Liječenje kroničnog rinosinuitisa s nazalnim polipima – kirurški ili neinvazivno
Abstract
Background
Nasal polyps cause nasal obstruction, discharge and reduction in or loss of sense of smell, but their aetiology is unknown. The management of chronic rhinosinusitis with nasal polyps, aimed at improving these symptoms, includes both surgical and medical treatments, but there is no universally accepted management protocol.
Objectives
To assess the effectiveness of endonasal/endoscopic surgery versus medical treatment in chronic rhinosinusitis with nasal polyps.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; Cambridge Scientific Abstracts; ICTRP and additional sources for published and unpublished trials. The date of the search was 20 February 2014.
Selection criteria
Randomised controlled trials of any surgical intervention (e.g. polypectomy, endoscopic sinus surgery) versus any medical treatment (e.g. intranasal and/or systemic steroids), including placebo, in adult patients with chronic rhinosinusitis with nasal polyps.
Data collection and analysis
We used the standard methodological procedures expected by The Cochrane Collaboration. Meta‐analysis was not possible due to the heterogeneity of the studies and the selective (incomplete) outcome reporting by the studies.
Main results
Four studies (231 participants randomised) are included in the review. No studies were at low risk of bias. The studies compared different types of surgery versus various types and doses of systemic and topical steroids and antibiotics. There were three comparison pairs: (1) endoscopic sinus surgery (ESS) versus systemic steroids (one study, n = 109), (2) polypectomy versus systemic steroids (two studies, n = 87); (3) ESS plus topical steroid versus antibiotics plus high‐dose topical steroid (one study, n = 35). All participants also received topical steroids but doses and types were the same between the treatment arms of each study, except for the study using antibiotics. In that study, the medical treatment arm had higher doses than the surgical arm. In two of the studies, the authors failed to report the outcomes of interest. Although there were important differences in the types of treatments and comparisons used in these studies, the results were similar.
Primary outcomes: symptom scores and quality of life scores
There were no important differences between groups in either the patient‐reported disease‐specific symptom scores or the health‐related quality of life scores. Two studies (one comparing ESS plus topical steroid versus antibiotics plus high‐dose topical steroid, the other ESS versus systemic steroids) failed to find a difference in generic health‐related quality of life scores. The quality of this evidence is low or very low.
Endoscopic scores and other secondary outcomes
Two studies reported endoscopic scores. One study (ESS versus systemic steroids) reported a large, significant effect size in the surgical group, with a mean difference (MD) in score of ‐1.5 (95% confidence interval (CI) ‐1.78 to ‐1.22, n = 95) on a scale of 0 to 3 (0 = no polyposis, 3 = severe polyposis). In the other study (ESS plus topical steroid versus antibiotics plus high‐dose topical steroid) no difference was found between the groups (MD 2.3%, 95% CI ‐17.4% to 12.8%, n = 34). None of the included studies reported recurrence rates. No differences were found for any objective measurements or olfactory tests in those studies in which they were measured.
Complications
Complication rates were not reported in all studies, but rates of up to 21% for medical treatment and 14.3% for surgical treatment are described. Epistaxis was the most commonly reported complication with both medical and surgical treatments, with severe complications reported rarely.
Authors' conclusions
The evidence relating to the effectiveness of different types of surgery versus medical treatment for adults with chronic rhinosinusitis with nasal polyps is of very low quality. The evidence does not show that one treatment is better than another in terms of patient‐reported symptom scores and quality of life measurements. The one positive finding from amongst the several studies examining a number of different comparisons must be treated with appropriate caution, in particular when the clinical significance of the measure is uncertain.
As the overall evidence is of very low quality (serious methodological limitations, reporting bias, indirectness and imprecision) and insufficient to draw firm conclusions, further research to investigate this problem, which has significant implications for quality of life and healthcare service usage, is justified.
PICO
Laički sažetak
Liječenje kroničnog rinosinuitisa s nazalnim polipima – kirurški ili neinvazivno
Dosadašnje spoznaje
Nosni polipi su česta pojava i to su dobroćudne izrasline sluznice nosa. Ne stvaraju poteškoće, ali mogu uzrokovati začepljenost nosa, kongestiju (oteklina sluznice nosa), osjet pritiska na licu i anosmiju (gubitak osjeta mirisa). Učestalost simptomatskih nosnih polipa je u porastu s životnom dobi i češći su u muškaraca nego u žena. Uzrok njihovog nastanka nije poznat, ali je moguće da nastanu uslijed kronične (dugotrajne) upale sluznice nosa i sinusa (tj. kroničnog rinosinuitisa s nosnim polipima). Ovo stanje se može liječiti konzervativno tj. kortikosteroidnim lijekovima koji se primjenjuju lokalno u obliku intranazalnog raspršivača ili kirurški (uklanjanje polipa tj. polipektomija sa ili bez endoskopske kirurgije sinusa (engl. endoscopic sinus surgery, ESS). Nije poznato koji je oblik liječenja učinkovitiji.
Značajke studija
Četiri randomizirane kontrolirane kliničke studije su obuhvatile ukupno 231 bolesnika s dijagnozom kroničnog rinosinuitisa s nosnim polipima. U pojedinačnim studijama je bilo od 34 do 109 bolesnika i sve su provedene na ORL odjelima u nekoliko europskih zemalja. Bolesnici su bili odrasli i bilo je više muškaraca nego žena. U svim studijama je vrsta liječenja randomizirana (nasumično određeno), tj. kirurško ili konzervativno (antibiotici ili kortikosteroidi ili injekcije) uz lokalnu primjenu kortikosteroida u obliku raspršivača ili kapi. Različite vrste kirurških zahvata i konzervativnog liječenja primijenjene su u ovim studijama, a što je otežavalo usporedbu. Posljedično su studije raspoređene u četiri skupine na temelju sličnosti vrste liječenja.
Ključni rezultati
Najvažniji ishod je bodovanje simptoma vezanih uz osnovnu bolest i kvaliteta života vezanu uz zdravlje prema procjeni samih bolesnika, a također i opće bodovanje kvalitete života. Nije bilo razlika između skupina obzirom na simptome vezane uz bolest prema izjavama bolesnika ili prema bodovanju kvalitete života vezane uz zdravlje. Dvije studije (u jednoj je uspoređeno kirurško liječenje ESS i lokalna primjena kortikosteroida s liječenjem antibioticima i visoke doze lokalne primjene kortikosteroida; u drugoj je uspoređena ESS i sustavna primjena kortikosteroida) nisu pokazale razlike u općoj procjeni kvalitete života povezane sa zdravljem.
U dvije studije je uspoređena veličina polipa (pri pregledu). U jednoj studiji (usporedba ESS i sustavne primjene kortikosteroida) je nađena značajno bolja procjena nakon kirurškog liječenja u odnosu na liječenje kortikosteroidima nakon 12 mjeseci. U drugoj studiji nije nađena razlika između kirurškog liječenja (ESS) i liječenja antibioticima uz visoke doze lokalne primjene kortikosteroida.
Nije bilo razlike između različitih kirurških i konzervativnih vrsta liječenja u nijednoj od studija za ispitivane ishode. Pojavnost komplikacija nije bila prikazana u studijama, ali krvarenje iz nosa (epistaksa) je bilo najčešće kod kirurškog i konzervativnog liječenja. Teške komplikacije su bile rijetka pojava bez obzira na liječenje.
Zaključak
Ovi dokazi ne omogućuju zaključivanje o prednosti liječenja na temelju simptoma na koje se žale bolesnici i procjeni kvalitete života. Pozitivan ishod u svezi veličine polipa treba oprezno tumačiti budući da su usporedbe u tim studijama bile raznolike, a kliničko značenje nesigurno. Za sada nema dovoljno dokaza za zaključivanje o najboljem liječenju ovog stanja. Kronični rinosinuitis s nazalnim polipima znatno utječe na kvalitetu života i korištenje usluga u zdravstvu. Daljnja istraživanja su potrebna za razjašnjavanje ove problematike.
Kvaliteta dokaza
Općenito su dokazi bili niske i vrlo niske kvalitete i pouzdanost studija je slaba, te će daljnja istraživanja moguće promijeniti sadašnje rezultate. Postojala su znatna ograničenja u provedbi i prikazu rezultata ovih studija, a broj bolesnika uključenih je bio mali. Dodatna poteškoća je da su neki oblici konzervativnog liječenja danas izvan standardne upotrebe za ovo stanje.
Dokazi su pregledani zaključno s 20. veljačom 2014.
Authors' conclusions
Summary of findings
| Endonasal surgery compared with medical treatment for chronic rhinosinusitis with nasal polyps | |||||
| Patients: adults with chronic rhinosinusitis with nasal polyps, receiving concurrent topical steroids Settings: otorhinolaryngology clinics and/or hospital departments Intervention: endoscopic sinus surgery Comparison: systemic steroids | |||||
| Outcomes (at 12 months) | Illustrative comparative risk | Relative effect | Number of participants | Quality of the evidence | Comments |
| Patient‐reported disease‐specific symptom scores
| N/A |
| 95 (1 study) | ⊕⊕⊝⊝ | Negative values indicate lower scores (less severe symptoms) in the surgical group. The range of values corresponds to effect sizes that are small to large (SMD 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect size):
|
| Health‐related quality of life scores, measured by SF‐36 (range 0 to 100) | N/A |
| 95 (1 study) | ⊕⊕⊝⊝ | These effect sizes are negligible and correspond to a SMD of ‐0.15 and 0.07 respectively (a SMD of 0.2 corresponds to a small effect size) |
| Endoscopic appearances
| N/A | MD ‐1.5 (95% CI ‐1.2 to ‐1.8) | 95 (1 study) | ⊕⊕⊝⊝ | Negative values indicate lower scores (less severe polyposis) in the surgical group. The SMD is ‐2.2 (95% CI ‐2.7 to ‐1.7), which corresponds to a large effect size |
| Complications | N/A | Epistaxis: 3.6% Orbital: 7.1% Intracranial: 1.8% | 95 (1 study) | ⊕⊝⊝⊝ | Orbital complications: orbital fat exposed Intracranial complications: CSF leak with meningitis |
| Objective physiological measures | N/A | Not reported | Other comparison pairs did not show an important difference | ||
| Olfactory tests | N/A | Not reported | Other comparison pairs did not show an important difference | ||
| GRADE Working Group grades of evidence CI: confidence interval; CSF: cerebrospinal fluid; MCS: mental component score; MD: mean difference; N/A: not applicable; PCS: physical component score; SMD: standardised mean difference | |||||
| 1Downgraded twice due to limitations in the study design (unclear randomisation, non‐blinded outcome assessment), and some concerns about imprecision (small sample sizes) and indirectness of evidence (the medical treatment regimes used in the study differ from current standards). Additional downgrading for imprecision and lack of comparative data for the complications outcome. | |||||
Background
Description of the condition
Nasal polyps are tumour‐like hyperplastic swellings of the nasal mucosa, most commonly originating from within the ostiomeatal complex (Larsen 2004). They are a common problem; the prevalence is estimated at 0.2% to 4% in worldwide studies (Johansson 2003; Lange 2013; Min 1996). There is at least a 2:1 male to female predominance. The incidence of symptomatic nasal polyps increases with age, reaching a peak in individuals aged 50 to 59 years, and then declines (Larsen 2004). While polyps can be asymptomatic, they may produce symptoms such as nasal obstruction, congestion, anosmia and facial pressure, and are associated with a significant reduction in quality of life (Radenne 1999). Nasal polyps are usually found in association with chronic rhinosinusitis, which is broadly divided into two phenotypes based on the presence or absence of nasal polyps on examination. Chronic rhinosinusitis with nasal polyps is a chronic disease, with a clinical course often lasting over 20 years (Vento 2000).
