Lithotritie par onde de choc extra‐corporelle (LOCE) comparée à la néphrolithotomie percutanée (NLPC) ou la chirurgie intrarénale rétrograde (CIRR) pour éliminer les calculs rénaux
Résumé scientifique
Contexte
La formation de calculs dans les voies urinaires est un trouble médical fréquent dans la population générale. Actuellement, la forte expansion des techniques peu invasives a entraîné une diminution des chirurgies ouvertes. La lithotritie par onde de choc extra‐corporelle (LOCE) a été introduite comme une approche alternative qui désintègre les calculs dans le rein et la voie urinaire supérieure par l'action d'ondes de choc. Cependant, comme le taux de réussite de la LOCE est limité, d'autres modalités peu invasives sont largement employées pour les calculs rénaux, tels que la néphrolithotomie percutanée (NLPC) et la chirurgie intrarénale rétrograde (CIRR). Ceci est la mise à jour d'une revue initialement publiée en 2009.
Objectifs
L'objectif de cette revue était d'évaluer l'efficacité et les complications de la LOCE pour les calculs rénaux, par rapport à la NLPC ou la CIRR.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le registre spécialisé du groupe Cochrane sur la néphrologie jusqu'au 3 mars 2014 en prenant contact avec le coordinateur des recherches d'essais et en utilisant des termes de recherche pertinents pour cette revue.
Critères de sélection
Essais contrôlés randomisés (ECR) qui évaluent l'utilisation de la LOCE par rapport à la NLPC ou la CIRR pour traiter les calculs rénaux.
Recueil et analyse des données
Deux auteurs ont évalué indépendamment toutes les études à inclure. Des analyses statistiques ont été effectuées en utilisant le modèle à effets aléatoires et les résultats ont été exprimés sous la forme de risques relatifs (RR) pour les résultats dichotomiques ou de différence moyenne (DM) pour les données continues avec des intervalles de confiance (IC) à 95 %.
Résultats principaux
Cinq études (338 patients) ont été incluses, dont quatre ont comparé la LOCE avec la NLPC et une a comparé la LOCE avec la CIRR. Trois études font état d'une génération de séquence aléatoire, les deux autres restant imprécises à ce sujet. Aucune des études n'a déclaré une assignation secrète. La mise en aveugle des participants et des enquêteurs n'a pas été possible en raison de la nature des interventions ; la mise en aveugle des évaluateurs des résultats n'a pas été signalée. Toutes les études ont été jugées à faible risque de biais de déclaration. Une étude a été financée par l'industrie et dans une étude, le nombre de participants dans chaque groupe était déséquilibré.
Le succès du traitement à trois mois était significativement plus élevé dans le groupe NLPC par rapport au groupe LOCE (3 études, 201 participants : RR 0,46, IC à 95 % de 0,35 au 0,62). En comparaison avec le groupe NLPC, une augmentation significative a été observée dans le groupe LOCE dans l'emploi d'un nouveau traitement (1 étude, 122 participants : RR 1,81, IC à 95 % de 0,66 à 4,99) et de procédures auxiliaires (2 études, 184 participants : RR 9,06, IC à 95 % de 1,20 à 68,64). Le quotient d'efficacité (QE, utilisé pour évaluer l'efficacité des procédures) était plus élevé pour la NLPC que pour la LOCE ; il diminuait cependant avec l'augmentation de la taille des calculs. La durée du traitement (DM ‐36,00 min, IC à 95 % de ‐54,10 à ‐17,90) et le séjour à l'hôpital (une étude, 49 participants : DM ‐3,30 jours, IC à 95 % de ‐5,45 à ‐1,15) étaient significativement plus courts dans le groupe LOCE. Globalement, davantage de complications ont été rapportées avec la NLPC, mais nous n'avons pas pu effectuer de méta‐analyse des études incluses en raison de différences dans les critères rapportés et le moment des mesures de résultats.
Une étude a comparé la LOCE et la CIRR pour les calculs rénaux du pôle inférieur. Le succès du traitement n'était pas significativement différent à la fin du troisième mois (58 participants : RR 0,91, IC à 95 % de 0,64 à 1,30). Les durées moyennes de la procédure et de l'hospitalisation rapportées étaient plus longues dans le groupe CIRR.
