استفاده از دهنده نیتریک اکسید برای آمادهسازی دهانه رحم و القای زایمان
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | A single‐blind randomised placebo‐controlled trial in an outpatient setting. Study conducted between February 2010 to January 2011 at Safdarjung hospital, New Delhi, India. | |
| Participants | Study conducted on 200 postdate pregnant women with unfavourable cervix. Inclusion criteria: included all women with singleton pregnancy more than 36 weeks with Bishop score less than 6 and no uterine contractions. Exclusion criteria: included pregnant women with malpresentation, previous caesarean section, diabetes, hypertension/ PET, vaginal bleeding, ruptured membranes, oligohydramnios, IUGR, women with heart disease or any contraindication to receive ISMN such as allergy to the drugs, bronchial asthma, hypotension and palpitations. | |
| Interventions | After recording a baseline Bishop score 200 participants were either given two 40 mg tablets of ISMN (100 women) or two, 40 mg tablets of pyridoxine as placebo (100 women). They were instructed to self‐administer at home, vaginally, 1 of the tablets at 9 AM and the other at 9 PM the same day, and to report to the hospital the next day at 9 AM for admission. Participants were also instructed to report immediately to the hospital if they had labour pains, vaginal bleeding or leakage, or decrease fetal movements. | |
| Outcomes | Maternal: caesarean section, uterine hyperstimulation with and without FHR changes, cervix unfavourable after 12‐24 hours, oxytocin augmentation, postpartum haemorrhage and headache. Fetal: meconium‐stained liquor, Apgar score < 7 at 5 minutes and NICU admission. | |
| Notes | The study used 1 dose of 0.5 mg intracervical PGE2 in both ISMN and placebo groups if the Bishop score was < 6 on admission. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence was used for randomisation. |
| Allocation concealment (selection bias) | High risk | Open allocation sequence used. |
| Incomplete outcome data (attrition bias) | Low risk | ITT was applied.100 women in ISMN group and 100 women in placebo group entered the study. 100 participants were included in the outcome analysis in each arm. No dropouts reported. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Unclear risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blind randomised control trial. Participants were blinded, but therapist were not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No evidence to the contrary. |
| Methods | Double‐blind randomised controlled trial in an outpatient setting. Recruitment between March 2005 and December 2006 at Princess Royal Maternity Hospital, Glasgow. | |
| Participants | Women scheduled for admission for cervical ripening and labour induction. Inclusion criteria: included all of the following: nulliparity, singleton fetus, cephalic presentation, ≥ 37 completed weeks' gestation, modified Bishop score < 7, and willingness to self‐administer vaginal tablets. Exclusion criteria: included women < 16 years of age, those who needed delivery within the next 48 hours in the fetal or maternal interest or who had ruptured membranes. | |
| Interventions | 350 were randomised. 177 were prescribed 40 mg ISMN tablets and 173 received placebo, with instructions to self‐administer the tablets vaginally at home at 48, 32 and 16 hours prior to scheduled time of admission. After admission to hospital, induction of labour was with vaginal prostaglandins until cervical ripening (described as Bishop score > 6) was achieved or 3 doses of prostaglandin tablets (3 mg each) were administered. Once the cervix was ripe fetal membranes were ruptured and oxytocin administered if required. | |
| Outcomes | Maternal: elapsed time from admission to delivery, operative delivery rates (caesarean section and instrumental vaginal delivery), vaginal delivery not achieved in 24 hours, cervix unfavourable/unchanged at 12 to 24 hours, oxytocin augmentation, epidural analgesia, maternal side effects, postpartum haemorrhage, requirement for additional inpatient cervical ripening agents. various outcomes relating to maternal satisfaction. Neonatal: serious neonatal morbidity/perinatal death, meconium‐stained liquor, admission and duration of NICU admission, 5‐minute Apgar score of less than 7. | |
| Notes | Detailed economic data also included. Protocol published previously. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally‐generated randomisation schedule in permuted blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Pharmacy at Western Infirmary in Glasgow prepared identical treatment packs, labelled with relevant unique study number. Allocation via automated interactive telephone response service. |
| Incomplete outcome data (attrition bias) | Low risk | 47 in ISMN arm and 46 in placebo arm withdrawn after randomisation. Majority went into spontaneous labour. 11 withdrawals in total, 2 diagnosed with breech presentations and hence excluded. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Low risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Low risk | Patients, therapists, outcome assessors and analysts blinded to allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Patients, therapists, outcome assessors and analysts blinded to allocation. |
| Methods | Double‐blind randomised controlled trial in an outpatient setting. Recruitment between November 2002 and April 2005, at the Department of Obstetrics and Gynecology, Sahlgrenska University Hospital, Gothenberg, Sweden. | |
| Participants | Inclusion criteria: uncomplicated pregnancy, singleton pregnancy, cephalic presentation, gestational age at least 42 weeks, Bishop score < 5, normal AFI > 5 cm, reactive fetal heart pattern, intact membranes. Exclusion criteria: regular uterine contractions, cardiorespiratory disease, history of headaches, history of alcohol abuse, intolerance to ISMN, serious disease defined as daily use of medication. | |
| Interventions | 200 women randomised, 100 received vaginally‐administered ISMN, 40 mg and 100 received placebo tablet. Subsequently in women where regular contractions were not established an amniotomy was performed or 1 mg of prostaglandin given. | |
| Outcomes | Maternal: caesarean section, maternal side effects, postpartum haemorrhage. Neonatal: Apgar score < 7 at 5 minutes, NICU admission. Non‐prespecified: cervix unfavourable after outpatient ripening. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Sealed sequentially numbered envelopes. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence to the contrary. |
| Selective reporting (reporting bias) | Unclear risk | Limited reporting in this area. |
| Other bias | Low risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Low risk | Suitable dummies used. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No evidence to the contrary. |
| Methods | Randomly allocated by computer programme. Inpatient setting. Recruitment between January 1999 and September 1999 in Ramathibodi Hospital, Mahidol University, Thailand. | |
| Participants | Inclusion criteria: singleton pregnancy, cephalic presentation, Bishop score < 6, reactive non‐stress test. Exclusion criteria: fetal malpresentations, previous scarred uterus or contraindications to receive nitric oxide donors or prostaglandins. | |
| Interventions | 112 women randomised, 110 analysed. 54 women received vaginal GTN (500 µg) versus 56 women who received vaginal PGE2 (3 mg). Both groups reviewed at 3, 6,12 and 24 hours. Both medications repeated after 6 hours if Bishop score < 6. At 24 hours (or earlier if possible) both groups had forewater amniotomy and oxytocin. | |
| Outcomes | Maternal: uterine hyperstimulation both with and without FHR changes, caesarean section, maternal headache, postpartum haemorrhage, serious maternal complications. Neonatal: Apgar score < 7 at 5 minutes, NICU admission. | |
| Notes | Data also presented within abstract from FIGO 2000. Also early data from study presented within Chanrachakul et all 2000, but not mentioned in final report. May represent salami slicing/duplicate publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) | Low risk | 2 women excluded due to incomplete data. |
| Selective reporting (reporting bias) | Unclear risk | Limited reporting in this area. |
| Other bias | Unclear risk | Limited reporting in this area. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Limited reporting in this area. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor unaware of allocation. |
| Methods | Randomly allocated by computer‐generated number. Inpatient setting. Recruitment between December 1999 and September 2000 in Ramathibodi Hospital, Mahidol University, Thailand. | |
| Participants | Inclusion criteria: singleton pregnancy, cephalic presentation, Bishop score < 6, reactive non‐stress test. Exclusion criteria: fetal malpresentations. contraindications to receive nitric oxide donors or prostaglandins. | |
| Interventions | 110 women randomised, 107 analysed. 55 women received vaginal ISMN tablet (40 mg) versus 52 women who received vaginal misoprostol (50 µg). Both groups reviewed at 6, 12 and 24 hours. At 24 hours (or earlier if possible) both groups had forewater amniotomy and oxytocin. | |
| Outcomes | Maternal: uterine hyperstimulation both with and without FHR changes, caesarean section, cervix unfavourable/unchanged after 12 to 24 hours, oxytocin augmentation, maternal nausea or headache and postpartum haemorrhage. Neonatal: Apgar score < 7 at 5 minutes, NICU admission. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) | Low risk | 2 sets of incomplete data and 1 woman withdrawn due to an undiagnosed breech presentation. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Low risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of suitable dummies used. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor not aware of treatment allocation. |
| Methods | An inpatient single‐centre randomised trial carried out at Rajshahi College Hospital, Bangledesh between January 2008 and June 2009. | |
| Participants | Inclusion criteria: nulliparous, singleton, term pregnancy, intact membranes, Bishop score 4 or less, cephalic presentation. Exclusion criteria: fetal compromise of sufficient severity, cephalopelvic disproportion, non‐cephalic presentation. | |
| Interventions | 200 women randomised. 100 women received vaginal 40 mg IMN tablets and 100 women received 50 mcg misoprostol (1/4 of 200 mcg tablet) administered into posterior vaginal fornix. All women were assessed every 6 hours and re‐administered the medication if Bishop score was not more than 6 or labour pains were established for a maximum of 4 doses. | |
| Outcomes | Maternal outcomes: maternal demographics, adverse outcomes, mode of delivery, maternal complications (hyperstimulation, tachysystole, fever, nausea and vomiting, headache, hypotension, postpartum atony), change in Bishop score after medication. Neonatal outcomes: general neonatal outcomes (not clearly specified). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly divided' but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence to the contrary. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes do not appear to be prespecified in text. |
| Other bias | Low risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details given. |
| Methods | A randomised double‐blind clinical trial, conducted between January 2009 and November 2010, in Shahid Akbar‐abadi Obstetrics and Gynaecology Centre, a University hospital in Tehran, Iran. | |
