Antibióticos para las complicaciones neurológicas de la enfermedad de Lyme
Resumen
Antecedentes
Diversos antibióticos que ingresan al sistema nervioso central son bactericidas in vitro e in vivo contra el agente causal de la neuroborreliosis de Lyme (NBL), Borrelia burgdorferi. Estos antibióticos son de uso clínico habitual para tratar la NBL, aunque no está clara su eficacia relativa.
Objetivos
Evaluar los efectos de los antibióticos para el tratamiento de la NBL.
Métodos de búsqueda
El 25 octubre 2016, se hicieron búsquedas en el registro especializado del Grupo Cochrane Neuromuscular (Cochrane Neuromuscular Specialised Register), en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE y en EMBASE. Se hicieron búsquedas en registros de ensayos clínicos el 26 de octubre de 2016. Se revisaron las bibliografías de los ensayos aleatorizados identificados y se estableció contacto con los autores y expertos conocidos en el área para identificar datos adicionales publicados o no publicados. No hubo restricciones de idioma en las búsquedas de estudios.
Criterios de selección
Ensayos clínicos aleatorizados sobre el tratamiento de la NBL con antibióticos en adultos y niños que compararon cualquier tratamiento con antibióticos, incluidas las combinaciones de tratamientos, versus otro tratamiento, placebo o ningún tratamiento. Se excluyeron los estudios de cuadros considerados como síndrome post Lyme.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por Cochrane.
Resultados principales
Se identificaron siete estudios aleatorizados que incorporaron a 450 pacientes europeos con NBL para inclusión en esta revisión sistemática. No se encontraron ensayos realizados en los Estados Unidos. La heterogeneidad significativa entre estos estudios impidió el metanálisis. Ninguno de los estudios incluyó un control placebo en el tratamiento inicial con antibióticos, y uno solo fue cegado. Ninguno de los estudios analizó el retraso del comienzo del tratamiento. Todos fueron estudios con comparador activo, y en la mayoría el poder estadístico no fue suficiente para la comparación de no inferioridad. En los ensayos se investigaron cuatro antibióticos: la penicilina G y la ceftriaxona en cuatro estudios, la doxiciclina en tres estudios y la cefotaxima en dos estudios. Un estudio analizó un ciclo de tres meses de amoxicilina oral versus placebo después del tratamiento inicial con ceftriaxona intravenosa. Un estudio se limitó a los niños. Los ensayos midieron la eficacia con el uso de resultados heterogéneos informados por el paciente o el médico, o ambos. En algunos casos, se incluyó el análisis del líquido cefalorraquídeo como biomarcador indirecto de la enfermedad y como resultado. Ninguno de los estudios informó el resultado primario propuesto de "Mejoría en una medida de discapacidad general a largo plazo (tres o más meses)". Ninguno de los ensayos mostró diferencias entre los grupos en cuanto a la resolución de los síntomas en respuesta al tratamiento activo. En general, el tratamiento fue bien tolerado. Sin embargo, la calidad del informe de los eventos adversos fue baja.
Conclusiones de los autores
En su mayoría, hay evidencia clínica de calidad baja a muy baja de un número limitado de ensayos principalmente pequeños y heterogéneos con medidas de resultado diversas, que compararon la eficacia relativa de los antibióticos que ingresan al sistema nervioso central para el tratamiento de la NBL. Los pocos estudios aleatorizados existentes tienen poder estadístico limitado y carecen de criterios de ingreso bien definidos y consistentes y de variables de evaluación de la eficacia. No es posible establecer conclusiones firmes sobre la eficacia relativa de los regímenes antibióticos aceptados para el tratamiento de la NBL. Se informa que la mayoría de los pacientes tiene buenos resultados, y los síntomas se resuelven en 12 meses, independientemente del antibiótico utilizado. Una minoría de pacientes no mostró un grado suficiente de mejoría, y algunos fueron tratados nuevamente. Estos estudios aleatorizados aportan alguna evidencia de que la doxiciclina, la penicilina G, la ceftriaxona y la cefotaxima son eficaces para el tratamiento de la NBL europea. No se hubo evidencia de una eficacia adicional cuando, en un estudio, un tratamiento inicial con ceftriaxona intravenosa fue seguido de un tratamiento adicional más prolongado con amoxicilina oral. A través de la estrategia de búsqueda de alta calidad no fue posible identificar evidencia sobre la eficacia de los antibióticos para el tratamiento de la NBL en los Estados Unidos.
PICO
Resumen en términos sencillos
Tratamiento para las complicaciones neurológicas de la enfermedad de Lyme
Pregunta de la revisión
¿Los antibióticos son efectivos para el tratamiento de la enfermedad de Lyme que compromete el sistema nervioso?
Antecedentes
En los seres humanos, la enfermedad de Lyme es causada por una bacteria llamada Borrelia burgdorferi. Las personas contraen la infección por picadura de garrapatas que transportan la bacteria. Las personas pueden presentar síntomas en las articulaciones, la piel, los músculos, y el sistema nervioso (nervios periféricos [los nervios que se encuentran por fuera del cerebro y la médula espinal], el cerebro y la médula espinal). Sin tratamiento con antibióticos, la enfermedad de Lyme neurológica se puede superar o causar problemas a largo plazo. Hay diferencias en la enfermedad de Lyme neurológica entre Europa y los Estados Unidos, probablemente debido a diferencias en la B. burgdorferi. Existe información limitada acerca de qué antibióticos son mejores para el tratamiento de la enfermedad de Lyme neurológica.
Características de los estudios
Se encontraron siete ensayos que analizaron tratamientos con antibióticos para la enfermedad de Lyme neurológica. Excepto uno, todos los ensayos compararon diferentes antibióticos. Otro ensayo comparó los efectos del tratamiento con amoxicilina oral versus placebo después del tratamiento inicial con ceftriaxona. Los ensayos incluyeron a 450 europeos. Los antibióticos estudiados fueron la penicilina G, la doxiciclina, la ceftriaxona y la cefotaxima. Uno de los ensayos incluyó a niños solamente, mientras que los otros incluyeron principalmente adultos. Sólo se seleccionaron los estudios en los que la asignación al tratamiento se determinó al azar, debido a que estos estudios aportan la mejor información para comparar los efectos de diferentes tratamientos. No hubo cegamiento en la mayoría de los estudios (lo que significa que los pacientes y el personal del estudio sabían el tratamiento que se administraba). No fue posible encontrar estudios de tratamientos con antibióticos para la enfermedad de Lyme neurológica realizados en Estados Unidos. Ningún estudio evaluó los efectos de retrasar el inicio del tratamiento.
Resultados clave y calidad de la evidencia
La diferencia entre los siete estudios fue demasiado grande como para combinar los resultados, por lo que se analizaron de forma individual.
Ninguno de los estudios aportó evidencia clara de que un antibiótico fuese mejor que otro. Un estudio no logró encontrar evidencia de que un segundo tratamiento más prolongado con un antibiótico oral (amoxicilina) brindara un efecto beneficioso adicional después del tratamiento intravenoso con ceftriaxona. Se desconoce el efecto beneficioso adicional del tratamiento con antibióticos sobre la recuperación natural ya que ninguno de los otros estudios utilizó un tratamiento inactivo (placebo). En general, el tratamiento fue bien tolerado, aunque la calidad del informe de los eventos adversos en la mayoría de los estudios pareció ser baja.
Los resultados indican que el tratamiento con cualquiera de los cuatro antibióticos produjo resultados igualmente buenos en el tratamiento de la enfermedad neurológica de Lyme en Europa. Un segundo tratamiento con amoxicilina no parece proporcionar un efecto beneficioso agregado a la ceftriaxona. No se encontraron ensayos de antibióticos para el tratamiento de la enfermedad de Lyme neurológica realizados en los Estados Unidos.
La evidencia está actualizada hasta octubre de 2016.
Authors' conclusions
Background
Description of the condition
Lyme neuroborreliosis (LNB) is a group of diseases that can affect the central nervous system (CNS) and the peripheral nervous system (PNS), or both, as a result of infection with or the postinfectious consequences of different species of the spirochete bacterium Borrelia burgdorferi. These organisms are transmitted by ixodid ticks in endemic areas in the United States and Europe. Although a multitude of clinical manifestations of LNB have been reported, the most common are radicular pains, facial paralysis, and meningitis, referred to as Bannwarth's syndrome in Europe (Bannwarth 1941; Bannwarth 1944). It was not until 1981 that entomologist Willy Burgdorfer and colleagues in the United States suspected that the cause of Lyme disease was a tick‐borne spirochete (Burgdorfer 1982). In the decades since the identification of B. burgdorferi, it has become clear that LNB is one of the most common and important complications of Lyme disease. The diagnosis of LNB requires confirmation of infection with B. burgdorferi plus evidence of involvement of the CNS, the PNS, or both. According to the Centers for Disease Control and Prevention, from the 154,405 cases of Lyme disease reported during 2001 to 2010 in the United States, 14% were identified with facial palsy, radiculoneuropathy, meningitis, or encephalitis (CDC 2011a). Looking at Lyme disease occurring in Europe, others have estimated that up to 12% of cases have neurological manifestations (Koedel 2015), and that approximately 5% of individuals with an untreated erythema migrans will develop LNB (Hansen 2013).
Knowledge of the natural course and prognosis of untreated LNB is limited, and both increases in severity of disease and spontaneous remissions may occur. A random review of medical records from the original Lyme disease investigation among people from Connecticut, United States, who were not treated with antibiotics because they were diagnosed before the infectious cause of the disease was known, revealed that when left untreated, LNB can result in long‐term sequelae (Kalish 2001). In this report, 31 people who had presented with facial palsy and meningism frequently went on to develop more disseminated manifestations of LNB, with two‐thirds being formally diagnosed with lymphocytic meningitis, radiculoneuritis, or both, and 1 in 5 developing atrioventricular block. In a German retrospective study of 72 people with untreated LNB, only 59 went into full remission, whereas 13 developed mild‐to‐moderate sequelae during 5 to 27 years of follow‐up. Importantly, all those participants were eventually judged as “having been cured without antibiotics” (Kruger 1989).
The incidence of LNB varies widely among European countries, with the highest incidences in central European and Scandinavian countries. Population‐based annual incidence rates of LNB in central Europe are 30 to 50 per million for acute LNB and less than 0.4 per million for chronic LNB (Hansen 2013).
Description of the intervention
People with the characteristic skin lesion of Lyme disease, erythema migrans, followed by manifestations of infection of the nervous system, referred to as LNB, were successfully treated with antibiotics (penicillin) as early as 1948 (Hollstrom 1951). Treatment with antibiotics capable of crossing the blood‐brain barrier is now the standard of care for people diagnosed with LNB. However, no placebo‐controlled trials have ever been performed, and the antibiotic of choice, route of administration, dose, and length of treatment for LNB remain controversial.
Why it is important to do this review
At the time that the protocol for this review was conceived, there had been several attempts at producing treatment guidelines for Lyme disease and no high‐quality systematic evidence reviews to synthesize the available evidence to feed those reviews. Guidelines have now been produced by the American Academy of Neurology (AAN) (Practice Parameter) (Halperin 2007), the European Federation of Neurological Societies (EFNS) (Mygland 2010), the German Neurological Society (Rauer 2012), and the International Lyme and Associated Diseases Society (ILADS) (Cameron 2014). American Academy of Neurology/American College of Rheumatology/Infectious Diseases Society of America as well as the German Neurological Society are currently working on updated guidelines. This review will continue to synthesize the evidence for the antibiotic treatment of LNB.
Objectives
To assess the effects of antibiotics for the treatment of Lyme neuroborreliosis.
Methods
Criteria for considering studies for this review
Types of studies
The review authors only considered quasi‐randomized and randomized, prospective, controlled trials of antibiotic treatment for Lyme neuroborreliosis (LNB) for inclusion in this review. We excluded non‐randomized and uncontrolled studies. We also excluded single‐case reports and case series.
Types of participants
We considered trials that evaluated individuals with clinically diagnosed LNB. The clinical syndrome of LNB included one or more of the following: meningitis, encephalitis, myelitis, radiculitis, cranial neuropathies (including facial nerve palsy or ocular motor palsy, or both), optic neuritis, peripheral neuropathies, and myopathies. We excluded studies of people with post‐Lyme disease syndrome, defined as people with persistent symptoms attributed to Lyme disease in the absence of ongoing infection following prior antibiotic treatment. We also excluded trials of LNB prevention through treatment of erythema migrans.
Diagnoses of LNB are supported by a number of approaches, including positive serologic testing (Stiernstedt 1988; Dressler 1993; Anonymous 1995; Engstrom 1995; Wilske 2000). Cerebrospinal fluid (CSF) culture of B. burgdorferi was also acceptable as supportive evidence of LNB, as well as a positive CSF Lyme polymerase chain reaction (PCR), the presence of CSF anti‐B. burgdorferi antibodies, Steere 1990, or CSF pleocytosis, or both (Halperin 1996). Peripheral neuropathy and myopathy required clinically detectable impairment of motor or sensory function confirmed by abnormal electrophysiological tests.
Types of interventions
We considered any antibiotic treatment, including combinations of treatments, versus any other treatment, placebo, or no treatment.
Types of outcome measures
When designing the review we prespecified several primary and secondary outcome measures to assess the efficacy of antibiotics for treatment of LNB, as follows.
Primary outcomes
-
Improvement in a measure of overall disability in the long term (three or more months) following treatment.
-
Improvement or resolution of the person's presenting neurological deficits in the long term (three or more months) following treatment. In general, we considered improvement as determined and defined by the original authors, provided that they included objective findings as criteria for the outcome.
Secondary outcomes
-
Improvement in a measure of overall disability in the short term (two weeks) following treatment.