Conclusions des auteurs
Les résultats de cinq études de petite envergure dont la qualité méthodologique était faible indiquent que la LOCE est moins efficace pour les calculs rénaux que la NLPC mais pas significativement différente de la CIRR. Le séjour à l'hôpital et la durée du traitement étaient plus courts avec la LOCE. Des ECR à plus grande échelle et de haute qualité méthodologique sont nécessaires pour évaluer l'efficacité et les complications de la LOCE pour les calculs rénaux par rapport à la NLPC si des progrès technologiques sont obtenus dans l'élimination non invasive des fragments résiduels. En outre, des recherches supplémentaires sont nécessaires pour évaluer les résultats de la LOCE et de la CIRR dans des études portant sur le pôle inférieur et non, y compris sur la NLPC par rapport à la CIRR.
PICO
Résumé simplifié
Lithotritie par onde de choc extra‐corporelle (LOCE) comparée à la néphrolithotomie percutanée (NLPC) ou la chirurgie intrarénale rétrograde (CIRR) pour éliminer les calculs rénaux
La formation de calculs dans les voies urinaires est un trouble médical fréquent. La moitié des patients qui présentent des calculs urinaires souffrent d'une récurrence au cours des 10 années suivantes. Les calculs rénaux peuvent causer des douleurs, la présence de sang dans l'urine, des infections, une diminution de la fonction rénale et l'insuffisance rénale. Le traitement consiste à retirer les calculs du rein. La lithotritie par onde de choc extra‐corporelle (LOCE), qui désintègre les calculs en utilisant des ondes de choc, est une technique peu invasive. D'autres méthodes peu invasives (la néphrolithotomie percutanée (NLPC) et la chirurgie intrarénale rétrograde (CIRR)) sont fréquemment utilisées pour traiter les calculs rénaux car le taux de réussite de la LOCE est limité. Le but de cette revue était de comparer l'efficacité et les complications entre la LOCE et l'élimination des calculs par l'intermédiaire de la néphroscopie à travers la peau à niveau du rein (NLPC) ou un urétéroscope à travers la vessie et l'uretère vers le rein (CIRR). Cinq essais randomisés de petite envergure (338 patients) ont été inclus. Quatre études ont comparé la LOCE avec la NLPC et une étude la LOCE avec la CIRR. Les patients avec des calculs rénaux qui se soumettent à une NLPC présentent un taux de réussite plus élevé que la LOCE, tandis que la CIRR n'a pas montré de différence significative vis‐à‐vis de la LOCE. Cependant, les patients sous LOCE ont passé moins de temps à l'hôpital, la durée du traitement était plus courte et les complications moins fréquentes.
Authors' conclusions
Background
Description of the condition
Urolithiasis (stones in the urinary tract) is a common medical problem with a prevalence of approximately 2% to 3% in the general population. It has been estimated that 50% of patients with previous urinary stones have a recurrence within 10 years (Portis 2001). Additionally, the overall probability of forming kidney stones varies according to age, gender, race, geographic location, climate, occupation. Men are more commonly found to have urinary stones than women (2 to 3:1), and Caucasians have the highest incidence of upper urinary tract stones compared with Asians, Hispanics and African Americans (Pearle 2007). Kidney stones can also cause serious morbidity, pain, haematuria, infection, decreased kidney function and kidney failure.
Description of the intervention
The great expansion in minimally invasive techniques has led to the decrease in open surgery. Extracorporeal shock wave lithotripsy (ESWL) has been introduced as an alternative approach and disintegrates stones in the kidney and upper urinary tract through the use of shock waves. The stone‐free rates at three months for stones are 86% to 89% (renal pelvis), 71% to 83% (upper calyx), 73% to 84% (middle calyx) and 37% to 68% (lower calyx) (Albala 2001; Coz 2000; Maggio 1992; Obek 2001; Turna 2007).
Contraindications for ESWL treatment are restricted to pregnancy, severe skeletal malformations, severe obesity, urinary tract obstruction distal to the stone, and aortic and/or renal artery aneurysms. ESWL should not be performed in patients with uncontrolled blood coagulation, uncontrolled hypertension or uncontrolled urinary tract infection (Tiselius 2006). The complications of ESWL are steinstrasse (obstruction due to fragments becoming lodged in the ureter), haematoma, infection, sepsis, hypertension and diabetes mellitus (Krambeck 2006; Riedler 2003).
Due to the limitations of the success rate and the complications of ESWL, other minimal invasive modalities for kidney stones such as percutaneous nephrolithotomy (PCNL) and retrograde intrarenal surgery (RIRS) are widely used. PCNL is indicated for patients with large kidney and upper ureteral stones. The main advantage of PCNL is the higher success rate for these larger stones (May 1998; Netto 1991) as it is not dependent on the stone burden or composition (Tiselius 2006). However, PCNL is more invasive and has a higher associated morbidity than ESWL (Cass 1996; Havel 1998).
