Liječenje zastojne dojke tijekom laktacije
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006946.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 28 junio 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Lindeka Mangesi and Irena Zakarija‐Grkovic independently assessed study eligibility and carried out data extraction and assessment of risk of bias. The two review authors equally contributed to the update of this review.
Sources of support
Internal sources
-
Cochrane Croatia, Croatia.
Irena Zakarija‐Grkovic was supported by Cochrane Croatia and the Campbell and Cochrane Equity Methods Group
External sources
-
South African Cochrane Centre, South Africa.
Lindeka Mangesi was supported by South African Cochrane Centre.
Declarations of interest
None known.
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Lindeka Mangesi was awarded a fellowship by the South African Cochrane Centre through a grant received from the Effective Health Care Research Consortium www.evidence4health.org, which is funded by UKaid from the UK Government for International Development.
We acknowledge Therese Dowswell for her contribution in the initial review.
We thank the following members of the Campbell and Cochrane Equity Methods Group: Peter Tugwell, Jordi Pardo, Elizabeth Tanjong‐Ghogomu and George Wells for their assistance with interpreting data and assessment of risk of bias. In particular we would like to acknowledge Siavash Ghazvinian for translating the Farsi report and assisting with data extraction.
We acknowledge Livia Puljak, from Cochrane Croatia, for her assistance with final editing of the review.
We thank the editorial staff and peer reviewers of the Cochrane Pregnancy and Childbirth Group for their patience, advice, support and guidance in improving the quality of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Sep 18 | Treatments for breast engorgement during lactation | Review | Irena Zakarija-Grkovic, Fiona Stewart | |
| 2016 Jun 28 | Treatments for breast engorgement during lactation | Review | Lindeka Mangesi, Irena Zakarija‐Grkovic | |
| 2010 Sep 08 | Treatments for breast engorgement during lactation | Review | Lindeka Mangesi, Therese Dowswell | |
| 2008 Jan 23 | Treatments for breast engorgement during lactation | Protocol | Lindeka Mangesi, Gibson Muzonzini | |
Differences between protocol and review
The protocol Methods section has been updated. The following outcomes, which were not in the original protocol, were added because they had not been adequately addressed in the previous version of the review, and were felt to be important for women with breast engorgement.
Primary outcomes: breast pain, breast induration/hardness, breast swelling, breast engorgement.
Secondary outcome: pyrexia (as defined by trial authors) was added to replace temperature higher than 38 degrees celsius.
Methods for GRADE assessment of the quality of the evidence have been added for this update and a 'Summary of findings' table has been incorporated.
Due to the lack of trials and the way in which outcomes were assessed and reported in these trials we were unable to carry out some of our pre‐specified methods.
Data were not presented in a way that allowed us to include them in the data tables. We were unable to combine results in meta‐analyses or investigate subgroup analysis and assessment of heterogeneity. The small number of included studies with diverse interventions prevented us from carrying out meaningful sensitivity analysis.
For this version of the review we did not identify any cluster‐randomised trials.
Notes
This review was not updated earlier due to scarce availability of new evidence; hence conducting a review earlier may have been unnecessary and wasteful.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acupuncture Therapy;
- Brassica;
- Breast Diseases [etiology, *therapy];
- Cryotherapy [methods];
- Lactation Disorders [*therapy];
- Massage;
- Mastodynia [therapy];
- Oxytocin [therapeutic use];
- Peptide Hydrolases [therapeutic use];
- Phytotherapy [methods];
- Plant Leaves;
- Randomized Controlled Trials as Topic;
- Ultrasonic Therapy [methods];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Acupuncture versus usual care, Outcome 1 Breast abscess.

Comparison 1 Acupuncture versus usual care, Outcome 2 Lowest Severity Index score (day 5) (combined measurement of breast erythema, tension and pain).
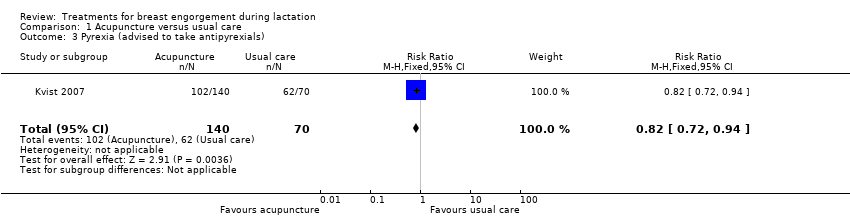
Comparison 1 Acupuncture versus usual care, Outcome 3 Pyrexia (advised to take antipyrexials).