Definition
Nasal polyps are considered a subgroup of chronic rhinosinusitis. The European Position Paper on Rhinosinusitis and Nasal Polyps defines these conditions clinically as: "inflammation of the nose and paranasal sinuses, associated with two or more of the following symptoms:
-
blockage/congestion; discharge (anterior or post‐nasal drip); facial pain/pressure; reduction of smell; and
-
either endoscopic evidence of polyps; mucopurulent discharge from the middle meatus or oedema/mucosal obstruction primarily in the middle meatus; and/or mucosal changes within the ostiomeatal complex or sinuses on computed tomography (CT) imaging" (Fokkens 2012).
Chronic rhinosinusitis with nasal polyps is then further defined by the endoscopic visualisation of polyps bilaterally in the middle meatus. The condition is defined as chronic when symptoms persist for more than 12 weeks.
Histology
Histologically, polyps consist of extracellular oedema with an associated inflammatory cell infiltrate, with a surface covering of respiratory epithelium, often with areas of metaplasia (Larsen 1989). The inflammatory cells found are characterised by type 2 T‐helper cell (Th2) inflammation, predominantly eosinophils (Stoop 1993), in addition to mast cells, lymphocytes, neutrophils and plasma cells (Pawliczak 2005). Numerous inflammatory mediators, growth factors and adhesion molecules are increased, including interleukins (IL), particularly IL‐5, interferon Y, RANTES, granulocyte macrophage colony‐stimulating factor, eosinophilic cationic protein and p‐selectin (Bateman 2003).
Aetiology
Nasal polyps are thought to be a manifestation of chronic inflammation, where they represent the final common pathway of several disease processes, the trigger for which is still unknown. There are numerous theories including hereditary factors, anatomical factors, systemic and local allergy, and infection.
A positive family history of nasal polyps has been found in 14% of patients (Greisner 1996), and nasal polyps have developed even when identical twins have been exposed to different environmental factors, suggesting a genetic link. Certain human leukocyte antigens (HLA), such as HLA‐DR7, have been associated with an increased susceptibility to developing nasal polyps (Molnar‐Gabor 2000).
Anatomically, mucosal contact within the nose has been postulated to cause an inflammatory reaction, with subsequent cytokine release leading to polyp formation (Stammberger 1991). However, mucosal contact also occurs without polyp formation (Jones 1997). Ostiomeatal occlusion secondary to an anatomical abnormality or mucosal oedema has also been suggested as an initiator of inflammation.
The eosinophilia, mast cell degranulation and high immunoglobulin (Ig) E levels found in nasal polyps suggest that allergy is a factor in polyp formation. However, when 3000 atopic patients were assessed only 0.5% had nasal polyps on anterior rhinoscopy (Caplin 1971). Similarly, objective measures of atopy (such as skin prick testing) were no more common in nasal polyp patients than in controls (Jamal 1987). A local allergic response within the nose may be responsible for nasal polyps, since it has been shown that total and specific IgE in polyp tissue correlates with the degree of eosinophilia, but is unrelated to positive skin prick tests (Bachert 2001). The allergens responsible may be bacterial, as bacterial‐specific IgE has been identified in patients with nasal polyps but not in those with allergic rhinitis (Calenoff 1993).
Infection may trigger immunologic events leading to the development of chronic rhinosinusitis with nasal polyps. Staphylococcus aureus has been found to colonise the nose in two‐thirds of patients with nasal polyps, compared with less than a third of controls and patients with chronic rhinosinusitis without nasal polyps (van Zele 2004). IgE antibodies specific to Staphylococcus aureus enterotoxins SE‐A and B have been found in nasal polyp tissue (Bachert 2001), indicating that superantigens could be involved in the pathogenesis. Superantigens are toxins of microbial or viral origin that target the immune system, triggering polyclonal T‐cell proliferation and activation. They have the ability to bypass conventional restrictions of the immune system triggering massive cytokine release. Therefore they could induce IgE synthesis leading to eosinophilic inflammation. Colonisation, however, does not always lead to production of superantigens, and other factors must be involved (Zhang 2005).
True type 1 allergic (eosinophilic) fungal sinusitis is characterised by diffuse nasal polyposis. The increasingly widespread demonstration of fungi in the nasal cavity and sinuses in patients with chronic rhinosinusitis has led to the suggestion that fungal colonisation may play an aetiological role in all polyps (Ponikau 1999), recruiting eosinophils and leading to release of inflammatory mediators. However, fungi may also be found in up to 100% of healthy control subjects (Cleland 2014).
There are clear associations between chronic rhinosinusitis with nasal polyps and other diseases. The prevalence of chronic rhinosinusitis with nasal polyps in asthmatic patients is reported to be as high as 13%. Conversely, in patients with chronic rhinosinusitis with nasal polyps, the prevalence of asthma is as high as 45% (Rugina 2002). There is an even stronger association in patients with aspirin hypersensitivity and bronchial asthma; more than 90% of these patients have severe nasal polyposis. This association is referred to as Samter's triad (Samter 1968). Nasal polyps are also associated with cystic fibrosis, ciliary dyskinesia syndromes (Kartagener's syndrome, Young's syndrome) and eosinophilic granulomatosis with polyangiitis (EGPA, Churg Strauss syndrome).
In summary, nasal polyps are likely to represent the end result of many different mechanisms and the search for a single aetiological factor may be in vain. Regardless of trigger, the end result is a failure to mount an appropriate immune response to antigens in the nose and sinuses, resulting in chronic inflammation (Chin 2013).
Description of the intervention
The management of chronic rhinosinusitis with nasal polyps involves both surgical and medical approaches and remains a controversial subject. A variety of intranasal corticosteroids form the mainstay of conservative management, with good evidence for their efficacy. A number of randomised, placebo‐controlled trials document statistically significant improvements in subjective symptom scores, polyp size and objective nasal flow rates following topical steroid use (Kalish 2012). Symptoms of nasal obstruction can be controlled in anywhere from 50% up to 80% of patients. However, clinical studies indicate that management of anosmia is poor, especially when compared with systemic steroids. Adverse effects from nasal steroids are few, and range from epistaxis to headaches and dizziness. Using the more modern formulations, such as fluticasone or mometasone, there is minimal systemic absorption and the dose is well below that required for adrenal suppression.
The use of systemic steroids (often termed a 'medical polypectomy') has also shown to be effective. Van Camp treated 25 patients with chronic rhinosinusitis with nasal polyps with oral steroids; 72% had relief of symptoms and 52% improved on computed tomography (CT) scan. Unlike topical therapy, systemic steroids appear to be effective in improving sense of smell (van Camp 1994). A recent systematic review identified three randomised controlled trials demonstrating benefit over placebo (Martinez‐Devesa 2011). However, there is a high rate of recurrence of symptoms once oral steroids are stopped (Lildholdt 1989). The use of oral steroids is limited by their toxicity, with adverse effects including weight gain, immunosuppression and adrenal suppression. Using a combination of topical and intermittent oral steroids, reasonable symptom control can be achieved in the majority of patients (Slavin 1997).
Alternative preparations used in the management of chronic rhinosinusitis with nasal polyps are reported in the literature, but lack the evidence base of corticosteroids. It has been suggested that treatment with leukotriene inhibitors has resulted in improvement and resolution of the polyps, particularly in patients with aspirin sensitivity (Parnes 2002). Macrolide antibiotics have been proposed to have therapeutic activity based on their anti‐inflammatory properties. Long‐term, low‐dose macrolide antibiotic treatment has been shown to reduce IL‐8 production in nasal polyps and decrease their size (Yamada 2000). However, subgroup analysis of a placebo‐controlled trial suggests benefit is limited to patients with normal levels of IgE (Cervin 2014). New approaches, particularly targeting eosinophilic recruitment, are evolving. Interleukins, particularly IL‐5 and eotaxin (but also IL‐13 and IL‐8) may play a major role in chronic rhinosinusitis with nasal polyps (Bachert 2000). IL‐5 is essential for maturation of eosinophils in the bone marrow and orchestrates their migration into the tissues. Blockage of IL‐5 and eotaxin production, chemokine receptors and other sites in the inflammatory pathway using neutralising monoclonal antibodies is currently being investigated in patients with asthma, rhinitis and nasal polyps. While these approaches may offer alternatives to corticosteroid treatment or surgery, large‐scale controlled trials are lacking at present.
In cases of marked mechanical obstruction of the airways or chronic disease unresponsive to maximal medical therapy, surgical intervention is the treatment of choice (Slavin 1997). Occlusion of the nasal passages by large polyps may be treated by simple polypectomy to restore patency of the nasal airway. The spectrum of surgical options ranges from simple polypectomy using a snare or forceps to surgery opening into the sinuses, or radical 'nasalisation' of the sinuses.