With the advance of endourologic technology, RIRS is considered as the second line therapy in the treatment of the ESWL‐resistant lower pole stones and for patients with co‐morbidities. RIRS management of the kidney stone is a reasonable alternative to ESWL or PCNL in patients with low volume stones (Auge 2001; Chung 2006; Grasso 1999; Holland 2006; Kourambas 2000; Preminger 2006a; Stav 2003).
How the intervention might work
ESWL produces the high‐energy shock wave from an external source and focus on the stone within the body. This energy disintegrates the stone into tiny fragments which can spontaneously pass through the urinary system. The shock wave is generated by a machine called a lithotriptor from either electrohydraulic, electromagnetic, or piezoelectric sources (Matlaga 2011).
Percutaneous nephrolithotomy (PCNL) is an operation to remove stones from the kidney. This surgical procedure must be obtained under anaesthesia. A small skin incision is made and a nephroscope is passed into the kidney to examine the stones. Stones are fragmented by either laser, ultrasonic or electrohydraulic through the nephroscope and then stones are removed. Finally, a nephrotomy tube is placed to drain fluid from the kidney (Matlaga 2011).
Retrograde intrarenal surgery (RIRS) is a minimally invasive surgical procedure using flexible ureteroscope enters the urethra through the bladder, the ureter, into the kidney. This procedure is a retrograde approach to the intrarenal urine‐collecting part and normally done under anaesthesia. The stones can be seen through the scope, then treated with intracorporeal lithotriptors and grasping devices. At present, RIRS is commonly used to remove stones from the kidney. The procedure had to be performed by special expertise in this field (Matlaga 2011).
Why it is important to do this review
Although, ESWL is a minimally invasive procedure for treating kidney stones, there are some factors that limit use of this procedure such as stone size, composition, and location. Thus, the effectiveness of ESWL treatments should be evaluated.
Objectives
This review aimed to assess the effectiveness and complications of ESWL for kidney stones compared with PCNL or RIRS.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at the outcomes between ESWL and PCNL or RIRS. The first period of randomised cross‐over studies were also to be included.
Types of participants
Inclusion criteria
Patients with kidney stones treated using ESWL compared with PCNL or RIRS.
Exclusion criteria
Pregnant women and children with kidney stones were excluded.
Types of interventions
Any patient with kidney stones treated with ESWL compared to PCNL or RIRS.
Types of outcome measures
Primary outcomes
-
Success of treatment: stone‐free, clinically insignificant residual fragments (residual fragment < 4 mm, or as reported by studies)
-
Re‐treatment rate
-
Auxiliary procedures
-
Efficiency quotient (EQ).
Secondary outcomes
-
Mean procedural or operating time
-
Mean hospital stay
-
Complications after treatment
-
Quality of life
-
Cost‐effectiveness.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register to 3 March 2014 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Cochrane Renal Group’s Specialised Register contains studies identified from the following sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of renal‐related journals and the proceedings of major renal conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected renal journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
-
Reference lists of clinical practice guidelines, review articles and relevant studies.
-
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The review was undertaken by five authors. The search strategy described was used to obtain titles and abstracts of studies that were potentially relevant to the review. The titles and abstracts were screened independently by three authors, who discarded studies that were not applicable; however studies and reviews that may have included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and the full text of these studies to identify studies which satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by the same authors using standard data extraction forms. Studies reported in non‐English language journals were to be translated before assessment but we did not find any non‐English language articles. Where more than one publication of one study existed, reports were to be grouped together and the most recent or most complete data set was to be used. Any discrepancy between published versions was to be highlighted. Disagreements were resolved by discussion. Data entry and analysis was performed by one author.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
-
Participants and personnel
-
Outcome assessors
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
The mean procedural or operating time and mean hospital stay were assessed using mean difference (MD) with 95% confidence intervals (CI). The risk ratio (RR) with 95% CI was used to assess the success of treatment (stone‐free rate, clinically insignificant residual fragments), re‐treatment, and use of auxiliary procedures. The success rate between ESWL and PCNL could be compared in meta‐analyses using random effects model. However, due to the difficulty of comparing the complications, quality of life and cost‐effectiveness of the interventions, we chose to describe these outcomes of each study.
Dealing with missing data
Participants were to be analysed according to the group to which they were randomised, regardless of whether or not they received the allocated intervention, but we could not restore them to the correct group and analyse because information of the missing data in the study reports were not clear.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
We could not use funnel plots of between‐treatment effect and its precision on individual studies for publication bias due to small numbers of studies.
Data synthesis
The success rates between ESWL and PCNL were pooled in meta‐analyses using random effects model when heterogeneity was not ruled out.
