授乳中の乳房緊満に対する治療
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial. | |
| Participants | 70 ˝lactating women with breast engorgement˝ who were referred to Gha'em Hospital in Fars, Iran. Exclusion criteria: mothers with a breast abscess, fever (defined as T > 38°C), sore/cracked nipples, heart disease, fracture in the shoulder region, history of breast surgery, use of traditional herbal remedies for breast engorgement and mothers who did not want to take part in the study. | |
| Interventions | Intervention group (35 participants): acupressure, using hand massage, was applied simultaneously to both breasts for 2 min, followed by a 30‐second rest. This was repeated for a total of 20 min and performed twice a day, on 2 consecutive days (a total of 4 times over 2 days). Control group (35 participants): hot (43‐46°C) and cold (10‐18° C) compresses were applied intermittently (2 min each) to both breasts simultaneously for 20 min, twice a day, on 2 consecutive days (a total of 4 times over 2 days). | |
| Outcomes | Breast engorgement severity index based on degree of breast tension, erythema and pain. | |
| Notes | The study uses individual breasts as the unit of analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper only states that the 70 women were randomly assigned to 1 intervention or the other in a way that would create 2 intervention groups of 35 each but method of sequence generation is not specified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not described. |
| Blinding (performance bias and detection bias) | High risk | The type of intervention did not allow blinding of women. |
| Blinding (performance bias and detection bias) | High risk | The type of intervention did not allow blinding of clinical staff. |
| Blinding of outcome assessment (detection bias) | High risk | The outcome assessor based results on a "breast engorgement checklist", but no blinding was done. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data ‐ all patients were followed up. |
| Selective reporting (reporting bias) | Low risk | The results are strictly based on the authors' pre‐made checklist. |
| Other bias | Low risk | No other bias identified. Baseline characteristics (education, career, parity, type of birth) of intervention and control groups very similar. |
| Methods | Quasi‐randomised controlled trial. | |
| Participants | 16 women who sought care for engorgement at the Human Milk Bank of the Hospital Universitario Evangelico de Curitiba, Curitiba, Brazil. Inclusion criteria: women aged 18 or over who were between 3 and 10 days postpartum with moderate and/or intense bilateral engorgement, regardless of location in the breast. Exclusion criteria: women with a history of mammoplasty and/or breast prosthesis; use of synthetic oxytocin; use of analgesics in the 6 hours prior to the study; use of cream or talc on the breasts on the exam day; having had a bath up until an hour before the study; exposure to sunlight or light in the 2 hours before the study; history of a palpable or non‐palpable breast lesion; previous history of lactational mastitis; obstructive glandular engorgement; tissue integrity impaired in any region of the breast; unwilling to participate. | |
| Interventions | Intervention group (8 participants): 1 min of electromechanical breast massage followed by mechanical pumping, if softening of the breast occurred. If no softening occurred following initial massage, then massager applied for a further 2 min before pumping. The domestically manufactured, vibro‐therapeutic massager under the trademark 'Physical' was used; whereas for milk expression a 'Medela' pump in high vibration mode, at maximum suction, was applied, first to the hands of the participant and subsequently, to their breasts. Control group (8 participants): 1 min of manual breast massage followed by manual pumping, if softening occurred. If no softening occurred following initial massage, then a further 2 min of manual massage performed prior to pumping. | |
| Outcomes | Temperature of the breasts measured with thermography. The degree of swelling, breast tenderness and intensity of symptoms were measured before the intervention, to determine inclusion eligibility, but, unfortunately, they were not measured post‐intervention. | |
| Notes | No information was provided on the massage technique used nor on the duration of pumping (milk expression). No statement on potential conflict of interest or source of funding is provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | ˝The investigator flipped a coin. With the result ˝face˝, the first lactating woman would be in the control group...If it was crown˝˝, the lactating woman belonged to the experimental group...Thus the two methods of treatment were alternated starting from the initial random selection.˝ |
| Allocation concealment (selection bias) | High risk | ˝The two methods of treatment were alternated.˝ |
| Blinding (performance bias and detection bias) | High risk | The types of interventions did not allow blinding of women. |
| Blinding (performance bias and detection bias) | High risk | The types of interventions did not allow blinding of clinicians. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors is not mentioned and it is not clear whether the outcome assessor was independent of the clinician performing the intervention. |
| Incomplete outcome data (attrition bias) | Unclear risk | A sample size of 196 women was calculated but only 16 were in the final sample. According to the authors the ˝sample was compromised due to the lack of availability of the instrumentation, the acclimatization period required for application of the thermography protocol, and the lack of signed consent forms˝. It is unclear whether women dropped out before or after study inclusion, and if afterwards, how many belonged to each group. |
| Selective reporting (reporting bias) | High risk | ˝In the evaluation, two methods were applied: clinical exam and thermographic exam...˝ but only breast temperature is reported pre‐and post‐intervention. According to the authors, the degree of breast swelling, breast tenderness and intensity of engorgement symptoms were measured pre‐intervention but they are not reported in the article. It is unclear whether the measurements were repeated post‐intervention. The latter outcomes would have been more useful for assessing the effectiveness of the intervention, as they are common symptoms of breast engorgement, unlike a rise in breast temperature. |