Staging and outcome assessment
As curative treatment is hard to achieve in chronic rhinosinusitis with nasal polyps, management is primarily aimed at reducing symptom severity. It is therefore important to include a measurement of health‐related quality of life when assessing the severity of disease or outcome of treatment. The duration and severity of individual symptoms may be measured numerically, or using visual analogue scales (VAS). In addition, there are now many validated questionnaires available that measure general health or disease‐specific quality of life. Several instruments have been designed and validated to measure disease‐specific quality of life in sinonasal disease; some examples are shown in Table 1.
| Instrument | Details |
| Rhinosinusitis Outcome Measure (RSOM‐31) (Piccirillo 1995) | 31 items in 7 domains: nasal, eye, ear, sleep, general, practical and emotional problems. 2 rating scales: magnitude and importance of each item. Each item is measured on a 0 to 10 cm visual analogue scale (VAS). The minimal important difference (MID) is greater than 1. Time‐consuming and complex scoring system |
| 20‐item Sino‐Nasal Outcome Test (SNOT‐20) (Piccirillo 2002) | 20‐item modification of RSOM‐31, in 5 domains. Patients rate the magnitude of each item and the 5 most important items. Each item is rated on a 6‐point scale (0 = no problem, 5 = most serious problem) (range 0 to 100). Complex scoring system because of weighting for important symptoms. Excludes nasal blockage and anosmia |
| 22‐item Sino‐Nasal Outcome Test (SNOT‐22) (Hopkins 2009) | Modification of SNOT‐20 including nasal blockage and anosmia. Patients rate the magnitude of each item. Each item is rated on a 6‐point scale (0 = no problem, 5 = most serious problem) (range 0 to 110). Excludes weighting for most important symptoms. The minimal important difference (MID) is greater than 8.9 |
Alternatively, severity may be measured using clinical indicators of disease. Polyps are graded by convention as: grade I ‐ confined to the middle meatus; grade II ‐ extending below the level of the middle turbinate; and grade III ‐ causing total obstruction. Endoscopic examination may assess the condition of the nasal mucosa and demonstrate residual or recurrent disease following treatment. The Lund‐Mackay staging system generates a simple numeric score from the CT scan (and hence is available only when such imaging is performed) and may be used to stage the extent of inflammatory disease within the sinuses on cross‐sectional imaging (Lund 1997). The total score ranges from 0 to 24. The Lund‐Mackay score in 'normal' individuals in the absence of symptoms or signs of chronic rhinosinusitis has been found to be 4. The score does not reflect the extent of polyps in the nasal cavity, but some studies have found an association between the score and the likelihood of recurrence following treatment (Kennedy 1992).
Nasal obstruction may be estimated using acoustic rhinometry (which measures the cross‐sectional area of the nasal cavity) or rhinomanometry (which measures nasal resistance) (Clement 2005), although the correlation between such measurements and subjective symptoms of blockage is poor in some studies (Thulesius 2012). Expired nasal nitric oxide (nNO) levels may be used to give a measure of sinus ventilation or obstruction. Revision surgery rates or topical steroid usage may be used as evidence of treatment failure.
A discrepancy between 'subjective' symptomatic improvement reported by patients following surgical treatment for polyposis and 'objective' endoscopic evidence of resolution of disease in response to surgery has been described (Kennedy 1992). The ideal study should therefore include both patient‐based and clinical measures of outcome.
Why it is important to do this review
In summary, both the aetiology and the most effective management of chronic rhinosinusitis with nasal polyps remains unknown. Studies have shown that patients with chronic rhinosinusitis report poorer generic quality of life scores than patients with other chronic conditions including angina, congestive heart failure and chronic back pain (Glicklich 1995). In the US, chronic rhinosinusitis has been shown to account for 12 million doctor visits and 70 million restricted activity days annually (Adams 1999). This is a chronic and common problem with a significant health burden, reducing both quality of life and normal activity, and hence merits review to identify the optimum treatment.
The comparison of different types of surgical intervention (simple polyp surgery versus more extensive surgical clearance) for chronic rhinosinusitis with nasal polyps is assessed in a separate Cochrane review (Sharma 2014).
Objectives
To assess the effectiveness of endonasal/endoscopic surgery versus medical treatment in chronic rhinosinusitis with nasal polyps.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials where the unit of randomisation is the patient.
We excluded any 'split‐nose' studies in which the patient acts as their own control, i.e. one side of the nose is treated surgically and the other side is treated medically (similar to 'split‐mouth' studies). It is difficult to limit the effects of intervention to one side of the nose (e.g. ongoing inflammation on one side may affect the rate of recurrence in the contralateral side), or to measure accurately and attribute outcomes that are important to patients to just one side of the nose.
As the mean time between revision procedures has been shown to be six years in a large, multicentre cohort study (Hopkins 2006), included studies should ideally have a follow‐up period of many years. However, we planned to include all studies but to state clearly the length of follow‐up and, where multiple time points were reported, we analysed only the longest follow‐up data in each study.
Types of participants
Patients over 16 with bilateral nasal polyps confirmed by direct visualisation (preferably, but not exclusively, with an endoscope). We did not consider duration of polyps as an inclusion criterion.
We excluded studies involving patients under 16 and patients undergoing revision surgery. We also excluded patients with known malignancy and those unilateral polyps shown to be inverting papillomas.
Types of interventions
Any surgical intervention, including simple polypectomy or more extensive endoscopic sinus surgery, versus any medical treatment (including placebo) as described above.
Types of outcome measures
We analysed the following outcomes in the review, but they were not used as a basis for including or excluding studies.
Primary outcomes
-
Disease severity, as measured by patient‐reported disease‐specific symptom scores. This includes rating of nasal obstruction and other sinonasal symptoms (using visual analogue scales or other methods).
-
Health‐related quality of life, using disease‐specific health‐related quality of life scores, such as the Sino‐Nasal Outcome Test‐22 (SNOT‐22) (Table 1).
-
Health‐related quality of life, using generic quality of life scores, such as the SF‐36.
Secondary outcomes
-
Endoscopic appearances (there is no single accepted endoscopic grading system).
-
Complications from surgical or medical treatment: epistaxis, infection, orbital complications, intracranial complications, intolerance to medication or other medication side effects.
-
Recurrence rate; if available we used the disease‐free interval.
-
Objective physiological measures: nasal peak flow, nasal volume, nasal cross‐sectional area, nasal nitric oxide (nNO), ciliary function (including saccharine clearance time).
-
Olfactory tests.
We did not consider postoperative medication use, as this may be either reactive to recurrent symptoms/disease or prophylactic.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the search was 20 February 2014.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 1); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; ISRCTN; ClinicalTrials.gov; ICTRP, Google Scholar and Google. In searches prior to 2013, we also searched BIOSIS Previews 1926 to 2012 and CNKI.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by The Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in theCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. We searched for conference abstracts using the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
Data collection and analysis
Selection of studies
The Cochrane ENT Group's Trials Search Co‐ordinator used reference management software to merge the search results and remove duplicate records of the same report. All three authors (Joanne Rimmer (JR), Wytske Fokkens (WF) and Claire Hopkins (CH)) independently examined titles and abstracts to remove obviously irrelevant reports. We retrieved the full text of any reports potentially meeting the inclusion criteria and examined these independently to determine study eligibility. Where necessary we contacted/attempted to contact study authors to obtain additional information to clarify study eligibility. We identified and linked multiple reports of the same study, or we excluded them if duplicated or not relevant. We discussed study selection and there were no differences to resolve.
Data extraction and management
Two authors (JR and WF) extracted data independently from the studies using a standardised data form. The data categories collected were:
-
source;
-
eligibility;
-
methods;
-
participants;
-
interventions;
-
outcomes;
-
results;
-
key conclusions of the study authors.
We discussed the extracted data; there were no disagreements between the authors involved.
Assessment of risk of bias in included studies
JR and WF undertook assessment of the risk of bias of the included trials independently, taking the following into consideration, as guided by theCochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
-
sequence generation;
-
allocation concealment;
-
blinding;
-
incomplete outcome data;
-
selective outcome reporting; and
-
other sources of bias.
We used the Cochrane 'Risk of bias' tool in RevMan 5 (RevMan 2014), which involved describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: high, low or unclear (or unknown) risk of bias.
Measures of treatment effect
Where data were comparable we planned to pool to give a summary measure of effect. We intended to use the risk ratio or odds ratio for dichotomous data and for continuous data the mean difference (for data from the same scale) or standardised mean difference (for data from different scales).
Unit of analysis issues
The units of randomisation and analysis in the included trials were at the level of the individual. We excluded studies where patients served as their own control ('split‐nose' studies). See Types of studies.
Where studies presented results at multiple time points during follow‐up (e.g. one month, six months and one year), we only analysed the longest follow‐up from each study, as we were looking at a chronic disease and wished to avoid the risk of multiplicity of analysis.
Dealing with missing data
Where data were missing, we attempted to contact the original investigators to request the missing data. Where this was not possible we assumed that the data were missing at random, as this is more likely to reflect real life when patients do not attend for follow‐up review. If data from all patients were not available, we conducted an available case analysis whenever possible (analysing all patients available at follow‐up according to group randomised).
Assessment of heterogeneity
We evaluated studies for clinical and methodological heterogeneity on the basis of the treatment protocols used and the outcomes measured in each. A meta‐analysis would be considered appropriate if treatment protocols were broadly comparable and the appropriate outcome data were available.
We planned to examine statistical heterogeneity visually, using confidence intervals where available, or using the I2 statistic and the Chi2 test.
Assessment of reporting biases
We addressed publication bias by including trial databases in the electronic search, looking for published, unpublished and ongoing trials. Where potentially eligible but unpublished trials were identified, we contacted the authors to ask for results (where available).
We addressed multiple publication bias by combining papers that described different results from the same study, and by excluding papers that reported results that had already been published. We addressed language bias by including all languages in the search strategy and obtaining a translation when necessary. We addressed outcome reporting bias by assessing the risk of bias from within‐study selective reporting and selective under‐reporting of data.
Data synthesis
If meta‐analysis was possible, we had planned to use the fixed‐effect model in the absence of significant heterogeneity and the random‐effects model if heterogeneity was present. If meta‐analysis was not possible, the systematic approaches we planned to use to evaluate the outcomes and findings of the different studies included summary tables and forest plots, where appropriate.
Subgroup analysis and investigation of heterogeneity
We intended to explore clinical heterogeneity by subgroup analysis as appropriate. Potential subgroups included:
-
simple polypectomy;
-
endoscopic sinus surgery;
-
topical medical treatment;
-
systemic medical treatment;
-
combined topical and systemic medical treatment.
Sensitivity analysis
We planned to use study risk of bias in a sensitivity analysis, if required.
