐group, placebo‐controlled, dose‐escalation study | |
| Participants | 5133 patients were screened at 194 centres in the USA and Europe. 3980 enrolled in 3‐4 weeks single‐blind placebo run ‐in period. 1797 patients were randomized to once‐daily oral administration of treatment for 4 weeks and all patients underwent a forced titration to double the dose of their treatments, and were treated for another 4 weeks. Inclusion criteria: Men and women aged 18 years or over with stage 1‐2 hypertension (MSDBP > 95 mmHg to < 110 mmHg) and an absolute difference in MSDBP of < 10 mmHg from the prior visit, as well as a mean 8‐hour daytime ABPM DBP > 90 mmHg. Exclusion criteria: Patients who previously entered an aliskiren study; severe hypertension (MSDBP > 110 mmHg and/or MSSBP > 180 mmHg); secondary hypertension; known Keith‐Waegner grade III or IV hypertensive retinopathy; history of hypertensive encephalopathy or cerebrovascular accident, TIA; MI, coronary bypass surgery, or PCI; serum sodium less than lower limit of normal; serum potassium > 5.3 mEq/L at visit 1; history of type I or II diabetes mellitus with poor glycaemic control HbA1C > 8% at visit 1; current angina pectoris requiring pharmacological treatment; second or third degree heart block without a pacemaker; potentially life‐threatening arrhythmia; clinically significant valvular disease; any surgical or medical condition that might significantly alter absorption, distribution, metabolism or excretion of the drug; history of malignancy including leukaemia or lymphoma; history of major gastrointestinal tract surgery such as gastrectomy, gastroenterostomy, or bowel resection; history of active inflammatory bowel disease; currently active gastritis, duodenal or gastric ulcer; gastrointestinal bleeding in the past 3months; any history of pancreatitis, pancreatic injury, or evidence of impaired pancreatic function or abnormal lipase or amylase during past 12 months of visit 1; Evidence of hepatic disease, history of hepatic encephalopathy; history of oesophageal varices; history of portocaval shunt; evidence of renal impairment or dialysis or nephrotic syndrome; Current treatment with cholestyramine or colestipol resins; history of hyper sensitivity to any of the study drugs or those belonging to the same therapeutic class; history of angioedema due to usage of ARB or ACE inhibitors; history of malignancy of any organ system treated or untreated in the past 5 years; history of drug or alcohol abuse within last 12 months; pregnant or nursing women; and history of non‐compliance to medical regimens or unwillingness to comply with study protocol were excluded. Baseline characteristics: Mean age was 52 + 10.4 years with 13.9% > 65 years in placebo group and 11.7% in aliskiren group, respectively; Males 58.4 to 61.2%; Caucasian 74.6 to 76%; 15 to 16% Black; 1.5% Asian and 7 to 9% others; Mean BMI 30.2 + 5.7 kg/m2;Baseline MSSBP was 154.1 + 12.8 mmHg and MSDBP was 100.4 + 4.2 mmHg. | |
| Interventions | Placebo: N = 459; aliskiren 150 mg to 300 mg: N = 437; valsartan 160 mg to 320 mg N = 455; aliskiren 150 mg/300 mg/valsartan 160 mg/320 mg: N = 446 Duration of treatment = 8 weeks | |
| Outcomes | Primary: change in MSDBP for baseline to week‐8 endpoint | |
| Notes | Study CSPP100A2327 is published in a journal as Oparil 2007. EudraCT no. 2005‐000039‐73 It is not registered on clinicalTrials.gov It is registered on Novartis Clinical Trial Results database (results are not posted). CSR without appendices was received from EMA on June 16th 2016. Data of this study was also reported in the FDA Medical Review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation list was generated by Novartis Drug Supply Management (Basel, Switzerland) with a validated system that automated the random assignment of treatment groups to randomisation numbers." |
| Allocation concealment (selection bias) | Low risk | The randomization scheme was reviewed by a biostatistics quality assurance group at Novartis and locked by them after approval. |
| Blinding (performance bias and detection bias) | Low risk | "Placebo and drug tablets and capsules were matched in size, shape, and colour to maintain blinding." Patients were required to take two tablets and two capsules. |
| Incomplete outcome data (attrition bias) | High risk | 63 (13.7%) withdrew from placebo group and 53 (12%) in aliskiren group. Reason for withdrawal differed between groups ‐ Unsatisfactory therapeutic effect ‐ 36 (8%) in placebo and 13 (3%) in aliskiren 150 mg group. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was available and primary and secondary outcomes were reported. Pulse rate and pulse pressure are not reported at baseline or endpoint. This information may be available in the appendices that were not provided with the CSR by the EMA. |
| Other bias | High risk | Manufacturer sponsored. Oparil is the recipient of grants‐in‐aid from Abbot Laboratories, Astra Zeneca, Aventis, Bioavail, Boehringher Ingelhiem, Bristol Meyers Squibb, Forest laboratories, GlaxoSmithkline, Novartis, Merck & Co, Pfizer, Sanofi Aventis, and the Salt Institute. Other authors are employees of Novartis and therefore eligible for stock and stock option Patients could have been selected based on their response to other drugs acting on the renin‐angiotensin system. Manufacturer sponsored. The overall incidence of major protocol violation was higher in placebo group (10.9%) than in other aliskiren monotherapy groups (5.4%). |
| Methods | Multicentre, randomized, double‐blind, parallel‐group, placebo‐controlled study. | |
| Participants | 1508 patients with essential hypertension were screened from 53 centres in the USA and 48 centres in the European Union. 642 eligible patients were randomized, of which 576 (89.7%) completed the double‐blind treatment period. Inclusion criteria: Patients who previously entered an aliskiren study; patients aged ≥18 years with stage 1 or 2 essential uncomplicated hypertension. MSDBP ≥95 and <110 mmHg, with a difference of ≤10 mmHg, on their last 2 visits and mean 8‐hour daytime ambulatory DBP ≥90 mmHg. Patients fulfilling these eligibility criteria then underwent ABPM at visit 5. Only patients with mean 8‐hour daytime ambulatory DBP (ADBP) ≥90 mmHg were entered in the study. Exclusion criteria: Patients with were severe hypertension (MSDBP ≥110 mmHg and/or MSSBP ≥180 mmHg); suspected secondary or malignant hypertension; type 1 diabetes mellitus (DM); type 2 DM, poorly controlled (glycosylated haemoglobin > 8.0%) or requiring insulin; serious cardiac, hepatic, pancreatic, renal, or cerebrovascular disease; serum sodium less than lower limit of normal and/or serum potassium > 5.3 mEq/L or dehydration at visit 1; atrial fibrillation or atrial flutter; clinically significant allergy; and malignant tumours; pregnant and breast‐feeding women were excluded. Baseline characteristics: Mean age 52 + 10.7 years; 13.1% > 65 years in placebo group and 9.8%, 15.8% and 14.6% in aliskiren 75 mg, 150 mg and 300 mg groups respectively; Males 60%; Caucasian 80.8%; Black 14.2%; Asian 2.3% and others 12.6%; Mean BMI 30.4 + 5.6 kg/m2; baseline MSSBP 153.5 + 12.8 mmHg and MSDBP 100.5 + 4.1 mmHg at baseline | |
| Interventions | N = 642 patients were randomized Placebo: N = 160; aliskiren 75 mg: N =1 53; aliskiren 150 mg: N = 171; aliskiren 300 mg: N = 158 Duration of treatment = 8 weeks | |
| Outcomes | Primary: Change from baseline in MSDBP at the week‐8 endpoint Secondary: Change from baseline in MSSBP at week‐4 and week‐8 endpoint; change in MSDBP at weeks 4 and 8; proportion of responders (MSDBP < 90 mmHg and/or at least a 10 mmHg reduction from baseline); proportion of patients achieving BP control (MSSBP/MSDBP <140/90 mmHG); dose‐response relationship; safety and tolerability of aliskiren compared to placebo; impact of treatment on plasma renin activity, plasma renin concentration and plasma aldosterone | |
| Notes | Study CSPP100A2328 is published in a journal as Puig 2009. It is not registered on clinicalTrials.gov It is registered on Novartis Clinical Trial Results database (results are posted). EMA does not possess CSR for this study. CSPP100A2328 synopsis has been used to report mean change from baseline in MSSBP and MSDBP with SD and adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was generated using a validated system that automated the random assignment of treatment groups to randomization numbers. At randomization, eligible patients were assigned the lowest available number on the randomization list and were supplied with a medication pack labelled with the relevant randomization number. The randomization scheme was reviewed by a biostatistics quality assurance group at Novartis Pharma AG and locked by them after approval." |