| Participants | 136 women scheduled for induction pf labour were recruited for the study. Inclusion criteria: primiparous, singleton, term or post‐term pregnant women with Bishop score < 5 and cephalic presentation were included in the study. Exclusion criteria: EFW > 4 kg, oligohydramnios, IUGR, non reassuring FHR, ruptured membranes, any contraindication to prostaglandins or ISDN, BMI > 30, placenta praevia, vaginal bleeding, uterine contractions, suspected chorioamnionitis. | |
| Interventions | 132 participants were randomly assigned to either misoprostol group or ISDN. 64 in misoprostol group and 66 in ISDN group. 2 women in misoprostol group had caesarean section on request and hence were excluded. Women in misoprostol group had 25 mcg PGE1 and women in ISDN group had 40 mg ISDN, maximum of 2 doses were inserted vaginally after 4 hours if the Bishop score was < 8 or uterine contractions < 3 in 10 min with duration of < 40 seconds. | |
| Outcomes | Maternal: changes in Bishop score after the drug administration, need for stimulation, time from initial dose to active phase of labour and to delivery, method of delivery, complications of ISDN. Fetal: 1 and 5 min Apgar score < 7. | |
| Notes | After 2 doses of the drug, oxytocin was used and women had caesarean section delivery if labour not established 6 hours after oxytocin infusion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation. |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes were used. |
| Incomplete outcome data (attrition bias) | Low risk | The study drop outs were explained and participants were analysed in their respective groups. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Low risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind trial. |
| Methods | Non‐blinded parallel randomised controlled trial conducted on the midwifery ward of Shahid Akbar Abadi Hospital, Tehran, Iran. Used block randomisation. | |
| Participants | 149 nulliparous women were recruited to this study Inclusion criteria: nulliparous women, singleton, cephalic presentation, gestation over 40 weeks and 4 days, Bishop score less than 5, no contraindications for ISDN, no previous caesarean section, no uterine scar, no underlying disease, not required to have reactive nonstress test or normal biophysical profile ultrasound. | |
| Interventions | 149 nulliparous women were randomised into 3 groups: 50 received 40 mg ISDN, maximum 2 doses inserted vaginally after 4 hours, 49 received 20 mg ISDN orally, maximum 2 doses 4 hourly, 50 were the control and received no medication. | |
| Outcomes | Suggested to be Bishop score change but prespecified outcomes are not explicit. | |
| Notes | Monitiored for 4 hours following administration of medication then discharged home for 24 hours. We combined the oral and vaginal ISDN groups to create a single pair‐wise comparison with the control. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'selected by simple random sampling method' |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) | Unclear risk | Total numbers of groups are not given in the results tables. |
| Selective reporting (reporting bias) | High risk | Outcomes not prespecified. |
| Other bias | Unclear risk | None noted. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Methods | Prospective study. Post Graduate Institute of Medical Sciences, India. Setting unclear. | |
| Participants | Inclusion criteria: primigravidae, singleton pregnancy, cephalic presentation unfavourable cervix. Exclusion criteria: unclear. | |
| Interventions | 400 women randomised 200 received intracervically administered ISDN, 40 mg and 200 received 0.5 mg PGE2 vaginal gel, which was repeated after 6 hours if Bishop score remained low. Subsequently oxytocin was started after 12 hours in both groups. | |
| Outcomes | Maternal: vaginal delivery not achieved in 24 hours, caesarean section. | |
| Notes | Limited data extraction as report in abstract format only. Authors contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomised.' |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) | Unclear risk | Full report awaited. |
| Selective reporting (reporting bias) | Unclear risk | Full report awaited. |
| Other bias | Unclear risk | Full report awaited. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention if suitable dummies used. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention if suitable dummies used. |
| Methods | Randomly allocated using computer generated random number table. Allocation concealed using sequentially numbered opaque envelopes.Inpatient setting in India. | |
| Participants | Inclusion criteria: primigravida, singleton, cephalic presentation, 38 weeks gestation or more, modified Bishop score of less than 6. Exclusion criteria: under 18 years old, uterine scar, ruptured membranes, uterine contractions, medical complications, contraindications to vaginal delivery or isosorbide mononitrite therapy. | |
| Interventions | 100 recruited, 100 randomised into 2 groups: 50 women received 40 mg isosorbide mononitrite inserted vaginally into posterior fornix, second dose given 12 hours later if Bishop score still less than 6, 50 received 40 mg placebo (pyridoxine) administered the same way as intervention. | |
| Outcomes | Maternal: change in modified Bishop Score at 12 and 24 hours after drug insertion, time from initiation of cervical ripening till delivery, labour duration, need of oxytocin augmentation, mode of delivery, uterine hyperstimulation, tachysystole, headache, tachycardia, palpitations, hypotension, nausea and vomiting, proportions of unripe cervix (Bishop Score < 6) at 24 hr after first drug insertion. Neonatal: Apgar scores < 7 at 1 min and 5 min, fetal distress, NICU admissions, length of neonatal stay in NICU. | |
| Notes | Absense of headache, nausea, vomiting and palpitations only mentioned referring to IMN group in text. No side effects mentioned for control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated into 2 groups using a computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | Opaque, sequentially numbered envelopes |
| Incomplete outcome data (attrition bias) | Low risk | No evidence to the contrary. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Unclear risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Low risk | 'drug insertion was done by a senior resident who was not part of the investigation'. Dummy used as placebo therefore assuming patients blinded as well. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Methods | Randomised clinical trial with 3 treatment arms. Recruitment period unclear, conducted at University Hospital in Qazvin, Iran. | |
| Participants | Inclusion criteria: nulliparous women, 39 weeks gestation or over with Bishop score less than 4. Exclusion criteria: vaginal bleeding, membrane rupture, active genital herpes infection, history of myomectomy, non‐reassuring fetal heart status, history of heart disease. | |
| Interventions | 75 women randomised into 3 groups: 25 women received ISMN, 25 received transvaginal catheter and 25 received Laminaria. | |
| Outcomes | Maternal: interval between time of induction and cervical ripening, interval between oxytocin administration and full cervical dilatation, duration of second and third labour phases, mode of delivery, maternal complications. Neonatal: complications. | |
| Notes | Non‐English language. Laminaria not eligible as an intervention for this review. No data suitable for analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants 'randomly divided' by choosing colourful cards. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence to the contrary. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Unclear risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of suitable dummies used. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details given. |
| Methods | Randomly allocated using random number tables in permuted blocks of 12. Concealment sealed, opaque sequentially numbered envelopes. Inpatient setting. Recruitment between August 1998 and July 1999 Dept of Obstetrics and Gynecology, University of Glasgow and Glasgow Royal Maternity Hospital. | |
| Participants | Inclusion criteria: not stated. Exclusion criteria: Bishop score > 7, multiple pregnancy, history of antepartum haemorrhage, pregnancy‐induced hypertension or pre‐eclampsia, breech presentation, fetal abdominal circumference < 5th percentile, AFI < 5th percentile, history of cardiorespiratory disease, history of headache. | |
| Interventions | 38 recruited, 36 women randomised into 3 groups 13 women received vaginally‐administered ISMN (20 mg), 11 women received vaginally‐administered ISMN (40 mg), 12 women received a vaginal examination only. Women who failed to achieve a Bishop score of > 7, 360 minutes after treatment allocation underwent an amniotomy. The women filled out a symptom questionnaire and had their cervical score assessed pretreatment administration and 360 minutes after administration. | |
| Outcomes | Maternal: caesarean section, instrumental vaginal delivery and maternal side effects (headache). Neonatal: NICU admission. | |
| Notes | Only data comparing the 40 mg ISMN group to placebo were analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated using random number tables in permuted blocks of 12. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque sequentially numbered envelopes. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence to the contrary. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Low risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Low risk | This was a double‐blinded study and independent observer administrated the treatment. The assessment of the cervix was carried out by the same assessor to reduce individual variation. The patient was not aware of the treatment given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The assessment of the cervix was carried out by the same assessor to reduce individual variation. |
| Methods | Double‐blind randomised controlled trial in an inpatient setting. Recruitment between September 2001 and November 2003 at Princess Royal Maternity Hospital, Glasgow. | |
| Participants | Women scheduled for admission for cervical ripening and labour induction. Inclusion criteria: included all of the following: nulliparity, singleton fetus, cephalic presentation, ≥ 38 completed weeks' gestation, modified Bishop score < 6, and normal admission CTG. Exclusion criteria: included women < 16 years of age, ≥ 1 birth at > 23 weeks' gestation, previous caesarean section, those who needed delivery within the next 48 hours in the fetal or maternal interest or who had ruptured membranes. | |
| Interventions | 400 were randomised. 200 were prescribed 40 mg ISMN tablets and 200 received 2 mg vaginal PGE2. After 24 hours if the Bishop score was < 6 then a 1 mg 'rescue' dose of PGE2 gel was given. | |
| Outcomes | Maternal: uterine hyperstimulation with FHR changes, caesarean section, epidural analgesia, instrumental vaginal delivery, maternal side effects (nausea and headache). Neonatal: Apgar score at 5 minutes, NICU admission. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated in permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Centrally‐dispensed sealed opaque sequentially numbered envelopes. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence to the contrary. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Low risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Low risk | Patient and outcome assessors unaware of allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Patient and outcome assessors unaware of allocation. |
| Methods | Double‐blind randomised controlled trial (setting unclear). Recruitment period not stated, at Urquinana Central Hospital, Maracaibo, Zuilia State, Venezuela. | |