GRADE and 'Summary of findings' table
We used the GRADE approach to rate the overall quality of evidence. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high quality of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs which do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of the these factors:
-
study limitations (risk of bias);
-
inconsistency;
-
Indirectness of evidence;
-
imprecision; and
-
publication bias.
We included a 'Summary of findings' (SOF) table (summary of findings Table for the main comparison), constructed according to the recommendations described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). There were several possible comparisons from the data we found for this review. We produced the SOF table for the comparison of endoscopic sinus surgery (ESS) versus systemic steroids, as this is the comparison most likely to reflect the most common clinical practice.
Results
Description of studies
Results of the search
The literature searches found 3014 articles using the outlined search terms, and we identified 35 articles from other sources including reference lists of systematic reviews. After removal of duplicates and screening the abstracts, only 36 papers matched the criteria closely enough to be potentially eligible for the review. Of these 36 papers, we discarded 24 after further review as they were retrospective studies or review articles rather than trials, and we formally excluded three after the full text was assessed. We included eight papers describing four studies in the final review (see Figure 1). There were no 'ongoing studies' or 'studies awaiting assessment'.
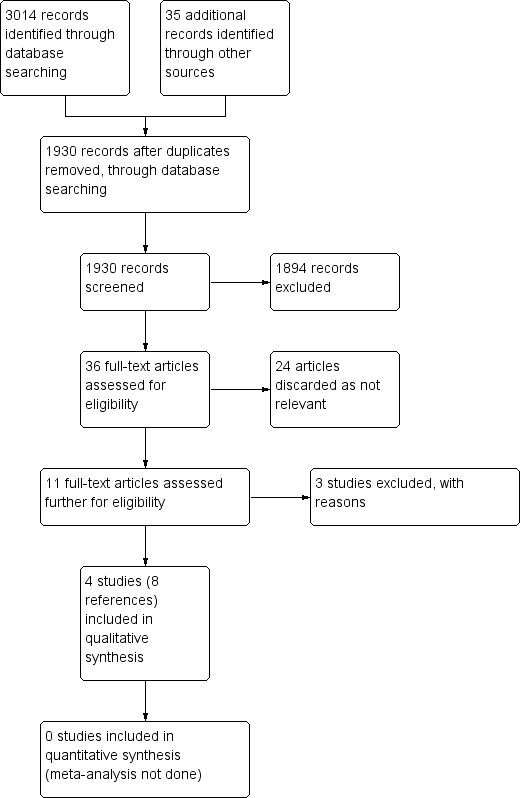
Process for sifting search results and selecting studies for inclusion.
Included studies
Please see the Characteristics of included studies table for further information.
We included four studies (Alobid 2005; Lildholdt 1988; Lildholdt 1997; Ragab 2004). Two separate papers reported the Lildholdt 1997 study; we therefore grouped them together. The 1995 paper (Clinical Otolaryngology) described phase one of the study in more detail, in particular relating to the definition of "failed medical treatment" that put patients into the "surgical verus medical treatment" part of the study before phase two. In total there were four papers by the same authors as Ragab 2004, all reporting on the same study; we therefore grouped these together. One 2006 paper (Allergy) describes nasal nitric oxide levels in further detail than the original study report but there was no new information. Another 2006 paper (European Respiratory Journal) reports asthma outcomes, which were not one of the outcome measures we reviewed. The 2010 paper discusses quality of life outcomes in more detail; again there was no new information.
Design
All four included studies were prospective, randomised controlled trials of medical versus surgical treatment in participants with nasal polyps (Alobid 2005; Lildholdt 1988; Lildholdt 1997; Ragab 2004).
Lildholdt 1997 was conducted over three phases; the first phase had three arms: two doses of budesonide (400 µg versus 800 µg daily) and a placebo group. After one month of treatment, all patients were randomised to either 400 µg or 800 µg daily of budesonide in phase two, for 11 months. However, patients who "failed treatment" in phase one (as judged by a lack of improvement in at least three of the following assessments: investigator's assessment of polyp size; patient's overall assessment of polyposis; patient's assessment of sense of smell; patient's overall assessment of treatment efficacy; peak expiratory flow (PEF) index improvement of at least 10%) were randomised to either surgery or a single dose of systemic steroids prior to entering phase two. Phase three represented a further 12‐month period with or without treatment "based on the clinical judgement of the physician".
Sample size
In Alobid 2005, 109 participants with nasal polyps were randomly allocated into two treatment groups; 56 were randomised into the surgical treatment group and 53 into the medical treatment group.
In Lildholdt 1988, 53 participants with nasal polyps were randomly allocated (by computer‐generated random numbers) into two treatment groups; 27 were randomised into the surgical treatment group and 26 into the medical treatment group.
Lildholdt 1997 included 126 consecutive participants with nasal polyps, but only 34 patients (18 surgical and 16 medical) who failed initial medical treatment in phase one were randomised into the trial of surgical versus medical treatment that preceded phase two (see 'Design' above).
In Ragab 2004, 327 consecutive participants with chronic rhinosinusitis with or without nasal polyps were approached: 131 participants enrolled and 41 were excluded during a run‐in period of "initial medical treatment". Ninety participants were therefore randomised (45 medical, 45 surgical), 35 of whom had nasal polyps (19 surgical, 16 medical).
Setting
The four included studies took place in a single university hospital otorhinolaryngology clinic (Barcelona, Spain) (Alobid 2005); four otorhinolaryngology clinics (Denmark) (Lildholdt 1988); a hospital otorhinolaryngology outpatient clinic or specialty private practice (Sweden) (Lildholdt 1997); and rhinology clinics at single hospital (London, UK) (Ragab 2004).
Participants
The surgical versus medical treatment phase of Lildholdt 1997 only included patients who had failed initial medical treatment in phase one. Ragab 2004 excluded patients who had responded to six weeks of initial medical treatment (consisting of topical steroid spray and alkaline nasal douche) prior to randomisation.
In Alobid 2005, polyps were diagnosed by endoscopic examination. The mean age overall was 50.1 ± 1.4 years (range 22 to 84 years). The mean age in the surgical group 49.6 ± 2.0 years and in the medical group was 50.7 ± 1.8 years. Thirty‐five participants (32%) were female and 74 (68%) were male; the surgical group included 39 men and 17 women, and the medical group 35 men and 18 women. Participants were excluded if they were found to have an inverted papilloma, antrochoanal polyp, cystic fibrosis, cerebrospinal fluid (CSF) leak or contraindication to oral steroid treatment. All participants had a four‐week washout period of intranasal and oral steroids, after which they were randomly allocated into treatment groups.
In Lildholdt 1988, polyps were diagnosed by "mini biopsy" (unclear, but have must included direct visualisation in order to biopsy). All participants were "eligible for surgical removal of nasal polyps" (unclear). Thirty‐four participants were 60 years old or less (19 in the surgical group and 16 in the medical group) and 19 were over 60 years (nine in the surgical group and 10 in the medical group); the lower age limit was not reported. Forty participants (75.5%) were male and 13 (24.5%) were female; the surgical group included 18 men and nine women, the medical group 22 men and four women. Exclusion criteria were not reported.
Participants in Lildholdt 1997 were diagnosed by biopsy (unclear but presumably this must have included direct visualisation). The median age was 48 years (range 22 to 64 years), but the demographics of the surgical and medical subgroups were not reported separately. Fifteen participants (46.9%) were female and 17 (53.1%) were male, but again surgical versus medical subgroups were not reported separately. Exclusion criteria were recent steroid treatment (sustained‐release within three months, systemic within two months or topical within one month), pregnancy or acute purulent sinusitis. However only participants who were assessed as having "failed" medical therapy after one month (in phase one patients were randomised to either placebo, 400 µg of budesonide or 800 µg of budesonide daily) qualified to be randomised to a separate "surgical versus medical treatment" part of the study. It was unclear how many of these patients had received budesonide or placebo therapy in phase one.
The diagnosis of chronic rhinosinusitis in Ragab 2004 was primarily based on criteria described by the Staging and Therapy Group (Lund 1995); nasal polyps were diagnosed by endoscopy. Exclusion criteria included pregnancy, lactation, significant psychological problems, inability to comply with study protocol, children under 18 years, systemic diseases affecting the nose (e.g. Wegener's granulomatosis, sarcoid, primary ciliary dyskinesia, cystic fibrosis, and acute upper or lower respiratory tract infections within two weeks of the inclusion visit), use of systemic corticosteroids within four weeks of the inclusion visit, systemic diseases preventing participation in the study, and surgical or medical treatments influencing the study. One hundred and thirty‐one participants met the initial inclusion criteria and consented to take part. All received six weeks of medical treatment consisting of Dexa‐Rhinaspray (DRS ‐ 40 µg dexamethasone and 240 µg tramazoline hydrochloride) twice daily into each nostril and an alkaline nasal douche. Out of 131, only 90 were subsequently randomised into the study: seven were lost to follow‐up, eight had only minimal disease on CT scanning, and 26 responded to medical treatment prior to the study. The overall mean age was 43 ± 13 years and there were 45 males and 45 females, but the 35 participants with nasal polyps were not described separately.
Interventions
The surgical and medical treatments used were not comparable between most studies, although two studies were similar (Lildholdt 1988; Lildholdt 1997). All patients in these studies also had concurrent topical steroids, with both arms receiving same doses, except for Ragab 2004. There are therefore three comparisons (see Table 2 for further details):
| Study | Surgical treatment | Medical treatment | Comparison description |
| GA endoscopic sinus surgery (variable extent) then topical budesonide 400 µg bd for 1 year | Oral prednisolone for 14 days (30 mg od 4 days then reduced by 5 mg every 2 days) then topical budesonide 400 µg bd for 1 year | Endoscopic sinus surgery versus systemic steroids | |
| LA polypectomy then topical beclomethasone dipropionate 100 µg bd for 1 year | 14 mg im betamethasone then topical beclomethasone dipropionate 100 µg bd for 1 year | Polypectomy versus systemic steroids | |
| LA snare polypectomy then topical budesonide 400 µg or 800 µg od for 11 months | 14mg im betamethasone then topical budesonide 400 µg or 800 µg od for 11 months | Polypectomy versus systemic steroids | |
| GA endoscopic sinus surgery (variable extent) then antibiotics for 2 weeks plus topical fluticasone propionate spray 100 µg bd for 12 weeks, then "tailored treatment" | Antibiotics plus topical fluticasone propionate drops 400 µg bd for 12 weeks, then "tailored treatment" | Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid |
bd: twice daily
GA: general anaesthesia
im: intramuscular
LA: local anaesthesia
od: once daily
-
Endoscopic sinus surgery (ESS) versus systemic steroids, plus topical steroids (Alobid 2005).
