نقش آنتیبیوتیکها برای درمان عوارض عصبی بیماری لایم
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Prospective, randomized, non‐blinded, parallel trial with an active comparator arm | |
| Participants | 54 participants with clinical signs and symptoms of meningoradiculitis, encephalomyelitis, or chronic meningitis and with elevated Borrelia burgdorferi‐specific antibody titers in serum or CSF, or both, or with B. burgdorferi cultured from CSF Doxycycline arm (22 women, 10 men); penicillin arm (13 women, 9 men) Other reported metrics comparable Exclusion criteria included age below 12 years, pregnancy, breast‐feeding, allergy to treatment compounds, and antibiotic treatment within the previous 4 weeks. See Table 2 and Table 3 for diagnostic criteria and additional baseline characteristics. | |
| Interventions | 14‐day course of:
| |
| Outcomes | Participant‐scoring daily self report form (0 = no symptoms, 1 = mild, 2 = moderate, 3 = severe). CSF analysis, serologic and clinical follow‐up for 1 year. It is unclear how symptoms were scored at later follow‐up visits. Symptoms scored included malaise, fatigue, nausea, vomiting, vertigo, headache, neck stiffness, muscular pain, arthralgia, visual and hearing disturbances, hypoesthesia or hyperesthesia, and paresis. CSF and serum samples were analyzed for antibodies against whole‐cell sonicate of B. burgdorferi by ELISA. Assessments were done at 14 days, 3, 6, and 12 months. | |
| Funding | Not disclosed | |
| Conflicts of interest | Not disclosed | |
| Notes | Study years 1987 to 1990 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
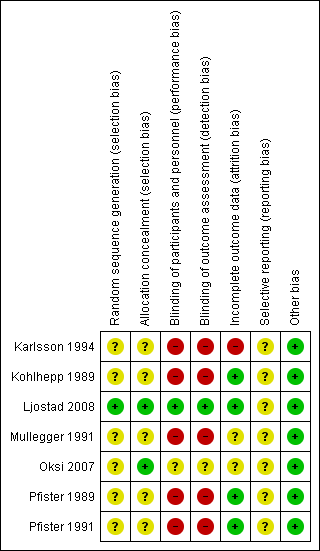
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to treatment groups, but the report does not describe the method of randomization. Though a small study, 31 participants in doxycycline arm and 23 in penicillin G arm would suggest a randomization problem. A sex imbalance was present, with many more women in the doxycycline group (N = 22) than in the penicillin G group (N = 13). |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded study |
| Incomplete outcome data (attrition bias) | High risk | Participant dropout was minimal (2 participants in penicillin arm, 1 participant in doxycycline arm). However, participants were randomized and dosed before the serology or culture results were available, and 17 were excluded. The timing of dropout was not specified. |
| Selective reporting (reporting bias) | Unclear risk | We found no evidence that all outcomes were prespecified; the pre‐study protocol was not available for review, and the study was not registered prior to initiation. |
| Other bias | Low risk | No other identified |
| Methods | Prospective, randomized, parallel‐group, open‐label study with active comparator, no placebo | |
| Participants | 75 participants with acute and chronic LNB. 12% of participants had a disease duration of ≥ 1 year with no statistically significant difference between treatment groups Participants had Borrelia burgdorferi‐specific antibodies in serum and at least 3 of the following diagnostic criteria: radicular pain; meningitic symptoms; cranial neuritis; sensory or motor radiculitis, or both; arthritis or carditis or encephalitis or myelitis or peripheral neuritis; tick bite or erythema migrans, or both. All participants were required to also have an elevated B. burgdorferi‐specific antibody titer in the serum. The following CSF laboratory parameters were analyzed:
See Table 2 and Table 3 for diagnostic criteria and additional baseline characteristics. | |
| Interventions | 10‐day treatment with either:
Participants with any or all of the following were considered a “treatment failure” and were eligible (procedure not clearly defined) for a second course of treatment with penicillin G (30 mega units per day for 10 days):
Participants with severe residual symptoms 3 months after therapy were offered the same therapeutic regimen. Participants with chronic encephalomyelitis were also treated with intravenous or intrathecal steroids, or both (N = 6) and cytarabine (N = 3). | |
| Outcomes | Clinical follow‐up examination (first outcome) was performed prior to the intervention, and 0, 5, 6, and 12 months after the intervention. Investigators graded the clinical status as “no remission,” “partial remission,” or “full remission.” Secondary outcomes included CSF findings (cell count, total protein, IgM index, and intrathecal B. burgdorferi‐specific Ab production) and B. burgdorferi‐specific IgG concentrations in serum. | |
| Funding | Not disclosed | |
| Conflicts of interest | Not disclosed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to treatment groups, but the report does not describe the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned, not clear if it was done |
| Blinding of participants and personnel (performance bias) | High risk | Not documented. We assume that neither participants nor investigators were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Not documented. We assume that outcome assessors were not blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | All participants returned to follow‐up at 1 year or longer. |
| Selective reporting (reporting bias) | Unclear risk | No evidence that all outcomes were prespecified; the pre‐study protocol was not available for review, and the study was not registered prior to initiation. |
| Other bias | Low risk | No other identified |
| Methods | Double‐blind, double‐dummy, randomized clinical non‐inferiority trial | |
| Participants | 118 consecutive adult participants in Norway with prospective newly diagnosed LNB were randomized. 102 were evaluable. Entry criteria were neurological symptoms without alternative explanation and 1 or more of the following criteria: CSF WBC > 5 cells/mL, intrathecal Borrelia burgdorferi‐specific antibody production, and/or verified acrodermatitis chronica atrophicans This study included both acute and chronic LNB, but groups were not analyzed separately, although chronic LNB comprised only 8% and 11% of each arm. See Table 3 for additional baseline characteristics. | |
| Interventions | 14‐day course of either:
| |
| Outcomes | The primary endpoint was a composite clinical score administered by experienced clinicians at baseline, 13 days, and 4 months after therapy. Secondary endpoints included the number of participants who had full recovery 4 months after treatment, reduction in CSF cell count at 4 months, and both reduction in CSF counts and clinical score at day 13. | |
| Funding | Sørlandet Kompetansefond (100%) | |
| Conflicts of interest | Authors reported no conflicts of interest. | |
| Notes | A large number of anticipated participants ended up not qualifying for inclusion and were excluded (N = 18). The study was not powered to investigate true differences in side effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized allocation performed in advance in blocks of 4 participants, stratified according to early disease (duration of symptoms < 6 months) and chronic disease (duration of symptoms > 6 months). |