| Allocation concealment (selection bias) | Low risk | "At randomization eligible patients were assigned the lowest available number on the randomization list and were supplied with a medication pack labelled with he relevant randomization number. Randomization codes were kept confidential until after database lock." |
| Blinding (performance bias and detection bias) | Low risk | "Blinding was maintained by providing the study drugs along with matching placebo tablets. All patients took 3 tablets daily at 8:00am, except on the day of visit, when the study drug was taken after the visit procedures were completed." |
| Incomplete outcome data (attrition bias) | Unclear risk | "Of the 642 randomized patients, 576 (89.7%) completed the double‐blind treatment period. The proportion of discontinuations was higher in the aliskiren 150 mg group 21 (12.3%) and the placebo group 19 (11.9%), and lower in the aliskiren 75 mg 12 (7.8%) and aliskiren 300 mg 14 (8.9%) groups." |
| Selective reporting (reporting bias) | Unclear risk | Could not be assessed as protocol is not available. SAE data detail not available. |
| Other bias | High risk | Study funded by manufacturer (Novartis). Medical writers from Novartis drafted manuscript. It is not registered on clinicalTrials.gov. EMA does not possess CSR for this study |
| Methods | Randomized, double‐blind, parallel‐group, placebo‐controlled study. Randomization was stratified by region and age group (≥ 65 to < 75 years and ≥ 75 years). | |
| Participants | 836 patients were enrolled at 95 centres in 8 countries (Argentina, Czech Republic, Germany, Iceland, Italy, the Netherlands, Poland and Slovakia). 754 (90.2%) completed the single‐blind, run‐in period. However, two patients were mistakenly randomized, creating a randomized set with two additional patients (N = 756). Though these two patients did not meet study criteria, they were included in the placebo group for the randomized set, but excluded from the full analysis set and safety set (N = 754). Inclusion criteria: Men and women in the outpatient setting aged ≥ 65 years with essential hypertension (defined as MSSBP ≥150 mmHg and < 180 mmHg and MSDBP <110 mmHg) and in addition, patients’ MSSBP had to differ by ≤15 mmHg between the last two visits of the placebo run‐in period for inclusion in the study. Exclusion criteria: Patients with severe hypertension; those with secondary hypertension; cardiac dysfunction; diabetes; malignancy including leukaemia and lymphoma within the past five years; surgical or medical conditions which might significantly alter the absorption, distribution, metabolism, or excretion of study medication; known or suspected contraindications to aliskiren including history of allergy to ACE‐inhibitors or ARBS; previous exposure to aliskiren within 3 months of visit 1 of the study; previous participation in an investigation clinical study within 1 month of visit 1 of the study; participants with laboratory serum potassium values ≥ 5.5 mEq/L; with an estimated GFR < 45 mL/min/1.73m2; Women who were pregnant, lactating or women of childbearing potential were also excluded. Baseline characteristics: Mean age was 72 + 6 with 30.6 to 32.3% patients > 75 years old; 43.2 to 47.1% patients were male; 98.4 to 100% were Caucasians; 1.1% Black; 0.5 to 1.6% were others; Mean BMI 29 + 5.0 kg/m2; 16 to 21% patients had diabetes; MSSBP at baseline was 160 + 8 mmHg and MSDBP was 90 + 8 mmHg. | |
| Interventions | Aliskiren 75 mg ( N = 192), 150 mg (N = 189), or 300 mg (N = 186) and placebo (N = 189) once daily Duration of treatment = 8 weeks. | |
| Outcomes | Primary: Change in MSSBP from baseline to week‐8 endpoint. Secondary: Change in MSDBP from baseline to week‐8 endpoint; changes in MSSBP and MSDBP at week 4 and 8; mean 24‐hour ambulatory systolic and diastolic blood pressures; the proportion of patients achieving BP control (MSSBP/DBP < 140 mmHg/90 mmHg) at the week‐8 endpoint; serious adverse events and total adverse events | |
| Notes | Study CSPP100A2405 is published in a journal as Villa 2012. It is registered on clinicalTrials.gov. Identifier: NCT00706134. It is registered on Novartis Clinical Trial Results database (results are posted). CSR without appendices was received from EMA on December 22nd 2015. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An Interactive Voice Response System (IVRS) was used to randomly assign patients to study medication. The medication randomization list was produced by Novartis Drug Supply Management that automates the random assignment of medication numbers to medication packs containing each of the study drugs. Randomization was stratified by region and by age group > 65 years and < 75 years and > 75 years of age. The treatment groups were generally well‐matched for baseline and demographic characteristics. |
| Allocation concealment (selection bias) | Low risk | The IVRS automated the random assignment of patient numbers to randomization numbers, which are linked to the different treatment arms. The treatment assignment is unbiased and concealed from patients and investigator staff. |
| Blinding (performance bias and detection bias) | Low risk | Placebo tablets were matched to the active study drug, and a double‐dummy design was used to ensure study blinding. Patients, investigator staff, persons performing assessments and data analysts will remain blind to the identity of the treatment from time of randomization until database lock. |
| Incomplete outcome data (attrition bias) | Unclear risk | 754 completed the placebo run‐in period and were randomized to study treatment. Overall, 700 patients completed double‐blind treatment, with higher completion rates in the aliskiren 150‐ and 300 mg groups than in the aliskiren 75 mg or placebo groups. 18 (9.5%) discontinued in placebo group; 19 (9.9%) in aliskiren 75 mg; 6 (3.2%) in 150 mg group and 11 (5.9%) in 300 mg group. Reason for withdrawal differed ‐ withdrawal due to lack of therapeutic effect was 4.8% in the placebo group compared to 1.6% in aliskiren 75 mg and 150 mg group. Last‐observation‐carried‐ forward approach was used for the week‐8 endpoint analyses. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was available and primary and secondary outcomes were reported. Pulse rate and pulse pressure were not reported at baseline or endpoint. This information may be available in the appendices that were not provided with the CSR by the EMA.. |
| Other bias | High risk | Study supported by Novartis. Most authors were employees of Novartis and therefore eligible for Novartis stock and stock options. |
ABPM: ambulatory blood pressure monitoring; ACE: angiotensin‐converting enzyme; ARB: angiotensin receptor blockers; BMI: body mass index; CRF: case report form; CSR: clinical study report; DBP: diastolic blood pressure; EMA: European Medicines Agency; GFR: glomerular filtration rate; HTCZ: hydrochlorothiazide; IVRS: Interactive Voice; Response System; MI: myocardial infarction; MSDBP: mean sitting diastolic blood pressure; MSSBP: mean sitting systolic blood pressure; PCI: percutaneous coronary intervention; PRA: plasma renin activity; SAE: serious adverse event; SBP: systolic blood pressure; TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| No placebo monotherapy arm. | |
| A randomized, four‐way, double‐blind, single‐centre, cross‐over study was performed in 31 adult white patients. Screening was followed by a 40‐week study period in which patients received each of four once‐daily monotherapies sequentially in a random order: aliskiren 300 mg, moxonidine 0.4 mg, HCTZ 25 mg and matching placebo. Each treatment period began with a 2‐week titration phase during which halved doses were used. Thereafter, patients were force titrated to full medication doses. The efficacy and tolerability of each treatment was assessed after 8 weeks, followed by a 1‐week tapering period and a 1‐week washout period. Therefore, each treatment cycle lasted 10 weeks in total. Patients subsequently crossed over to the next treatment period. The parallel group phase of aliskiren 300 mg monotherapy for 8 weeks fixed dose compared to placebo meets the inclusion criteria but data have not been provided for office systolic and diastolic blood pressure measurements during the first phase of treatment when patients were randomized to aliskiren monotherapy and placebo. | |
