Eficacia hipotensora de los inhibidores de la renina para la hipertensión primaria
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007066.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 abril 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Hipertensión
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
James Wright and Vijaya Musini formulated the idea for the review and developed the basis for the protocol.
Vijaya Musini and Patricia Fortin took the lead roles in searching, identifying, and assessing studies, in data abstraction and analyses, and in writing the review for the original version of this review in 2009 and for the 2011 update.
Vijaya Musini attended to 2017 update by performing a screen to updated search findings, data extraction from clinical study reports, analysis, carrying out a 'Risk of bias' assessment and preparing a 'Summary of findings' table, and editing/writing the review.
Kendra Lawrence attended to the 2017 update of this review by performing a screen to updated search findings, requesting and obtaining CSRs from EMA, data extraction from clinical study reports, analysis, carrying out a 'Risk of bias' assessment, and editing/writing the review.
Patricia Fortin contributed to screening, data extraction from published journal articles, and editing of the review for the 2017 update.
James Wright and Ken Bassett aided in confirming accuracy of data, settling discrepancies in inclusion criteria or data abstraction, and in editing the final version of the review.
Sources of support
Internal sources
-
Department of Anesthesiology, Pharmacology & Therapeutics, University of British Columbia, Canada.
External sources
-
Canadian Institues of Health Research, Canada.
Declarations of interest
None known.
Acknowledgements
The authors would like to acknowledge the contribution of Doug Salzwedel, the information specialist of the Hypertension Review Group in updating the search strategy and providing search findings from various databases; Stephen Adams in retrieving innumerable articles; Aaushi Rawat in helping with screening the search results; for identifying studies that meet the inclusion criteria; and doing data abstraction for the two additional studies identified in 2011. We would also like to thank Ciprian Jauca who assisted in editing this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Apr 05 | Blood pressure lowering efficacy of renin inhibitors for primary hypertension | Review | Vijaya M Musini, Kendra AK Lawrence, Patricia M Fortin, Ken Bassett, James M Wright | |
| 2008 Oct 08 | Blood pressure lowering efficacy of renin inhibitors for primary hypertension | Review | Vijaya M Musini, Patricia M Fortin, Ken Bassett, James M Wright | |
| 2008 Apr 23 | Blood pressure lowering efficacy of renin inhibitors for primary hypertension | Protocol | Vijaya M Musini, Patricia M Fortin, Ken Bassett, J M and Wright, James M Wright | |
Differences between protocol and review
The previous version of this review included information from only published journal articles of included studies. However, in the 2017 update we were able to obtain nine clinical study reports (CSRs) of included studies and therefore it was possible to report change in mean sitting systolic blood pressure (MSSBP) and mean sitting diastolic blood pressure (MSDBP) with standard deviation of change from 11 studies, and required to impute missing standard deviation data from only one included study. In all cases where there was a difference between the CSR and published report, data from the CSR was used. Therefore, magnitude of blood pressure reduction and standard deviation of change data may vary from previous versions of the review.
This update includes additional information regarding mortality, non‐fatal serious adverse events, total adverse events and any other common specific adverse events (headache, nasopharyngitis, and diarrhoea). We had already included cough and angioedema as secondary outcomes in the published protocol.
We were also able to reassess the risk of bias of each individual study as detailed information was available in the CSR obtained from EMA and therefore judgement may vary from the previous version of this review. The quality of evidence has been downgraded from high quality in the 2011 update to moderate quality for BP data and to low quality for adverse event data in this 2017 update.
We also graded overall evidence using GRADEpro software and included a 'Summary of findings' table.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Amides [administration & dosage, adverse effects, *therapeutic use];
- Antihypertensive Agents [administration & dosage, adverse effects, *therapeutic use];
- Blood Pressure [*drug effects];
- Diarrhea [chemically induced];
- Fumarates [administration & dosage, adverse effects, *therapeutic use];
- Randomized Controlled Trials as Topic;
- Renin [*antagonists & inhibitors];
Medical Subject Headings Check Words
Humans; Middle Aged;
PICO

PRISMA Study flow diagram.

Methodological quality summary: review authors' judgments about each methodological quality item for each included study.