-
Resolution of CSF pleocytosis following treatment.
-
For people with peripheral neuropathy or myopathy, improvement in electrophysiological abnormalities following treatment.
-
Occurrence of one or more adverse events. We considered serious adverse events, defined as those which required hospitalization or that were life‐threatening or fatal, adverse events requiring discontinuation of treatment or substitution of alternative treatment, and any other adverse events as defined and reported by the original authors.
If cost and cost‐effectiveness information was available, we planned to include it as part of the effects of the intervention analysis.
Search methods for identification of studies
Electronic searches
On 25 October 2016, we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Register of Studies Online), MEDLINE (1966 to October 2016), and Embase (1980 to October 2016). On 26 October 2016, we also searched trials registers: US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov, the EU Clinical Trials Register (www.clinicaltrialsregister.eu), the World Health Organization Clinical Trials Registry Platform (www.who.int/ictrp/en/), and ISRCTN Registry (www.isrctn.com/).
The detailed search strategies are in the appendices: MEDLINE (Appendix 1), Embase (Appendix 2), CENTRAL (Appendix 3), Cochrane Neuromuscular Specialised Register (Appendix 4), and trials registers (Appendix 5).
Searching other resources
We reviewed the bibliographies of the randomized trials identified, and contacted authors and known experts in the field to identify additional published or unpublished data. We handsearched conference proceedings for additional trials. We applied no language restriction when searching for studies.
Data collection and analysis
Selection of studies
All review authors checked titles and abstracts identified from the searches to determine which studies met the eligibility criteria. When the review authors could not determine eligibility from the title and abstract, they obtained the full text of all potentially relevant studies for independent assessment. Two review authors independently assessed and decided which of the trials identified from the preliminary searches fitted the inclusion and exclusion criteria and graded the risk of bias of the trials. The review authors resolved disagreements about study inclusion by consensus. Two systematic review specialists conducted a duplicate study selection process. The review authors assessed any discrepancies in comparison with their selection.
Data extraction and management
Two review authors independently extracted data from all studies that met the inclusion and exclusion criteria onto a specially designed data extraction form. One of the review authors entered data into the Cochrane Review Manager 5 software (RevMan 2014), and a second review author checked the data extraction. In the case of missing data, the review authors attempted to contact the trial authors. Review authors were not blinded to trial authors, journal, or institution. To assist the review authors, two systematic review specialists conducted an independent data extraction. The Cochrane Neuromuscular Managing Editor created analysis tables and added numerical data to the Results using this data extraction. A review author checked the outcome data entry.
Assessment of risk of bias in included studies
Two review authors independently assessed all of the included studies for risk of bias. In the event of disagreement, all of the review authors achieved consensus through discussion. We used the Cochrane 'Risk of bias' tool to assess risk of bias of the included studies (Higgins 2011). This tool applies the following criteria: random sequence generation; concealment of allocation; blinding of participants and personnel; blinding of outcome assessors; incomplete outcome data (numbers of participants lost to follow‐up and use of intention‐to‐treat analysis); selective reporting; and other sources of bias, such as baseline differences in study populations (other than imbalances caused by inadequate randomization, lack of allocation concealment, or exclusion of participants, which we considered under other criteria). We assessed all included studies under each criterion as at high, low, or unclear risk of bias (we used 'unclear' when there was insufficient information to permit judgement or when what occurred in the study was known but the implications in terms of bias were unclear). To assist the review authors, two systematic review specialists provided by Cochrane conducted an independent 'Risk of bias' assessment, and the review authors addressed any discrepancies in assessments.
Measures of treatment effect
We used Review Manager 5 to calculate risk ratios with 95% confidence intervals for dichotomous outcomes. For continuous data, we reported the mean difference and corresponding 95% confidence intervals. Missing standard deviations were calculated from confidence interval using the Review Manager 5 calculator tool during data entry.
The review authors originally planned to divide the analysis according to whether the LNB studies were North American or European, since infecting strains of Borrelia species as well as clinical manifestations of LNB may differ between the two regions. If the division between European and North American studies did not reveal important differences, we planned to pool all studies. However, we did not identify any eligible study from North America; the review analyses are therefore based only on available European studies until studies from other world areas become available.
Assessment of heterogeneity
Significant heterogeneity of inclusion and exclusion criteria and primary and secondary outcome measures was evident by simple examination. To illustrate this heterogeneity, we have presented a detailed comparison of the study characteristics in Table 1.
| Study | Population | Length of follow‐up | Interventions | Antibiotic naïve | Clinical remission measurement | Time to remission | Patient‐reported outcomes | CSF remission | Serology response |
| Yes | Up to 3 years | Penicillin Doxycycline | Unknown | Complete/partial/no | No | No | Yes | No | |
| Yes | Average of 7 months | Penicillin Cefotaxime | Unknown | Yes/no | No | Yes: VAS, 0 to 10 | Yes | No | |
| Yes (children only) | >6 months, <12 months | Penicillin Ceftriaxone | Yes | Yes/no | Yes; from treatment onset to complete remission | No | No | Yes | |
| Yes | Average of 7.7 months | Ceftriaxone Cefotaxime | Unknown | Yes/no | No | No | Yes | No | |
| Yes | 12 months | Penicillin Doxycycline | Past 4 weeks | Yes/no; by specific sign/symptom | No | Yes: Likert‐like scale, 0 to 3 | Yes, at 2 weeks and 12 months | Yes | |
| No (but large subgroup of LNB enrolled) | 12 months | Ceftriaxone followed by amoxicillin or placebo | Past 1 month | Physician VAS 0 to 100; scored as excellent/good, poor/none, controversial | No | Yes: VAS | Lumbar puncture and measurement of CSF antibody levels and PCR for Borrelia burgdorferi was repeated in selected cases during or after treatment. | Yes: scored as strong decline, mild, none | |
| Yes | Up to 4 months | Ceftriaxone Doxycycline | Past 14 days | No/mild/more than mild; also change in baseline deficits in past 3 months using own composite clinical score | No | Yes: 6 items, each scored 0 to 2 | Yes | No |
CSF: cerebrospinal fluid
LNB: Lyme neuroborreliosis
PCR: polymerase chain reaction
VAS: visual analogue scale
Data synthesis
We performed a narrative review of the evidence. In Appendix 6 we provide additional methods relating to the originally planned meta‐analysis described in the protocol (Cadavid 2008).
Sensitivity analysis
We did not perform any formal sensitivity analysis because of the small sample size and heterogeneity among the studies.
'Summary of findings' table
We included a 'Summary of findings' table according to recommendations in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to determine the quality of the body of evidence for each outcome, and to draw conclusions about the quality of evidence within the text of the review. We used footnotes to justify any decision to downgrade the quality of evidence. We reported the following outcomes whether or not they were measured or reported in the included studies.
-
Improvement in a measure of overall disability in the long term (three or more months) following treatment.
-
Improvement or resolution of the person's presenting neurological deficits in the long term (three or more months) following treatment.
-
Improvement in a measure of overall disability in the short term (two weeks) following treatment.
-
Resolution of CSF pleocytosis following treatment.
-
All adverse events.
We presented outcome number two as one outcome, or two if the included studies reported improvement and resolution separately. We created 'Summary of findings' tables for comparisons unless very little data were available for a comparison. We chose to report adverse events in the 'Summary of findings' table as 'All adverse events' with a comment on serious adverse events, as the data were inconsistently reported in the included studies.
Results
Description of studies
Results of the search
The search strategies listed in the appendices identified 34 studies from the Cochrane Neuromuscular Specialised Register, 35 from CENTRAL, 75 from MEDLINE, and 116 from Embase. The number of references found in each database, the number of studies remaining after removal of duplicates, the number of studies selected for further review, and the number of studies meeting the inclusion criteria are shown in Figure 1. We identified seven trials eligible for inclusion in the review. We identified one ongoing study (NCT02553473).

Study flow diagram.
Included studies
Seven studies fulfilled our predefined inclusion criteria. Table 1 shows an overview of the included studies. All were randomized studies of participants with LNB comparing initial treatment with two different antibiotics, except for one that compared treatment with a second antibiotic to placebo following initial antibiotic treatment. One study only enrolled children. Table 2 shows details of clinical and laboratory eligibility criteria supporting the diagnosis of LNB in the seven studies; all were consistent with current case definitions for LNB (CDC 2011b). Table 3 shows baseline demographics and laboratory findings for enrolled participants by treatment group. The evaluation period in all but one study was a year or less. There was marked heterogeneity in the efficacy assessments used. Baseline characteristics among the seven studies varied widely; disease duration prior to treatment ranged from as short as five days to as long as several months. The number of study participants varied from 22 to 145, with only two studies enrolling over 100 participants. Reporting of concomitant treatment with corticosteroids was incomplete in most studies.
| Criteria | (N = 75) | (N = 21) | (N = 23) | (N = 30) | (N = 54) | (N = 145; 72% to 75% definite, 25% to 27% possiblea) | (N = 102) |
| Clinical | Radicular pain, meningitic symptoms, cranial neuritis, sensory and/or motor radiculitis, arthritis, carditis, myelitis or peripheral neuritis, tick bite and/or erythema migrans | Radicular pain (15/21), headache (2/21), facial palsy (8/21), unilateral VI palsy (1/21), lower limb muscle weakness (9/21), sensory disturbance (12/21) | Presence of neurological signs and symptoms indicative of LNB | Radiculopathy (motor or sensory, or both), cranial neuropathy (facial palsy, ocular motor) | Headache (71% to 74%), subjective stiff neck (65%), paresis (55% to 57%) including facial palsy in 35% to 43% | Lymphocytic meningitis without radiculitis in 18 (all definite), meningoradiculitis (16 definite) or radiculitis (11 definite), paresis in 5, encephalomyelitis in 4, encephalopathy in 6, facial paresis in 21, sudden deafness in 6, tinnitus in 8, other cranial nerve involvement in 13, peripheral neuritis in 6, and other peripheral nervous system manifestations (9 peripheral mononeuropathy or polyneuropathy, 15 paresthesia, 39 with headache without meningitis, 29 with dizziness or vertigo, and 11 with memory impairment) | 25% to 33% Bannwarth's syndrome, 19% to 22% facial palsy, 24% to 38% radiculopathy, various others (other cranial neuropathies, ataxia, myelopathy, limb paresis, paresthesias, cognitive deficits) |
| Laboratory | B. burgdorferi‐specific antibody titer in serum, B. burgdorferi‐specific antibody titer in CSF, lymphocytic pleocytosis, elevated CSF protein (>50 mg/dL), elevated CSF IgM‐, IgA‐, and/or IgG‐index, oligoclonal bands in CSF. Only group‐level information is given. | Elevated B. burgdorferi‐specific IgG and IgM antibody titers in serum (1:64 to 1:512): found in 11, of whom 4 had both elevated IgG and IgM, 6 had only elevated IgG, and 1 had only elevated IgM. 4 were seropositive in CSF but not in blood; 6 had negative serology in both serum and CSF (seronegative LNB), but 4 had EM and 2 a history of insect bites. | 1 or more of the following specific CSF laboratory parameters: elevated B. burgdorferi‐specific IgG antibody titer, intrathecally produced B. burgdorferi‐specific antibodies, and/or direct cultivation of B. burgdorferi from the CSF in a modified Barbour‐Stoenner‐Kelly medium | Elevated B. burgdorferi‐specific IgG and IgM antibody titers in serum: found in 22 and 8, respectively. 13 had positive B. burgdorferi‐specific CSF/serum antibody index. | Elevated B. burgdorferi‐specific IgM or IgG concentration, or both in 83% to 90%; all had positive serology or B. burgdorferi‐specific CSF antibodies, except for 1 participant who had a positive CSF culture. | Only 3 of the 145 study participants were seronegative. Presence of inflammatory changes in the CSF or intrathecal antibodies against B. burgdorferi, or both supported a diagnosis of definite LNB; 124/145 participants had lumbar puncture performed at diagnosis. | Intrathecal production of B. burgdorferi‐specific antibodies or B. burgdorferi‐specific antibodies in serum, or both were required for enrollment. |
aLNB considered possible if clinical presentation was an uncommon manifestation, but serum antibodies against Borrelia burgdorferi were positive and other causes were excluded.