| Participants | Women scheduled for admission for cervical ripening and labour induction. Inclusion criteria: included all of the following: singleton fetus, term pregnancies, modified Bishop score < 6, and not in labour. Exclusion criteria: Bishop score > 7, ruptured membranes, chorioamnionitis, bleeding. | |
| Interventions | 60 were randomised. 30 were prescribed 40 mg ISMN tablets and 30 received 50 mcg vaginal misoprostol. These medications were repeated every 4 hours for 24 hours. no further details of subsequent treatments were given. | |
| Outcomes | Oxytocin augmentation, Apgar score < 7 at 5 minutes, maternal side effects. | |
| Notes | Original trial report in Spanish and translated prior to extraction. The authors are grateful to Luciana Figuera for her translation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Limited reporting unable to make judgement. |
| Selective reporting (reporting bias) | Unclear risk | Limited reporting unable to make judgement. |
| Other bias | Unclear risk | Limited reporting unable to make judgement. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Limited reporting unable to make judgement. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Limited reporting unable to make judgement. |
| Methods | Double‐blind randomised controlled trial (stratified by parity) in an inpatient setting. Recruitment between August 2003 and April 2004 at the University Obstetric Unit, Teaching Hospital, Galle, Sri Lanka. | |
| Participants | Women scheduled for admission for cervical ripening and labour induction. Inclusion criteria: included all of the following: uncomplicated singleton fetus, cephalic presentation, ≥ 41 completed weeks' gestation, modified Bishop score < 5. Exclusion criteria: any medical or obstetric problems or contraindications to ISMN. | |
| Interventions | 156 were randomised. 78 were prescribed 60 mg ISMN tablets and 78 received placebo (vitamin C) re‐examined after 48 hours. If cervix favourable (Bishop score ≥ 7) then they were induced the same day with amniotomy and oxytocin. if unfavourable then an intracervical extra amniotic Foley catheter was used to induce further ripening. | |
| Outcomes | Maternal: caesarean section, additional induction agents used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation (stratified by parity). |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque sequentially numbered envelopes. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence to the contrary. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Low risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Low risk | Outcome assessor unaware of allocation. Suitable dummies used so patient blinded as well. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor unaware of allocation. Suitable dummies used so patient blinded as well. |
| Methods | Prospective randomised control trial carried out at Al‐Elwiya Maternity Teaching Hospital, Baghdad. | |
| Participants | 150 pregnant women were randomised. Inclusion criteria: primiparous women, singleton fetus with uncomplicated pregnancy, admitted for post‐dates induction. Exclusion criteria: obstetric, gynaecological or medical problems. | |
| Interventions | Out of 150 women randomised, 75 received 40 mg IMN vaginally in the form of two 20 mg tablets and 75 received 50 mcg misoprostol vaginally. The process was repeated in the misoprostol group every 6 hours if the Bishop scores did not improve for a maximum of 3 doses. | |
| Outcomes | Maternal: delivery interval, mode of delivery, adverse effects. Neonatal: general outcomes (not prespecified). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomised', no further details given. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence to the contrary. |
| Selective reporting (reporting bias) | High risk | Outcomes not clearly specified in the text. |
| Other bias | Low risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear. |
| Methods | A single‐centre balanced randomised parallel group study carried out at Menoufia University Hospital, Egypt between January 2013 and January 2014 in an outpatient setting. | |
| Participants | 80 pregnant women with previous 1 caesarean section were randomised. Inclusion criteria: women with 37 weeks and beyond gestation, intact membranes, Bishop score less than 6, reactive non‐stress test, normal umbilical artery dopplers indices, absence of labour and willingness to participate were included. Exclusion criteria: women with intrauterine fetal death, twin pregnancy, polyhydramnios, placenta praevia, severe anaemia, severe hypertension, uncontrolled diabetes, coagulopathy and any contraindication to labour induction were excluded from the study. | |
| Interventions | Out of 80 women who were recruited for the study, 40 had Foley catheter inserted (control) and 40 received single‐dose 40 mg ISMN vaginally. Foley catheter was either removed at 12 hours or expelled spontaneously. Women in ISMN group were examined every 3 hours for the next 24 hours. | |
| Outcomes | Maternal: caesarean section, oxytocin augmentation, uterine rupture, epidural analgesia, instrumental delivery, nausea and vomiting, headache, puerperal pyrexia and women not satisfied with the treatment. Fetal: meconium‐stained liquor, Apgar score less than 7 at 5 minutes and NICU admission. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence was used for randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence to the contrary. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of the patient and therapist is not feasible. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not feasible. |
| Methods | Double‐blind randomised controlled trial using random number tables. Trial conducted at Mexican Social Security Institute's High speciality medical unit number 48 in Leon, Guanajuato. Patients were recruited between February 1 to August 31, 2009. | |
| Participants | Total number of participants in the study were 66. Divided in to intervention and control group. Each group had 33 participants. Inclusion criteria: women with singleton pregnancy, cephalic presentation at 41 weeks and 6 days gestation with Bishop score less than 6 were recruited for the study. Exclusion criteria: women with acute fetal distress, cephalopelvic disproportion, allergy to Isosorbide or dinoprostone, cardiothoracic condition, placenta praevia, oligohydramnios with AFI < 5, caesarean section and premature membrane rupture were excluded. | |
| Interventions | 66 were randomised. 33 received 0.5 mcg of dinoprostone (control group) and another 33 received 20 mg of ISDN (experimental group). In both groups the medication was applied vaginally at 6 hours intervals for a maximum of 3 doses. | |
| Outcomes | Maternal: length of labour and caesarean section. Fetal: meconium liquor, NICU admission, Apgar score to the minute and at 5 minutes. | |
| Notes | Spanish paper. Available information limited by language. Cost‐analysis for both the drugs administered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables were used. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Article did not indicate whether any patients withdrew or dropped out. |
| Selective reporting (reporting bias) | Unclear risk | No information available. |
| Other bias | Unclear risk | Limited information. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Patients were blinded but it is unclear if therapist was blinded as well. |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear. |
| Methods | Double‐blind multicentre randomised controlled trial conducted in 11 French university hospital referral maternity units. Trial conducted between June 25, 2009, and November 14, 2012 in an outpatient setting. | |
| Participants | Total of 1373 women were randomised and included in the trial. 11 women were later excluded as they did not meet the inclusion criteria. ITT analysis was applied. Inclusion criteria: nulliparous women with singleton pregnancy, cephalic presentation, intact membranes, Bishop score less than 6 and at 41 + 0 weeks of gestation were included in the study. Exclusion criteria: women less than 18 years, with no social security coverage, on antihypertensive treatment, fetal death and known to have contraindication to ISMN were excluded from the study. | |
| Interventions | 1373 women were randomised into 2 groups. 684 women were given placebo and 678 women were given 40 mg ISMN. 11 women were excluded and ITT analysis was applied. In each group women received 3 doses of the medication vaginally at 48 hours interval. | |
| Outcomes | Maternal: caesarean section, serious maternal morbidity or death, oxytocin augmentation, instrumental vaginal delivery, maternal side effects, nausea, vomiting, diarrhoea, headache, postpartum haemorrhage, severe postpartum haemorrhage and women not satisfied with the treatment. We have assumed that the data of severe postpartum haemorrhage are included in the postpartum haemorrhage and hence have only considered postpartum haemorrhage data. Fetal: serious neonatal morbidity or perinatal death and Apgar score less than 7 at 5 minutes. We did not include the data on NICU as the trial only mentioned the number of NICU admissions for 5 days or more and data for all admissions are not available. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence was used for randomisation in permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Central randomisation: a web‐based application was used to assign women and the allocation was available to any of the research team. |
| Incomplete outcome data (attrition bias) | Low risk | Low risk. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Unclear risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Low risk | Patient and therapist both blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor and analyst blinded. |
| Methods | 'Randomised' Inpatient setting. Recruitment between November 2001 and November 2003, Deptartment of Obstetrics and Gynecology, at the All India Institute of Medical Sciences, New Delhi. | |
| Participants | Women scheduled for admission for cervical ripening and labour induction. Inclusion criteria: included all of the following: nulliparity, singleton fetus, modified Bishop score < 6. Exclusion criteria: previous caesarean section and ruptured membranes. | |
| Interventions | 65 were randomised into 3 groups. 21 were prescribed 500 mcg GTN (misoprostol) tablets, 21 received 0.5 mg intracervical PGE2 and 23 received 50 mcg of vaginal misoprostol. Women were reassessed at 6 hours and if possible amniotomy was performed. If Bishop score < 6 then further dose of same drug was given. | |
| Outcomes | Maternal: uterine hyperstimulation with and without FHR changes, caesarean section, cervix unfavourable at 12 to 24 hours, oxytocin augmentation, instrumental vaginal delivery and maternal side effects (headache). Neonatal: perinatal death, serious neonatal morbidity or death. | |
| Notes | 2 patients (1 from GTN and 1 from misoprostol group excluded due to being delivered by caesarean section after first dose of medication. Not clear if included in final data on caesarean section. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised." |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) | Unclear risk | May be concern over 2 post randomisation exclusions. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Unclear risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Methods | A prospective double‐blind randomised clinical trial conducted in Tanta University Hospital, Egypt between April 2010 and March 2012. | |