Forest plot of comparison: 1 Aliskiren vs. placebo, outcome: 1.1 Systolic BP.
Highly significant subgroup differences were observed therefore mean overall effect size across all doses is not shown. The number of placebo group patients are divided equally in dose ranging studies when more than one dose of aliskiren was used.
CSPP100A2308 study the SBP reduction in the treatment and placebo group are reported from the CSR page 61.
CSPP100A2405 study the SD for all treatment groups are calculated from SEM reported on page 7 in the CSR .
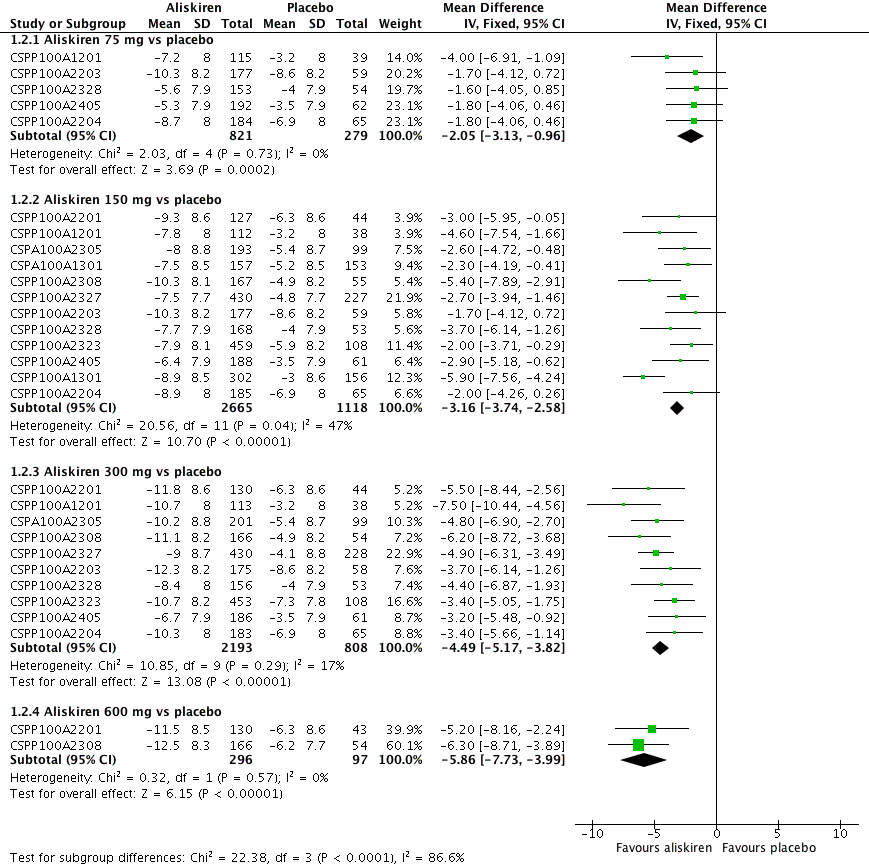
Forest plot of comparison: 1 Aliskiren vs. placebo, outcome: 1.2 Diastolic BP.
Highly significant subgroup differences were observed, therefore mean overall effect size across all doses is not shown. The number of placebo group patients are divided equally in dose ranging studys when more than one dose of aliskiren was used.
CSPP100A2308 study the DBP reduction in the treatment and placebo group are reported from the CSR which differ from those reported in the published article. The previous version of this review used data from the published article.
CSPP100A2405 study the SD for all treatment groups are calculated from SEM reported from the CSR on page 8.

Comparison 1 Aliskiren vs. placebo, Outcome 1 Systolic BP.

Comparison 1 Aliskiren vs. placebo, Outcome 2 Diastolic BP.

Comparison 1 Aliskiren vs. placebo, Outcome 3 Withdrawals due to adverse event.

Comparison 1 Aliskiren vs. placebo, Outcome 4 Cough.

Comparison 1 Aliskiren vs. placebo, Outcome 5 Diarrhoea.

Comparison 2 Aliskiren150 mg vs. Aliskiren 75 mg, Outcome 1 Systolic BP.

Comparison 2 Aliskiren150 mg vs. Aliskiren 75 mg, Outcome 2 Diastolic BP.

Comparison 3 Aliskiren 300 mg Vs. Aliskiren 75 mg, Outcome 1 SBP.

Comparison 3 Aliskiren 300 mg Vs. Aliskiren 75 mg, Outcome 2 DBP.

Comparison 4 Aliskiren 300 mg vs. Aliskiren 150 mg, Outcome 1 Systolic BP.

Comparison 4 Aliskiren 300 mg vs. Aliskiren 150 mg, Outcome 2 Diastolic BP.

Comparison 5 Aliskiren 600 mg vs. Aliskiren 150 mg, Outcome 1 SBP.

Comparison 5 Aliskiren 600 mg vs. Aliskiren 150 mg, Outcome 2 DBP.

Comparison 6 Aliskiren 600 mg vs. Aliskiren 300 mg, Outcome 1 Systolic BP.