| A randomized double blind cross‐over study meets the inclusion criteria but is available as abstract only with no details of methodology to assess risk of bias. Also the study does not provide data on SBP or DBP. | |
| No aliskiren monotherapy arm. | |
| No placebo monotherapy arm. | |
| No aliskiren monotherapy arm. | |
| Although this study is a double‐blind randomized placebo‐controlled study it included patients untreated for hypertension as well as those patients on antihypertensive medication other than aliskiren and whose systolic BP (SBP) was 115 mmHg to 159 mmHg and diastolic BP (DBP) was 60 mmHg to 99 mmHg at screening. Therefore all patients were not on aliskiren monotherapy. | |
| No aliskiren monotherapy arm. | |
| Aliskiren in combination with losartan compared to losartan on the regression of left ventricular hypertrophy in overweight patients with essential hypertension (ALLAY). There is no parallel placebo group. | |
| Comparison of aliskiren and amlodipine on insulin resistance and endothelial dysfunction in patients with hypertension and metabolic syndrome. There is no parallel placebo group. | |
| This study compared the efficacy and safety of once daily dosing of aliskiren (300 mg (qd) once a day) to twice‐daily dosing of aliskiren (150 mg (twice a day) in treating moderate hypertension. There is no parallel placebo group. | |
| This study evaluated the efficacy and safety of combination aliskiren/amlodipine in patients not adequately responding to aliskiren alone. There is no parallel placebo group. | |
| Efficacy and safety of aliskiren in patients with mild‐to‐moderate hypertension during exercise. No details of the study are provided. | |
| This study compared efficacy and safety of aliskiren 300 mg compared to telmisartan 80 mg after 1 week of treatment withdrawal (ASSERTIVE). There is no parallel placebo group. | |
| This study compared aliskiren and valsartan vs valsartan alone in patients with stage II systolic hypertension and type II diabetes mellitus. There is no parallel placebo group. | |
| Efficacy of aliskiren compared to ramipril in the treatment of moderate systolic hypertensive patients (ALIAS). No other detail provided. | |
| Treatment of Adiposity Related hypErTension (TARGET). No details of the study are provided. | |
| This is a randomized, double‐blind, active‐controlled, parallel study to analyze effects of the combination of aliskiren and valsartan on the vascular structure and function of retinal vessels. It compares aliskiren 150 mg for 1 week then 300 mg/day for 7 weeks to placebo once per day. Results have not been reported. | |
| No aliskiren monotherapy arm. | |
| Dose selection/different formulation of drug than one marketed used in this study. | |
| Open‐label combination therapy. | |
| It is a double‐blind randomized cross‐over study. Patients were randomized to placebo, aliskiren 300 mg or irbasartan 300 mg or combination therapy. However all patients also received furosemide in a stable dose throughout the study so it was excluded as there is no aliskiren monotherapy arm. | |
| Combination therapy study. | |
| No aliskiren monotherapy arm. | |
| No aliskiren monotherapy arm. | |
| Also identified as NCT00414609. No aliskiren or placebo monotherapy arm as these drugs were added to standard therapy in patients with acute myocardial infarction. | |
| Dose selection study with no placebo control. | |
| No placebo control. | |
| No aliskiren monotherapy arm. Patients were given additional antihypertensives HCTZ 25 mg or amlodipine 5 mg or respective placebos | |
| No placebo control. | |
| No placebo control. |
DBP: diastolic blood pressure;HTCZ: hydrochlorothiazide; SBP: systolic blood pressure
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 12 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 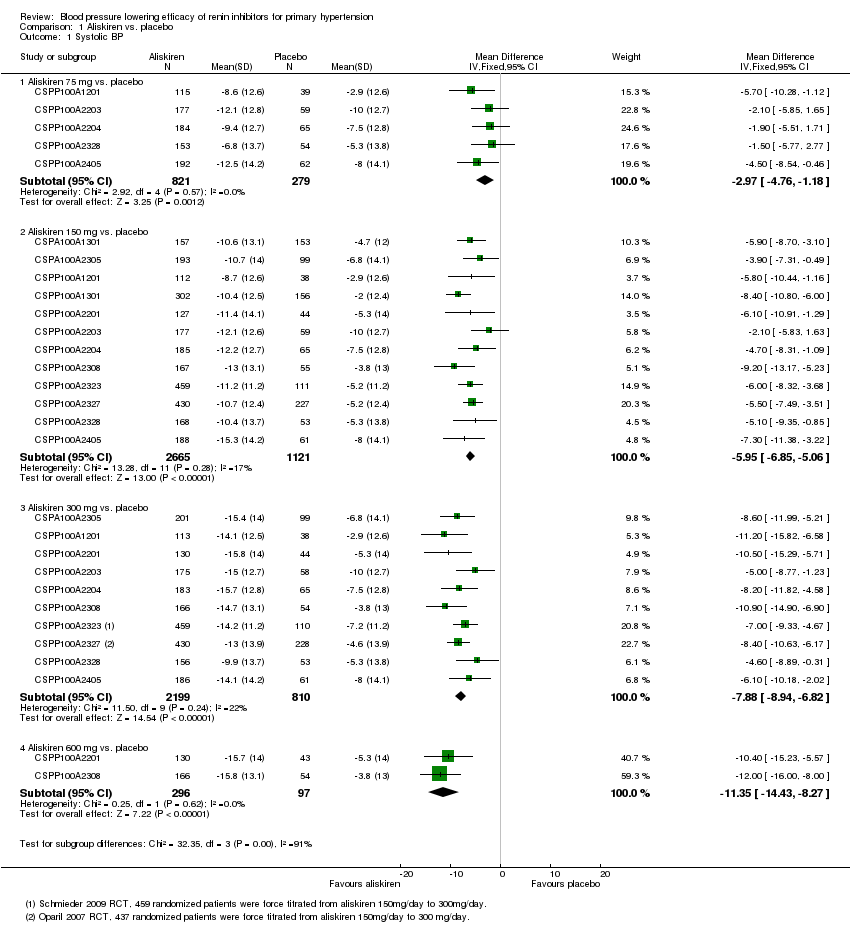 Comparison 1 Aliskiren vs. placebo, Outcome 1 Systolic BP. | ||||
| 1.1 Aliskiren 75 mg vs. placebo | 5 | 1100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.97 [‐4.76, ‐1.18] |
| 1.2 Aliskiren 150 mg vs. placebo | 12 | 3786 | Mean Difference (IV, Fixed, 95% CI) | ‐5.95 [‐6.85, ‐5.06] |
| 1.3 Aliskiren 300 mg vs. placebo | 10 | 3009 | Mean Difference (IV, Fixed, 95% CI) | ‐7.88 [‐8.94, ‐6.82] |
| 1.4 Aliskiren 600 mg vs. placebo | 2 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐11.35 [‐14.43, ‐8.27] |
| 2 Diastolic BP Show forest plot | 12 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Aliskiren vs. placebo, Outcome 2 Diastolic BP. | ||||
| 2.1 Aliskiren 75 mg vs placebo | 5 | 1100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐3.13, ‐0.96] |
| 2.2 Aliskiren 150 mg vs placebo | 12 | 3783 | Mean Difference (IV, Fixed, 95% CI) | ‐3.16 [‐3.74, ‐2.58] |
| 2.3 Aliskiren 300 mg vs placebo | 10 | 3001 | Mean Difference (IV, Fixed, 95% CI) | ‐4.49 [‐5.17, ‐3.82] |
| 2.4 Aliskiren 600 mg vs placebo | 2 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐5.86 [‐7.73, ‐3.99] |
| 3 Withdrawals due to adverse event Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 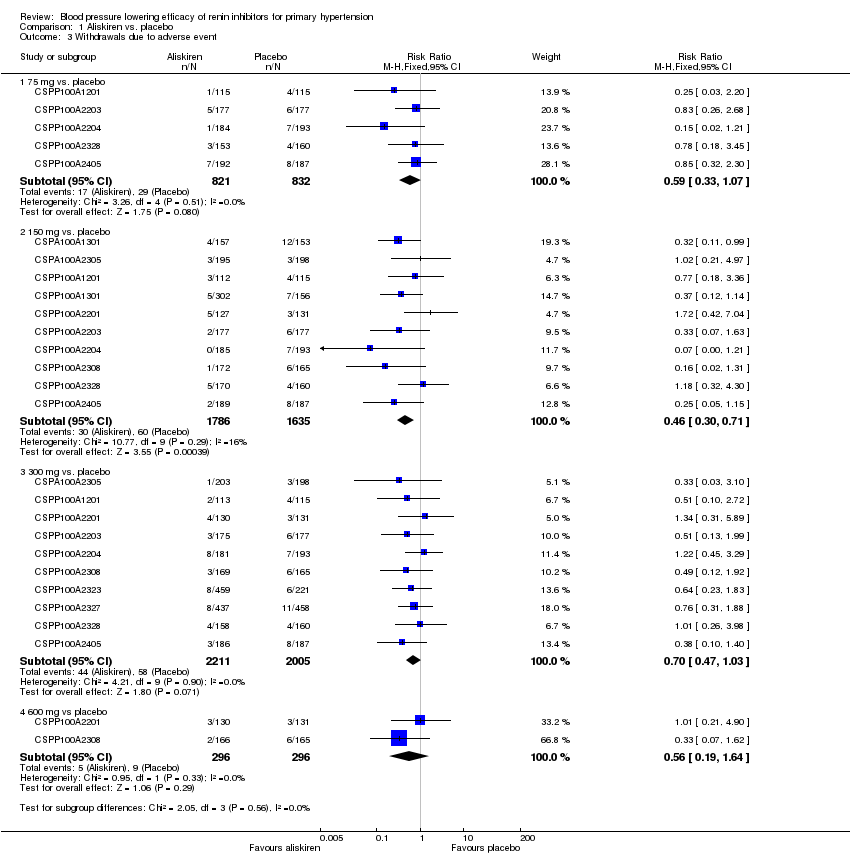 Comparison 1 Aliskiren vs. placebo, Outcome 3 Withdrawals due to adverse event. | ||||
| 3.1 75 mg vs. placebo | 5 | 1653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.33, 1.07] |
| 3.2 150 mg vs. placebo | 10 | 3421 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.30, 0.71] |
| 3.3 300 mg vs. placebo | 10 | 4216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.47, 1.03] |
| 3.4 600 mg vs placebo | 2 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.64] |
| 4 Cough Show forest plot | 5 | 2886 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.49, 2.64] |
| Analysis 1.4  Comparison 1 Aliskiren vs. placebo, Outcome 4 Cough. | ||||
| 5 Diarrhoea Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5 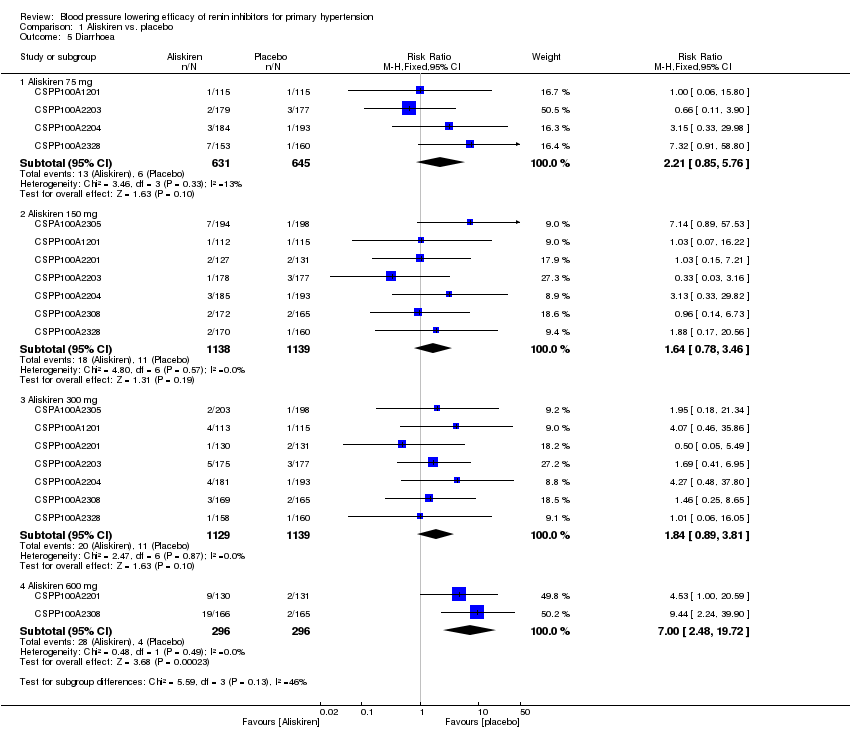 Comparison 1 Aliskiren vs. placebo, Outcome 5 Diarrhoea. | ||||
| 5.1 Aliskiren 75 mg | 4 | 1276 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.85, 5.76] |
| 5.2 Aliskiren 150 mg | 7 | 2277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.78, 3.46] |
| 5.3 Aliskiren 300 mg | 7 | 2268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.89, 3.81] |
| 5.4 Aliskiren 600 mg | 2 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.00 [2.48, 19.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 5 | 1651 | Mean Difference (IV, Fixed, 95% CI) | ‐1.89 [‐3.16, ‐0.62] |
| Analysis 2.1  Comparison 2 Aliskiren150 mg vs. Aliskiren 75 mg, Outcome 1 Systolic BP. | ||||
| 2 Diastolic BP Show forest plot | 5 | 1651 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.58, ‐0.03] |
| Analysis 2.2  Comparison 2 Aliskiren150 mg vs. Aliskiren 75 mg, Outcome 2 Diastolic BP. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 3 | 904 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐6.79, ‐3.40] |
| Analysis 3.1  Comparison 3 Aliskiren 300 mg Vs. Aliskiren 75 mg, Outcome 1 SBP. | ||||
| 2 DBP Show forest plot | 3 | 904 | Mean Difference (IV, Fixed, 95% CI) | ‐2.49 [‐3.53, ‐1.45] |
| Analysis 3.2  Comparison 3 Aliskiren 300 mg Vs. Aliskiren 75 mg, Outcome 2 DBP. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 10 | 4405 | Mean Difference (IV, Fixed, 95% CI) | ‐2.62 [‐3.38, ‐1.87] |
| Analysis 4.1  Comparison 4 Aliskiren 300 mg vs. Aliskiren 150 mg, Outcome 1 Systolic BP. | ||||
| 2 Diastolic BP Show forest plot | 10 | 4405 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.28, ‐1.32] |
| Analysis 4.2  Comparison 4 Aliskiren 300 mg vs. Aliskiren 150 mg, Outcome 2 Diastolic BP. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 2 | 590 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.58, ‐1.23] |
| Analysis 5.1  Comparison 5 Aliskiren 600 mg vs. Aliskiren 150 mg, Outcome 1 SBP. | ||||
| 2 DBP Show forest plot | 2 | 590 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐3.55, ‐0.85] |
| Analysis 5.2  Comparison 5 Aliskiren 600 mg vs. Aliskiren 150 mg, Outcome 2 DBP. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 2 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐2.78, 1.56] |
| Analysis 6.1  Comparison 6 Aliskiren 600 mg vs. Aliskiren 300 mg, Outcome 1 Systolic BP. | ||||
| 2 Diastolic BP Show forest plot | 2 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐2.03, 0.67] |
| Analysis 6.2  Comparison 6 Aliskiren 600 mg vs. Aliskiren 300 mg, Outcome 2 Diastolic BP. | ||||