| Participants | 196 women participated in this study. Inclusion criteria: nulliparous women, gestational age of at least 37 weeks, with singleton fetus and vertex presentation, Bishop score less than 6 and intact membranes, reactive non‐stress test, normal umbilical artery doppler indices, absence of labour and willingness to participate were included in the study. Exclusion criteria: women excluded from study were multiparous, with multiple pregnancy, fetal malpresentation, premature rupture of membranes, regular uterine contractions, major cephalopelvic disproportion and with contraindications to ISMN or misoprostol. | |
| Interventions | 200 women were randomised into 3 groups and 196 women were analysed. 4 dropouts noted, 2 each in ISMN and misoprostol group as they did not meet the inclusion criteria. 65 women received 50 mcg of misoprostol vaginally. 65 women received 40 mg ISMN and 66 women had both 40 mg ISMN and 50 mcg misoprostol. 3 doses of the medication was inserted vaginally at 0, 6 and 12 hours. | |
| Outcomes | Maternal: caesarean section, uterine hyperstimulation without FHR changes, oxytocin augmentation, epidural analgesia, analgesia required, nausea and vomiting, headache and postpartum haemorrhage. Fetal: meconium‐stained liquor, Apgar score less than 7 in 5 minutes and NICU admission. | |
| Notes | In this review we have not used the combination treatment data because it is a complex intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence used for randomisation. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque and sequentially numbered envelopes were used. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence to the contrary. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) | Low risk | Patient and therapist were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear. |
| Methods | A double blind randomised controlled trial conducted between March 2006 and April 2007 at the University Obstetic Unit, Teaching Hopital Mahamodara Galle, Sri Lanka. The study was conducted an inpatient setting. | |
| Participants | Women with post‐term pregnancy and unfavourable cervix. Incusion criteria: singleton pregnancy with cephalic presentation, gestation between 40 weeks + 5 days and 41 weeks and Bishop score < 5. Exclusion criteria: women with any medical or obstetrics problems and with any contraindication to the use of ISMN were excluded from the study. | |
| Interventions | 156 women were recruited to the study and randomised into 3 groups. 52 women in group A received ISMN 40 mg tablets, 52 women in group B received 60 mg ISMN‐SR tab, and rest 52 women in group C received 100 mg vitamin C tablets. | |
| Outcomes | Maternal: changes in the mean Bishop score at 6 hours and 48 hours, caesarean section rate, uterine hyperstimulation without FHR changes. Fetal: mean 5 minute Apgar score of babies delivered within 72 hours of the intervention. | |
| Notes | Randomised controlled trial with 3 study arms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence to the contrary. |
| Selective reporting (reporting bias) | Low risk | No evidence to the contrary. |
| Other bias | Low risk | No evidence to the contrary. |
| Blinding of participants and personnel (performance bias) | High risk | Therapist aware of the intervention given. |
| Blinding of outcome assessment (detection bias) | Low risk | No evidence to the contrary. |
| Methods | A randomised double‐blind, placebo‐controlled trial conducted in Sina Hospital (an education hospital in Ahvaz, Iran) between June to October 2010. Trial was conducted in an outpatient setting. | |
| Participants | 90 primiparous women presenting to the hospital with any sign of labour were recruited for the study. Inclusion criteria: primiparous women, between age 18‐35 years, Bishop score < 6, BMI between 19.8‐26, cephalic presentation, singleton fetus, normal stress test or biophysical profile in last 48 hours and gestation age of 40‐42 weeks. Exclusion criteria: women with headache, alcohol abuse, polyhydramnios, placenta praevia or abruption and with any contraindication to induction of labour were excluded from the study. | |
| Interventions | 90 women recruited in the study were randomised into 2 groups. ISMN group received 2 doses of 40 mg ISMN vaginally at 0 and 12 hours. The other group received placebo tablets vaginally at 0 and 12 hours. | |
| Outcomes | Maternal: changes in Bishop score, duration between drug administration to active phase of labour, induction to delivery interval, amount of oxytocin used, length of second and third stages of labour and caesarean section rates. Fetal: Apgar scores at 1st and 5th minute after birth. | |
| Notes | This trial recruited women coming with signs of labour. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Coded drug boxes. |
| Incomplete outcome data (attrition bias) | Unclear risk | 10 dropouts from the study not explained. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) | Low risk | Both patient and therapist blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | No evidence to the contrary. |
AFI: amniotic fluid index
BMI: body mass index
CTG: cardiotocograph
EFW: estimated fetal weight
FHR: fetal heart rate
GTN: glyceral trinitrate
ISDN: isosorbide dinitrate
ISMN: isosorbide mononitrate
ITT: intention‐to‐treat
IUGR: intrauterine growth restriction
mcg: microgram
mg: milligram
NICU: neonatal intensive care unit
PET: pre‐eclamptic toxaemia
PGE2: prostaglandin E2
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This study compared used complex intervention. An ISMN and misoprostol combination was compared against placebo and misoprostol combination. Hence was excluded. | |
| This study was excluded as there were no extractable data. It has not been possible to contact the authors for clarification. | |
| Study outcome measures relate to pharmacokinetic characteristics (serum concentration) of NO donor (ISMN) administration, rather than to clinical outcomes. | |
| Study intervention involves the use of ISMN with or without oral misoprostol. Hence as a complex intervention is excluded from review. | |
| Study setting inappropriate. Participants received NO donor (ISMN) before elective caesarean section rather than for the indication of post‐term pregnancy and thus induction of labour. | |
| Randomised trial comparing misoprostol with intracervical Foley catheter plus NO donor (IMN). Complex intervention which does not allow direct comparison for NO donor efficiency. | |
| Randomised trial comparing ISMN to placebo. Subsequent treatment was dependent on Bishop score and if less than 6 the patients received up to 3 doses of vaginal PGE2 (3 mg) if more than 6 patients received an amniotomy and intravenous oxytocin. Hence intervention is complex and it is not possible to separate out those women who did or did not have prostaglandins in addition to oxytocin. | |
| The published study has large sections that appears to be similar to a previous paper (Nicoll et al 2000: study ID 11517) which is already included in our review. In particular the entire introduction section, large sections of the methods, and parts of the comments section does not appear to be original work. | |
| Study intervention involved use of ISMN with concurrent oxytocin compared to extra‐amniotic saline as complex intervention is excluded from review. | |
| Randomised trial comparing GTN (500 micrograms) with concomitant vaginal PGE2 (2 mg) to GTN alone. Hence excluded as is a complex intervention. | |
| The primary focus of this study is to examine NO levels. No relevant data are extractable. | |
| Study comparison inappropriate for review criteria. Study compared NO donor (ISMN) and PGE2 (dinoprostone) to PGE2 (dinoprostone) alone. This study design does not allow a direct comparison for NO donor efficacy. | |
| Randomised trial comparing 2 regimens of ISMN administration which is an inappropriate comparison for this review. |
GTN: glyceral trinitrate
ISMN: isosorbide mononitrate
NO: nitric oxide
PGE2: prostaglandin E2
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Non‐English language ‐ in Farsi. Awaiting translation. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| Analysis 1.1 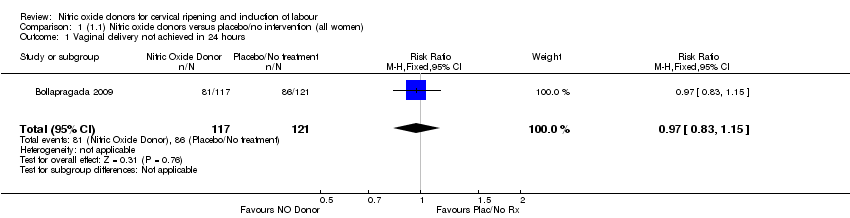 Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.62] |
| Analysis 1.2  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 3 Caesarean section Show forest plot | 9 | 2624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.11] |
| Analysis 1.3  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 3 Caesarean section. | ||||
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 2 | 1712 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.08, 33.26] |
| Analysis 1.4  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 4 Serious neonatal morbidity/perinatal death. | ||||
| 5 Serious maternal morbidity or death Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.5  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 5 Serious maternal morbidity or death. | ||||
| 6 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 4 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.67, 0.90] |
| Analysis 1.6  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 6.1 Standard release | 4 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.69, 0.94] |
| 6.2 Slow release | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.82] |
| 7 Oxytocin augmentation Show forest plot | 4 | 1916 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| Analysis 1.7 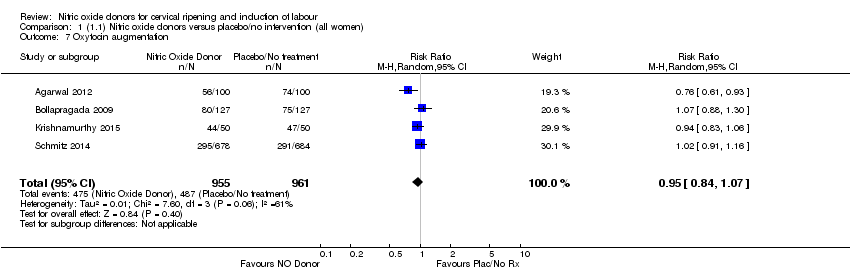 Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 7 Oxytocin augmentation. | ||||
| 8 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.80] |
| Analysis 1.8 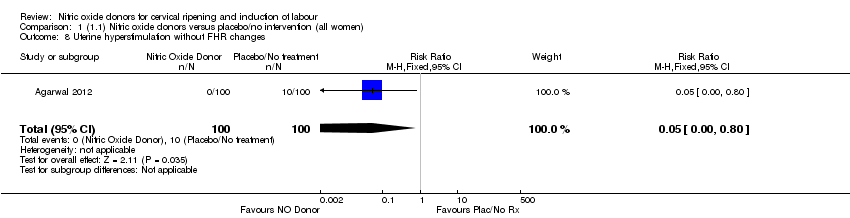 Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 8 Uterine hyperstimulation without FHR changes. | ||||
| 9 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 1.9  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 9 Epidural analgesia. | ||||
| 10 Instrumental vaginal delivery Show forest plot | 4 | 1835 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.10] |
| Analysis 1.10  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 10 Instrumental vaginal delivery. | ||||
| 11 Meconium‐stained liquor Show forest plot | 3 | 699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.14] |
| Analysis 1.11 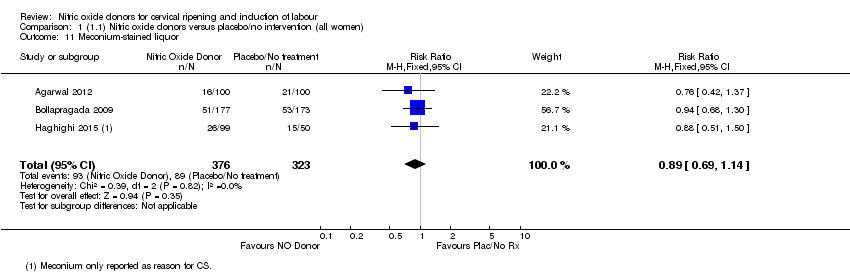 Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 11 Meconium‐stained liquor. | ||||
| 12 Apgar score < 7 at 5 minutes Show forest plot | 5 | 2212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.54, 2.07] |
| Analysis 1.12 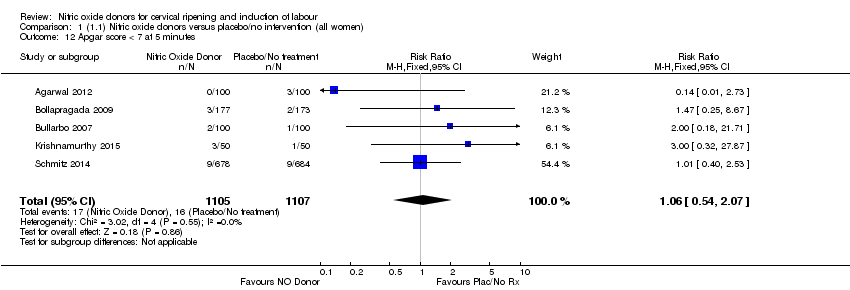 Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 12 Apgar score < 7 at 5 minutes. | ||||
| 13 Neonatal intensive care unit admission Show forest plot | 5 | 873 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.47, 1.46] |
| Analysis 1.13  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 13 Neonatal intensive care unit admission. | ||||
| 14 Perinatal death Show forest plot | 2 | 1712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| Analysis 1.14 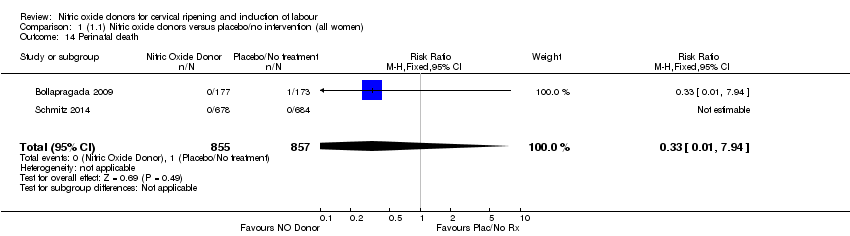 Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 14 Perinatal death. | ||||
| 15 Maternal side effects (all) Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [2.49, 3.20] |
| Analysis 1.15 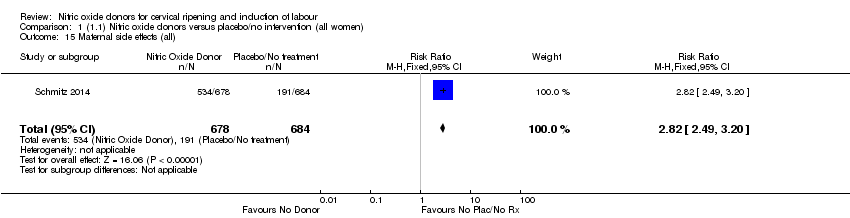 Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 15 Maternal side effects (all). | ||||
| 16 Maternal side effects (nausea) Show forest plot | 3 | 1782 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.47, 4.05] |