Comparison 6 Aliskiren 600 mg vs. Aliskiren 300 mg, Outcome 2 Diastolic BP.
| Aliskiren compared to placebo for primary hypertension | ||||||
| Patient or population: primary hypertension Setting: Outpatient Intervention: Aliskiren Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI)mmHg | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Effect with placebo1 | Effect with Aliskiren2 | |||||
| Systolic BP ‐ Aliskiren 75 mg vs. placebo | 2.9 lower to 10.0 lower | 2.97 lower (4.76 lower to 1.18 lower) | ‐ | 1100 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 150 mg vs. placebo | 2.0 lower to 10.0 lower | 5.95 lower (6.85 lower to 5.06 lower) | ‐ | 3786 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 300 mg vs. placebo | 2.9 lower to 10.0 lower | 7.88 lower (8.94 lower to 6.82 lower) | ‐ | 3009 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 600 mg vs. placebo | 3.8 lower to 5.3 lower | 11.35 lower (14.43 lower to 8.27 lower) | ‐ | 393 | ⊕⊕⊝⊝ | |
| Diastolic BP ‐ Aliskiren 75 mg vs placebo | 3.2 lower to 8.6 lower | 2.05 lower (3.13 lower to 0.96 lower) | ‐ | 1100 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 150 mg vs placebo | 3.0 lower to 8.6 lower | 3.16 lower (3.74 lower to 2.58 lower) | ‐ | 3783 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 300 mg vs placebo | 3.2 lower to 8.6 lower | 4.49 lower (5.17 lower to 3.82 lower) | ‐ | 3001 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 600 mg vs placebo | 6.2 lower to 6.3 lower | 5.86 lower (7.73 lower to 3.99 lower) | ‐ | 393 | ⊕⊕⊝⊝ | |
| Diarrhoea ‐ Aliskiren 600 mg vs placebo | 14 per 1,000 | 95 per 1,000 | RR 7.00 | 592 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Range of mean decrease in SBP and DBP mmHg as compared to baseline in the individual trials in the placebo group. 2. Effects in Aliskiren group represent the blood pressure lowering effect in excess of that with placebo. 3. Downgraded by 1 level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 5 included studies). Also study CSPP100A2204 had high likelihood of selection bias. 4. Downgraded by one level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 12 included studies). 5. Downgraded by one level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 10 included studies). 6. Downgraded by 1 level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in both included studies). 7. Downgraded by 1 more level due to wide confidence interval. | ||||||
| Dose | Female | Male | ||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐1.3 | ‐1.1 | ‐3.6 | ‐2.9 |
| 150 mg | ‐5.5 | ‐2.9 | ‐5.9 | ‐3.5 |
| 300 mg | ‐9.4 | ‐4.8 | ‐9.0 | ‐5.4 |
| 600 mg | ‐12.6 | ‐6.4 | ‐10.7 | ‐6.5 |
| 75 mg | ‐1.3 | ‐1.1 | ‐3.6 | ‐2.9 |
| Dose | Age < 65 years | Age > 65 years | ||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐2.7 | ‐1.9 | ‐3.6 | ‐3.4 |
| 150 mg | ‐5.5 | ‐2.9 | ‐6.9 | ‐4.8 |
| 300 mg | ‐9.7 | ‐5.2 | ‐7.1 | ‐4.4 |
| 600 mg | ‐11.5 | ‐6.6 | ‐11.6 | ‐6.4 |
| 75 mg | ‐2.7 | ‐1.9 | ‐3.6 | ‐3.4 |
| Dose | White | Black | Asian | |||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐2.1 | ‐1.7 | ‐2.8 | 0.2 | ‐8.8 | ‐3.2 |
| 150 mg | ‐6.5 | ‐3.5 | ‐5.5 | ‐1.4 | ‐3.7 | ‐2.9 |
| 300 mg | ‐9.6 | ‐4.8 | ‐6.1 | ‐2.6 | ‐12.7 | ‐6.3 |
| 600 mg | ‐12.3 | ‐6.7 | ‐8.7 | ‐3.5 | ‐11.2 | ‐9.4 |
| Dose of Aliskiren mg/day | Previous version without access to information from CSR | Present version with access to information from CSR | ||
| MSSBP mmHg MD with 95% CI | MSDBP mmHg MD with 95% CI | MSSBP mmHg MD with 95% CI | MSDBP mmHg MD with 95% CI | |
| Aliskiren 75 mg vs. Placebo | ‐2.64 (‐4.06 to ‐1.23) | ‐2.07 (‐2.94 to ‐1.20) | ‐2.97 (‐4.76 to ‐1.18) | ‐2.05 (‐3.13 to ‐0.96) |
| Aliskiren 150 mg vs. Placebo | ‐5.55 (‐6.39 to ‐4.71) | ‐2.91 (‐3.46 to ‐2.37) | ‐5.95 (‐6.85 to ‐5.06) | ‐3.16 (‐3.74 to ‐2.58) |
| Aliskiren 300 mg vs. Placebo | ‐7.93 (‐8.77 to ‐7.08) | ‐4.76 (‐5.33 to ‐4.19) | ‐7.88 (‐8.94 to ‐6.82) | ‐4.49 (‐5.17 to ‐3.82) |
| Aliskiren 600 mg vs. Placebo | ‐11.36 (‐13.53 to ‐9.19) | ‐6.57 (‐7.92 to ‐5.23) | ‐11.35 (‐14.43 to ‐8.27) | ‐5.86 (‐7.73 to ‐3.99) |
| MSSBP: mean sitting systolic blood pressure; MSDBP: mean sitting diastolic blood pressure; | ||||