PRISMA Study flow diagram.

Methodological quality summary: review authors' judgments about each methodological quality item for each included study.

Forest plot of comparison: 1 Aliskiren vs. placebo, outcome: 1.1 Systolic BP.
Highly significant subgroup differences were observed therefore mean overall effect size across all doses is not shown. The number of placebo group patients are divided equally in dose ranging studies when more than one dose of aliskiren was used.
CSPP100A2308 study the SBP reduction in the treatment and placebo group are reported from the CSR page 61.
CSPP100A2405 study the SD for all treatment groups are calculated from SEM reported on page 7 in the CSR .
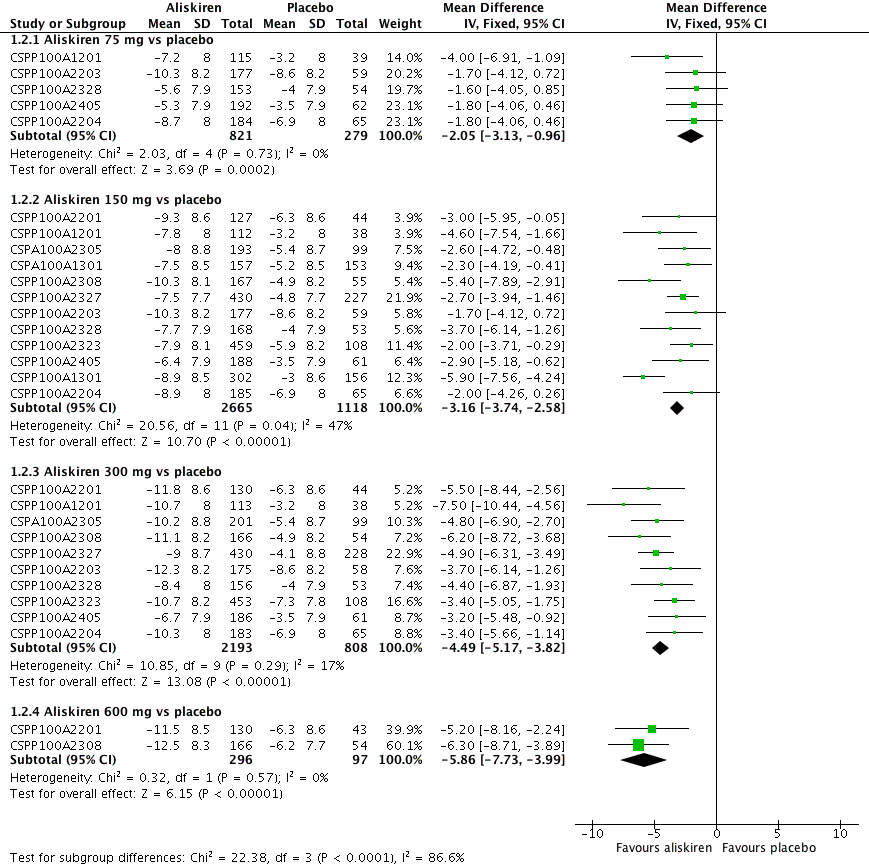
Forest plot of comparison: 1 Aliskiren vs. placebo, outcome: 1.2 Diastolic BP.
Highly significant subgroup differences were observed, therefore mean overall effect size across all doses is not shown. The number of placebo group patients are divided equally in dose ranging studys when more than one dose of aliskiren was used.
CSPP100A2308 study the DBP reduction in the treatment and placebo group are reported from the CSR which differ from those reported in the published article. The previous version of this review used data from the published article.
CSPP100A2405 study the SD for all treatment groups are calculated from SEM reported from the CSR on page 8.