-
Polypectomy versus systemic steroids, plus topical steroids (Lildholdt 1988; Lildholdt 1997).
-
ESS plus topical steroid versus antibiotics plus high‐dose topical steroid (Ragab 2004).
In Alobid 2005, the surgical group underwent ESS consisting of polypectomy and anterior ethmoidectomy in all 56 patients; 49 participants also underwent posterior ethmoidectomy and 45 had more extensive surgery. Two weeks after surgery, the participants commenced topical budesonide 400 µg twice daily for one year. The medical group were treated with oral prednisolone for 14 days (30 mg for four days, then the dose was reduced by 5 mg every two days), after which these participants also commenced topical budesonide 400 µg twice daily for one year.
In Lildholdt 1988, surgical treatment involved removal of visible polyps under local anaesthesia. The medical group were treated with a single dose of 10 mg betamethasone dipropionate and 4 mg betamethasone disodium phosphate (Diprospan) intramuscularly. Both groups then commenced topical beclomethasone dipropionate nasal spray 100 µg twice daily for one year.
Phase one of Lildholdt 1997 was a double‐blind, placebo‐controlled trial with all 126 participants randomised (method not specified) to either placebo or a 400 µg or 800 µg dose of budesonide daily for one month. After one month, the results of treatment were assessed as described above to determine whether treatment had been a "success" or a "failure". Those deemed to have "failed" were randomised (method not specified) to an additional treatment of either surgery involving a snare polypectomy under local anaesthetic or a single injection of 10 mg betamethasone dipropionate and 4 mg betamethasone disodium phosphate (Diprospan) intramuscularly. Phase two was started after completion of phase one; all participants were randomised to either 400 µg or 800 µg of budesonide for a further 11 months. Phase three followed on from phase two and did not involve any further treatment unless deemed necessary by the physician.
In Ragab 2004, participants in the surgical group underwent ESS under general anaesthesia after the Messerklinger technique by two senior surgeons, the extent of which was tailored to the disease. After surgery, all participants were prescribed a two‐week course of erythromycin (500 mg twice daily), Dexa‐Rhinaspray (DRS ‐ 40 µg dexamethasone and 240 µg tramazoline hydrochloride twice daily into each nostril) and an alkaline nasal douche, followed by a three‐month course of fluticasone propionate intranasal spray (100 µg twice daily into each nostril) and an alkaline nasal douche. After that, medical treatment was tailored to the symptoms, which comprised a topical corticosteroid spray in most instances. Participants in the medical group received a 12‐week course of erythromycin (500 mg twice daily for two weeks, then 250 mg twice daily for 10 weeks), an alkaline nasal douche and intranasal corticosteroid; participants with nasal polyps received a 12‐week course of fluticasone propionate drops (400 µg twice daily into each nostril). In addition, three participants with polyps were prescribed a nine‐day course of oral prednisolone tablets (30 mg for three days, 20 mg for three days and 10 mg for three days) after failure of the above regimen to control their manifestations. After that, medical treatment was tailored to the symptoms. Therefore, in this study, there was a nine‐month period in both arms where patients' treatment was based on symptoms, rather than a standardised protocol.
Outcomes
All participants in Alobid 2005 had an initial baseline assessment, then further assessments at six and 12 months post‐treatment. The outcomes consisted of nasal symptom scores (0 to 3), endoscopic polyp size scores (0 to 3) and a quality of life assessment using the short form 36 (SF‐36) health questionnaire (both physical and mental components). Lund‐Mackay CT scores (0 to 24) were reported before treatment began.
All patients in Lildholdt 1988 had an initial assessment with "recording of symptoms and signs" (methods not specified) and baseline measurements of smell (method not specified) and nasal expiratory peak flow, then further assessments regularly for one year (at two weeks, and two, four, six, nine and 12 months). Results for olfactory tests were reported at two to 12 months.
All participants in Lildholdt 1997 were assessed at three, six, nine and 12 months and then every three months for a further year. At every visit participants were assessed with symptom scores (0 to 3), endoscopic polyp size score (0 to 3), peak expiratory flow rate (PEFR) index (nasal PEFR/oral PEFR) and a semi‐quantitative smell test (0 to 3); an overall subjective assessment of treatment efficacy was made at the end of phase two.
All participants in Ragab 2004 had an initial assessment then further assessments at six and 12 months, and outcomes for participants with nasal polyps were reported separately. Each assessment included patient‐reported symptom scores using a visual analogue scale (VAS) (0 to 10) and disease‐specific quality of life using the Sino‐Nasal Outcome Test‐20 (SNOT‐20, 0 to 100), generic health‐related quality of life SF‐36 (eight domains, 0 to 100 each), endoscopic scoring, nasal nitric oxide (NO) levels (a gas produced within the sinuses, levels of which may be used as an indicator of sinus disease), acoustic rhinometry and the saccharine clearance time (a measure of ciliary function within the nose, in which the time taken to taste a saccharine tablet placed just inside the nose is recorded).
For the main outcomes of interest for our review, data from validated scores were only available for SF‐36 and SNOT‐20. Other than Lildholdt 1988 (where the method was not stated), all had used patient‐reported symptom scores but these scores were not reported as validated; it is unclear whether these can reliably detect changes in the outcomes of interest and are sensitive enough to differentiate the difference between two groups.
In Alobid 2005, surgical complications were recorded. Lildholdt 1988 mentioned complications in both groups, but not all were quantified. Lildholdt 1997 recorded adverse events in both groups. In Ragab 2004, complications in both groups were recorded.
Excluded studies
We excluded three studies after reviewing the full text: one was a randomised controlled trial with no surgical treatment group, one was a non‐randomised trial and one was a randomised controlled trial that was planned and registered on www.controlled‐trials.com, but had not been performed (information obtained from the trial's first author). Please see the Characteristics of excluded studies table for further information.
Risk of bias in included studies
Please see the 'Risk of bias' tables for further information (Characteristics of included studies). See also Figure 2 ('Risk of bias' graph) and Figure 3 ('Risk of bias' summary).
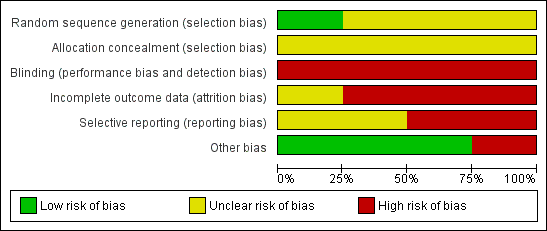
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
One study used computer‐generated random numbers for randomisation, giving a low risk of bias (Lildholdt 1988). One study used random blocks, but did not describe the size of blocks or any further details so the risk was unclear (Ragab 2004). The remaining studies did not describe the randomisation process and so the risk was unclear (Alobid 2005; Lildholdt 1997).
Allocation concealment
Allocation was concealed from both participants and the investigator in one study at the point of randomisation, although it was not stated how this was done; this was viewed as an unclear risk (Ragab 2004). Concealment procedures were not mentioned in the other studies, giving an unclear risk of bias (Alobid 2005; Lildholdt 1988; Lildholdt 1997).
Blinding
It is not possible to blind participants to medical versus surgical intervention. Whilst assessors may be blinded when reviewing the results of investigations or subjective scores, evidence of surgery may be apparent on endoscopic examination. No study reported blinding either the participants or assessors and, taking into account that most of the outcomes of interest are subjective measures, we therefore considered them all at high risk. In addition, the treatment protocol for Ragab 2004 only lasted 12 weeks post‐randomisation. Between the end of this treatment protocol and the time point measured (12 months), the "medical treatment was tailored to patients' manifestations". For one arm to be treated more intensively than the other is a very serious risk of bias, since blinding is not possible.
Incomplete outcome data
Alobid 2005 reported a drop‐out rate of 16% (data available for 95 out of 109 patients), but did not specify the drop‐out rates for each treatment arm. It also did not specify how the missing data were handled in the analysis, giving a high risk of bias. Ragab 2004 had an overall drop‐out rate of 13% from the whole study; the drop‐out rate for the subgroup with nasal polyps is not clearly stated but appears to include 0% of the surgical group and 18.8% of the medical group. Of these studies, only Ragab 2004 gave a reason for attrition (which was "lost to follow‐up"), but it remained at high risk of bias despite this due to the differential drop‐out for the nasal polyps subgroup. Lildholdt 1988 did not report whether or not there was any attrition so the risk of bias was unclear. It is also unclear how many of the patients randomised to the surgery versus systemic steroids group in Lildholdt 1997 were available; the study does not report the outcomes from this part of study separately. The only information available is combined with that of phase two; only 75 out of 124 patients (60%) who entered this phase of the study were available at 12 months. We therefore suspect that the risk of attrition bias is high for this study.
Selective reporting
Two studies had a high risk of selective reporting. The study protocol for Lildholdt 1988 is not available but it is clear that the published report did not include all expected outcomes; nasal symptoms were not reported, apart from subjective "intact smell", and objective appearances were not recorded. Exact data were not given and would be insufficient for meta‐analysis. Lildholdt 1997 does not report most outcomes for their surgical versus medical subgroups separately. When reported, these were presented in graph form with insufficient data available for meta‐analysis.
The remaining two studies had an unclear risk. In Alobid 2005, the study protocol is not available; the published report included all expected outcomes but did not provide full data. Results were presented in tables as mean ± standard error of mean (SEM). The study protocol is not available for Ragab 2004, but it is again clear that the published report included all expected outcomes but incomplete data. Outcomes were expressed as mean value ± standard deviation.
Other potential sources of bias
Lildholdt 1997 was supported by grants from a pharmaceutical company, but it is unclear what the risk of bias from this is.
Ragab 2004 recruited patients with chronic sinusitis, but reported the results of patients with polyps (40.5% of all participants available for analyses) and without polyps as subgroup analyses. Without access to the actual protocol, it is unclear whether this subgroup analysis was pre‐planned or post hoc.
Apart from Lildholdt 1997, which reported funding from Astra Draco, none of the studies provided information on sources of funding or conflicts of interest.