| Other bias | High risk | Varying degrees of engorgement among women prior to treatment are alluded to but no data specifically given for study groups. A sample size of 196 women was calculated but only 16 were in the final sample suggesting that the study is severely underpowered. |
| Methods | Randomised controlled trial. Computer‐generated block randomisation list, with block sizes of 4 and 8, used to ensure even distribution of participants (30 in each group). | |
| Participants | 60 breastfeeding women recruited from a medical centre in central Taiwan. Inclusion criteria: a) breast engorgement (diagnosed as having hot, painful, hard breasts; non‐flow of milk; abnormal thirst levels; and breast tenderness); b) no high‐risk complications both before or following childbirth (˝high risk˝ not defined); and c) willingness to participate. | |
| Interventions | Intervention group (27 participants): short and soft Gua‐Sha scraping therapy was applied to acupoints ST16, ST18 and SP17, in the direction of the nipples. In addition, scraping therapy was applied between the engorged breasts to acupoints CV17. Each position was lightly scraped 7 times in 2 cycles before the next breastfeed. Intervention time was 2 +/‐ 0.5 min. Control group (27 participants): small towels were immersed in hot water, of 43 ± 2 °C, and then applied to the breasts. This was followed by massage, done using the index and middle fingers in a spiral motion towards the nipples. Intervention time in the control group was 20 ± 2 min. | |
| Outcomes | Breast engorgement symptoms based on SBES measured at 5 min and 30 min post‐treatment. SBES addresses pain, engorgement and discomfort, measured with a visual analogue scale (0 to 10). Breast and body temperatures (measured with digital infrared thermal imaging system) and vital signs (BP) were recorded at 5 min and 30 min post‐treatment. | |
| Notes | The standard deviation of changes from baseline was missing for all variables so we used a correlation coefficient of 0.80 to impute the change‐from‐baseline standard deviation according to the formula provided in the Cochrane Handbook for Systematic Reviews of Interventions (Ch. 16.1.3.2). Mild skin redness and elevation was noted in the intervention group but no discomfort was expressed by study participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ˝Computer‐ generated block randomisation list.˝ |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Author contacted, additional information not provided. |
| Blinding (performance bias and detection bias) | High risk | ˝Open trial, i.e., all participants knew to which group they had been assigned.˝ |
| Blinding (performance bias and detection bias) | High risk | ˝Open trial˝; ˝the primary investigator handled all interventions.˝ |
| Blinding of outcome assessment (detection bias) | Low risk | ˝All data were collected by a nurse who was blinded to patient group assignments.˝ |
| Incomplete outcome data (attrition bias) | Low risk | Author contacted to clarify inclusions/exclusions: 60 participants initially recruited of which 30 in the experimental group and 30 in the control group. 6 women were ˝removed˝ from the study due to: fever (n = 2), early discharge (n = 2) and fatigue (n = 2). Final number of participants: 54 (27 in each group). Hence, attrition appears balanced in number and reason across groups. |
| Selective reporting (reporting bias) | Low risk | BP results not reported, but least relevant to study objectives. |
| Other bias | Low risk | ˝The groups showed no statistically significant differences in any variables except for age. No significant differences were found between groups in terms of pretest variables.˝ |
| Methods | Quasi‐randomised trial. Allocation by folder numbers. | |
| Participants | 45 women with pronounced signs of engorgement during the second to the 4th day postpartum. Women were located on a private hospital ward in Stockholm, Sweden. | |
| Interventions | Intervention group (20 participants): oxytocin 2.5 IU given subcutaneously daily to women until breasts became soft. Control group (25 participants): a corresponding amount of physiological saline was given similarly. In both groups the baby was allowed to breastfeed from the first day after delivery. | |
| Outcomes | Amount of breast milk produced. Duration of treatment before the engorgement disappeared. | |
| Notes | There were only limited data we were able to use in data tables. The authors state that the baby was allowed to suckle from the first day after delivery and the volume of milk was measured. The results state that the daily amount of milk produced was the same in both groups, although it was not clear how the amount of milk produced was measured. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Odd or even folder numbers. |
| Allocation concealment (selection bias) | High risk | There was no allocation concealment. Women were allocated into different groups based on their hospital records. |
| Blinding (performance bias and detection bias) | Low risk | "It was concealed from both patient and doctor whether oxytocin or saline was being used." |
| Blinding (performance bias and detection bias) | Low risk | "It was concealed from both patient and doctor whether oxytocin or saline was being used." |
| Blinding of outcome assessment (detection bias) | Unclear risk | The article does not mention blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study does not mention how incomplete outcome data were addressed. |
| Selective reporting (reporting bias) | Unclear risk | The authors report their outcomes in percentages not in numbers out of the totals; which makes it difficult to determine the denominators. |
| Other bias | Low risk | No other bias identified. |
| Methods | Double‐blind randomised controlled trial. | |
| Participants | 70 women recruited from a postpartum hospital ward in urban Singapore. Inclusion criteria: postpartum women with breast engorgement. Diagnosis of breast engorgement was based on some or all of the following: subjective complaint of pain in the breast and objective evidence of breast swelling, induration and impaired lactation. | |
| Interventions | Intervention group (35 women): oral serrapeptase (Danzen), an anti‐inflammatory proteolytic enzyme drug derived from serratia E15 (isolated from the silk worm intestine) was administered in a dose of 2 tablets (5 mg per tablet) 3 times a day for 3 days. Control group (35 women) specially made tablets that were identical in appearance to the Danzen tablets were given according to the same regime. During the study breastfeeding was encouraged and concomitant breast massage and milk expression was allowed. | |