Abbreviations:
B. burgdorferi: Borrelia burgdorferi
CSF: cerebrospinal fluid
EM: erythema migrans
Ig: immunoglobulin
LNB: Lyme neuroborreliosis
| Study | Kohlhepp 1989 Penicillin | Kohlhepp 1989 Doxycycline | Pfister 1989 Penicillin G | Pfister 1989 Cefotaxime | Pfister 1991 Ceftriaxone | Pfister 1991 Cefotaxime | Mullegger 1991 Penicillin G | Mullegger 1991 Ceftriaxone | Penicillin G | Karlsson 1994 Doxycycline | Oksi 2007 Amoxicillin post‐ceftriaxone | Oksi 2007 Placebo post‐ceftriaxone | Ljostad 2008 Doxycycline | Ljostad 2008 Ceftriaxone |
| Number of participants (evaluable) | 36 | 39 | 10 | 11 | 14 | 16 | 11 | 12 | 23 | 31 | 73 | 72 | 54 | 48 |
| Age mean (SD) unless specified | Men 55 (12.6); women 54.1 (16.3) | Men 49.6 (14); women 55.7 (14.3) | 56.7 (15) | 55.4 (10.8) | 58.7 (19.5) | 53.7 (16.8) | 8.1 (3.1) | Median 55 (range 16 to 88) | Median 49 (range 18 to 74) | Mean 52.3, range 19 to 87 | Mean 50.5, range 16 to 80 | 54 (13) | 52 (13) | |
| Percentage males | 44% | 51% | 50% | 64% | 64% | 44% | 36% | 42% | 44% | 29% | 48% | 50% | 52% | 65% |
| History of erythema migrans | 36% | 31% | 80% | 45% | 50% | 56% | Not reported | 61% | 42% | 26% (probable) | 31% | 10% | ||
| Mean (SD) time from onset of LNB to treatment | 5.2 (13.6) months | 4.1 (11.1) months | 28.7 (33.8) days | 23.5 (16.3) days | 64.5 (84.7) days | 38.6 (23.1) days | All included children were admitted to the hospital within 5 ± 1.8 days from onset of symptoms. | 3.5 weeks (1 week to 25 months) | 4 weeks (1 week to 18 months) | Unknown | 10 (19) weeks | 8 (13) weeks | ||
| Previous treatment with antibiotics | 11% | 8% | Not reported | Not reported | Not reported (mentioned for 1 participant) | Was an exclusion criteria | None for the 4 weeks prior to enrollment | Yes, in all participants with EM, 24 adequately and 14 not adequately | Treatment with cephalosporin, penicillin, tetracycline in past 14 days exclusion criterion | |||||
| Concomitant treatment with steroids | 28% | 26% | None | Not reported | Not reported | Not reported | Not reported | Not reported | ||||||
| CSF leukocytes mean (SD) cells/uL | 186 (75) | 145 (58) | 280.9 (212) | 435.7 (528) | 86.4 (128.4) | 135.3 (299.2) | Not reported | Median 96, range 6 to 1190 | Median 117, range 8 to 910 | 59% with available CSF showed lymphocytic pleocytosis. | 194 (237) | 178 (187) | ||
| CSF total protein mean (SD) in mg/dL | 133 (110) | 119 (112) | 115 (69) | 136 (67.4) | 72.7 (42) | 79.1 (48.4) | Not reported | Median 110, range 40 to 360 | Median 120, range 50 to 580 | Not reported | 120 (70) | 130 (80) | ||
| Presence of CSF oligoclonal bands | 78% | 62% | 70% | 64% | 64% | Not reported | Not reported | Not reported | Detected in 24/58 (41%) participants with definite LNB and CSF examined | Not reported | ||||
CSF: cerebrospinal fluid
EM: erythema migrans
LNB: Lyme neuroborreliosis
SD: standard deviation
See Characteristics of included studies.
Physician‐reported measures of efficacy
In all but one of the included trials, investigators reported neurologic findings on clinical examination, allowing for objective clinical evaluation of efficacy. Physician tools for quantifying efficacy as primary or secondary outcome varied between trials. In the two largest trials, final grading by the treating physician combined objective clinical examination and patient report of subjective symptoms (Oksi 2007; Ljostad 2008). Only the trial by Karlsson 1994 did not report a physician‐based judgement.
Patient‐reported measures of efficacy
Studies largely used clinical evaluations, which often depended on unspecified symptoms to judge cure, improvement, or failure. These symptoms were typically not systematically tracked. Some studies included people with LNB who were only a subset of participants with disseminated Lyme disease. Subjective patient outcome measures were not usually outlined rigorously but were included within global clinical assessments, or at least inferred. One study, Karlsson 1994, did track specific individual complaints, such as fatigue, during follow‐up time points. Ljostad 2008 incorporated subjective symptoms into a clinical composite score, but with a minority weighting compared to objective findings. Oksi 2007 directly incorporated subjective assessments by participants and independent assessments by physicians in a visual analogue scale to assess the success or failure of antibiotic therapy. Pfister 1989 individually tracked radicular pain in all participants.
Table 4 shows an overview of the clinical efficacy assessments used in each study and whether any treatment difference was reported.
| Study | Tool | Signs assessed | Subjective symptoms elicited |
| Visual analogue scale | Yes | Possibly | |
| Composite clinical score | Yes | Yes | |
| Change of clinical symptoms | Yes | No | |
| Disease duration | Yes | No | |
| Change of clinical symptoms (3‐level classification) | Yes | Unclear | |
| Change of clinical symptoms | Yes | Yes | |
| Change of clinical symptoms | Yes | Unclear | |
| Change of clinical symptoms | Yes | No |
*The efficacy of interventions was quantified by diverse tools in each study assessing the change in objective findings (signs) or subjective complaints (symptoms), or both, as reported by participants or judged by the study physician.
CSF parameters
Most trials measured CSF parameters, although there was significant heterogeneity in the percentage of participants whose CSF was examined, the timing of the CSF sampling, and the parameters that were reported, as well as the specific interventions compared (Table 5). The CSF parameter initially prespecified as an outcome measure for this review was resolution of CSF pleocytosis at three or more months following treatment. Of the included trials, Ljostad 2008, Karlsson 1994, Pfister 1989, and Pfister 1991 provided meaningful information on this prespecified parameter. No data were available to determine the effect of any of the antibiotics versus placebo on CSF parameters, except for retreatment with amoxicillin in Oksi 2007. Only two studies looked at the ability to achieve minimum inhibitory concentrations of antibiotics in the CSF.
| Study | Parameter |
| Decrease of B. burgdorferi‐specific antibody concentrations at 12 months of at least 20% ("moderate decline") or 50% ("strong decline") | |
| Resolution of CSF pleocytosis | |
| Cell count, protein, antibody index, B. burgdorferi‐specific antibody production | |
| Abnormal CSF on repeated lumbar puncture1 | |
| Abnormal CSF on repeated lumbar puncture2 | |
| Cell count, B. burgdorferi‐specific antibody production | |
| Changes in intrathecally produced specific antibodies against B. burgdorferi |
1One or more of lymphocytic pleocytosis, protein elevation, oligoclonal bands, B. burgdorferi‐specific antibody production.
2One or more of lymphocytic pleocytosis, protein elevation, oligoclonal bands, culture positive for B. burgdorferi.
Abbreviations:
B. burgdorferi: Borrelia burgdorferi
CSF: cerebrospinal fluid
Excluded studies
The Characteristics of excluded studies section refers to relevant randomized studies that were not included in the review and the reasons for their exclusion. We excluded a total of seven studies, one because it tested a non‐antibiotic intervention and six that did not specifically address the LNB population.
Risk of bias in included studies
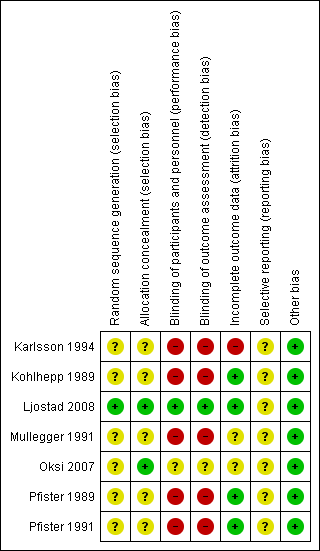
Figure 2 summarizes the review authors' judgements for each 'Risk of bias' domain in the seven included studies.
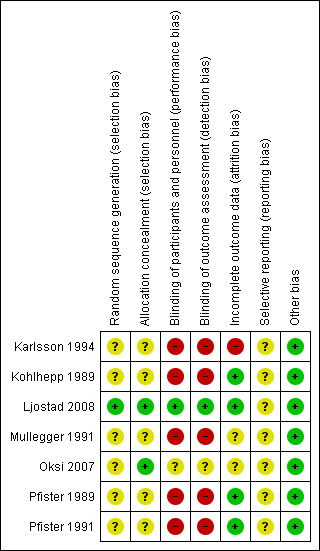
With the exception of Ljostad 2008 and Oksi 2007 (amoxicillin phase only), all trials were unblinded.
All but one of the studies were at an overall high risk of bias (i.e. at high risk of bias in at least one domain). Only the study by Ljostad 2008 was at low risk of bias, although as no protocol was available, we judged the risk of selective reporting unclear. The risk of bias in Oksi 2007 was mostly unclear, as the information provided on the subgroup of participants with LNB was quite limited. The remaining five included studies were at high risk of bias due to lack of blinding of study participants and outcome assessments. All studies suffered from poor or incomplete reporting in at least one domain, which prevented an adequate assessment of the risk of bias affecting in particular random sequence generation, allocation concealment, and selective reporting in all but Ljostad 2008.
Allocation
Ljostad 2008 was the only study at low risk of selection bias.
Blinding
Ljostad 2008 and Oksi 2007 were the only two studies at low risk of performance and detection bias.
Incomplete outcome data
This domain had the lowest general risk of bias; only Karlsson 1994 was judged to have incomplete outcome data.
Selective reporting
We could not evaluate the risk of bias for this domain for any of the included studies, as we did not have final study protocols or statistical analyses plans, or both for any of them.
Other potential sources of bias
As the reporting of efficacy and safety followed the standard of medical care in the various countries, and none of the studies was identified as having potential financial or other conflicts of interest, we did not consider any of the seven included studies to have other potential sources of bias.
Effects of interventions
Oral antibiotic therapy with amoxicillin versus placebo after previous treatment with 21 days of ceftriaxone for disseminated LNB
Studied in Oksi 2007. See Table 6.
| Oral amoxicillin versus placebo for people previously treated with ceftriaxone for Lyme neuroborreliosis (acute and late) | ||||||
| Patient or population: people previously treated with ceftriaxone for disseminated Lyme neuroborreliosis (acute and chronic)1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Placebo | Oral amoxicillin | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Improvement or resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment2 | Separate information on the LNB subgroup (N = 62), but not for intervention groups within this subgroup was provided at the review authors' request. 59/62 participants were classified as experiencing improvement of presenting neurological deficits at month 12 (dichotomous assessment: 'excellent or good' based on investigator VAS values and medical record information).3 | Not estimable | 624 | Low5 | ||
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment4 | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| All adverse events ‐ 12 months | 24 adverse events for all 145 Lyme disease participants, mostly diarrhea and fever with no need for discontinuation. No serious adverse events reported. Attribution of adverse events to either pretreatment with ceftriaxone, or to amoxicillin or placebo, or both, is unclear. | Not estimable | 145 | Very low6 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
1Subpopulation with LNB (N = 62) within study of people with definite or possible disseminated Lyme borreliosis (N = 145).
2Month 12: dichotomous outcome: excellent or good, based on investigator VAS values and medical record information.
3No transparency on the influence of subjective symptoms on investigator VAS values, no standardization of inclusion of medical record information on LNB subgroup (N = 62). No transparency of date in publication. Only in the larger center did the same physician rate participants during the whole study.
4Trialists reported no statistically significant differences in VAS values between amoxicillin and placebo groups at 0, 1, 3, 6, and 12 months without providing numerical data for analysis.
5Downgraded twice: for study limitations (unclear risk of bias for all domains) and imprecision (small study size). We did not downgrade the quality of evidence for indirectness as, although flawed, the measure is likely to reflect clinical reality.
6Downgraded three times: twice for study limitations (lack of blinding and adverse events not ascribed to interventions) and once for indirectness (participants not limited to those with LNB; separate data not available for the LNB subgroup of 62 participants). In the absence of comprehensive adverse event reporting in the included trials, the table presents ‘all adverse events’ with a comment on severe adverse events when these data are presented in the trial.
This was the only study to compare amoxicillin treatment with placebo after a three‐week course of ceftriaxone. The study report did not distinguish between participants with disseminated Lyme disease and those with LNB. We contacted the trial authors in an effort to obtain separate information on the LNB group and they supplied the supplementary information.
This study provides low‐ or very low‐quality evidence. Despite author transparency, the study remains at high risk of bias overall (unclear risk of bias in most domains), and data for amoxicillin and placebo groups were not reported separately.
Improvement in a measure of overall disability in the long term (three or more months)
Not reported.
Improvement or resolution of the person's presenting neurological deficits in the long term (three or more months)
Oksi 2007 investigated whether 100 days of oral antibiotic therapy with amoxicillin provided any additional benefit after previous treatment with 21 days of ceftriaxone for disseminated Lyme disease. Investigators enrolled 152 participants with definite (74%) or possible (26%) Lyme disease, of whom five were withdrawn due to discontinuation of the study drug and two due to diagnosis other than disseminated Lyme disease. Sixty‐two of those enrolled had LNB (Jarmo Oksi, supplementary information). Trialists measured objective efficacy using an investigator‐rated visual analogue scale (VAS) that ranged from 0 to 100, administered at baseline (prior to randomization), after completion of ceftriaxone treatment, and at 1, 3, 6, and 12 months thereafter. A value of 50 was attributed to average baseline severity, 0 for full remission of symptoms, and 100 for a certain poor outcome. A similar VAS completed by participants was used for assessment of efficacy. Only in the largest center were participants rated by the same physician throughout the whole study. The degree to which subjective symptoms influenced the investigator VAS judgement was not documented. Also, non‐neurologists judged severity of symptoms at some sites. Furthermore, participants with definite and possible LNB were combined. Participants in the definite LNB group had objective signs, making remission of objective neurological signs a more relevant criterion for efficacy assessment in this subgroup. For the disease severity rating, the outcome was categorized as “excellent or good,” “controversial,” or “poor,” using VAS values plus information obtained from participant medical records, but without any standardization.
