Хирургические против медикаментозных вмешательств при хроническом риносинусите с назальными полипами
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006991.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 01 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades de oído, nariz y garganta
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
JR: data search and analysis; author of results, discussion and conclusion text; author of tables and figures.
WF: data search and analysis; author of results tables.
LYC: data analysis, author of results, discussion and conclusion text and 'Summary of findings' table.
CH: background literature search; development of protocol; data search and analysis; author of background text.
Sources of support
Internal sources
-
NIHR/Cochrane Incentive Scheme Award 2013, UK.
External sources
-
None, Other.
Declarations of interest
Joanne Rimmer has no conflicts of interest to declare.
Wytske Fokkens has no conflicts of interest to declare.
Lee Yee Chong has no conflicts of interest to declare.
Claire Hopkins has no conflicts of interest to declare.
Acknowledgements
The authors would like to acknowledge Jenny Bellorini and Samantha Faulkner of the Cochrane Ear, Nose & Throat Disorders Group for their invaluable support, advice and assistance with the literature search, analysis and writing of the review. We also wish to acknowledge Ben Hunter for undertaking partial review and analysis of the earliest search.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Dec 01 | Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps | Review | Joanne Rimmer, Wytske Fokkens, Lee Yee Chong, Claire Hopkins | |
| 2008 Jan 23 | Medical versus surgical interventions for nasal polyps | Protocol | Claire Hopkins, Chris Loh, David Roberts | |
Differences between protocol and review
The title has changed from 'Medical versus surgical interventions for nasal polyps' to 'Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps', in accordance with current terminology (Fokkens 2012), and usual practice (less invasive/first‐line treatments as control).
We have further developed the Methods section regarding study selection. Initially, the primary outcome was "symptom scores"; we have expanded this into "disease severity, as measured by patient‐reported disease‐specific symptom scores", "health‐related quality of life, using disease‐specific health‐related quality of life scores" and "health‐related quality of life, using generic quality of life scores". The fourth secondary outcome was originally "physiological measures of nasal airway resistance, cross‐sectional area, olfaction or ciliary function"; we have divided this into two separate secondary outcomes. The first is "objective physiological measures: nasal peak flow, nasal volume, nasal cross‐sectional area, nasal nitric oxide (nNO), ciliary function" and the second is "olfactory tests". This was done because olfactory tests are by definition subjective.
We have also added the clarifying statement, "We analysed the following outcomes in the review, but they were not used as a basis for including or excluding studies", reflecting current standards.
We have explicitly stated that studies with a 'split‐nose' design (i.e. one side of the nose is treated with one surgical technique and the other side is treated with another) were excluded from the review due to their high risk of bias, as ongoing inflammation on one side may affect the rate of recurrence on the contralateral side, thus confounding a particular surgical technique.
We have adopted the Cochrane 'Risk of bias' tool for study quality assessment (Handbook 2011).
We have provided more detail on data synthesis methods.
We have included a 'Summary of findings' table and described the method used.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Aged; Aged, 80 and over; Female; Humans; Male; Middle Aged;
PICO

Process for sifting search results and selecting studies for inclusion.
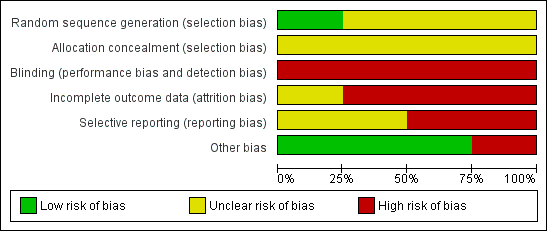
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
| Endonasal surgery compared with medical treatment for chronic rhinosinusitis with nasal polyps | |||||
| Patients: adults with chronic rhinosinusitis with nasal polyps, receiving concurrent topical steroids Settings: otorhinolaryngology clinics and/or hospital departments Intervention: endoscopic sinus surgery Comparison: systemic steroids | |||||
| Outcomes (at 12 months) | Illustrative comparative risk | Relative effect | Number of participants | Quality of the evidence | Comments |
| Patient‐reported disease‐specific symptom scores
| N/A |
| 95 (1 study) | ⊕⊕⊝⊝ | Negative values indicate lower scores (less severe symptoms) in the surgical group. The range of values corresponds to effect sizes that are small to large (SMD 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect size):
|
| Health‐related quality of life scores, measured by SF‐36 (range 0 to 100) | N/A |
| 95 (1 study) | ⊕⊕⊝⊝ | These effect sizes are negligible and correspond to a SMD of ‐0.15 and 0.07 respectively (a SMD of 0.2 corresponds to a small effect size) |