| Analysis 1.16 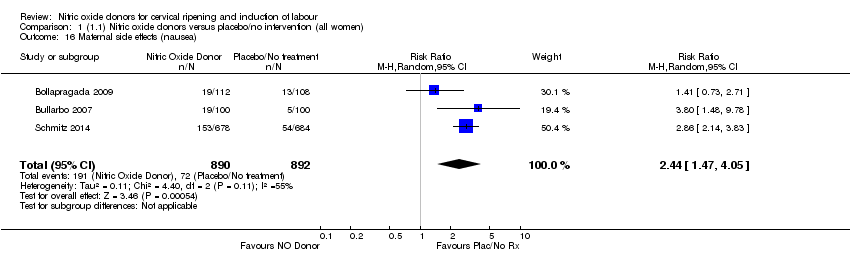 Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 16 Maternal side effects (nausea). | ||||
| 17 Maternal side effects (headache) Show forest plot | 6 | 2085 | Risk Ratio (M‐H, Random, 95% CI) | 6.59 [3.97, 10.95] |
| Analysis 1.17  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 17 Maternal side effects (headache). | ||||
| 18 Maternal side effects (vomiting) Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.54, 3.81] |
| Analysis 1.18  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 18 Maternal side effects (vomiting). | ||||
| 19 Maternal side effects (diarrhoea) Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.95, 2.19] |
| Analysis 1.19 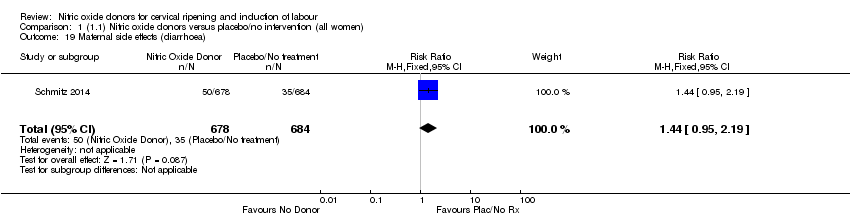 Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 19 Maternal side effects (diarrhoea). | ||||
| 20 Postpartum haemorrhage Show forest plot | 2 | 1562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.90, 1.40] |
| Analysis 1.20  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 20 Postpartum haemorrhage. | ||||
| 21 Women not satisfied Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.82, 1.38] |
| Analysis 1.21  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 21 Women not satisfied. | ||||
| 22 Additional induction agents used Show forest plot | 5 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.88] |
| Analysis 1.22  Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 22 Additional induction agents used. | ||||
| 22.1 Standard release | 5 | 2077 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.58, 0.92] |
| 22.2 Slow release | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| Analysis 2.1 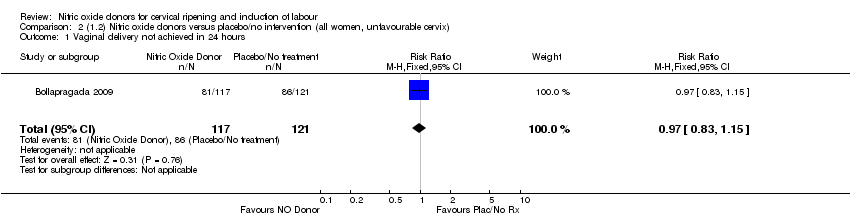 Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.62] |
| Analysis 2.2  Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 3 Caesarean section Show forest plot | 8 | 1262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| Analysis 2.3  Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 3 Caesarean section. | ||||
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| Analysis 2.4 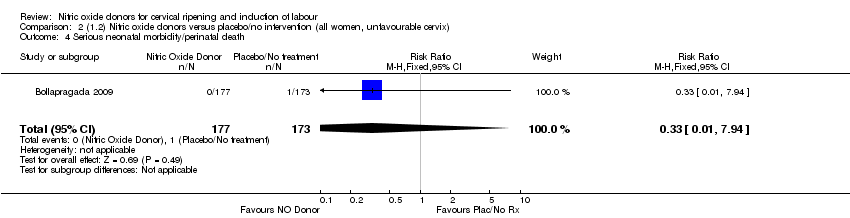 Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death. | ||||
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 3 | 557 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.73, 0.89] |
| Analysis 2.5  Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 6 Oxytocin augmentation Show forest plot | 3 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.82, 1.03] |
| Analysis 2.6  Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 6 Oxytocin augmentation. | ||||
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.80] |
| Analysis 2.7  Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 7 Uterine hyperstimulation without FHR changes. | ||||
| 8 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 2.8  Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 8 Epidural analgesia. | ||||
| 9 Instrumental vaginal delivery Show forest plot | 3 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.28] |
| Analysis 2.9  Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 9 Instrumental vaginal delivery. | ||||
| 10 Meconium‐stained liquor Show forest plot | 3 | 699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.14] |
| Analysis 2.10 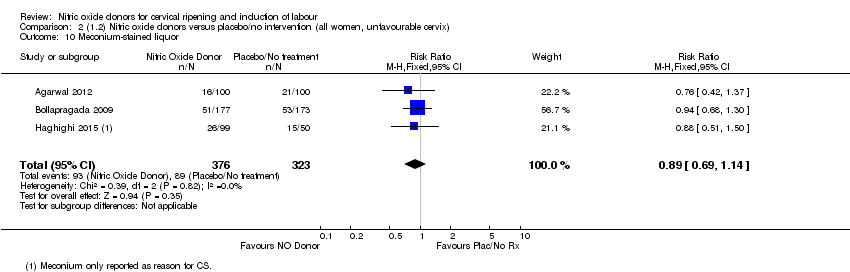 Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 10 Meconium‐stained liquor. | ||||
| 11 Apgar score < 7 at 5 minutes Show forest plot | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.42, 2.98] |
| Analysis 2.11 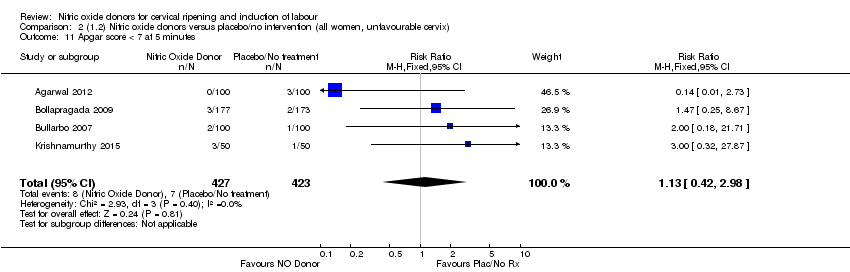 Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 11 Apgar score < 7 at 5 minutes. | ||||
| 12 Neonatal intensive care unit admission Show forest plot | 5 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.30] |
| Analysis 2.12  Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 12 Neonatal intensive care unit admission. | ||||
| 13 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| Analysis 2.13  Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 13 Perinatal death. | ||||
| 14 Maternal side effects (nausea) Show forest plot | 2 | 420 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.22, 3.50] |
| Analysis 2.14 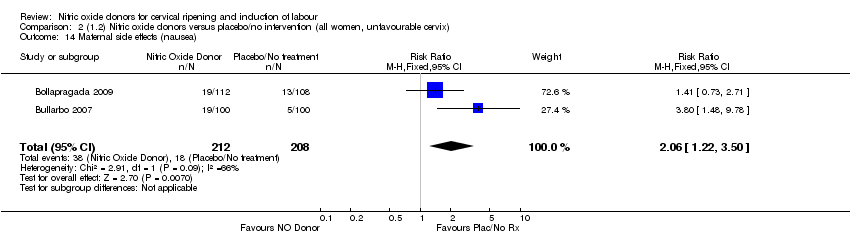 Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 14 Maternal side effects (nausea). | ||||
| 15 Maternal side effects (headache) Show forest plot | 5 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.04 [5.13, 9.66] |
| Analysis 2.15  Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 15 Maternal side effects (headache). | ||||
| 16 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.57, 2.40] |
| Analysis 2.16 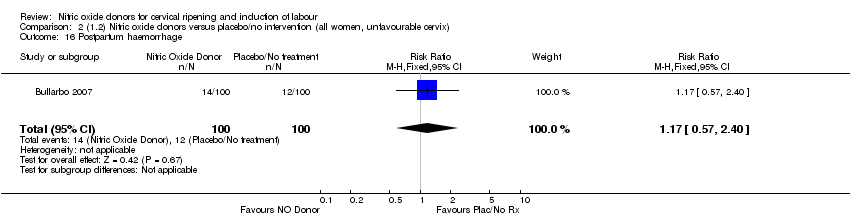 Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 16 Postpartum haemorrhage. | ||||
| 17 Additional induction agents used Show forest plot | 2 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.57, 0.77] |
| Analysis 2.17 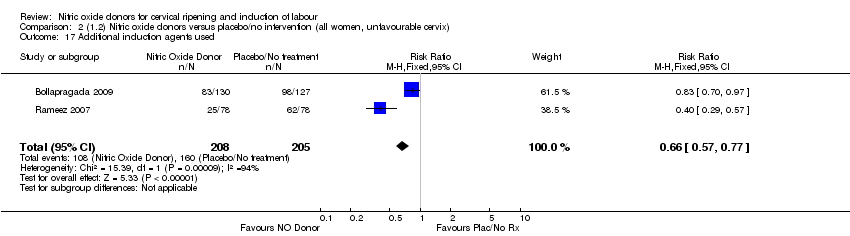 Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 17 Additional induction agents used. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| Analysis 3.1 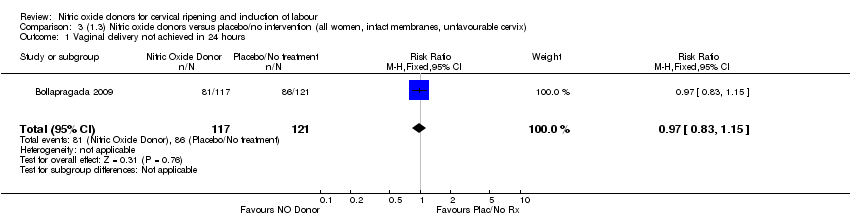 Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.62] |
| Analysis 3.2 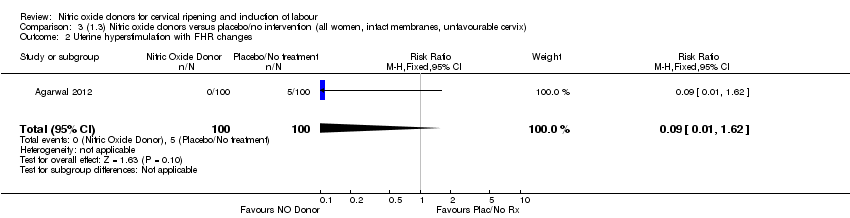 Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 3 Caesarean section Show forest plot | 3 | 754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.22] |
| Analysis 3.3  Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 3 Caesarean section. | ||||
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| Analysis 3.4  Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death. | ||||
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.61, 0.85] |
| Analysis 3.5 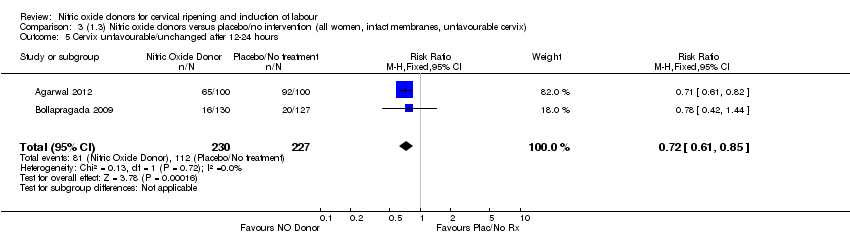 Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 6 Oxytocin augmentation Show forest plot | 2 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.05] |
| Analysis 3.6 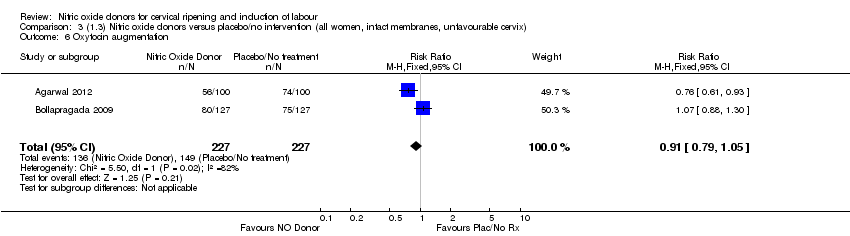 Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 6 Oxytocin augmentation. | ||||
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.80] |
| Analysis 3.7  Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 7 Uterine hyperstimulation without FHR changes. | ||||
| 8 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 3.8  Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 8 Epidural analgesia. | ||||
| 9 Instrumental vaginal delivery Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| Analysis 3.9 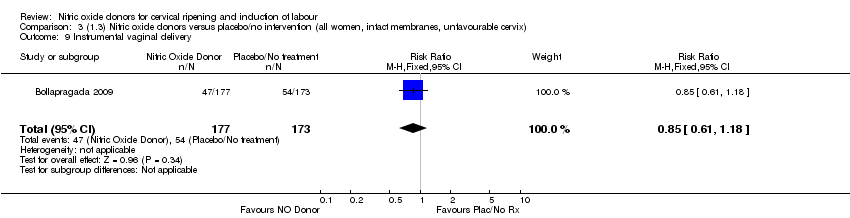 Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 9 Instrumental vaginal delivery. | ||||
| 10 Meconium‐stained liquor Show forest plot | 2 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.67, 1.18] |
| Analysis 3.10  Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 10 Meconium‐stained liquor. | ||||
| 11 Apgar score < 7 at 5 minutes Show forest plot | 3 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.27, 2.59] |
| Analysis 3.11 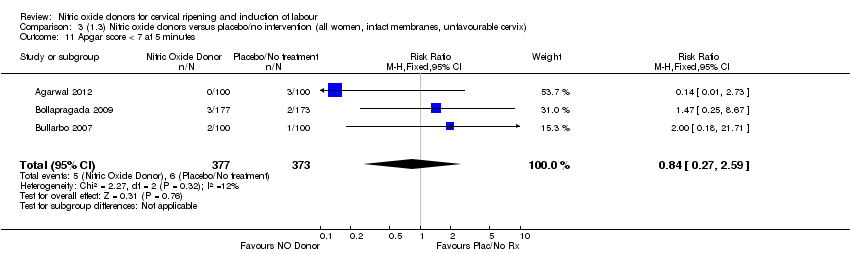 Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 11 Apgar score < 7 at 5 minutes. | ||||
| 12 Neonatal intensive care unit admission Show forest plot | 3 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.42, 1.84] |
| Analysis 3.12  Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 12 Neonatal intensive care unit admission. | ||||
| 13 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| Analysis 3.13  Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 13 Perinatal death. | ||||
| 14 Maternal side effects (nausea) Show forest plot | 2 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.82, 5.77] |
| Analysis 3.14  Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 14 Maternal side effects (nausea). | ||||
| 15 Maternal side effects (headache) Show forest plot | 3 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 9.27 [2.47, 34.73] |
| Analysis 3.15 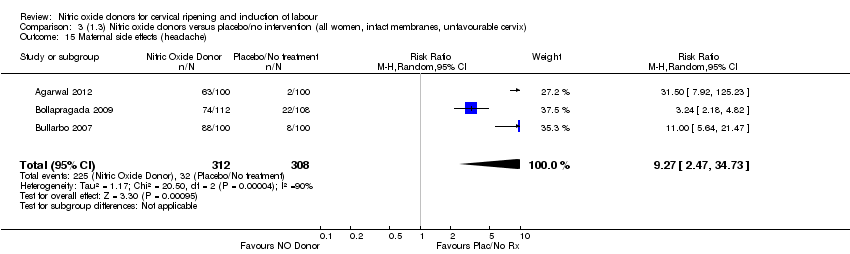 Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 15 Maternal side effects (headache). | ||||
| 16 Postpartum haemorrhage Show forest plot | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.55, 2.07] |
| Analysis 3.16 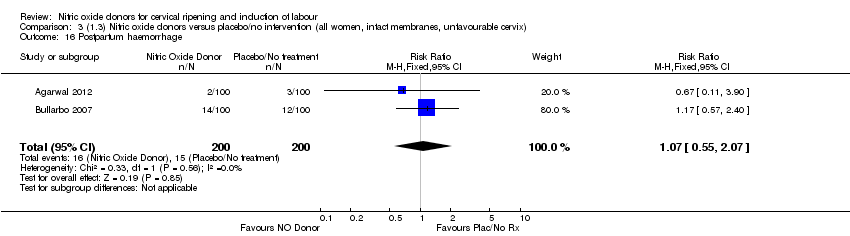 Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 16 Postpartum haemorrhage. | ||||
| 17 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Analysis 3.17  Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 17 Additional induction agents used. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| Analysis 4.1  Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.2  Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 3 Caesarean section Show forest plot | 4 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.31] |
| Analysis 4.3  Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 3 Caesarean section. | ||||
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| Analysis 4.4 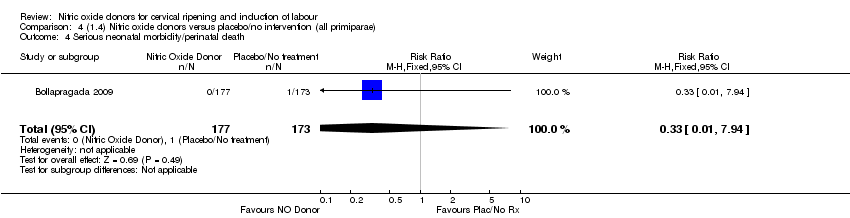 Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 4 Serious neonatal morbidity/perinatal death. | ||||
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 0.99] |
| Analysis 4.5  Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 6 Oxytocin augmentation Show forest plot | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| Analysis 4.6  Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 6 Oxytocin augmentation. | ||||
| 7 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 4.7  Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 7 Epidural analgesia. | ||||
| 8 Instrumental vaginal delivery Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.25] |
| Analysis 4.8  Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 8 Instrumental vaginal delivery. | ||||
| 9 Meconium‐stained liquor Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.22] |
| Analysis 4.9 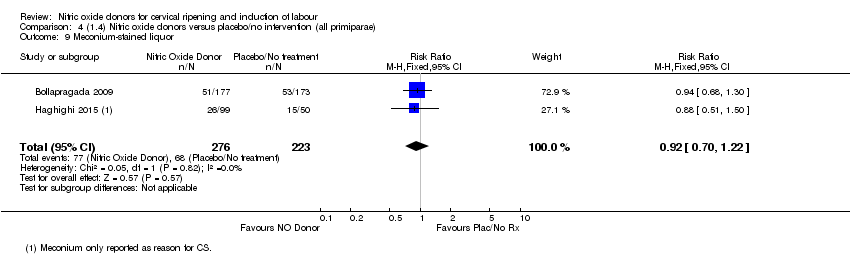 Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 9 Meconium‐stained liquor. | ||||
| 10 Apgar score < 7 at 5 minutes Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.50, 7.77] |
| Analysis 4.10 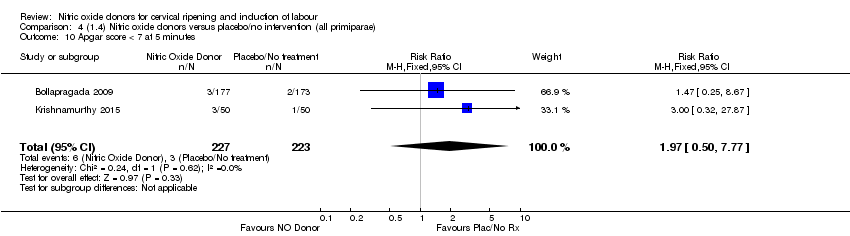 Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 10 Apgar score < 7 at 5 minutes. | ||||
| 11 Neonatal intensive care unit admission Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.53, 1.80] |
| Analysis 4.11 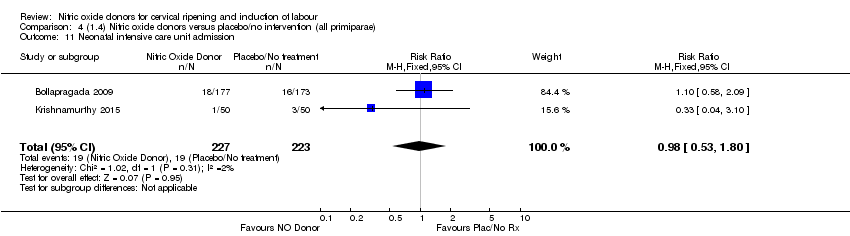 Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 11 Neonatal intensive care unit admission. | ||||
| 12 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| Analysis 4.12 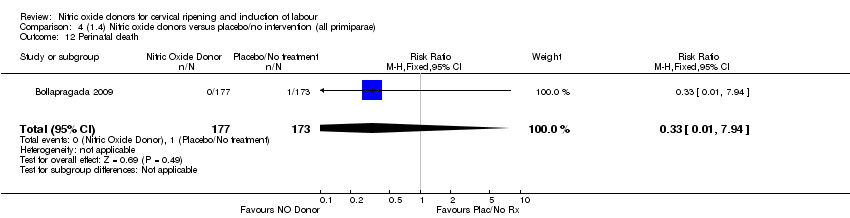 Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 12 Perinatal death. | ||||
| 13 Maternal side effects (nausea) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.73, 2.71] |
| Analysis 4.13  Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 13 Maternal side effects (nausea). | ||||
| 14 Maternal side effects (headache) Show forest plot | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 4.10 [1.97, 8.56] |
| Analysis 4.14 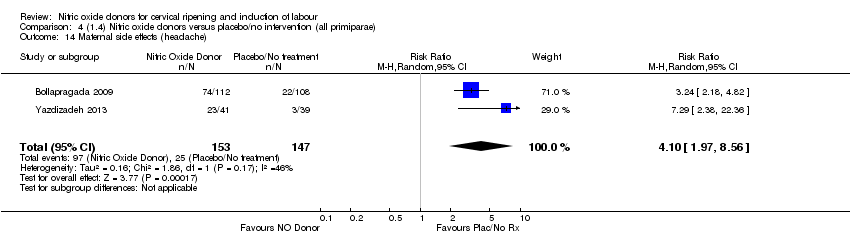 Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 14 Maternal side effects (headache). | ||||
| 15 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Analysis 4.15  Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 15 Additional induction agents used. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| Analysis 5.1  Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 5.2  Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 3 Caesarean section Show forest plot | 4 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.31] |