In participants with definite Lyme disease, the outcome was excellent or good in 49 (92.5%) amoxicillin‐treated participants and 47 (87%) placebo‐treated participants; the outcome was poor in 3 (5.7%) amoxicillin‐treated and 6 (11.1%) placebo‐treated participants. In participants with possible Lyme disease treated with amoxicillin, the outcome was excellent or good in 11 (55%) participants and poor in 4 participants (20%); in the placebo group with possible Lyme disease, the outcome was excellent or good in 8 participants (44.4%) and poor in 4 participants (22.2%). In the whole group of 145 participants, the mean differences (MDs) in patient‐reported VAS at 3, 6, and 12 months for amoxicillin versus placebo were 4.20 (95% confidence interval (CI) 3.39 to 5.01), ‐0.50 (95% CI ‐1.38 to 0.38), and 0.60 (95% CI ‐0.21 to 1.41). The corresponding MDs for investigator‐rated VAS at 3, 6, and 12 months were 0.50 (95% CI ‐0.28 to 1.28), ‐2.40 (95% CI ‐3.18 to ‐1.62), and ‐0.40 (95% CI ‐1.13 to 0.33) (Analysis 1.1). Comparison of all VAS values did not differ significantly between participant and investigator assessments, including the subset of participants with definite LNB (62 of the total 145 participants). The results showed no clinically important differences between amoxicillin and placebo groups on the participant or investigator VAS scores in either the whole group (Analysis 1.2), or the definite Lyme disease subgroup. The report does not provide separate results for the possible LNB subgroup. The trialists reported that there were no statistically significant differences in the definite LNB subgroup between amoxicillin and placebo groups in mean patient or investigator VAS scores at 0, 1, 3, 6, and 12 months. We did not obtain numerical data for analysis.
The risk ratio (RR) for improvement of symptoms (excellent or good according to investigator VAS) at 12 months with amoxicillin versus placebo was 1.06, 95% CI 0.93 to 1.21 in the 107 participants with definite Lyme disease (Analysis 1.3). Investigators concluded that additional amoxicillin therapy was not beneficial, with most participants (59/62) in the LNB subgroup having an excellent or good response regardless of treatment arm. Data from participants with LNB were not reported for amoxicillin and placebo groups separately. Less than 5% of the LNB subgroup ended up with a controversial or poor response on the investigator ratings. The investigators did not find any correlation between the clinical outcomes and persistence without decline of B. burgdorferi‐specific antibodies in the serum.
A comparison of the overall response to the additional treatment between the definite and possible LNB subgroups showed that the response was better for amoxicillin in the subgroup with definite LNB than in the subgroup with possible LNB, but this was not the case for placebo.
Improvement in a measure of overall disability in the short term (two weeks)
Not reported.
Resolution of CSF pleocytosis following treatment
Not reported.
Other CSF parameters
Antibody titers
Dr Oksi provided additional information for the number of participants in the LNB subgroup with a decline in B. burgdorferi‐specific antibodies at 12 months; however, the text is unclear whether serum or CSF levels were reported. Among participants who had LNB, a strong antibody decline (a decrease of over 50%) occurred in 17/30 in the amoxicillin group and 18/32 in the placebo group (RR 1.01, 95% CI 0.65 to 1.56), with a partial decline in 3/30 participants in the amoxicillin group and 2/32 participants in the placebo group (RR 1.60, 95% CI 0.29 to 8.92).
Improvement in electrophysiological abnormalities following treatment
Not reported.
Adverse events
No serious adverse effects of antibiotic treatment occurred in any of the 145 trial participants (Analysis 1.4). Diarrhea was reported in 33 participants (22.8%) during intravenous ceftriaxone treatment and in 19 participants (13.1%) during the second phase of oral treatment, higher with amoxicillin than placebo (15/73 versus 4/72, respectively; RR 3.70, 95% CI 1.29 to 10.61). The diarrhea was usually mild and resolved spontaneously over about two weeks. No participant had to discontinue treatment due to diarrhea. Clostridium difficile colitis was reported in two participants, one in the placebo group and one after “discontinuation of study drug” (amoxicillin or placebo). Cholecystitis or biliary sludging was not observed in any participant. None of the participants developed urticaria or other allergic reactions. Seventeen episodes of fever were reported in 15 participants. Of these, 14 episodes were not attributable to an infection other than B. burgdorferi (2 on placebo, 3 on amoxicillin, and 9 on ceftriaxone). One participant developed facial paresis three days after the onset of ceftriaxone treatment, and about half of the participants reported transient intensification of symptoms during ceftriaxone treatment.
Oral doxycycline versus intravenous ceftriaxone for LNB (acute and chronic)
Studied in Ljostad 2008. See Table 7.
| Oral doxycycline compared to intravenous ceftriaxone for Lyme neuroborreliosis (LNB) (acute and chronic) | ||||||
| Patient or population: Lyme neuroborreliosis (acute and chronic) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous ceftriaxone | Oral doxycycline | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment | 333 per 1000 | 480 per 1000 (297 to 783) | RR 1.44 (0.89 to 2.35) | 102 | Moderate1 | Symptom resolution; composite clinical score of neurological signs and symptoms at 12 months2 |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | CSF was analyzed in 88/102 participants; authors state that no significant between‐group difference was present at 13 days and 4 months, but data are not available for verification. | Not estimable | 88 | Low3 | Resolution of CSF pleocytosis in all participants | |
| All adverse events | 464 per 1000 | 367 per 1000 | RR 0.79 (0.51 to 1.23) | 113 | Moderate4 | 48 adverse events in all participants randomized to study drug. 3 participants on ceftriaxone and 1 on doxycycline experienced serious adverse events (as defined by trial authors); RR 0.33 (95% CI 0.04 to 3.05). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
1Downgraded once for imprecision (small study). We did not downgrade the quality of evidence for indirectness as, although flawed, the measure is likely to reflect clinical reality.
2Participants had predominantly acute LNB, although people with an acute or chronic course of LNB were eligible. Investigators included subjective symptoms in the overall neurologic deficit assessment but with a higher maximum score for objective neurologic findings. No long‐term assessment was performed, however worsening after near‐resolution in the majority of participants is unlikely.
3Downgraded twice: for indirectness of pleocytosis as an outcome measure and imprecision (small study).
4Downgraded once for imprecision (few events, small study). In the absence of comprehensive adverse event reporting in the included trials, the table presents ‘all adverse events’ with a comment on severe adverse events when these data are presented in the trial.
The investigators included 118 consecutive participants from nine hospitals in coastal areas of Southern Norway.
Improvement in a measure of overall disability in the long term (three or more months)
Not reported.
Improvement or resolution of the person's presenting neurological deficits in the long term (three or more months)
The primary outcome was a custom‐made composite score measured four months after randomization. To calculate this score, the severity of 27 multiple objective neurologic signs (maximum score of 54) and six subjective symptoms (maximum score of 12) were graded from 0 to 2 (total maximum score of 66). Secondary outcomes were full recovery (composite score = 0) at four months and reduction of the composite score two weeks after randomization.
There was no significant difference in reduction in clinical score at 4 months (MD 0.10, 95% CI ‐1.20 to 1.40; N = 102; Analysis 2.1); the RR for complete resolution of symptoms at 12 months favored doxycyline, but the result was imprecise, with CI including no difference (RR 1.44, 95% CI 0.89 to 2.35; N = 102; moderate‐quality evidence; Analysis 2.2).
Improvement in a measure of overall disability in the short term (two weeks)
Not reported.
Resolution of CSF pleocytosis following treatment
In Ljostad 2008, investigators obtained CSF at inclusion and at 13 days and 4 months after the start of antibiotic treatment. No significant difference was found between oral doxycycline and intravenous ceftriaxone for reduction in CSF cell count at 13 days (P = 0.89) or 4 months (P = 0.56) after the start of treatment (data not provided; low‐quality evidence).
Improvement in electrophysiological abnormalities following treatment
Not reported.
Adverse events
The safety population included 113 participants with available data. The RR of adverse event between the two groups favored doxycycline, but the data were very imprecise and allowed for the possibility of no difference (RR 0.79, 95% CI 0.51 to 1.23; N = 113; moderate‐quality evidence; Analysis 2.3). Three participants discontinued ceftriaxone treatment due to adverse events: one with cholecystitis, one with stomatitis and proctitis, and one with allergy. There were no other serious adverse events. There was one serious adverse event but no withdrawals in the doxycycline group. Results for adverse events leading to discontinuation (RR 0.14, 95% CI 0.01 to 2.71; N = 118; Analysis 2.4) and serious adverse events (RR 0.33, 95% CI 0.04 to 3.05; N = 113; Analysis 2.5) also favored doxycycline but with serious imprecision. Diarrhea, nausea, and urticaria were reported for 19, 15, and 3 participants, respectively; all were generally mild. Emergence of new symptoms compatible with LNB or intensification of symptoms during treatment was not reported.
Ceftriaxone versus penicillin G for acute LNB in children
Studied in Mullegger 1991.
Improvement in a measure of overall disability in the long term (three or more months)
Not measured.
Improvement or resolution of the person's presenting neurological deficits in the long term (three or more months)
No documentation was provided on how clinical status was assessed or whether subjective complaints were incorporated into the assessment in Mullegger 1991. All children (N = 23) initially had objective neurologic findings and recovered completely. Median recovery times differed between interventions: 20 days for penicillin and 33 days for ceftriaxone, with no other statistical information provided. The authors concluded that their study did not show any differences in efficacy between the two treatments.
Improvement in a measure of overall disability in the short term (two weeks)
Not measured.
Resolution of CSF pleocytosis following treatment
This study provided no useful information on CSF parameters with regard to the effect of antibiotic treatment in children, because it did not routinely collect CSF for analysis of parameters after treatment; some were studied but not all reported.
Improvement in electrophysiological abnormalities following treatment
Not reported.
Adverse events
In the ceftriaxone group, transient elevation of serum transaminases (once) and drug‐induced toxic skin reaction (twice) were seen. No side effects were noticed in the penicillin G group. Intensification of symptoms or emergence of new symptoms compatible with LNB during treatment was not reported.
Intravenous penicillin G versus oral doxycycline for LNB (acute and chronic)
Studied in Karlsson 1994. See Table 8.
| Intravenous penicillin G compared to oral doxycyline for Lyme neuroborreliosis (acute and chronic) | ||||||
| Patient or population: Lyme neuroborreliosis (acute and chronic) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Oral doxycycline | Intravenous penicillin G | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Improvement of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 1000 per 1000 | 1000 per 1000 (920 to 1000) | RR 1.0 | 51 | Low2 | Investigators rating symptom composite on Likert scale from 1 to 3 (no, mild, moderate to severe)2 |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 900 per 1000 | 855 per 1000 (693 to 1000) | RR 0.95 | 51 | Low2 | Investigators rating symptom composite on Likert scale from 1 to 3 (no, mild, moderate to severe)2 |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | 1000 per 1000 | 930 per 1000 | RR 0.93 | 29 | Very low3 | |
| All adverse events | 129 per 1000 | 130 per 1000 | RR 1.01 | 54 | Very low4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
1Measured at 3, 6, and 12 months; reported here at 12 months.
2Downgraded twice: for study limitations (unclear risk of selection bias and lack of blinding) and imprecision (small number of participants). Although judgement of objective findings is implied, the assessment approach does not allow a distinction between participant‐ and physician‐based judgement on the basis of subjective and objective findings. We did not downgrade the quality of evidence for indirectness as, although flawed, the measure is likely to reflect clinical reality.
3Downgraded three times: for study limitations (unclear risk of selection bias and incomplete outcome data), imprecision (small number of participants), and indirectness of pleocytosis as an outcome measure.
4Downgraded three times: twice for study limitations (unclear risk of selection bias and lack of blinding) and once for imprecision (small number of participants, few events, and wide CI). In the absence of comprehensive adverse event reporting in the included trials, the table presents ‘all adverse events’ with a comment on severe adverse events when these data are presented in the trial.
Improvement in a measure of overall disability in the long term (three or more months)
Not reported.
Improvement or resolution of the person's presenting neurological deficits in the long term (three or more months)
Karlsson 1994 compared a 14‐day treatment with penicillin G to 14 days of oral doxycycline in 54 participants with LNB. The study included only participants with objective findings and a positive serology or evidence of abnormal CSF. Investigators used a rating of subjective and objective findings on a Likert scale from 0 to 3 (no symptoms, mild symptoms, moderate or severe symptoms) for primary efficacy. The RRs for improvement and resolution with penicillin G versus oral doxycycline at 12 months were 1.0 (95% CI 0.92 to 1.08) and 0.95 (95% CI 0.77 to 1.18), respectively (N = 51; low‐quality evidence; Analysis 3.1; Analysis 3.2). Participants were followed for 12 months, with no difference found between the two treatment arms except for the fact that more participants treated with doxycycline reported vertigo at the end of treatment but not at one month. One participant in each treatment group was retreated because of residual symptoms. Subjective symptoms were completely absent at 12 months except for 1 penicillin G participant with neuromuscular pain and hypoesthesia and 1 doxycycline participant with arthralgia. Selection bias is a concern in this study due to a considerable imbalance in the number of participants randomized to each treatment arm. The report provided few statistics and did not allow a distinction to be made between participant‐ and physician‐based judgements. Objective judgement of findings at the end of follow‐up was implied but not well documented, thus this study did not use a well‐characterized objective measure of efficacy by a physician.
Improvement in a measure of overall disability in the short term (two weeks)
Not reported.
Resolution of CSF pleocytosis following treatment
CSF was obtained at inclusion, at 13 days, and in some participants at one year after the start of treatment. All participants had positive B. burgdorferi‐specific antibodies in serum, CSF, or both, or had a positive CSF culture (one participant had a positive culture but no specific antibodies) at study entry. The CSF cell count in all 9 participants in the penicillin group and 18 of the 20 participants in the doxycycline group had returned to normal at 1 year (RR 0.93, 95% CI 0.75 to 1.15; N = 29; very low‐quality evidence; Analysis 3.3).
Other CSF parameters
All participants in both treatment arms were negative for immunoglobulin M antibodies in the CSF at one year. B. burgdorferi‐specific CSF immunoglobulin G antibodies were negative in only 5/9 penicillin participants and 9/20 doxycycline participants (56% versus 45%, difference not significant). No significant differences were found between oral doxycycline and intravenous penicillin for any CSF parameter.
Improvement in electrophysiological abnormalities following treatment
Not reported.