| Endoscopic appearances
| N/A | MD ‐1.5 (95% CI ‐1.2 to ‐1.8) | 95 (1 study) | ⊕⊕⊝⊝ | Negative values indicate lower scores (less severe polyposis) in the surgical group. The SMD is ‐2.2 (95% CI ‐2.7 to ‐1.7), which corresponds to a large effect size |
| Complications | N/A | Epistaxis: 3.6% Orbital: 7.1% Intracranial: 1.8% | 95 (1 study) | ⊕⊝⊝⊝ | Orbital complications: orbital fat exposed Intracranial complications: CSF leak with meningitis |
| Objective physiological measures | N/A | Not reported | Other comparison pairs did not show an important difference | ||
| Olfactory tests | N/A | Not reported | Other comparison pairs did not show an important difference | ||
| GRADE Working Group grades of evidence CI: confidence interval; CSF: cerebrospinal fluid; MCS: mental component score; MD: mean difference; N/A: not applicable; PCS: physical component score; SMD: standardised mean difference | |||||
| 1Downgraded twice due to limitations in the study design (unclear randomisation, non‐blinded outcome assessment), and some concerns about imprecision (small sample sizes) and indirectness of evidence (the medical treatment regimes used in the study differ from current standards). Additional downgrading for imprecision and lack of comparative data for the complications outcome. | |||||
| Instrument | Details |
| Rhinosinusitis Outcome Measure (RSOM‐31) (Piccirillo 1995) | 31 items in 7 domains: nasal, eye, ear, sleep, general, practical and emotional problems. 2 rating scales: magnitude and importance of each item. Each item is measured on a 0 to 10 cm visual analogue scale (VAS). The minimal important difference (MID) is greater than 1. Time‐consuming and complex scoring system |
| 20‐item Sino‐Nasal Outcome Test (SNOT‐20) (Piccirillo 2002) | 20‐item modification of RSOM‐31, in 5 domains. Patients rate the magnitude of each item and the 5 most important items. Each item is rated on a 6‐point scale (0 = no problem, 5 = most serious problem) (range 0 to 100). Complex scoring system because of weighting for important symptoms. Excludes nasal blockage and anosmia |
| 22‐item Sino‐Nasal Outcome Test (SNOT‐22) (Hopkins 2009) | Modification of SNOT‐20 including nasal blockage and anosmia. Patients rate the magnitude of each item. Each item is rated on a 6‐point scale (0 = no problem, 5 = most serious problem) (range 0 to 110). Excludes weighting for most important symptoms. The minimal important difference (MID) is greater than 8.9 |
| Study | Surgical treatment | Medical treatment | Comparison description |
| GA endoscopic sinus surgery (variable extent) then topical budesonide 400 µg bd for 1 year | Oral prednisolone for 14 days (30 mg od 4 days then reduced by 5 mg every 2 days) then topical budesonide 400 µg bd for 1 year | Endoscopic sinus surgery versus systemic steroids | |
| LA polypectomy then topical beclomethasone dipropionate 100 µg bd for 1 year | 14 mg im betamethasone then topical beclomethasone dipropionate 100 µg bd for 1 year | Polypectomy versus systemic steroids | |
| LA snare polypectomy then topical budesonide 400 µg or 800 µg od for 11 months | 14mg im betamethasone then topical budesonide 400 µg or 800 µg od for 11 months | Polypectomy versus systemic steroids | |
| GA endoscopic sinus surgery (variable extent) then antibiotics for 2 weeks plus topical fluticasone propionate spray 100 µg bd for 12 weeks, then "tailored treatment" | Antibiotics plus topical fluticasone propionate drops 400 µg bd for 12 weeks, then "tailored treatment" | Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | |
| bd: twice daily | |||
| Study | Intervention/comparison | Outcome measure (scale) | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | Nasal obstruction (0 to 3) | 12 months | MD ‐0.3 (95% CI ‐0.6 to 0.0); standardised mean difference (SMD) ‐0.4 (95% CI ‐0.8 to 0.0) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Endoscopic sinus surgery versus systemic steroids | Rhinorrhoea/nasal discharge (0 to 3) | 12 months | MD ‐0.2 (95% CI ‐0.5 to 0.1); SMD ‐0.3 (95% CI ‐0.7 to 0.1) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Endoscopic sinus surgery versus systemic steroids | Sneezing (0 to 3) | 12 months | MD ‐0.2 (95% CI ‐0.5 to 0.1); SMD ‐0.3 (95% CI ‐0.7 to 0.1) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Endoscopic sinus surgery versus systemic steroids | Loss of smell (0 to 3) | 12 months | MD ‐0.4 (95% CI ‐0.7 to ‐0.1); SMD ‐0.6 (95% CI ‐1.0 to ‐0.2) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Polypectomy versus systemic steroids | Sense of smell (not known) | 12 months | Improved with medical and surgical treatment (P value > 0.05); no statistical difference between groups (exact data not given) | |
