腎素抑制劑(renin‑inhibitors)對原發性高血壓的降血壓療效
Abstract
Background
Hypertension is a chronic condition associated with an increased risk of mortality and morbidity. Renin is the enzyme responsible for converting angiotensinogen to angiotensin I, which is then converted to angiotensin II. Renin inhibitors are a new class of drugs that decrease blood pressure (BP) by preventing the formation of both angiotensin I and angiotensin II.
Objectives
To quantify the dose‐related BP lowering efficacy of renin inhibitors compared to placebo in the treatment of primary hypertension.
To determine the change in BP variability, pulse pressure, and heart rate and to evaluate adverse events (mortality, non‐fatal serious adverse events, total adverse events, withdrawal due to adverse effects and specific adverse events such as dry cough, diarrhoea and angioedema).
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomized controlled trials (RCTs) up to February 2017: the Cochrane Hypertension Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (2017, Issue 2), MEDLINE (from 1946), Embase (from 1974), the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. There was no restriction by language or publication status. We also searched the European Medicines Agency (EMA) for clinical study reports, the Novartis Clinical Study Results Database, bibliographic citations from retrieved references, and contacted authors of relevant papers regarding further published and unpublished work.
Selection criteria
We included randomized, double‐blinded, placebo‐controlled studies evaluating BP lowering efficacy of fixed‐dose monotherapy with renin inhibitor compared with placebo for a minimum duration of three to 12 weeks in adult patients with primary hypertension.
Data collection and analysis
This systematic review is a comprehensive update which includes four additional studies and extensive detail from nine clinical study reports (CSRs) of previously included studies obtained from EMA. The remaining three CSRs are not available.
Two review authors independently assessed study eligibility and extracted data. In all cases where there was a difference between the CSR and the published report, data from the CSR was used. Dichotomous outcomes were reported as risk ratio (RR) with 95% confidence intervals (CIs) and continuous outcomes as mean difference (MD) with 95% CIs.
Main results
12 studies (mean duration of eight weeks) in 7439 mostly Caucasian patients (mean age 54 years) with mild‐to‐moderate uncomplicated hypertension were eligible for inclusion in the review. Aliskiren was the only renin inhibitor evaluated. All included studies were assessed to have high likelihood of attrition, reporting and funding bias.
Aliskiren has a dose‐related systolic/diastolic blood pressure (SBP/DBP) lowering effect as compared with placebo MD with 95% CI: aliskiren 75 mg (MD ‐2.97, 95% CI ‐4.76 to ‐1.18)/(MD ‐2.05, 95% CI ‐3.13 to ‐0.96) mm Hg (moderate‐quality evidence), aliskiren 150 mg (MD ‐5.95, 95% CI ‐6.85 to ‐5.06)/ (MD ‐3.16, 95% CI ‐3.74 to ‐2.58) mm Hg (moderate‐quality evidence), aliskiren 300 mg (MD ‐7.88, 95% CI ‐8.94 to ‐6.82)/ (MD ‐4.49, 95% CI ‐5.17 to ‐3.82) mm Hg (moderate‐quality evidence), aliskiren 600 mg (MD ‐11.35, 95% CI ‐14.43 to ‐8.27)/ (MD ‐5.86, 95% CI ‐7.73 to ‐3.99) mm Hg (low‐quality evidence). There was a dose‐dependent decrease in blood pressure for aliskiren 75 mg, 150 mg and 300 mg. The blood pressure lowering effect of aliskiren 600 mg was not different from 300 mg (MD ‐0.61, 95% CI ‐2.78 to 1.56)/(MD ‐0.68, 95% CI ‐2.03 to 0.67). Aliskiren had no effect on blood pressure variability. Due to very limited information available regarding change in heart rate and pulse pressure, it was not possible to meta‐analyze these outcomes.
Mortality and non‐fatal serious adverse events were not increased. This review found that in studies of eight week duration aliskiren may not increase withdrawal due to adverse events (low‐quality evidence). Diarrhoea was increased in a dose‐dependent manner (RR 7.00, 95% CI 2.48 to 19.72) with aliskiren 600 mg (low‐quality evidence). The most frequent adverse events reported were headache, nasopharyngitis, diarrhoea, dizziness and fatigue.
Authors' conclusions
Compared to placebo, aliskiren lowered BP and this effect is dose‐dependent. This magnitude of BP lowering effect is similar to that for angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). There is no difference in mortality, nonfatal serious adverse events or withdrawal due to adverse effects with short term aliskiren monotherapy. Diarrhoea was considerably increased with aliskiren 600 mg.
PICO
Plain language summary
Aliskiren的降血壓療效
文獻回顧問題
我們想要確定aliskiren相較於安慰劑,在有高血壓的成人病人身上,是否有較好的降血壓效果,以及是否會增加藥物的不良反應發生。我們也想確定是否會引起血壓變異性、脈壓、心率的變化和因副作用而停用藥物等變化。
我們從可取得的醫學文獻中,搜尋了所有評估這些問題的試驗。本文獻回顧納入的數據是截至2017年二月的最新資料。
背景
血壓高或有高血壓疾病可能會引起心臟病發作和中風。新一類藥物「腎素抑制劑」被用來治療高血壓。Aliskiren是目前唯一在這類腎素抑制劑中,經研究並被核准用來治療高血壓的藥物。
研究特性
我們找到12個為期8週的研究,總共隨機分派了7439位有中度至重度的非複雜性高血壓成年患者,使用aliskiren劑量範圍在75mg至600mg或安慰劑。所有的研究由諾華藥廠(Novartis)資助。從9個提交給監管機關的臨床試驗報告資料中,取得關於不良反應的詳細報告,亦納入於此次的文獻更新中。
主要結論
我們的結論是aliskiren在降低血壓的療效優於安慰劑,當使用到建議的最高劑量300mg時,該藥物的效果與其他類別的藥物效果相似。劑量300mg的Aliskrien降低了8個點的上壓值〈稱為收縮壓〉及5個點的下壓值〈稱為舒張壓〉Aliskrien對於血壓可變性沒有影響。因為有關心率和脈壓變化〈上壓與下壓值的差異〉的資訊非常有限,所以沒有辦法分析這些結果。
這些研究的研究時間都太短,不足以評估副作用。Aliskiren不會增加死亡、非致命性的嚴重不良事件,或因副作用而停用藥物。觀察到最常見的副作用有頭痛、腹瀉、暈眩和疲倦。與安慰劑相比,劑量600mg的Aliskiren在腹瀉症狀有顯著的增加。
證據品質:使用建議劑量來降低血壓被評等為中度證據等級,不良反應的資料被評為低品質證據,因為納入研究的評估很有可能有報告及資助偏誤。
Authors' conclusions
Summary of findings
| Aliskiren compared to placebo for primary hypertension | ||||||
| Patient or population: primary hypertension Setting: Outpatient Intervention: Aliskiren Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI)mmHg | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Effect with placebo1 | Effect with Aliskiren2 | |||||
| Systolic BP ‐ Aliskiren 75 mg vs. placebo | 2.9 lower to 10.0 lower | 2.97 lower (4.76 lower to 1.18 lower) | ‐ | 1100 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 150 mg vs. placebo | 2.0 lower to 10.0 lower | 5.95 lower (6.85 lower to 5.06 lower) | ‐ | 3786 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 300 mg vs. placebo | 2.9 lower to 10.0 lower | 7.88 lower (8.94 lower to 6.82 lower) | ‐ | 3009 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 600 mg vs. placebo | 3.8 lower to 5.3 lower | 11.35 lower (14.43 lower to 8.27 lower) | ‐ | 393 | ⊕⊕⊝⊝ | |
| Diastolic BP ‐ Aliskiren 75 mg vs placebo | 3.2 lower to 8.6 lower | 2.05 lower (3.13 lower to 0.96 lower) | ‐ | 1100 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 150 mg vs placebo | 3.0 lower to 8.6 lower | 3.16 lower (3.74 lower to 2.58 lower) | ‐ | 3783 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 300 mg vs placebo | 3.2 lower to 8.6 lower | 4.49 lower (5.17 lower to 3.82 lower) | ‐ | 3001 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 600 mg vs placebo | 6.2 lower to 6.3 lower | 5.86 lower (7.73 lower to 3.99 lower) | ‐ | 393 | ⊕⊕⊝⊝ | |
| Diarrhoea ‐ Aliskiren 600 mg vs placebo | 14 per 1,000 | 95 per 1,000 | RR 7.00 | 592 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Range of mean decrease in SBP and DBP mmHg as compared to baseline in the individual trials in the placebo group. 2. Effects in Aliskiren group represent the blood pressure lowering effect in excess of that with placebo. 3. Downgraded by 1 level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 5 included studies). Also study CSPP100A2204 had high likelihood of selection bias. 4. Downgraded by one level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 12 included studies). 5. Downgraded by one level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 10 included studies). 6. Downgraded by 1 level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in both included studies). 7. Downgraded by 1 more level due to wide confidence interval. | ||||||
Background
Description of the condition
Hypertension is defined as a systolic blood pressure (SBP) of 140 mmHg or greater and/or diastolic blood pressure (DBP) of 90 mmHg or greater in people who are not taking antihypertensive medication. (Poulter 2015). It is a chronic condition associated with an increased risk of mortality and morbidity from stroke, coronary heart disease, congestive heart failure, and renal disease. For patients with established hypertension, blood pressure should first be managed with life‐style and behaviour modification. However, if these measures prove inadequate then pharmacotherapy is indicated.
Description of the intervention
The renin‐angiotensin‐aldosterone system (RAAS) is a hormone system that regulates blood pressure and fluid balance. When renal blood flow is reduced, the kidney converts prorenin to renin and secretes it directly into the circulation. Plasma renin then converts angiotensinogen released by the liver to angiotensin I. Angiotensin I is subsequently converted to angiotensin II by the angiotensin‐converting enzyme (ACE) found in the lungs. This causes blood vessels to constrict, resulting in increased blood pressure. Angiotensin II also stimulates the secretion of the hormone aldosterone from the adrenal cortex, which causes the tubules of the kidney to increase the reabsorption of sodium and water into the blood. This increases the volume of the extracellular fluid in the body, which also increases blood pressure.
RAAS is an important target site for five distinctive antihypertensive drug classes: beta blockers, renin inhibitors, angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) and aldosterone inhibitors.
How the intervention might work
The RAAS is a major regulator of cardiovascular homeostasis, in which the vasoactive peptide, angiotensin II, plays a central role. ACEIs and ARBs block this system by inhibiting the generation and action of angiotensin II, respectively. Although ACEIs and ARBs have proven effective, these agents do not block the RAAS completely. Whereas ACEIs and ARBs block the RAAS further downstream, renin inhibitors prevent the formation of renin, the enzyme that catalyses the conversion of angiotensinogen to angiotensin I, the rate‐limiting step in this cascade. Renin is responsible for all "downstream" events leading to production of angiotensin II and subsequent stimulation of its receptors.
Attempts to block renin began in the 1950s with the use of renin antibodies (Helmer 1958). Issues of potency, bioavailability, duration of action and cost of synthesis have marred the drug development of renin inhibitors. For example, potent renin inhibitors such as remikiren and enalkiren had low oral bioavailability (Frishman 1994). Newer drugs such as zankiren and terlakiren looked more promising, but further development was halted in the mid 1990s with the development of ARBs.
More recent programmes to develop renin inhibitors have been based on X‐ray crystallography of renin's active site with computational modelling rather than based on the structure of angiotensinogen (Krum 2007). This has led to aliskiren, a new non‐peptide, low molecular weight, orally active renin inhibitor, which has been approved in the USA, Canada and other countries for the treatment of hypertension.
Why it is important to do this review
ACEIs and ARBs block the RAAS downstream whereas renin inhibitors prevent the formation of renin, which is responsible for all "downstream" events leading to production of angiotensin II and subsequent stimulation of its receptors. It has been speculated that renin inhibitors might provide a more effective means of blockade of the RAAS than is possible with ACEIs or ARBs (Duprez 2006).
Two Cochrane reviews (Heran 2008a; Heran 2008b) have quantified the dose ranging blood pressure lowering efficacy of both ACEIs and ARBs as compared to placebo using similar methodology. This review was done to determine the dose ranging blood pressure lowering efficacy and adverse effects of renin inhibitors as compared to placebo and to compare the magnitude of reduction in blood pressure with ACEIs and ARBs.
It is important to note that a new methodological approach was undertaken to update this review by actively seeking out information available from all possible sources instead of relying on the very limited information available in the journal‐published trials included in the previous update of this review. For this update, a formal request for all relevant clinical study reports (CSRs) was made to the European Medicines Agency (EMA) under the Access to Documents Policy (0043). Information regarding an individual study was obtained from additional web sites: ClinicalTrials.gov, EU Clinical Trial Register, and Novartis Clinical Trial Results Database.
Cochrane reviews typically rely on journal‐published trials for critical appraisal of study conduct, methods and, if appropriate, for meta‐analysis of study data. However, reliance on journal‐published studies may pose a threat to the validity of a meta‐analysis due to levels of bias introduced by the published versions of these trials (Dwan 2013). Reporting bias is particularly relevant with respect to our decision to use data from regulatory sources, rather than solely rely on journal‐published studies, for this systematic review. At the study level reporting bias may occur when studies are not submitted or are rejected for publication (Chalmers 1990); at the outcome level reporting bias can occur as a result of selective non‐reporting of outcomes. The latter in particular has been described as an under‐recognized level of bias that serves to undermine the validity of systematic reviews (Hodkinson 2013; Kirkham 2010; McGauran 2010). Despite being considered one of the highest forms of evidence, systematic reviews may merely further the misrepresentation of journal‐published study data.
In an effort to correct for reporting bias, there has been an increased effort to access clinical study reports (Gotzsche 2011), which have been described as "the most complete synthesis of the planning, execution, and results of a clinical study" (Doshi 2012). These documents are required by regulatory authorities for market approval purposes and may also be produced for ongoing safety evaluations. Compared to journal‐published trials, CSRs do not require compression for journal publication, but are composed of thousands of pages that include multiple data sets on all pre‐specified outcome measures, numerous tables, figures and patient narratives (Jefferson 2015). A growing number of systematic reviews that rely on CSRs suggest that the data reported in journal‐published trials, as a result of discrepant reporting or non‐reporting, do indeed often lead to a misinformed representation of the evidence (Eyding 2010; Jefferson 2014; Sharma 2016). Additionally, studies that directly compare the reporting of harms between CSRs and journal‐published trials reveal the extent of reporting bias, and the distorted representation of safety within journal publications (Hodkinson 2016; Maund 2014; Wieseler 2012). Lacking access to the full complement of evidence, systematic reviews may serve to influence health policy and prescribing practices that result in harm or lack of benefit, a burden directly borne by the patient.
In light of these considerations, we took a relatively new methodological approach to rely more heavily on regulatory sources of data. We chose to include data from the US Food and Drug Administration medical review (FDA Medical Review 2007) within the 2017 update of this systematic review (Hart 2012; Rising 2008), and CSRs obtained through the European Medicine Agency's Access to Documents policy (0043). Though the process of obtaining CSRs and navigating the large volume of these documents is time‐consuming (Doshi 2016), the motivation to do so stems from a desire to create a review that is based upon transparent and complete data, and is essentially of greater value for our readers.
Objectives
Primary objective
To determine the dose ranging blood pressure lowering efficacy of fixed dose renin inhibitor monotherapy as compared with placebo over a period of three to 12 weeks in adult patients with primary hypertension.
Secondary objectives
To determine whether renin inhibitors affect blood pressure variability, pulse pressure, and heart rate as compared with placebo.
To document adverse events of renin inhibitors as compared with placebo (including mortality, non‐fatal serious adverse events, total adverse events, withdrawal due to adverse effects and specific adverse events such as dry cough, diarrhoea and angioedema).
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished study reports of double‐blind, randomized, placebo‐controlled trials that randomly allocated patients to a fixed dose of renin inhibitor monotherapy or parallel placebo with duration of follow‐up of at least three weeks in adult patients with primary hypertension. There were no limits on language or publication status. Studies reported blood pressure measurements at baseline (following washout) and at one or more time points between three and 12 weeks post‐treatment on a fixed dose of drug.
Types of participants
Using a standard method of measurement, such as a calibrated standard mercury sphygmomanometer, adult participants had to have a baseline blood pressure of at least 140 mm Hg systolic and/or a diastolic blood pressure of at least 90 mm Hg. Participants could not have a creatinine levels greater than 1.5 times the normal level. Participants were not restricted by age, gender, baseline risk or any other co‐morbid conditions.
Types of interventions
Intervention included monotherapy with different fixed doses of renin inhibitor. Control group received a placebo. Data from study or arms of study in which titration to a higher dose was based on blood pressure response were not eligible. However, if a lower fixed dose was used for minimum of three weeks and then in all patients aliskiren was force titrated to a fixed higher dose for at least three weeks duration, then data at both doses were used from the same study to evaluate dose response.
Types of outcome measures
Primary outcomes
1. Change from baseline of trough and/or peak systolic and diastolic blood pressure over a minimum time frame of three weeks.
Secondary outcomes
-
Change in standard deviation of blood pressure.
-
Change in pulse pressure.
-
Change in heart rate.
-
Adverse events (including mortality, non‐fatal serious adverse events, total adverse events, withdrawal due to adverse effects and specific adverse events such as dry cough, diarrhoea and angioedema).
All secondary outcomes were assessed over a minimum time frame of three weeks and measured at the end of follow‐up period.
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist conducted systematic searches in the following databases for randomized controlled trials without language, publication year or publication status restrictions:
-
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 12 February 2017);
-
the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) via the Cochrane Register of Studies (CRS‐Web) (searched 12 February 2017);
-
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 12 February 2017);
-
Embase Ovid (searched 12 February 2017);
-
ClinicalTrials.gov (www.clinicaltrials.gov) searched 12 February 2017);
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch) searched 12 February 2017).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomized controlled (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Higgins 2011)). Search strategies for major databases are provided in Appendix 1.
Searching other resources
-
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE and Epistemonikos for systematic reviews) to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
-
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
-
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials.
-
We did not perform a separate search for adverse effects of interventions used for the treatment of hypertension. We considered adverse effects described in included studies only.
In preparation for the 2017 update, a formal request for all relevant clinical study reports (CSRs) was made to the European Medicines Agency (EMA) under the Access to Documents Policy (0043). Additionally, an enquiry for all relevant CSRs was submitted to Clinical Study Data Request (CSDR) (https://clinicalstudydatarequest.com), the data sharing platform to which the manufacturer, Novartis, belongs. We also searched the Novartis Clinical Trial Results Database (https://www.novctrd.com).
Review author KL submitted an enquiry to Novartis on the data sharing platform to which this company belongs, www.clinicalstudydatarequest.com (CSDR) on 17 October 2015 with respect to 12 studies that met the inclusion criteria for this review. Placing an enquiry is a step recommended if studies are not listed on the platform for data‐sharing. The Novartis data holder is expected to respond to the enquiry regarding potential for data‐sharing privileges to be granted. Two separate attempts were made to request a response from the data holder using the CSDR platform on 11 November 2015 and 20 January 2016. Unfortunately, we have not received a response from Novartis to date. CSDR Support has also made repeated attempts to elicit a response from the Novartis data holder to our enquiry without success.
Data collection and analysis
Selection of studies
Two review authors (VM and PF) independently screened the titles and the abstracts resulting from the search strategies from 2008 until October 2014. Two review authors (VM and KL) independently screened the titles and the abstracts resulting from the search strategies from October 2014 until February 2017. Articles were rejected on initial screening if it was judged from the title or the abstract that the article was not a report of a randomized placebo‐controlled study. We retrieved the full text of the remaining articles. The bibliographies of pertinent articles, reviews and texts were searched for additional citations.
Two review authors independently (VM and KL or PF) assessed the eligibility of the studies using a study selection form. We resolved discrepancies by discussion, and when necessary by a third author (JMW or KB).
Data extraction and management
Two review authors (VM and PF or KL) extracted data independently and cross‐checked them. All numeric calculations and graphic interpolations were confirmed by a second person.
This update relied on data from nine CSRs (not including appendices) received to date instead of their respective journal‐published study: 1) CSPP100A2201, 2) CSPP100A1201, 3) CSPP100A2308, 4) CSPP100A2203, 5) CSPP100A2405 6) CSPP100A2204, 7) CSPP100A2327, 8) CSPP100A2323 and 9) CSPP100A1301. The 9th CSR is of the study CSPP100A1301, which to date has no corresponding journal‐published trial.
The EMA does not possess CSRs of the three remaining studies meeting the inclusion criteria.
1. CSPA100A1301: Provided this trial does not have a corresponding journal‐published trial, we have relied on the results available on the ClinicalTrials.gov web site for this study.
2. CSPA100A2305: We have relied on the journal‐published report; additionally, results have been posted on the Novartis Clinical Trial Results Database, as well as results posted on ClinicalTrials.gov web site.
3. CSPP100A2328: We have relied on the journal‐published report; additionally, results have been posted on Novartis Clinical Trial Results database. This study was not registered on ClinicalTrials.gov web site.
In all cases where there was a difference between the CSR and published report, data from the CSR was used.
Assessment of risk of bias in included studies
Two review authors independently (VM and PF or KL) assessed the risk of bias of all included studies and prepared a 'Risk of bias' table.
Since our access to information for each included study differed based on the availability of CSRs, and/or results posted on Novartis Clinical Trial Results Database and/or results posted on ClinicalTrials.gov web site, our approach to evaluating the risk of bias of each study is based on differing availability of information.
1. Within CSRs, we found that the details on methods are generally reported more extensively than in journal‐published article. Since appendices were not included with the CSRs received by the EMA (their arrival is pending), we chose to assess a level of bias as 'unclear risk' when details on methods were insufficiently reported or not reported at all within a CSR. In the future update of this review, we will include information from the CSR appendices which are being requested from the EMA. Provided that Jefferson 2014 had access to CSRs with appendices for review, we could not follow their approach to assess a level of bias as 'high risk' of bias instead of 'unclear risk' when details on methods are insufficiently reported or not reported at all within a CSR.
2. For situations where we did not have access to the corresponding CSR of a journal‐published trial, the 'Risk of bias' assessment was conducted according to the journal‐published trial in accordance with the criteria established in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
3. As a result of not having access to either the CSR or journal‐published study, we relied on clinical study synopses available on ClinicalTrials.gov for one study that met our inclusion criteria (CSPA100A1301). As these synopses are even more compressed in terms of reporting than journal‐published studies, we did not assume 'high risk' of bias for random sequence generation, allocation concealment or blinding if the methods were not described; rather, we rated them as 'unclear', or if described sufficiently, as 'low risk' of bias.
Measures of treatment effect
Mean difference was used in the meta‐analyses in this version and the previous version to report continuous outcomes such as change in SBP and DBP and the effect size is reported as MD with 95% confidence intervals (CIs).
Relative risk was used in the meta‐analyses in this version and the previous version to report dichotomous outcomes such as mortality, non‐fatal serious adverse events, withdrawal due to adverse effects and specific adverse events and the effect size is reported as RR with 95% CIs.
Unit of analysis issues
In the 12 included studies, although sitting trough SBP and DBP measurements were reported at different time points within each study, we have included data at the longest period of follow‐up on the monotherapy dose. Ten of the 12 included studies were of eight weeks duration and data at this time point was included in the analyses. From one study CSPP100A2323, 26 weeks in duration, data reported at three weeks on aliskiren monotherapy were used. Study CSPP100A1201 was 13 weeks in duration, but data reported at the end of the double‐blind period at eight weeks were included in the analyses.
Dealing with missing data
In the original review (Musini 2008b) and the first update in 2011, in the case of missing values for standard deviation of the change in blood pressure, the standard deviation was imputed based on the information in the same study or from other studies using the same dose. The following hierarchy (listed from high to low preference) was used to impute standard deviation values.
-
Standard deviation of change in blood pressure data from a different position (standing or supine).
-
Standard deviation of blood pressure at the end of treatment.
-
Standard deviation of blood pressure at baseline (except if this measure is used for entry criteria) (Musini 2009).
-
Mean standard deviation of change in blood pressure from other studies using the same class of drug.
In this 2017 update, we were able to include full reporting of mean sitting SBP (MSSBP) and mean sitting DBP (MSDBP) data with standard deviation (SD), least square means (LSMs) of change in MSSBP and MSDBP with standard error of the mean (SEM) from eight CSRs of published studies (CSPP100A1201; CSPP100A2201; CSPP100A2203; CSPP100A2204; CSPP100A2308; CSPP100A2327; CSPP100A2405; CSPP100A2323), and from one CSR of an unpublished study CSPP100A1301. Therefore, the reduction in LSM of change in SBP and DBP data including SD or SEM reported in this update may vary from previous versions of the review.
In study CSPP100A2323 patients were on aliskiren 150 mg dose until week 3 after which patients were force titrated to 300 mg until week 6. After week 6, patients initially randomized to placebo were re‐randomized to receive aliskiren 300 mg or hydrochlorothiazide (HCTZ) 25 mg in a 1:1 ratio. As there is no parallel placebo group after week 6, only data for change in MSDBP and MSSBP at week 3 for aliskiren 150 mg dose and at week 6 for aliskiren 300 mg dose is useful. The data for mean change in MSDBP with SEM was obtained from (CSR Page 84). The CSR did not report this information at week 3. Change in MSDBP and MSSBP at week 3 and for MSSBP at week 6 were estimated from the graphs (figure 3 in the published journal article). The SD's were imputed from the baseline SD for MSSBP (SD at baseline =11.2) and since the SD's were unusually low as reported in the baseline characteristics for MSDBP SD (3.3) the SD's were imputed from the average SD for all studies using aliskiren 150 mg dose or aliskiren 300 mg dose respectively .
Since CSRs are not available for two published studies CSPP100A2328 and CSPA100A2305 the missing values for standard error were imputed as follows:
In study CSPP100A2328, the change in MSDBP and MSSBP from baseline with standard deviation of change for placebo and aliskiren 75 mg, 150 mg and 300 mg at week 8 are reported from Novartis Clinical Trial Results Database.
In study CSPA100A2305, the change in MSDBP and MSSBP from baseline with standard deviation of change for placebo, aliskiren 150 mg and 300 mg at week 8 are reported from the clinical study synopses as reported on ClinicalTrials.gov (NCT00739973).
For the unpublished study CSPA100A1301 for which CSR is not available with EMA, the change in MSDBP and MSSBP from baseline with standard deviation of change for placebo and aliskiren 150 mg at week 8 are reported from clinical synopses posted on ClinicalTrials.gov (NCT01237223).
Assessment of heterogeneity
Test for heterogeneity of treatment effect between the studies was done using a standard Chi2 statistic for heterogeneity. The fixed‐effect model was applied to obtain summary statistics of pooled studies, unless significant between‐study heterogeneity was present, in which case the random‐effects model was used to test statistical significance.
Assessment of reporting biases
We planned to assess publication bias using a funnel plot if at least 10 studies met the inclusion criteria. In this version of the review we did not use a funnel plot to assess publication bias.
Data synthesis
Data synthesis and analyses were carried out using the Cochrane Review Manager software, RevMan 5.3.
The least square means (LSMs) reporting of blood pressure data is non‐robust to data outliers, particularly if outliers have a skewed distribution. This can cause an increase in type 2 errors, however it is not known if there were significant outliers in the data.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were planned a priori.
-
Race: black, white, other (Asian)
-
Age: adults (18 to 64 years), elderly (65 years or years old)
-
Gender
-
Baseline severity of hypertension: mild, moderate, severe
Since none of the included studies provided data for specific age groups, gender, race or baseline severity of hypertension, we could not perform these analyses. However, the FDA Medical Review 2007 provided additional information regarding mean placebo‐corrected change from baseline in BP by dose and gender (seeTable 1); dose and age (seeTable 2); and dose and race (seeTable 3) from five placebo‐controlled studies (CSPP100A1201; CSPP100A2201; CSPP100A2203; CSPP100A2204; CSPP100A2308). This information is described in Effects of interventions.
| Dose | Female | Male | ||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐1.3 | ‐1.1 | ‐3.6 | ‐2.9 |
| 150 mg | ‐5.5 | ‐2.9 | ‐5.9 | ‐3.5 |
| 300 mg | ‐9.4 | ‐4.8 | ‐9.0 | ‐5.4 |
| 600 mg | ‐12.6 | ‐6.4 | ‐10.7 | ‐6.5 |
| 75 mg | ‐1.3 | ‐1.1 | ‐3.6 | ‐2.9 |
| Dose | Age < 65 years | Age > 65 years | ||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐2.7 | ‐1.9 | ‐3.6 | ‐3.4 |
| 150 mg | ‐5.5 | ‐2.9 | ‐6.9 | ‐4.8 |
| 300 mg | ‐9.7 | ‐5.2 | ‐7.1 | ‐4.4 |
| 600 mg | ‐11.5 | ‐6.6 | ‐11.6 | ‐6.4 |
| 75 mg | ‐2.7 | ‐1.9 | ‐3.6 | ‐3.4 |
| Dose | White | Black | Asian | |||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐2.1 | ‐1.7 | ‐2.8 | 0.2 | ‐8.8 | ‐3.2 |
| 150 mg | ‐6.5 | ‐3.5 | ‐5.5 | ‐1.4 | ‐3.7 | ‐2.9 |
| 300 mg | ‐9.6 | ‐4.8 | ‐6.1 | ‐2.6 | ‐12.7 | ‐6.3 |
| 600 mg | ‐12.3 | ‐6.7 | ‐8.7 | ‐3.5 | ‐11.2 | ‐9.4 |
When heterogeneity was significant, we planned to investigate the factors contributing to heterogeneity such as differences in population characteristics or response to placebo that would possibly explain the reason for heterogeneity. However, no significant heterogeneity (I2 > 50%) was found for any outcome measure.
Sensitivity analysis
To test the robustness of the results several sensitivity analyses were planned a priori to be performed.
-
Studies of high risk of bias versus low risk of bias
-
Studies that were industry‐sponsored versus non‐industry sponsored
-
Studies that assess the drug as primary drug of investigation versus those that assess the drug as comparator
-
Studies with blood pressure data measured in the sitting position versus other measurement positions
-
Studies with published standard deviations of blood pressure change versus imputed standard deviation
Sensitivity analyses could not be done as all studies had similar risks of bias; all studies were industry sponsored; aliskiren was the primary drug of investigation; all studies reported mean sitting trough SBP and DBP; and standard deviation was imputed only in one study (CSPP100A2323).
We conducted a sensitivity analyses to look for differences in the magnitude of SBP and DBP reduction due to inclusion of information from CSRs in this update compared to previous update in 2011 which included information only from published journal articles. Additional information obtained from CSRs in this update did not result in altering the magnitude of reduction in MSSBP and MSDBP at any dose as compared to placebo from the previous 2011 update as the 95% CIs overlapped. See Table 4
| Dose of Aliskiren mg/day | Previous version without access to information from CSR | Present version with access to information from CSR | ||
| MSSBP mmHg MD with 95% CI | MSDBP mmHg MD with 95% CI | MSSBP mmHg MD with 95% CI | MSDBP mmHg MD with 95% CI | |
| Aliskiren 75 mg vs. Placebo | ‐2.64 (‐4.06 to ‐1.23) | ‐2.07 (‐2.94 to ‐1.20) | ‐2.97 (‐4.76 to ‐1.18) | ‐2.05 (‐3.13 to ‐0.96) |
| Aliskiren 150 mg vs. Placebo | ‐5.55 (‐6.39 to ‐4.71) | ‐2.91 (‐3.46 to ‐2.37) | ‐5.95 (‐6.85 to ‐5.06) | ‐3.16 (‐3.74 to ‐2.58) |
| Aliskiren 300 mg vs. Placebo | ‐7.93 (‐8.77 to ‐7.08) | ‐4.76 (‐5.33 to ‐4.19) | ‐7.88 (‐8.94 to ‐6.82) | ‐4.49 (‐5.17 to ‐3.82) |
| Aliskiren 600 mg vs. Placebo | ‐11.36 (‐13.53 to ‐9.19) | ‐6.57 (‐7.92 to ‐5.23) | ‐11.35 (‐14.43 to ‐8.27) | ‐5.86 (‐7.73 to ‐3.99) |
MSSBP: mean sitting systolic blood pressure; MSDBP: mean sitting diastolic blood pressure;
Results
Description of studies
Results of the search
From our search strategy we identified 862 study reports: 250 when the original search was run in 2008, another 172 when the review was updated in June 2011 and another 440 for the update in February 2017. Of these, 634 were duplicates or not relevant to this review.
We retrieved 46 articles for detailed evaluation: 8 for the initial version of this review in 2008, another 10 for the June 2011 update and another 28 for the current update in February 2017.
A total of 12 studies met the inclusion criteria: six published studies for the initial version of this review in 2008 (CSPP100A2201, CSPP100A1201, CSPP100A2308, CSPP100A2327, CSPP100A2203, CSPP100A2204), another two published studies for the June 2011 update (CSPP100A2328, and CSPP100A2323), and another four for the most recent update in February 2017, two of which are published studies (CSPP100A2405, CSPA100A2305), and two are unpublished studies (CSPP100A1301 and CSPA100A1301). See PRISMA Study flow diagram (Figure 1).