Adverse events
Emergence of new symptoms compatible with LNB during treatment was not reported. There was intensification of symptoms during treatment in one participant. One penicillin G‐treated participant had a transitory rise of temperature and increased vertigo during treatment. Two participants had thrombophlebitis. Two doxycycline‐treated participants had skin rash at the end of treatment; another two reported transient diarrhea (RR for adverse events 1.01, 95% CI 0.25 to 4.08; N = 54; very low‐quality evidence; Analysis 3.4).
Intravenous doxycycline versus intravenous penicillin G for LNB (acute and chronic)
Studied in Kohlhepp 1989. See Table 9.
| Intravenous doxycycline compared to intravenous penicillin G for Lyme neuroborreliosis (acute and chronic) | ||||||
| Patient or population: Lyme neuroborreliosis (acute and chronic) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous penicillin G | Intravenous doxycycline | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Improvement of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 833 per 1000 | 817 per 1000 | RR 0.98 (0.80 to 1.21) | 75 (1 study) | Low2 | Clinical findings were classified as no remission, partial remission, or full remission. |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 694 per 1000 | 667 per 1000 | RR 0.96 (0.70 to 1.31) | 75 (1 study) | Low2 | Clinical findings were classified as no remission, partial remission, or full remission. |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | See comment | See comment | Not estimable | ‐ | ‐ | Measured but not reported in detail |
| All adverse events3 | See comment | See comment | Not estimable | 75 | ‐ | 'Adverse events' not reported. No serious adverse events occurred. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
1Measured at 6 and 12 months; 12‐month results reported here.
2Downgraded twice: for study limitations (unclear risk of selection bias, lack of blinding) and imprecision (small sample size). We did not downgrade the quality of evidence for indirectness as, although flawed, the measure is likely to reflect clinical reality.
3In the absence of comprehensive adverse event reporting in the included trials, the table presents ‘all adverse events’ with a comment on severe adverse events when these data are presented in the trial.
Improvement in a measure of overall disability in the long term (three or more months)
Not reported.
Improvement or resolution of the person's presenting neurological deficits in the long term (three or more months)
Kohlhepp 1989 randomized a clinically well‐defined cohort of 75 participants with predominantly acute (n = 67) but also chronic (n = 8) LNB to a 10‐day course of intravenous doxycycline or intravenous penicillin G. Follow‐up was 12 months, but for cases with residual symptoms the follow‐up was three years. The primary outcome was the treating physicians’ categorical grading of the clinical status as “no remission,” “partial remission,” or “full remission,” based on objective and subjective signs and symptoms with no specification given. At the end of treatment, over 80% of participants had responded to some degree in both groups. Early responders were usually asymptomatic after six months. Pain, meningitic symptoms, and acute cranial neuritis began to remit within days. A slower improvement was observed in symptoms of radiculitis, myelitis, encephalitis, and peripheral neuropathy. According to data in Figure 2 of the study report, at six months the RR for "partial remission" (improvement) showed no clear difference between interventions (RR 1.10, 95% CI 0.95 to 1.28), whereas the RR for "full remission" (resolution) favored doxycycline, but with the possibility of no effect (RR 1.42, 95% CI 0.83 to 2.42; low‐quality evidence; Analysis 4.1 and Analysis 4.2). At 12 months, the RRs for "partial remission" and "full remission" were 0.98, 95% CI 0.80 to 1.21 and 0.96, 95% CI 0.70 to 1.31, respectively (low‐quality evidence; Analysis 4.1 and Analysis 4.2). Of the 22 participants with only partial remission after 6 months, 10 chose to receive retreatment with penicillin G, 6 from the penicillin arm and 4 from the doxycycline arm. Three years after randomization, the recovery rate was 94% in the doxycycline and 91% in the penicillin G group if the retreatment group was excluded. In the “partial remission" group, 7/10 participants who chose retreatment recovered completely, compared to 7/12 of those who did not choose retreatment (no significant difference). Participants with partial remission had central nervous system involvement, a disseminated clinical picture, and/or a longer disease duration. The authors concluded that there was no clinically relevant difference between doxycycline and penicillin G. The number of participants with chronic LNB was too low for any subgroup analysis. In addition, the majority of these chronic LNB cases were also treated with immunosuppressants.
Improvement in a measure of overall disability in the short term (two weeks)
Not reported.
Resolution of CSF pleocytosis following treatment
Kohlhepp 1989 did not report resolution of CSF pleocytosis with sufficient detail for reporting in the review.
Improvement in electrophysiological abnormalities following treatment
Not reported.
Adverse events
None of the participants experienced serious side effects such as a Jarisch‐Herxheimer reaction or developed an allergic reaction. Intensification of symptoms or emergence of new symptoms compatible with LNB during treatment was not reported.
Intravenous cefotaxime versus intravenous penicillin G for acute LNB
Studied in Pfister 1989. See Table 10.
| Intravenous cefotaxime compared to intravenous penicillin G for Lyme neuroborreliosis (acute) | ||||||
| Patient or population: Lyme neuroborreliosis (acute) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous penicillin G | Intravenous cefotaxime | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 800 per 1000 | 816 per 1000 (536 to 1000) | RR 1.02 | 21 | Low2 | Investigators' nonstandardized judgement of improvement or resolution of symptoms reported at 7.7 months. |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | 1000 per 1000 | 920 per 1000 | RR 0.92 | 21 | Very low3 | Follow‐up: mean 7.7 months |
| All adverse events | See comment | See comment | Not estimable | 21 | Low4 | No adverse events occurred. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
1Improvement in all participants at an average of 7.7 months.
2Downgraded twice: for study limitations (unclear risk of selection bias and lack of blinding) and imprecision (small sample size). We did not downgrade the quality of evidence for indirectness as, although flawed, the measure is likely to reflect clinical reality.
3Downgraded three times: for study limitations (unclear risk of selection bias), imprecision (small sample size), and indirectness (limitation to acute Lyme neuroborreliosis, indirectness of pleocytosis as an outcome measure). One participant in the cefotaxime group had mild residual pleocytosis. Resolution reported in 20/21 participants.
4Downgraded twice: for study limitations (unclear risk of selection bias and lack of blinding) and imprecision (small sample size). In the absence of comprehensive adverse event reporting in the included trials, the table presents ‘all adverse events’ with a comment on severe adverse events when these data are presented in the trial.
Improvement in a measure of overall disability in the long term (three or more months)
Not reported.
Improvement or resolution of the person's presenting neurological deficits in the long term (three or more months)
Pfister 1989, in an open‐label study, randomized 21 participants with acute LNB (Bannwarth’s syndrome, meningitis) to a 10‐day treatment with either cefotaxime or penicillin G. Neurologic examination was performed daily during treatment or at follow‐up (mean 7.7 +/‐ 2.4 months). In addition, the study authors scored radicular pain daily during therapy. Most participants improved by day 3 or 4 of therapy. Eight of the 10 participants in the penicillin G group and 9/11 in the cefotaxime group had complete remission at follow‐up, with slight residual findings like mild radicular hypoesthesia or mild paresis in the rest (RR 1.02, 95% CI 0.67 to 1.55; N = 21; low‐quality evidence; Analysis 5.1). Pain subsided in all participants within nine days, with the exception of recurrent radicular pain lasting five weeks in one penicillin‐treated participant. While on therapy, four participants developed facial palsy, radicular symptoms, or bilateral proximal arm paresis, which subsided by the end of therapy. The authors stated that there was no difference in neurological examinations or pain scorings between groups during treatment and at follow‐up, with no statistics given.
Improvement in a measure of overall disability in the short term (two weeks)
Not reported.
Resolution of CSF pleocytosis following treatment
CSF was obtained prior to treatment and 8 to 10 days after the start of treatment (treatment duration was 10 days). There was no significant difference between the two treatment arms for abnormal CSF on repeated lumbar puncture on any of the CSF parameters, including pleocytosis, protein elevation, oligoclonal bands, or positive culture.
CSF cell counts normalized in almost all participants (10/10 in the penicillin G group and 10/11 in the cefotaxime group; RR 0.92, 95% CI 0.71 to 1.18; N = 21; very low‐quality evidence; Analysis 5.2), while CSF oligoclonal bands persisted in 6 participants from each treatment group, and intrathecal antibody production was still present in 1 participant from each group.
Other CSF parameters
CSF cefotaxime concentrations reached the minimum inhibitory concentration (MIC) in all participants, while none of the participants treated with penicillin G had CSF concentrations above the MIC.
Improvement in electrophysiological abnormalities following treatment
Not reported.
Adverse events
No side effects of antibiotic treatment were observed (N = 21; low‐quality evidence). Two participants per group developed a new symptom compatible with LNB during treatment. Intensification of symptoms during treatment was not reported.
Intravenous ceftriaxone versus intravenous cefotaxime for acute LNB
Studied in Pfister 1991. See Table 11.
| Intravenous ceftriaxone compared to intravenous cefotaxime for Lyme neuroborreliosis (acute) | ||||||
| Patient or population: acute Lyme neuroborreliosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous cefotaxime | Intravenous ceftriaxone | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment | 600 per 1000 | 666 per 1000 (378 to 1000) | RR 1.11 (0.63 to 1.97) | 27 | Low1 | Outcome reported at a mean of 8.1 months' follow‐up. |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | 867 per 1000 | 988 per 1000 | RR 1.14 (0.90 to 1.44) | 27 | Very low2 | |
| All adverse events | 188 per 1000 | 71 per 1000 | RR 0.38 | 30 | Low3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
1Downgraded twice: for study limitations (unclear risk of selection bias and lack of blinding) and imprecision (small sample size). We did not downgrade the quality of evidence for indirectness as, although flawed, the measure is likely to reflect clinical reality.
2Downgraded three times: for study limitations (unclear risk of selection bias), imprecision (small sample size), and indirectness of pleocytosis as an outcome measure.
3Downgraded twice: for study limitations (unclear risk of selection bias and lack of blinding) and imprecision (small sample size, few events). In the absence of comprehensive adverse event reporting in the included trials, the table presents ‘all adverse events’ with a comment on severe adverse events when these data are presented in the trial.
Improvement in a measure of overall disability in the long term (three or more months)
Not reported.
Improvement or resolution of the person's presenting neurological deficits in the long term (three or more months)
Pfister and colleagues compared a 10‐day treatment with ceftriaxone to cefotaxime in acute LNB (Bannwarth’s syndrome in 28/33 participants, 30 of whom could be evaluated). They reported no clinical difference between groups as judged by objective and subjective neurologic signs and symptoms during treatment or at follow‐up (mean 8.1 +/‐ 1.9 months). In most participants clinical improvement was observed on days 3 to 5 of therapy, while two participants in each group did not respond or deteriorated during treatment. Sixty‐three per cent of participants were asymptomatic at follow‐up, while 37% remained symptomatic, although all but 1 participant in each arm had improved to mild symptoms; 1 participant in each treatment arm had recurrent radicular pain with either pleocytosis or isolation of B. burgdorferi from the CSF. Symptom resolution at follow‐up occurred in 8/12 participants receiving ceftriaxone and 9/15 participants receiving cefotaxime (RR 1.11, 95% CI 0.63 to 1.97; low‐quality evidence; Analysis 6.1).
Improvement in a measure of overall disability in the short term (two weeks)
Not reported.
Resolution of CSF pleocytosis following treatment
CSF was obtained prior to treatment and very early at 2 to 4 days after the start of treatment (treatment duration was 10 days). No significant difference was found between the two treatment arms for abnormal CSF parameters on repeated lumbar puncture, including pleocytosis, protein elevation, oligoclonal bands, or positive culture. Cell counts normalized in all 10 participants in the ceftriaxone group and in 11/13 participants in the cefotaxime group with only mild pleocytosis, while oligoclonal bands persisted in 5 participants from the ceftriaxone group and 7 from the cefotaxime group, and culture was positive in 1 participant from the ceftriaxone group. The RR for resolution of pleocytosis for ceftriaxone versus cefotaxime was 1.14 (95% CI 0.90 to 1.44; N = 27; very low‐quality evidence; Analysis 6.2).
Other CSF parameters
There was no significant difference in the ability of the two antibiotics to reach MIC in the CSF.
Improvement in electrophysiological abnormalities following treatment
Not reported.
Adverse events
Both drugs were generally well tolerated. Intensification of symptoms during treatment was reported for one participant per group. One cefotaxime recipient developed allergic exanthema on day 9, so therapy was stopped. One ceftriaxone recipient developed fever and diarrhea and had mildly elevated liver enzymes on day 5. All symptoms and laboratory abnormalities resolved after his therapy was terminated on day 9. Two cefotaxime recipients had a worsening soon after antibiotic infusion consistent with the Jarisch‐Herxheimer reaction. The RR for adverse events with ceftriaxone versus cefotaxime was 0.38 (95% CI 0.04 to 3.26; N = 30; low‐quality evidence; Analysis 6.3).
Cost‐effectiveness
We found no cost‐effectiveness information in any included study.
Discussion
In clinical practice, almost all people with identified symptomatic bacterial infections are treated with antibiotics. This is also true of people diagnosed with Lyme neuroborreliosis (LNB) (Halperin 2015). However, uncertainty exists about the absolute and relative efficacy of the available antibiotic regimens for LNB. This review did not address the prevention of LNB following initial antibiotic treatment of erythema migrans, the optimal duration of treatment with the various antibiotics, or the efficacy of antibiotics for treatment of late neurological manifestations of Lyme disease, sometimes referred to as late‐ or third‐stage LNB. Also, this review did not address the efficacy of antibiotics for treatment of post‐Lyme disease syndrome. We included only randomized comparative trials for quality reasons. The majority of the 450 participants from the 7 randomized treatment trials included in this review had typical manifestations of early disseminated (acute or stage II) LNB. Only a minority had objective, defined central nervous system parenchymal involvement consistent with late disseminated (chronic or stage III) LNB (4/145 participants in Oksi 2007 and 6/75 participants in Kohlhepp 1989). In Oksi 2007, three participants had a disease duration of one year or longer.