| Novartis Clinical Trial Results Database identifier used for study identification in this review | Journal‐Published Author /year | Registered on Novartis Clinical Trial Results Database | Registered on ClinicalTrials.gov | CSR* received from EMA | Request to EMA was made on November 15th 2015. Weeks waited to obtain CSR |
| No journal publication | Results NOT posted | NCT01237223 Results posted | EMA does NOT possess | ‐ | |
| Results posted | NCT00739973 Results posted | EMA does NOT possess | ‐ | ||
| Results NOT posted | NOT registered | Mar 11, 2016 | 16 | ||
| No journal publication | Results posted | NCT00344110 Results NOT posted | May 23, 2016 | 27 | |
| Results NOT posted | NOT registered | Jan 25, 2016 | 10 | ||
| NOT FOUND | NOT registered | Apr 12, 2016 | 21 | ||
| Results NOT posted | NCT00219024 Results posted | Apr 6, 2016 | 20 | ||
| Results NOT posted | NCT00219128 Results NOT posted | Feb 20, 2016 | 14 | ||
| Results posted | NCT00219154 Results NOT posted | May 27, 2016 | 27 | ||
| Results NOT posted | NOT registered | June 16, 2016 | 30 | ||
| Results posted | NOT registered | EMA does NOT possess | ‐ | ||
| Results posted | NCT00706134 Results posted | December 22, 2015 | 5 |
| Dose comparison | SBP WMD with 95% CI | DBP WMD with 95% CI | Comment |
| Aliskiren 150 mg vs 75 mg | ‐1.89 (‐3.16 to ‐0.62) | ‐0.80 (‐1.58 to ‐0.03) | Aliskiren 150 mg reduces SBP and DBP more than 75 mg by 2/1 mmHg |
| Aliskiren 300 mg vs 75 mg | ‐5.10 (‐6.79 to ‐3.40) | ‐2.49 (‐3.53 to ‐1.45) | Aliskiren 300 mg reduces SBP and DBP more than 75 mg 5/3 mmHg |
| Aliskiren 300 mg vs 150 mg | ‐2.62 (‐3.38 to ‐1.87) | ‐1.80 (‐2.28 to ‐1.32) | Aliskiren 300 mg reduces SBP and DBP more than 150 mg 3/2 mmHg |
| Aliskiren 600 mg vs 150 mg | ‐3.40 (‐5.58 to ‐1.23) | ‐2.20 (‐3.55 to ‐0.85) | Aliskiren 600 mg reduces SBP and DBP more than 150 mg by 3/2mmHg |
| Aliskiren 600 mg vs 300 mg | ‐0.61 (‐2.78 to 1.56) | ‐0.68 (‐2.03 to 0.67) | No significant difference was observed |
| Study identifier | Single‐blind period | Double‐blind period | Withdrawal period |
| No deaths | No deaths | No deaths | |
| No deaths | No deaths | No deaths | |
| One death in placebo group during observation period due to pancreatic carcinoma and metastases to liver. | One death occurred in aliskiren 150 mg monotherapy on day 41 of the study, due to drug intoxication. The overdose was attributed to a psychiatric drug prescribed prior to start of the study, and a diagnosis of manic depressive psychosis was recorded. | No deaths | |
| No deaths | No deaths | No deaths | |
| No deaths | No deaths | No deaths | |
| No deaths | One death occurred in the placebo arm on day 16 from "natural causes". One death occurred in the valsartan 160 mg arm (day 26) as a result of motor vehicle accident. | No deaths | |
| No deaths | One death was reported in the Aliskiren/HCTZ 150/25 mg group due to thoracic trauma from a motor vehicle accident. | No deaths | |
| No deaths | No deaths | No deaths | |
| 1 death occurred in placebo run‐in phase. Patient hospitalized due to supraventricular tachycardia and abdominal pain and died due to stroke after discontinuing from the study. | No deaths | One death occurred in the aliskiren/HCTZ 150/300 mg group from acute bronchopneumonia and associated sepsis during the 30‐day follow‐up period after discontinuing the study. | |
| No deaths | 2 deaths reported. 1 death in aliskiren group on day 41 due to myocardial infarction. 1 death in valsartan group on day 13 and the cause was reported as hypertensive arteriosclerotic cardiovascular disease with SAE of sudden death. There were no deaths in placebo arm or combination therapy group. | No deaths | |
| No deaths | No deaths | No deaths | |
| One death occurred during the follow‐up period of the study from pneumonia leading to respiratory failure (day of death not provided). Further information regarding this death is described in the patient narratives of the CSR, which we do not have access to yet. Within the CSR body, the arm of the study that this participant belongs to has been redacted. In the 2008 FDA Medical Review report, a death due to colon cancer is reported as occurring within CSPP100A2204 (N = 1, aliskiren 300 mg). The medical review states "no other details were provided". For our comments on this please see Discussion. | |||