Comparison 1 Aliskiren vs. placebo, Outcome 1 Systolic BP.

Comparison 1 Aliskiren vs. placebo, Outcome 2 Diastolic BP.

Comparison 1 Aliskiren vs. placebo, Outcome 3 Withdrawals due to adverse event.

Comparison 1 Aliskiren vs. placebo, Outcome 4 Cough.

Comparison 1 Aliskiren vs. placebo, Outcome 5 Diarrhoea.

Comparison 2 Aliskiren150 mg vs. Aliskiren 75 mg, Outcome 1 Systolic BP.

Comparison 2 Aliskiren150 mg vs. Aliskiren 75 mg, Outcome 2 Diastolic BP.

Comparison 3 Aliskiren 300 mg Vs. Aliskiren 75 mg, Outcome 1 SBP.

Comparison 3 Aliskiren 300 mg Vs. Aliskiren 75 mg, Outcome 2 DBP.

Comparison 4 Aliskiren 300 mg vs. Aliskiren 150 mg, Outcome 1 Systolic BP.

Comparison 4 Aliskiren 300 mg vs. Aliskiren 150 mg, Outcome 2 Diastolic BP.

Comparison 5 Aliskiren 600 mg vs. Aliskiren 150 mg, Outcome 1 SBP.

Comparison 5 Aliskiren 600 mg vs. Aliskiren 150 mg, Outcome 2 DBP.

Comparison 6 Aliskiren 600 mg vs. Aliskiren 300 mg, Outcome 1 Systolic BP.