| Analysis 5.3  Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 3 Caesarean section. | ||||
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| Analysis 5.4 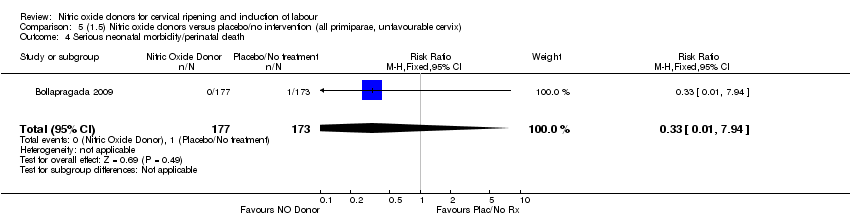 Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death. | ||||
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 0.99] |
| Analysis 5.5  Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 6 Oxytocin augmentation Show forest plot | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| Analysis 5.6  Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 6 Oxytocin augmentation. | ||||
| 7 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 5.7 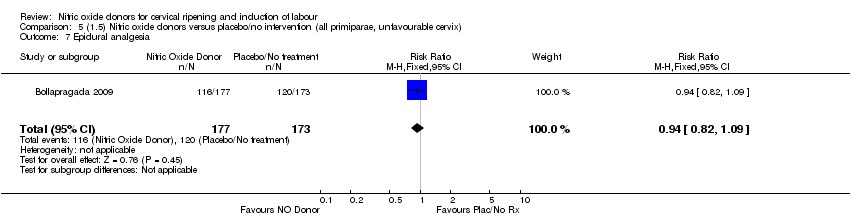 Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 7 Epidural analgesia. | ||||
| 8 Instrumental vaginal delivery Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.25] |
| Analysis 5.8  Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 8 Instrumental vaginal delivery. | ||||
| 9 Meconium‐stained liquor Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.22] |
| Analysis 5.9  Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 9 Meconium‐stained liquor. | ||||
| 10 Apgar score < 7 at 5 minutes Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.50, 7.77] |
| Analysis 5.10  Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 10 Apgar score < 7 at 5 minutes. | ||||
| 11 Neonatal intensive care unit admission Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.53, 1.80] |
| Analysis 5.11  Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 11 Neonatal intensive care unit admission. | ||||
| 12 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| Analysis 5.12 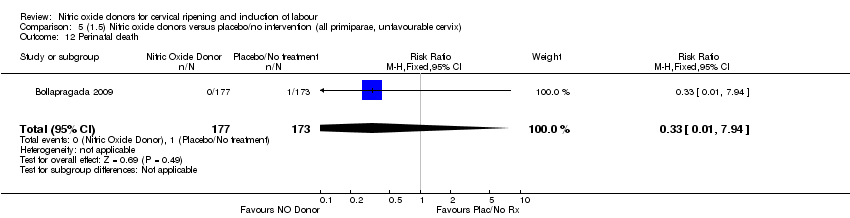 Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 12 Perinatal death. | ||||
| 13 Maternal side effects (nausea) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.73, 2.71] |
| Analysis 5.13 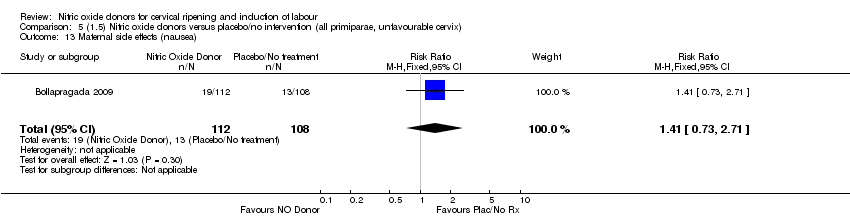 Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 13 Maternal side effects (nausea). | ||||
| 14 Maternal side effects (headache) Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.73 [2.56, 5.43] |
| Analysis 5.14 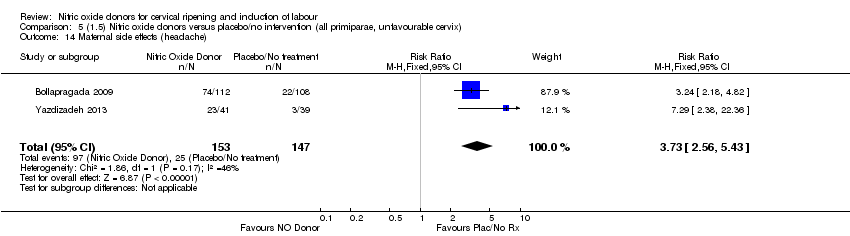 Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 14 Maternal side effects (headache). | ||||
| 15 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Analysis 5.15 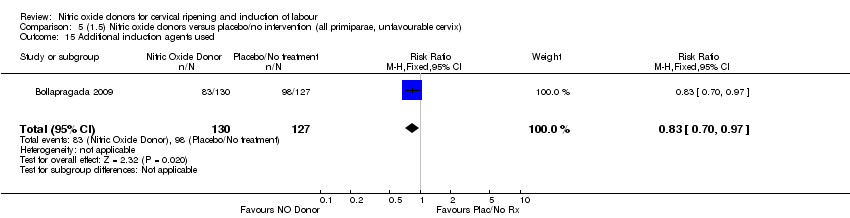 Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 15 Additional induction agents used. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| Analysis 6.1  Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 2 Caesarean section Show forest plot | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.55] |
| Analysis 6.2 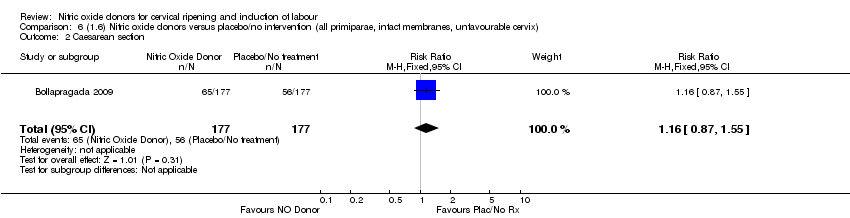 Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 2 Caesarean section. | ||||
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| Analysis 6.3 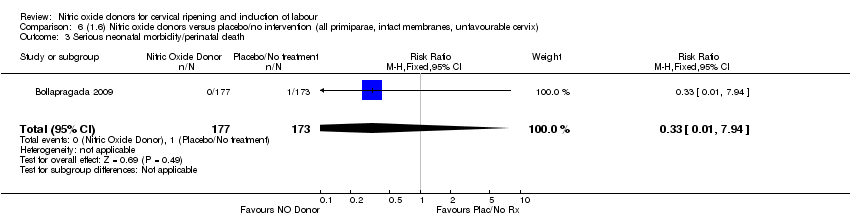 Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death. | ||||
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.42, 1.44] |
| Analysis 6.4 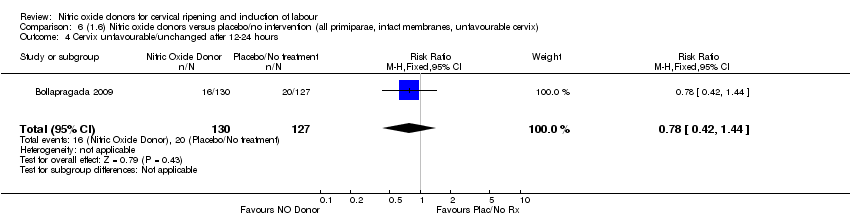 Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 5 Oxytocin augmentation Show forest plot | 1 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.30] |
| Analysis 6.5  Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 5 Oxytocin augmentation. | ||||
| 6 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 6.6 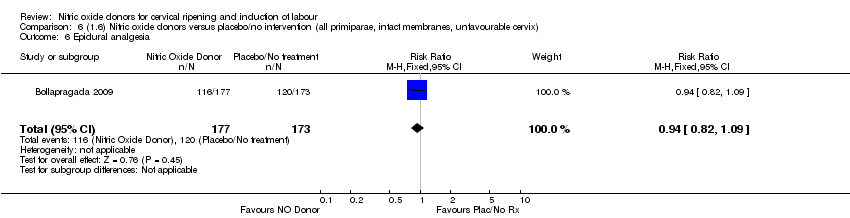 Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 6 Epidural analgesia. | ||||
| 7 Instrumental vaginal delivery Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| Analysis 6.7  Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 7 Instrumental vaginal delivery. | ||||
| 8 Meconium‐stained liquor Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.30] |
| Analysis 6.8  Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 8 Meconium‐stained liquor. | ||||
| 9 Apgar score < 7 at 5 minutes Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.67] |
| Analysis 6.9  Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 9 Apgar score < 7 at 5 minutes. | ||||
| 10 Neonatal intensive care unit admission Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.58, 2.09] |
| Analysis 6.10  Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 10 Neonatal intensive care unit admission. | ||||
| 11 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| Analysis 6.11  Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 11 Perinatal death. | ||||
| 12 Maternal side effects (nausea) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.73, 2.71] |
| Analysis 6.12  Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 12 Maternal side effects (nausea). | ||||
| 13 Maternal side effects (headache) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [2.18, 4.82] |
| Analysis 6.13 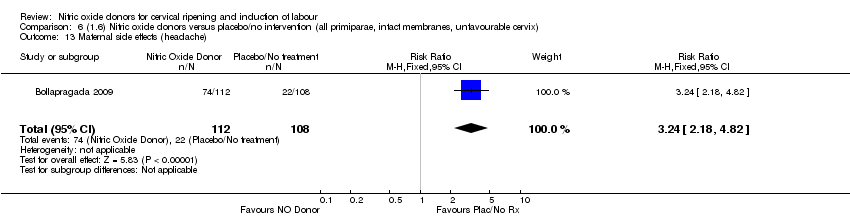 Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 13 Maternal side effects (headache). | ||||
| 14 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Analysis 6.14  Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 14 Additional induction agents used. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| Analysis 7.1 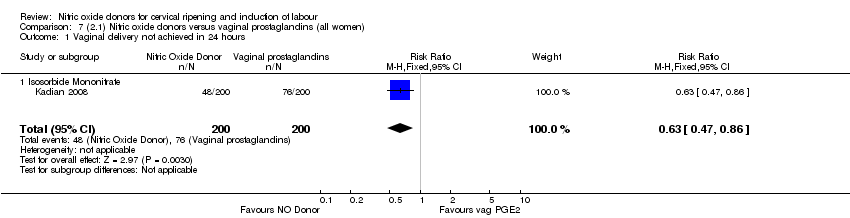 Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 1.1 Isosorbide Mononitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| Analysis 7.2 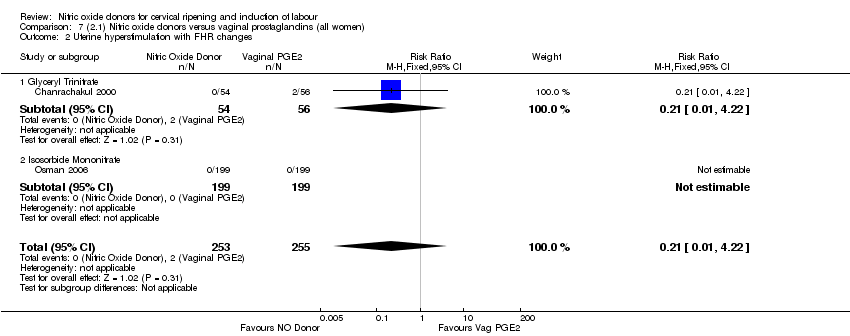 Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 2.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 2.2 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| Analysis 7.3  Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 3 Uterine hyperstimulation without FHR changes. | ||||
| 3.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| 4 Caesarean section Show forest plot | 3 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| Analysis 7.4  Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 4 Caesarean section. | ||||
| 4.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.59, 1.63] |
| 4.2 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 4.3 Isosorbide Dinitrate | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.06] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| Analysis 7.5  Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 5 Instrumental vaginal delivery. | ||||
| 5.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.32, 2.28] |
| Analysis 7.6  Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 6 Meconium‐stained liquor. | ||||
| 6.1 Isosorbide Dinitrate | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.32, 2.28] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 2 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 1.98] |
| Analysis 7.7  Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 7 Apgar score < 7 at 5 minutes. | ||||
| 7.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 7.2 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 8 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| Analysis 7.8 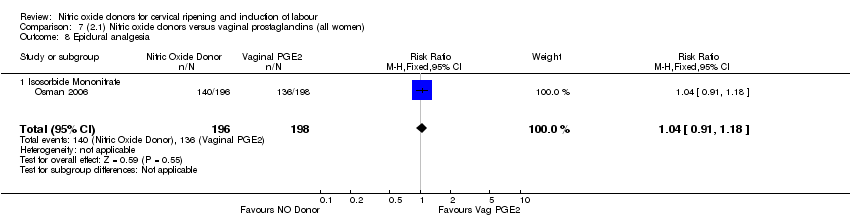 Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 8 Epidural analgesia. | ||||
| 8.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 9 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| Analysis 7.9  Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 9 Maternal side effects (nausea). | ||||
| 9.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 10 Maternal side effects (headache) Show forest plot | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.79 [5.75, 13.45] |
| Analysis 7.10 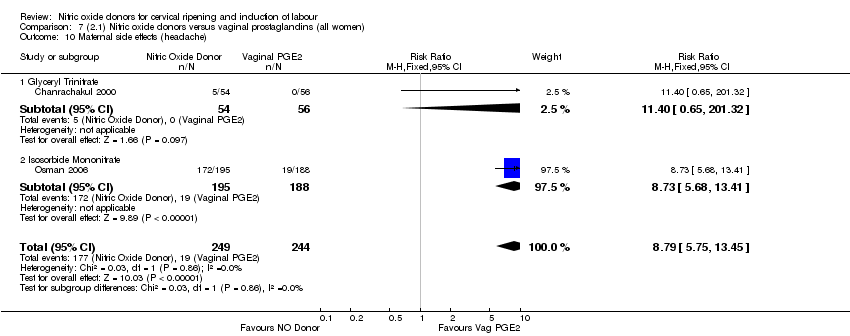 Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 10 Maternal side effects (headache). | ||||
| 10.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.4 [0.65, 201.32] |
| 10.2 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| Analysis 7.11 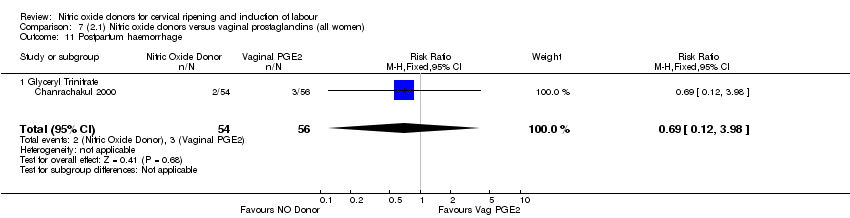 Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 11 Postpartum haemorrhage. | ||||
| 11.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 12 Serious maternal complications Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| Analysis 7.12  Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 12 Serious maternal complications. | ||||
| 12.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 13 Neonatal intensive care unit admission Show forest plot | 3 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.78] |
| Analysis 7.13  Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 13 Neonatal intensive care unit admission. | ||||
| 13.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 13.2 Isosorbide Dinitrate | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| Analysis 8.1 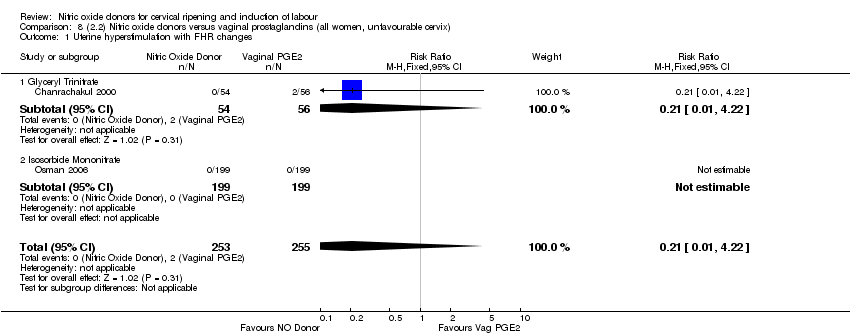 Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes. | ||||
| 1.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 1.2 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 2 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.82, 1.35] |
| Analysis 8.2  Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 2 Caesarean section. | ||||
| 2.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.59, 1.63] |
| 2.2 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| Analysis 8.3  Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 3 Uterine hyperstimulation without FHR changes. | ||||
| 3.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| 4 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| Analysis 8.4  Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 4 Epidural analgesia. | ||||
| 4.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| Analysis 8.5  Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 5 Instrumental vaginal delivery. | ||||
| 5.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 2 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 1.98] |
| Analysis 8.6  Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 6 Apgar score < 7 at 5 minutes. | ||||
| 6.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 6.2 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 7 Neonatal intensive care unit admission Show forest plot | 2 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.78] |
| Analysis 8.7  Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 7 Neonatal intensive care unit admission. | ||||
| 7.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 7.2 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 8 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| Analysis 8.8  Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 8 Maternal side effects (nausea). | ||||
| 8.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 9 Maternal side effects (headache) Show forest plot | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.79 [5.75, 13.45] |
| Analysis 8.9 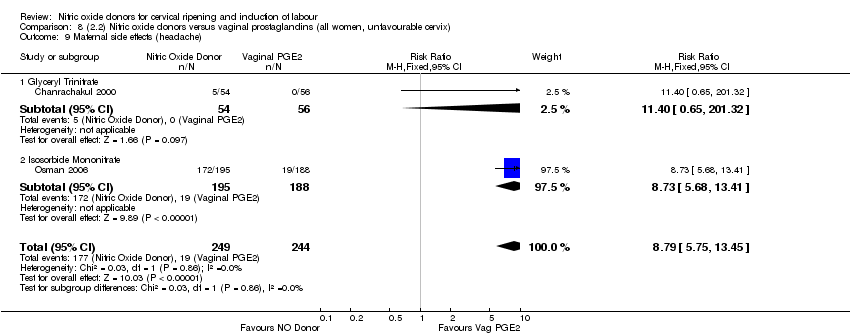 Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 9 Maternal side effects (headache). | ||||
| 9.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.4 [0.65, 201.32] |
| 9.2 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 10 Postpartum haemorrhage Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| Analysis 8.10 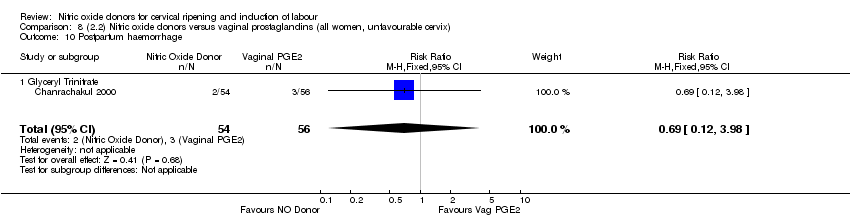 Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 10 Postpartum haemorrhage. | ||||
| 10.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 11 Serious maternal complications Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| Analysis 8.11 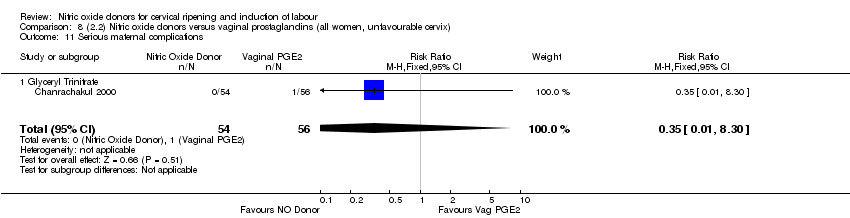 Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 11 Serious maternal complications. | ||||