| CSR: clinical study report; HTCZ: hydrochlorothiazide; SAE: serious adverse event | |||
| Study identifier | Wash out period | Single blind period | Double blind period | Withdrawal period |
| Not reported | Not reported | No SAEs were reported in the aliskiren monotherapy group. 2 in amlodipine 2.5 mg; 1 in amlodipine 5 mg; and 1 in aliskiren 150/amlodipine 5 mg combination arm (detail regarding SAE was not reported) | Not reported | |
| Not reported | Not reported | 9 SAEs reported. 2 in placebo and 7 in other treatment groups (retinal detachment, abdominal mass, bronchitis, calculus ureteric, gastroenteritis, hand fracture, hydronephrosis, pneumonia, and cerebrovascular accident). None occurred in aliskiren 150 mg or 300 mg groups. | Not reported | |
| Not reported | Reported 1 SAE in the single‐blind period ‐ infectious enterocolitis/dehydration (N = 1, 55M, placebo). | 2 SAEs in the double‐blind period: cerebral infarction (N = 1, 69F, placebo); acute myocardial infarction (N = 1, 58F, aliskiren 75 mg). | Not reported | |
| Not reported | Not reported | 2 SAEs reported. 1 SAE in placebo due to myocardial infarction. The other in the losartan 50 mg group due to right medullary infarction. None in aliskiren 150 mg group. | Not reported | |
| One in washout period ‐ transient Ischaemic attack | Two in the single‐blind run‐in phase ‐ anxiety; and diverticulitis and | 2 SAEs in irbesartan group ‐ Intravertebral disc protrusion; and bipolar depression. | Two in the withdrawal period in placebo group ‐ Left ventricular failure; and gout and infective arthritis). | |
| Not reported | Reported 6 SAE. 1 bile duct cancer, 1 diverticulitis, 1 pregnancy, 1 mild melanorrhagia and gastric pain, 1 myocardial infarction, and 1 squamous cell carcinoma. | 8 SAEs reported. 2 in Placebo: 1 due to natural cause and 1 due to myocardial infarction requiring hospitalization on day 46 resulting in study drug discontinuation. 1 in aliskiren 75 mg on day 4 coronary artery disease requiring hospitalization resulting in study discontinuation. Patient required quadruple bypass surgery. 1 in aliskiren 300 mg on day 42 pregnancy resulting in study discontinuation. 1 in valsartan 160 mg on day 28 ‐ angioneurotic oedema resulting in study discontinuation, dyspnoea and chest pressure. Aliskiren 150 mg/valsartan 160 mg (N = 1, Day 29) motor vehicle accident requiring hospitalization; not discontinued from study. | 1 in aliskiren 75 mg/valsartan 80 mg on day 3 post‐study. | |
| Not reported | 4 SAE due to (lung cancer, fractured leg, angina and urinary tract infection) | 28 patients experienced at least 1 SAE in the monotherapy and combination therapy treatment groups. 1 in A 75 mg ‐ due to renal colic; 1 in aliskiren 150 mg due to haemorrhagic diarrhoea; none in aliskiren 300 mg; 1 in HCTZ 6.25 mg due to neoplasm of skin; 3 in HCTZ 12.5 mg ‐ due to breast cancer, joint injury and pregnancy; 2 in HCTZ 25 mg due to deep vein thrombosis and lymphadenopathy; 5 in aliskiren 75 mg/HCTZ 12.5 mg due to (diplopia, IIIrd nerve paresis, mood disorder, phlebothrombosis, psychotic disorder, small intestinal obstruction and syncope); 4 in aliskiren 75 mg/HCTZ 25 mg due to (cerebral infarction, dysarthria, physical disability, pregnancy and renal colic); 2 in aliskiren 150 mg/ HCTZ 6.25 mg ulcerative colitis, pregnancy); 3 in aliskiren 150 mg/ HCTZ 12.5 mg (non‐cardiac chest pain, pregnancy, syncope); 2 in aliskiren 150 mg/ HCTZ 25 mg (lung neoplasm, road traffic accident); 2 in aliskiren 300 mg/ HCTZ 12.5 mg (diabetes mellitus, lung disorder); 1 in aliskiren 300 mg/HCTZ 25 mg (coronary artery disease). There were no SAEs in the placebo group. | Not reported | |
| 2 SAEs reported during washout period ‐ 1 due to subarachnoid haemorrhage due to rupture of cerebral aneurysm on day 8; 1 due to partial small bowel obstruction on day 13 | One SAE ‐ bladder carcinoma prior to randomization and before receiving double‐blind study drug. Patient was randomized to placebo but later discontinued due to unsatisfactory therapeutic effect; | 4 SAEs reported. 1 SAE in aliskiren 150 mg on day 27‐ unstable angina and increased blood pressure; 2 SAEs in aliskiren 300 mg ‐ hospitalization for acute appendicitis on day 34; hospitalization for depression on day 51; 1 SAE in aliskiren 600 mg of hospitalization for pain/bodily injury on day 35. No SAE reported in the placebo group. | One SAE was reported during withdrawal period in aliskiren 150 mg of serious venous occlusion and thrombosis of the right eye on day 8 withdrawal period). | |