Subgroup analysis and investigation of heterogeneity
Initially we planned to conduct subgroup analyses for kidney stone sizes (< 10 mm, 10 to 20 mm, > 20 mm) and/or stone location (renal pelvis, upper pole, middle pole, and lower pole) but we could obtain for stone size between 10 to 20 mm and lower pole kidney stone (≤ 20 mm). For the comparison of ESWL and RIRS, subgroup analyses could not be performed because of insufficient data.
Sensitivity analysis
Sensitivity analyses were not conducted to explore the effect of study quality as there were too few studies and some studies used different criteria for measuring outcomes.
Results
Description of studies
Results of the search
In our 2009 review we retrieved 2903 reports and after initial screening we identified 996 potentially relevant titles. Further screening via abstract review identified 11 potentially relevant studies. We retrieved the full‐text articles for further evaluation. Eight articles were excluded, six were not RCTs, one was a time series and the other was an editorial comment. Three studies (214 patients) fulfilled our inclusion criteria (Albala 2001; Carlsson 1992; Pearle 2005). Two were from the USA (Albala 2001; Pearle 2005) and one from Sweden (Carlsson 1992).
For this update we screened 16 reports and identified two new studies (Deem 2010; Yuruk 2010), making a total of five included studies. Four studies compared ESWL and PCNL (Albala 2001; Carlsson 1992; Deem 2010; Yuruk 2010) and one compared ESWL and ureteroscopy for lower pole kidney stones (RIRS) (Pearle 2005). One editorial comment previously excluded has been added to Carlsson 1992.
We identified two ongoing studies (NCT01604304; TOP‐Stone Study) which will be included in a future update of this review.
See Figure 1 for study selection procedure.
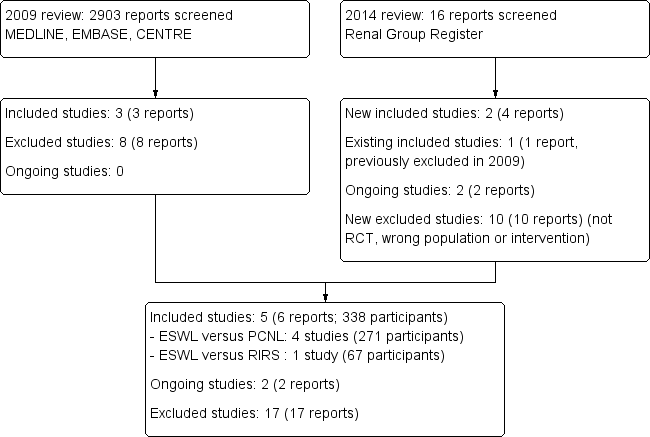
Flow chart of study selection procedure
Included studies
ESWL versus PCNL
Albala 2001 compared ESWL with PCNL for symptomatic lower pole kidney stones ≤ 30 mm. This study was supported by a grant from Microvasive. A total of 160 patients aged over 18 were initially randomised however 32 patients were excluded post‐randomisation but pre‐intervention (withdrew after randomisation (21); stones moved away or passed before surgery (7); violated study protocol (4)). Sixty were enrolled in the ESWL group and 68 in the PCNL group. The mean stone size was 14.43 mm in the ESWL group and 13.59 mm in the PCNL group. Stratified randomisation according to stone sizes of 1 to 10 mm, 11 to 20 mm, and 21 to 30 mm was performed. For ESWL, all lithotriptors were used according to recognised standards. Stone fragments with diameters ≤ 3 mm were defined as successful ESWL treatment. Most PCNL were performed using a single stage procedure and one centre used a two stage technique. The initial outcome measurements were assessed by the end of the third month. They were stone‐free status using nephrotomograms, re‐treatment, auxiliary procedures, complications, stone burden, stone composition, quality of life, EQ, and hospital stay.
Carlsson 1992 compared ESWL with PCNL for medium‐sized kidney stones (4 to 30 mm in diameter). Fifty five consecutive patients from one single hospital were randomised, 25 to PCNL (performed at the Karolinska Hospital) and 30 to ESWL (performed at the Lingköping University Hospital). There were six exclusions post‐randomisation but pre‐intervention (PCNL (4), ESWL (2)). Twenty eight patients (mean age 49 years) were treated with ESWL and 21 (mean age 48.2 years) with PCNL. ESWL treatment used an unmodified Dornier HM‐3 lithotriptor. PCNL was done under epidural anaesthesia with nephrostomy. The outcomes were success rate, hospital stay, duration of treatment, complications and cost/successful treatment. Stone‐free or stone fragments ≤ 5 mm using radiographic examination were considered successful treatment. Follow‐up for stone‐free rates were reported by the end of the fourth week and at one year.