| 11.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 9.1  Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes. | ||||
| 1.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| Analysis 9.2 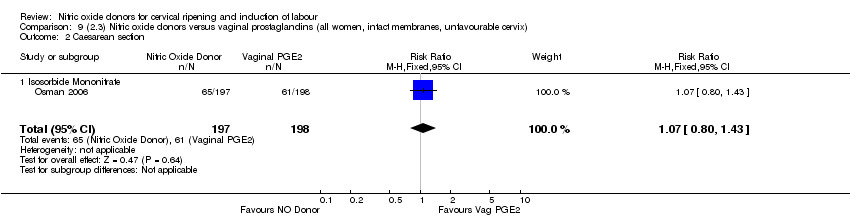 Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 2 Caesarean section. | ||||
| 2.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| Analysis 9.3  Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 3 Epidural analgesia. | ||||
| 3.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| Analysis 9.4  Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 4 Instrumental vaginal delivery. | ||||
| 4.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| Analysis 9.5 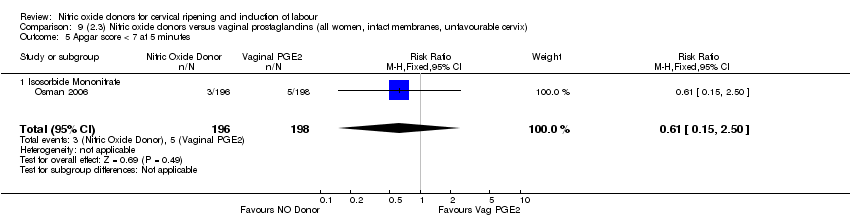 Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes. | ||||
| 5.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 6 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| Analysis 9.6 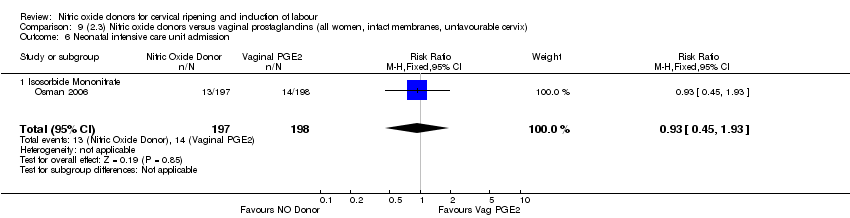 Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 6 Neonatal intensive care unit admission. | ||||
| 6.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 7 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| Analysis 9.7  Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 7 Maternal side effects (nausea). | ||||
| 7.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Analysis 9.8 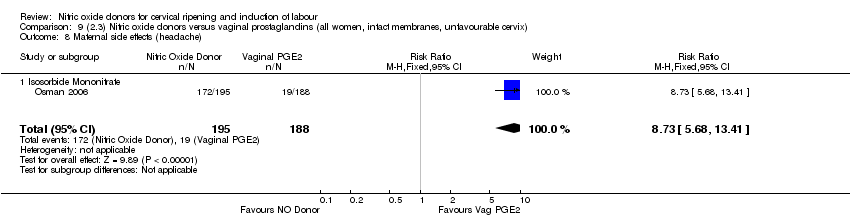 Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 8 Maternal side effects (headache). | ||||
| 8.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| Analysis 10.1  Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 1.1 Isosorbide Mononitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 10.2  Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 2.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section Show forest plot | 2 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| Analysis 10.3  Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 3 Caesarean section. | ||||
| 3.1 Isosorbide Mononitrate | 2 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| 4 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| Analysis 10.4  Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 4 Epidural analgesia. | ||||
| 4.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| Analysis 10.5 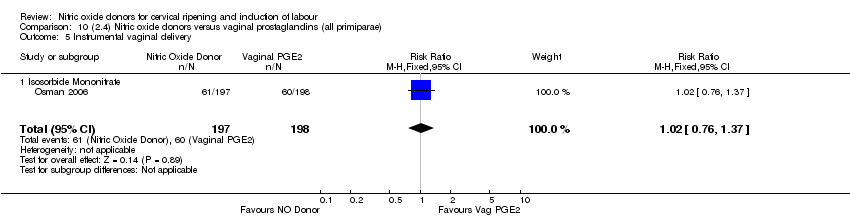 Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 5 Instrumental vaginal delivery. | ||||
| 5.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| Analysis 10.6  Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 6 Apgar score < 7 at 5 minutes. | ||||
| 6.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 7 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| Analysis 10.7  Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 7 Neonatal intensive care unit admission. | ||||
| 7.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 8 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| Analysis 10.8 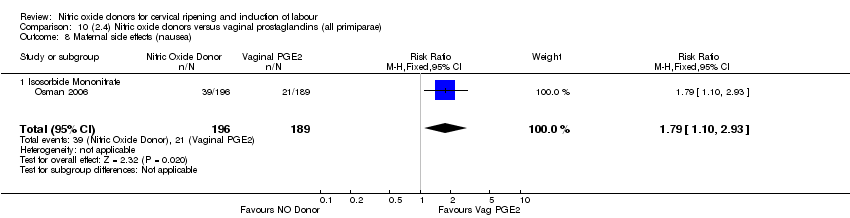 Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 8 Maternal side effects (nausea). | ||||
| 8.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Analysis 10.9 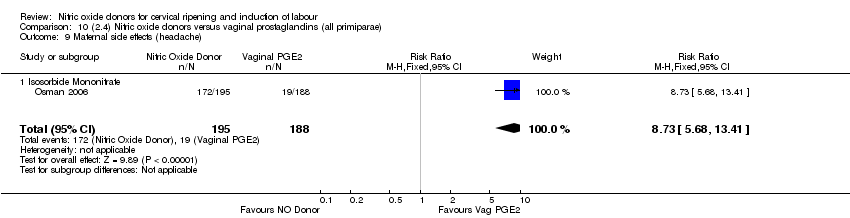 Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 9 Maternal side effects (headache). | ||||
| 9.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 11.1  Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes. | ||||
| 1.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| Analysis 11.2  Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 2 Caesarean section. | ||||
| 2.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| Analysis 11.3  Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 3 Epidural analgesia. | ||||
| 3.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| Analysis 11.4  Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 4 Instrumental vaginal delivery. | ||||
| 4.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| Analysis 11.5  Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes. | ||||
| 5.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 6 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| Analysis 11.6  Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 6 Neonatal intensive care unit admission. | ||||
| 6.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 7 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| Analysis 11.7  Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 7 Maternal side effects (nausea). | ||||
| 7.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Analysis 11.8  Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 8 Maternal side effects (headache). | ||||
| 8.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 12.1  Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes. | ||||
| 1.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| Analysis 12.2  Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 2 Caesarean section. | ||||
| 2.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| Analysis 12.3  Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 3 Epidural analgesia. | ||||
| 3.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| Analysis 12.4  Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 4 Instrumental vaginal delivery. | ||||
| 4.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| Analysis 12.5 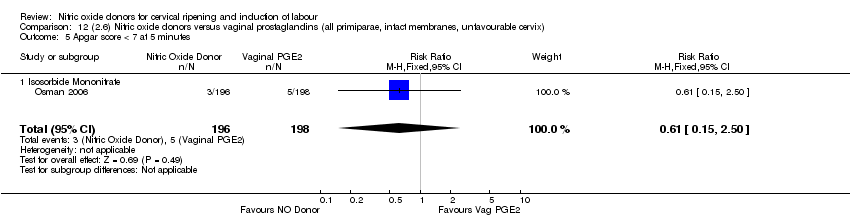 Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes. | ||||
| 5.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 6 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| Analysis 12.6  Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 6 Neonatal intensive care unit admission. | ||||
| 6.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 7 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| Analysis 12.7  Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 7 Maternal side effects (nausea). | ||||
| 7.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Analysis 12.8 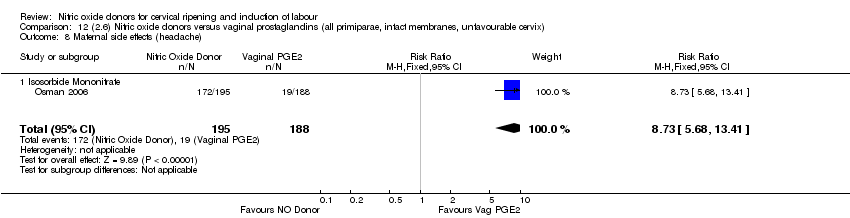 Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 8 Maternal side effects (headache). | ||||
| 8.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| Analysis 13.1  Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 1.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 13.2 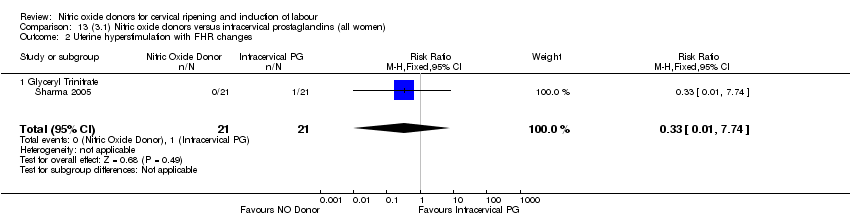 Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 2.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3 Caesarean section Show forest plot | 2 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.90] |
| Analysis 13.3  Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 3 Caesarean section. | ||||
| 3.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.38, 0.89] |
| 3.2 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 13.4  Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 4 Serious neonatal morbidity/perinatal death. | ||||
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| Analysis 13.5  Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 6 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| Analysis 13.6  Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 6 Oxytocin augmentation. | ||||
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| Analysis 13.7 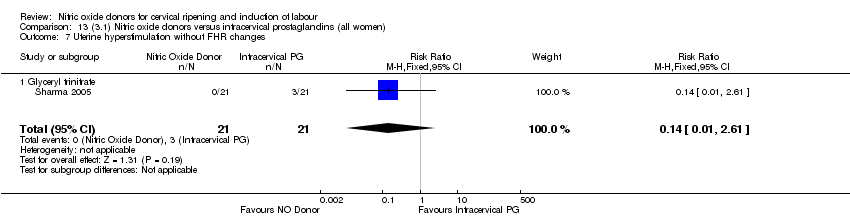 Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 7 Uterine hyperstimulation without FHR changes. | ||||
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| Analysis 13.8  Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 8 Instrumental vaginal delivery. | ||||
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 9 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 13.9  Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 9 Perinatal death. | ||||
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Analysis 13.10  Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 10 Maternal side effects (headache). | ||||
| 10.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| Analysis 14.1  Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 1.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 14.2 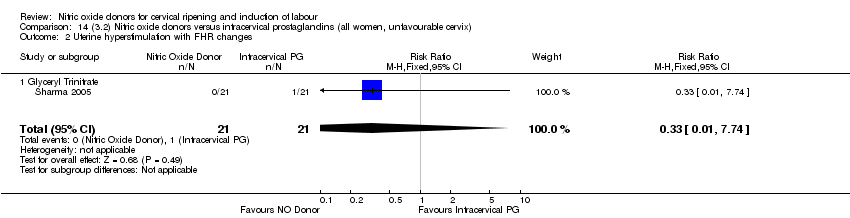 Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 2.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| Analysis 14.3  Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 3 Caesarean section. | ||||
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 14.4 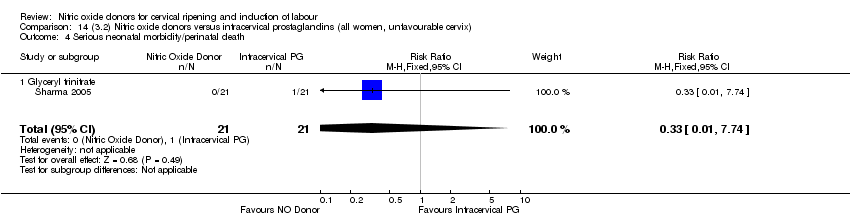 Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death. | ||||
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| Analysis 14.5 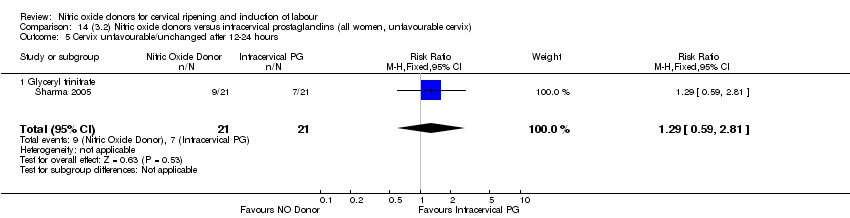 Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 6 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| Analysis 14.6 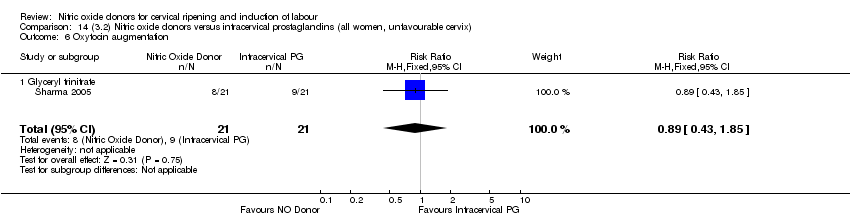 Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 6 Oxytocin augmentation. | ||||
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| Analysis 14.7  Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 7 Uterine hyperstimulation without FHR changes. | ||||
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| Analysis 14.8 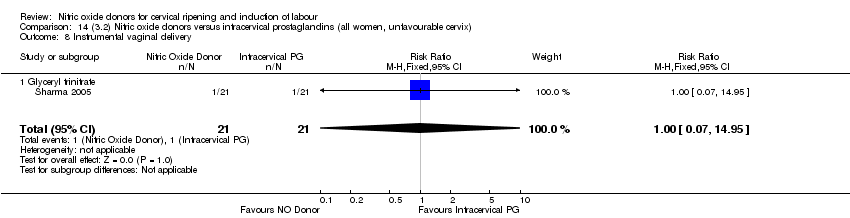 Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 8 Instrumental vaginal delivery. | ||||
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 9 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 14.9  Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 9 Perinatal death. | ||||
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Analysis 14.10 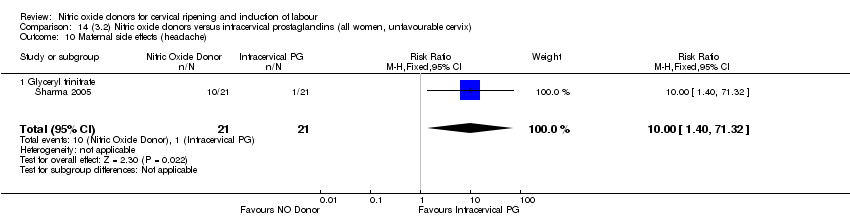 Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 10 Maternal side effects (headache). | ||||
| 10.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| Analysis 15.1  Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 1.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 15.2  Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 2.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| Analysis 15.3  Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 3 Caesarean section. | ||||
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 15.4  Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 4 Serious neonatal morbidity/perinatal death. | ||||
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| Analysis 15.5 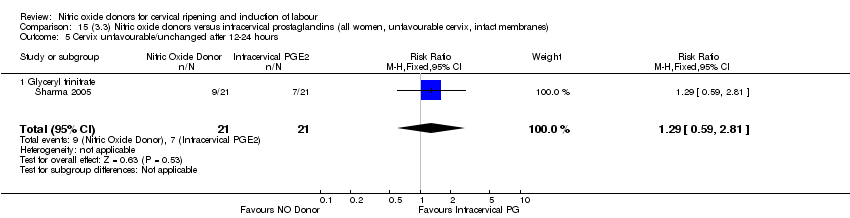 Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 6 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| Analysis 15.6 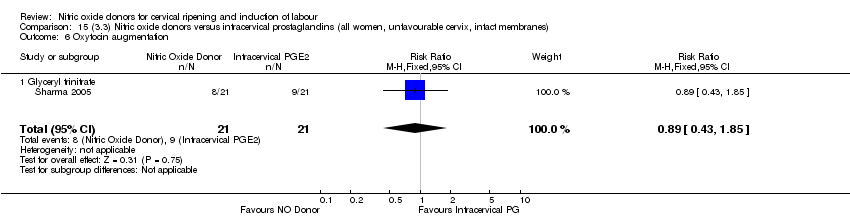 Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 6 Oxytocin augmentation. | ||||
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| Analysis 15.7  Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 7 Uterine hyperstimulation without FHR changes. | ||||
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| Analysis 15.8  Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 8 Instrumental vaginal delivery. | ||||
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 9 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 15.9 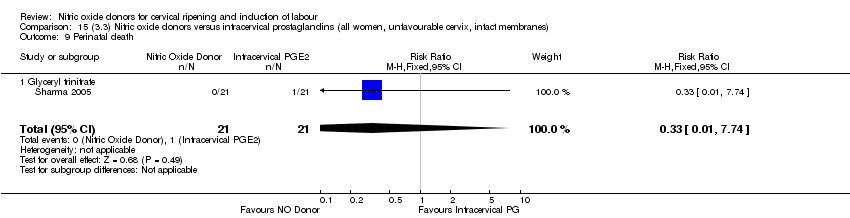 Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 9 Perinatal death. | ||||
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Analysis 15.10  Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 10 Maternal side effects (headache). | ||||
| 10.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 16.1 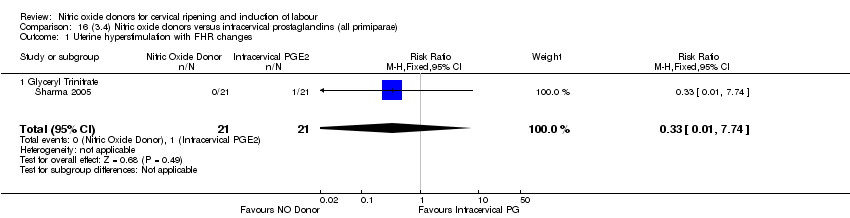 Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 1 Uterine hyperstimulation with FHR changes. | ||||