| Allocation concealment (selection bias) | Low risk | Generally comparable baseline characteristics by treatment allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Investigators who participated in randomization were not further involved in study. A double‐blind, double‐dummy trial design was used. |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians were unaware of assignment groups. |
| Incomplete outcome data (attrition bias) | Low risk | 7/55 in ceftriaxone arm and 5/59 in doxycycline arm were excluded due to either loss to follow‐up or new diagnosis. All participants who received doxycycline completed a 14‐day course; 4 in the ceftriaxone arm did not complete (1 owing to late delivery, 3 owing to adverse reactions). |
| Selective reporting (reporting bias) | Unclear risk | No evidence that all outcomes were prespecified; the pre‐study protocol was not available for review, and the study was not registered prior to initiation. Relevant outcomes were reported in the paper, but some with insufficient detail for numerical analysis. |
| Other bias | Low risk | The study provided separate results for cases of definite and probable LNB both at randomization and by efficacy outcomes. |
| Methods | Prospective, randomized, parallel‐group, open‐label study with active comparator, no placebo | |
| Participants | 23 children with acute neurologic symptoms of LNB, i.e. 1 or more of the following symptoms: meningism (n = 17), peripheral facial palsy (n = 14), torticollis (n = 1), VI cranial nerve paresis (n = 1), pseudotumor cerebri (n = 1) Inclusion criteria were:
All children had to be antibiotic treatment‐naïve. See Table 2 and Table 3 for diagnostic criteria and additional baseline characteristics. | |
| Interventions | 14 days of treatment with either:
| |
| Outcomes |
B. burgdorferi‐specific IgG in serum was the primary outcome and was measured by ELISA prior to randomization, at the end of treatment, and 3, 6, and 12 months after the end of treatment. | |
| Funding | Not disclosed | |
| Conflicts of interest | Not disclosed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly allocated to treatment groups, but the report does not describe the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned, not clear if it was done |
| Blinding of participants and personnel (performance bias) | High risk | Neither participants nor investigators were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Not documented. We assume that outcome assessors were not blinded to treatment group. |
| Incomplete outcome data (attrition bias) | Unclear risk | All children in both groups recovered completely. Some children did not participate in the final control visit (12 months), which could have resulted in missed detection of late relapse in these children. |
| Selective reporting (reporting bias) | Unclear risk | No evidence that all outcomes were prespecified; the pre‐study protocol was not available for review, and the study was not registered prior to initiation |
| Other bias | Low risk | No other bias identified |
| Methods | Prospective, randomized, double‐blind, parallel‐group multicenter study with placebo | |
| Participants | 152 consecutive adults (145 evaluable) from 3 tertiary hospitals in Finland who had just completed treatment for Lyme disease with ceftriaxone. 62 participants had definite LNB. Criteria for a definite LNB diagnosis were: a classical manifestation (e.g. facial paresis, meningitis, or meningoradiculitis, along with exclusion of other causes) and inflammatory changes in the CSF or Borrelia burgdorferi‐specific intrathecal antibodies, or both. Criteria for possible LNB were less common manifestations of LNB and presence of serum B. burgdorferi‐specific antibodies. Diagnosis of definite or probable LNB required exclusion of other causes. Among all Lyme disease participants, 52/73 (71.2%) amoxicillin‐treated group and 54/72 (75%) placebo group had a definite diagnosis. See Table 2 and Table 3 for diagnostic criteria and additional baseline characteristics. | |
| Interventions | All participants received:
| |
| Outcomes | 1‐year follow‐up, with outcome measurement at the end of ceftriaxone treatment and 1, 3, 6, and 12 months later. Outcome measured by 0 to 100 VAS, where 50 = baseline before intravenous treatment, 0 = symptom free, 100 = "definitely poor outcome". | |
| Funding | Bristol–Myers Squibb provided amoxicillin tablets, and Roche covered part of the costs of the study; any other funding was not disclosed. | |
| Conflicts of interest | Not disclosed | |
| Notes | Study conducted 1998 to 2003. Location: Finland The study may have been underpowered to permit a definite conclusion about the lack of efficacy of the adjunctive treatment, as a total of 200 participants would have been needed to show a 10% difference with an 80% power to detect a significant (P < 0.05, 2‐sided) difference. Dr. Oksi provided us with unpublished data separating the LNB from the other Lyme disease participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to treatment group in a pharmacy, but the report does not describe the exact method. Baseline comparison of treatment groups was not adequately described. |
| Allocation concealment (selection bias) | Low risk | The enrolled participants received labeled containers marked with a code. The investigators had no access to the codes before the end of the study. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding for the initial antibiotic treatment, but there was blinding for the second. Although the investigators had no access to the codes until the end of the study, the possibility that side effects unblinded participants was not addressed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No blinding for the initial antibiotic treatment, only for the second. Although the investigators had no access to the study codes until the end of the study, the possibility that side effects unblinded investigators was not addressed. |
| Incomplete outcome data (attrition bias) | Unclear risk | 7 participants were withdrawn and not included in analysis (5 for discontinuing the study drug and 2 who received an alternate diagnosis). No intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | No evidence that all outcomes were prespecified; the pre‐study protocol was not available for review, and the study was not registered prior to initiation. Reporting for the LNB group was complete but never published. |
| Other bias | Low risk | The study provided separate results for cases of definite and probable LNB both at randomization and by efficacy outcomes. |
| Methods | Prospective, randomized, parallel‐group, open‐label study with an active comparator, no placebo | |
| Participants | Participants had acute painful LNB radiculitis (n = 18) or LNB meningitis (n = 3). The diagnostic criteria included the following1:
See Table 2 and Table 3 for diagnostic criteria and additional baseline characteristics. | |
| Interventions | 10‐day treatment with either:
| |