Deem 2010 compared ESWL with PCNL for moderate sized (10 to 20 mm in largest dimension) upper and middle pole kidney stones. Initially eighty patients (40 for each group) were estimated. Patients were randomised in block randomisation using statistical software SAS®. Thirty five patients were enrolled but 32 completed in the study, The three incomplete patients were in the ESWL group because of pregnant immediately after treatment (1), loss of insurance (1) and loss of follow‐up (1). Twelve patients (mean age 52.2 years) were treated with ESWL and 20 (mean age 47.2 years) with PCNL. The Medispec Lithotripter (Montgomery Village, MD) was used with fluoroscopy for ESWL treatment. For PCNL, fluoroscopic guidance, renal mapping were performed using a flexible cystoscopy, fluoroscopy and the "eye of the needle technique". The stone was retrieved with graspers or fragmented in situ with a combined ultrasonic and pneumatic device. The outcomes were stone‐free status using CT scan at three months, complications, need for additional procedures, and quality of life.
Yuruk 2010 compared ESWL with PCNL and observation for asymptomatic lower pole kidney stone size ≤ 20 mm in greatest diameter. 99 patients were randomised to ESWL (33), PCNL (33) and observation (33). Five dropped out (ESWL (2), PCNL (2) and observation (1)) because of no follow‐up data. Among these groups, age, stone size, sex, follow‐up time, and numbers of renal scarring on dimercaptosuccinic acid renal scintigraphy (DMSA) were compared. The mean stone size was 139.4 mm for ESWL, 153.3 mm for PCNL, and 136.7 mm for observation group. Patients underwent ESWL using a Compact Sigma® electromagnetic lithotriptor without anaesthesia. For PCNL, patients was performed and stone fragmented using a Swiss Lithoclast® Master combined pneumatic and ultrasonic lithotriptor. The outcomes were stone‐free rate on non‐contrast abdominal helical computerised tomography at 3 and 12 months after intervention.
ESWL versus RIRS
Pearle 2005 compared ESWL with RIRS using ureteroscopy for lower pole kidney stones ≤ 10 mm. This study was supported by Boston Scientific, Natick, Massachusetts. Seventy eight patients were randomised in blocks of 10 based on a random number table to undergo ESWL or RIRS. Eleven dropped out before treatment, leaving 67 (ESWL (32), RIRS (35)). The two treatment groups were comparable in age, sex, body mass index, stone size, operative time, hospital stay and complications (intra‐and postoperative). Recognised standards for each ESWL machine were used with power settings and number of shock waves used left to the discretion of the individual physician. Ureteroscopes were used for RIRS, including 7.5 Fr and Flex‐X™, ACMI Dur 8™ and Dur 8‐Elite™ and URF‐P3. The use of a ureteral access sheath, intact stone retrieval versus intracorporeal lithotripsy and stent placement were left to the discretion of the individual physician. Stone‐free status was determined by plain X‐ray by the end of third month or sooner if stone‐free. Non‐contrast, thin cut computed tomography (5 mm) was required if there was no evidence of residual stones on plain X‐ray. Success rate was being stone‐free or fragments < 4 mm.
Excluded studies
Seventeen studies were excluded from this updated review. Seven were not studies in kidney stones (Arrabal‐Polo 2009;Karlsen 2007;Lee 2006;Pearle 2001; Peschel 1999; Sun 2008; Zeng 2002), seven were not RCTs (Carr 1996; Eterovic 2005; Liou 2001; Mays 1988; Preminger 2006; Turna 2007; You 2006), two did not have comparison of ESWL with PCNL or RIRS (Bryniarski 2012; Meretyk 1997), and one was a time series (Charig 1986).
Risk of bias in included studies
See Figure 2, Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
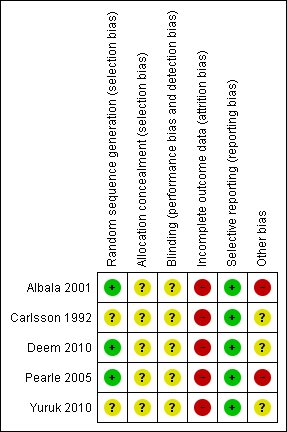
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Three studies clearly described the appropriate method of random sequence generation (Albala 2001; Deem 2010; Pearle 2005) and two did not report the information (Carlsson 1992; Yuruk 2010). Information of allocation concealment was not provided by any of the included studies.
Blinding
Patients and investigators could not be blinded to the interventions. Blinding of the outcome and data assessors were not described by any of the included studies. However, risk of detection bias should be low for this situation.