| Polypectomy versus systemic steroids | Nasal obstruction (0 to 3) | 12 months | Improved with medical and surgical treatment (P value > 0.05); no statistical difference between groups (exact data not given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Nasal obstruction Nasal discharge Facial pain/pressure Headache Overall discomfort (VAS 0 to 10 for each) | 12 months | Improved with medical treatment (61.2% change from baseline (SD = 19.1), P value < 0.01) and surgical treatment (54.7% change from baseline (SD = 27.3), P value < 0.01); no statistical difference between groups (data not given separately) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Olfactory disturbance | 12 months | Improved with medical and surgical treatment (P value < 0.05); no statistical difference between groups (exact data not given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | SNOT‐20 | 12 months | Improved with medical and surgical treatment (P value < 0.01); no statistical difference between groups (exact data not given) | |
| CI: confidence interval | ||||
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | SF‐36 (0 to 100 each domain) | 12 months | The MD was ‐1.4 (95% CI ‐5.0 to 2.2) for the physical component summary score, and 0.6 (95% CI ‐2.9 to 4.1) for the mental component scores. Effect sizes are negligible and correspond to a SMD of 0.07 and ‐0.15 respectively (a SMD of 0.2 corresponds to a small effect size) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | SF‐36 (0 to 100 each domain) | 12 months | Improved with surgical and medical treatment (P value < 0.01), except physical domain (P value > 0.05); no statistical difference between groups (exact data not given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | SNOT‐20 | 12 months | Actual results not reported; only stated that there were no statistically significant differences between groups | |
| CI: confidence interval | ||||
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | Polyp size score (0 to 3) | 12 months | Polyp size scores improved significantly in both groups at 12 months, and were significantly better in the endoscopic sinus surgery group (P value < 0.05): MD ‐1.5 (95% CI ‐1.8 to ‐1.2, n = 95). This corresponds to a large effect size | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Endoscopic score (0 to 3) | 12 months | Polyp size scores improved significantly in both groups at 12 months, but there was no important difference between groups (P value > 0.05): MD ‐2.3% (95% CI ‐17.4 to 12.8, n = 34) | |
| CI: confidence interval | ||||
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | Epistaxis (nosebleed) | 12 months | 3.6% (endoscopic sinus surgery) | |
| Polypectomy versus systemic steroids | Epistaxis | 12 months | "1 patient failed the study because of bleeding" (no further information) | |
| Polypectomy versus systemic steroids | Epistaxis | 12 months | 21% (all patients were on medical treatment throughout; polypectomy versus systemic steroids comparison not reported separately) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Epistaxis | 12 months | 2.2% (endoscopic sinus surgery plus topical steroid) versus 4.4% (antibiotics plus high‐dose topical steroid) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Infection | 12 months | 4.4% (endoscopic sinus surgery plus topical steroid) | |
| Endoscopic sinus surgery versus systemic steroids | Orbital complications | 12 months | 7.1% (endoscopic sinus surgery ‐ exposure of orbital fat) | |
| Endoscopic sinus surgery versus systemic steroids | Intracranial complications | 12 months | 1.8% (endoscopic sinus surgery ‐ CSF leak with meningitis) | |
| CSF: cerebrospinal fluid | ||||
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Polypectomy versus systemic steroids | Nasal expiratory peak flow | 12 months | Improved with surgical and medical treatment; no statistical difference between groups (P value > 0.05) (exact data not given) | |
| Polypectomy versus systemic steroids | Peak expiratory flow rate index | 12 months | Improved in all groups; no statistical difference between groups (surgical versus medical groups not reported separately, no exact data given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Total nasal volume | 12 months | Improved with surgical (58.8% change from baseline, SD = 40) and medical treatment (50.3% change from baseline, SD = 50.7), P value < 0.01; no statistical difference between groups although medical group tended towards greater improvement (P value > 0.05) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Nasal cross‐sectional area | 12 months | No statistical difference between groups (P value > 0.05, Mann Whitney‐U test, not normally distributed data, median not reported) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Nasal nitric oxide levels | 12 months | No statistical difference between groups (P value > 0.05, Mann Whitney‐U test, not normally distributed data, median not reported) | |
| SD: standard deviation | ||||
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Polypectomy versus systemic steroids | Proportion of patients "expressing intact smell" | 2 to 12 months | No important difference between groups: risk ratio 0.96 (95% CI 0.71 to 1.31, n = 53) | |
| Polypectomy versus systemic steroids | Semi‐quantitative smell test | 12 months | No statistical difference between any treatment groups (surgical versus medical groups not reported separately, exact data not given) |