Effects of interventions
See: Summary of findings for the main comparison
All studies reported data at 12 months of follow‐up. For many of the outcomes, the studies only reported whether the results were statistically significant or not (P value > 0.05 or P value < 0.05); exact values were not reported. There was more emphasis on reporting of whether the treatment arms improved (statistically) compared to baseline (a significant improvement in most cases, since both treatment and comparison arms were active interventions). We estimated the effect sizes for the Alobid 2005 study using the standard error of the mean (SEM) reported by the studies.
The quality of evidence for each of the outcomes for all comparisons was either low orvery low quality. There were serious limitations in the methods used for study conduct or data collection, and there was imprecision (wide confidence intervals and/or small sample sizes). There were some serious concerns about indirectness (treatment protocols did not reflect current clinical practice, or surrogate outcomes were used) and publication bias for some comparisons and/or outcomes.
See summary of findings Table for the main comparison for a summary of key findings for the comparison that is most commonly used in clinical practice (endoscopic sinus surgery versus systemic steroids in patients on topical steroids).
Primary outcomes
Disease severity, as measured by patient‐reported disease‐specific symptom scores
Table 3 provides symptom score results for each study (where reported).
| Study | Intervention/comparison | Outcome measure (scale) | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | Nasal obstruction (0 to 3) | 12 months | MD ‐0.3 (95% CI ‐0.6 to 0.0); standardised mean difference (SMD) ‐0.4 (95% CI ‐0.8 to 0.0) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Endoscopic sinus surgery versus systemic steroids | Rhinorrhoea/nasal discharge (0 to 3) | 12 months | MD ‐0.2 (95% CI ‐0.5 to 0.1); SMD ‐0.3 (95% CI ‐0.7 to 0.1) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Endoscopic sinus surgery versus systemic steroids | Sneezing (0 to 3) | 12 months | MD ‐0.2 (95% CI ‐0.5 to 0.1); SMD ‐0.3 (95% CI ‐0.7 to 0.1) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Endoscopic sinus surgery versus systemic steroids | Loss of smell (0 to 3) | 12 months | MD ‐0.4 (95% CI ‐0.7 to ‐0.1); SMD ‐0.6 (95% CI ‐1.0 to ‐0.2) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Polypectomy versus systemic steroids | Sense of smell (not known) | 12 months | Improved with medical and surgical treatment (P value > 0.05); no statistical difference between groups (exact data not given) | |
| Polypectomy versus systemic steroids | Nasal obstruction (0 to 3) | 12 months | Improved with medical and surgical treatment (P value > 0.05); no statistical difference between groups (exact data not given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Nasal obstruction Nasal discharge Facial pain/pressure Headache Overall discomfort (VAS 0 to 10 for each) | 12 months | Improved with medical treatment (61.2% change from baseline (SD = 19.1), P value < 0.01) and surgical treatment (54.7% change from baseline (SD = 27.3), P value < 0.01); no statistical difference between groups (data not given separately) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Olfactory disturbance | 12 months | Improved with medical and surgical treatment (P value < 0.05); no statistical difference between groups (exact data not given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | SNOT‐20 | 12 months | Improved with medical and surgical treatment (P value < 0.01); no statistical difference between groups (exact data not given) |
CI: confidence interval
MD: mean difference
SD: standard deviation
SNOT‐20: Sino‐Nasal Outcome Test‐20
VAS: visual analogue scale
Endoscopic sinus surgery (ESS) versus systemic steroids
Data from 95 patients (out of 109 randomised) were available at the end of the study (12 months) (Alobid 2005). Symptoms were scored on a 0‐ to 3‐point scale where 0 = no symptoms and 3 = severe symptoms. It was unclear if these scales were validated. The following are the mean differences (MD) between the surgical and medical groups at 12 months; the negative values indicate lower scores (less severe symptoms) in the surgical group:
-
nasal obstruction: MD ‐0.3 (95% confidence interval (CI) 0.6 to 0.0); standardised mean difference (SMD) ‐0.4 (95% CI ‐0.8 to 0.0);
-
nasal discharge: MD ‐0.2 (95% CI ‐0.5 to 0.1); SMD ‐0.3 (95% CI ‐0.7 to 0.1);
-
sneezing: MD ‐0.2 (95% CI ‐0.5 to 0.1); SMD ‐0.3 (95% CI ‐0.7 to 0.1);
-
loss of smell: MD ‐0.4 (95% CI ‐0.7 to 0.1); SMD ‐0.6 (95% CI ‐1.0 to ‐0.2).
Polypectomy versus systemic steroids
Lildholdt 1988 reported no statistically significant difference in symptom scores between groups at 12 months (on a 0‐ to 3‐point scale where 0 = no symptoms and 3 = severe symptoms). Exact data were not given and it was unclear if this scale was validated.
ESS plus topical steroid versus antibiotics plus high‐dose topical steroid
Ragab 2004 reported that the difference in percentage improvement from baseline in symptom scores was ‐6.5% (95% CI ‐22.58 to 8.58, n = 32) (using a visual analogue scale (VAS) of 0 to 10; it is unclear whether the scale was validated). The negative numbers suggest a smaller improvement in the surgery group.
Health‐related quality of life, using disease‐specific health‐related quality of life scores
ESS versus systemic steroids
A disease‐specific health‐related quality of life measure was not employed for this comparison.
Polypectomy versus systemic steroids
A disease‐specific health‐related quality of life measure was not employed for this comparison.
ESS plus topical steroid versus antibiotics plus high‐dose topical steroid
Ragab 2004 measured health‐related quality of life using SNOT‐20, but did not report the results. The authors only stated that there was no statistically significant difference between groups.
Health‐related quality of life, using generic health‐related quality of life scores
Table 4 provides quality of life score results for each study (where reported). See also summary of findings Table for the main comparison.
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | SF‐36 (0 to 100 each domain) | 12 months | The MD was ‐1.4 (95% CI ‐5.0 to 2.2) for the physical component summary score, and 0.6 (95% CI ‐2.9 to 4.1) for the mental component scores. Effect sizes are negligible and correspond to a SMD of 0.07 and ‐0.15 respectively (a SMD of 0.2 corresponds to a small effect size) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | SF‐36 (0 to 100 each domain) | 12 months | Improved with surgical and medical treatment (P value < 0.01), except physical domain (P value > 0.05); no statistical difference between groups (exact data not given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | SNOT‐20 | 12 months | Actual results not reported; only stated that there were no statistically significant differences between groups |
CI: confidence interval
MD: mean difference
SF‐36: Short Form‐36 Health Survey
SMD: standardised mean difference
ESS versus systemic steroids
The MD was ‐1.4 (95% CI ‐5.0 to 2.2) for the physical component summary score, and 0.6 (95% CI ‐2.87 to 4.07) for the mental component scores of the SF‐36 (Alobid 2005). These effect sizes are negligible and correspond to a SMD of 0.07 and ‐0.15 respectively (a SMD of 0.2 corresponds to a small effect size). The numerical values of the individual scales were not given, but the study reported and showed using graphs that there were no important differences between groups across all scales.
Polypectomy versus systemic steroids
A quality of life measure was not employed for this comparison.
ESS plus topical steroid versus antibiotics plus high‐dose topical steroid
There was no statistical difference between the two groups in SF‐36 scores (P value < 0.05); the exact data were not provided (Ragab 2004).
Secondary outcomes
Endoscopic appearances
Table 5 shows the results for endoscopic appearances. See also summary of findings Table for the main comparison.
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | Polyp size score (0 to 3) | 12 months | Polyp size scores improved significantly in both groups at 12 months, and were significantly better in the endoscopic sinus surgery group (P value < 0.05): MD ‐1.5 (95% CI ‐1.8 to ‐1.2, n = 95). This corresponds to a large effect size | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Endoscopic score (0 to 3) | 12 months | Polyp size scores improved significantly in both groups at 12 months, but there was no important difference between groups (P value > 0.05): MD ‐2.3% (95% CI ‐17.4 to 12.8, n = 34) |
CI: confidence interval
MD: mean difference
SD: standard deviation
ESS versus systemic steroids
Polyp size scores (on a 0‐ to 3‐point scale) improved significantly in both groups at 12 months, and were significantly better in the ESS group (P value < 0.05): MD ‐1.5 (95% CI ‐1.8 to ‐1.2, n = 95) (Alobid 2005). This corresponds to a large effect size.
Polypectomy versus systemic steroids
Endoscopic appearances for this comparison were either not reported (Lildholdt 1988), or not reported separately for the 'surgical versus medical treatment' part of the study (Lildholdt 1997).
ESS plus topical steroid versus antibiotics plus high‐dose topical steroid
Endoscopic scores were measured on a 0‐ to 3‐point scale but reported as percentage improvement compared to baseline. At 12 months, the percentage improvement from baseline was similar between the two groups (MD 2.3%, 95% CI ‐17.4 to 12.8, n = 34) (Ragab 2004). However, the estimate is very imprecise due to the small sample size.
Complications from surgery or medical treatment
Complications from surgery or medical treatment are shown in Table 6. See also summary of findings Table for the main comparison.
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | Epistaxis (nosebleed) | 12 months | 3.6% (endoscopic sinus surgery) | |
| Polypectomy versus systemic steroids | Epistaxis | 12 months | "1 patient failed the study because of bleeding" (no further information) | |
| Polypectomy versus systemic steroids | Epistaxis | 12 months | 21% (all patients were on medical treatment throughout; polypectomy versus systemic steroids comparison not reported separately) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Epistaxis | 12 months | 2.2% (endoscopic sinus surgery plus topical steroid) versus 4.4% (antibiotics plus high‐dose topical steroid) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Infection | 12 months | 4.4% (endoscopic sinus surgery plus topical steroid) | |
| Endoscopic sinus surgery versus systemic steroids | Orbital complications | 12 months | 7.1% (endoscopic sinus surgery ‐ exposure of orbital fat) | |
| Endoscopic sinus surgery versus systemic steroids | Intracranial complications | 12 months | 1.8% (endoscopic sinus surgery ‐ CSF leak with meningitis) |
CSF: cerebrospinal fluid
ESS versus systemic steroids
There was an overall 14.3% complication rate in the ESS group: epistaxis was reported in 3.6% of this group, orbital fat was exposed in 7.1% and a postsurgical cerebrospinal fluid leak with meningitis occurred in one patient (1.8%). No complications were reported in the systemic steroids group (Alobid 2005).