| data at week 3 and week 6 not available | Not reported | Not reported | During 26 weeks double‐blind treatment period 19 SAEs reported. 10 in aliskiren group and 9 in HCTZ group. | Not reported |
| not reported | Not reported | 20 SAEs reported. 5 in placebo group ‐ atrial flutter, cerebrovascular accident, headache, hypertension, hypertensive crisis and ventricular tachycardia); 8 in aliskiren group ‐ 1 gastritis, 1 grand mal convulsions, 1 intestinal poly, 2 myocardial infarction, 1 non‐cardiac chest pain, 1 peripheral vascular disease, 1 acute renal failure); 6 in valsartan group ‐ 1 angina pectoris, 1 arteriosclerosis, 1 breast cancer, 1 bronchitis, 1 COPD, 1 facial paresis, 1 ovarian cancer, 1 pneumonia, 1 pulmonary oedema); 3 in aliskiren/valsartan group ‐ 1 aortic aneurysm, 1 intravertebral disc protrusion, 1 prostate cancer and 1 thyroidectomy). The FDA medical review (2007) also reported two cases of renal carcinoma occurred (N = 1, placebo, day 20 post‐study; N = 1, aliskiren, day 44 post‐study). For our comments on this, please see Discussion. | Not reported | |
| 11 patients had serious AEs during the washout and placebo run‐in periods (detail of which are not provided). | Not reported | 1 SAE was reported in aliskiren 75 mg group ‐ cardiac chest pain; 2 in aliskiren 150 mg group ‐ rectal bleeding due to anal ulcer and episode of secondary anaemia; basal cell carcinoma; 1 in the 300 mg group ‐TIA with concomitant nausea and dyspnoea; and 2 in the placebo group ‐ prostate cancer; accelerated hypertension with left facial numbness. | Not reported | |
| Reported 2 SAEs in the washout period; Detail not provided in CSR. | Three in the single‐blind placebo run‐in period. Detail not provided in CSR. | 6 SAEs were reported. 2 in aliskiren 75 mg group ‐ erysipelas (skin infection) and osteoarthritis; 3 in aliskiren 150 mg group ‐ severe glaucoma; moderate haemorrhoids; and mild hemorrhagic stroke; none in aliskiren 300 mg group; and 1 in the placebo group ‐ vertigo, wrist fracture, concussion, and head contusion subsequent to a fall. | Not reported | |
| COPD: chronic obstructive pulmonary disease; CSR: clinical study report; HTCZ: hydrochlorothiazide; SAE: serious adverse event; TIA: transient ischaemic attack | ||||
| Study Identifier | Total AEs and Common AEs as reported in the corresponding available CSR A = Aliskiren; P = Placebo |
| Total AEs: A150 =15(7.8%); A 300 = 18(8.9%) and P = 22(7.7%) Headache: A150 =13( 6.7%); A 300 =15(7.4%) and P = 20(10.1%) Periphaeral edema: A150 = 2(1.0%); A 300 = 3(1.5%) and P = 2(1%) | |
| Total AEs: A150 = 40.1%; A 300 = 46.7%; A 600 = 52.4%; P = 43% Headache: A150 = 12(7%); A300 = 13(7.7%); A600 = 9(5.4%); P = 16(9.7%) Nasopharyngitis: A 150 = 5(2.9%); A 300 = 6(3.6%); A 600 = 3(1.8%); P = 10(6.1%) Dizziness: A 150 = 2(1.2%); A 300 = 9(5.3%); A 600 = 5(3%); P = 7(4.2%) Diarrhoea:A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 19 (11.4%); P = 2(1.2%) Nausea: A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 0%; P = 4(2.4%) | |
| Total AEs: A75 = 61(53%); A150 = 58(51.8%); A 300 = 62(54.9%); P = 58(50.4)% Headache: A75 = 3(2.6%); A 150 = 3(2.7%); A 300 = 6 (5.3%) and P = 4(3.5%) Nasopharyngitis:A75 = 24(20.9%); A150 = 20(17.9%); A300 = 20(17.7%); P = 16(13.9%) Back pain:A75 = 2(1.7%); A150 = 0%; A300 = 0% and P = 0% Diarrhoea: A75 = 1(0.9%); A150 = 1(0.9%); A300 = 4(3.5%) and P = 1(0.9%) Vertigo: A75 =0%; A150 = 0%); A300 = 1(0.9%) and P = 2(1.7%) | |
| Total AEs: A150 = 152(50.3%), P = 66(42.3%) Nasopharyngitis: A150 = 48(15.9%), P = 13(8.3%) Headache: A150 = 9(3.0%), P = 6(3.8%) Occult blood positive: A150 = 9 (3.0%), P =5 (3.2%) | |