Incomplete outcome data
The analysis was not done under intention‐to‐treat basis. Patients discontinued their follow‐up after treatment in all studies.
Selective reporting
All studies reported the primary outcomes of interest for this review.
Other potential sources of bias
Two studies were supported by medical devices companies (Albala 2001; Pearle 2005). One author of Deem 2010 was a consultant of a company and subjects of each group after recruitment was not balanced.
Effects of interventions
Success of treatment
ESWL versus PCNL
The success of treatment was significantly greater in the PCNL group compared to the ESWL group at three months (Analysis 1.1.3 (3 studies, 201 participants): RR 0.46, 95% CI 0.35 to 0.62; I² = 28%) and one year (Analysis 1.1.4 (2 studies, 107 participants): RR 0.72, 95% CI 0.54 to 0.95; I² = 57%). Heterogeneity was moderated at one year and this could be attributed to the different sizes of stone fragments measured at the end of treatment. Deem 2010 reported significantly greater success in the PCNL group at the end of the first week (Analysis 1.1.1 (32 participants): RR 0.18, 95% CI 0.05 to 0.62), and Carlsson 1992 reported a significantly greater success in the PCNL group at the end of four weeks ((Analysis 1.1.2 (40 participants): RR 0.77, 95% CI 0.61 to 0.98)
When success was analysed at three months according to initial stone size, there was significantly better success in the PCNL group for stones sized 1 to 10 mm (Analysis 1.1.5 (1 study, 39 participants): RR 0.64, 95% CI 0.45 to 0.90), 10 to 22 mm (Analysis 1.1.6 (2 studies, 86 participants): RR 0.30, 95% CI 0.18 to 0.52; I² = 0%), and 21 to 30 mm (Analysis 1.1.7 (2 studies, 46 participants): RR 0.34, 95% CI 0.16 to 0.72; I² = 0%).
The success of treatment for of lower pole kidney stones (size ≤ 20 mm) was significantly better with PCNL compared to ESWL (Analysis 1.1.8 (2 studies, 155 participants): RR 0.49, 95% CI 0.36 to 0.67; I² = 37%).
ESWL versus RIRS
Pearle 2005 reported no significant difference in success of treatment at three months between ESWL and RIRS (Analysis 2.1 (58 participants): RR 0.91, 95% CI 0.64 to 1.30).
Re‐treatment
ESWL versus PCNL
Albala 2001 reported no significant difference between ESWL and PCNL in overall need for re‐treatment (Analysis 1.2.1 (122 participants): RR 1.81, 95% CI 0.66 to 4.99) or based on initial stone size (Analysis 1.2.2 (1 to 10 mm; 42 participants): RR 0.91, 95% CI 0.14 to 5.86; Analysis 1.2.3 (11 to 20 mm; 62 participants): RR 5.27, 95% CI 0.67 to 41.26; Analysis 1.2.4 (21 to 30 mm; 18 participants): RR 1.00, 95% CI 0.18 to 5.63).
Carlsson 1992; Deem 2010; Yuruk 2010 did not report re‐treatment data.
ESWL versus RIRS
Pearle 2005 reported secondary treatments were necessary for five ESWL patients and two RIRS patients (Analysis 2.2 (67 participants): RR 2.73, 95% CI 0.57 to 13.12) (see Characteristics of included studies).
Auxiliary procedures
There was a significant increase in overall auxiliary procedures in the ESWL group compared to PCNL group (Analysis 1.3.1 (2 studies, 184 participants): RR 8.33, 95% CI 1.58 to 44.02; I² = 0%) however there were no differences between ESWL or PCNL based on initial stone size (Analysis 1.3.2 (1 to 10 mm; 42 participants): RR 6.39, 95% CI 0.35 to 116.57; Analysis 1.3.3 (11 to 20 mm): RR 6.15, 95% CI 0.80 to 47.07.
There was a significant increase in auxiliary procedures for lower pole stones (≤ 20 mm) in the ESWL group (Analysis 1.3.4 (2 studies, 166 participants): RR 8.24, 95% CI 1.56 to 43.40; I² = 0%).
Carlsson 1992, Deem 2010 and Pearle 2005 did not report auxiliary procedures.
Efficiency quotient (EQ)
EQ was calculated to assess the effectiveness of procedures with formula of the stone‐free % / (100% + re‐treatment rate % + auxiliary procedures %). We calculated overall EQ for Albala 2001 which was 28% for ESWL and 86% for PCNL. However, EQ decreased when stone size increased. It accounted for 51%, 17% and 12% in the ESWL group and 91%, 88% and 71% in the PCNL group for stone sizes 1 to 10 mm, 11 to 20 mm and 21 to 30 mm respectively. There was no EQ shown in the studies of Carlsson 1992; Deem 2010; Pearle 2005; Yuruk 2010.