Comparison 6 Aliskiren 600 mg vs. Aliskiren 300 mg, Outcome 2 Diastolic BP.
| Aliskiren compared to placebo for primary hypertension | ||||||
| Patient or population: primary hypertension Setting: Outpatient Intervention: Aliskiren Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI)mmHg | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Effect with placebo1 | Effect with Aliskiren2 | |||||
| Systolic BP ‐ Aliskiren 75 mg vs. placebo | 2.9 lower to 10.0 lower | 2.97 lower (4.76 lower to 1.18 lower) | ‐ | 1100 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 150 mg vs. placebo | 2.0 lower to 10.0 lower | 5.95 lower (6.85 lower to 5.06 lower) | ‐ | 3786 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 300 mg vs. placebo | 2.9 lower to 10.0 lower | 7.88 lower (8.94 lower to 6.82 lower) | ‐ | 3009 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 600 mg vs. placebo | 3.8 lower to 5.3 lower | 11.35 lower (14.43 lower to 8.27 lower) | ‐ | 393 | ⊕⊕⊝⊝ | |
| Diastolic BP ‐ Aliskiren 75 mg vs placebo | 3.2 lower to 8.6 lower | 2.05 lower (3.13 lower to 0.96 lower) | ‐ | 1100 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 150 mg vs placebo | 3.0 lower to 8.6 lower | 3.16 lower (3.74 lower to 2.58 lower) | ‐ | 3783 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 300 mg vs placebo | 3.2 lower to 8.6 lower | 4.49 lower (5.17 lower to 3.82 lower) | ‐ | 3001 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 600 mg vs placebo | 6.2 lower to 6.3 lower | 5.86 lower (7.73 lower to 3.99 lower) | ‐ | 393 | ⊕⊕⊝⊝ | |
| Diarrhoea ‐ Aliskiren 600 mg vs placebo | 14 per 1,000 | 95 per 1,000 | RR 7.00 | 592 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Range of mean decrease in SBP and DBP mmHg as compared to baseline in the individual trials in the placebo group. 2. Effects in Aliskiren group represent the blood pressure lowering effect in excess of that with placebo. 3. Downgraded by 1 level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 5 included studies). Also study CSPP100A2204 had high likelihood of selection bias. 4. Downgraded by one level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 12 included studies). 5. Downgraded by one level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 10 included studies). 6. Downgraded by 1 level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in both included studies). 7. Downgraded by 1 more level due to wide confidence interval. | ||||||
| Dose | Female | Male | ||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐1.3 | ‐1.1 | ‐3.6 | ‐2.9 |
| 150 mg | ‐5.5 | ‐2.9 | ‐5.9 | ‐3.5 |
| 300 mg | ‐9.4 | ‐4.8 | ‐9.0 | ‐5.4 |
| 600 mg | ‐12.6 | ‐6.4 | ‐10.7 | ‐6.5 |
| 75 mg | ‐1.3 | ‐1.1 | ‐3.6 | ‐2.9 |
| Dose | Age < 65 years | Age > 65 years | ||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐2.7 | ‐1.9 | ‐3.6 | ‐3.4 |
| 150 mg | ‐5.5 | ‐2.9 | ‐6.9 | ‐4.8 |
| 300 mg | ‐9.7 | ‐5.2 | ‐7.1 | ‐4.4 |
| 600 mg | ‐11.5 | ‐6.6 | ‐11.6 | ‐6.4 |
| 75 mg | ‐2.7 | ‐1.9 | ‐3.6 | ‐3.4 |
| Dose | White | Black | Asian | |||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐2.1 | ‐1.7 | ‐2.8 | 0.2 | ‐8.8 | ‐3.2 |
| 150 mg | ‐6.5 | ‐3.5 | ‐5.5 | ‐1.4 | ‐3.7 | ‐2.9 |
| 300 mg | ‐9.6 | ‐4.8 | ‐6.1 | ‐2.6 | ‐12.7 | ‐6.3 |
| 600 mg | ‐12.3 | ‐6.7 | ‐8.7 | ‐3.5 | ‐11.2 | ‐9.4 |
| Dose of Aliskiren mg/day | Previous version without access to information from CSR | Present version with access to information from CSR | ||
| MSSBP mmHg MD with 95% CI | MSDBP mmHg MD with 95% CI | MSSBP mmHg MD with 95% CI | MSDBP mmHg MD with 95% CI | |
| Aliskiren 75 mg vs. Placebo | ‐2.64 (‐4.06 to ‐1.23) | ‐2.07 (‐2.94 to ‐1.20) | ‐2.97 (‐4.76 to ‐1.18) | ‐2.05 (‐3.13 to ‐0.96) |
| Aliskiren 150 mg vs. Placebo | ‐5.55 (‐6.39 to ‐4.71) | ‐2.91 (‐3.46 to ‐2.37) | ‐5.95 (‐6.85 to ‐5.06) | ‐3.16 (‐3.74 to ‐2.58) |
| Aliskiren 300 mg vs. Placebo | ‐7.93 (‐8.77 to ‐7.08) | ‐4.76 (‐5.33 to ‐4.19) | ‐7.88 (‐8.94 to ‐6.82) | ‐4.49 (‐5.17 to ‐3.82) |
| Aliskiren 600 mg vs. Placebo | ‐11.36 (‐13.53 to ‐9.19) | ‐6.57 (‐7.92 to ‐5.23) | ‐11.35 (‐14.43 to ‐8.27) | ‐5.86 (‐7.73 to ‐3.99) |
| MSSBP: mean sitting systolic blood pressure; MSDBP: mean sitting diastolic blood pressure; | ||||
| Novartis Clinical Trial Results Database identifier used for study identification in this review | Journal‐Published Author /year | Registered on Novartis Clinical Trial Results Database | Registered on ClinicalTrials.gov | CSR* received from EMA | Request to EMA was made on November 15th 2015. Weeks waited to obtain CSR |
| No journal publication | Results NOT posted | NCT01237223 Results posted | EMA does NOT possess | ‐ | |
| Results posted | NCT00739973 Results posted | EMA does NOT possess | ‐ | ||
| Results NOT posted | NOT registered | Mar 11, 2016 | 16 | ||
| No journal publication | Results posted | NCT00344110 Results NOT posted | May 23, 2016 | 27 | |
| Results NOT posted | NOT registered | Jan 25, 2016 | 10 | ||
| NOT FOUND | NOT registered | Apr 12, 2016 | 21 | ||
| Results NOT posted | NCT00219024 Results posted | Apr 6, 2016 | 20 | ||
| Results NOT posted | NCT00219128 Results NOT posted | Feb 20, 2016 | 14 | ||
| Results posted | NCT00219154 Results NOT posted | May 27, 2016 | 27 | ||
| Results NOT posted | NOT registered | June 16, 2016 | 30 | ||
| Results posted | NOT registered | EMA does NOT possess | ‐ | ||
| Results posted | NCT00706134 Results posted | December 22, 2015 | 5 |
| Dose comparison | SBP WMD with 95% CI | DBP WMD with 95% CI | Comment |
| Aliskiren 150 mg vs 75 mg | ‐1.89 (‐3.16 to ‐0.62) | ‐0.80 (‐1.58 to ‐0.03) | Aliskiren 150 mg reduces SBP and DBP more than 75 mg by 2/1 mmHg |
| Aliskiren 300 mg vs 75 mg | ‐5.10 (‐6.79 to ‐3.40) | ‐2.49 (‐3.53 to ‐1.45) | Aliskiren 300 mg reduces SBP and DBP more than 75 mg 5/3 mmHg |
| Aliskiren 300 mg vs 150 mg | ‐2.62 (‐3.38 to ‐1.87) | ‐1.80 (‐2.28 to ‐1.32) | Aliskiren 300 mg reduces SBP and DBP more than 150 mg 3/2 mmHg |
| Aliskiren 600 mg vs 150 mg | ‐3.40 (‐5.58 to ‐1.23) | ‐2.20 (‐3.55 to ‐0.85) | Aliskiren 600 mg reduces SBP and DBP more than 150 mg by 3/2mmHg |
| Aliskiren 600 mg vs 300 mg | ‐0.61 (‐2.78 to 1.56) | ‐0.68 (‐2.03 to 0.67) | No significant difference was observed |
| Study identifier | Single‐blind period | Double‐blind period | Withdrawal period |
| No deaths | No deaths | No deaths | |
| No deaths | No deaths | No deaths | |
| One death in placebo group during observation period due to pancreatic carcinoma and metastases to liver. | One death occurred in aliskiren 150 mg monotherapy on day 41 of the study, due to drug intoxication. The overdose was attributed to a psychiatric drug prescribed prior to start of the study, and a diagnosis of manic depressive psychosis was recorded. | No deaths | |
| No deaths | No deaths | No deaths | |
| No deaths | No deaths | No deaths | |
| No deaths | One death occurred in the placebo arm on day 16 from "natural causes". One death occurred in the valsartan 160 mg arm (day 26) as a result of motor vehicle accident. | No deaths | |
| No deaths | One death was reported in the Aliskiren/HCTZ 150/25 mg group due to thoracic trauma from a motor vehicle accident. | No deaths | |
| No deaths | No deaths | No deaths | |
| 1 death occurred in placebo run‐in phase. Patient hospitalized due to supraventricular tachycardia and abdominal pain and died due to stroke after discontinuing from the study. | No deaths | One death occurred in the aliskiren/HCTZ 150/300 mg group from acute bronchopneumonia and associated sepsis during the 30‐day follow‐up period after discontinuing the study. | |
| No deaths | 2 deaths reported. 1 death in aliskiren group on day 41 due to myocardial infarction. 1 death in valsartan group on day 13 and the cause was reported as hypertensive arteriosclerotic cardiovascular disease with SAE of sudden death. There were no deaths in placebo arm or combination therapy group. | No deaths | |
| No deaths | No deaths | No deaths | |
| One death occurred during the follow‐up period of the study from pneumonia leading to respiratory failure (day of death not provided). Further information regarding this death is described in the patient narratives of the CSR, which we do not have access to yet. Within the CSR body, the arm of the study that this participant belongs to has been redacted. In the 2008 FDA Medical Review report, a death due to colon cancer is reported as occurring within CSPP100A2204 (N = 1, aliskiren 300 mg). The medical review states "no other details were provided". For our comments on this please see Discussion. | |||