PRISMA Study flow diagram.
See Table 5 for detailed information regarding identifier for individual studies meeting the inclusion criteria. All included study have been identified in this update using Novartis Clinical Trial Results Database study identifier number as opposed to the journal‐published author name and year, which were used in the previous two versions of this review.
| Novartis Clinical Trial Results Database identifier used for study identification in this review | Journal‐Published Author /year | Registered on Novartis Clinical Trial Results Database | Registered on ClinicalTrials.gov | CSR* received from EMA | Request to EMA was made on November 15th 2015. Weeks waited to obtain CSR |
| No journal publication | Results NOT posted | NCT01237223 Results posted | EMA does NOT possess | ‐ | |
| Results posted | NCT00739973 Results posted | EMA does NOT possess | ‐ | ||
| Results NOT posted | NOT registered | Mar 11, 2016 | 16 | ||
| No journal publication | Results posted | NCT00344110 Results NOT posted | May 23, 2016 | 27 | |
| Results NOT posted | NOT registered | Jan 25, 2016 | 10 | ||
| NOT FOUND | NOT registered | Apr 12, 2016 | 21 | ||
| Results NOT posted | NCT00219024 Results posted | Apr 6, 2016 | 20 | ||
| Results NOT posted | NCT00219128 Results NOT posted | Feb 20, 2016 | 14 | ||
| Results posted | NCT00219154 Results NOT posted | May 27, 2016 | 27 | ||
| Results NOT posted | NOT registered | June 16, 2016 | 30 | ||
| Results posted | NOT registered | EMA does NOT possess | ‐ | ||
| Results posted | NCT00706134 Results posted | December 22, 2015 | 5 |
Two studies were not published in journals but were identified on the Novartis Clinical Trial Results Database.
One published study CSPP100A2203 was not found on the Novartis Clinical Trial Results Database (CSPP100A2203).
Six studies, which are registered on the Novartis Clinical Trial Results Database, did not provide results (CSPP100A1201, CSPP100A2201, CSPP100A1301, CSPP100A2308, CSPP100A2327, and CSPP100A2204).
Five of the included studies are not registered on ClinicalTrials.gov web site ( CSPP100A1201, CSPP100A2201, CSPP100A2203, CSPP100A2327, CSPP100A2328).
Three studies are registered on ClinicalTrials.gov web site (CSPA100A1301, CSPP100A2308, and CSPP100A2323), but the results are not posted.
We have received only partial clinical study reports (CSRs) as appendices and individual patient data were not included. We need to make separate specific requests to the European Medicines Agency (EMA) to obtain this information.
Included studies
All 12 included studies were sponsored by the manufacturer (Novartis).
The 12 included studies had a total of 7439 patients (placebo = 2319; aliskiren = 5120). The majority of included studies were randomized , double‐blind, parallel group multicentre studies of eight weeks duration. The exceptions include the three trials: CSPP100A1201 (13 weeks duration), CSPP100A2327 (four weeks duration per dose), and CSPP100A2323 (three weeks duration per dose).
Six studies (CSPA100A2305CSPA100A1301; CSPP100A2203; CSPP100A2204; CSPP100A2327; CSPP100A2323) included active treatment arms in addition to placebo, comparing arms of aliskiren, combination therapy with valsartan, hydrochlorothiazide (HCTZ), and the respective monotherapies. Details of each included study are provided in Characteristics of included studies.
Following a one‐ to two‐week washout period from any prior antihypertensive medications, all studies had a two ‐to four‐week single‐blind placebo‐controlled run‐in period, followed by three to 13 weeks of randomized treatment. In most published studies, baseline mean sitting diastolic blood pressure (MSDBP) and mean sitting systolic blood pressure (MSSBP) were provided, but no endpoint MSDBP and MSSBP were provided. Consequently, comparisons were based on least square means (LSMs) and standard error (SE), which were converted to standard deviations (SDs). Only two published studies provided baseline and endpoint BP data (CSPP100A2201 and CSPP100A2203). The differences in the change in MSDBP and MSSBP in these two studies were similar to the LSMs provided.
In five of the studies (CSPP100A1201; CSPP100A2203; CSPP100A2204; CSPP100A2328; CSPP100A2405), patients received aliskiren once daily 75 mg, 150 mg and 300 mg.
In two studies (CSPP100A2201; CSPP100A2308), patients received once daily aliskiren 150 mg, 300 mg or 600 mg.
In CSPP100A2327, patients received once daily aliskiren 150 mg and then increased it to 300 mg in all patients after four weeks.
In CSPP100A2323, study patients received aliskiren 150 mg, HCTZ 12.5 mg or placebo for three weeks and all patients were then force titrated to double the dose for an additional three weeks. After six weeks from the start of the study, the placebo group was given active treatment so only data from aliskiren 150 mg data at week three and aliskiren 300 mg data at week six have been included.
In CSPA100A2305, patients were randomized to aliskiren 150 mg, 300 mg and placebo monotherapy as well as several combination therapy groups for a duration of eight weeks.
In CSPA100A1301, study patients were randomized to aliskiren 150 mg and placebo for a duration of eight weeks.
In CSPP100A1301, study patients were randomized to aliskiren 150 mg and placebo for a duration of eight weeks.
Population Characteristics
All studies had similar inclusion and exclusion criteria (see Characteristics of included studies).
Inclusion criteria: Patients were at least 18 years of age with mild‐to‐moderate essential hypertension defined as MSDBP > 95 mm Hg and < 110 mm Hg at baseline. Two studies (CSPP100A1201 and CSPP100A1301) were conducted in Japanese centres with only Asian adult patients (20 to 80 years of age for CSPP100A1201; 20 to 75 years of age for CSPP100A1301). All 12 studies had similar SBP and DBP at baseline ranging from 152 mmHg to 160 mmHg for SBP and 90 mmHg to 100 mmHg DBP. Most studies had Caucasian patients, ranging from 61% to 99% of randomized patients. The mean age in all studies ranged from 52 to 56 years of age except in CSPP100A2405. This study was conducted in elderly hypertensive patients ≥ 65 years of age; mean age of participants was 72 years.
Exclusion criteria: Patients were excluded if pregnant or breast feeding, had MSDBP >110 mm Hg and/or MSSBP > 180 mm Hg, had a history or evidence of secondary hypertension, type 1 or type 2 diabetes with poor glycaemic control, or any surgical or medical condition that might alter the absorption, distribution, metabolism or excretion of study drugs. Patients were also excluded if they had a history of severe cardiovascular, cerebrovascular, hepatic, or renal disease.
Blood Pressure Measurements
The position of the patient during blood pressure measurement may affect the blood pressure lowering effect. When blood pressure measurement data was available in more than one position, data was extracted in accordance with the following order of preference: 1) sitting; 2) standing; and 3) supine. In all studies, MSSBP and MSDBP were available for baseline measurements, and LSMs and SE were available for end of treatment reductions in MSSBP and MSDBP.
If blood pressure measurements were available at more than one time during the 24‐hour period, the trough measurement was used. Peak level is defined as within 12 hours of the dose and trough level is defined as between 12 and 24 hours. All studies reported trough MSSBP and MSDBP levels.
In all studies, sitting blood pressure (BP) measurements were recorded at baseline and regular intervals throughout studies (for example, at weeks one, two, four, six and eight). As well, sitting BP was measured using a calibrated standard mercury sphygmomanometer or an alternative calibrated method, in accordance with the 1988 AHA Committee Report on Blood Pressure Determination. Sitting blood pressure was measured after the patient had rested in sitting position for at least five minutes. The measurement was repeated a total of three times at an interval of one to two minutes. The three values were averaged to obtain a mean sitting blood pressure.
BP was measured at trough (24 + 3 hour post dose). At the first study visit, the patient's arm with the highest sitting DBP became the arm used for all subsequent readings in the study. At each study visit BP was measured three times at one‐ to two‐minute intervals, with the mean used as the BP for that visit. As well, one BP measurement was taken in standing position after the patient stood for two minutes.
Power
In five studies (CSPP100A2201; CSPP100A2203; CSPP100A2308; CSPP100A2328; CSPA100A2305), sample size was calculated to ensure sufficient power for both a primary intention‐to‐treat (ITT) analysis and a per‐protocol analysis. The study assumed a dropout rate of 10% and a standard deviation of 8 mm Hg for MSDBP. The sample size provided a 90% power to detect a treatment difference of at least 3.5 mm Hg for pair‐wise comparisons at a two‐sided significance level of 0.05.
In CSPP100A1201, the sample size provided an 80% power to detect a 3.5 mm Hg in MSDBP.
The study CSPP100A2327 provided 90% power, and CSPP100A2323 provided 95% power, to detect a treatment difference of at least 2 mm Hg in MSDBP.
In CSPP100A2405, the sample size was calculated to provide at least 97% power to detect a difference of 5 mm Hg in MSSBP between at least one aliskiren dose and placebo.
In CSPP100A2204, the sample size provided an 90% power to detect a 3.3 mm Hg in MSDBP and a SD of 8 mmHg.
The synopses of CSPA100A1301 and CSPP100A1301 studies do not provide details of how sample size was calculated.
Outcome Characteristics
The primary outcome of all studies was reduction in MSDBP.
Secondary efficacy variables of the studies included the following.
-
Mean reduction in MSSBP
-
Diastolic responder rates (defined as DBP less than 90 mm Hg or equal to 10 mm Hg reduction in DBP)
-
BP control rates (defined as a BP less than 140/90 mm Hg)
-
Safety and tolerability of aliskiren
-
Effects on plasma renin activity and renin concentration.
Excluded studies
Of the 46 articles retrieved for detail reading of study methodology, 31 studies did not meet our inclusion criteria and were excluded: two in 2008, eight in 2011 and 21 in 2017. The reasons for exclusion are reported in Characteristics of excluded studies. The main reasons form exclusion were lack of a parallel placebo control group or no aliskiren monotherapy treatment arm.
Risk of bias in included studies
See Figure 2
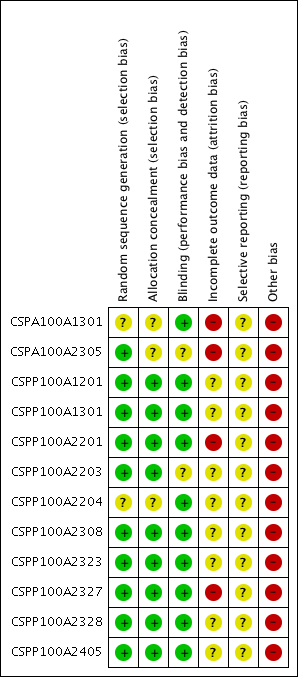
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
Allocation
Random sequence generation (selection bias)
We considered 10 studies to have low risk of bias CSPA100A2305; CSPP100A1201; CSPP100A1301; CSPP100A2201; CSPP100A2203; CSPP100A2308; CSPP100A2323; CSPP100A2327; CSPP100A2328; CSPP100A2405), because they reported adequate sequence generation using computer‐generated random numbers and patients were assigned to treatment groups via central allocation or using validated interactive voice response system that automates random assignment.
We considered two studies to have 'unclear' risk of bias:
-
Study CSPA100A1301: random sequence generation was not reported within the clinical study synopses. Also, lack of access to their respective CSRs renders us unable to rate this level of bias. We have rated it as 'unclear' at this time.
-
Study CSPP100A2204: was rated to have unclear risk of bias as randomization was performed by Covance Inc. using validated system, however the detail of the method used was not reported in the CSR and we did not have access to the appendices for further details.
Allocation concealment (selection bias)
We considered nine studies to have low risk of bias for allocation concealment as randomization data were kept strictly confidential until completion of the study and patients, investigators, collaborators and the sponsor were unaware of the treatment assignments throughout the study until the database was locked (CSPP100A1201; CSPP100A1301; CSPP100A2201; CSPP100A2203; CSPP100A2308; CSPP100A2405; CSPP100A2327; CSPP100A2328; CSPP100A2323).
We considered three studies to have unclear risk of bias:
-
CSPA100A1301: as method of allocation concealment was not described in the clinical synopsis.
-
CSPP100A2204: randomization number was provided along with a unique medication number for the packages of study drug to be dispensed. We did not have access to the appendices for more detail.
-
CSPA100A2305: method of allocation concealment was not described in the clinical synopsis. The CSR is not available with EMA.
Blinding
We considered 10 studies to have low risk of bias for blinding as the identity of the treatment was concealed from patients, investigators and staff performing the assessment by the use of drugs that were identical in packaging, labelling, appearance, odour and schedule of administration (CSPA100A1301; CSPP100A1201; CSPP100A1301; CSPP100A2201; CSPP100A2204; CSPP100A2308; CSPP100A2323; CSPP100A2327; CSPP100A2328; CSPP100A2405 ).
We considered two studies to have unclear risk of bias as blinding of outcome assessors was not described (CSPA100A2305 and CSPP100A2203).
-
Study CSPA100A2305: is described as double‐blind. In order to adequately blind the study, patients were required to take a total of three tablets and two capsules of study medication throughout the study. No further details are provided.
-
Study CSPP100A2203: aliskiren was supplied as capsule for two doses (75 mg and 150 mg) and as tablet for 300 mg dose and there was insufficient information to determine if blinding was successful.
Incomplete outcome data
We considered four studies to have high risk of bias for incomplete outcome reporting because total withdrawal of randomized patients significantly differed in placebo and aliskiren treatment groups. Also last observation carried forward (LOCF) analysis was done for missing data at study endpoint. CSPA100A1301; CSPA100A2305; CSPP100A2201; CSPP100A2327.
We considered eight studies to have unclear risk of bias as reasons for withdrawal differed between placebo and treatment groups particularly due to unsatisfactory therapeutic effect. Also LOCF analysis was done for missing data at study endpoint (CSPP100A1201; CSPP100A1301; CSPP100A2203; CSPP100A2204; CSPP100A2308; CSPP100A2328; CSPP100A2405; CSPP100A2323.
Selective reporting
We meticulously looked for all possible studies conducted in various databases as well as the manufacturer's web site Novartis Clinical Trial Results Database, FDA web site U.S. Food and Drug Administration 2016 and ClinicalTrials.gov and found reporting bias for two studies (CSPA100A1301; CSPP100A1301), which had no journal publication but were listed on the manufacturer's web site.
We considered all 12 studies to have unclear risk of selective reporting bias. Protocols and CSRs (without appendices) were available for nine included studies. Most primary and secondary outcomes in these studies were reported. However for outcomes such as pulse pressure and heart rate, data were available in appendices which were not provided within CSRs from EMA. For the remaining three studies, since protocols were not available, we were unable to determine selective reporting risk of bias.
Other potential sources of bias
We considered all 12 studies to have high risk of other bias as they were sponsored by the manufacturer. Authors of six studies are employees of Novartis Pharmaceuticals Corporation and are therefore eligible for Novartis stock and stock options (CSPP100A2201; CSPA100A2305; CSPP100A2308; CSPP100A2327; CSPP100A2323; CSPP100A2405).
There was no description available with respect to the trialists' conflict of interest for three studies (CSPA100A1301; CSPP100A2328; CSPP100A2204). From the FDA Medical Review 2007 the regulator comments that financial disclosure forms were obtained for CSPP100A2201, CSPP100A2203, CSPP100A2204, and CSPP100A2308, but the financial disclosure form for CSPP100A1201 was not obtained. Moreover, the returned forms identified "Grants, Honoraria, Travel Expenses" exceeding $25,000 for Study (Study identifier redacted in FDA medical review) and "Consultant, speaker" exceeding $25,000 for Study (Study identifier redacted in FDA medical review). The regulator comments that "the two potential conflicts of interest could not prejudice the results greatly even if there were overt manipulation" due to the fact that the study takes place over multiple centres with small percentages of total study participants, and the study is double‐blinded.
Effects of interventions
See: Summary of findings for the main comparison
Mean change from baseline of trough systolic blood pressure
Seesummary of findings Table for the main comparison
Aliskiren monotherapy was superior to placebo in lowering mean sitting systolic blood pressure (MSSBP). The additional magnitude of blood pressure lowering minus the placebo effect can be seen in Analysis 1.1.
Aliskiren 75 mg versus placebo (mean difference (MD) ‐2.97, 95% confidence interval (CI) ‐4.76 to ‐1.18; participants = 1100; studies = 5; I2 = 0%); aliskiren 150 mg versus placebo (MD ‐5.95, 95% CI ‐6.85 to ‐5.06; participants = 3786; studies = 12; I2 = 17%); aliskiren 300 mg versus placebo (MD ‐7.88, 95% CI ‐8.94 to ‐6.82; participants = 3009; studies = 10; I2 = 22%); aliskiren 600 mg versus placebo (MD ‐11.35, 95% CI ‐14.43 to ‐8.27; participants = 393; studies = 2; I2 = 0%) see Figure 3. Quality of evidence was graded as moderate for 75 mg, 150 mg and 300 mg dose and as low for 600 mg dose.