| Outcomes | Total improvement of breast engorgement. Improvement of individual symptoms: ◦ improvement of breast induration; ∘ improvement of breast swelling; and ∘ improvement of breast pain. | |
| Notes | The authors gave cumulative percentages in the results section, which the review authors corrected. The study authors reported that breastfeeding was encouraged during the study but they report that only 4 patients in the treatment group and 8 in the placebo breastfed their babies during the study period. No adverse reactions were reported by any of the patients given Danzen. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial but random sequence generation not adequately described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not defined. |
| Blinding (performance bias and detection bias) | Low risk | "The placebo tablets were specially manufactured for the study and were identical in appearance to Danzen tablets." |
| Blinding (performance bias and detection bias) | Low risk | "None of the research team was aware of the respective identification during the duration of the study." |
| Blinding of outcome assessment (detection bias) | Low risk | "An independent observer, unaware of the groups the patients were in, assessed each symptom and sign daily." |
| Incomplete outcome data (attrition bias) | Low risk | The authors reported on all outcomes. |
| Selective reporting (reporting bias) | Low risk | The authors reported on all outcomes and all participants. |
| Other bias | Unclear risk | Role of sponsor unclear. Presumably provided tested drugs. Possible vested interest may have lead to a risk of bias in favour of tested drug. |
| Methods | Randomised controlled trial. | |
| Participants | 88 women attending breastfeeding clinics in the South of Sweden with at least 2 of the following symptoms of breast inflammation: erythema, tension, resistance, pain or pyrexia. Half of the women were within 2 weeks of giving birth. Exclusion criteria (contraindications for acupuncture treatment): psychiatric illness, haemorrhagic disease, prosthetic heart valves, infections of the skin, hepatitis B or HIV. | |
| Interventions | Group 1 (28 women): usual care, including oxytocin nasal spray at the discretion of attending midwives. Group 2 (35 women): acupuncture to points HT 3 (heart) and GB 21 (gall bladder). Group 3 (25 women): acupuncture to points HT 3, GB 21 and SP 6 (spleen). Acupuncture was carried out by midwives with acupuncture experience. All 3 groups received advice on interval and duration of breastfeeds, breast emptying and application of unrefined cotton wool. | |
| Outcomes | Severity of symptoms on day 3 expressed as severity index (sum of scores for breast tension, erythema and pain); maternal satisfaction with breastfeeding; breast tissue resistance. | |
| Notes | Published results were not reported in a way that we were able to use in data tables. Results state that there were no differences between groups at day 3, but no original data were presented. We contacted the author for further information; data from the study are no longer available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A total of 150 opaque envelopes, 50 for each group, were prepared with a paper denoting the intervention group and sealed. These were then randomly mixed and the envelopes numbered. The envelopes were identical in weight. For those wishing to take part, the midwife opened an envelope in correct numerical order, in the mothers’ presence." |
| Allocation concealment (selection bias) | Low risk | Described as sealed opaque envelopes opened by midwives in order. |
| Blinding (performance bias and detection bias) | High risk | Not feasible. "Blinding of participants was not attempted in this study because the practice of sham acupuncture has been questioned for its reliability." |
| Blinding (performance bias and detection bias) | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) | High risk | ˝The treating midwife completed protocols for the mothers' initial visit to the clinic and for every follow‐up contact until the mother reported that symptoms had subsided.˝ |
| Incomplete outcome data (attrition bias) | High risk | Cannot be measured. 88 women randomised. Denominators for results not clear. No post‐intervention tables provided. Study was ended prematurely partly due to ˝the realization that the number of patients referred to the doctor for prescription of antibiotics was small". |
| Selective reporting (reporting bias) | High risk | Breast tissue resistance was not reported in the results even though a scale was devised to measure it. Non‐significant results are mentioned but not reported adequately. |
| Other bias | Unclear risk | Study report not very clear to allow identification of other potential sources of bias. |
| Methods | Randomised, non‐blinded 3‐arm controlled trial. | |
| Participants | Women attending a hospital breastfeeding clinic in the South of Sweden. 210 cases randomised. Inclusion criteria: at least 2 of the following symptoms: breast erythema, tension, resistance, pain or pyrexia. Exclusion criteria (contraindications for acupuncture treatment): psychiatric illness, haemorrhagic disease, prosthetic heart valves, infections of the skin, hepatitis B or HIV. | |
| Interventions | Essential care to everyone: advice on duration and frequency of breastfeeds, advice on breast emptying (manual expression, pumping or warm shower) and application of unrefined cotton wool. Group 1 (70 women): essential care and oxytocin nasal spray, at the discretion of the clinical staff. Group 2 (70 women): essential care and acupuncture, avoiding the SP6 site which stimulates oxytocin. Group 3 (70 women): essential care and acupuncture, including the SP6 site. The acupuncture was performed by midwives who had completed a course in obstetrical acupuncture and had at least 5 years experience in its use. | |
| Outcomes | Severity index (sum of scores for breast tension, erythema and pain) on days 3, 4 and 5; number of contact days till recovery; maternal satisfaction with breastfeeding on days 3, 4 and 5; need for antipyretics, number of contact days till recovery; residual symptoms after 6 weeks, occurrence of breast abscess, need for antibiotics. | |