| Total AEs: A150 = 127 (26.8%); A300 = 130 (36.2%); A600 = 43 (33.1%); P = 32 (32.1%) Headache: A150 = 3 (2.4%); A300 = 8 (6.2%); A600 = 6 (4.6%); P = 7 (5.3%) Diarrhoea: A150 = 2 (1.6%); A300 = 1 (0.8%); A600 = 9 (6.9%); P = 2 (1.5%) Dizziness: A150 = 2 (1.6%); A300 = 4 (3.1%); A600 = 3 (2.3%); P = 5 (3.8%) Fatigue: A150 = 1 (0.8%); A300 = 5 (3.8%); A600 = 2 (1.5%); P = 4 (3.1%) | |
| Total AEs: A75 = 63(35.2%); A150 = 59(33.1%); A300 = 50(28.6%) and P = 57(32.2%) Headache: A75 = 15(8.4%); A150 = 9(5.1%;) A 300 = 7(4.0%) and P = 15(8.5%) Fatigue: A75 = 7(3.9%); A150 = 4(2.2%;) A 300 = 4(2.3%) and P = 4(2.3%) Back pain: A75 = 2(1.1%); A150 = 4(202%;) A 300 = 3(1.7%) and P = 2(1.1%) Diarrhoea: A75 = 2(1.1%); A150 = 1(0.6%;) A 300 = 5(2.9%) and P = 3(1.7%) Dizziness: A75 = 4(2.2%); A150 = 4(2.2%;) A 300 = 3(1.7%) and P = 2(1.1%) | |
| Total AEs: A75 = 69(37.5%); A150 = 69(37.3%); A300 = 71 (39.2%); P = 85(44%) Headache: A75 = 13(7.1%); A 150 = 13(7%); A 300 = 10 (5.5%); P = 26 (13.5%) Nasopharyngitis: A75 = 9( 4.9%); A150 = 5(2.7%;) A300 = 3 (1.7%); P = 10 (5.2%) Diarrhoea: A75 = 3(1.6%); A150 = 3(1.6%); A300 = 4(2.2%); P = 1(0.5%) Cough: A75 = 1(0.5%); A150 = 2(1.1%); A300 = 1(0.6%); P = 1(0.5%) Dizziness: A75 = 1(0.5%); A150 = 1(0.5%); A300 = 3(1.7%); P = 2(1.0%) Peripheral edema: A75 = 4(2.2%); A150 = 3(1.6%); A300 = 2(1.1%); P = 1(0.5%) | |
| Total AEs: A150 = 40.1%; A 300 = 46.7%; A 600 = 52.4%; P = 43% Headache: A150 = 12(7%); A300 = 13(7.7%); A600 = 9(5.4%); P = 16(9.7%) Nasopharyngitis: A 150 = 5(2.9%); A 300 = 6(3.6%); A 600 = 3(1.8%); P = 10(6.1%) Dizziness: A 150 = 2(1.2%); A 300 = 9(5.3%); A 600 = 5(3%); P = 7(4.2%) Diarrhoea: A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 19 (11.4%); P = 2(1.2%) Nausea: A 150 =2 (1.2%); A 300 = 3(1.8%); A 600 =0%; P = 4(2.4%) | |
| Total AEs at 6 weeks : A 26.4%; P = 28.5% (numbers not reported) Other AE were not reported at week 6. | |
| Total AEs: A150 to 300 = 149(34%) and P = 168(37%) Headache: A150 to 300 = 14(3.2%); P = 41(9%) Diarrhoea*: A150 to 300 = 2.3%; P = not reported Nasopharyngitis: A150 to 300 = 16(3.7%) ; P = 9(2%) Dizziness: A 150 to 300 = 8(1.8%) ; P = 9(2%) Fatigue:A 150 to 300 = 4(0.9%) ; P = 5(1.1%) Nausea: A 150 to 300 = 6(1.4%) ; P = 11(2.4%) | |
| Total AEs: A75 = 57(37.3%); A150 = 59(34.7%); A300 = 56(35.4%); P = 60(37.5%) Headache: A75 = 12(7.8%); A150 = 7(4.1%); A300 = 10(6.3%) and P = 9(5.6%) Nasopharyngitis: A75 = 2(1.3%); A150 = 2(1.2%); A300 = 5(3.2%) and P = 3(1.9%) Diarrhoea: A75 = 7(4.6%); A150 = 2(1.2%); A300 = 1(0.6%) and P = 1(0.6%) Cough: A75 = 1(0.7%); A150 = 4(2.4%); A300 = 1(0.6%) and P = 3(1.9%) Dizziness: A75 = 1(0.7%); A150 = 5(2.9%); A300 = 1(0.6%) and P = 3(1.9%) | |
| Total AEs: A75 = 36(18.8%); A150 = 41(21.7%); A300 = 36(19.1%); P = 39(21.0%) Headache: A75 = 4(2.1%);A150 = 2(1.1%); A300 = 1(1.1%); P = 4(2.1%) Influenza: A75 = 1(0.5%); A150 = 4(2.1%); A300 = 1(1.1%); P = 1(0.5%) |
| Study Identifier | Reasons for increased withdrawal due to adverse events |
| Not reported. | |
| Not reported. | |
| Significant adverse events occurred for N = 7 patients, leading to discontinuation from study, as described below.
| |
|
| |
| A total of N = 18 patients discontinued from the study due to AEs. It was suspected that 2/3 of these discontinuations were for reasons drug‐related. Headaches resulted in the discontinuation for N = 3 patients. | |
| Most frequent reasons for 12 discontinuations are: fatigue (N = 5, 0.4%), headache (N = 3, 0.3%), diarrhoea (N = 2, 0.2%), and peripheral oedema (N = 2, 0.2%). Reasons for 14 discontinuations from AE (safety population).
The FDA medical review reports facial oedema (N = 1, 0.6%) that appears to be angioedema, resulting in discontinuation from study; however, this is not reported in CSR 2203.
| |
| The range of AEs resulting in study discontinuation is 0% is the aliskiren 150 mg arm of the study to 4.4% in the aliskiren 300 mg arm (N = 7). The most frequent AE resulting in study discontinuation is headache (N = 12, 0.4%). Reasons for discontinuations from adverse events ‐ N = 62 (2.2%).
Additionally four patients discontinued from the study due to abnormal laboratory values.
| |
| Most frequent reasons for 9 discontinuation from study: headache (N = 4), dizziness (N = 3), and diarrhoea (N = 2). Reasons for discontinuations from AE (safety population).