Forest plot of comparison: 1 Aliskiren vs. placebo, outcome: 1.1 Systolic BP.
Highly significant subgroup differences were observed therefore mean overall effect size across all doses is not shown. The number of placebo group patients are divided equally in dose ranging studies when more than one dose of aliskiren was used.
CSPP100A2308 study the SBP reduction in the treatment and placebo group are reported from the CSR page 61.
CSPP100A2405 study the SD for all treatment groups are calculated from SEM reported on page 7 in the CSR .
Note: Since there was highly significant subgroup differences between aliskiren 75 mg, 150 mg, 300 mg and 600 mg dose for MSSBP reduction (I2 = 90%), effect size is only presented as subtotals for each dose and overall MSSBP reduction is not shown. The number of patients in the placebo group is divided between various aliskiren dose comparisons if the study used more than one dose of aliskiren as compared to placebo.
There was no statistically significant heterogeneity observed for reduction in MSSBP for aliskiren 75 mg, 150 mg, 300 mg or the 600 mg dose. The I2 was zero for the 75 mg and 600 mg doses. For aliskiren 150 mg, one unpublished study which had a low MSSBP reduction in the placebo group ( ‐2.0 mmHg in CSPP100A1301) when removed from the analysis, reduced the I2 from 17% to 0%.
For aliskiren 300 mg, when two published studies, which had a low MSSBP reduction in the placebo group ( ‐2.9 mmHg in CSPP100A1201 and ‐3.8 mmHg in CSPP100A2308) were removed from the analysis, the I2 was reduced from 22% to 0%. Baseline MSSBPs were similar in all studies.
Mean change from baseline of trough diastolic blood pressure
Seesummary of findings Table for the main comparison
Aliskiren monotherapy was superior to placebo in lowering mean sitting diastolic blood pressure (MSDBP). The additional magnitude of blood pressure lowering minus the placebo effect can be seen in Analysis 1.2.
Aliskiren 75 mg versus placebo (MD ‐2.05, 95% CI ‐3.13 to ‐0.96; participants = 1100; studies = 5; I2 = 0%); aliskiren 150 mg versus placebo (MD ‐3.16, 95% CI ‐3.74 to ‐2.58; participants = 3783; studies = 12; I2 = 47%); aliskiren 300 mg versus placebo (MD ‐4.49, 95% CI ‐5.17 to ‐3.82; participants = 3001; studies = 10; I2 = 17%); aliskiren 600 mg versus placebo (MD ‐5.86, 95% CI ‐7.73 to ‐3.99; participants = 393; studies = 2; I2 = 0%), see Figure 4. Quality of evidence was graded as moderate for 75 mg, 150 mg and 300 mg dose, and as low for 600 mg dose.