| CSR: clinical study report; HTCZ: hydrochlorothiazide; SAE: serious adverse event | |||
| Study identifier | Wash out period | Single blind period | Double blind period | Withdrawal period |
| Not reported | Not reported | No SAEs were reported in the aliskiren monotherapy group. 2 in amlodipine 2.5 mg; 1 in amlodipine 5 mg; and 1 in aliskiren 150/amlodipine 5 mg combination arm (detail regarding SAE was not reported) | Not reported | |
| Not reported | Not reported | 9 SAEs reported. 2 in placebo and 7 in other treatment groups (retinal detachment, abdominal mass, bronchitis, calculus ureteric, gastroenteritis, hand fracture, hydronephrosis, pneumonia, and cerebrovascular accident). None occurred in aliskiren 150 mg or 300 mg groups. | Not reported | |
| Not reported | Reported 1 SAE in the single‐blind period ‐ infectious enterocolitis/dehydration (N = 1, 55M, placebo). | 2 SAEs in the double‐blind period: cerebral infarction (N = 1, 69F, placebo); acute myocardial infarction (N = 1, 58F, aliskiren 75 mg). | Not reported | |
| Not reported | Not reported | 2 SAEs reported. 1 SAE in placebo due to myocardial infarction. The other in the losartan 50 mg group due to right medullary infarction. None in aliskiren 150 mg group. | Not reported | |
| One in washout period ‐ transient Ischaemic attack | Two in the single‐blind run‐in phase ‐ anxiety; and diverticulitis and | 2 SAEs in irbesartan group ‐ Intravertebral disc protrusion; and bipolar depression. | Two in the withdrawal period in placebo group ‐ Left ventricular failure; and gout and infective arthritis). | |
| Not reported | Reported 6 SAE. 1 bile duct cancer, 1 diverticulitis, 1 pregnancy, 1 mild melanorrhagia and gastric pain, 1 myocardial infarction, and 1 squamous cell carcinoma. | 8 SAEs reported. 2 in Placebo: 1 due to natural cause and 1 due to myocardial infarction requiring hospitalization on day 46 resulting in study drug discontinuation. 1 in aliskiren 75 mg on day 4 coronary artery disease requiring hospitalization resulting in study discontinuation. Patient required quadruple bypass surgery. 1 in aliskiren 300 mg on day 42 pregnancy resulting in study discontinuation. 1 in valsartan 160 mg on day 28 ‐ angioneurotic oedema resulting in study discontinuation, dyspnoea and chest pressure. Aliskiren 150 mg/valsartan 160 mg (N = 1, Day 29) motor vehicle accident requiring hospitalization; not discontinued from study. | 1 in aliskiren 75 mg/valsartan 80 mg on day 3 post‐study. | |
| Not reported | 4 SAE due to (lung cancer, fractured leg, angina and urinary tract infection) | 28 patients experienced at least 1 SAE in the monotherapy and combination therapy treatment groups. 1 in A 75 mg ‐ due to renal colic; 1 in aliskiren 150 mg due to haemorrhagic diarrhoea; none in aliskiren 300 mg; 1 in HCTZ 6.25 mg due to neoplasm of skin; 3 in HCTZ 12.5 mg ‐ due to breast cancer, joint injury and pregnancy; 2 in HCTZ 25 mg due to deep vein thrombosis and lymphadenopathy; 5 in aliskiren 75 mg/HCTZ 12.5 mg due to (diplopia, IIIrd nerve paresis, mood disorder, phlebothrombosis, psychotic disorder, small intestinal obstruction and syncope); 4 in aliskiren 75 mg/HCTZ 25 mg due to (cerebral infarction, dysarthria, physical disability, pregnancy and renal colic); 2 in aliskiren 150 mg/ HCTZ 6.25 mg ulcerative colitis, pregnancy); 3 in aliskiren 150 mg/ HCTZ 12.5 mg (non‐cardiac chest pain, pregnancy, syncope); 2 in aliskiren 150 mg/ HCTZ 25 mg (lung neoplasm, road traffic accident); 2 in aliskiren 300 mg/ HCTZ 12.5 mg (diabetes mellitus, lung disorder); 1 in aliskiren 300 mg/HCTZ 25 mg (coronary artery disease). There were no SAEs in the placebo group. | Not reported | |
| 2 SAEs reported during washout period ‐ 1 due to subarachnoid haemorrhage due to rupture of cerebral aneurysm on day 8; 1 due to partial small bowel obstruction on day 13 | One SAE ‐ bladder carcinoma prior to randomization and before receiving double‐blind study drug. Patient was randomized to placebo but later discontinued due to unsatisfactory therapeutic effect; | 4 SAEs reported. 1 SAE in aliskiren 150 mg on day 27‐ unstable angina and increased blood pressure; 2 SAEs in aliskiren 300 mg ‐ hospitalization for acute appendicitis on day 34; hospitalization for depression on day 51; 1 SAE in aliskiren 600 mg of hospitalization for pain/bodily injury on day 35. No SAE reported in the placebo group. | One SAE was reported during withdrawal period in aliskiren 150 mg of serious venous occlusion and thrombosis of the right eye on day 8 withdrawal period). | |
| data at week 3 and week 6 not available | Not reported | Not reported | During 26 weeks double‐blind treatment period 19 SAEs reported. 10 in aliskiren group and 9 in HCTZ group. | Not reported |
| not reported | Not reported | 20 SAEs reported. 5 in placebo group ‐ atrial flutter, cerebrovascular accident, headache, hypertension, hypertensive crisis and ventricular tachycardia); 8 in aliskiren group ‐ 1 gastritis, 1 grand mal convulsions, 1 intestinal poly, 2 myocardial infarction, 1 non‐cardiac chest pain, 1 peripheral vascular disease, 1 acute renal failure); 6 in valsartan group ‐ 1 angina pectoris, 1 arteriosclerosis, 1 breast cancer, 1 bronchitis, 1 COPD, 1 facial paresis, 1 ovarian cancer, 1 pneumonia, 1 pulmonary oedema); 3 in aliskiren/valsartan group ‐ 1 aortic aneurysm, 1 intravertebral disc protrusion, 1 prostate cancer and 1 thyroidectomy). The FDA medical review (2007) also reported two cases of renal carcinoma occurred (N = 1, placebo, day 20 post‐study; N = 1, aliskiren, day 44 post‐study). For our comments on this, please see Discussion. | Not reported | |
| 11 patients had serious AEs during the washout and placebo run‐in periods (detail of which are not provided). | Not reported | 1 SAE was reported in aliskiren 75 mg group ‐ cardiac chest pain; 2 in aliskiren 150 mg group ‐ rectal bleeding due to anal ulcer and episode of secondary anaemia; basal cell carcinoma; 1 in the 300 mg group ‐TIA with concomitant nausea and dyspnoea; and 2 in the placebo group ‐ prostate cancer; accelerated hypertension with left facial numbness. | Not reported | |
| Reported 2 SAEs in the washout period; Detail not provided in CSR. | Three in the single‐blind placebo run‐in period. Detail not provided in CSR. | 6 SAEs were reported. 2 in aliskiren 75 mg group ‐ erysipelas (skin infection) and osteoarthritis; 3 in aliskiren 150 mg group ‐ severe glaucoma; moderate haemorrhoids; and mild hemorrhagic stroke; none in aliskiren 300 mg group; and 1 in the placebo group ‐ vertigo, wrist fracture, concussion, and head contusion subsequent to a fall. | Not reported | |
| COPD: chronic obstructive pulmonary disease; CSR: clinical study report; HTCZ: hydrochlorothiazide; SAE: serious adverse event; TIA: transient ischaemic attack | ||||
| Study Identifier | Total AEs and Common AEs as reported in the corresponding available CSR A = Aliskiren; P = Placebo |
| Total AEs: A150 =15(7.8%); A 300 = 18(8.9%) and P = 22(7.7%) Headache: A150 =13( 6.7%); A 300 =15(7.4%) and P = 20(10.1%) Periphaeral edema: A150 = 2(1.0%); A 300 = 3(1.5%) and P = 2(1%) | |
| Total AEs: A150 = 40.1%; A 300 = 46.7%; A 600 = 52.4%; P = 43% Headache: A150 = 12(7%); A300 = 13(7.7%); A600 = 9(5.4%); P = 16(9.7%) Nasopharyngitis: A 150 = 5(2.9%); A 300 = 6(3.6%); A 600 = 3(1.8%); P = 10(6.1%) Dizziness: A 150 = 2(1.2%); A 300 = 9(5.3%); A 600 = 5(3%); P = 7(4.2%) Diarrhoea:A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 19 (11.4%); P = 2(1.2%) Nausea: A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 0%; P = 4(2.4%) | |
| Total AEs: A75 = 61(53%); A150 = 58(51.8%); A 300 = 62(54.9%); P = 58(50.4)% Headache: A75 = 3(2.6%); A 150 = 3(2.7%); A 300 = 6 (5.3%) and P = 4(3.5%) Nasopharyngitis:A75 = 24(20.9%); A150 = 20(17.9%); A300 = 20(17.7%); P = 16(13.9%) Back pain:A75 = 2(1.7%); A150 = 0%; A300 = 0% and P = 0% Diarrhoea: A75 = 1(0.9%); A150 = 1(0.9%); A300 = 4(3.5%) and P = 1(0.9%) Vertigo: A75 =0%; A150 = 0%); A300 = 1(0.9%) and P = 2(1.7%) | |
| Total AEs: A150 = 152(50.3%), P = 66(42.3%) Nasopharyngitis: A150 = 48(15.9%), P = 13(8.3%) Headache: A150 = 9(3.0%), P = 6(3.8%) Occult blood positive: A150 = 9 (3.0%), P =5 (3.2%) | |