| Notes | The authors included 5 women, who were randomised twice because they developed residual symptoms after 6 weeks, to the original 205 participants to give 210 episodes of inflammatory symptoms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes prepared in advance. These were then randomly mixed and the envelopes numbered. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes were used to allocate women into the 3 groups. "The sequence of group allocation was not known to anyone.˝ |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial did not allow blinding. Women who were getting acupuncture would know their intervention. |
| Blinding (performance bias and detection bias) | High risk | The authors do not mention blinding of clinical staff but the nature of the study would not allow blinding of clinical staff. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The authors do not mention blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | The authors report about drop outs and they report that they did ITT analysis. |
| Selective reporting (reporting bias) | Low risk | The authors reported on all pre‐specified outcomes. |
| Other bias | Low risk | No other bias identified. |
| Methods | Randomised double‐blind, placebo‐controlled trial. Analysis for breasts rather than women. | |
| Participants | 197 engorged breasts from 109 women who were referred to the physiotherapist for treatment of breast engorgement. Exclusion criteria: spoken or written English insufficient for informed consent; breast implants. | |
| Interventions | Intervention breast: Medtron model P300 ultrasound machine used with aquasonic ultrasound transmission gel as coupling agent. Intensity was adjusted to give a comfortable warmth; application head massaged over breast towards areola; firmer pressure used on inwards stroke; duration of treatment ranged from 8 min for A cup to 15 min for a breast of DD or greater cup size. Control breast: ultrasound machine of identical appearance used in the same way as described above; the control machine had the crystal removed and replaced with a resistor to produce surface heat only. Participants were divided into 3 groups: group 1 (22 women) ‐ both breasts received ultrasound; group 2 (23 women) ‐ both breasts received sham treatment; group 3 (64 women) ‐ 1 breast received ultrasound and 1 breast received sham treatment. | |
| Outcomes | Pain using a visual analogue scale, hardness using a visual analogue scale, hardness using a digital tonometer. Outcomes measured before and after treatment, prior to breastfeed. | |
| Notes | Each breast, instead of an individual woman was the unit of analysis. The machines were labelled as A and B and were changed weekly by someone blind to allocation of women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Balanced block randomisation sequence." |
| Allocation concealment (selection bias) | Unclear risk | The authors do not mention allocation concealment. |
| Blinding (performance bias and detection bias) | Low risk | Women did not know which treatment they were getting. "The serial numbers of the machines were covered and the machines were labelled A and B. Labels were changed weekly by the Head of Department who had no role in the ultrasound treatment and did not hold the trial log book." |
| Blinding (performance bias and detection bias) | Low risk | "The serial numbers of the machines were covered and the machines were labelled A and B. Labels were changed weekly by the Head of Department who had no role in the ultrasound treatment and did not hold the trial log book. The woman's name was given to the clerical officer who held the trial log book. She informed the treating physiotherapist which machine to use. (A or B)." |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessor was blinded to the groups the women were in. |
| Incomplete outcome data (attrition bias) | High risk | The authors mentioned that 3 women were lost to follow‐up but they did not say how that was handled. |
| Selective reporting (reporting bias) | Low risk | The authors reported all the pre‐specified outcomes. |
| Other bias | High risk | Results were very difficult to interpret as analysis was by breast. The authors also state that when the visual analogue scale was used, it was not always easy for women to make a clear distinction between the left and the right breast. |
| Methods | Quasi‐randomised trial. Women allocated to different groups on alternate days. Experimental group on even number days and placebo on odd number days. | |
| Participants | 59 women presenting with breast engorgement (˝mammal swelling or induration˝...˝complaining of pain or tenderness˝) on 3rd to 5th day post‐delivery. | |
| Interventions | Intervention group (35 women): day 1: 2 tablets of protease complex, an enteric‐coated tablet consisting of bromelain and trypsin, taken 4 times a day (after each meal and before bed time); day 2 and 3: 1 tablet 4 times a day; total of 16 tablets. Control group (24 women): lactose containing placebo tablets given according to the above regime. | |
| Outcomes | Swelling and pain on the afternoon of the 4th day; maternal opinion of treatment; size of breast; shape of nipple; coagulation, prothrombin and bleeding time. | |
| Notes | It was not clear whether all women were breastfeeding. The outcomes were measured using grades according to the degree of improvement of symptoms. In situations where there was no change the grade allocated was 0, where the symptoms became worse, the grade was ‐ 1, in cases where there was a 1 stage improvement the grade given was 1 and where there was a 2 stage improvement, the grade was 2. No change was detected before and after treatment in regard to coagulation, prothrombin and bleeding time. No complaints were made in regards to gastro‐intestinal troubles or poor uterine involution. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation, allocation by day of the week. This method introduced bias in the way the participants were allocated to different groups. |
| Allocation concealment (selection bias) | High risk | Group allocation could be anticipated in advance as it was known which arm of the study was allocated to which day of the week. |
| Blinding (performance bias and detection bias) | Unclear risk | The study was placebo‐controlled but the authors do not mention whether the placebo was identical to the treatment or if it was easy for women to see what they were getting. |