Forest plot of comparison: 1 Aliskiren vs. placebo, outcome: 1.2 Diastolic BP.
Highly significant subgroup differences were observed, therefore mean overall effect size across all doses is not shown. The number of placebo group patients are divided equally in dose ranging studys when more than one dose of aliskiren was used.
CSPP100A2308 study the DBP reduction in the treatment and placebo group are reported from the CSR which differ from those reported in the published article. The previous version of this review used data from the published article.
CSPP100A2405 study the SD for all treatment groups are calculated from SEM reported from the CSR on page 8.
Note: Since there was highly significant subgroup differences between aliskiren 75 mg, 150 mg, 300 mg and 600 mg dose for MSSBP reduction (I2 = 86.6%), effect size is only presented as subtotals for each dose and overall MSDBP reduction is not shown. The number of patients in the placebo group is divided between various aliskiren dose comparisons if the study used more than one dose of aliskiren.
There was no statistically significant heterogeneity observed for MSDBP for aliskiren 75 mg, and 600 mg dose. For aliskiren 150 mg, when one unpublished study which had a low MSDBP reduction in the placebo group (‐3.0 mmHg in CSPP100A1301) was removed from the analysis, the I2 was reduced from 47% to 0%. For aliskiren 300 mg , removing either CSPP100A1201 or CSPP100A2405, which had a low MSDBP reduction in the placebo group ( ‐3.0 mmHg in CSPP100A1201 or ‐3.5 mmHg in CSPP100A2405) from the analysis, reduced the I2 from 4% to 0%. Baseline MSDBPs were similar in all studies.
Comparing MSSBP and MSDBP reduction between aliskiren doses from the same study
Refer Table 6
| Dose comparison | SBP WMD with 95% CI | DBP WMD with 95% CI | Comment |
| Aliskiren 150 mg vs 75 mg | ‐1.89 (‐3.16 to ‐0.62) | ‐0.80 (‐1.58 to ‐0.03) | Aliskiren 150 mg reduces SBP and DBP more than 75 mg by 2/1 mmHg |
| Aliskiren 300 mg vs 75 mg | ‐5.10 (‐6.79 to ‐3.40) | ‐2.49 (‐3.53 to ‐1.45) | Aliskiren 300 mg reduces SBP and DBP more than 75 mg 5/3 mmHg |
| Aliskiren 300 mg vs 150 mg | ‐2.62 (‐3.38 to ‐1.87) | ‐1.80 (‐2.28 to ‐1.32) | Aliskiren 300 mg reduces SBP and DBP more than 150 mg 3/2 mmHg |
| Aliskiren 600 mg vs 150 mg | ‐3.40 (‐5.58 to ‐1.23) | ‐2.20 (‐3.55 to ‐0.85) | Aliskiren 600 mg reduces SBP and DBP more than 150 mg by 3/2mmHg |
| Aliskiren 600 mg vs 300 mg | ‐0.61 (‐2.78 to 1.56) | ‐0.68 (‐2.03 to 0.67) | No significant difference was observed |
Aliskiren 150 mg lowered MSSBP and MSSDP more than aliskiren 75 mg SBP: (MD ‐1.89, 95% CI ‐3.16 to ‐0.62; participants = 1651; studies = 5; I2 = 23%) Analysis 2.1 DBP: (MD ‐0.80, 95% CI ‐1.58 to ‐0.03; participants = 1651; studies = 5; I2 = 0%) Analysis 2.2
Aliskiren 300 mg lowered MSSBP and MSSDP more than aliskiren 75 mg SBP: (MD ‐5.10, 95% CI ‐6.79 to ‐3.40; participants = 904; studies = 3; I2 = 21%) Analysis 3.1 DBP: (MD ‐2.49, 95% CI ‐3.53 to ‐1.45; participants = 904; studies = 3; I2 = 7%) Analysis 3.2
Aliskiren 300 mg lowered MSSBP and MSSDP more than aliskiren 150 mg SBP: (MD ‐2.62, 95% CI ‐3.38 to ‐1.87; participants = 4405; studies = 10; I2 = 51%) Analysis 4.1 DBP: ((MD ‐1.80, 95% CI ‐2.28 to ‐1.32; participants = 4405; studies = 10; I2 = 33%) Analysis 4.2
Aliskiren 600 mg lowered MSSBP and MSSDP more than aliskiren 150 mg SBP: (MD ‐3.40, 95% CI ‐5.58 to ‐1.23; participants = 590; studies = 2; I2 = 0%) Analysis 5.1 DBP: (MD ‐2.20, 95% CI ‐3.55 to ‐0.85; participants = 590; studies = 2; I2 = 0%) Analysis 5.2
However, there were no differences in MSSBP (MD ‐0.61, 95% CI ‐2.78 to 1.56; participants = 592; studies = 2; I2 = 0%) Analysis 6.1 or MSDBP (MD ‐0.68, 95% CI ‐2.03 to 0.67; participants = 592; studies = 2; I2 = 33%) Analysis 6.2 for aliskiren 600 mg as compared to aliskiren 300 mg.
Change in standard deviation
End of treatment standard deviation of SBP and DBP was similar in the placebo and aliskiren arms in the 12 included studies. Standard deviation of the change in MSSBP ranged from 11.2 to 14.2 in the aliskiren treatment arm as compared to 11.2 to 14.1 in the placebo treatment arm. Standard deviation of the change in MSDBP ranged from 7.7 to 8.8 in aliskiren treatment arm and from 7.7 to 8.7 in the placebo treatment arm. This is consistent with aliskiren having no effect on BP variability.
Due to limited information available on standard error of the mean change in SD, this outcome has not been meta‐analyzed.
Change in pulse pressure
Limited information on pulse pressure is available in the bodies of the clinical study reports (CSRs); rather, this outcome is reported within the CSR appendices to which we do not yet have access.
However, CSPP100A2201 reports a reduction in pulse pressure at endpoint of ‐1.34, ‐3.5 and ‐3.91 for aliskiren 150 mg, 300 mg, and 600 mg, respectively versus +1.4 for placebo. At all study visits, aliskiren 600 mg produced the greatest reduction in sitting pulse pressure.
CSPP100A2203 reports pulse pressure increased (aliskiren 150 mg n = 1) as an adverse event, but the magnitude of this value is reported only in the appendices which are not yet accessible at this time.
No journal‐published study reported on pulse pressure at both baseline or endpoint. Therefore due to very limited information available on change in pulse pressure, this data has not been meta‐analyzed.
Change in heart rate
Five CSRs report on baseline sitting pulse rate but no data has been reported at the end of treatment. This data may be available in appendices which are not yet accessible at this time.
CSPP100A1201 study reports baseline sitting pulse rate (SD) as 72.0 (9.80), 75.0 (9.73), 72.0 (9.68), and 73.8 (8.91) for placebo, aliskiren 75 mg, 150 mg, and 300 mg.
CSPP100A2201 reports baseline sitting pulse as 72.8 (9.24), 72.9 (9.43), 72.2 (8.21) and 73.2 (8.51) for placebo, aliskiren 150 mg, 300 mg, and 600 mg, respectively. Also reported is baseline standing pulse rate as 76.1 (9.00), 76.3 (9.32), 76.0 (8.38) and 76.1 (8.95) for placebo, aliskiren 150 mg, 300 mg, and 600 mg, respectively. Aliskiren 600 mg produced a greater increase in trough sitting pulse than placebo at week eight (1.23 bpm versus ‐0.96 bpm; P = 0.0423). No other statistically significant differences between treatments were observed in either within‐treatment or between‐treatment analyses of trough sitting pulse at any time point.
CSPP100A2203 reports baseline sitting pulse as 71.6 (9.83), 72.0 (9.39), 72.3 (9.48) and 72.7 (9.22) for placebo, aliskiren 75 mg, aliskiren 150 mg, and aliskiren 300 mg, respectively. Also reported is baseline standing pulse rate as 76.3 (10.56), 77.0 (10.11), 76.4 (10.20, and 77.4 (10.02) for placebo, aliskiren 75 mg, aliskiren 150 mg, and aliskiren 300 mg respectively. Changes from baseline were minim al and generally similar across treatment groups.
CSPP100A2204 reports average baseline sitting and standing pulse rate as 72.2 bpm and 75.6bpm, respectively (page 60 of CSR). Summary statistics for baseline average sitting and standing pulse are provided in appendices, but not yet ascertained for the randomized and per protocol population.
CSPP100A2405 reports baseline sitting pulse rate as 73.8 (9.32), 72.8 (9.00), 73.8 (9.07) and 73.3 (9.03) for placebo, aliskiren 75 mg, 150 mg, and 300 mg, respectively (page 67 of CSR). There were no clinically meaningful differences in mean change from baseline in sitting pulse.
The journal‐published studies and clinical study synopses did not report on pulse rate. Therefore, due to very limited information available, these data have not been meta‐analyzed.
Mortality
No deaths were reported in six studies during single‐blind, double‐blind or withdrawal period (CSPA100A1301; CSPA100A2305; CSPP100A1301; CSPP100A2201; CSPP100A2308; CSPP100A2328.
Refer to Table 7 for detail information on cause of death in the following studies:
| Study identifier | Single‐blind period | Double‐blind period | Withdrawal period |
| No deaths | No deaths | No deaths | |
| No deaths | No deaths | No deaths | |
| One death in placebo group during observation period due to pancreatic carcinoma and metastases to liver. | One death occurred in aliskiren 150 mg monotherapy on day 41 of the study, due to drug intoxication. The overdose was attributed to a psychiatric drug prescribed prior to start of the study, and a diagnosis of manic depressive psychosis was recorded. | No deaths | |
| No deaths | No deaths | No deaths | |
| No deaths | No deaths | No deaths | |
| No deaths | One death occurred in the placebo arm on day 16 from "natural causes". One death occurred in the valsartan 160 mg arm (day 26) as a result of motor vehicle accident. | No deaths | |
| No deaths | One death was reported in the Aliskiren/HCTZ 150/25 mg group due to thoracic trauma from a motor vehicle accident. | No deaths | |
| No deaths | No deaths | No deaths | |
| 1 death occurred in placebo run‐in phase. Patient hospitalized due to supraventricular tachycardia and abdominal pain and died due to stroke after discontinuing from the study. | No deaths | One death occurred in the aliskiren/HCTZ 150/300 mg group from acute bronchopneumonia and associated sepsis during the 30‐day follow‐up period after discontinuing the study. | |
| No deaths | 2 deaths reported. 1 death in aliskiren group on day 41 due to myocardial infarction. 1 death in valsartan group on day 13 and the cause was reported as hypertensive arteriosclerotic cardiovascular disease with SAE of sudden death. There were no deaths in placebo arm or combination therapy group. | No deaths | |
| No deaths | No deaths | No deaths | |
| One death occurred during the follow‐up period of the study from pneumonia leading to respiratory failure (day of death not provided). Further information regarding this death is described in the patient narratives of the CSR, which we do not have access to yet. Within the CSR body, the arm of the study that this participant belongs to has been redacted. In the 2008 FDA Medical Review report, a death due to colon cancer is reported as occurring within CSPP100A2204 (N = 1, aliskiren 300 mg). The medical review states "no other details were provided". For our comments on this please see Discussion. |
CSR: clinical study report; HTCZ: hydrochlorothiazide; SAE: serious adverse event
During the single‐blind period, there was one death in the placebo group in two studies, CSPP100A1201 and CSPP100A2323.
During the double‐blind period, there was one death in aliskiren 150 mg group in study CSPP100A1201; one death in placebo group in study CSPP100A2203; one death in aliskiren/HCTZ 150 mg/25 mg group in study CSPP100A2204; and one death in aliskiren group in study CSPP100A2327.
During the withdrawal period there was one death in the aliskiren/HCTZ 150 mg/300 mg group in study CSPP100A2323 and one death in aliskiren 300 mg in study CSPP100A2405.
Due to the short duration of these studies and a very low incidence of death, the outcome of death was not meta analyzed.
Non‐fatal serious adverse events (SAE)
During the double‐blind treatment period two SAEs were reported in the aliskiren monotherapy group in study CSPP100A2203; two SAEs in the aliskiren monotherapy group in study CSPP100A2204; four SAEs in aliskiren 150 mg to 600 mg aliskiren monotherapy arms in study CSPP100A2308; eight SAEs in aliskiren monotherapy arms in study CSPP100A2327; four SAEs in aliskiren monotherapy arm in study CSPP100A2328; and five SAEs in aliskiren monotherapy arm in study CSPP100A2405. Refer to Table 8 for detailed information.
| Study identifier | Wash out period | Single blind period | Double blind period | Withdrawal period |
| Not reported | Not reported | No SAEs were reported in the aliskiren monotherapy group. 2 in amlodipine 2.5 mg; 1 in amlodipine 5 mg; and 1 in aliskiren 150/amlodipine 5 mg combination arm (detail regarding SAE was not reported) | Not reported | |
| Not reported | Not reported | 9 SAEs reported. 2 in placebo and 7 in other treatment groups (retinal detachment, abdominal mass, bronchitis, calculus ureteric, gastroenteritis, hand fracture, hydronephrosis, pneumonia, and cerebrovascular accident). None occurred in aliskiren 150 mg or 300 mg groups. | Not reported | |
| Not reported | Reported 1 SAE in the single‐blind period ‐ infectious enterocolitis/dehydration (N = 1, 55M, placebo). | 2 SAEs in the double‐blind period: cerebral infarction (N = 1, 69F, placebo); acute myocardial infarction (N = 1, 58F, aliskiren 75 mg). | Not reported | |
| Not reported | Not reported | 2 SAEs reported. 1 SAE in placebo due to myocardial infarction. The other in the losartan 50 mg group due to right medullary infarction. None in aliskiren 150 mg group. | Not reported | |
| One in washout period ‐ transient Ischaemic attack | Two in the single‐blind run‐in phase ‐ anxiety; and diverticulitis and | 2 SAEs in irbesartan group ‐ Intravertebral disc protrusion; and bipolar depression. | Two in the withdrawal period in placebo group ‐ Left ventricular failure; and gout and infective arthritis). | |
| Not reported | Reported 6 SAE. 1 bile duct cancer, 1 diverticulitis, 1 pregnancy, 1 mild melanorrhagia and gastric pain, 1 myocardial infarction, and 1 squamous cell carcinoma. | 8 SAEs reported. 2 in Placebo: 1 due to natural cause and 1 due to myocardial infarction requiring hospitalization on day 46 resulting in study drug discontinuation. 1 in aliskiren 75 mg on day 4 coronary artery disease requiring hospitalization resulting in study discontinuation. Patient required quadruple bypass surgery. 1 in aliskiren 300 mg on day 42 pregnancy resulting in study discontinuation. 1 in valsartan 160 mg on day 28 ‐ angioneurotic oedema resulting in study discontinuation, dyspnoea and chest pressure. Aliskiren 150 mg/valsartan 160 mg (N = 1, Day 29) motor vehicle accident requiring hospitalization; not discontinued from study. | 1 in aliskiren 75 mg/valsartan 80 mg on day 3 post‐study. | |
| Not reported | 4 SAE due to (lung cancer, fractured leg, angina and urinary tract infection) | 28 patients experienced at least 1 SAE in the monotherapy and combination therapy treatment groups. 1 in A 75 mg ‐ due to renal colic; 1 in aliskiren 150 mg due to haemorrhagic diarrhoea; none in aliskiren 300 mg; 1 in HCTZ 6.25 mg due to neoplasm of skin; 3 in HCTZ 12.5 mg ‐ due to breast cancer, joint injury and pregnancy; 2 in HCTZ 25 mg due to deep vein thrombosis and lymphadenopathy; 5 in aliskiren 75 mg/HCTZ 12.5 mg due to (diplopia, IIIrd nerve paresis, mood disorder, phlebothrombosis, psychotic disorder, small intestinal obstruction and syncope); 4 in aliskiren 75 mg/HCTZ 25 mg due to (cerebral infarction, dysarthria, physical disability, pregnancy and renal colic); 2 in aliskiren 150 mg/ HCTZ 6.25 mg ulcerative colitis, pregnancy); 3 in aliskiren 150 mg/ HCTZ 12.5 mg (non‐cardiac chest pain, pregnancy, syncope); 2 in aliskiren 150 mg/ HCTZ 25 mg (lung neoplasm, road traffic accident); 2 in aliskiren 300 mg/ HCTZ 12.5 mg (diabetes mellitus, lung disorder); 1 in aliskiren 300 mg/HCTZ 25 mg (coronary artery disease). There were no SAEs in the placebo group. | Not reported | |
| 2 SAEs reported during washout period ‐ 1 due to subarachnoid haemorrhage due to rupture of cerebral aneurysm on day 8; 1 due to partial small bowel obstruction on day 13 | One SAE ‐ bladder carcinoma prior to randomization and before receiving double‐blind study drug. Patient was randomized to placebo but later discontinued due to unsatisfactory therapeutic effect; | 4 SAEs reported. 1 SAE in aliskiren 150 mg on day 27‐ unstable angina and increased blood pressure; 2 SAEs in aliskiren 300 mg ‐ hospitalization for acute appendicitis on day 34; hospitalization for depression on day 51; 1 SAE in aliskiren 600 mg of hospitalization for pain/bodily injury on day 35. No SAE reported in the placebo group. | One SAE was reported during withdrawal period in aliskiren 150 mg of serious venous occlusion and thrombosis of the right eye on day 8 withdrawal period). | |
| data at week 3 and week 6 not available | Not reported | Not reported | During 26 weeks double‐blind treatment period 19 SAEs reported. 10 in aliskiren group and 9 in HCTZ group. | Not reported |
| not reported | Not reported | 20 SAEs reported. 5 in placebo group ‐ atrial flutter, cerebrovascular accident, headache, hypertension, hypertensive crisis and ventricular tachycardia); 8 in aliskiren group ‐ 1 gastritis, 1 grand mal convulsions, 1 intestinal poly, 2 myocardial infarction, 1 non‐cardiac chest pain, 1 peripheral vascular disease, 1 acute renal failure); 6 in valsartan group ‐ 1 angina pectoris, 1 arteriosclerosis, 1 breast cancer, 1 bronchitis, 1 COPD, 1 facial paresis, 1 ovarian cancer, 1 pneumonia, 1 pulmonary oedema); 3 in aliskiren/valsartan group ‐ 1 aortic aneurysm, 1 intravertebral disc protrusion, 1 prostate cancer and 1 thyroidectomy). The FDA medical review (2007) also reported two cases of renal carcinoma occurred (N = 1, placebo, day 20 post‐study; N = 1, aliskiren, day 44 post‐study). For our comments on this, please see Discussion. | Not reported | |
| 11 patients had serious AEs during the washout and placebo run‐in periods (detail of which are not provided). | Not reported | 1 SAE was reported in aliskiren 75 mg group ‐ cardiac chest pain; 2 in aliskiren 150 mg group ‐ rectal bleeding due to anal ulcer and episode of secondary anaemia; basal cell carcinoma; 1 in the 300 mg group ‐TIA with concomitant nausea and dyspnoea; and 2 in the placebo group ‐ prostate cancer; accelerated hypertension with left facial numbness. | Not reported | |
| Reported 2 SAEs in the washout period; Detail not provided in CSR. | Three in the single‐blind placebo run‐in period. Detail not provided in CSR. | 6 SAEs were reported. 2 in aliskiren 75 mg group ‐ erysipelas (skin infection) and osteoarthritis; 3 in aliskiren 150 mg group ‐ severe glaucoma; moderate haemorrhoids; and mild hemorrhagic stroke; none in aliskiren 300 mg group; and 1 in the placebo group ‐ vertigo, wrist fracture, concussion, and head contusion subsequent to a fall. | Not reported |
COPD: chronic obstructive pulmonary disease; CSR: clinical study report; HTCZ: hydrochlorothiazide; SAE: serious adverse event; TIA: transient ischaemic attack
In addition, the FDA Medical Review 2007 reports further details for the following studies.
CSPP100A1201 : The FDA medical review reports events of cancer in aliskiren monotherapy arms, and lists two SAEs of rectal cancer (n = 1, in 59 Asian women, aliskiren 75 mg, day 75) and (n = 1, in 54 Asian men, aliskiren 75 mg, day 330). However, the study identifiers within the FDA medical review have been redacted.
The clinical study synopsis for CSR 1202, an open‐label, extension study of CSPP100A1201, lists two events of rectal cancer as reasons for study discontinuation (n = 1, aliskiren monotherapy; n = 1, aliskiren + diuretic), and the corresponding journal‐published study Kushiro 2006 also reports two events of rectal cancer, but does not report the arm of the study in which they occurred, and states "these were considered by the investigators to be present at the commencement of the study." Provided that the regulator reviewed only one study of Japanese participants (CSPP100A1201), and given the rectal cancer outcomes reported in Kushiro 2006, we believe the SAEs pertain to the extension study of CSPP100A1201. Unfortunately, due to the redactions in the FDA medical review and the lack of access to the unabridged CRSs (including patient narratives), we cannot confirm this. These SAEs are not described in the body of CSR 1201.