| Outcomes | Neurologic examination was performed daily. Improvement or resolution in the neurological history and physical exam (cranial nerve palsies, pareses of extremities and abdominal muscles, headache, and sensory disturbances) was recorded on day 10 (early outcome) and on average 7.7 months later (longer‐term outcome). The severity of radicular pain was scored daily with a 0‐to‐10 rating system. For evaluation, the medians of the corresponding maximum daily pain scores in the penicillin group (n = 7) were compared with those in the cefotaxime group (n = 8). Trialists recorded the daily dose of analgesics during the 10‐day treatment period and measured the total amount of analgesics taken during the 10‐day treatment period. Investigators performed lumbar puncture prior to (n = 21) and on the 8th to 10th day of treatment (n = 17) and quantified CSF‐WBC, CSF protein, and intrathecal IgG synthesis. They visualized oligoclonal bands and cultured CSF in BSK media. In addition, the investigators measured B. burgdorferi‐specific antibody concentration in serum at randomization and follow‐up. | |
| Funding | Not disclosed | |
| Conflicts of interest | Not disclosed | |
| Notes | 1At the time of the onset of therapy, radicular pain and headache had already subsided in 3 participants with radiculitis and in 1 participant with meningitis, respectively. 6 of 21 participants were seronegative: 6 participants had normal (n = 5) or marginal (n = 1) B. burgdorferi‐specific antibody titers in the serum and normal B. burgdorferi‐specific CSF antibody titers. 4 of these 6 participants had a history of erythema migrans, which was still present in 2 participants at the time of hospital admission. The other 2 seronegative participants (1 participant from each treatment group) had no history of erythema migrans but reported multiple "insect bites" and bites by horseflies within a few weeks prior to the onset of the neurologic disease. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to treatment groups, but the report does not describe the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Neither participants nor investigators were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The majority of participants returned for longer‐term follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No evidence that all outcomes were prespecified; the pre‐study protocol was not available for review, and the study was not registered prior to initiation. |
| Other bias | Low risk | No other bias identified |
| Methods | Prospective, randomized, parallel‐group, open‐label study with an active comparator, no placebo | |
| Participants | 33 participants with predominantly acute LNB 32 of the 33 participants had clinical LNB; 1 was asymptomatic. Trialists excluded 3 of the 33 participants because they were never symptomatic (N = 1) or because the symptoms had resolved prior to randomization (N = 2). 28 participants had typical Bannwarth's syndrome with intense radicular pain and lymphocytic pleocytosis in the CSF. These participants usually also had paresis of the extremities or cranial nerve palsies. 4 had lymphocytic meningitis with detectable Borrelia burgdorferi‐specific antibodies in the serum or CSF, or both. The remaining participant had no clinical symptoms due to B. burgdorferi infection, but the B. burgdorferi‐specific IgG titer in the serum was elevated and B. burgdorferi was isolated from CSF. A history of an arthropod bite or typical erythema migrans within 3 months before the onset of the neurologic disease was found in 18 and 16 participants, respectively. See Table 2 and Table 3 for diagnostic criteria and additional baseline characteristics. | |
| Interventions | 10 days' treatment with either:
| |
| Outcomes | The primary outcome was the number of participants whose symptoms improved (improvement of symptoms attributable to LNB including radicular pain, headache, cranial nerve palsies, pareses of extremities, and sensory disturbances). Study authors also reported tolerability. | |
| Funding | Not disclosed | |
| Conflicts of interest | Not disclosed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to treatment groups, but the report does not describe the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Neither study participants nor study personnel were blinded to treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The majority of participants returned for follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No evidence that all outcomes were prespecified; the pre‐study protocol was not available for review, and the study was not registered prior to initiation. |
| Other bias | Low risk | No other bias identified |
Ab: antibody
BSK media: Barbour‐Stoenner‐Kelly media
CSF: cerebrospinal fluid
ELISA: enzyme‐linked immunosorbent assay
Ig: immunoglobulin
LNB: Lyme neuroborreliosis
VAS: visual analogue scale
WBC: white blood cells
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This study focused only on late LB (all types, not specifically neurological). | |
| This open randomized clinical trial compared 14 and 28 days of ceftriaxone treatment in people with LB including LNB. As LNB participants were not separately documented, we excluded the trial from the review. | |
| Excluded for 2 main reasons: clear objective evidence of neurological disease was lacking, and participants were a mixed population with manifestations of joint involvement with or without peripheral nerve involvement, which was poorly documented. We contacted the author, who was unable to retrieve study data from an outdated digital storing system for further analysis. | |
| Participants had erythema migrans, not untreated LNB. Excluded as the objective of the review was not to determine the efficacy of antibiotic treatment of erythema migrans to prevent the development of LNB. | |
|
| |
| This was a randomized study of treatment of LNB with a non‐antibiotic intervention (corticosteroid). |
Ab: antibody
CSF: cerebrospinal fluid
LB: Lyme borreliosis
LNB: Lyme neuroborreliosis
PCR: polymerase chain reaction
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Six versus two weeks treatment with doxycycline in Lyme neuroborreliosis |
| Methods | Multicenter, non‐inferiority, randomized, penta‐blind (participant, caregiver, investigator, outcomes assessor), placebo‐controlled study |
| Participants | 250 adults (18 years and older) with neuroborreliosis (Borrelia burgdorferi) from Norwegian hospitals Inclusion criteria: neurological symptoms suggestive of Lyme neuroborreliosis without other obvious causes, one or both of a) cerebrospinal fluid pleocytosis (> 5 leukocytes/mm3), b) intrathecal B. burgdorferi antibody production |
| Interventions | Doxycycline 200 mg once daily for 6 weeks versus doxycycline 200 mg once daily for 2 weeks + placebo for 4 weeks |
| Outcomes | Primary: composite clinical score at 6 months after the end of treatment Secondary: Fatigue Severity Scale (FSS), Patient Health Questionnaire (PHQ‐15), 36‐Item Short‐Form Health Survey (SF‐36), and blood and cerebrospinal fluid findings at inclusion and after 6 and 12 months Safety |
| Starting date | October 2015 |
| Contact information | Sorlandet Hospital |
| Notes | Estimated completion 2020 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptoms (patient‐rated VAS, scale 0 to 100, higher worse) in all participants (definite and possible Lyme disease) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 1 Symptoms (patient‐rated VAS, scale 0 to 100, higher worse) in all participants (definite and possible Lyme disease). | ||||