| Blinding (performance bias and detection bias) | Unclear risk | There is no mention of blinding of the clinician who administered the treatment. |
| Blinding of outcome assessment (detection bias) | Low risk | 2 outcome assessors recorded the change in swelling and pain on the 4th day and were not informed as to which participant belonged to which group. |
| Incomplete outcome data (attrition bias) | Low risk | There were no exclusions and no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The authors reported on all pre‐specified outcomes. |
| Other bias | Unclear risk | Protease complex tablet was supplied by Mochida Pharmaceutical Company under the trademark 'Kimotab'. Possible vested interest may have lead to a risk of bias in favour of tested drug. |
| Methods | Random assignment to 2 treatment groups. | |
| Participants | 28 lactating women with breast engorgement. Inpatients recruited from 2 hospitals in Darwin, Australia, usually on the 3rd day postpartum. Inclusion criteria: lactation and engorgement defined as ˝hard, very warm, painful breasts, with difficulty feeding", according to the professional judgment of the midwives caring for them. Exclusion criteria: Aboriginal women. | |
| Interventions | Group 1: chilled cabbage leaves were placed on the right breast and room temperature cabbage leaves were placed on the left breast. Group 2: cabbage leaves placed in reverse order. Cabbage leaves applied between feedings and left on for 2 hours. Cabbage leaves, from common green cabbages (Brassica oleracea), were prepared by stripping out the large vein, cutting a hole for the nipple, rinsing, and chilling or leaving at room temperature. | |
| Outcomes | Pain: pre‐treatment measurement; post‐treatment measurement 2 hours later. | |
| Notes | This was a convenience sample of lactating women with breast engorgement. All women had both treatments and analyses were for individual breasts rather than for women. As breasts are not independent, results are very difficult to interpret. Pre‐test assessments were for 28 women whereas post‐test assessments were for 56 breasts. Data were not in a form in which we were able to enter them into data tables. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | The authors did not state how allocation concealment was done. |
| Blinding (performance bias and detection bias) | High risk | Blinding of participants was not feasible. All women had both treatments ‐ 1 on each breast and the treatments were not identical as 1 was cold and 1 was room temperature cabbage leaves. |
| Blinding (performance bias and detection bias) | High risk | Blinding was not feasible as women either received cold or room temperature cabbage leaves. |
| Blinding of outcome assessment (detection bias) | High risk | Women were assessing their breast on a visual analogue scale and midwives were supervising. |
| Incomplete outcome data (attrition bias) | Low risk | The authors reported on all participants included in the study. |
| Selective reporting (reporting bias) | Low risk | The authors reported on all outcomes. |
| Other bias | High risk | Analysis was by breast rather than by women. Breast are unlikely to be independent, especially in terms of outcomes reported in this study. As mentioned in McLachlan 1991, when a visual analogue scale was used, it was not always easy for women to make a clear distinction between the left and the right breast. |
| Methods | Quasi‐randomised trial (breasts rather than women were the unit of analysis). | |
| Participants | 34 lactating women located on postnatal wards in 2 Australian hospitals. Inclusion criteria: non‐Aboriginal, lactating, suffering from breast engorgement (hard, warm, painful breasts, with difficulty feeding), according to the professional judgement of the midwives caring for them. | |
| Interventions | Group 1 (even hospital registration numbers): chilled gel pack on the right breast and chilled cabbage leaves on the left breast. Group 2 (odd hospital registration numbers): opposite to above. Leaves from common green cabbages were prepared by stripping out the large vein, cutting a hole for the nipple, rinsing and chilling. Breast‐shaped gel pack in small, medium, and large sizes were designed by the researcher to fit under the brassiere, covering the breast except for the nipple area. Treatment left on breasts for up to 8 hours, with mothers renewing the cabbage leaves and gel packs ad lib, usually every 2 to 4 hours. | |
| Outcomes | Pre and post‐test pain rating for each breast rated on a "pain ruler" (a visual analogue scale with numbers from 0‐10, labelled with descriptions 0 = no pain, 5 = moderate pain, and 10 = excruciating pain). Descriptive data about engorgement were also collected. | |
| Notes | Analysis was at the breast level and results were at high risk of bias and difficult to interpret. We have not been able to included data in the data tables. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation (by hospital number). |
| Allocation concealment (selection bias) | Unclear risk | The authors did not mention allocation concealment. |
| Blinding (performance bias and detection bias) | High risk | The nature of the study did not allow blinding of women. |
| Blinding (performance bias and detection bias) | High risk | The nature of the study did not allow blinding of clinicians. |
| Blinding of outcome assessment (detection bias) | High risk | Participants rated outcomes in a self‐administered questionnaire. Given that visibly different interventions were placed on each breast blinding of participants/outcome assessors was not possible. |
| Incomplete outcome data (attrition bias) | Low risk | The authors reported on all outcomes. |
| Selective reporting (reporting bias) | Low risk | No other identified bias. |
| Other bias | High risk | The data were analysed at the breast level with no adjustment for the non‐independence of breasts. As mentioned in McLachlan 1991, when the visual analogue scale was used, it was not always easy for women to make a clear distinction between the left and right breast. |
| Methods | Double‐blind randomised controlled trial. | |
| Participants | 39 lactating, postpartum women with breast engorgement recruited from postnatal wards at Royal Darwin Hospital and Darwin Private Hospital, Australia. Breast engorgement defined as hard, warm, painful breasts with difficulty feeding. Exclusion criteria: Aboriginal women (tend to have less breast engorgement), women allergic to roses or the cabbage family of plants. Majority were multiparas with prior breastfeeding experience, who reported the appearance of engorgement symptoms on day 3 postpartum. Significantly more primiparas were in the intervention group. | |