CSPP100A2327: The FDA Medical Review (2007) reported a case of an isolated seizure (n = 1, aliskiren, day 3). The participant was hospitalized with a diagnosis of grand mal seizure, despite no previous history of seizures. The regulator notes that imaging studies and EEG were not provided in the initial report provided. The FDA medical review (2007) also reported two cases of renal carcinoma occurred (n = 1, placebo, day 20 post‐study; n = 1, aliskiren, day 44 post‐study). For our comments on this, please see Discussion.
CSPP100A2204: The three aliskiren monotherapy groups each had one patient with SAEs. Though these are reported within the CSR body, they are described in greater detail in the 2007 FDA medical review: renal colic necessitating hospitalization (n = 1 in 44 men, aliskiren 75 mg, day 19); hematuria and urinary retention necessitating catheterization to unblock a clot (n = 1 in 80 men, aliskiren 150 mg, day 19); and rectal bleeding from a stenosing tumour, which was confirmed as adenocarcinoma (n = 1 in 69 men, aliskiren 300 mg, day 46) aliskiren 300 mg group). Study medication was not discontinued. We note that CSPP100A2204 reports this latter SAE only as "hemorrhagic diarrhoea". This explains the reason that the FDA medical review (2007) reports an event of colon cancer (n = 1 in 69 men, aliskiren 300 mg, day 59), though this event is not reported in CSPP100A2204. It is unfortunate that we cannot confirm at this time whether this patient is the same patient or different from the reported event of death due to colon cancer within the 2008 FDA medical review.
During the single‐blind period, the FDA medical review (2007) reports chest pain and headache (n = 1 in 42 women, aliskiren 300 mg, day 25), which developed into cardiac ischaemia with diagnosis based on ST depression in V3‐5 not present on the baseline ECG after one dose of blinded study medication. The study drug was discontinued. The patient was subsequently hospitalized for unstable angina. HCTZ monotherapy had oneSAE in the 6.25 mg group; three SAEs in the 12.5 mg group and two SAEs in the 25 mg group. An event of colon cancer is reported in the FDA medical review (2007) (n = 1, HCTZ 25 mg, day 57), though this is not described in CSPP100A2204. Combination treatment groups had a total of 19 SAEs. The FDA medical review (2007) reports a stroke (n = 1, in 67 men, aliskiren 150 mg/HCTZ 6.25 mg) 19 days after the participant discontinued from the study. This SAE is not described within the body of CSPP100A2204.
Any adverse event
Reporting of total adverse events varies between studies since journal‐published studies did not report details regarding adverse events. In the 12 included studies, total adverse events in aliskiren groups ranged from 7.8% to 55% and from 21% to 50% in the placebo group. The most frequent events being ‐ headache ‐ ranged from 1.7% to 7.8% in aliskiren treatment groups and from 3.4 % to 13.5% in the placebo group; diarrhoea ‐ ranged from 0.8% to 11.4% in aliskiren treatment groups and from 0.5 % to 1.7% in the placebo group; dizziness ‐ ranged from 1.2% to 5.3% in aliskiren treatment groups and from 2% to 4.2% in the placebo group; and fatigue ‐ ranged from 0.8% to 3.8% in aliskiren treatment groups and from 1% to 3.1% in the placebo group. Refer Table 9 for additional details.
| Study Identifier | Total AEs and Common AEs as reported in the corresponding available CSR A = Aliskiren; P = Placebo |
| Total AEs: A150 =15(7.8%); A 300 = 18(8.9%) and P = 22(7.7%) Headache: A150 =13( 6.7%); A 300 =15(7.4%) and P = 20(10.1%) Periphaeral edema: A150 = 2(1.0%); A 300 = 3(1.5%) and P = 2(1%) | |
| Total AEs: A150 = 40.1%; A 300 = 46.7%; A 600 = 52.4%; P = 43% Headache: A150 = 12(7%); A300 = 13(7.7%); A600 = 9(5.4%); P = 16(9.7%) Nasopharyngitis: A 150 = 5(2.9%); A 300 = 6(3.6%); A 600 = 3(1.8%); P = 10(6.1%) Dizziness: A 150 = 2(1.2%); A 300 = 9(5.3%); A 600 = 5(3%); P = 7(4.2%) Diarrhoea:A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 19 (11.4%); P = 2(1.2%) Nausea: A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 0%; P = 4(2.4%) | |
| Total AEs: A75 = 61(53%); A150 = 58(51.8%); A 300 = 62(54.9%); P = 58(50.4)% Headache: A75 = 3(2.6%); A 150 = 3(2.7%); A 300 = 6 (5.3%) and P = 4(3.5%) Nasopharyngitis:A75 = 24(20.9%); A150 = 20(17.9%); A300 = 20(17.7%); P = 16(13.9%) Back pain:A75 = 2(1.7%); A150 = 0%; A300 = 0% and P = 0% Diarrhoea: A75 = 1(0.9%); A150 = 1(0.9%); A300 = 4(3.5%) and P = 1(0.9%) Vertigo: A75 =0%; A150 = 0%); A300 = 1(0.9%) and P = 2(1.7%) | |
| Total AEs: A150 = 152(50.3%), P = 66(42.3%) Nasopharyngitis: A150 = 48(15.9%), P = 13(8.3%) Headache: A150 = 9(3.0%), P = 6(3.8%) Occult blood positive: A150 = 9 (3.0%), P =5 (3.2%) | |
| Total AEs: A150 = 127 (26.8%); A300 = 130 (36.2%); A600 = 43 (33.1%); P = 32 (32.1%) Headache: A150 = 3 (2.4%); A300 = 8 (6.2%); A600 = 6 (4.6%); P = 7 (5.3%) Diarrhoea: A150 = 2 (1.6%); A300 = 1 (0.8%); A600 = 9 (6.9%); P = 2 (1.5%) Dizziness: A150 = 2 (1.6%); A300 = 4 (3.1%); A600 = 3 (2.3%); P = 5 (3.8%) Fatigue: A150 = 1 (0.8%); A300 = 5 (3.8%); A600 = 2 (1.5%); P = 4 (3.1%) | |
| Total AEs: A75 = 63(35.2%); A150 = 59(33.1%); A300 = 50(28.6%) and P = 57(32.2%) Headache: A75 = 15(8.4%); A150 = 9(5.1%;) A 300 = 7(4.0%) and P = 15(8.5%) Fatigue: A75 = 7(3.9%); A150 = 4(2.2%;) A 300 = 4(2.3%) and P = 4(2.3%) Back pain: A75 = 2(1.1%); A150 = 4(202%;) A 300 = 3(1.7%) and P = 2(1.1%) Diarrhoea: A75 = 2(1.1%); A150 = 1(0.6%;) A 300 = 5(2.9%) and P = 3(1.7%) Dizziness: A75 = 4(2.2%); A150 = 4(2.2%;) A 300 = 3(1.7%) and P = 2(1.1%) | |
| Total AEs: A75 = 69(37.5%); A150 = 69(37.3%); A300 = 71 (39.2%); P = 85(44%) Headache: A75 = 13(7.1%); A 150 = 13(7%); A 300 = 10 (5.5%); P = 26 (13.5%) Nasopharyngitis: A75 = 9( 4.9%); A150 = 5(2.7%;) A300 = 3 (1.7%); P = 10 (5.2%) Diarrhoea: A75 = 3(1.6%); A150 = 3(1.6%); A300 = 4(2.2%); P = 1(0.5%) Cough: A75 = 1(0.5%); A150 = 2(1.1%); A300 = 1(0.6%); P = 1(0.5%) Dizziness: A75 = 1(0.5%); A150 = 1(0.5%); A300 = 3(1.7%); P = 2(1.0%) Peripheral edema: A75 = 4(2.2%); A150 = 3(1.6%); A300 = 2(1.1%); P = 1(0.5%) | |
| Total AEs: A150 = 40.1%; A 300 = 46.7%; A 600 = 52.4%; P = 43% Headache: A150 = 12(7%); A300 = 13(7.7%); A600 = 9(5.4%); P = 16(9.7%) Nasopharyngitis: A 150 = 5(2.9%); A 300 = 6(3.6%); A 600 = 3(1.8%); P = 10(6.1%) Dizziness: A 150 = 2(1.2%); A 300 = 9(5.3%); A 600 = 5(3%); P = 7(4.2%) Diarrhoea: A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 19 (11.4%); P = 2(1.2%) Nausea: A 150 =2 (1.2%); A 300 = 3(1.8%); A 600 =0%; P = 4(2.4%) | |
| Total AEs at 6 weeks : A 26.4%; P = 28.5% (numbers not reported) Other AE were not reported at week 6. | |
| Total AEs: A150 to 300 = 149(34%) and P = 168(37%) Headache: A150 to 300 = 14(3.2%); P = 41(9%) Diarrhoea*: A150 to 300 = 2.3%; P = not reported Nasopharyngitis: A150 to 300 = 16(3.7%) ; P = 9(2%) Dizziness: A 150 to 300 = 8(1.8%) ; P = 9(2%) Fatigue:A 150 to 300 = 4(0.9%) ; P = 5(1.1%) Nausea: A 150 to 300 = 6(1.4%) ; P = 11(2.4%) | |
| Total AEs: A75 = 57(37.3%); A150 = 59(34.7%); A300 = 56(35.4%); P = 60(37.5%) Headache: A75 = 12(7.8%); A150 = 7(4.1%); A300 = 10(6.3%) and P = 9(5.6%) Nasopharyngitis: A75 = 2(1.3%); A150 = 2(1.2%); A300 = 5(3.2%) and P = 3(1.9%) Diarrhoea: A75 = 7(4.6%); A150 = 2(1.2%); A300 = 1(0.6%) and P = 1(0.6%) Cough: A75 = 1(0.7%); A150 = 4(2.4%); A300 = 1(0.6%) and P = 3(1.9%) Dizziness: A75 = 1(0.7%); A150 = 5(2.9%); A300 = 1(0.6%) and P = 3(1.9%) | |
| Total AEs: A75 = 36(18.8%); A150 = 41(21.7%); A300 = 36(19.1%); P = 39(21.0%) Headache: A75 = 4(2.1%);A150 = 2(1.1%); A300 = 1(1.1%); P = 4(2.1%) Influenza: A75 = 1(0.5%); A150 = 4(2.1%); A300 = 1(1.1%); P = 1(0.5%) |
Withdrawals due to adverse events
Withdrawals due to adverse effects between placebo and aliskiren at 75 mg, 300 mg and 600 mg dose did not differ (see Analysis 1.3). Aliskiren 75 mg versus placebo ‐ (RR 0.59, 95% CI 0.33 to 1.07; participants = 1653; studies = 5; I2 = 0%); aliskiren 300 mg versus placebo ‐ (RR 0.70, 95% CI 0.47 to 1.03; participants = 4216; studies = 10; I2 = 0%); and aliskiren 600 mg versus placebo ‐ (RR 0.56, 95% CI 0.19 to 1.64; participants = 592; studies = 2; I2 = 0%).
Compared to aliskiren 150 mg group, withdrawals due to adverse effects were greater in the placebo group mostly due to lack of therapeutic effect. Aliskiren 150 mg versus placebo ‐ (RR 0.46, 95% CI 0.30 to 0.71; participants = 3421; studies = 10; I2 = 16%). CSPP100A2323 was not included in this comparison as the authors only report a combined total withdrawal rate n = 22 (3.9%) for both aliskiren and placebo treatment groups.
For withdrawals due to adverse effects, there was no statistically significant heterogeneity at 75 mg, 150 mg, 300 mg or 600 mg dose. For 75 mg, 300 mg and 600 mg dose, the I2 was 0%. For the 150 mg dose, when CSPP100A2201 was removed from the analyses, the I2 was reduced from 16% to 0%. The reason for heterogeneity could not be determined.
Refer to Table 10 for detail regarding reasons for withdrawal from included studies.
| Study Identifier | Reasons for increased withdrawal due to adverse events |
| Not reported. | |
| Not reported. | |
| Significant adverse events occurred for N = 7 patients, leading to discontinuation from study, as described below.
| |
|
| |
| A total of N = 18 patients discontinued from the study due to AEs. It was suspected that 2/3 of these discontinuations were for reasons drug‐related. Headaches resulted in the discontinuation for N = 3 patients. | |
| Most frequent reasons for 12 discontinuations are: fatigue (N = 5, 0.4%), headache (N = 3, 0.3%), diarrhoea (N = 2, 0.2%), and peripheral oedema (N = 2, 0.2%). Reasons for 14 discontinuations from AE (safety population).
The FDA medical review reports facial oedema (N = 1, 0.6%) that appears to be angioedema, resulting in discontinuation from study; however, this is not reported in CSR 2203.
| |
| The range of AEs resulting in study discontinuation is 0% is the aliskiren 150 mg arm of the study to 4.4% in the aliskiren 300 mg arm (N = 7). The most frequent AE resulting in study discontinuation is headache (N = 12, 0.4%). Reasons for discontinuations from adverse events ‐ N = 62 (2.2%).
Additionally four patients discontinued from the study due to abnormal laboratory values.
| |
| Most frequent reasons for 9 discontinuation from study: headache (N = 4), dizziness (N = 3), and diarrhoea (N = 2). Reasons for discontinuations from AE (safety population).
We question that a participant withdrew for the reason specified as "unsatisfactory effect" as opposed to being categorized as a discontinuation from an SAE as the event necessitated hospitalization. On Day 27 in CSPP100A2308, the participant (N = 1, 64F, aliskiren 150 mg) was hospitalized for unstable angina and increased blood pressure. The unstable angina was treated with concomitant medication and her condition resolved within 3 days. | |
| We do not have data at week 3 and week 6 for this study. | |
| The range of AEs resulting in study discontinuation is 8 (1.8%) is the aliskiren arm; 8 (2.6%) in valsartan arm; 6 (1.3%) in aliskiren/valsartan combination arm; and 11 (2.4%) in the placebo arm. Reasons for discontinuations from adverse events ‐ N = 37 (2%).
| |
| Aliskiren 75 mg = 3 (2.0%); aliskiren 150 mg = 5 (2.9%); aliskiren 300 mg = 4(2.5%) . Placebo = 4 (2.5%). Reasons for withdrawal were not reported. | |
| Reasons for 18 discontinuations from AE (safety population)
|
Specific adverse events
Dry cough as an adverse event was reported in the following five studies: refer Analysis 1.4.
-
CSPP100A1201: aliskiren 150 mg n = 1 (0.9%)
-
CSPP100A2201: placebo n = 1 (0.8%), aliskiren 150 mg n = 2 (1.6%), aliskiren 300 mg n = 4 (3.1%)
-
CSPP100A2203: placebo n = 2 (1.1%), aliskiren 75 mg n = 2 (1.1%), aliskiren 150 mg n = 5 (2.8%), aliskiren 300 mg n = 1 (0.6%)
-
CSPP100A2204: aliskiren 300 mg n = 1 (0.6%)
-
CSPP100A2328: placebo: n = 3 (1.9%), aliskiren 75 mg n = 1 (0.7%), aliskiren 150 mg: n = 4 (2.4%), aliskiren 300 mg: n = 1 (0.6%).
Cough was not reported in the other seven studies (CSPA100A2305; CSPP100A2308; CSPP100A2405; CSPA100A1301;CSPP100A1301; CSPP100A2327; and CSPP100A2323).
Based on five studies in 2886 patients, the incidence of cough did not differ between aliskiren (75 mg to 600 mg dose) as compared to placebo (RR 1.14, 95% CI 0.49 to 2.64; I2 = 0%).
Diarrhoea
Refer to Analysis 1.5 and to summary of findings Table for the main comparison graded as low‐quality evidence.
Aliskiren at 75 mg to 300 mg dose as compared to placebo showed a trend towards increase in incidence of diarrhoea. However at a dose of 600 mg, a substantial increase was observed (from 14 per 1000 in placebo group to 95 per 1000 in aliskiren 600 mg group):
-
aliskiren 75 mg: (RR 2.21, 95% CI 0.85 to 5.76; participants = 1276; studies = 4; I2 = 13%);
-
aliskiren 150 mg: (RR 1.64, 95% CI 0.78 to 3.46; participants = 2277; studies = 7; I2 = 0%);
-
aliskiren 300 mg: (RR 1.84, 95% CI 0.89 to 3.81; participants = 2268; studies = 7; I2 = 0%); and
-
aliskiren 600 mg: (RR 7.00, 95% CI 2.48 to 19.72; participants = 592; studies = 2; I2 = 0%).
Angioedema:
No journal‐published study reported angioedema. The FDA Medical Review 2007 reporting angioedema are the following.
-
CSPP100A2203 reports angioneurotic oedema occurred in valsartan 160 mg (n = 1, 1.7%). In the FDA medical review, this is described as "moderate facial edema diagnosed as angioedema. Drug was discontinued and the edema resolved. This patient had a similar episode with an ACE inhibitor." The FDA medical review also reports an event of facial oedema (n = 1) that appears to be angioedema, beginning with treatment and resolving eight days after discontinuing treatment. A third event of ankle and periorbital oedema (n = 1) is reported in the aliskiren 300 mg/valsartan 320 mg group beginning five days into treatment and resolving three days after discontinuing treatment.
-
CSPP100A2204 reports 'peripheral oedema' (n = 1, aliskiren 300 mg) as a reason for study discontinuation. However, the FDA medical review (2007) reports a separate event in greater detail: "A 61 year‐old diabetic white male in the aliskiren 75mg group developed dyspnoea and edema of the lower limb to the knee, hands and face (eyelid but no lip or tongue) on day 22. He also had purplish‐blue lesions with angiomatous aspect on the upper part of his trunk. He completed the study with continuation of the edema and also developed anorexia, weight loss, tachycardia and elevated CRP." The regulator comments that "the participant was diagnosed with scleredema, and that the investigator confirmed that there was no evidence of heart failure at baseline and suspected angioedema."
-
CSPP100A2308 ‐The FDA medical review describes that a patient in the aliskiren 300 mg group reported "Oedema hands and feet" on day 53. The regulator suggests this case may represent angioedema.
Subgroup analysis from FDA medical review
The FDA Medical Review 2007 provided additional information regarding mean placebo‐corrected change from baseline in BP by dose and gender (Table 1); dose and age (Table 2); and dose and race (Table 3) from five placebo‐controlled studies (CSPP100A1201; CSPP100A2201; CSPP100A2203; CSPP100A2204; CSPP100A2308).
The FDA medical reviewer comments: "In the multivariate regressions aliskiren shows a clear dose‐response from 75 through 600 mg for both SBP and DBP. Blacks show a reduced response and Asians an increased response with statistically significant interaction term with aliskiren use for Asians for DBP and almost statistically significant for Blacks for DBP. The interaction term for age > 65 years and drug is statistically significant for SBP. There appear to be differences in response in various sub groups with aliskiren use. Blacks respond poorly to aliskiren monotherapy while Asians and the elderly may respond to lower doses."
Discussion
To our knowledge this is the first systematic review to use clinical study reports (CSRs) and other regulatory documents to report on a more comprehensive set of efficacy and safety data regarding dose‐related blood pressure lowering efficacy of renin inhibitors versus placebo. As a result, this updated review provides more detailed information regarding mortality, non‐fatal serious adverse events, total adverse events and specific adverse events. Though we found outcome reporting to be far more extensive within CSRs as compared to journal‐published studies, even access to these regulatory documents remain insufficient as a means to correct for reporting bias. Our attention was drawn to the discrepant reporting or non‐reporting of important safety outcomes, discovered by closely reading the FDA medical review of Dr. Thomas Marciniak. These findings have increased our awareness for the need to access the patient narratives on adverse events located within the appendices of CSRs. Further to this, individual patient data and access to all case reports and narratives are likely needed to report more completely on the harms and efficacy of aliskiren. Given the EMA can provide de‐identified patient narratives on serious and non‐serious adverse events, we question the reasons the sponsor, Novartis, has for not providing any case narratives to researchers who are granted data‐sharing privileges through the CSDR platform. Though obtaining the data from CSR bodies has increased our ability to report more extensively on efficacy and safety outcomes, we remain unable to accurately depict the clinical safety, usefulness and value of aliskiren. As to whether aliskiren results in greater benefit than harm to patients remains unclear. This is a direct result of not having access to the entire complement of evidence.
We have not identified any randomized controlled trials (RCTs) evaluating the effectiveness of renin inhibitors as compared to placebo in reducing mortality and morbidity, as well as documenting the long‐term reduction in systolic blood pressure (SBP) and diastolic blood pressure (DBP), which is the goal of antihypertensive therapy.
Zhang 2015 conducted a systematic review of RCTS comparing the effect of aliskiren versus non‐aliskiren therapy on major cardiovascular outcomes in clinically diverse patients with hypertension. Based on six studies (ALTITUDE 2012; AVOID 2011; AVANT GARDE ‐ TIMI 43 2010; ASPIRE 2011 ; ASTRONAUT 2013; and AQUARIUS 2013), reporting data in 12,465 patients, the authors concluded that aliskiren had no effect (RR with 95% CI) on major cardiovascular events 0.93 (0.77 to 1.13), total mortality 1.00 (0.77 to 1.29), cardiac death 1.01 (0.79 to 1.29), myocardial infarction 0.71 (0.36 to 1.38), or stroke 0.87 (0.48 to 1.58).
Summary of main results
Aliskiren at all doses lowered both SBP and DBP as compared to placebo ranging from ‐3.0 to ‐11.4 mmHg for SBP and ‐2.1 to ‐5.9 mmHg for DBP. There was a significant dose response for the 75 mg, 150 mg and 300 mg doses. The effect of 600 mg of aliskiren was not different from 300 mg. Information in this update from nine available CSRs obtained from EMEA did not result in altering the magnitude of reduction in mean sitting systolic blood pressure (MSSBP) and mean sitting diastolic blood pressure (MSDBP) at any dose as compared to placebo from the previous 2011 update as the 95% confidence intervals (CIs) overlapped.