We question that a participant withdrew for the reason specified as "unsatisfactory effect" as opposed to being categorized as a discontinuation from an SAE as the event necessitated hospitalization. On Day 27 in CSPP100A2308, the participant (N = 1, 64F, aliskiren 150 mg) was hospitalized for unstable angina and increased blood pressure. The unstable angina was treated with concomitant medication and her condition resolved within 3 days. | |
| We do not have data at week 3 and week 6 for this study. | |
| The range of AEs resulting in study discontinuation is 8 (1.8%) is the aliskiren arm; 8 (2.6%) in valsartan arm; 6 (1.3%) in aliskiren/valsartan combination arm; and 11 (2.4%) in the placebo arm. Reasons for discontinuations from adverse events ‐ N = 37 (2%).
| |
| Aliskiren 75 mg = 3 (2.0%); aliskiren 150 mg = 5 (2.9%); aliskiren 300 mg = 4(2.5%) . Placebo = 4 (2.5%). Reasons for withdrawal were not reported. | |
| Reasons for 18 discontinuations from AE (safety population)
|
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 12 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Aliskiren 75 mg vs. placebo | 5 | 1100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.97 [‐4.76, ‐1.18] |
| 1.2 Aliskiren 150 mg vs. placebo | 12 | 3786 | Mean Difference (IV, Fixed, 95% CI) | ‐5.95 [‐6.85, ‐5.06] |
| 1.3 Aliskiren 300 mg vs. placebo | 10 | 3009 | Mean Difference (IV, Fixed, 95% CI) | ‐7.88 [‐8.94, ‐6.82] |
| 1.4 Aliskiren 600 mg vs. placebo | 2 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐11.35 [‐14.43, ‐8.27] |
| 2 Diastolic BP Show forest plot | 12 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Aliskiren 75 mg vs placebo | 5 | 1100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐3.13, ‐0.96] |
| 2.2 Aliskiren 150 mg vs placebo | 12 | 3783 | Mean Difference (IV, Fixed, 95% CI) | ‐3.16 [‐3.74, ‐2.58] |
| 2.3 Aliskiren 300 mg vs placebo | 10 | 3001 | Mean Difference (IV, Fixed, 95% CI) | ‐4.49 [‐5.17, ‐3.82] |
| 2.4 Aliskiren 600 mg vs placebo | 2 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐5.86 [‐7.73, ‐3.99] |
| 3 Withdrawals due to adverse event Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 75 mg vs. placebo | 5 | 1653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.33, 1.07] |
| 3.2 150 mg vs. placebo | 10 | 3421 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.30, 0.71] |
| 3.3 300 mg vs. placebo | 10 | 4216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.47, 1.03] |
| 3.4 600 mg vs placebo | 2 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.64] |
| 4 Cough Show forest plot | 5 | 2886 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.49, 2.64] |
| 5 Diarrhoea Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Aliskiren 75 mg | 4 | 1276 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.85, 5.76] |
| 5.2 Aliskiren 150 mg | 7 | 2277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.78, 3.46] |
| 5.3 Aliskiren 300 mg | 7 | 2268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.89, 3.81] |
| 5.4 Aliskiren 600 mg | 2 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.00 [2.48, 19.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 5 | 1651 | Mean Difference (IV, Fixed, 95% CI) | ‐1.89 [‐3.16, ‐0.62] |
| 2 Diastolic BP Show forest plot | 5 | 1651 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.58, ‐0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 3 | 904 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐6.79, ‐3.40] |
| 2 DBP Show forest plot | 3 | 904 | Mean Difference (IV, Fixed, 95% CI) | ‐2.49 [‐3.53, ‐1.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 10 | 4405 | Mean Difference (IV, Fixed, 95% CI) | ‐2.62 [‐3.38, ‐1.87] |
| 2 Diastolic BP Show forest plot | 10 | 4405 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.28, ‐1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 2 | 590 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.58, ‐1.23] |
| 2 DBP Show forest plot | 2 | 590 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐3.55, ‐0.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 2 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐2.78, 1.56] |
| 2 Diastolic BP Show forest plot | 2 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐2.03, 0.67] |