Comparison 2 Cabbage leaf extract versus placebo, Outcome 1 Breast engorgement (Hill and Humenich Breast engorgement scale).
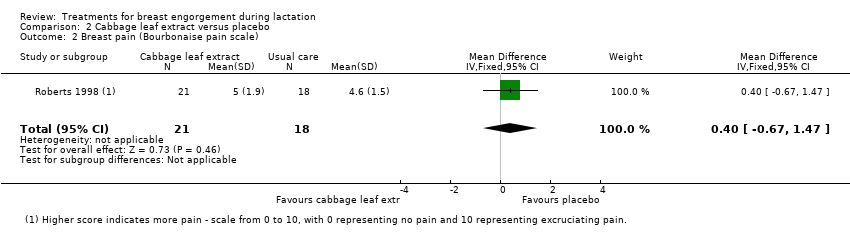
Comparison 2 Cabbage leaf extract versus placebo, Outcome 2 Breast pain (Bourbonaise pain scale).
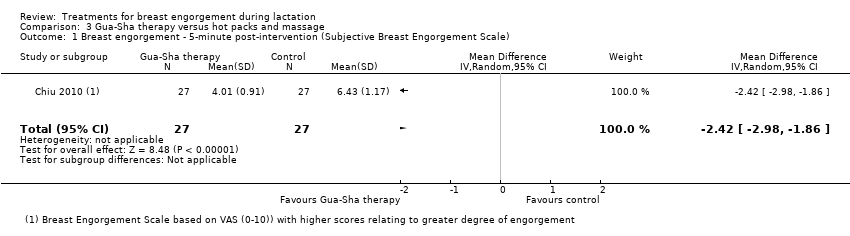
Comparison 3 Gua‐Sha therapy versus hot packs and massage, Outcome 1 Breast engorgement ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale).

Comparison 3 Gua‐Sha therapy versus hot packs and massage, Outcome 2 Breast pain ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale).
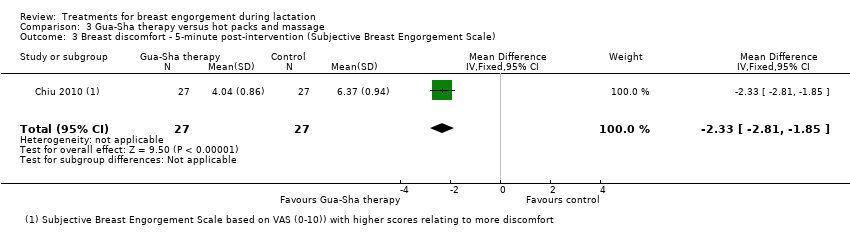
Comparison 3 Gua‐Sha therapy versus hot packs and massage, Outcome 3 Breast discomfort ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale).

Comparison 4 Ultrasound versus sham treatment, Outcome 1 Analgesic requirement.

Comparison 5 Protease complex versus placebo, Outcome 1 Pain not improved.
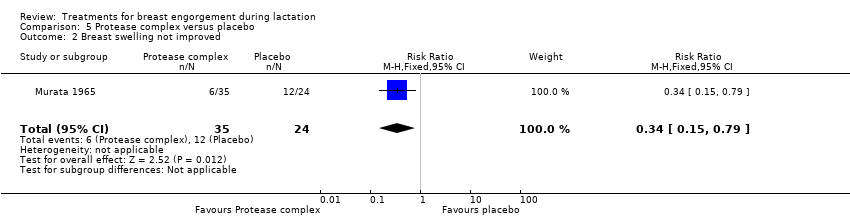
Comparison 5 Protease complex versus placebo, Outcome 2 Breast swelling not improved.
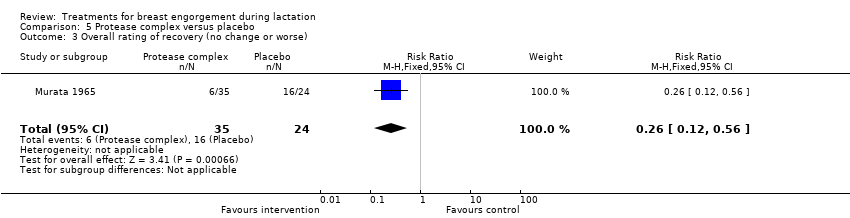
Comparison 5 Protease complex versus placebo, Outcome 3 Overall rating of recovery (no change or worse).