Mean procedural and operating times
ESWL versus PCNL
Carlsson 1992 reported the mean duration of treatment was significantly longer for PCNL (Analysis 1.4 (49 participants): MD ‐36.0 min, 95% CI ‐54.1 to ‐17.9).
ESWL versus RIRS
Pearle 2005 reported the mean operating time was significantly longer for RIRS (Analysis 2.3 (67 participants): MD ‐24.9 min, 95% CI ‐42.34 to ‐7.46).
Mean hospital stay
ESWL versus PCNL
Carlsson 1992 reported the mean hospital stay was significantly shorter for ESWL (Analysis 1.5 (49 participants): MD ‐3.30 days, 95% CI ‐5.45 to ‐1.15).
Albala 2001 reported a mean hospital stay of 0.55 days (range 0 to 9) for ESWL and 2.66 days (range 1 to 7) for PCNL. We could not determine the MD between the two interventions because SDs were not reported. Deem 2010 and Yuruk 2010 did not report mean hospital stay.
ESWL versus RIRS
In Pearle 2005, all patients were discharged home on the day of treatment in the ESWL group, whereas 94% of patients had a mean stay of 0.06 days in hospital after RIRS. We could not determine the MD between the two interventions because the SDs were not reported.
Complications after treatment
ESWL versus PCNL
Albala 2001 reported the complications for ESWL were; urinary tract infection (1), obstruction (2), colic (2), haematoma (1) and steinstrasse (1). For PCNL the reported complications were; urinary tract infection (1), ileus (3), sepsis (1), haematoma (2), obstruction (1), perforation (3), transfusion (1) and arteriovenous fistula (1).
Deem 2010 reported the minor complications (renal colic and fever) in both the ESWL and the PCNL group. Urinary tract infection and inability to tolerate pain (2) were found for PCNL.
The complications in Carlsson 1992 were haematuria and fever (over 38°C). Pain was not considered a complication. They measured complications at days 1, 3, 5, 7, and 9. The period of haematuria in ESWL group was longer than in the PCNL group. Haematuria was still present at day 9 in the ESWL group but not in the PCNL group. Fever was reported less in the ESWL group than the PCNL group.
There were lower pole haematuria (1), lower pole scarring (5) for ESWL and postoperatively fever (1), bleeding necessitating blood transfusion (1), lower pole scarring (1) for PCNL in Yuruk 2010.
ESWL versus RIRS
Pearle 2005 reported intra‐ and postoperative complications. There were 1/32 (3%) Intraoperative complications in the ESWL group and 7/35 (20%) in the RIRS group. There were 7/30 (23%) postoperative complications in the ESWL group and 7/33 (21%) in RIRS group.
Quality of life
There was no important differences of the quality of life data using SF‐36 questionnaire between patients underwent ESWL or PCNL from a patient perspective in Albala 2001 despite PCNL is more invasive.
Deem 2010 reported the improvement of quality of life data at three months after treatment. Physical health scores were significant increase for PCNL while did not for ESWL. However, mental health scores were not different in patients treated with either ESWL or PCNL.
Carlsson 1992; Pearle 2005; Yuruk 2010 did not reported the quality of life data.
Cost‐effectiveness
Carlsson 1992 reported the mean total cost per patient for treated with ESWL (£1394) was significantly lower than PCNL (£2063). The cost per one successful treatment increased in both interventions (ESWL (£1810), PCNL (£2196)). However, patients treated with ESWL were more cost‐effective than PCNL.
Albala 2001; Deem 2010; Pearle 2005 and Yuruk 2010 did not report the cost‐effectiveness data
Discussion
Summary of main results
The success of treatment at three months was significantly greater in the PCNL compared to the ESWL group. Re‐treatment and using auxiliary procedures was significantly increased with ESWL group compared to PCNL. The EQ was higher for PCNL than ESWL; however EQ decreased when stone size increased. Duration of treatment and hospital stay were significantly shorter in the ESWL group. Overall more complications were reported with PCNL, however we were unable to meta‐analyse the included studies due to the differing outcomes reported and the timing of the outcome measurements.
One study compared ESWL versus RIRS for lower pole kidney stones. The success of treatment was not significantly different at the end of the third month between the two treatments; however mean procedural time and mean hospital stay was reported to be longer in the RIRS group.