Summary of main results
We found no high‐quality evidence on the absolute or relative efficacy of antibiotics for the treatment of LNB. Although there was some evidence on the relative efficacy of antibiotic treatment for LNB, it was mostly of low or very low quality. Although we selected only randomized studies for inclusion in this review, most lacked consistent, standardized, and well‐defined efficacy outcomes. The heterogeneity of eligibility criteria, interventions, and assessment of outcomes meant that we could not perform a meta‐analysis.
None of the studies reported on our proposed primary outcome, 'Improvement in a measure of overall disability in the long term (three or more months).' None of the trials reported a significant difference between antibiotic treatments by physician‐ or patient‐reported measures of efficacy. In the studies that reported lack of efficacy or partial response separately, there were no differences between randomized treatment regimens. However, differences in eligibility, the assessments used, and the duration of follow‐up among trials made any assessment of differences in outcomes difficult. In all seven trials, the majority of participants were reported as having recovered completely. The lack of a placebo group for the initial antibiotic treatment prevented assessment of the efficacy of antibiotic treatment versus natural recovery.
The majority of participants enrolled in these seven studies presumably had European LNB, predominantly acute LNB, and appear to have had good clinical outcomes (resolution of presenting signs and symptoms) regardless of whether the initial antibiotic treatment was ceftriaxone, cefotaxime, penicillin G, or oral or intravenous doxycycline. In a single study (Kohlhepp 1989), the need for retreatment (as determined by the incomplete resolution of symptoms) was 29%, but this was not addressed in most studies. Retreatment with antibiotics usually occurred in people with chronic or stage III LNB, supporting the need for antibiotic treatment as early as possible to prevent disease progression to stage III LNB. A single trial that examined the question of prolonged treatment with a second antibiotic found no evidence of additional efficacy when extending ceftriaxone treatment with amoxicillin, where improvement occurred with antibiotic and placebo at the same rate (small numbers) (Oksi 2007). In Oksi 2007, participants with "probable" Lyme disease did not respond to antibiotic treatment as well, in general, as participants with definite LNB, with the implication that some "probable" participants may have had alternative causation for their symptoms.
In summary:
-
We found no clinical trials to evaluate the absolute efficacy of initial treatment with antibiotics for LNB. The lack of placebo‐controlled studies prevented us from assessing the extent to which antibiotics improve spontaneous recovery from LNB or prevent further complications.
-
Seven randomized studies mostly at high risk of bias and of marked heterogeneity provided some relative efficacy data for antibiotic treatment of LNB, only one of which was blinded (Ljostad 2008). All studies were from Europe.
-
Marked heterogeneity among the eligible studies in terms of differences in inclusion and exclusion criteria, supportive laboratory diagnostic criteria, primary and secondary outcome measures, treatment regimens, prior antibiotic treatment, and duration of disease and follow‐up prevented incorporation of results into a systematic meta‐analysis. The quality of the evidence was mostly low to very low; we have formally presented this in 'Summary of findings' tables.
-
All studies reported improvement in the majority of participants following antibiotic treatment, and the majority had complete resolution of their symptoms in long‐term follow‐up, irrespective of the antibiotic regimen received and in the case of doxycycline regardless of whether it was given orally or intravenously.
-
Only three studies provided information on the need for retreatment (Kohlhepp 1989; Karlsson 1994; Ljostad 2008).
-
Where measured, objective biomarkers of response (CSF pleocytosis) recovered in almost all participants examined at follow‐up.
-
Incomplete or poor treatment responses for the efficacy outcomes used in the trials were reported in only a minority of participants, regardless of the antibiotic used. Where no or partial response was recorded, there were no obvious differences in the rates of partial or lack of response between treatments.
-
All of the antibiotics studied appear to have been generally well tolerated as judged by all adverse event reporting. Only four studies provided information on discontinuation due to adverse events (Pfister 1989; Mullegger 1991; Pfister 1991; Ljostad 2008), which can have a major impact on the outcome of the treatment.
-
The single study conducted in children treated very early in their disease reported full remission in all children. This study had a high risk of bias, with qualitative outcomes and incomplete follow‐up data.
Overall completeness and applicability of evidence
As placebo‐controlled studies have never been performed, the extent to which antibiotic treatment contributes to the natural recovery (absolute efficacy) of LNB is not known. Our review was therefore only able to summarize outcome data for the comparative effectiveness of antibiotics (relative efficacy) when used for the initial treatment of LNB. This review did not address the prevention of LNB following initial antibiotic treatment of erythema migrans, and we identified only one randomized controlled trial (RCT) that investigated the efficacy of retreatment following initial antibiotic treatment with ceftriaxone.
The participants in the seven trials included in the review are likely to all have been representative of LNB from Europe. We did not find any randomized studies that included participants with LNB from the United States. LNB is caused by a number of different Borrelia burgdorferi sensu lato species. In Europe it is caused by B. garinii and B. afzelii and only infrequently by B. burgdorferi sensu stricto, while in the United States all cases are caused by B. burgdorferi sensu stricto. Possible differences in clinical presentation and disease course between Europe and the United States are grounds for caution in applying the findings of this review to the development of guidance for treatment of LNB in the United States. For this reason, a straightforward extrapolation of conclusions from this review to people with LNB from the United States has limitations.
All studies used diagnostic criteria for LNB consistent with currently accepted case definitions (CDC 2011b). Owing to the paucity of data from late disseminated LNB, the evidence applies mostly to early disseminated (acute or stage II) LNB rather than to late disseminated (chronic or stage III) LNB.
One challenging aspect of LNB therapy concerns the definition of initial antibiotic treatment failure. Lyme immunoglobulin G antibodies postinfection may persist for many years despite complete clinical response, and on its own is not a diagnostic test of active infection with B. burgdorferi or a reliable criterion for treatment failure. A prospective Danish study of 187 consecutive LNB participants with intrathecal B. burgdorferi‐specific antibody synthesis who were treated with penicillin G between 1985 and 1990 could not find a single case of treatment failure based on the clinical outcome and normalization of CSF (Hansen 1992). In contrast, in the studies included in this review, there were reported instances of treatment failure or only partial response, mostly for late disseminated (chronic or stage III) LNB. A second course of treatment with a different antibiotic in a single study was associated with a response in no more participants than placebo (Oksi 2007). Objective quantitative measures of treatment 'failure' are few. The resolution of CSF pleocytosis in 99/106 participants in whom measurements were available provide biomarker evidence of a treatment response in most cases. Qualitative symptom reports and measures of cognitive impairments are non‐specific and error prone. Proper assessment of the response to antibiotic treatment in LNB requires a uniform definition of diagnostic criteria, a better understanding of the natural and treated history of the disease, and an understanding of what constitutes a treatment failure from both the patient and physician. Inclusion of subjective symptomatology is patient focused but makes it difficult to distinguish symptoms from other origins. All but one of the seven trials included in this review included both objective and subjective assessments of efficacy, and these correlated relatively well on the improvement from baseline. Further research is needed to prove the link between infection, ongoing versus pre‐existing damage, presenting versus residual symptoms, signs, and other comorbid conditions.
Quality of the evidence
We identified limitations in the design of all the included studies, in particular the lack of blinding in the majority of studies. We found a lack of standardization of entry criteria and of efficacy assessments across trials. All studies had a small sample size, with variable duration of follow‐up. The confidence in our estimates on the efficacy of the studied antibiotics for LNB is limited, and the true effects may well be substantially different. We thus graded the quality of the evidence as mostly low to very low, with the GRADE definition of 'low' being "further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate" (GRADE Working Group 2004).
Potential biases in the review process
The review process extended over eight years from initial protocol to completion of the systematic review. The review team included specialists in neurology and infectious diseases and experts in the diagnosis, treatment, and pathogenesis of LNB from both the United States and Europe, who worked as unpaid volunteers. To minimize the risk of publication bias, we performed a comprehensive search for studies and also solicited information from investigators of included trials for unpublished data. We also extended requests for unpublished RCT data to known specialists in the field and to leaders of patient organizations to allow their membership to respond (International Lyme and Associated Diseases Society). Outcome measures differed in the trials, and recording of adverse drug reactions was limited, therefore it was difficult for the review authors to interpret the effect of antibiotic intervention positively or negatively except in general terms. The limited number of participants studied in individual trials also meant that this review could not detect less common or rare adverse events.
We revised the scope of the review to focus on antibiotic treatment for LNB, the usual treatment for Lyme disease, which resulted in the exclusion of one RCT that assessed the effect of steroids (Pfister 1988). We revised our methods section to include current Cochrane methodology, including 'Summary of findings' tables, implemented since publication of our protocol (Cadavid 2008),
We attempted to reduce potential biases in the review process and analytical biases by reselecting studies and extracting data with an independent team.
Agreements and disagreements with other studies or reviews
In their recent systematic review, Dersch and colleagues considered both randomized and non‐randomized trials investigating all pharmacological treatments and focused on adults with acute LNB (Dersch 2015). We identified one RCT of non‐antibiotic treatment (Pfister 1988), which they listed as a reference but did not include. We agree with their conclusion that the heterogeneity among the selected RCTs and the overall bias precludes conclusion on the preference of any antibiotic regimen investigated. However, unlike Dersch and colleagues, we considered the studies to be heterogeneous to a degree that prevented pooling of data from different studies into a meta‐analysis. Differing from the study selection of Dersch and colleagues, we excluded two studies, mainly due to the lack of objective evidence of neurological disease (Hassler 1990; Oksi 1998). We did, however, include two studies not included by Dersch and colleagues, one in adults, Oksi 2007, and one in children (Mullegger 1991). We were able to include Oksi 2007 because the trial authors provided separate information on the LNB participants.

Study flow diagram.

Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 1 Symptoms (patient‐rated VAS, scale 0 to 100, higher worse) in all participants (definite and possible Lyme disease).
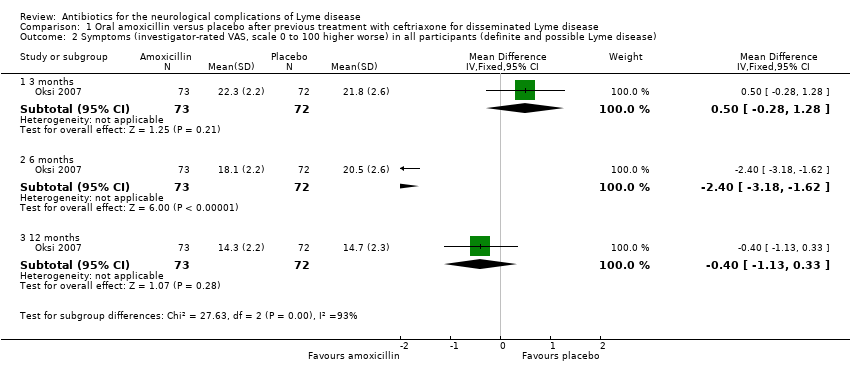
Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 2 Symptoms (investigator‐rated VAS, scale 0 to 100 higher worse) in all participants (definite and possible Lyme disease).
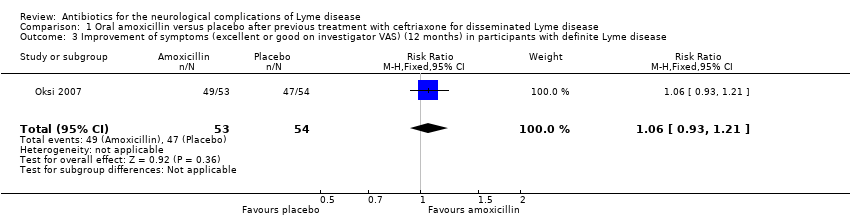
Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 3 Improvement of symptoms (excellent or good on investigator VAS) (12 months) in participants with definite Lyme disease.
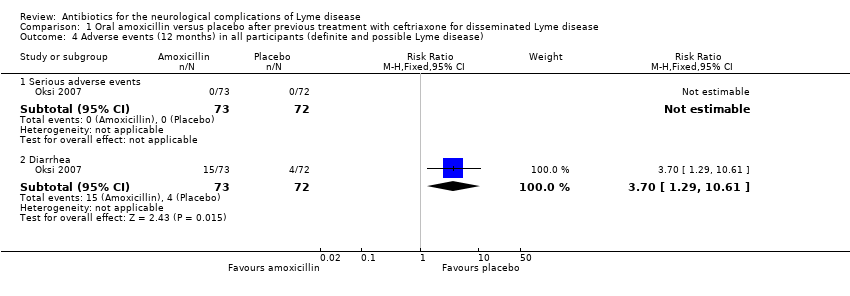
Comparison 1 Oral amoxicillin versus placebo after previous treatment with ceftriaxone for disseminated Lyme disease, Outcome 4 Adverse events (12 months) in all participants (definite and possible Lyme disease).
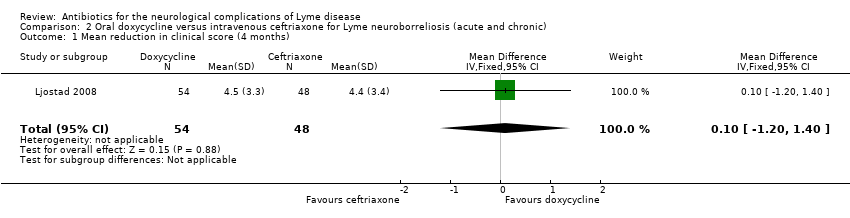
Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 1 Mean reduction in clinical score (4 months).