| Total AEs: A150 = 127 (26.8%); A300 = 130 (36.2%); A600 = 43 (33.1%); P = 32 (32.1%) Headache: A150 = 3 (2.4%); A300 = 8 (6.2%); A600 = 6 (4.6%); P = 7 (5.3%) Diarrhoea: A150 = 2 (1.6%); A300 = 1 (0.8%); A600 = 9 (6.9%); P = 2 (1.5%) Dizziness: A150 = 2 (1.6%); A300 = 4 (3.1%); A600 = 3 (2.3%); P = 5 (3.8%) Fatigue: A150 = 1 (0.8%); A300 = 5 (3.8%); A600 = 2 (1.5%); P = 4 (3.1%) | |
| Total AEs: A75 = 63(35.2%); A150 = 59(33.1%); A300 = 50(28.6%) and P = 57(32.2%) Headache: A75 = 15(8.4%); A150 = 9(5.1%;) A 300 = 7(4.0%) and P = 15(8.5%) Fatigue: A75 = 7(3.9%); A150 = 4(2.2%;) A 300 = 4(2.3%) and P = 4(2.3%) Back pain: A75 = 2(1.1%); A150 = 4(202%;) A 300 = 3(1.7%) and P = 2(1.1%) Diarrhoea: A75 = 2(1.1%); A150 = 1(0.6%;) A 300 = 5(2.9%) and P = 3(1.7%) Dizziness: A75 = 4(2.2%); A150 = 4(2.2%;) A 300 = 3(1.7%) and P = 2(1.1%) | |
| Total AEs: A75 = 69(37.5%); A150 = 69(37.3%); A300 = 71 (39.2%); P = 85(44%) Headache: A75 = 13(7.1%); A 150 = 13(7%); A 300 = 10 (5.5%); P = 26 (13.5%) Nasopharyngitis: A75 = 9( 4.9%); A150 = 5(2.7%;) A300 = 3 (1.7%); P = 10 (5.2%) Diarrhoea: A75 = 3(1.6%); A150 = 3(1.6%); A300 = 4(2.2%); P = 1(0.5%) Cough: A75 = 1(0.5%); A150 = 2(1.1%); A300 = 1(0.6%); P = 1(0.5%) Dizziness: A75 = 1(0.5%); A150 = 1(0.5%); A300 = 3(1.7%); P = 2(1.0%) Peripheral edema: A75 = 4(2.2%); A150 = 3(1.6%); A300 = 2(1.1%); P = 1(0.5%) | |
| Total AEs: A150 = 40.1%; A 300 = 46.7%; A 600 = 52.4%; P = 43% Headache: A150 = 12(7%); A300 = 13(7.7%); A600 = 9(5.4%); P = 16(9.7%) Nasopharyngitis: A 150 = 5(2.9%); A 300 = 6(3.6%); A 600 = 3(1.8%); P = 10(6.1%) Dizziness: A 150 = 2(1.2%); A 300 = 9(5.3%); A 600 = 5(3%); P = 7(4.2%) Diarrhoea: A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 19 (11.4%); P = 2(1.2%) Nausea: A 150 =2 (1.2%); A 300 = 3(1.8%); A 600 =0%; P = 4(2.4%) | |
| Total AEs at 6 weeks : A 26.4%; P = 28.5% (numbers not reported) Other AE were not reported at week 6. | |
| Total AEs: A150 to 300 = 149(34%) and P = 168(37%) Headache: A150 to 300 = 14(3.2%); P = 41(9%) Diarrhoea*: A150 to 300 = 2.3%; P = not reported Nasopharyngitis: A150 to 300 = 16(3.7%) ; P = 9(2%) Dizziness: A 150 to 300 = 8(1.8%) ; P = 9(2%) Fatigue:A 150 to 300 = 4(0.9%) ; P = 5(1.1%) Nausea: A 150 to 300 = 6(1.4%) ; P = 11(2.4%) | |
| Total AEs: A75 = 57(37.3%); A150 = 59(34.7%); A300 = 56(35.4%); P = 60(37.5%) Headache: A75 = 12(7.8%); A150 = 7(4.1%); A300 = 10(6.3%) and P = 9(5.6%) Nasopharyngitis: A75 = 2(1.3%); A150 = 2(1.2%); A300 = 5(3.2%) and P = 3(1.9%) Diarrhoea: A75 = 7(4.6%); A150 = 2(1.2%); A300 = 1(0.6%) and P = 1(0.6%) Cough: A75 = 1(0.7%); A150 = 4(2.4%); A300 = 1(0.6%) and P = 3(1.9%) Dizziness: A75 = 1(0.7%); A150 = 5(2.9%); A300 = 1(0.6%) and P = 3(1.9%) | |
| Total AEs: A75 = 36(18.8%); A150 = 41(21.7%); A300 = 36(19.1%); P = 39(21.0%) Headache: A75 = 4(2.1%);A150 = 2(1.1%); A300 = 1(1.1%); P = 4(2.1%) Influenza: A75 = 1(0.5%); A150 = 4(2.1%); A300 = 1(1.1%); P = 1(0.5%) |
| Study Identifier | Reasons for increased withdrawal due to adverse events |
| Not reported. | |
| Not reported. | |
| Significant adverse events occurred for N = 7 patients, leading to discontinuation from study, as described below.
| |
|
| |
| A total of N = 18 patients discontinued from the study due to AEs. It was suspected that 2/3 of these discontinuations were for reasons drug‐related. Headaches resulted in the discontinuation for N = 3 patients. | |
| Most frequent reasons for 12 discontinuations are: fatigue (N = 5, 0.4%), headache (N = 3, 0.3%), diarrhoea (N = 2, 0.2%), and peripheral oedema (N = 2, 0.2%). Reasons for 14 discontinuations from AE (safety population).
The FDA medical review reports facial oedema (N = 1, 0.6%) that appears to be angioedema, resulting in discontinuation from study; however, this is not reported in CSR 2203.
| |
| The range of AEs resulting in study discontinuation is 0% is the aliskiren 150 mg arm of the study to 4.4% in the aliskiren 300 mg arm (N = 7). The most frequent AE resulting in study discontinuation is headache (N = 12, 0.4%). Reasons for discontinuations from adverse events ‐ N = 62 (2.2%).
Additionally four patients discontinued from the study due to abnormal laboratory values.
| |
| Most frequent reasons for 9 discontinuation from study: headache (N = 4), dizziness (N = 3), and diarrhoea (N = 2). Reasons for discontinuations from AE (safety population).
We question that a participant withdrew for the reason specified as "unsatisfactory effect" as opposed to being categorized as a discontinuation from an SAE as the event necessitated hospitalization. On Day 27 in CSPP100A2308, the participant (N = 1, 64F, aliskiren 150 mg) was hospitalized for unstable angina and increased blood pressure. The unstable angina was treated with concomitant medication and her condition resolved within 3 days. | |
| We do not have data at week 3 and week 6 for this study. | |
| The range of AEs resulting in study discontinuation is 8 (1.8%) is the aliskiren arm; 8 (2.6%) in valsartan arm; 6 (1.3%) in aliskiren/valsartan combination arm; and 11 (2.4%) in the placebo arm. Reasons for discontinuations from adverse events ‐ N = 37 (2%).
| |
| Aliskiren 75 mg = 3 (2.0%); aliskiren 150 mg = 5 (2.9%); aliskiren 300 mg = 4(2.5%) . Placebo = 4 (2.5%). Reasons for withdrawal were not reported. | |
| Reasons for 18 discontinuations from AE (safety population)
|
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 12 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Aliskiren 75 mg vs. placebo | 5 | 1100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.97 [‐4.76, ‐1.18] |
| 1.2 Aliskiren 150 mg vs. placebo | 12 | 3786 | Mean Difference (IV, Fixed, 95% CI) | ‐5.95 [‐6.85, ‐5.06] |
| 1.3 Aliskiren 300 mg vs. placebo | 10 | 3009 | Mean Difference (IV, Fixed, 95% CI) | ‐7.88 [‐8.94, ‐6.82] |
| 1.4 Aliskiren 600 mg vs. placebo | 2 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐11.35 [‐14.43, ‐8.27] |
| 2 Diastolic BP Show forest plot | 12 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Aliskiren 75 mg vs placebo | 5 | 1100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐3.13, ‐0.96] |
| 2.2 Aliskiren 150 mg vs placebo | 12 | 3783 | Mean Difference (IV, Fixed, 95% CI) | ‐3.16 [‐3.74, ‐2.58] |
| 2.3 Aliskiren 300 mg vs placebo | 10 | 3001 | Mean Difference (IV, Fixed, 95% CI) | ‐4.49 [‐5.17, ‐3.82] |
| 2.4 Aliskiren 600 mg vs placebo | 2 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐5.86 [‐7.73, ‐3.99] |
| 3 Withdrawals due to adverse event Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 75 mg vs. placebo | 5 | 1653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.33, 1.07] |
| 3.2 150 mg vs. placebo | 10 | 3421 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.30, 0.71] |
| 3.3 300 mg vs. placebo | 10 | 4216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.47, 1.03] |
| 3.4 600 mg vs placebo | 2 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.64] |
| 4 Cough Show forest plot | 5 | 2886 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.49, 2.64] |
| 5 Diarrhoea Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Aliskiren 75 mg | 4 | 1276 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.85, 5.76] |
| 5.2 Aliskiren 150 mg | 7 | 2277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.78, 3.46] |
| 5.3 Aliskiren 300 mg | 7 | 2268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.89, 3.81] |
| 5.4 Aliskiren 600 mg | 2 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.00 [2.48, 19.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 5 | 1651 | Mean Difference (IV, Fixed, 95% CI) | ‐1.89 [‐3.16, ‐0.62] |
| 2 Diastolic BP Show forest plot | 5 | 1651 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.58, ‐0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 3 | 904 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐6.79, ‐3.40] |
| 2 DBP Show forest plot | 3 | 904 | Mean Difference (IV, Fixed, 95% CI) | ‐2.49 [‐3.53, ‐1.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 10 | 4405 | Mean Difference (IV, Fixed, 95% CI) | ‐2.62 [‐3.38, ‐1.87] |
| 2 Diastolic BP Show forest plot | 10 | 4405 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.28, ‐1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 2 | 590 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.58, ‐1.23] |
| 2 DBP Show forest plot | 2 | 590 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐3.55, ‐0.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 2 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐2.78, 1.56] |
| 2 Diastolic BP Show forest plot | 2 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐2.03, 0.67] |