Comparison between various doses within the same study showed that aliskiren 150 mg lowered SBP/DBP more than aliskiren 75 mg (‐1.9/‐0.8 mmHg); aliskiren 300 mg lowered SBP/DBP more than aliskiren 150 mg (‐2.6/‐1.8 mmHg); and aliskiren 600 mg lowered SBP/DBP more than aliskiren 150 mg ‐3.4/‐2.2 mmHg, However, SBP/DBP lowering did not differ between aliskiren 600 mg versus aliskiren 300 mg.
The magnitude of the BP lowering with aliskiren at the maximum recommended dose of 300 mg daily ‐7.9/‐4.5 mm Hg is similar to what has been determined using similar methods in systematic reviews of angiotensin‐converting‐enzyme inhibitors (ACEIs), ‐8/‐5 mm Hg and angiotensin receptor blockers (ARBs) ‐8/‐5 mm Hg (Heran 2008a; Heran 2008b; Law 2003). Direct comparison of renin inhibitor with ARBs from the three placebo‐controlled studies included in this systematic review that compared aliskiren 300 mg with ARBs also support this finding.
-
CSPP100A2327 study: aliskiren 300 mg lowered MSSBP as well as MSDBP as compared to baseline to a similar extent as valsartan 320 mg at week eight (SBP : ‐13.0 mm Hg versus ‐12.8 mm Hg, respectively and DBP: ‐9.0 mm Hg versus ‐9.7 mm Hg, respectively).
-
CSPP100A2203 study: aliskiren 300 mg lowered MSSBP as well as MSDBP as compared to baseline to a similar extent as valsartan 320 mg at week eight (SBP: ‐5.08 mm Hg versus ‐6.54 mm Hg and DBP: ‐ 3.67mm Hg versus ‐2.69 mm Hg).
-
CSPP100A2201 study: aliskiren 150 mg lowered MSSBP as well as MSDBP as compared to baseline to a similar extent as irbesartan 150 mg at week eight (SBP: ‐6.01 mm Hg versus ‐7.21 mm Hg and DBP: ‐2.94 mm Hg versus ‐2.54 mm Hg).
This suggests that inhibiting the renin angiotensin system at different sites does not lead to any clinically different BP lowering effects. Adequately powered RCTs assessing different classes of drugs in head‐to‐head studies are the best way to look for clinically significant differences in BP lowering effect.
The AHRQ review 2011 update by Powers 2011 concluded that studies evaluating the renin inhibitors aliskiren versus ACEIs and ARBs on mortality and morbidity outcomes were relatively short, and few deaths or cardiovascular events occurred, resulting in insufficient evidence to discern differences. They also reported that a random‐effects meta‐analysis of 23 RCTs comparing ACEIs and ARBs found no significant difference in the proportion of patients who achieved successful BP control on a single antihypertensive agent.
This update provides additional details on adverse events from CSRs. During the double‐blind period, there were two (0.04%) deaths and 27 (0.5%) non‐fatal serious adverse events in 5120 patients in the aliskiren monotherapy arm as compared to one (0.04%) death and 14 (0.6%) non‐fatal serious adverse events in 2319 patients in the placebo group. There were no differences in all‐cause mortality and non‐fatal serious adverse events. The durations (eight to 13 weeks) of the included studies were too short and were not adequately powered to detect differences in adverse events between aliskiren and placebo. Journal‐published studies and clinical study summaries listed only a few events, generally those with an incidence greater than 1% to 2.5% in any group, while others reported by broad systems affected by the drugs: cardiac, immune system, respiratory, etc. The most common adverse events reported were headache, nasopharyngitis, dizziness, and back pain, which were similar in placebo groups and aliskiren groups at all doses. Consistent with the FDA medical review, we have determined a statistically significant, dose‐dependent relationship between aliskiren 600 mg and diarrhoea. Due to the limited duration of the studies, it is impossible to determine whether diarrhoea is merely a marker for more serious adverse outcomes that may require longer periods of time to develop. This review found low‐quality evidence that with short‐term use, aliskiren does not increase withdrawal due to adverse effects as compared to placebo.
Overall completeness and applicability of evidence
There are numerous ways we have experienced a lack of access to the entire complement of evidence and encountered limitations accordingly. Although this update is based primarily on CSRs, which are far more comprehensive than journal‐published trials, we were only able to obtain nine CSRs of the 12 studies that met the inclusion criteria of this review, provided the EMA does not possess the other three studies. In addition, of the nine CSRs we have obtained, we do not yet possess the appendices, which contain valuable and revealing information (e.g. patient narratives), as has been previously discussed. Overt publication bias can be noted as two studies do not have a corresponding journal publications (CSPA100A1301 and CSPP100A1301), but can also be demonstrated by a lack of public trial registration. Five of 12 studies (42%) are not registered on ClinicalTrials.gov (CSPP100A1201; CSPP100A2201; CSPP100A2203; CSPP100A2327; CSPP100A2328). Of the seven studies that are registered, three trials (43%) do not have any study results posted on the ClinicalTrials.gov web site. These findings cause us to question the degree to which the 2007 US Food and Drug Administration Amendments Act is followed, as it mandates that all drugs and devices approved for use must be publicly registered at ClinicalTrials.gov within 24 months of trial completion, regardless of whether the trial is published. Of the studies included within this review, we have observed trials that are not journal‐published, not registered publicly, and are not possessed by the European regulatory authority. A combination of all three situations would effectively hide the existence of a trial, which could distort the evidence, mislead health policy and prescribing practices. Although we have tried to diligently include all available information from multiple sources, we have encountered various obstacles that limit our ability to evaluate the safety and efficacy of aliskiren as compared to placebo.
Safety Warning
Although aliskiren has shown a good safety and tolerability profile in short‐term studies in adult patients with hypertension, the Food and Drug Administration (FDA) has recently issued a warning about combining aliskiren with ACEIs and ARBs in patients diagnosed with diabetes or renal impairment.
A recent RCT (ALTITUDE 2012) was halted prematurely due to safety concerns when aliskiren 300 mg daily was used as an adjunctive therapy with an ACEI or an ARB. This study enrolled adults 35 years of age or older with type 2 diabetes and evidence of microalbuminuria, macroalbuminuria, or cardiovascular disease.
-
A second interim efficacy analyses of the data from 8561 participants found that adjunct therapy of aliskiren with ARBs or ACEIs (n = 4274) contributed to an increased rate of adverse events and did not reduce cardiovascular or renal outcomes as compared to placebo (n = 4287) after a median follow up of 32.9 months.
-
-
The primary endpoint was cardiovascular death or a first occurrence of cardiac arrest with resuscitation, myocardial infarction, stroke, unplanned hospitalization for heart failure, end stage renal disease, death attributable to kidney failure, or loss of kidney function, and doubling of baseline serum creatinine. It occurred in 783 (18.3%) of patients assigned to aliskiren as compared with 732 (17.1%) in the placebo arm (hazard ratio (HR), 1.08, 95% CI, 0.98 to 1.20; P = 0.12).
-
Within the primary endpoint, 19 participants experienced cardiac arrest requiring resuscitation versus eight patients assigned to placebo (HR, 2.40, 95% CI 1.05 to 5.48; P = 0.04).
-
-
-
The proportion of patients with hyperkalaemia (serum potassium level, ≥6 mmol/L) was significantly higher in the aliskiren group than in the placebo group (11.2% versus 7.2%), as was the proportion with reported hypotension (12.1% versus 8.3%) and diarrhoea (9.8% versus 7.3%) (P < 0.001 for all comparisons).
-
During the study, 1445 patients assigned to aliskiren (33.8%) and 1218 assigned to placebo (28.4%) discontinued the study drug permanently for a reason other than death (P = 0.001).
-
In the aliskiren group, 563 patients (13.2%) discontinued the study drug because of an adverse event, as compared with 437 patients (10.2%) in the placebo group (P < 0.001).
-
Favourable results for the aliskiren group were seen with respect to surrogate markers such as lower SBP and DBP measurements (1.3 and 0.6 mm Hg, respectively), and reduced urinary albumin excretion as compared to placebo.
However, the authors concluded that "A number of studies of various drugs have shown favourable changes in surrogate markers of disease progression, with subsequent studies of morbidity and mortality documenting a lack of clinical benefit or even harm. The present study documented more adverse events in the aliskiren group than in the placebo group without clinical benefits to offset them, which underscores the need to go beyond surrogate bio‐markers and obtain risk‐benefit data from clinical endpoint studies to better inform clinical decisions" (ALTITUDE 2012).
Harel 2012 conducted a systematic review comparing combined treatment using aliskiren and ACEIs or ARBs with monotherapy in 4814 participants from 10 RCTS. Harel also concluded that use of aliskiren in combination with ACEIs or ARBs is associated with an:
-
increased risk for hyperkalaemia as compared to ACEI or ARB monotherapy RR with 95% CI 1.58 (1.24 to 2.02) as well as aliskiren monotherapy RR with 95% CI 1.67 (1.01 to 2.79).
-
The risk of acute kidney injury did not differ significantly between the combined therapy and monotherapy groups (RR 1.14, 0.68 to 1.89).
Makani 2013 conducted a similar systematic review of combined treatment using aliskiren and ACEIs or ARBs with monotherapy with these agents in 68,405 participants from 33 RCTs. Makani concluded that although dual blockade of the renin‐angiotensin system may have seemingly beneficial effects on certain surrogate endpoints, it failed to reduce mortality and was associated with an excessive risk of adverse events such as hyperkalaemia, hypotension, and renal failure compared with monotherapy. The authors concluded that the risk to benefit ratio argues against the use of dual therapy.
Regulatory Marketing Authorization
Rasilez (aliskiren) remains approved with the EMA, but Tekturna (aliskiren) has been withdrawn. Enviage (aliskiren) and Sprimeo (aliskiren) have also been withdrawn with the EMA. But Tekturna (aliskiren) remains approved for marketing with the FDA. Additionally, for both the FDA and EMA, a number of combination treatments have had their marketing authorization withdrawn, through a combination of voluntary withdrawal and forced withdrawal from the regulatory body. U.S. Food and Drug Administration 2016.
Quality of the evidence
We used the GRADE approach to build a 'Summary of Findings' table as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We used the GRADE approach to rate the quality of the evidence as 'high', 'moderate' 'low', or 'very low' using the five GRADE considerations.
-
Risk of bias: not serious; serious; or very serious.
-
Inconsistency: not serious; serious; or very serious.
-
Indirectness: not serious; serious; or very serious.
-
Imprecision: not serious; serious; or very serious.
-
Publication bias: undetected; or strongly suspected.
We reported 'Summary of Findings' tables for SBP, DBP and diarrhoea. Although the inclusion criteria of the 12 studies was high quality to begin with ‐ randomized double‐blind studies, the overall quality of evidence is graded as moderate‐quality evidence for BP outcome and low‐quality evidence for adverse event outcomes because of a number of limitations in this systematic review. Refer to the 'Risk of bias' assessment of individual studies Figure 2 as well as reasons for downgrading evidence in the summary of findings Table for the main comparison.
Potential biases in the review process
Publication bias is evident as it has come to our attention that several studies were not registered on ClinicalTrials.gov (CSPP100A2201; CSPP100A1201; CSPP100A2327; CSPP100A2203; CSPP100A2328). The EMA does not possess CSRs of CSPP100A2328, CSPA100A2305, or CSPA100A1301.
CSPP100A1301 and CSPA100A1301 do not have corresponding journal‐published studies.
With respect to the dose‐ranging BP lowering effect size as reported above and shown in forest plots, this could be an overestimate of the magnitude of BP lowering due to publication bias and also because wide confidence intervals in effect size for some doses were observed. All included studies were sponsored by the manufacturer, Novartis. Requests for the unpublished studies have not been granted. It is possible that negative studies exist, which have not been published and therefore are not included in this meta‐analysis. In addition, the BP lowering efficacy estimate is over a short duration (eight to 13 weeks).
No study is available as yet evaluating effectiveness of long‐term renin inhibitor monotherapy on clinically relevant outcomes such as (mortality, cardiovascular and cerebrovascular morbidity).
Agreements and disagreements with other studies or reviews
Comparison with other systematic reviews
Several systematic reviews published by Weir 2007; White 2010; Gradman 2010; and Chen 2013 were limited to a smaller subset of included studies and provide very limited information regarding adverse events.
Weir 2007 published pooled analysis of antihypertensive efficacy, safety, and tolerability of the oral direct renin inhibitor aliskiren alone, and in combination with other antihypertensive agents, from seven randomized, multicentre studies in patients with hypertension. Data were available for 7045 patients (mean age 52.5 to 59.8 years, 50.2% to 72.5% men) with mild‐to‐moderate hypertension (mean sitting diastolic blood pressure (MSDBP) 95 mm Hg to 109 mm Hg) over treatment durations of six to eight weeks.
-
In placebo‐controlled studies, aliskiren reduced MSDBP from baseline by 8.6 to 12.1/7.2 to 10.3 mm Hg (75 mg), 8.7 to 13.0/7.8 to 10.3 mm Hg (150 mg), 14.1 to 15.8/10.3 to 12.3 mm Hg (300 mg), and 15.7 to 15.8/11.5 to 12.5 mm Hg (600 mg), compared with 2.9 to 10.0/3.3 to 8.6 mm Hg for placebo.
-
Aliskiren demonstrated comparable efficacy in patients aged > 65 years old or < 65 years old, in men and women, and it lowered BP effectively in all racial subgroups.
-
Rates of discontinuation due to adverse events were low (1.7% to 2.6%).
-
The most frequently reported adverse events with aliskiren were headache (5.7%), nasopharyngitis (4.4%), diarrhoea (2.6%), dizziness (1.8%) and fatigue (1.6%).
-
The overall incidence of adverse events with aliskiren monotherapy was similar to placebo (39.8% versus 40.2%, respectively).
-
The incidence of diarrhoea with aliskiren was higher than placebo due to a significantly higher rate with aliskiren 600 mg (P = .0001 versus placebo).
White 2010 published pooled analysis of safety and tolerability of aliskiren in more than 12,000 patients with hypertension. Twelve randomized double‐blind clinical studies in patients with hypertension were evaluated: eight short‐term (8‐week) placebo‐controlled studies and four long‐term (26‐ to 52‐week) active‐controlled studies.
In short‐term studies:
-
adverse events occurred in similar proportions of aliskiren 150 mg and 300 mg (33.6% and 31.6%, respectively) and placebo treatment groups (36.8%);
-
in placebo‐controlled studies, the incidence of angioedema ⁄ urticaria was lower in patients receiving aliskiren 150 mg (0.2%) and aliskiren 300 mg (0.3%) than in the placebo group (0.5%; RR, 0.31; 95% CI, 0.07 to 1.47 for 150 mg; RR, 0.57; 95% CI, 0.17 to 1.89 for 300 mg);
-
cough was not significantly different for aliskiren (0.7% to 1.4%) versus placebo (0.7%);
-
hyperkalaemia was 0.1% in aliskiren 300 mg group and 0.1% in the placebo group.
Analysis by individual aliskiren monotherapy dose demonstrated no significant increase in diarrhoea compared with placebo for patients receiving aliskiren 150 mg (RR, 1.18; 95% CI, 0.62 to 2.24) or 300 mg (RR, 1.62; 95% CI, 0.91 to 2.90). No patient receiving short‐term treatment with aliskiren 150 mg or 300 mg discontinued due to diarrhoea. There was no evidence for an increased risk of gastrointestinal bleeding or ulceration with aliskiren treatment over placebo in the short‐term studies (RR, 0.62; 95% CI, 0.06 to 6.87 for aliskiren 150 mg and RR, 0.57; 95% CI, 0.05 to 6.28 for aliskiren 300 mg).
Gradman 2010 published a pooled analysis of efficacy, safety and tolerability of monotherapy with the direct renin inhibitor aliskiren (150 mg and 300 mg) over eight to 12 weeks in women with mild‐to‐moderate hypertension with MSDBP > 95 and < 110 mmHg across eight randomized and double‐blind studies. Safety and tolerability were assessed in the five placebo‐controlled studies in the analysis.
-
Women participating in the studies tended to be slightly older than men (range of means 55.1–57.3 years versus 52.9 to 55.3 years), with a longer duration of hypertension (7.8 to 8.2 years versus 6.5 to 7.8 years).
-
There was a higher proportion of Black women (5.0% to 17.7%) than Black men (2.2% to 8.5%); conversely the proportion of Asian women (7.7% to 10.4%) was lower than the proportion of Asian men (12.8% to 25.5%).
-
Slightly more women than men were obese (40.8% to 42.8% versus 31.1% to 37.7%).
-
Baseline BP was similar in women and men.
In the 1527 women enrolled in these studies:
-
aliskiren 150 mg and 300 mg produced significantly greater BP reductions (14.1/11.0 and 16.1/12.3 mmHg, respectively) compared with placebo (7.2/7.6 mmHg; P < 0.0001);
-
BP reductions with aliskiren monotherapy in women were similar to those observed in men, and consistent across subgroups of age, metabolic syndrome and obesity;
-
the overall incidence of adverse events in women was similar with aliskiren treatment (150 mg, 42.3%; 300 mg, 46.0%); and placebo (39.0%);
-
adverse events with aliskiren were more frequent in women than in men, consistent with previous studies of gender differences in drug tolerability;
-
few patients in this analysis discontinued aliskiren treatment and the incidence was similar in women (150 mg, 7.3%; 300 mg, 5.8%); and men (150 mg, 6.8%; 300 mg, 6.6%);
-
discontinuation rates with placebo were higher than with aliskiren therapy for both women (12.5%) and men (12.9%), primarily because of the higher numbers of discontinuations due to unsatisfactory therapeutic effect with placebo (5.0% to 5.6%) than with active therapy (1.5% to 2.3%). The reasons for discontinuation from aliskiren treatment were similar across doses and genders, with adverse events (0.8% to 2.7%) and withdrawal of consent (0.9% to 2.3%) being the most commonly reported reasons.
Chen 2013 investigated the antihypertensive effects and tolerability of aliskiren in comparison with other antihypertensive drugs and placebo in patients with hypertension. Nine studies included study arms with placebo, involving 3541 patients. They did not provide dose‐ranging decrease in SBP and DBP. Results were only shown for aliskiren at 300 mg dose and it was significantly superior to placebo in lowering both DBP and SBP WMD ‐4.75, 95% CI ‐5.59 to ‐3.92, P < 0.00001; MD ‐8.12, 95% CI ‐9.61 to ‐6.63, P < 0.00001, respectively). The Chen 2013 meta‐analysis indicates that aliskiren is associated with a similar incidence of adverse events and discontinuation due to adverse events to placebo.