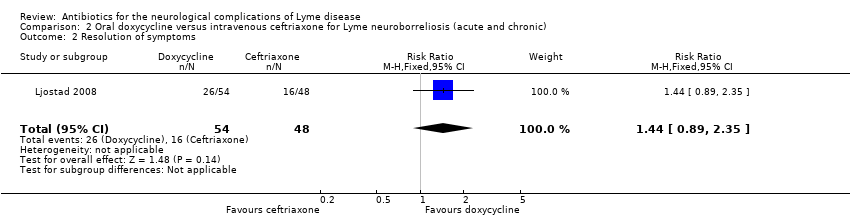
Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 2 Resolution of symptoms.
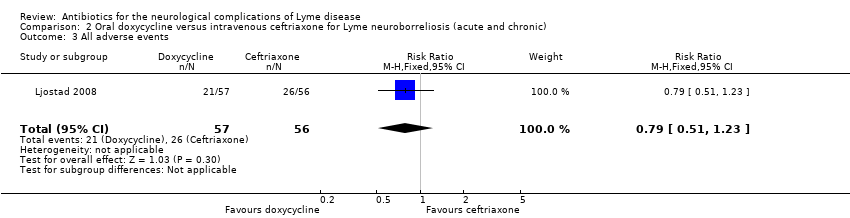
Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 3 All adverse events.
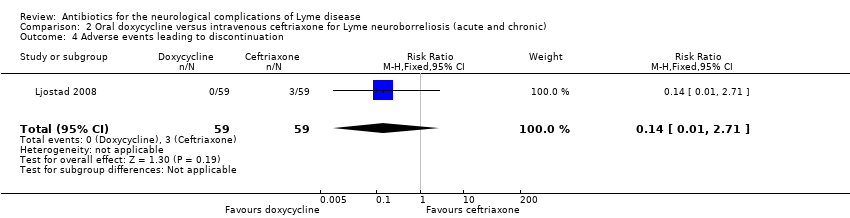
Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 4 Adverse events leading to discontinuation.
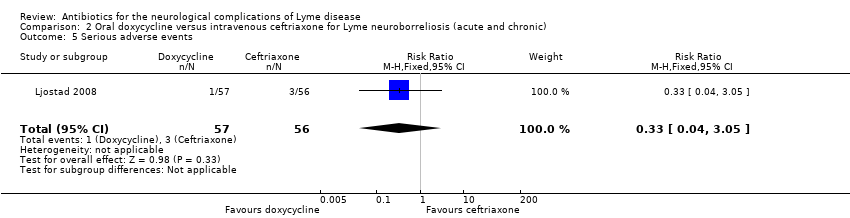
Comparison 2 Oral doxycycline versus intravenous ceftriaxone for Lyme neuroborreliosis (acute and chronic), Outcome 5 Serious adverse events.
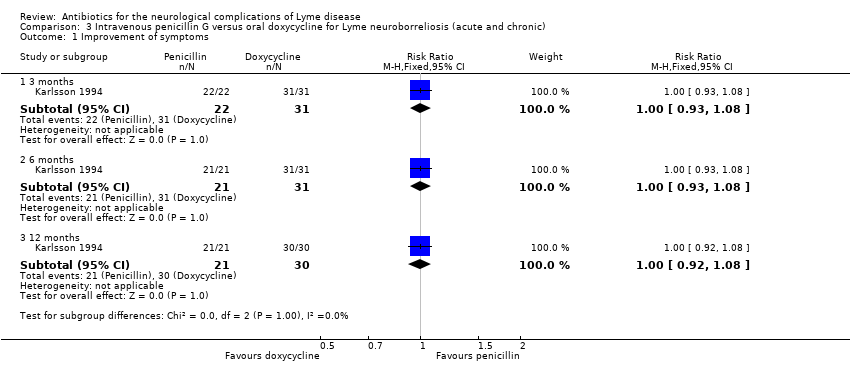
Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 1 Improvement of symptoms.

Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 2 Resolution of symptoms.
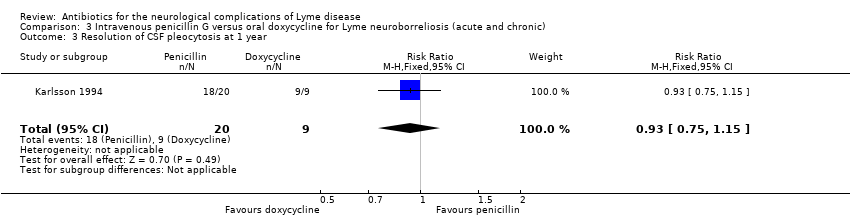
Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 3 Resolution of CSF pleocytosis at 1 year.

Comparison 3 Intravenous penicillin G versus oral doxycycline for Lyme neuroborreliosis (acute and chronic), Outcome 4 All adverse events.
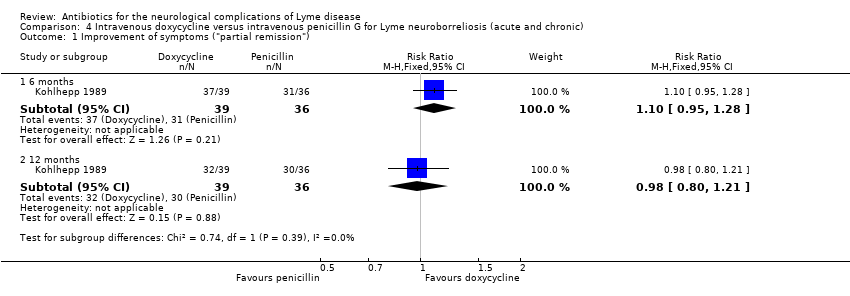
Comparison 4 Intravenous doxycycline versus intravenous penicillin G for Lyme neuroborreliosis (acute and chronic), Outcome 1 Improvement of symptoms ("partial remission").

Comparison 4 Intravenous doxycycline versus intravenous penicillin G for Lyme neuroborreliosis (acute and chronic), Outcome 2 Resolution of symptoms ("full remission").

Comparison 4 Intravenous doxycycline versus intravenous penicillin G for Lyme neuroborreliosis (acute and chronic), Outcome 3 Serious adverse events.

Comparison 5 Intravenous cefotaxime versus intravenous penicillin G for acute Lyme neuroborreliosis, Outcome 1 Resolution of symptoms (mean 7.7 months' follow‐up).
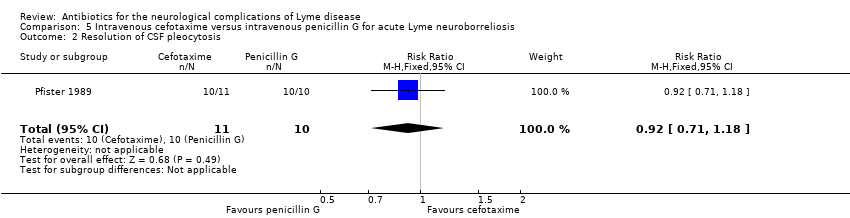
Comparison 5 Intravenous cefotaxime versus intravenous penicillin G for acute Lyme neuroborreliosis, Outcome 2 Resolution of CSF pleocytosis.
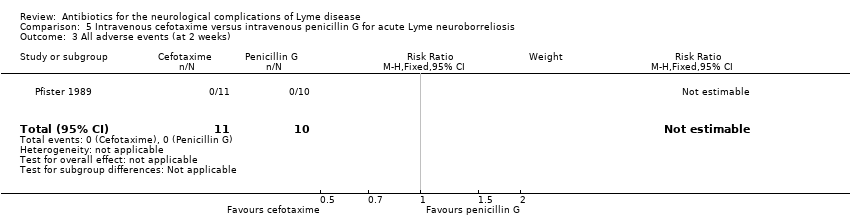
Comparison 5 Intravenous cefotaxime versus intravenous penicillin G for acute Lyme neuroborreliosis, Outcome 3 All adverse events (at 2 weeks).
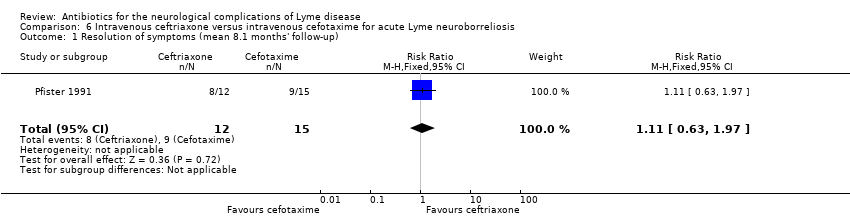
Comparison 6 Intravenous ceftriaxone versus intravenous cefotaxime for acute Lyme neuroborreliosis, Outcome 1 Resolution of symptoms (mean 8.1 months' follow‐up).

Comparison 6 Intravenous ceftriaxone versus intravenous cefotaxime for acute Lyme neuroborreliosis, Outcome 2 Resolution of CSF pleocytosis.

Comparison 6 Intravenous ceftriaxone versus intravenous cefotaxime for acute Lyme neuroborreliosis, Outcome 3 All adverse events.
| Study | Population | Length of follow‐up | Interventions | Antibiotic naïve | Clinical remission measurement | Time to remission | Patient‐reported outcomes | CSF remission | Serology response |
| Yes | Up to 3 years | Penicillin Doxycycline | Unknown | Complete/partial/no | No | No | Yes | No | |
| Yes | Average of 7 months | Penicillin Cefotaxime | Unknown | Yes/no | No | Yes: VAS, 0 to 10 | Yes | No | |
| Yes (children only) | >6 months, <12 months | Penicillin Ceftriaxone | Yes | Yes/no | Yes; from treatment onset to complete remission | No | No | Yes | |
| Yes | Average of 7.7 months | Ceftriaxone Cefotaxime | Unknown | Yes/no | No | No | Yes | No | |
| Yes | 12 months | Penicillin Doxycycline | Past 4 weeks | Yes/no; by specific sign/symptom | No | Yes: Likert‐like scale, 0 to 3 | Yes, at 2 weeks and 12 months | Yes | |
| No (but large subgroup of LNB enrolled) | 12 months | Ceftriaxone followed by amoxicillin or placebo | Past 1 month | Physician VAS 0 to 100; scored as excellent/good, poor/none, controversial | No | Yes: VAS | Lumbar puncture and measurement of CSF antibody levels and PCR for Borrelia burgdorferi was repeated in selected cases during or after treatment. | Yes: scored as strong decline, mild, none | |
| Yes | Up to 4 months | Ceftriaxone Doxycycline | Past 14 days | No/mild/more than mild; also change in baseline deficits in past 3 months using own composite clinical score | No | Yes: 6 items, each scored 0 to 2 | Yes | No | |
| CSF: cerebrospinal fluid | |||||||||
| Criteria | (N = 75) | (N = 21) | (N = 23) | (N = 30) | (N = 54) | (N = 145; 72% to 75% definite, 25% to 27% possiblea) | (N = 102) |
| Clinical | Radicular pain, meningitic symptoms, cranial neuritis, sensory and/or motor radiculitis, arthritis, carditis, myelitis or peripheral neuritis, tick bite and/or erythema migrans | Radicular pain (15/21), headache (2/21), facial palsy (8/21), unilateral VI palsy (1/21), lower limb muscle weakness (9/21), sensory disturbance (12/21) | Presence of neurological signs and symptoms indicative of LNB | Radiculopathy (motor or sensory, or both), cranial neuropathy (facial palsy, ocular motor) | Headache (71% to 74%), subjective stiff neck (65%), paresis (55% to 57%) including facial palsy in 35% to 43% | Lymphocytic meningitis without radiculitis in 18 (all definite), meningoradiculitis (16 definite) or radiculitis (11 definite), paresis in 5, encephalomyelitis in 4, encephalopathy in 6, facial paresis in 21, sudden deafness in 6, tinnitus in 8, other cranial nerve involvement in 13, peripheral neuritis in 6, and other peripheral nervous system manifestations (9 peripheral mononeuropathy or polyneuropathy, 15 paresthesia, 39 with headache without meningitis, 29 with dizziness or vertigo, and 11 with memory impairment) | 25% to 33% Bannwarth's syndrome, 19% to 22% facial palsy, 24% to 38% radiculopathy, various others (other cranial neuropathies, ataxia, myelopathy, limb paresis, paresthesias, cognitive deficits) |
| Laboratory | B. burgdorferi‐specific antibody titer in serum, B. burgdorferi‐specific antibody titer in CSF, lymphocytic pleocytosis, elevated CSF protein (>50 mg/dL), elevated CSF IgM‐, IgA‐, and/or IgG‐index, oligoclonal bands in CSF. Only group‐level information is given. | Elevated B. burgdorferi‐specific IgG and IgM antibody titers in serum (1:64 to 1:512): found in 11, of whom 4 had both elevated IgG and IgM, 6 had only elevated IgG, and 1 had only elevated IgM. 4 were seropositive in CSF but not in blood; 6 had negative serology in both serum and CSF (seronegative LNB), but 4 had EM and 2 a history of insect bites. | 1 or more of the following specific CSF laboratory parameters: elevated B. burgdorferi‐specific IgG antibody titer, intrathecally produced B. burgdorferi‐specific antibodies, and/or direct cultivation of B. burgdorferi from the CSF in a modified Barbour‐Stoenner‐Kelly medium | Elevated B. burgdorferi‐specific IgG and IgM antibody titers in serum: found in 22 and 8, respectively. 13 had positive B. burgdorferi‐specific CSF/serum antibody index. | Elevated B. burgdorferi‐specific IgM or IgG concentration, or both in 83% to 90%; all had positive serology or B. burgdorferi‐specific CSF antibodies, except for 1 participant who had a positive CSF culture. | Only 3 of the 145 study participants were seronegative. Presence of inflammatory changes in the CSF or intrathecal antibodies against B. burgdorferi, or both supported a diagnosis of definite LNB; 124/145 participants had lumbar puncture performed at diagnosis. | Intrathecal production of B. burgdorferi‐specific antibodies or B. burgdorferi‐specific antibodies in serum, or both were required for enrollment. |
| aLNB considered possible if clinical presentation was an uncommon manifestation, but serum antibodies against Borrelia burgdorferi were positive and other causes were excluded. Abbreviations: | |||||||
| Study | Kohlhepp 1989 Penicillin | Kohlhepp 1989 Doxycycline | Pfister 1989 Penicillin G | Pfister 1989 Cefotaxime | Pfister 1991 Ceftriaxone | Pfister 1991 Cefotaxime | Mullegger 1991 Penicillin G | Mullegger 1991 Ceftriaxone | Penicillin G | Karlsson 1994 Doxycycline | Oksi 2007 Amoxicillin post‐ceftriaxone | Oksi 2007 Placebo post‐ceftriaxone | Ljostad 2008 Doxycycline | Ljostad 2008 Ceftriaxone |
| Number of participants (evaluable) | 36 | 39 | 10 | 11 | 14 | 16 | 11 | 12 | 23 | 31 | 73 | 72 | 54 | 48 |
| Age mean (SD) unless specified | Men 55 (12.6); women 54.1 (16.3) | Men 49.6 (14); women 55.7 (14.3) | 56.7 (15) | 55.4 (10.8) | 58.7 (19.5) | 53.7 (16.8) | 8.1 (3.1) | Median 55 (range 16 to 88) | Median 49 (range 18 to 74) | Mean 52.3, range 19 to 87 | Mean 50.5, range 16 to 80 | 54 (13) | 52 (13) | |
| Percentage males | 44% | 51% | 50% | 64% | 64% | 44% | 36% | 42% | 44% | 29% | 48% | 50% | 52% | 65% |
| History of erythema migrans | 36% | 31% | 80% | 45% | 50% | 56% | Not reported | 61% | 42% | 26% (probable) | 31% | 10% | ||
| Mean (SD) time from onset of LNB to treatment | 5.2 (13.6) months | 4.1 (11.1) months | 28.7 (33.8) days | 23.5 (16.3) days | 64.5 (84.7) days | 38.6 (23.1) days | All included children were admitted to the hospital within 5 ± 1.8 days from onset of symptoms. | 3.5 weeks (1 week to 25 months) | 4 weeks (1 week to 18 months) | Unknown | 10 (19) weeks | 8 (13) weeks | ||
| Previous treatment with antibiotics | 11% | 8% | Not reported | Not reported | Not reported (mentioned for 1 participant) | Was an exclusion criteria | None for the 4 weeks prior to enrollment | Yes, in all participants with EM, 24 adequately and 14 not adequately | Treatment with cephalosporin, penicillin, tetracycline in past 14 days exclusion criterion | |||||
| Concomitant treatment with steroids | 28% | 26% | None | Not reported | Not reported | Not reported | Not reported | Not reported | ||||||
| CSF leukocytes mean (SD) cells/uL | 186 (75) | 145 (58) | 280.9 (212) | 435.7 (528) | 86.4 (128.4) | 135.3 (299.2) | Not reported | Median 96, range 6 to 1190 | Median 117, range 8 to 910 | 59% with available CSF showed lymphocytic pleocytosis. | 194 (237) | 178 (187) | ||
| CSF total protein mean (SD) in mg/dL | 133 (110) | 119 (112) | 115 (69) | 136 (67.4) | 72.7 (42) | 79.1 (48.4) | Not reported | Median 110, range 40 to 360 | Median 120, range 50 to 580 | Not reported | 120 (70) | 130 (80) | ||
| Presence of CSF oligoclonal bands | 78% | 62% | 70% | 64% | 64% | Not reported | Not reported | Not reported | Detected in 24/58 (41%) participants with definite LNB and CSF examined | Not reported | ||||
| CSF: cerebrospinal fluid | ||||||||||||||
| Study | Tool | Signs assessed | Subjective symptoms elicited |
| Visual analogue scale | Yes | Possibly | |
| Composite clinical score | Yes | Yes | |
| Change of clinical symptoms | Yes | No | |
| Disease duration | Yes | No | |
| Change of clinical symptoms (3‐level classification) | Yes | Unclear | |
| Change of clinical symptoms | Yes | Yes | |
| Change of clinical symptoms | Yes | Unclear | |
| Change of clinical symptoms | Yes | No | |
| *The efficacy of interventions was quantified by diverse tools in each study assessing the change in objective findings (signs) or subjective complaints (symptoms), or both, as reported by participants or judged by the study physician. | |||
| Study | Parameter |
| Decrease of B. burgdorferi‐specific antibody concentrations at 12 months of at least 20% ("moderate decline") or 50% ("strong decline") | |
| Resolution of CSF pleocytosis | |
| Cell count, protein, antibody index, B. burgdorferi‐specific antibody production | |
| Abnormal CSF on repeated lumbar puncture1 | |
| Abnormal CSF on repeated lumbar puncture2 | |
| Cell count, B. burgdorferi‐specific antibody production | |
| Changes in intrathecally produced specific antibodies against B. burgdorferi | |
| 1One or more of lymphocytic pleocytosis, protein elevation, oligoclonal bands, B. burgdorferi‐specific antibody production. Abbreviations: | |