Comparison 6 Oxytocin versus placebo, Outcome 1 Symptoms not subsided after three days of treatment.

Comparison 7 Serrapeptase versus placebo, Outcome 1 Slight or no improvement in breast engorgement.
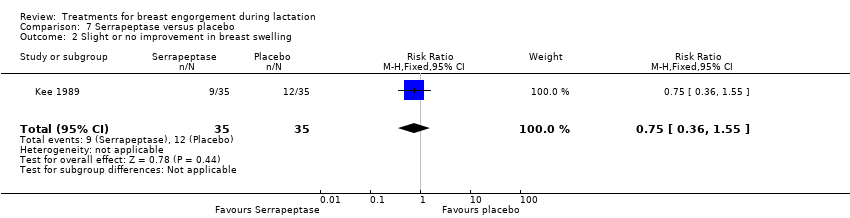
Comparison 7 Serrapeptase versus placebo, Outcome 2 Slight or no improvement in breast swelling.
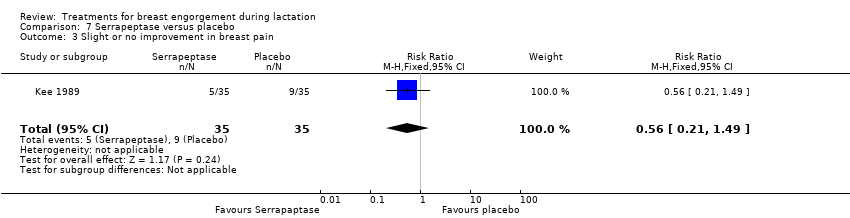
Comparison 7 Serrapeptase versus placebo, Outcome 3 Slight or no improvement in breast pain.
| Cabbage cream for breast engorgement during lactation | ||||||
| Patient or population: women with breast engorgement during lactation Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cabbage cream | |||||
| Breast pain | The mean breast pain in the intervention groups was | 39 | ⊕⊕⊝⊝ low1,2 | Higher score indicates more pain ‐ Bourbonaise pain scale ranks pain on a scale from 0 to 10, with 0 representing no pain and 10 representing excruciating pain. | ||
| Breast induration/hardness | This outcome was not reported in the trial. | |||||
| Breast swelling | This outcome was not reported in the trial. | |||||
| Breast engorgement | The mean engorgement in the intervention groups was | 39 | ⊕⊕⊝⊝ low1,2 | Higher score indicates more engorgement ‐ Hill and Humenich Breast engorgement scale ranks engorgement on a scale from 0 to 6, with 0 representing soft, no change in breasts and 6 representing very firm, very tender. | ||
| Analgesic requirement | This outcome was not reported in the trial. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The number of participants was even smaller than the pre‐determined sample size. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast abscess Show forest plot | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.04, 1.01] |
| 2 Lowest Severity Index score (day 5) (combined measurement of breast erythema, tension and pain) Show forest plot | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 0.99] |
| 3 Pyrexia (advised to take antipyrexials) Show forest plot | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast engorgement (Hill and Humenich Breast engorgement scale) Show forest plot | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.18, 0.58] |
| 2 Breast pain (Bourbonaise pain scale) Show forest plot | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.67, 1.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast engorgement ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale) Show forest plot | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐2.98, ‐1.86] |
| 2 Breast pain ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.01 [‐2.60, ‐1.42] |
| 3 Breast discomfort ‐ 5‐minute post‐intervention (Subjective Breast Engorgement Scale) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.33 [‐2.81, ‐1.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Analgesic requirement Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.63, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain not improved Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.74] |
| 2 Breast swelling not improved Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.79] |
| 3 Overall rating of recovery (no change or worse) Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.12, 0.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptoms not subsided after three days of treatment Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.68, 14.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Slight or no improvement in breast engorgement Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.14, 0.88] |
| 2 Slight or no improvement in breast swelling Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.55] |
| 3 Slight or no improvement in breast pain Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.21, 1.49] |