PRISMA Study flow diagram.

Methodological quality summary: review authors' judgments about each methodological quality item for each included study.

Forest plot of comparison: 1 Aliskiren vs. placebo, outcome: 1.1 Systolic BP.
Highly significant subgroup differences were observed therefore mean overall effect size across all doses is not shown. The number of placebo group patients are divided equally in dose ranging studies when more than one dose of aliskiren was used.
CSPP100A2308 study the SBP reduction in the treatment and placebo group are reported from the CSR page 61.
CSPP100A2405 study the SD for all treatment groups are calculated from SEM reported on page 7 in the CSR .
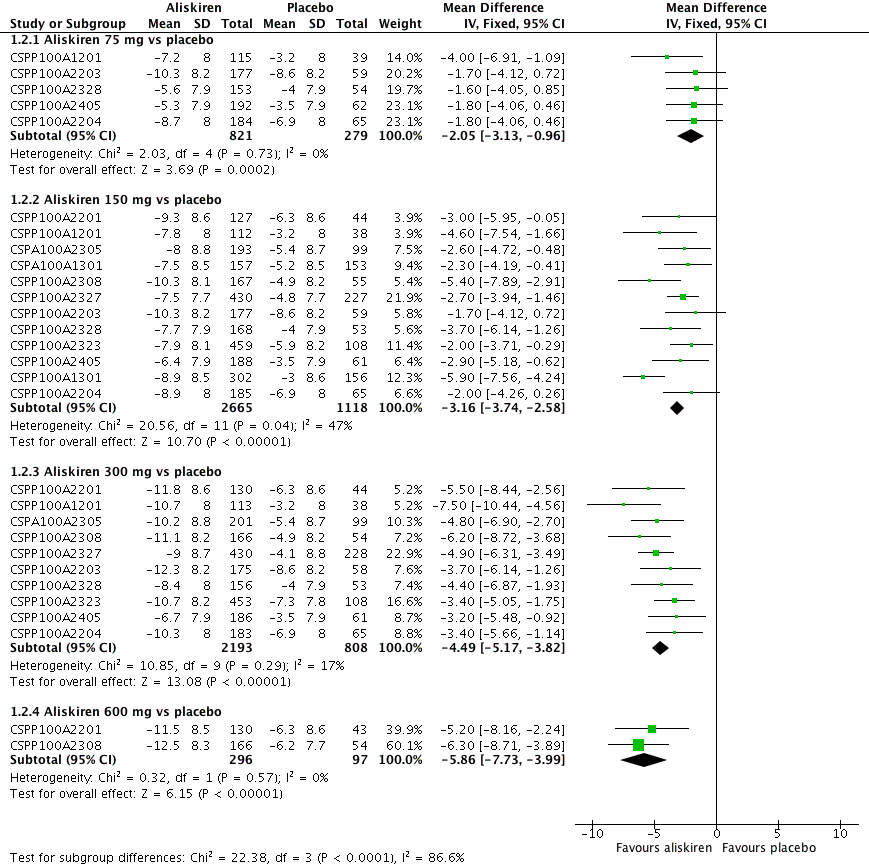
Forest plot of comparison: 1 Aliskiren vs. placebo, outcome: 1.2 Diastolic BP.
Highly significant subgroup differences were observed, therefore mean overall effect size across all doses is not shown. The number of placebo group patients are divided equally in dose ranging studys when more than one dose of aliskiren was used.
CSPP100A2308 study the DBP reduction in the treatment and placebo group are reported from the CSR which differ from those reported in the published article. The previous version of this review used data from the published article.
CSPP100A2405 study the SD for all treatment groups are calculated from SEM reported from the CSR on page 8.

Comparison 1 Aliskiren vs. placebo, Outcome 1 Systolic BP.

Comparison 1 Aliskiren vs. placebo, Outcome 2 Diastolic BP.

Comparison 1 Aliskiren vs. placebo, Outcome 3 Withdrawals due to adverse event.

Comparison 1 Aliskiren vs. placebo, Outcome 4 Cough.

Comparison 1 Aliskiren vs. placebo, Outcome 5 Diarrhoea.

Comparison 2 Aliskiren150 mg vs. Aliskiren 75 mg, Outcome 1 Systolic BP.

Comparison 2 Aliskiren150 mg vs. Aliskiren 75 mg, Outcome 2 Diastolic BP.

Comparison 3 Aliskiren 300 mg Vs. Aliskiren 75 mg, Outcome 1 SBP.

Comparison 3 Aliskiren 300 mg Vs. Aliskiren 75 mg, Outcome 2 DBP.

Comparison 4 Aliskiren 300 mg vs. Aliskiren 150 mg, Outcome 1 Systolic BP.

Comparison 4 Aliskiren 300 mg vs. Aliskiren 150 mg, Outcome 2 Diastolic BP.

Comparison 5 Aliskiren 600 mg vs. Aliskiren 150 mg, Outcome 1 SBP.

Comparison 5 Aliskiren 600 mg vs. Aliskiren 150 mg, Outcome 2 DBP.

Comparison 6 Aliskiren 600 mg vs. Aliskiren 300 mg, Outcome 1 Systolic BP.