| Oral amoxicillin versus placebo for people previously treated with ceftriaxone for Lyme neuroborreliosis (acute and late) | ||||||
| Patient or population: people previously treated with ceftriaxone for disseminated Lyme neuroborreliosis (acute and chronic)1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Placebo | Oral amoxicillin | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Improvement or resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment2 | Separate information on the LNB subgroup (N = 62), but not for intervention groups within this subgroup was provided at the review authors' request. 59/62 participants were classified as experiencing improvement of presenting neurological deficits at month 12 (dichotomous assessment: 'excellent or good' based on investigator VAS values and medical record information).3 | Not estimable | 624 | Low5 | ||
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment4 | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| All adverse events ‐ 12 months | 24 adverse events for all 145 Lyme disease participants, mostly diarrhea and fever with no need for discontinuation. No serious adverse events reported. Attribution of adverse events to either pretreatment with ceftriaxone, or to amoxicillin or placebo, or both, is unclear. | Not estimable | 145 | Very low6 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Subpopulation with LNB (N = 62) within study of people with definite or possible disseminated Lyme borreliosis (N = 145). | ||||||
| Oral doxycycline compared to intravenous ceftriaxone for Lyme neuroborreliosis (LNB) (acute and chronic) | ||||||
| Patient or population: Lyme neuroborreliosis (acute and chronic) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous ceftriaxone | Oral doxycycline | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment | 333 per 1000 | 480 per 1000 (297 to 783) | RR 1.44 (0.89 to 2.35) | 102 | Moderate1 | Symptom resolution; composite clinical score of neurological signs and symptoms at 12 months2 |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | CSF was analyzed in 88/102 participants; authors state that no significant between‐group difference was present at 13 days and 4 months, but data are not available for verification. | Not estimable | 88 | Low3 | Resolution of CSF pleocytosis in all participants | |
| All adverse events | 464 per 1000 | 367 per 1000 | RR 0.79 (0.51 to 1.23) | 113 | Moderate4 | 48 adverse events in all participants randomized to study drug. 3 participants on ceftriaxone and 1 on doxycycline experienced serious adverse events (as defined by trial authors); RR 0.33 (95% CI 0.04 to 3.05). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Downgraded once for imprecision (small study). We did not downgrade the quality of evidence for indirectness as, although flawed, the measure is likely to reflect clinical reality. | ||||||
| Intravenous penicillin G compared to oral doxycyline for Lyme neuroborreliosis (acute and chronic) | ||||||
| Patient or population: Lyme neuroborreliosis (acute and chronic) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Oral doxycycline | Intravenous penicillin G | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Improvement of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 1000 per 1000 | 1000 per 1000 (920 to 1000) | RR 1.0 | 51 | Low2 | Investigators rating symptom composite on Likert scale from 1 to 3 (no, mild, moderate to severe)2 |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 900 per 1000 | 855 per 1000 (693 to 1000) | RR 0.95 | 51 | Low2 | Investigators rating symptom composite on Likert scale from 1 to 3 (no, mild, moderate to severe)2 |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | 1000 per 1000 | 930 per 1000 | RR 0.93 | 29 | Very low3 | |
| All adverse events | 129 per 1000 | 130 per 1000 | RR 1.01 | 54 | Very low4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Measured at 3, 6, and 12 months; reported here at 12 months. | ||||||
| Intravenous doxycycline compared to intravenous penicillin G for Lyme neuroborreliosis (acute and chronic) | ||||||
| Patient or population: Lyme neuroborreliosis (acute and chronic) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous penicillin G | Intravenous doxycycline | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Improvement of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 833 per 1000 | 817 per 1000 | RR 0.98 (0.80 to 1.21) | 75 (1 study) | Low2 | Clinical findings were classified as no remission, partial remission, or full remission. |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 694 per 1000 | 667 per 1000 | RR 0.96 (0.70 to 1.31) | 75 (1 study) | Low2 | Clinical findings were classified as no remission, partial remission, or full remission. |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | See comment | See comment | Not estimable | ‐ | ‐ | Measured but not reported in detail |
| All adverse events3 | See comment | See comment | Not estimable | 75 | ‐ | 'Adverse events' not reported. No serious adverse events occurred. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Measured at 6 and 12 months; 12‐month results reported here. | ||||||
| Intravenous cefotaxime compared to intravenous penicillin G for Lyme neuroborreliosis (acute) | ||||||
| Patient or population: Lyme neuroborreliosis (acute) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous penicillin G | Intravenous cefotaxime | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment1 | 800 per 1000 | 816 per 1000 (536 to 1000) | RR 1.02 | 21 | Low2 | Investigators' nonstandardized judgement of improvement or resolution of symptoms reported at 7.7 months. |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | 1000 per 1000 | 920 per 1000 | RR 0.92 | 21 | Very low3 | Follow‐up: mean 7.7 months |
| All adverse events | See comment | See comment | Not estimable | 21 | Low4 | No adverse events occurred. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Improvement in all participants at an average of 7.7 months. | ||||||
| Intravenous ceftriaxone compared to intravenous cefotaxime for Lyme neuroborreliosis (acute) | ||||||
| Patient or population: acute Lyme neuroborreliosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk or score/value | Corresponding risk or score/value | |||||
| Intravenous cefotaxime | Intravenous ceftriaxone | |||||
| Improvement in a measure of overall disability in the long term (3 or more months) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Resolution of the person's presenting neurological deficits in the long term (3 or more months) following treatment | 600 per 1000 | 666 per 1000 (378 to 1000) | RR 1.11 (0.63 to 1.97) | 27 | Low1 | Outcome reported at a mean of 8.1 months' follow‐up. |
| Improvement in a measure of overall disability in the short term (2 weeks) following treatment | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Resolution of CSF pleocytosis following treatment | 867 per 1000 | 988 per 1000 | RR 1.14 (0.90 to 1.44) | 27 | Very low2 | |
| All adverse events | 188 per 1000 | 71 per 1000 | RR 0.38 | 30 | Low3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Evidence based on randomized controlled trials begins as high‐quality evidence, but confidence in the evidence was decreased for several reasons, including the following.
| ||||||
| 1Downgraded twice: for study limitations (unclear risk of selection bias and lack of blinding) and imprecision (small sample size). We did not downgrade the quality of evidence for indirectness as, although flawed, the measure is likely to reflect clinical reality. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptoms (patient‐rated VAS, scale 0 to 100, higher worse) in all participants (definite and possible Lyme disease) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 3 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [3.39, 5.01] |
| 1.2 6 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.38, 0.38] |
| 1.3 12 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.21, 1.41] |
| 2 Symptoms (investigator‐rated VAS, scale 0 to 100 higher worse) in all participants (definite and possible Lyme disease) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 3 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.28, 1.28] |
| 2.2 6 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐3.18, ‐1.62] |
| 2.3 12 months | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.13, 0.33] |
| 3 Improvement of symptoms (excellent or good on investigator VAS) (12 months) in participants with definite Lyme disease Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.21] |
| 4 Adverse events (12 months) in all participants (definite and possible Lyme disease) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Serious adverse events | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Diarrhea | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.70 [1.29, 10.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean reduction in clinical score (4 months) Show forest plot | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.20, 1.40] |
| 2 Resolution of symptoms Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.89, 2.35] |
| 3 All adverse events Show forest plot | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.23] |
| 4 Adverse events leading to discontinuation Show forest plot | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.71] |
| 5 Serious adverse events Show forest plot | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement of symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 3 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.93, 1.08] |
| 1.2 6 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.93, 1.08] |
| 1.3 12 months | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.92, 1.08] |
| 2 Resolution of symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 3 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.64, 1.61] |
| 2.2 6 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.10, 2.54] |
| 2.3 12 months | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.18] |
| 3 Resolution of CSF pleocytosis at 1 year Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.15] |
| 4 All adverse events Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.25, 4.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement of symptoms ("partial remission") Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.28] |
| 1.2 12 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 2 Resolution of symptoms ("full remission") Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.83, 2.42] |
| 2.2 12 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.31] |
| 3 Serious adverse events Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of symptoms (mean 7.7 months' follow‐up) Show forest plot | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.55] |
| 2 Resolution of CSF pleocytosis Show forest plot | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.18] |
| 3 All adverse events (at 2 weeks) Show forest plot | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of symptoms (mean 8.1 months' follow‐up) Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.63, 1.97] |
| 2 Resolution of CSF pleocytosis Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.90, 1.44] |
| 3 All adverse events Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.04, 3.26] |