| 1.1 3 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [3.39, 5.01] |
| 1.2 6 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.38, 0.38] |
| 1.3 12 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.21, 1.41] |
| 2 Symptoms (investigator‐rated VAS, scale 0 to 100 higher worse) in all participants (definite and possible Lyme disease) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 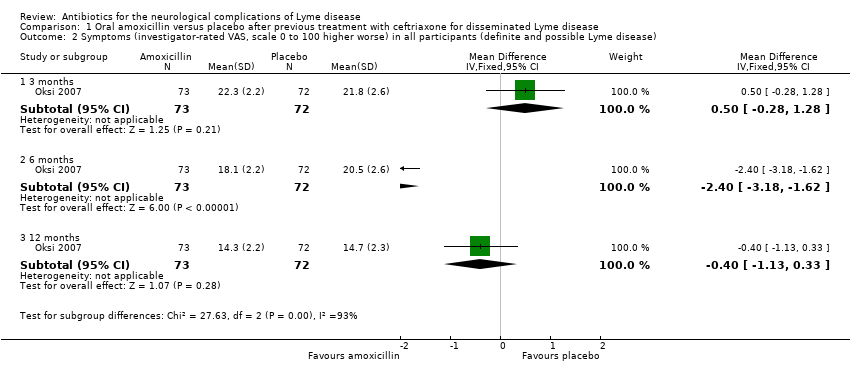 Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 2 Symptoms (investigator‐rated VAS, scale 0 to 100 higher worse) in all participants (definite and possible Lyme disease). | ||||
| 2.1 3 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.28, 1.28] |
| 2.2 6 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐3.18, ‐1.62] |
| 2.3 12 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.13, 0.33] |
| 3 Improvement of symptoms (excellent or good on investigator VAS) (12 months) in participants with definite Lyme disease Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.21] |
| Analysis 1.3  Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 3 Improvement of symptoms (excellent or good on investigator VAS) (12 months) in participants with definite Lyme disease. | ||||
| 4 Adverse events (12 months) in all participants (definite and possible Lyme disease) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 4 Adverse events (12 months) in all participants (definite and possible Lyme disease). | ||||
| 4.1 Serious adverse events | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Diarrhea | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.70 [1.29, 10.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean reduction in clinical score (4 months) Show forest plot | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.20, 1.40] |
| Analysis 2.1  Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 1 Mean reduction in clinical score (4 months). | ||||
| 2 Resolution of symptoms Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.89, 2.35] |
| Analysis 2.2 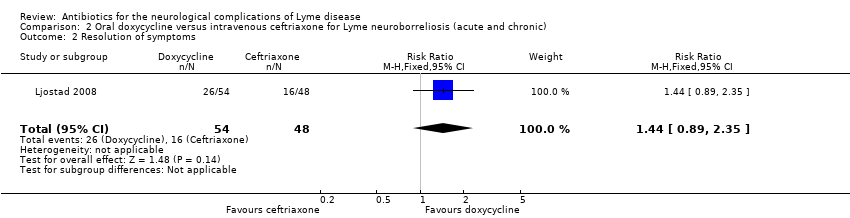 Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 2 Resolution of symptoms. | ||||
| 3 All adverse events Show forest plot | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.23] |
| Analysis 2.3  Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 3 All adverse events. | ||||
| 4 Adverse events leading to discontinuation Show forest plot | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.71] |
| Analysis 2.4 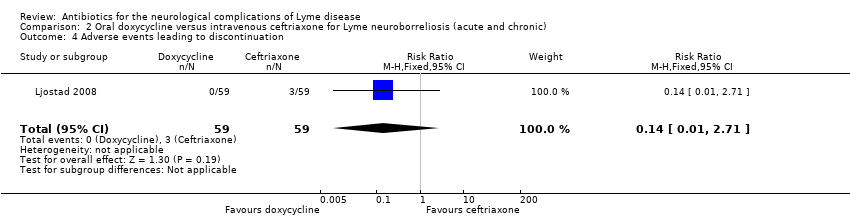 Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 4 Adverse events leading to discontinuation. | ||||
| 5 Serious adverse events Show forest plot | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.05] |
| Analysis 2.5  Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 5 Serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement of symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 1 Improvement of symptoms. | ||||
| 1.1 3 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.93, 1.08] |
| 1.2 6 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.93, 1.08] |
| 1.3 12 months | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.92, 1.08] |
| 2 Resolution of symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 2 Resolution of symptoms. | ||||
| 2.1 3 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.64, 1.61] |
| 2.2 6 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.10, 2.54] |
| 2.3 12 months | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.18] |
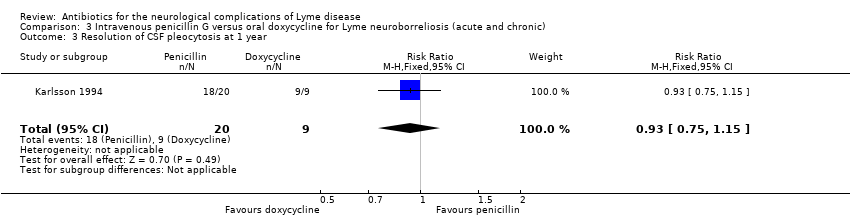
| 3 Resolution of CSF pleocytosis at 1 year Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.15] |
| Analysis 3.3 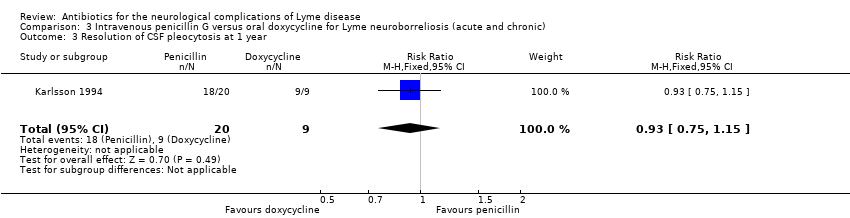 Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 3 Resolution of CSF pleocytosis at 1 year. | ||||
| 4 All adverse events Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.25, 4.08] |
| Analysis 3.4  Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 4 All adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement of symptoms ("partial remission") Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Intravenous doxycycline versus intravenous penicillin G for Lyme neuroborreliosis (acute and chronic), Outcome 1 Improvement of symptoms ("partial remission"). | ||||
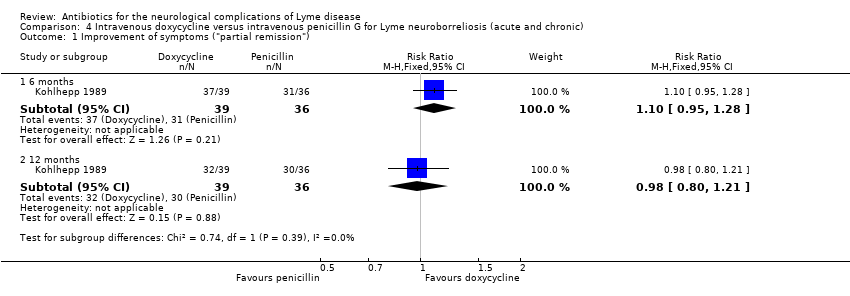
| 1.1 6 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.28] |
| 1.2 12 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 2 Resolution of symptoms ("full remission") Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Intravenous doxycycline versus intravenous penicillin G for Lyme neuroborreliosis (acute and chronic), Outcome 2 Resolution of symptoms ("full remission"). | ||||
| 2.1 6 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.83, 2.42] |
| 2.2 12 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.31] |
| 3 Serious adverse events Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.3  Comparison 4 Intravenous doxycycline versus intravenous penicillin G for Lyme neuroborreliosis (acute and chronic), Outcome 3 Serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of symptoms (mean 7.7 months' follow‐up) Show forest plot | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.55] |
| Analysis 5.1  Comparison 5 Intravenous cefotaxime versus intravenous penicillin G for acute Lyme neuroborreliosis, Outcome 1 Resolution of symptoms (mean 7.7 months' follow‐up). | ||||
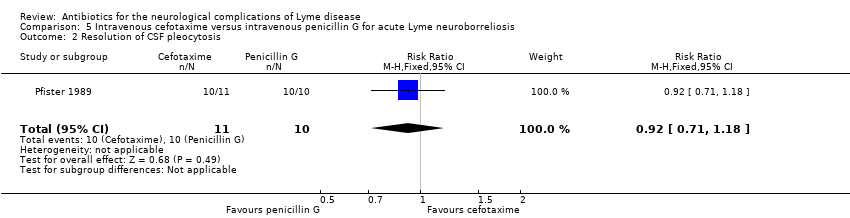
| 2 Resolution of CSF pleocytosis Show forest plot | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.18] |
| Analysis 5.2 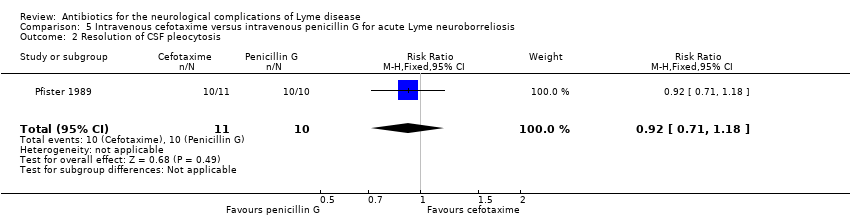 Comparison 5 Intravenous cefotaxime versus intravenous penicillin G for acute Lyme neuroborreliosis, Outcome 2 Resolution of CSF pleocytosis. | ||||
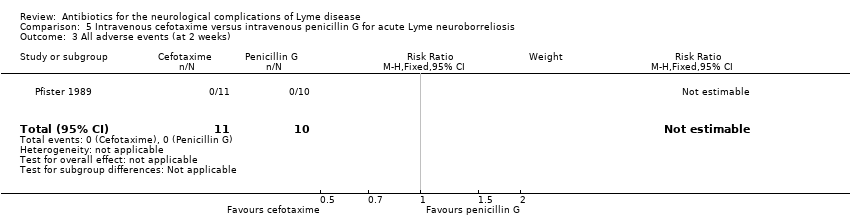
| 3 All adverse events (at 2 weeks) Show forest plot | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 5.3  Comparison 5 Intravenous cefotaxime versus intravenous penicillin G for acute Lyme neuroborreliosis, Outcome 3 All adverse events (at 2 weeks). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of symptoms (mean 8.1 months' follow‐up) Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.63, 1.97] |
| Analysis 6.1  Comparison 6 Intravenous ceftriaxone versus intravenous cefotaxime for acute Lyme neuroborreliosis, Outcome 1 Resolution of symptoms (mean 8.1 months' follow‐up). | ||||
| 2 Resolution of CSF pleocytosis Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.90, 1.44] |
| Analysis 6.2 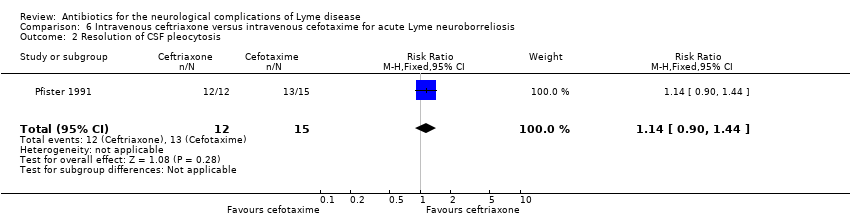 Comparison 6 Intravenous ceftriaxone versus intravenous cefotaxime for acute Lyme neuroborreliosis, Outcome 2 Resolution of CSF pleocytosis. | ||||
| 3 All adverse events Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.04, 3.26] |
| Analysis 6.3  Comparison 6 Intravenous ceftriaxone versus intravenous cefotaxime for acute Lyme neuroborreliosis, Outcome 3 All adverse events. | ||||

Study flow diagram.

Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 1 Symptoms (patient‐rated VAS, scale 0 to 100, higher worse) in all participants (definite and possible Lyme disease).
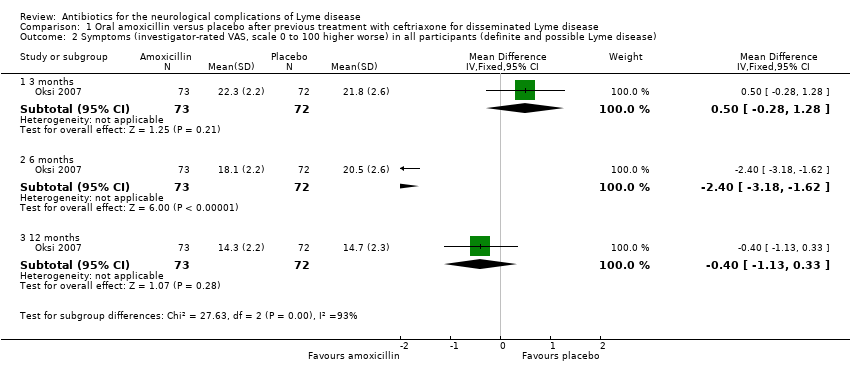
Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 2 Symptoms (investigator‐rated VAS, scale 0 to 100 higher worse) in all participants (definite and possible Lyme disease).
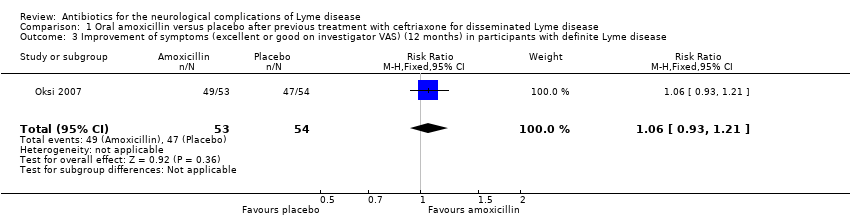
Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 3 Improvement of symptoms (excellent or good on investigator VAS) (12 months) in participants with definite Lyme disease.
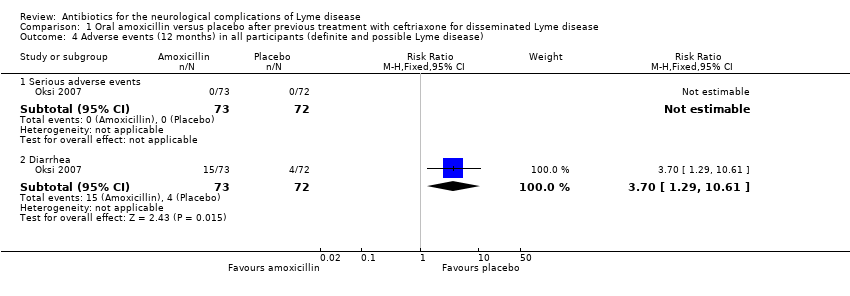
Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 4 Adverse events (12 months) in all participants (definite and possible Lyme disease).
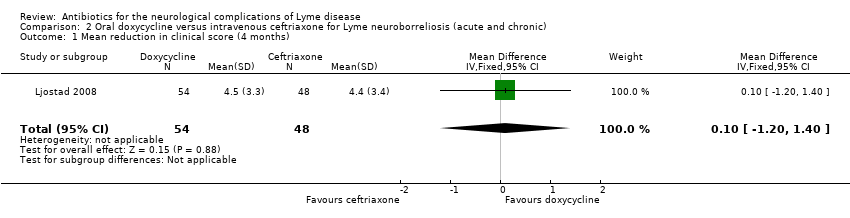
Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 1 Mean reduction in clinical score (4 months).
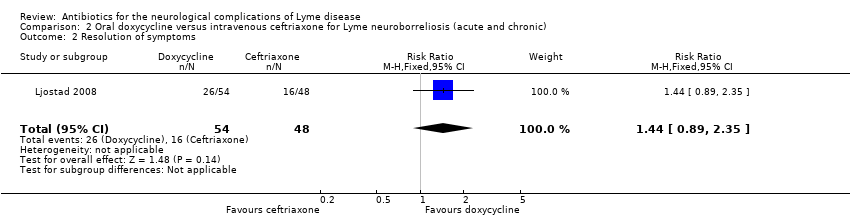
Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 2 Resolution of symptoms.
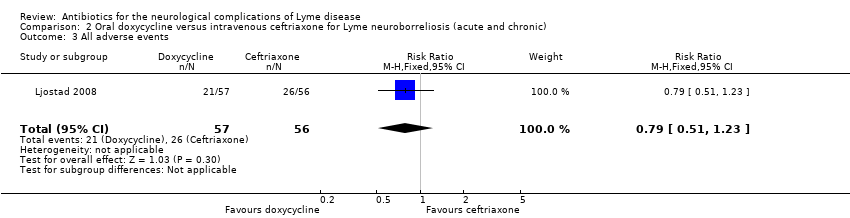
Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 3 All adverse events.
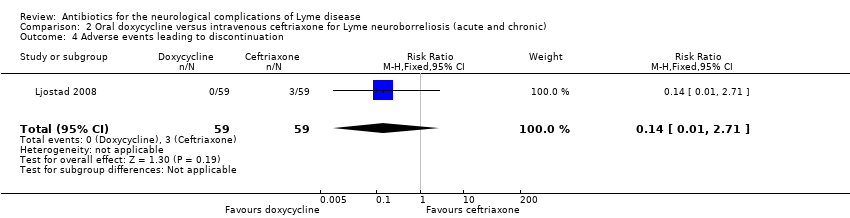
Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 4 Adverse events leading to discontinuation.
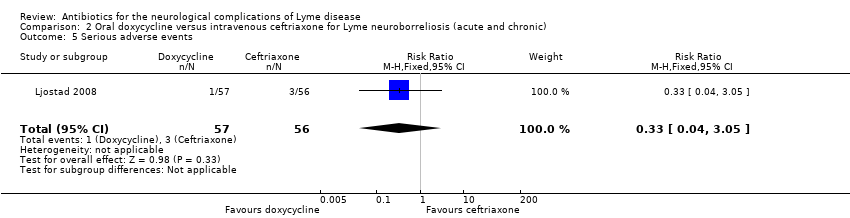
Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 5 Serious adverse events.