Polypectomy versus systemic steroids
One patient "failed the study because of bleeding", but it is not reported which comparison group this patient was in (Lildholdt 1988). Epistaxis was reported in 21% of all patients using systemic and/or topical steroids, but the 'surgical versus medical treatment' patients were not reported separately (Lildholdt 1997). Lildholdt 1988 reported that "a few patients complained of headache some days after the injection of systemic steroids" and that "some experienced local reaction to the nasal spray"; no further information was given regarding exact numbers or which groups these patients were in. Lildholdt 1997 reported five "serious adverse events" (4%), one of which was a peptic ulcer, but do not state which treatment group the patients were in. The investigators judged that the adverse events were "unlikely to have been caused by either medication or surgery", although four of the five patients had medication stopped as a result.
ESS plus topical steroid versus antibiotics plus high‐dose topical steroid
Epistaxis was reported in 4.4% of the antibiotics plus high‐dose topical steroid group and 2.2% of the ESS plus topical steroid group; all settled with conservative treatment and statistical significance was not discussed. Infection occurred postoperatively in 4.4% of the ESS plus topical steroid group.
Recurrence rate
Polypectomy versus systemic steroids
"Residual or recurrent polyps necessitated the initial treatment to be repeated" in 11.1% of the polypectomy groups and 15.4% of those in the systemic steroids group. One participant (3.9%) from the systemic steroids group underwent surgery, and one patient in each group "required repeated surgery". No further information regarding residual disease versus recurrence was given and no statistical comparisons were made (Lildholdt 1988).
Recurrence rates were not reported by the studies included in the other two comparisons.
Objective physiological measures
Results for objective physiological measures are shown in Table 7.
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Polypectomy versus systemic steroids | Nasal expiratory peak flow | 12 months | Improved with surgical and medical treatment; no statistical difference between groups (P value > 0.05) (exact data not given) | |
| Polypectomy versus systemic steroids | Peak expiratory flow rate index | 12 months | Improved in all groups; no statistical difference between groups (surgical versus medical groups not reported separately, no exact data given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Total nasal volume | 12 months | Improved with surgical (58.8% change from baseline, SD = 40) and medical treatment (50.3% change from baseline, SD = 50.7), P value < 0.01; no statistical difference between groups although medical group tended towards greater improvement (P value > 0.05) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Nasal cross‐sectional area | 12 months | No statistical difference between groups (P value > 0.05, Mann Whitney‐U test, not normally distributed data, median not reported) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Nasal nitric oxide levels | 12 months | No statistical difference between groups (P value > 0.05, Mann Whitney‐U test, not normally distributed data, median not reported) |
SD: standard deviation
ESS versus systemic steroids
Objective measurements were not performed.
Polypectomy versus systemic steroids
Lildholdt 1997 reported no statistical difference between groups for nasal expiratory peak flow, but exact data were not reported.
ESS plus topical steroid versus antibiotics plus high‐dose topical steroid
Percentage change compared to baseline was reported at 12 months for total nasal volume, nasal cross‐sectional area, nasal nitric oxide levels and the saccharine clearance time (Ragab 2004). However, the data were not normally distributed. The authors reported no statistically significant difference (P value > 0.05) using the Mann Whitney U test.
Olfactory tests
Table 8 provides the results for olfactory tests, where available.
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Polypectomy versus systemic steroids | Proportion of patients "expressing intact smell" | 2 to 12 months | No important difference between groups: risk ratio 0.96 (95% CI 0.71 to 1.31, n = 53) | |
| Polypectomy versus systemic steroids | Semi‐quantitative smell test | 12 months | No statistical difference between any treatment groups (surgical versus medical groups not reported separately, exact data not given) |
Only two studies in one of the three comparisons reported any tests of olfaction; one did not describe the test used (Lildholdt 1988), whilst the other reported a semi‐quantitative subjective test (Lildholdt 1997).
Polypectomy versus systemic steroids
Lildholdt 1988 reported the proportion of patients "expressing intact smell" for "2 to 12 months". There was no important difference between groups: risk ratio 0.96 (95% CI 0.71 to 1.31, n = 53). Lildholdt 1997 did not report the olfactory outcomes for the relevant comparison.
Discussion
Summary of main results
Although the treatments were not standardised across studies and there were three different pairs of comparisons (endoscopic sinus surgery (ESS) versus systemic steroids, polypectomy versus systemic steroids, and ESS plus topical steroid versus antibiotics plus high‐dose topical steroid), the studies consistently reported that no important difference was found between groups for patient‐reported disease‐specific symptom scores or disease‐specific health‐related quality of life scores, such as Sino‐Nasal Outcome Test‐20 (SNOT‐20) (Alobid 2005; Lildholdt 1997; Ragab 2004), or generic health‐related quality of life scores (SF‐36) (Alobid 2005; Ragab 2004), suggesting that medical and surgical treatment are of equivalent efficacy (low to very low quality evidence).
Only two studies employed patient‐reported quality of life measures (Alobid 2005; Ragab 2004). Significant improvement in quality of life was reported across both comparisons (ESS versus systemic steroids, and ESS plus topical steroid versus antibiotics plus high‐dose topical steroid), but there was no important difference found between groups. See summary of findings Table for the main comparison for further details.
The same two studies reported improvements in endoscopic appearances in both comparisons (Alobid 2005; Ragab 2004). In the former (ESS versus systemic steroids), the ESS group were significantly better at 12 months. There were no significant differences found between any other comparisons for any other objective measurements.
The surgical and medical treatments used in the included studies are all effective for chronic rhinosinusitis with nasal polyps; most studies do not report a significant difference between the treatment groups. However, there is some limited evidence to suggest that surgery may convey an additional benefit over medical treatment, as far as patient‐reported symptom scores and endoscopic appearances are concerned. No studies reported any medical treatment producing better outcomes than any surgical treatment at 12 months.
The potential complications of both surgical and medical treatment also need to be taken into account; these have not generally been well documented. Complication rates of up to 14.3% for all surgical treatment and 21% for all medical treatment are described, but these data are incomplete and cannot be used to make a judgement regarding the possibility of harm. See summary of findings Table for the main comparison for further details.
Overall completeness and applicability of evidence
The evidence reviewed is relevant to the study question and incorporates all available evidence from the randomised controlled trials (RCTs) currently available.
Whilst the different treatment regimes used may reflect clinical practice, they do not follow recent European evidence‐based guidelines (Fokkens 2012), and the evidence is therefore indirect. The variety of treatments used also makes it difficult to pool results together and draw any specific conclusions or make firm recommendations about best practice. It must also be recognised that all studies gave postoperative medical treatment, usually in the form of topical intranasal steroids, in line with standard clinical practice, so any surgical treatment given cannot be considered in isolation; rather it should be considered as an adjunct to long‐term medical treatment.
We cannot be confident about the completeness of the data, as there were serious reporting biases in two of these studies (Lildholdt 1988; Lildholdt 1997); outcomes were either not reported or reported in a way that provided insufficient data for meta‐analysis. Lildholdt 1997 admits that "in general, the present analysis considers the entire study as a clinical entity".
Quality of the evidence
Four studies, including 231 participants, have been included in this review. They all have different methodology, use different surgical techniques and varying medical treatment regimes, and employ a variety of non‐standardised subjective and objective outcome measurements. They all include relatively small numbers of patients with relatively short‐term follow‐up (12 months) for what is a chronic condition.
Whilst all the surgical and medical treatments used led to improvements in patient‐related symptom scores and some objective measures, overall the quality of the evidence to support the use of surgical or medical treatment is low or very low (using the GRADE criteria), as can be seen from summary of findings Table for the main comparison. The quality of the evidence does not allow a robust conclusion regarding the effectiveness of endonasal/endoscopic surgery or medical treatment in chronic rhinosinusitis with nasal polyps. The quality of the evidence for patient‐reported disease‐specific symptom scores is low due to limitations in the design of studies and indirectness of evidence. The quality of evidence regarding patient‐reported quality of life scores is low, with indirectness of the evidence and publication bias affecting this. There is very low quality evidence for endoscopic appearances based on limitations in the design of studies, indirectness of the evidence and publication bias. The quality of evidence for complications is very low because of limitations in the design of studies, indirectness of the evidence and publication bias. There is low quality evidence for objective physiological measures due to limitations in the design of studies and indirectness of the evidence. The quality of evidence for olfactory tests is very low, with limitations in the design of studies, indirectness of the evidence and imprecise results.
Potential biases in the review process
The search strategy should have identified all relevant studies as broad search terms were used, all languages were included and trial databases were included to ensure any unpublished studies were found. We contacted authors where necessary to obtain unpublished data. Three authors independently reviewed the search results to minimise selection bias. Two authors extracted data independently and were in agreement throughout, limiting bias. However, the authors were not blinded to the authors of the studies, which is a potential source of bias. The lack of data reported in a way that is suitable for (meta‐)analysis is a form of reporting bias; we have tried to contact the authors of the studies to obtain further information but have not been successful.
There were no marginal decisions during the review process. We did make some minor changes to the protocol: we further developed the methods section regarding study selection and clarified our outcome measures (see Differences between protocol and review).
We were unable to combine the studies for meta‐analysis, as the interventions and outcomes used in each were not directly comparable. The type and dose of both systemic and topical steroids differed in three of the four studies; one study included a prolonged course of antibiotics as part of its medical treatment. The type and extent of surgery was also different in three of the four studies, hence the need to describe the outcomes with reference to three different pairs of treatment comparisons. In two studies, it was also not possible to extract numerical results; outcomes were given only as P values and exact figures were not reported (Alobid 2005; Lildholdt 1988). Despite attempting to contact the study authors we were unable to obtain any exact data to use in a meta‐analysis. These same studies either do not describe what statistical method was used or use non‐parametric tests, which precluded us from obtaining the standard deviation for analysis. The selective reporting biases in these individual studies meant that this review was unable to carry out meta‐analysis. In addition, the lack of comparability means that meta‐analysis would have been difficult to justify for most outcomes even with all the data available.
Agreements and disagreements with other studies or reviews
The 2012 European Position Paper on Rhinosinusitis and Nasal Polyps is the only recent evidence‐based systematic review on the aetiology, management and treatment of chronic rhinosinusitis with and without nasal polyps, and it agrees that the efficacy of ESS is equivalent to that of medical therapy in chronic rhinosinusitis with nasal polyps (Fokkens 2012).

Process for sifting search results and selecting studies for inclusion.