| Interventions | Intervention group (21 women): base cream with 1% cabbage leaf extract (according to British Pharmacopoeia formulation). Control group (18 women): base/placebo cream only. Rosewater added to both creams to camouflage residual odour of cabbage. 1 tube of cream was applied liberally to both breasts and left on for 2 hours. The 2‐hour period was chosen since cabbage leaves had been shown to act within this period of time, and it could be done within the inter‐feeding period. Mothers were asked to refrain from showers, analgesia and feeding the baby during this period. | |
| Outcomes | Pain, using Bourbonnais pain scale (a visual analogue scale). Chest circumference, using plastic tape measure. Degree of hardness, using Roberts durometer. Degree of engorgement, using Hill and Humenick Breast Engorgement Scale. Outcomes measured at baseline, 2 hours after application of cream and following subsequent breastfeed. | |
| Notes | Authors were contacted to clarify method of cream application, i.e. to elucidate whether application method (e.g. massage) contributed to treatment effect. According to authors ˝the cream was lightly rubbed in, not massaged, just enough pressure to have the cream absorbed˝. Authors were also contacted to confirm that results shown in Table 3 refer to post‐test measurements (not stated in manuscript), and to check whether there were any significant differences between experimental and control groups for pretest values (given as a combined measure, in Table 4). Precise data on the latter could not be provided, since Australian regulations require data be kept for 5 years only post‐publication, but according to trial authors no differences were detected. The authors report that breastfeeding had a better effect than application of cream in relieving discomfort and decreasing tissue hardness. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ˝Randomised assignment list generated using coin toss.˝ |
| Allocation concealment (selection bias) | Unclear risk | ˝Group assignments were placed in sealed envelopes˝ but type of envelope not stated (transparent or opaque). |
| Blinding (performance bias and detection bias) | Low risk | Women did not know which cream they were using as the creams were identical in colour and odour. |
| Blinding (performance bias and detection bias) | Low risk | The same midwife applied the cream and performed the measurements. The midwife was however blinded to the groups the women were in. |
| Blinding of outcome assessment (detection bias) | Low risk | The midwife who assessed the outcomes was blinded to the allocations. |
| Incomplete outcome data (attrition bias) | Low risk | No loss of data. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported but in a form that could not be easily interpreted. Pre‐test measurements for each outcome were given as a single value, i.e. for both groups combined, so change in outcome measures could not be accurately calculated. |
| Other bias | High risk | A significant imbalance in primiparas at baseline (P = 0.047) may have been due to chance but may also have been due to possible allocation concealment bias or compromised blinding. |
| Methods | Randomised controlled trial. | |
| Participants | 88 breastfeeding mothers with "varying degrees" of breast engorgement, all mothers had a caesarean section. Exclusion criteria: oriental ethnic background (it is not clear how many women were excluded for this reason). | |
| Interventions | Intervention group: breast‐shaped cold packs worn 15‐20 min after 2 consecutive feeds. Control group: routine care which encouraged the use of supportive bras, warm compresses. manual expression/pumping of breasts, demand feeding and night time feeding, with intervals between feeds not being longer than 5 hours and each feed taking 30 min to 1 hour. | |
| Outcomes | Pre‐ versus post‐test pain scores. Scores were not reported in a way in which we were able to include them in data tables. We have briefly summarised the results in the text of the review. Transfer of milk. Degree of engorgement. | |
| Notes | This study is at high risk of bias. Women in the intervention group who were most distressed were moved into the control group, and those in the control group who wanted packs were moved to the intervention group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Table of random numbers generated but randomisation sequence was not observed. 8/88 women were not allocated according to randomisation schedule but according to preferred treatment arm. |
| Allocation concealment (selection bias) | Unclear risk | The authors did not make mention of allocation concealment. |
| Blinding (performance bias and detection bias) | High risk | The nature of the study did not allow blinding of women. |
| Blinding (performance bias and detection bias) | High risk | The nature of the study did not allow blinding of clinical staff. |
| Blinding of outcome assessment (detection bias) | High risk | A record of each participant was kept so that data could be analysed without their results. |
| Incomplete outcome data (attrition bias) | High risk | There were serious protocol deviations and no ITT analysis. |
| Selective reporting (reporting bias) | Low risk | The authors reported on all pre‐specified outcomes. |
| Other bias | High risk | There was considerable baseline imbalance. Women in the control groups had much lower pretest pain scores. This may be due to the fact that 3 women with the most severe symptoms were moved out of the control group and into the intervention group. There was no ITT analysis. |
BP: blood pressure
ITT: intention‐to‐treat
IU: international unit(s)
min: minutes
SBES: subjective breast engorgement scale
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This study focused on the suppression of lactation in women who did not intend to breastfeed. | |
| This study was examining interventions to prevent breast engorgement. Women in 3 villages were assigned 3 different treatments. | |
| This study focused on an intervention for "drying up breasts" in women who did not intend to breastfeed. | |
| This study focused on an intervention to suppress lactation in women who did not intend to breastfeed. | |