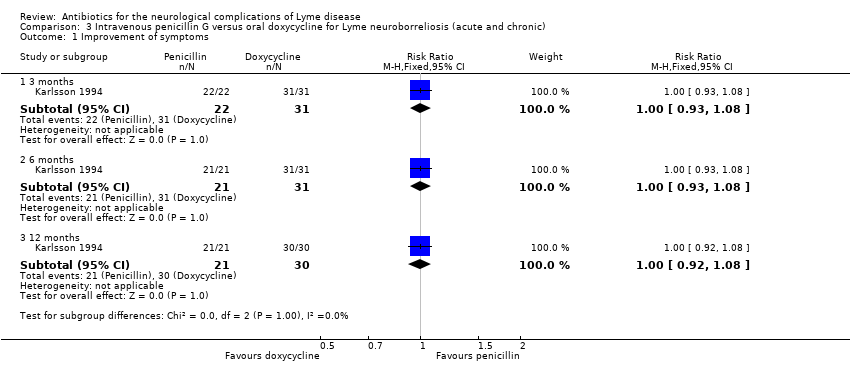
Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 1 Improvement of symptoms.

Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 2 Resolution of symptoms.
Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 3 Resolution of CSF pleocytosis at 1 year.

Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 4 All adverse events.
Comparison 4 Intravenous doxycycline versus intravenous penicillin G for Lyme neuroborreliosis (acute and chronic), Outcome 1 Improvement of symptoms ("partial remission").

Comparison 4 Intravenous doxycycline versus intravenous penicillin G for Lyme neuroborreliosis (acute and chronic), Outcome 2 Resolution of symptoms ("full remission").

Comparison 4 Intravenous doxycycline versus intravenous penicillin G for Lyme neuroborreliosis (acute and chronic), Outcome 3 Serious adverse events.

Comparison 5 Intravenous cefotaxime versus intravenous penicillin G for acute Lyme neuroborreliosis, Outcome 1 Resolution of symptoms (mean 7.7 months' follow‐up).
Comparison 5 Intravenous cefotaxime versus intravenous penicillin G for acute Lyme neuroborreliosis, Outcome 2 Resolution of CSF pleocytosis.
Comparison 5 Intravenous cefotaxime versus intravenous penicillin G for acute Lyme neuroborreliosis, Outcome 3 All adverse events (at 2 weeks).
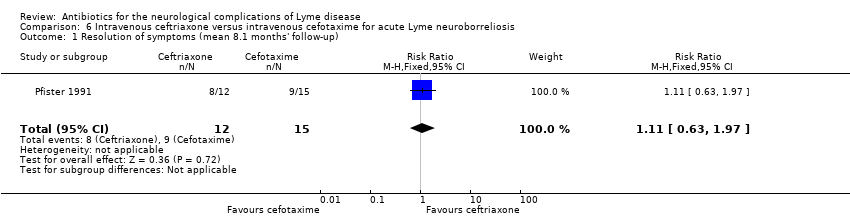
Comparison 6 Intravenous ceftriaxone versus intravenous cefotaxime for acute Lyme neuroborreliosis, Outcome 1 Resolution of symptoms (mean 8.1 months' follow‐up).

Comparison 6 Intravenous ceftriaxone versus intravenous cefotaxime for acute Lyme neuroborreliosis, Outcome 2 Resolution of CSF pleocytosis.

Comparison 6 Intravenous ceftriaxone versus intravenous cefotaxime for acute Lyme neuroborreliosis, Outcome 3 All adverse events.
| Study | Population | Length of follow‐up | Interventions | Antibiotic naïve | Clinical remission measurement | Time to remission | Patient‐reported outcomes | CSF remission | Serology response |
| Yes | Up to 3 years | Penicillin Doxycycline | Unknown | Complete/partial/no | No | No | Yes | No | |
| Yes | Average of 7 months | Penicillin Cefotaxime | Unknown | Yes/no | No | Yes: VAS, 0 to 10 | Yes | No | |
| Yes (children only) | >6 months, <12 months | Penicillin Ceftriaxone | Yes | Yes/no | Yes; from treatment onset to complete remission | No | No | Yes | |
| Yes | Average of 7.7 months | Ceftriaxone Cefotaxime | Unknown | Yes/no | No | No | Yes | No | |
| Yes | 12 months | Penicillin Doxycycline | Past 4 weeks | Yes/no; by specific sign/symptom | No | Yes: Likert‐like scale, 0 to 3 | Yes, at 2 weeks and 12 months | Yes | |
| No (but large subgroup of LNB enrolled) | 12 months | Ceftriaxone followed by amoxicillin or placebo | Past 1 month | Physician VAS 0 to 100; scored as excellent/good, poor/none, controversial | No | Yes: VAS | Lumbar puncture and measurement of CSF antibody levels and PCR for Borrelia burgdorferi was repeated in selected cases during or after treatment. | Yes: scored as strong decline, mild, none | |
| Yes | Up to 4 months | Ceftriaxone Doxycycline | Past 14 days | No/mild/more than mild; also change in baseline deficits in past 3 months using own composite clinical score | No | Yes: 6 items, each scored 0 to 2 | Yes | No | |
| CSF: cerebrospinal fluid | |||||||||
| Criteria | (N = 75) | (N = 21) | (N = 23) | (N = 30) | (N = 54) | (N = 145; 72% to 75% definite, 25% to 27% possiblea) | (N = 102) |
| Clinical | Radicular pain, meningitic symptoms, cranial neuritis, sensory and/or motor radiculitis, arthritis, carditis, myelitis or peripheral neuritis, tick bite and/or erythema migrans | Radicular pain (15/21), headache (2/21), facial palsy (8/21), unilateral VI palsy (1/21), lower limb muscle weakness (9/21), sensory disturbance (12/21) | Presence of neurological signs and symptoms indicative of LNB | Radiculopathy (motor or sensory, or both), cranial neuropathy (facial palsy, ocular motor) | Headache (71% to 74%), subjective stiff neck (65%), paresis (55% to 57%) including facial palsy in 35% to 43% | Lymphocytic meningitis without radiculitis in 18 (all definite), meningoradiculitis (16 definite) or radiculitis (11 definite), paresis in 5, encephalomyelitis in 4, encephalopathy in 6, facial paresis in 21, sudden deafness in 6, tinnitus in 8, other cranial nerve involvement in 13, peripheral neuritis in 6, and other peripheral nervous system manifestations (9 peripheral mononeuropathy or polyneuropathy, 15 paresthesia, 39 with headache without meningitis, 29 with dizziness or vertigo, and 11 with memory impairment) | 25% to 33% Bannwarth's syndrome, 19% to 22% facial palsy, 24% to 38% radiculopathy, various others (other cranial neuropathies, ataxia, myelopathy, limb paresis, paresthesias, cognitive deficits) |
| Laboratory | B. burgdorferi‐specific antibody titer in serum, B. burgdorferi‐specific antibody titer in CSF, lymphocytic pleocytosis, elevated CSF protein (>50 mg/dL), elevated CSF IgM‐, IgA‐, and/or IgG‐index, oligoclonal bands in CSF. Only group‐level information is given. | Elevated B. burgdorferi‐specific IgG and IgM antibody titers in serum (1:64 to 1:512): found in 11, of whom 4 had both elevated IgG and IgM, 6 had only elevated IgG, and 1 had only elevated IgM. 4 were seropositive in CSF but not in blood; 6 had negative serology in both serum and CSF (seronegative LNB), but 4 had EM and 2 a history of insect bites. | 1 or more of the following specific CSF laboratory parameters: elevated B. burgdorferi‐specific IgG antibody titer, intrathecally produced B. burgdorferi‐specific antibodies, and/or direct cultivation of B. burgdorferi from the CSF in a modified Barbour‐Stoenner‐Kelly medium | Elevated B. burgdorferi‐specific IgG and IgM antibody titers in serum: found in 22 and 8, respectively. 13 had positive B. burgdorferi‐specific CSF/serum antibody index. | Elevated B. burgdorferi‐specific IgM or IgG concentration, or both in 83% to 90%; all had positive serology or B. burgdorferi‐specific CSF antibodies, except for 1 participant who had a positive CSF culture. | Only 3 of the 145 study participants were seronegative. Presence of inflammatory changes in the CSF or intrathecal antibodies against B. burgdorferi, or both supported a diagnosis of definite LNB; 124/145 participants had lumbar puncture performed at diagnosis. | Intrathecal production of B. burgdorferi‐specific antibodies or B. burgdorferi‐specific antibodies in serum, or both were required for enrollment. |
| aLNB considered possible if clinical presentation was an uncommon manifestation, but serum antibodies against Borrelia burgdorferi were positive and other causes were excluded. Abbreviations: | |||||||
| Study | Kohlhepp 1989 Penicillin | Kohlhepp 1989 Doxycycline | Pfister 1989 Penicillin G | Pfister 1989 Cefotaxime | Pfister 1991 Ceftriaxone | Pfister 1991 Cefotaxime | Mullegger 1991 Penicillin G | Mullegger 1991 Ceftriaxone | Penicillin G | Karlsson 1994 Doxycycline | Oksi 2007 Amoxicillin post‐ceftriaxone | Oksi 2007 Placebo post‐ceftriaxone | Ljostad 2008 Doxycycline | Ljostad 2008 Ceftriaxone |
| Number of participants (evaluable) | 36 | 39 | 10 | 11 | 14 | 16 | 11 | 12 | 23 | 31 | 73 | 72 | 54 | 48 |