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
| Endonasal surgery compared with medical treatment for chronic rhinosinusitis with nasal polyps | |||||
| Patients: adults with chronic rhinosinusitis with nasal polyps, receiving concurrent topical steroids Settings: otorhinolaryngology clinics and/or hospital departments Intervention: endoscopic sinus surgery Comparison: systemic steroids | |||||
| Outcomes (at 12 months) | Illustrative comparative risk | Relative effect | Number of participants | Quality of the evidence | Comments |
| Patient‐reported disease‐specific symptom scores
| N/A |
| 95 (1 study) | ⊕⊕⊝⊝ | Negative values indicate lower scores (less severe symptoms) in the surgical group. The range of values corresponds to effect sizes that are small to large (SMD 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect size):
|
| Health‐related quality of life scores, measured by SF‐36 (range 0 to 100) | N/A |
| 95 (1 study) | ⊕⊕⊝⊝ | These effect sizes are negligible and correspond to a SMD of ‐0.15 and 0.07 respectively (a SMD of 0.2 corresponds to a small effect size) |
| Endoscopic appearances
| N/A | MD ‐1.5 (95% CI ‐1.2 to ‐1.8) | 95 (1 study) | ⊕⊕⊝⊝ | Negative values indicate lower scores (less severe polyposis) in the surgical group. The SMD is ‐2.2 (95% CI ‐2.7 to ‐1.7), which corresponds to a large effect size |
| Complications | N/A | Epistaxis: 3.6% Orbital: 7.1% Intracranial: 1.8% | 95 (1 study) | ⊕⊝⊝⊝ | Orbital complications: orbital fat exposed Intracranial complications: CSF leak with meningitis |
| Objective physiological measures | N/A | Not reported | Other comparison pairs did not show an important difference | ||
| Olfactory tests | N/A | Not reported | Other comparison pairs did not show an important difference | ||
| GRADE Working Group grades of evidence CI: confidence interval; CSF: cerebrospinal fluid; MCS: mental component score; MD: mean difference; N/A: not applicable; PCS: physical component score; SMD: standardised mean difference | |||||
| 1Downgraded twice due to limitations in the study design (unclear randomisation, non‐blinded outcome assessment), and some concerns about imprecision (small sample sizes) and indirectness of evidence (the medical treatment regimes used in the study differ from current standards). Additional downgrading for imprecision and lack of comparative data for the complications outcome. | |||||
| Instrument | Details |
| Rhinosinusitis Outcome Measure (RSOM‐31) (Piccirillo 1995) | 31 items in 7 domains: nasal, eye, ear, sleep, general, practical and emotional problems. 2 rating scales: magnitude and importance of each item. Each item is measured on a 0 to 10 cm visual analogue scale (VAS). The minimal important difference (MID) is greater than 1. Time‐consuming and complex scoring system |
| 20‐item Sino‐Nasal Outcome Test (SNOT‐20) (Piccirillo 2002) | 20‐item modification of RSOM‐31, in 5 domains. Patients rate the magnitude of each item and the 5 most important items. Each item is rated on a 6‐point scale (0 = no problem, 5 = most serious problem) (range 0 to 100). Complex scoring system because of weighting for important symptoms. Excludes nasal blockage and anosmia |
| 22‐item Sino‐Nasal Outcome Test (SNOT‐22) (Hopkins 2009) | Modification of SNOT‐20 including nasal blockage and anosmia. Patients rate the magnitude of each item. Each item is rated on a 6‐point scale (0 = no problem, 5 = most serious problem) (range 0 to 110). Excludes weighting for most important symptoms. The minimal important difference (MID) is greater than 8.9 |
| Study | Surgical treatment | Medical treatment | Comparison description |
| GA endoscopic sinus surgery (variable extent) then topical budesonide 400 µg bd for 1 year | Oral prednisolone for 14 days (30 mg od 4 days then reduced by 5 mg every 2 days) then topical budesonide 400 µg bd for 1 year | Endoscopic sinus surgery versus systemic steroids | |
| LA polypectomy then topical beclomethasone dipropionate 100 µg bd for 1 year | 14 mg im betamethasone then topical beclomethasone dipropionate 100 µg bd for 1 year | Polypectomy versus systemic steroids | |
| LA snare polypectomy then topical budesonide 400 µg or 800 µg od for 11 months | 14mg im betamethasone then topical budesonide 400 µg or 800 µg od for 11 months | Polypectomy versus systemic steroids | |
| GA endoscopic sinus surgery (variable extent) then antibiotics for 2 weeks plus topical fluticasone propionate spray 100 µg bd for 12 weeks, then "tailored treatment" | Antibiotics plus topical fluticasone propionate drops 400 µg bd for 12 weeks, then "tailored treatment" | Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | |
| bd: twice daily | |||
| Study | Intervention/comparison | Outcome measure (scale) | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | Nasal obstruction (0 to 3) | 12 months | MD ‐0.3 (95% CI ‐0.6 to 0.0); standardised mean difference (SMD) ‐0.4 (95% CI ‐0.8 to 0.0) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Endoscopic sinus surgery versus systemic steroids | Rhinorrhoea/nasal discharge (0 to 3) | 12 months | MD ‐0.2 (95% CI ‐0.5 to 0.1); SMD ‐0.3 (95% CI ‐0.7 to 0.1) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Endoscopic sinus surgery versus systemic steroids | Sneezing (0 to 3) | 12 months | MD ‐0.2 (95% CI ‐0.5 to 0.1); SMD ‐0.3 (95% CI ‐0.7 to 0.1) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Endoscopic sinus surgery versus systemic steroids | Loss of smell (0 to 3) | 12 months | MD ‐0.4 (95% CI ‐0.7 to ‐0.1); SMD ‐0.6 (95% CI ‐1.0 to ‐0.2) (negative values indicate lower scores (less severe symptoms) in the surgical group) | |
| Polypectomy versus systemic steroids | Sense of smell (not known) | 12 months | Improved with medical and surgical treatment (P value > 0.05); no statistical difference between groups (exact data not given) | |
| Polypectomy versus systemic steroids | Nasal obstruction (0 to 3) | 12 months | Improved with medical and surgical treatment (P value > 0.05); no statistical difference between groups (exact data not given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Nasal obstruction Nasal discharge Facial pain/pressure Headache Overall discomfort (VAS 0 to 10 for each) | 12 months | Improved with medical treatment (61.2% change from baseline (SD = 19.1), P value < 0.01) and surgical treatment (54.7% change from baseline (SD = 27.3), P value < 0.01); no statistical difference between groups (data not given separately) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Olfactory disturbance | 12 months | Improved with medical and surgical treatment (P value < 0.05); no statistical difference between groups (exact data not given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | SNOT‐20 | 12 months | Improved with medical and surgical treatment (P value < 0.01); no statistical difference between groups (exact data not given) | |
| CI: confidence interval | ||||
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | SF‐36 (0 to 100 each domain) | 12 months | The MD was ‐1.4 (95% CI ‐5.0 to 2.2) for the physical component summary score, and 0.6 (95% CI ‐2.9 to 4.1) for the mental component scores. Effect sizes are negligible and correspond to a SMD of 0.07 and ‐0.15 respectively (a SMD of 0.2 corresponds to a small effect size) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | SF‐36 (0 to 100 each domain) | 12 months | Improved with surgical and medical treatment (P value < 0.01), except physical domain (P value > 0.05); no statistical difference between groups (exact data not given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | SNOT‐20 | 12 months | Actual results not reported; only stated that there were no statistically significant differences between groups | |
| CI: confidence interval | ||||
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | Polyp size score (0 to 3) | 12 months | Polyp size scores improved significantly in both groups at 12 months, and were significantly better in the endoscopic sinus surgery group (P value < 0.05): MD ‐1.5 (95% CI ‐1.8 to ‐1.2, n = 95). This corresponds to a large effect size | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Endoscopic score (0 to 3) | 12 months | Polyp size scores improved significantly in both groups at 12 months, but there was no important difference between groups (P value > 0.05): MD ‐2.3% (95% CI ‐17.4 to 12.8, n = 34) | |
| CI: confidence interval | ||||
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Endoscopic sinus surgery versus systemic steroids | Epistaxis (nosebleed) | 12 months | 3.6% (endoscopic sinus surgery) | |
| Polypectomy versus systemic steroids | Epistaxis | 12 months | "1 patient failed the study because of bleeding" (no further information) | |
| Polypectomy versus systemic steroids | Epistaxis | 12 months | 21% (all patients were on medical treatment throughout; polypectomy versus systemic steroids comparison not reported separately) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Epistaxis | 12 months | 2.2% (endoscopic sinus surgery plus topical steroid) versus 4.4% (antibiotics plus high‐dose topical steroid) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Infection | 12 months | 4.4% (endoscopic sinus surgery plus topical steroid) | |
| Endoscopic sinus surgery versus systemic steroids | Orbital complications | 12 months | 7.1% (endoscopic sinus surgery ‐ exposure of orbital fat) | |
| Endoscopic sinus surgery versus systemic steroids | Intracranial complications | 12 months | 1.8% (endoscopic sinus surgery ‐ CSF leak with meningitis) | |
| CSF: cerebrospinal fluid | ||||
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Polypectomy versus systemic steroids | Nasal expiratory peak flow | 12 months | Improved with surgical and medical treatment; no statistical difference between groups (P value > 0.05) (exact data not given) | |
| Polypectomy versus systemic steroids | Peak expiratory flow rate index | 12 months | Improved in all groups; no statistical difference between groups (surgical versus medical groups not reported separately, no exact data given) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Total nasal volume | 12 months | Improved with surgical (58.8% change from baseline, SD = 40) and medical treatment (50.3% change from baseline, SD = 50.7), P value < 0.01; no statistical difference between groups although medical group tended towards greater improvement (P value > 0.05) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Nasal cross‐sectional area | 12 months | No statistical difference between groups (P value > 0.05, Mann Whitney‐U test, not normally distributed data, median not reported) | |
| Endoscopic sinus surgery plus topical steroid versus antibiotics plus high‐dose topical steroid | Nasal nitric oxide levels | 12 months | No statistical difference between groups (P value > 0.05, Mann Whitney‐U test, not normally distributed data, median not reported) | |
| SD: standard deviation | ||||
| Study | Intervention/comparison | Outcome measure | Timing | Outcome |
| Polypectomy versus systemic steroids | Proportion of patients "expressing intact smell" | 2 to 12 months | No important difference between groups: risk ratio 0.96 (95% CI 0.71 to 1.31, n = 53) | |
| Polypectomy versus systemic steroids | Semi‐quantitative smell test | 12 months | No statistical difference between any treatment groups (surgical versus medical groups not reported separately, exact data not given) |