Overall completeness and applicability of evidence
Four RCTs fulfilled our inclusion criteria and presented the clinical effects between ESWL and PCNL (Albala 2001; Carlsson 1992; Deem 2010; Yuruk 2010) and one study compared ESWL and RIRS for lower pole kidney stones (Pearle 2005). The study by Carlsson 1992 was performed in the early period of ESWL technology and the success criteria for treating kidney stones was measured differently to the later study by Albala 2001; Deem 2010; Yuruk 2010. In term of hospital stay, Carlsson 1992 reported the 4.1 days from ESWL which have been used over a long time period from 1980s. Currently ESWL is accepted as an outpatient procedure. The different criteria of measuring the outcomes, follow‐up period and the different generation types of ESWL affect the results of treatment and make the comparison difficult. Albala 2001; Yuruk 2010 focused on lower pole kidney stones and Deem 2010 attended to upper and middle pole kidney while Carlsson 1992 did not specify stone location. We could combine the success rates of the two interventions at the end of the third month from Albala 2001; Deem 2010; Yuruk 2010. Subgroup analyses were conducted for kidney stones size 10 to 20 mm and lower pole location (stones ≤ 20 mm).
The present technology for ESWL reduces the time required to stay in hospital. As a result, the information on hospital stay for ESWL compared to PCNL and RIRS seems less relevant.
Quality of the evidence
Three studies presented the methods of random sequence generation (Albala 2001; Deem 2010; Pearle 2005) while two did not report sequence generation (Carlsson 1992; Yuruk 2010). None of the included studies provided any information about allocation concealment. Patients and investigators could not be blinded and there were no information about blinding of either the outcome or data assessors in these studies. There were missing patients during the follow‐up of all studies but the reasons were not reported. Thus, intention‐to‐treat was not used to analyse the outcome results. Two studies were supported by the medical device companies (Albala 2001; Pearle 2005) and one of authors in Deem 2010 was a consultant of a company. These factors limited the methodological strength of the evidence.
Potential biases in the review process
All authors had no conflict of interest in this review. Screening of titles and abstracts, assessing of risks of bias, data extraction and data analyses was independently performed (without financial interest of the results) following the Cochrane Handbook suggestions. Disagreements were discussed and resolved among authors. Therefore, there were no potential biases in the update review process.
Agreements and disagreements with other studies or reviews
We founded no systematic review or any other reviews comparing ESWL versus PCNL or RIRS for kidney stones.

Flow chart of study selection procedure

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 ESWL versus PCNL, Outcome 1 Success of treatment.

Comparison 1 ESWL versus PCNL, Outcome 2 Re‐treatment.

Comparison 1 ESWL versus PCNL, Outcome 3 Auxiliary procedures.

Comparison 1 ESWL versus PCNL, Outcome 4 Procedural and operating time.

Comparison 1 ESWL versus PCNL, Outcome 5 Hospital stay.

Comparison 2 ESWL versus RIRS, Outcome 1 Success of treatment.

Comparison 2 ESWL versus RIRS, Outcome 2 Re‐treatment.

Comparison 2 ESWL versus RIRS, Outcome 3 Procedural and operating time.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Success of treatment Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Success at 1 week | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.05, 0.62] |
| 1.2 Success at 4 weeks | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| 1.3 Success at 3 months | 3 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.62] |
| 1.4 Success at 1 year | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.54, 0.95] |
| 1.5 Success at 3 months according to stone size (1‐10 mm) | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.45, 0.90] |
| 1.6 Success at 3 months according to stone size (10‐20 mm) | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.18, 0.52] |
| 1.7 Success at 3 months according to stone size (21‐30 mm) | 2 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.16, 0.72] |
| 1.8 Success of treating lower pole stones ≤ 20 mm | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.36, 0.67] |
| 2 Re‐treatment Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Overall re‐treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Re‐treatment according to stone size (1‐10 mm) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Re‐treatment according to stone size (11‐20 mm) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Re‐treatment according to stone size (21‐30 mm) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Auxiliary procedures Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Overall auxiliary procedures | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 8.33 [1.58, 44.02] |
| 3.2 Auxiliary procedures according to stone size (1‐10 mm) | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 6.39 [0.35, 116.57] |
| 3.3 Auxiliary procedures according to stone size (11‐20 mm) | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 6.15 [0.80, 47.07] |
| 3.4 Auxiliary procedures according to stone size (21‐30 mm) | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Auxiliary procedures of lower pole stones (stone size ≤ 20 mm) | 2 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 8.24 [1.56, 43.40] |
| 4 Procedural and operating time Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Hospital stay Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Success of treatment Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Success at 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Re‐treatment Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Procedural and operating time Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