| This study included 120 women on postnatal wards of a Johannesburg hospital, South Africa. Women were recruited 72 hrs after delivery. Women in the intervention group received cabbage leaves to their breasts versus routine care (˝breast exercises˝) in the control group. The study was excluded as only approximately half of the sample perceived that they had symptoms of breast engorgement at baseline assessment. Cabbage leaves were therefore used as an intervention to prevent, as well as to treat, engorgement. Separate figures were not available for those women that had engorgement at the outset and were treated for symptoms. Results of this study suggested that women in the intervention group were more likely than those in the usual care group to be exclusively breastfeeding at 6 weeks (76% versus 58%). | |
| This study only included women who had chosen not to breastfeed. | |
| It was not clear whether this study was an RCT. This study focused on an intervention to suppress lactation in women who did not intend to breastfeed; the treatment was commenced during labour, before the onset of any symptoms of breast engorgement. | |
| In this study women that were breastfeeding were excluded.The study focused on an intervention to suppress lactation in women who did not intend to breastfeed. | |
| This study focused on an intervention to suppress lactation in women who did not intend to breastfeed. |
hrs: hours
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast abscess Show forest plot | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.04, 1.01] |
| Analysis 1.1  Comparison 1 Acupuncture versus usual care, Outcome 1 Breast abscess. | ||||
| 2 Lowest Severity Index score (day 5) (combined measurement of breast erythema, tension and pain) Show forest plot | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 0.99] |
| Analysis 1.2  Comparison 1 Acupuncture versus usual care, Outcome 2 Lowest Severity Index score (day 5) (combined measurement of breast erythema, tension and pain). | ||||
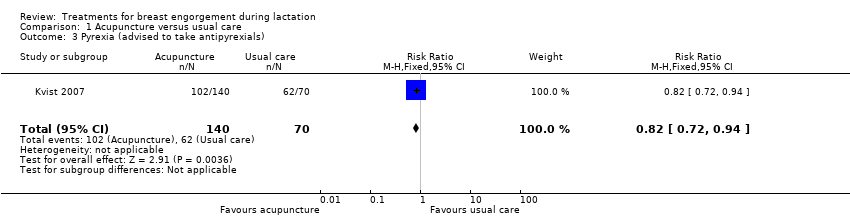
| 3 Pyrexia (advised to take antipyrexials) Show forest plot | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.94] |
| Analysis 1.3  Comparison 1 Acupuncture versus usual care, Outcome 3 Pyrexia (advised to take antipyrexials). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast engorgement (Hill and Humenich Breast engorgement scale) Show forest plot | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.18, 0.58] |
| Analysis 2.1  Comparison 2 Cabbage leaf extract versus placebo, Outcome 1 Breast engorgement (Hill and Humenich Breast engorgement scale). | ||||
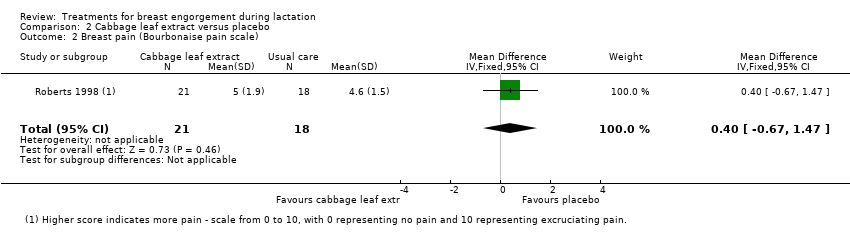
| 2 Breast pain (Bourbonaise pain scale) Show forest plot | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.67, 1.47] |
| Analysis 2.2  Comparison 2 Cabbage leaf extract versus placebo, Outcome 2 Breast pain (Bourbonaise pain scale). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
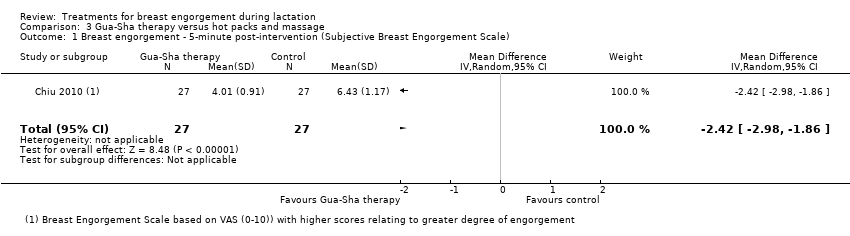
| 1 Breast engorgement ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale) Show forest plot | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐2.98, ‐1.86] |
| Analysis 3.1  Comparison 3 Gua‐Sha therapy versus hot packs and massage, Outcome 1 Breast engorgement ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale). | ||||
| 2 Breast pain ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.01 [‐2.60, ‐1.42] |
| Analysis 3.2  Comparison 3 Gua‐Sha therapy versus hot packs and massage, Outcome 2 Breast pain ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale). | ||||
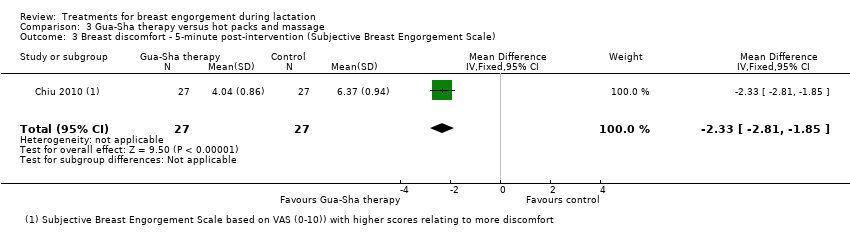
| 3 Breast discomfort ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.33 [‐2.81, ‐1.85] |
| Analysis 3.3  Comparison 3 Gua‐Sha therapy versus hot packs and massage, Outcome 3 Breast discomfort ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Analgesic requirement Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.63, 1.51] |
| Analysis 4.1  Comparison 4 Ultrasound versus sham treatment, Outcome 1 Analgesic requirement. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain not improved Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.74] |
| Analysis 5.1  Comparison 5 Protease complex versus placebo, Outcome 1 Pain not improved. | ||||
| 2 Breast swelling not improved Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.79] |
| Analysis 5.2  Comparison 5 Protease complex versus placebo, Outcome 2 Breast swelling not improved. | ||||
| 3 Overall rating of recovery (no change or worse) Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.12, 0.56] |
| Analysis 5.3  Comparison 5 Protease complex versus placebo, Outcome 3 Overall rating of recovery (no change or worse). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptoms not subsided after three days of treatment Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.68, 14.44] |
| Analysis 6.1  Comparison 6 Oxytocin versus placebo, Outcome 1 Symptoms not subsided after three days of treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Slight or no improvement in breast engorgement Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.14, 0.88] |
| Analysis 7.1  Comparison 7 Serrapeptase versus placebo, Outcome 1 Slight or no improvement in breast engorgement. | ||||
| 2 Slight or no improvement in breast swelling Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.55] |
| Analysis 7.2  Comparison 7 Serrapeptase versus placebo, Outcome 2 Slight or no improvement in breast swelling. | ||||
| 3 Slight or no improvement in breast pain Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.21, 1.49] |
| Analysis 7.3  Comparison 7 Serrapeptase versus placebo, Outcome 3 Slight or no improvement in breast pain. | ||||

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Acupuncture versus usual care, Outcome 1 Breast abscess.

Comparison 1 Acupuncture versus usual care, Outcome 2 Lowest Severity Index score (day 5) (combined measurement of breast erythema, tension and pain).
Comparison 1 Acupuncture versus usual care, Outcome 3 Pyrexia (advised to take antipyrexials).

Comparison 2 Cabbage leaf extract versus placebo, Outcome 1 Breast engorgement (Hill and Humenich Breast engorgement scale).
Comparison 2 Cabbage leaf extract versus placebo, Outcome 2 Breast pain (Bourbonaise pain scale).
Comparison 3 Gua‐Sha therapy versus hot packs and massage, Outcome 1 Breast engorgement ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale).