Comparison 6 Aliskiren 600 mg vs. Aliskiren 300 mg, Outcome 2 Diastolic BP.
| Aliskiren compared to placebo for primary hypertension | ||||||
| Patient or population: primary hypertension Setting: Outpatient Intervention: Aliskiren Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI)mmHg | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Effect with placebo1 | Effect with Aliskiren2 | |||||
| Systolic BP ‐ Aliskiren 75 mg vs. placebo | 2.9 lower to 10.0 lower | 2.97 lower (4.76 lower to 1.18 lower) | ‐ | 1100 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 150 mg vs. placebo | 2.0 lower to 10.0 lower | 5.95 lower (6.85 lower to 5.06 lower) | ‐ | 3786 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 300 mg vs. placebo | 2.9 lower to 10.0 lower | 7.88 lower (8.94 lower to 6.82 lower) | ‐ | 3009 | ⊕⊕⊕⊝ | |
| Systolic BP ‐ Aliskiren 600 mg vs. placebo | 3.8 lower to 5.3 lower | 11.35 lower (14.43 lower to 8.27 lower) | ‐ | 393 | ⊕⊕⊝⊝ | |
| Diastolic BP ‐ Aliskiren 75 mg vs placebo | 3.2 lower to 8.6 lower | 2.05 lower (3.13 lower to 0.96 lower) | ‐ | 1100 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 150 mg vs placebo | 3.0 lower to 8.6 lower | 3.16 lower (3.74 lower to 2.58 lower) | ‐ | 3783 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 300 mg vs placebo | 3.2 lower to 8.6 lower | 4.49 lower (5.17 lower to 3.82 lower) | ‐ | 3001 | ⊕⊕⊕⊝ | |
| Diastolic BP ‐ Aliskiren 600 mg vs placebo | 6.2 lower to 6.3 lower | 5.86 lower (7.73 lower to 3.99 lower) | ‐ | 393 | ⊕⊕⊝⊝ | |
| Diarrhoea ‐ Aliskiren 600 mg vs placebo | 14 per 1,000 | 95 per 1,000 | RR 7.00 | 592 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Range of mean decrease in SBP and DBP mmHg as compared to baseline in the individual trials in the placebo group. 2. Effects in Aliskiren group represent the blood pressure lowering effect in excess of that with placebo. 3. Downgraded by 1 level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 5 included studies). Also study CSPP100A2204 had high likelihood of selection bias. 4. Downgraded by one level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 12 included studies). 5. Downgraded by one level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in all 10 included studies). 6. Downgraded by 1 level for serious risk of bias (due to high likelihood of attrition, selective reporting and funding bias in both included studies). 7. Downgraded by 1 more level due to wide confidence interval. | ||||||
| Dose | Female | Male | ||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐1.3 | ‐1.1 | ‐3.6 | ‐2.9 |
| 150 mg | ‐5.5 | ‐2.9 | ‐5.9 | ‐3.5 |
| 300 mg | ‐9.4 | ‐4.8 | ‐9.0 | ‐5.4 |
| 600 mg | ‐12.6 | ‐6.4 | ‐10.7 | ‐6.5 |
| 75 mg | ‐1.3 | ‐1.1 | ‐3.6 | ‐2.9 |
| Dose | Age < 65 years | Age > 65 years | ||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐2.7 | ‐1.9 | ‐3.6 | ‐3.4 |
| 150 mg | ‐5.5 | ‐2.9 | ‐6.9 | ‐4.8 |
| 300 mg | ‐9.7 | ‐5.2 | ‐7.1 | ‐4.4 |
| 600 mg | ‐11.5 | ‐6.6 | ‐11.6 | ‐6.4 |
| 75 mg | ‐2.7 | ‐1.9 | ‐3.6 | ‐3.4 |
| Dose | White | Black | Asian | |||
| SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | SBP mmHg | DBP mmHg | |
| 75 mg | ‐2.1 | ‐1.7 | ‐2.8 | 0.2 | ‐8.8 | ‐3.2 |
| 150 mg | ‐6.5 | ‐3.5 | ‐5.5 | ‐1.4 | ‐3.7 | ‐2.9 |
| 300 mg | ‐9.6 | ‐4.8 | ‐6.1 | ‐2.6 | ‐12.7 | ‐6.3 |
| 600 mg | ‐12.3 | ‐6.7 | ‐8.7 | ‐3.5 | ‐11.2 | ‐9.4 |
| Dose of Aliskiren mg/day | Previous version without access to information from CSR | Present version with access to information from CSR | ||
| MSSBP mmHg MD with 95% CI | MSDBP mmHg MD with 95% CI | MSSBP mmHg MD with 95% CI | MSDBP mmHg MD with 95% CI | |
| Aliskiren 75 mg vs. Placebo | ‐2.64 (‐4.06 to ‐1.23) | ‐2.07 (‐2.94 to ‐1.20) | ‐2.97 (‐4.76 to ‐1.18) | ‐2.05 (‐3.13 to ‐0.96) |
| Aliskiren 150 mg vs. Placebo | ‐5.55 (‐6.39 to ‐4.71) | ‐2.91 (‐3.46 to ‐2.37) | ‐5.95 (‐6.85 to ‐5.06) | ‐3.16 (‐3.74 to ‐2.58) |
| Aliskiren 300 mg vs. Placebo | ‐7.93 (‐8.77 to ‐7.08) | ‐4.76 (‐5.33 to ‐4.19) | ‐7.88 (‐8.94 to ‐6.82) | ‐4.49 (‐5.17 to ‐3.82) |
| Aliskiren 600 mg vs. Placebo | ‐11.36 (‐13.53 to ‐9.19) | ‐6.57 (‐7.92 to ‐5.23) | ‐11.35 (‐14.43 to ‐8.27) | ‐5.86 (‐7.73 to ‐3.99) |
| MSSBP: mean sitting systolic blood pressure; MSDBP: mean sitting diastolic blood pressure; | ||||
| Novartis Clinical Trial Results Database identifier used for study identification in this review | Journal‐Published Author /year | Registered on Novartis Clinical Trial Results Database | Registered on ClinicalTrials.gov | CSR* received from EMA | Request to EMA was made on November 15th 2015. Weeks waited to obtain CSR |
| No journal publication | Results NOT posted | NCT01237223 Results posted | EMA does NOT possess | ‐ | |
| Results posted | NCT00739973 Results posted | EMA does NOT possess | ‐ | ||
| Results NOT posted | NOT registered | Mar 11, 2016 | 16 | ||
| No journal publication | Results posted | NCT00344110 Results NOT posted | May 23, 2016 | 27 | |
| Results NOT posted | NOT registered | Jan 25, 2016 | 10 | ||
| NOT FOUND | NOT registered | Apr 12, 2016 | 21 | ||
| Results NOT posted | NCT00219024 Results posted | Apr 6, 2016 | 20 | ||
| Results NOT posted | NCT00219128 Results NOT posted | Feb 20, 2016 | 14 | ||
| Results posted | NCT00219154 Results NOT posted | May 27, 2016 | 27 | ||
| Results NOT posted | NOT registered | June 16, 2016 | 30 | ||
| Results posted | NOT registered | EMA does NOT possess | ‐ | ||
| Results posted | NCT00706134 Results posted | December 22, 2015 | 5 |
| Dose comparison | SBP WMD with 95% CI | DBP WMD with 95% CI | Comment |
| Aliskiren 150 mg vs 75 mg | ‐1.89 (‐3.16 to ‐0.62) | ‐0.80 (‐1.58 to ‐0.03) | Aliskiren 150 mg reduces SBP and DBP more than 75 mg by 2/1 mmHg |
| Aliskiren 300 mg vs 75 mg | ‐5.10 (‐6.79 to ‐3.40) | ‐2.49 (‐3.53 to ‐1.45) | Aliskiren 300 mg reduces SBP and DBP more than 75 mg 5/3 mmHg |
| Aliskiren 300 mg vs 150 mg | ‐2.62 (‐3.38 to ‐1.87) | ‐1.80 (‐2.28 to ‐1.32) | Aliskiren 300 mg reduces SBP and DBP more than 150 mg 3/2 mmHg |
| Aliskiren 600 mg vs 150 mg | ‐3.40 (‐5.58 to ‐1.23) | ‐2.20 (‐3.55 to ‐0.85) | Aliskiren 600 mg reduces SBP and DBP more than 150 mg by 3/2mmHg |
| Aliskiren 600 mg vs 300 mg | ‐0.61 (‐2.78 to 1.56) | ‐0.68 (‐2.03 to 0.67) | No significant difference was observed |
| Study identifier | Single‐blind period | Double‐blind period | Withdrawal period |
| No deaths | No deaths | No deaths | |
| No deaths | No deaths | No deaths | |
| One death in placebo group during observation period due to pancreatic carcinoma and metastases to liver. | One death occurred in aliskiren 150 mg monotherapy on day 41 of the study, due to drug intoxication. The overdose was attributed to a psychiatric drug prescribed prior to start of the study, and a diagnosis of manic depressive psychosis was recorded. | No deaths | |
| No deaths | No deaths | No deaths | |
| No deaths | No deaths | No deaths | |
| No deaths | One death occurred in the placebo arm on day 16 from "natural causes". One death occurred in the valsartan 160 mg arm (day 26) as a result of motor vehicle accident. | No deaths | |
| No deaths | One death was reported in the Aliskiren/HCTZ 150/25 mg group due to thoracic trauma from a motor vehicle accident. | No deaths | |
| No deaths | No deaths | No deaths | |
| 1 death occurred in placebo run‐in phase. Patient hospitalized due to supraventricular tachycardia and abdominal pain and died due to stroke after discontinuing from the study. | No deaths | One death occurred in the aliskiren/HCTZ 150/300 mg group from acute bronchopneumonia and associated sepsis during the 30‐day follow‐up period after discontinuing the study. | |
| No deaths | 2 deaths reported. 1 death in aliskiren group on day 41 due to myocardial infarction. 1 death in valsartan group on day 13 and the cause was reported as hypertensive arteriosclerotic cardiovascular disease with SAE of sudden death. There were no deaths in placebo arm or combination therapy group. | No deaths | |
| No deaths | No deaths | No deaths | |
| One death occurred during the follow‐up period of the study from pneumonia leading to respiratory failure (day of death not provided). Further information regarding this death is described in the patient narratives of the CSR, which we do not have access to yet. Within the CSR body, the arm of the study that this participant belongs to has been redacted. In the 2008 FDA Medical Review report, a death due to colon cancer is reported as occurring within CSPP100A2204 (N = 1, aliskiren 300 mg). The medical review states "no other details were provided". For our comments on this please see Discussion. | |||
| CSR: clinical study report; HTCZ: hydrochlorothiazide; SAE: serious adverse event | |||
| Study identifier | Wash out period | Single blind period | Double blind period | Withdrawal period |
| Not reported | Not reported | No SAEs were reported in the aliskiren monotherapy group. 2 in amlodipine 2.5 mg; 1 in amlodipine 5 mg; and 1 in aliskiren 150/amlodipine 5 mg combination arm (detail regarding SAE was not reported) | Not reported | |
| Not reported | Not reported | 9 SAEs reported. 2 in placebo and 7 in other treatment groups (retinal detachment, abdominal mass, bronchitis, calculus ureteric, gastroenteritis, hand fracture, hydronephrosis, pneumonia, and cerebrovascular accident). None occurred in aliskiren 150 mg or 300 mg groups. | Not reported | |
| Not reported | Reported 1 SAE in the single‐blind period ‐ infectious enterocolitis/dehydration (N = 1, 55M, placebo). | 2 SAEs in the double‐blind period: cerebral infarction (N = 1, 69F, placebo); acute myocardial infarction (N = 1, 58F, aliskiren 75 mg). | Not reported | |
| Not reported | Not reported | 2 SAEs reported. 1 SAE in placebo due to myocardial infarction. The other in the losartan 50 mg group due to right medullary infarction. None in aliskiren 150 mg group. | Not reported | |
| One in washout period ‐ transient Ischaemic attack | Two in the single‐blind run‐in phase ‐ anxiety; and diverticulitis and | 2 SAEs in irbesartan group ‐ Intravertebral disc protrusion; and bipolar depression. | Two in the withdrawal period in placebo group ‐ Left ventricular failure; and gout and infective arthritis). | |
| Not reported | Reported 6 SAE. 1 bile duct cancer, 1 diverticulitis, 1 pregnancy, 1 mild melanorrhagia and gastric pain, 1 myocardial infarction, and 1 squamous cell carcinoma. | 8 SAEs reported. 2 in Placebo: 1 due to natural cause and 1 due to myocardial infarction requiring hospitalization on day 46 resulting in study drug discontinuation. 1 in aliskiren 75 mg on day 4 coronary artery disease requiring hospitalization resulting in study discontinuation. Patient required quadruple bypass surgery. 1 in aliskiren 300 mg on day 42 pregnancy resulting in study discontinuation. 1 in valsartan 160 mg on day 28 ‐ angioneurotic oedema resulting in study discontinuation, dyspnoea and chest pressure. Aliskiren 150 mg/valsartan 160 mg (N = 1, Day 29) motor vehicle accident requiring hospitalization; not discontinued from study. | 1 in aliskiren 75 mg/valsartan 80 mg on day 3 post‐study. | |
| Not reported | 4 SAE due to (lung cancer, fractured leg, angina and urinary tract infection) | 28 patients experienced at least 1 SAE in the monotherapy and combination therapy treatment groups. 1 in A 75 mg ‐ due to renal colic; 1 in aliskiren 150 mg due to haemorrhagic diarrhoea; none in aliskiren 300 mg; 1 in HCTZ 6.25 mg due to neoplasm of skin; 3 in HCTZ 12.5 mg ‐ due to breast cancer, joint injury and pregnancy; 2 in HCTZ 25 mg due to deep vein thrombosis and lymphadenopathy; 5 in aliskiren 75 mg/HCTZ 12.5 mg due to (diplopia, IIIrd nerve paresis, mood disorder, phlebothrombosis, psychotic disorder, small intestinal obstruction and syncope); 4 in aliskiren 75 mg/HCTZ 25 mg due to (cerebral infarction, dysarthria, physical disability, pregnancy and renal colic); 2 in aliskiren 150 mg/ HCTZ 6.25 mg ulcerative colitis, pregnancy); 3 in aliskiren 150 mg/ HCTZ 12.5 mg (non‐cardiac chest pain, pregnancy, syncope); 2 in aliskiren 150 mg/ HCTZ 25 mg (lung neoplasm, road traffic accident); 2 in aliskiren 300 mg/ HCTZ 12.5 mg (diabetes mellitus, lung disorder); 1 in aliskiren 300 mg/HCTZ 25 mg (coronary artery disease). There were no SAEs in the placebo group. | Not reported | |
| 2 SAEs reported during washout period ‐ 1 due to subarachnoid haemorrhage due to rupture of cerebral aneurysm on day 8; 1 due to partial small bowel obstruction on day 13 | One SAE ‐ bladder carcinoma prior to randomization and before receiving double‐blind study drug. Patient was randomized to placebo but later discontinued due to unsatisfactory therapeutic effect; | 4 SAEs reported. 1 SAE in aliskiren 150 mg on day 27‐ unstable angina and increased blood pressure; 2 SAEs in aliskiren 300 mg ‐ hospitalization for acute appendicitis on day 34; hospitalization for depression on day 51; 1 SAE in aliskiren 600 mg of hospitalization for pain/bodily injury on day 35. No SAE reported in the placebo group. | One SAE was reported during withdrawal period in aliskiren 150 mg of serious venous occlusion and thrombosis of the right eye on day 8 withdrawal period). | |
| data at week 3 and week 6 not available | Not reported | Not reported | During 26 weeks double‐blind treatment period 19 SAEs reported. 10 in aliskiren group and 9 in HCTZ group. | Not reported |
| not reported | Not reported | 20 SAEs reported. 5 in placebo group ‐ atrial flutter, cerebrovascular accident, headache, hypertension, hypertensive crisis and ventricular tachycardia); 8 in aliskiren group ‐ 1 gastritis, 1 grand mal convulsions, 1 intestinal poly, 2 myocardial infarction, 1 non‐cardiac chest pain, 1 peripheral vascular disease, 1 acute renal failure); 6 in valsartan group ‐ 1 angina pectoris, 1 arteriosclerosis, 1 breast cancer, 1 bronchitis, 1 COPD, 1 facial paresis, 1 ovarian cancer, 1 pneumonia, 1 pulmonary oedema); 3 in aliskiren/valsartan group ‐ 1 aortic aneurysm, 1 intravertebral disc protrusion, 1 prostate cancer and 1 thyroidectomy). The FDA medical review (2007) also reported two cases of renal carcinoma occurred (N = 1, placebo, day 20 post‐study; N = 1, aliskiren, day 44 post‐study). For our comments on this, please see Discussion. | Not reported | |
| 11 patients had serious AEs during the washout and placebo run‐in periods (detail of which are not provided). | Not reported | 1 SAE was reported in aliskiren 75 mg group ‐ cardiac chest pain; 2 in aliskiren 150 mg group ‐ rectal bleeding due to anal ulcer and episode of secondary anaemia; basal cell carcinoma; 1 in the 300 mg group ‐TIA with concomitant nausea and dyspnoea; and 2 in the placebo group ‐ prostate cancer; accelerated hypertension with left facial numbness. | Not reported | |
| Reported 2 SAEs in the washout period; Detail not provided in CSR. | Three in the single‐blind placebo run‐in period. Detail not provided in CSR. | 6 SAEs were reported. 2 in aliskiren 75 mg group ‐ erysipelas (skin infection) and osteoarthritis; 3 in aliskiren 150 mg group ‐ severe glaucoma; moderate haemorrhoids; and mild hemorrhagic stroke; none in aliskiren 300 mg group; and 1 in the placebo group ‐ vertigo, wrist fracture, concussion, and head contusion subsequent to a fall. | Not reported | |
| COPD: chronic obstructive pulmonary disease; CSR: clinical study report; HTCZ: hydrochlorothiazide; SAE: serious adverse event; TIA: transient ischaemic attack | ||||
| Study Identifier | Total AEs and Common AEs as reported in the corresponding available CSR A = Aliskiren; P = Placebo |
| Total AEs: A150 =15(7.8%); A 300 = 18(8.9%) and P = 22(7.7%) Headache: A150 =13( 6.7%); A 300 =15(7.4%) and P = 20(10.1%) Periphaeral edema: A150 = 2(1.0%); A 300 = 3(1.5%) and P = 2(1%) | |
| Total AEs: A150 = 40.1%; A 300 = 46.7%; A 600 = 52.4%; P = 43% Headache: A150 = 12(7%); A300 = 13(7.7%); A600 = 9(5.4%); P = 16(9.7%) Nasopharyngitis: A 150 = 5(2.9%); A 300 = 6(3.6%); A 600 = 3(1.8%); P = 10(6.1%) Dizziness: A 150 = 2(1.2%); A 300 = 9(5.3%); A 600 = 5(3%); P = 7(4.2%) Diarrhoea:A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 19 (11.4%); P = 2(1.2%) Nausea: A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 0%; P = 4(2.4%) | |
| Total AEs: A75 = 61(53%); A150 = 58(51.8%); A 300 = 62(54.9%); P = 58(50.4)% Headache: A75 = 3(2.6%); A 150 = 3(2.7%); A 300 = 6 (5.3%) and P = 4(3.5%) Nasopharyngitis:A75 = 24(20.9%); A150 = 20(17.9%); A300 = 20(17.7%); P = 16(13.9%) Back pain:A75 = 2(1.7%); A150 = 0%; A300 = 0% and P = 0% Diarrhoea: A75 = 1(0.9%); A150 = 1(0.9%); A300 = 4(3.5%) and P = 1(0.9%) Vertigo: A75 =0%; A150 = 0%); A300 = 1(0.9%) and P = 2(1.7%) | |
| Total AEs: A150 = 152(50.3%), P = 66(42.3%) Nasopharyngitis: A150 = 48(15.9%), P = 13(8.3%) Headache: A150 = 9(3.0%), P = 6(3.8%) Occult blood positive: A150 = 9 (3.0%), P =5 (3.2%) | |
| Total AEs: A150 = 127 (26.8%); A300 = 130 (36.2%); A600 = 43 (33.1%); P = 32 (32.1%) Headache: A150 = 3 (2.4%); A300 = 8 (6.2%); A600 = 6 (4.6%); P = 7 (5.3%) Diarrhoea: A150 = 2 (1.6%); A300 = 1 (0.8%); A600 = 9 (6.9%); P = 2 (1.5%) Dizziness: A150 = 2 (1.6%); A300 = 4 (3.1%); A600 = 3 (2.3%); P = 5 (3.8%) Fatigue: A150 = 1 (0.8%); A300 = 5 (3.8%); A600 = 2 (1.5%); P = 4 (3.1%) | |
| Total AEs: A75 = 63(35.2%); A150 = 59(33.1%); A300 = 50(28.6%) and P = 57(32.2%) Headache: A75 = 15(8.4%); A150 = 9(5.1%;) A 300 = 7(4.0%) and P = 15(8.5%) Fatigue: A75 = 7(3.9%); A150 = 4(2.2%;) A 300 = 4(2.3%) and P = 4(2.3%) Back pain: A75 = 2(1.1%); A150 = 4(202%;) A 300 = 3(1.7%) and P = 2(1.1%) Diarrhoea: A75 = 2(1.1%); A150 = 1(0.6%;) A 300 = 5(2.9%) and P = 3(1.7%) Dizziness: A75 = 4(2.2%); A150 = 4(2.2%;) A 300 = 3(1.7%) and P = 2(1.1%) | |
| Total AEs: A75 = 69(37.5%); A150 = 69(37.3%); A300 = 71 (39.2%); P = 85(44%) Headache: A75 = 13(7.1%); A 150 = 13(7%); A 300 = 10 (5.5%); P = 26 (13.5%) Nasopharyngitis: A75 = 9( 4.9%); A150 = 5(2.7%;) A300 = 3 (1.7%); P = 10 (5.2%) Diarrhoea: A75 = 3(1.6%); A150 = 3(1.6%); A300 = 4(2.2%); P = 1(0.5%) Cough: A75 = 1(0.5%); A150 = 2(1.1%); A300 = 1(0.6%); P = 1(0.5%) Dizziness: A75 = 1(0.5%); A150 = 1(0.5%); A300 = 3(1.7%); P = 2(1.0%) Peripheral edema: A75 = 4(2.2%); A150 = 3(1.6%); A300 = 2(1.1%); P = 1(0.5%) | |
| Total AEs: A150 = 40.1%; A 300 = 46.7%; A 600 = 52.4%; P = 43% Headache: A150 = 12(7%); A300 = 13(7.7%); A600 = 9(5.4%); P = 16(9.7%) Nasopharyngitis: A 150 = 5(2.9%); A 300 = 6(3.6%); A 600 = 3(1.8%); P = 10(6.1%) Dizziness: A 150 = 2(1.2%); A 300 = 9(5.3%); A 600 = 5(3%); P = 7(4.2%) Diarrhoea: A 150 = 2(1.2%); A 300 = 3(1.8%); A 600 = 19 (11.4%); P = 2(1.2%) Nausea: A 150 =2 (1.2%); A 300 = 3(1.8%); A 600 =0%; P = 4(2.4%) | |
| Total AEs at 6 weeks : A 26.4%; P = 28.5% (numbers not reported) Other AE were not reported at week 6. | |
| Total AEs: A150 to 300 = 149(34%) and P = 168(37%) Headache: A150 to 300 = 14(3.2%); P = 41(9%) Diarrhoea*: A150 to 300 = 2.3%; P = not reported Nasopharyngitis: A150 to 300 = 16(3.7%) ; P = 9(2%) Dizziness: A 150 to 300 = 8(1.8%) ; P = 9(2%) Fatigue:A 150 to 300 = 4(0.9%) ; P = 5(1.1%) Nausea: A 150 to 300 = 6(1.4%) ; P = 11(2.4%) | |
| Total AEs: A75 = 57(37.3%); A150 = 59(34.7%); A300 = 56(35.4%); P = 60(37.5%) Headache: A75 = 12(7.8%); A150 = 7(4.1%); A300 = 10(6.3%) and P = 9(5.6%) Nasopharyngitis: A75 = 2(1.3%); A150 = 2(1.2%); A300 = 5(3.2%) and P = 3(1.9%) Diarrhoea: A75 = 7(4.6%); A150 = 2(1.2%); A300 = 1(0.6%) and P = 1(0.6%) Cough: A75 = 1(0.7%); A150 = 4(2.4%); A300 = 1(0.6%) and P = 3(1.9%) Dizziness: A75 = 1(0.7%); A150 = 5(2.9%); A300 = 1(0.6%) and P = 3(1.9%) | |
| Total AEs: A75 = 36(18.8%); A150 = 41(21.7%); A300 = 36(19.1%); P = 39(21.0%) Headache: A75 = 4(2.1%);A150 = 2(1.1%); A300 = 1(1.1%); P = 4(2.1%) Influenza: A75 = 1(0.5%); A150 = 4(2.1%); A300 = 1(1.1%); P = 1(0.5%) |
| Study Identifier | Reasons for increased withdrawal due to adverse events |
| Not reported. | |
| Not reported. | |
| Significant adverse events occurred for N = 7 patients, leading to discontinuation from study, as described below.
| |
|
| |
| A total of N = 18 patients discontinued from the study due to AEs. It was suspected that 2/3 of these discontinuations were for reasons drug‐related. Headaches resulted in the discontinuation for N = 3 patients. | |
| Most frequent reasons for 12 discontinuations are: fatigue (N = 5, 0.4%), headache (N = 3, 0.3%), diarrhoea (N = 2, 0.2%), and peripheral oedema (N = 2, 0.2%). Reasons for 14 discontinuations from AE (safety population).
The FDA medical review reports facial oedema (N = 1, 0.6%) that appears to be angioedema, resulting in discontinuation from study; however, this is not reported in CSR 2203.
| |
| The range of AEs resulting in study discontinuation is 0% is the aliskiren 150 mg arm of the study to 4.4% in the aliskiren 300 mg arm (N = 7). The most frequent AE resulting in study discontinuation is headache (N = 12, 0.4%). Reasons for discontinuations from adverse events ‐ N = 62 (2.2%).
Additionally four patients discontinued from the study due to abnormal laboratory values.
| |
| Most frequent reasons for 9 discontinuation from study: headache (N = 4), dizziness (N = 3), and diarrhoea (N = 2). Reasons for discontinuations from AE (safety population).
We question that a participant withdrew for the reason specified as "unsatisfactory effect" as opposed to being categorized as a discontinuation from an SAE as the event necessitated hospitalization. On Day 27 in CSPP100A2308, the participant (N = 1, 64F, aliskiren 150 mg) was hospitalized for unstable angina and increased blood pressure. The unstable angina was treated with concomitant medication and her condition resolved within 3 days. | |
| We do not have data at week 3 and week 6 for this study. | |
| The range of AEs resulting in study discontinuation is 8 (1.8%) is the aliskiren arm; 8 (2.6%) in valsartan arm; 6 (1.3%) in aliskiren/valsartan combination arm; and 11 (2.4%) in the placebo arm. Reasons for discontinuations from adverse events ‐ N = 37 (2%).
| |
| Aliskiren 75 mg = 3 (2.0%); aliskiren 150 mg = 5 (2.9%); aliskiren 300 mg = 4(2.5%) . Placebo = 4 (2.5%). Reasons for withdrawal were not reported. | |
| Reasons for 18 discontinuations from AE (safety population)
|
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 12 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Aliskiren 75 mg vs. placebo | 5 | 1100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.97 [‐4.76, ‐1.18] |
| 1.2 Aliskiren 150 mg vs. placebo | 12 | 3786 | Mean Difference (IV, Fixed, 95% CI) | ‐5.95 [‐6.85, ‐5.06] |
| 1.3 Aliskiren 300 mg vs. placebo | 10 | 3009 | Mean Difference (IV, Fixed, 95% CI) | ‐7.88 [‐8.94, ‐6.82] |
| 1.4 Aliskiren 600 mg vs. placebo | 2 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐11.35 [‐14.43, ‐8.27] |
| 2 Diastolic BP Show forest plot | 12 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Aliskiren 75 mg vs placebo | 5 | 1100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐3.13, ‐0.96] |
| 2.2 Aliskiren 150 mg vs placebo | 12 | 3783 | Mean Difference (IV, Fixed, 95% CI) | ‐3.16 [‐3.74, ‐2.58] |
| 2.3 Aliskiren 300 mg vs placebo | 10 | 3001 | Mean Difference (IV, Fixed, 95% CI) | ‐4.49 [‐5.17, ‐3.82] |
| 2.4 Aliskiren 600 mg vs placebo | 2 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐5.86 [‐7.73, ‐3.99] |
| 3 Withdrawals due to adverse event Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 75 mg vs. placebo | 5 | 1653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.33, 1.07] |
| 3.2 150 mg vs. placebo | 10 | 3421 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.30, 0.71] |
| 3.3 300 mg vs. placebo | 10 | 4216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.47, 1.03] |
| 3.4 600 mg vs placebo | 2 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.64] |
| 4 Cough Show forest plot | 5 | 2886 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.49, 2.64] |
| 5 Diarrhoea Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Aliskiren 75 mg | 4 | 1276 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.85, 5.76] |
| 5.2 Aliskiren 150 mg | 7 | 2277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.78, 3.46] |
| 5.3 Aliskiren 300 mg | 7 | 2268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.89, 3.81] |
| 5.4 Aliskiren 600 mg | 2 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.00 [2.48, 19.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 5 | 1651 | Mean Difference (IV, Fixed, 95% CI) | ‐1.89 [‐3.16, ‐0.62] |
| 2 Diastolic BP Show forest plot | 5 | 1651 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.58, ‐0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 3 | 904 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐6.79, ‐3.40] |
| 2 DBP Show forest plot | 3 | 904 | Mean Difference (IV, Fixed, 95% CI) | ‐2.49 [‐3.53, ‐1.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 10 | 4405 | Mean Difference (IV, Fixed, 95% CI) | ‐2.62 [‐3.38, ‐1.87] |
| 2 Diastolic BP Show forest plot | 10 | 4405 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.28, ‐1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 2 | 590 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.58, ‐1.23] |
| 2 DBP Show forest plot | 2 | 590 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐3.55, ‐0.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic BP Show forest plot | 2 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐2.78, 1.56] |
| 2 Diastolic BP Show forest plot | 2 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐2.03, 0.67] |