| Age mean (SD) unless specified | Men 55 (12.6); women 54.1 (16.3) | Men 49.6 (14); women 55.7 (14.3) | 56.7 (15) | 55.4 (10.8) | 58.7 (19.5) | 53.7 (16.8) | 8.1 (3.1) | Median 55 (range 16 to 88) | Median 49 (range 18 to 74) | Mean 52.3, range 19 to 87 | Mean 50.5, range 16 to 80 | 54 (13) | 52 (13) | |
| Percentage males | 44% | 51% | 50% | 64% | 64% | 44% | 36% | 42% | 44% | 29% | 48% | 50% | 52% | 65% |
| History of erythema migrans | 36% | 31% | 80% | 45% | 50% | 56% | Not reported | 61% | 42% | 26% (probable) | 31% | 10% | ||
| Mean (SD) time from onset of LNB to treatment | 5.2 (13.6) months | 4.1 (11.1) months | 28.7 (33.8) days | 23.5 (16.3) days | 64.5 (84.7) days | 38.6 (23.1) days | All included children were admitted to the hospital within 5 ± 1.8 days from onset of symptoms. | 3.5 weeks (1 week to 25 months) | 4 weeks (1 week to 18 months) | Unknown | 10 (19) weeks | 8 (13) weeks | ||
| Previous treatment with antibiotics | 11% | 8% | Not reported | Not reported | Not reported (mentioned for 1 participant) | Was an exclusion criteria | None for the 4 weeks prior to enrollment | Yes, in all participants with EM, 24 adequately and 14 not adequately | Treatment with cephalosporin, penicillin, tetracycline in past 14 days exclusion criterion | |||||
| Concomitant treatment with steroids | 28% | 26% | None | Not reported | Not reported | Not reported | Not reported | Not reported | ||||||
| CSF leukocytes mean (SD) cells/uL | 186 (75) | 145 (58) | 280.9 (212) | 435.7 (528) | 86.4 (128.4) | 135.3 (299.2) | Not reported | Median 96, range 6 to 1190 | Median 117, range 8 to 910 | 59% with available CSF showed lymphocytic pleocytosis. | 194 (237) | 178 (187) | ||
| CSF total protein mean (SD) in mg/dL | 133 (110) | 119 (112) | 115 (69) | 136 (67.4) | 72.7 (42) | 79.1 (48.4) | Not reported | Median 110, range 40 to 360 | Median 120, range 50 to 580 | Not reported | 120 (70) | 130 (80) | ||
| Presence of CSF oligoclonal bands | 78% | 62% | 70% | 64% | 64% | Not reported | Not reported | Not reported | Detected in 24/58 (41%) participants with definite LNB and CSF examined | Not reported | ||||
| CSF: cerebrospinal fluid | ||||||||||||||
| Study | Tool | Signs assessed | Subjective symptoms elicited |
| Visual analogue scale | Yes | Possibly | |
| Composite clinical score | Yes | Yes | |
| Change of clinical symptoms | Yes | No | |
| Disease duration | Yes | No | |
| Change of clinical symptoms (3‐level classification) | Yes | Unclear | |
| Change of clinical symptoms | Yes | Yes | |
| Change of clinical symptoms | Yes | Unclear | |
| Change of clinical symptoms | Yes | No | |
| *The efficacy of interventions was quantified by diverse tools in each study assessing the change in objective findings (signs) or subjective complaints (symptoms), or both, as reported by participants or judged by the study physician. | |||
| Study | Parameter |
| Decrease of B. burgdorferi‐specific antibody concentrations at 12 months of at least 20% ("moderate decline") or 50% ("strong decline") | |
| Resolution of CSF pleocytosis | |
| Cell count, protein, antibody index, B. burgdorferi‐specific antibody production | |
| Abnormal CSF on repeated lumbar puncture1 | |
| Abnormal CSF on repeated lumbar puncture2 | |
| Cell count, B. burgdorferi‐specific antibody production | |
| Changes in intrathecally produced specific antibodies against B. burgdorferi | |
| 1One or more of lymphocytic pleocytosis, protein elevation, oligoclonal bands, B. burgdorferi‐specific antibody production. Abbreviations: | |
| Oral amoxicillin versus placebo for people previously treated with ceftriaxone for Lyme neuroborreliosis (acute and late) | ||||||
| Patient or population: people previously treated with ceftriaxone for disseminated Lyme neuroborreliosis (acute and chronic)1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Placebo | Oral amoxicillin | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Improvement or resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment2 | Separate information on the LNB subgroup (N = 62), but not for intervention groups within this subgroup was provided at the review authors' request. 59/62 participants were classified as experiencing improvement of presenting neurological deficits at month 12 (dichotomous assessment: 'excellent or good' based on investigator VAS values and medical record information).3 | Not estimable | 624 | Low5 | ||
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment4 | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| All adverse events ‐ 12 months | 24 adverse events for all 145 Lyme disease participants, mostly diarrhea and fever with no need for discontinuation. No serious adverse events reported. Attribution of adverse events to either pretreatment with ceftriaxone, or to amoxicillin or placebo, or both, is unclear. | Not estimable | 145 | Very low6 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Subpopulation with LNB (N = 62) within study of people with definite or possible disseminated Lyme borreliosis (N = 145). | ||||||
| Oral doxycycline compared to intravenous ceftriaxone for Lyme neuroborreliosis (LNB) (acute and chronic) | ||||||
| Patient or population: Lyme neuroborreliosis (acute and chronic) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous ceftriaxone | Oral doxycycline | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment | 333 per 1000 | 480 per 1000 (297 to 783) | RR 1.44 (0.89 to 2.35) | 102 | Moderate1 | Symptom resolution; composite clinical score of neurological signs and symptoms at 12 months2 |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | CSF was analyzed in 88/102 participants; authors state that no significant between‐group difference was present at 13 days and 4 months, but data are not available for verification. | Not estimable | 88 | Low3 | Resolution of CSF pleocytosis in all participants | |
| All adverse events | 464 per 1000 | 367 per 1000 | RR 0.79 (0.51 to 1.23) | 113 | Moderate4 | 48 adverse events in all participants randomized to study drug. 3 participants on ceftriaxone and 1 on doxycycline experienced serious adverse events (as defined by trial authors); RR 0.33 (95% CI 0.04 to 3.05). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Downgraded once for imprecision (small study). We did not downgrade the quality of evidence for indirectness as, although flawed, the measure is likely to reflect clinical reality. | ||||||
| Intravenous penicillin G compared to oral doxycyline for Lyme neuroborreliosis (acute and chronic) | ||||||
| Patient or population: Lyme neuroborreliosis (acute and chronic) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Oral doxycycline | Intravenous penicillin G | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Improvement of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 1000 per 1000 | 1000 per 1000 (920 to 1000) | RR 1.0 | 51 | Low2 | Investigators rating symptom composite on Likert scale from 1 to 3 (no, mild, moderate to severe)2 |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 900 per 1000 | 855 per 1000 (693 to 1000) | RR 0.95 | 51 | Low2 | Investigators rating symptom composite on Likert scale from 1 to 3 (no, mild, moderate to severe)2 |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | 1000 per 1000 | 930 per 1000 | RR 0.93 | 29 | Very low3 | |