Comparison 3 Gua‐Sha therapy versus hot packs and massage, Outcome 2 Breast pain ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale).
Comparison 3 Gua‐Sha therapy versus hot packs and massage, Outcome 3 Breast discomfort ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale).

Comparison 4 Ultrasound versus sham treatment, Outcome 1 Analgesic requirement.

Comparison 5 Protease complex versus placebo, Outcome 1 Pain not improved.
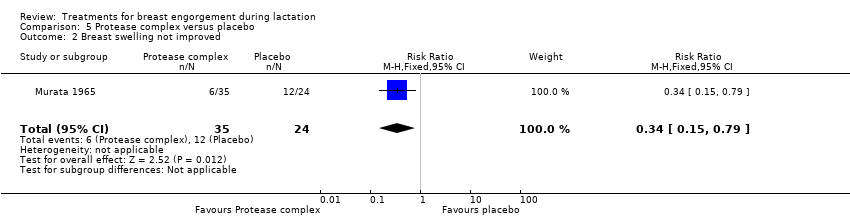
Comparison 5 Protease complex versus placebo, Outcome 2 Breast swelling not improved.
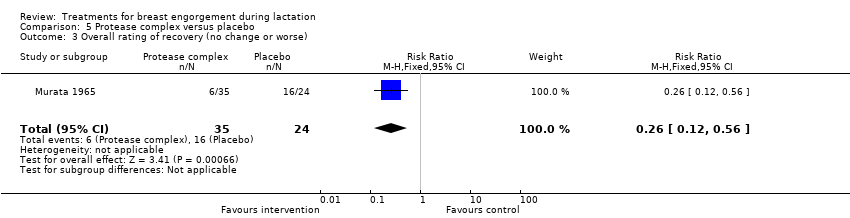
Comparison 5 Protease complex versus placebo, Outcome 3 Overall rating of recovery (no change or worse).

Comparison 6 Oxytocin versus placebo, Outcome 1 Symptoms not subsided after three days of treatment.

Comparison 7 Serrapeptase versus placebo, Outcome 1 Slight or no improvement in breast engorgement.
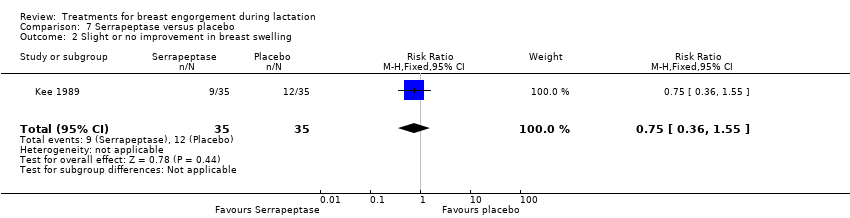
Comparison 7 Serrapeptase versus placebo, Outcome 2 Slight or no improvement in breast swelling.
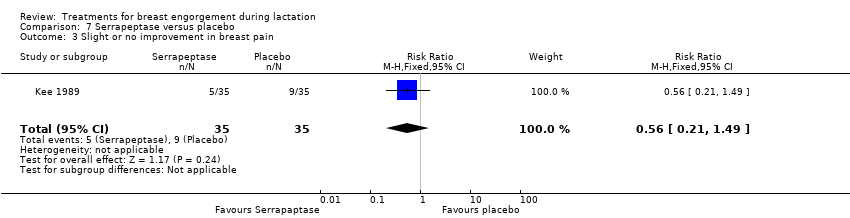
Comparison 7 Serrapeptase versus placebo, Outcome 3 Slight or no improvement in breast pain.
| Cabbage cream for breast engorgement during lactation | ||||||
| Patient or population: women with breast engorgement during lactation Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cabbage cream | |||||
| Breast pain | The mean breast pain in the intervention groups was | 39 | ⊕⊕⊝⊝ low1,2 | Higher score indicates more pain ‐ Bourbonaise pain scale ranks pain on a scale from 0 to 10, with 0 representing no pain and 10 representing excruciating pain. | ||
| Breast induration/hardness | This outcome was not reported in the trial. | |||||
| Breast swelling | This outcome was not reported in the trial. | |||||
| Breast engorgement | The mean engorgement in the intervention groups was | 39 | ⊕⊕⊝⊝ low1,2 | Higher score indicates more engorgement ‐ Hill and Humenich Breast engorgement scale ranks engorgement on a scale from 0 to 6, with 0 representing soft, no change in breasts and 6 representing very firm, very tender. | ||
| Analgesic requirement | This outcome was not reported in the trial. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The number of participants was even smaller than the pre‐determined sample size. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast abscess Show forest plot | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.04, 1.01] |
| 2 Lowest Severity Index score (day 5) (combined measurement of breast erythema, tension and pain) Show forest plot | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 0.99] |
| 3 Pyrexia (advised to take antipyrexials) Show forest plot | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast engorgement (Hill and Humenich Breast engorgement scale) Show forest plot | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.18, 0.58] |
| 2 Breast pain (Bourbonaise pain scale) Show forest plot | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.67, 1.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast engorgement ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale) Show forest plot | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐2.98, ‐1.86] |
| 2 Breast pain ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.01 [‐2.60, ‐1.42] |
| 3 Breast discomfort ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.33 [‐2.81, ‐1.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Analgesic requirement Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.63, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain not improved Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.74] |
| 2 Breast swelling not improved Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.79] |
| 3 Overall rating of recovery (no change or worse) Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.12, 0.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptoms not subsided after three days of treatment Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.68, 14.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Slight or no improvement in breast engorgement Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.14, 0.88] |
| 2 Slight or no improvement in breast swelling Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.55] |
| 3 Slight or no improvement in breast pain Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.21, 1.49] |