| All adverse events | 129 per 1000 | 130 per 1000 | RR 1.01 | 54 | Very low4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Measured at 3, 6, and 12 months; reported here at 12 months. | ||||||
| Intravenous doxycycline compared to intravenous penicillin G for Lyme neuroborreliosis (acute and chronic) | ||||||
| Patient or population: Lyme neuroborreliosis (acute and chronic) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous penicillin G | Intravenous doxycycline | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Improvement of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 833 per 1000 | 817 per 1000 | RR 0.98 (0.80 to 1.21) | 75 (1 study) | Low2 | Clinical findings were classified as no remission, partial remission, or full remission. |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 694 per 1000 | 667 per 1000 | RR 0.96 (0.70 to 1.31) | 75 (1 study) | Low2 | Clinical findings were classified as no remission, partial remission, or full remission. |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | See comment | See comment | Not estimable | ‐ | ‐ | Measured but not reported in detail |
| All adverse events3 | See comment | See comment | Not estimable | 75 | ‐ | 'Adverse events' not reported. No serious adverse events occurred. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Measured at 6 and 12 months; 12‐month results reported here. | ||||||
| Intravenous cefotaxime compared to intravenous penicillin G for Lyme neuroborreliosis (acute) | ||||||
| Patient or population: Lyme neuroborreliosis (acute) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous penicillin G | Intravenous cefotaxime | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 800 per 1000 | 816 per 1000 (536 to 1000) | RR 1.02 | 21 | Low2 | Investigators' nonstandardized judgement of improvement or resolution of symptoms reported at 7.7 months. |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | 1000 per 1000 | 920 per 1000 | RR 0.92 | 21 | Very low3 | Follow‐up: mean 7.7 months |
| All adverse events | See comment | See comment | Not estimable | 21 | Low4 | No adverse events occurred. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Improvement in all participants at an average of 7.7 months. | ||||||
| Intravenous ceftriaxone compared to intravenous cefotaxime for Lyme neuroborreliosis (acute) | ||||||
| Patient or population: acute Lyme neuroborreliosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous cefotaxime | Intravenous ceftriaxone | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment | 600 per 1000 | 666 per 1000 (378 to 1000) | RR 1.11 (0.63 to 1.97) | 27 | Low1 | Outcome reported at a mean of 8.1 months' follow‐up. |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | 867 per 1000 | 988 per 1000 | RR 1.14 (0.90 to 1.44) | 27 | Very low2 | |
| All adverse events | 188 per 1000 | 71 per 1000 | RR 0.38 | 30 | Low3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Downgraded twice: for study limitations (unclear risk of selection bias and lack of blinding) and imprecision (small sample size). We did not downgrade the quality of evidence for indirectness as, although flawed, the measure is likely to reflect clinical reality. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptoms (patient‐rated VAS, scale 0 to 100, higher worse) in all participants (definite and possible Lyme disease) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 3 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [3.39, 5.01] |
| 1.2 6 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.38, 0.38] |
| 1.3 12 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.21, 1.41] |
| 2 Symptoms (investigator‐rated VAS, scale 0 to 100 higher worse) in all participants (definite and possible Lyme disease) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 3 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.28, 1.28] |
| 2.2 6 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐3.18, ‐1.62] |
| 2.3 12 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.13, 0.33] |
| 3 Improvement of symptoms (excellent or good on investigator VAS) (12 months) in participants with definite Lyme disease Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.21] |
| 4 Adverse events (12 months) in all participants (definite and possible Lyme disease) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Serious adverse events | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Diarrhea | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.70 [1.29, 10.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean reduction in clinical score (4 months) Show forest plot | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.20, 1.40] |
| 2 Resolution of symptoms Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.89, 2.35] |
| 3 All adverse events Show forest plot | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.23] |
| 4 Adverse events leading to discontinuation Show forest plot | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.71] |
| 5 Serious adverse events Show forest plot | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement of symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 3 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.93, 1.08] |
| 1.2 6 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.93, 1.08] |
| 1.3 12 months | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.92, 1.08] |
| 2 Resolution of symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 3 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.64, 1.61] |
| 2.2 6 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.10, 2.54] |
| 2.3 12 months | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.18] |
| 3 Resolution of CSF pleocytosis at 1 year Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.15] |
| 4 All adverse events Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.25, 4.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement of symptoms ("partial remission") Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.28] |
| 1.2 12 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 2 Resolution of symptoms ("full remission") Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.83, 2.42] |
| 2.2 12 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.31] |
| 3 Serious adverse events Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of symptoms (mean 7.7 months' follow‐up) Show forest plot | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.55] |
| 2 Resolution of CSF pleocytosis Show forest plot | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.18] |
| 3 All adverse events (at 2 weeks) Show forest plot | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of symptoms (mean 8.1 months' follow‐up) Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.63, 1.97] |
| 2 Resolution of CSF pleocytosis Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.90, 1.44] |
| 3 All adverse events Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.04, 3.26] |