| 1.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| Analysis 16.2  Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 2 Caesarean section. | ||||
| 2.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 16.3  Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 3 Serious neonatal morbidity/perinatal death. | ||||
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| Analysis 16.4  Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| Analysis 16.5  Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 5 Oxytocin augmentation. | ||||
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| Analysis 16.6 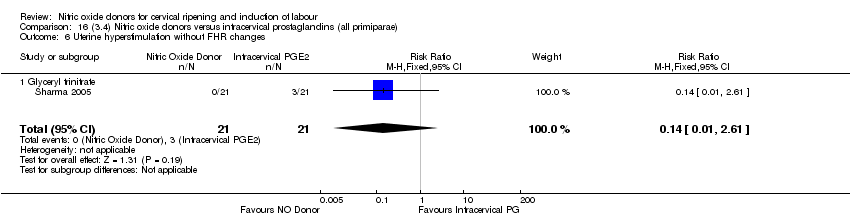 Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 6 Uterine hyperstimulation without FHR changes. | ||||
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| Analysis 16.7  Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 7 Instrumental vaginal delivery. | ||||
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 16.8  Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 8 Perinatal death. | ||||
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Analysis 16.9 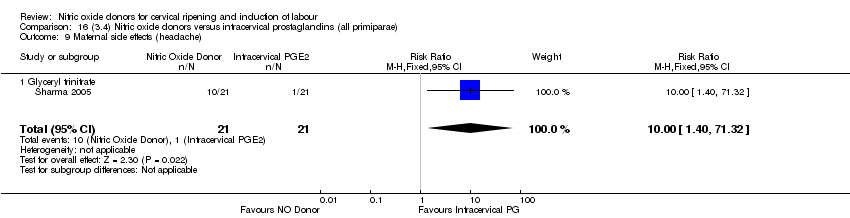 Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 9 Maternal side effects (headache). | ||||
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 17.1  Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes. | ||||
| 1.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| Analysis 17.2  Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 2 Caesarean section. | ||||
| 2.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 17.3  Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death. | ||||
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| Analysis 17.4  Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| Analysis 17.5  Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 5 Oxytocin augmentation. | ||||
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| Analysis 17.6 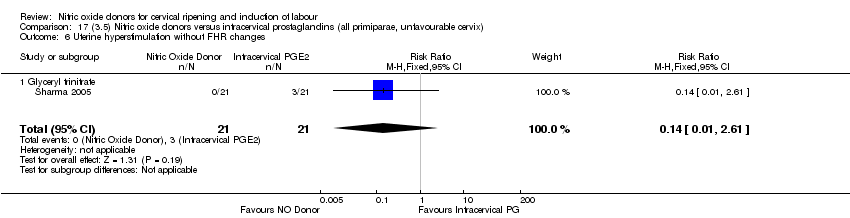 Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 6 Uterine hyperstimulation without FHR changes. | ||||
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| Analysis 17.7 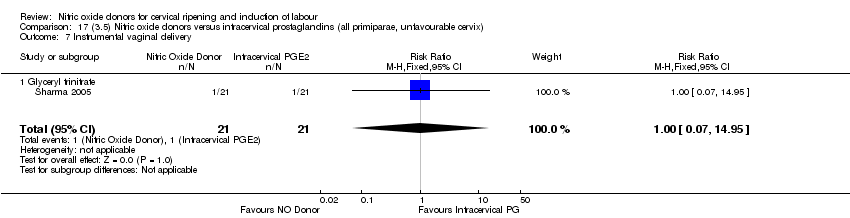 Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 7 Instrumental vaginal delivery. | ||||
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 17.8  Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 8 Perinatal death. | ||||
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Analysis 17.9  Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 9 Maternal side effects (headache). | ||||
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 18.1  Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 1 Uterine hyperstimulation with FHR changes. | ||||
| 1.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| Analysis 18.2  Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 2 Caesarean section. | ||||
| 2.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 18.3  Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 3 Serious neonatal morbidity/perinatal death. | ||||
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| Analysis 18.4  Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| Analysis 18.5 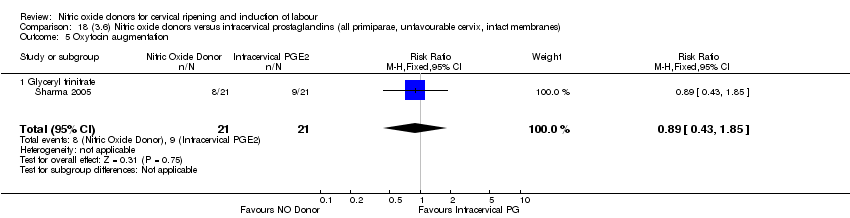 Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 5 Oxytocin augmentation. | ||||
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| Analysis 18.6  Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 6 Uterine hyperstimulation without FHR changes. | ||||
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| Analysis 18.7 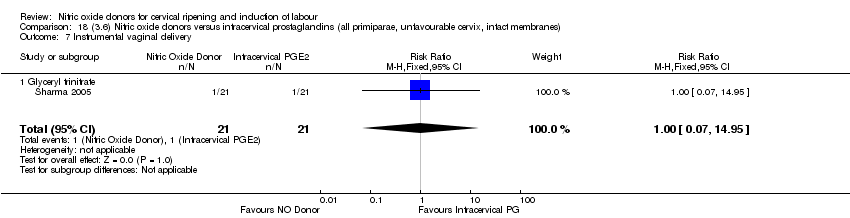 Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 7 Instrumental vaginal delivery. | ||||
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| Analysis 18.8  Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 8 Perinatal death. | ||||
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Analysis 18.9  Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 9 Maternal side effects (headache). | ||||
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| Analysis 19.1 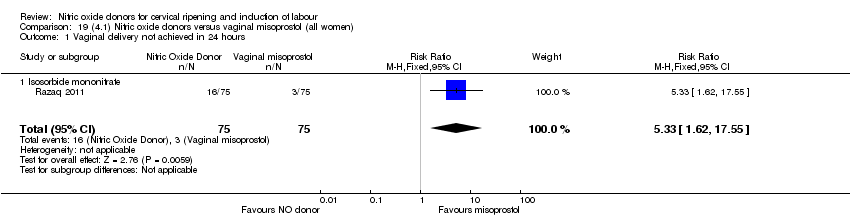 Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 1.1 Isosorbide mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 3 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.37] |
| Analysis 19.2  Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2.2 Isosorbide Mononitrate | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.40] |
| 3 Caesarean section Show forest plot | 6 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.82, 1.21] |
| Analysis 19.3 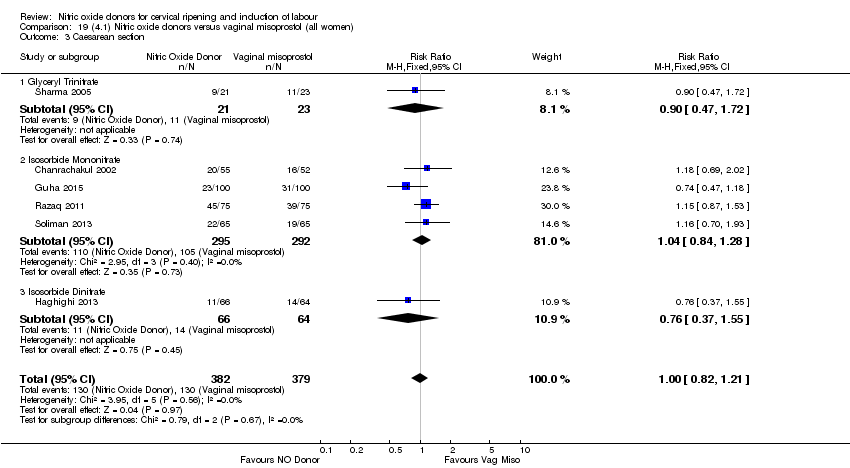 Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 3 Caesarean section. | ||||
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 3.2 Isosorbide Mononitrate | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.28] |
| 3.3 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 19.4  Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 4 Serious neonatal morbidity/perinatal death. | ||||
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [2.07, 5.66] |
| Analysis 19.5  Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5.2 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [2.12, 6.75] |
| 6 Oxytocin augmentation Show forest plot | 7 | 767 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [1.31, 5.45] |
| Analysis 19.6 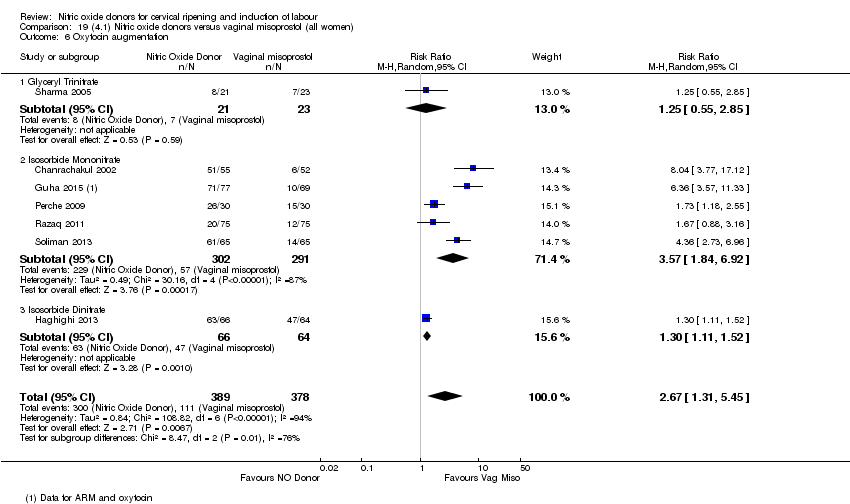 Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 6 Oxytocin augmentation. | ||||
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.85] |
| 6.2 Isosorbide Mononitrate | 5 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 3.57 [1.84, 6.92] |
| 6.3 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.11, 1.52] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.32] |
| Analysis 19.7  Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 7 Uterine hyperstimulation without FHR changes. | ||||
| 7.1 Isosorbide Mononitrate | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.34] |
| 7.2 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.96] |
| 8 Epidural analgesia Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.31] |
| Analysis 19.8  Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 8 Epidural analgesia. | ||||
| 8.1 Isosorbide Mononitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.31] |
| 9 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| Analysis 19.9 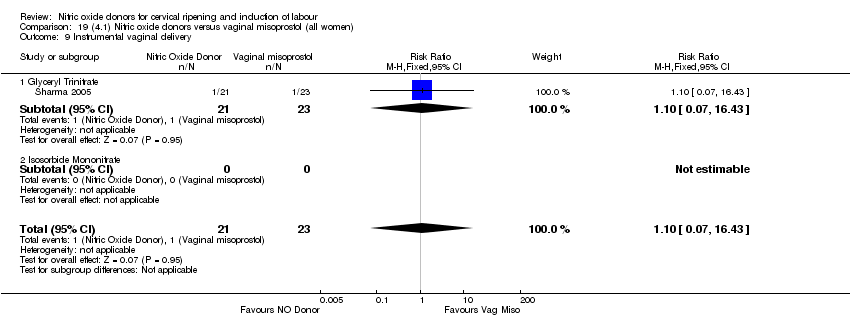 Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 9 Instrumental vaginal delivery. | ||||
| 9.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 9.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Meconium‐stained liquor Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.65] |
| Analysis 19.10  Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 10 Meconium‐stained liquor. | ||||
| 10.1 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.56] |
| 10.2 Isosorbide mononitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.15, 0.84] |
| 11 Apgar score < 7 at 5 minutes Show forest plot | 6 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.07, 0.38] |
| Analysis 19.11  Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 11 Apgar score < 7 at 5 minutes. | ||||
| 11.1 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Isosorbide Mononitrate | 5 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.07, 0.38] |
| 12 Neonatal intensive care unit admission Show forest plot | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.43] |
| Analysis 19.12  Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 12 Neonatal intensive care unit admission. | ||||
| 12.1 Isosorbide Mononitrate | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.43] |
| 13 Perinatal death Show forest plot | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 19.13 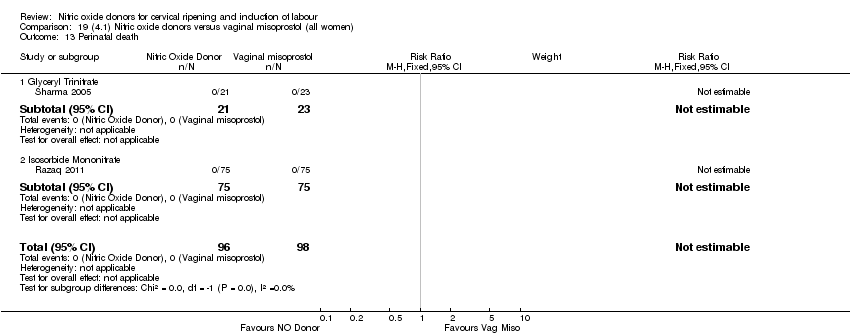 Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 13 Perinatal death. | ||||
| 13.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Isosorbide Mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal side effects (nausea) Show forest plot | 5 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.63, 2.17] |
| Analysis 19.14 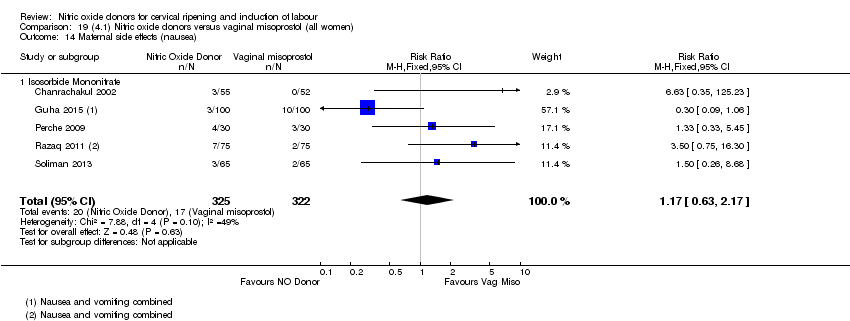 Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 14 Maternal side effects (nausea). | ||||
| 14.1 Isosorbide Mononitrate | 5 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.63, 2.17] |
| 15 Maternal side effects (headache) Show forest plot | 4 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.98 [4.05, 29.73] |
| Analysis 19.15  Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 15 Maternal side effects (headache). | ||||
| 15.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 15.2 Isosorbide Mononitrate | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.46 [2.69, 67.43] |
| 15.3 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 24.25 [1.47, 401.26] |
| 16 Postpartum haemorrhage Show forest plot | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.57, 3.06] |
| Analysis 19.16 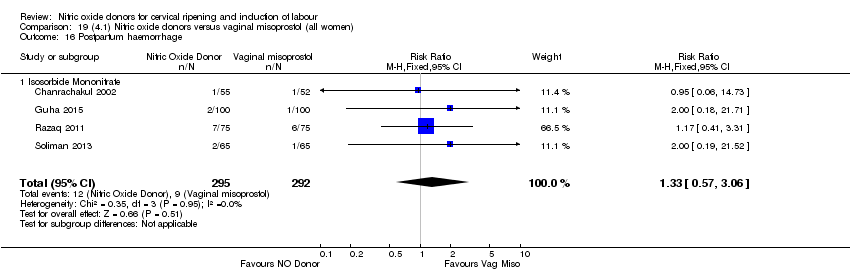 Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 16 Postpartum haemorrhage. | ||||
| 16.1 Isosorbide Mononitrate | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.57, 3.06] |
| 17 Analgesia requirement Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.49] |
| Analysis 19.17  Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 17 Analgesia requirement. | ||||
| 17.1 Isosorbide Mononitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.49] |
| 18 Additional induction agents required Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| Analysis 19.18 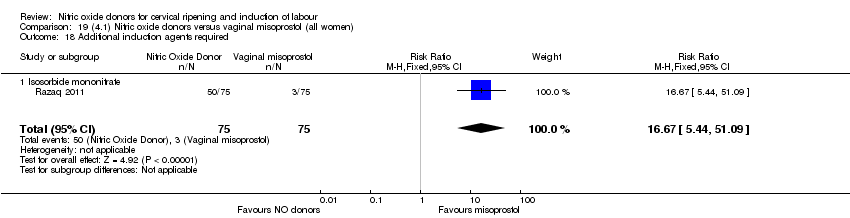 Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 18 Additional induction agents required. | ||||
| 18.1 Isosorbide mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.67] |
| Analysis 20.1  Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes. | ||||
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 1.2 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.94] |
| 2 Caesarean section Show forest plot | 3 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.66, 1.22] |
| Analysis 20.2  Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 2 Caesarean section. | ||||
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide Mononitrate | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.27] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 20.3  Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death. | ||||
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [2.07, 5.66] |
| Analysis 20.4 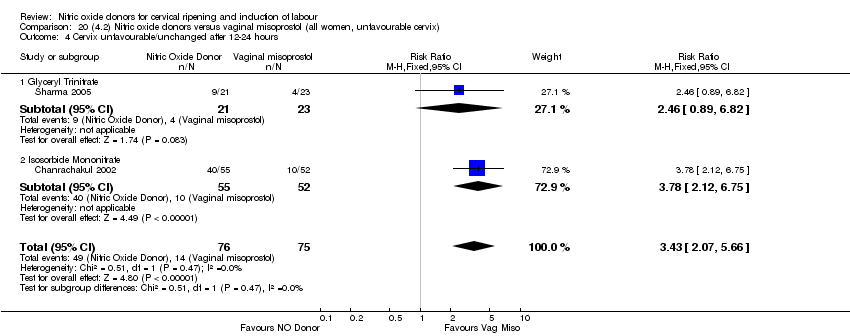 Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 4.2 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [2.12, 6.75] |
| 5 Oxytocin augmentation Show forest plot | 4 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [1.23, 8.55] |
| Analysis 20.5  Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 5 Oxytocin augmentation. | ||||
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide Mononitrate | 3 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 4.35 [1.32, 14.27] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.75] |
| Analysis 20.6 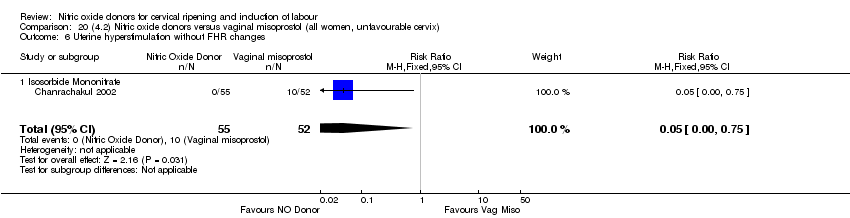 Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 6 Uterine hyperstimulation without FHR changes. | ||||
| 6.1 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.75] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| Analysis 20.7 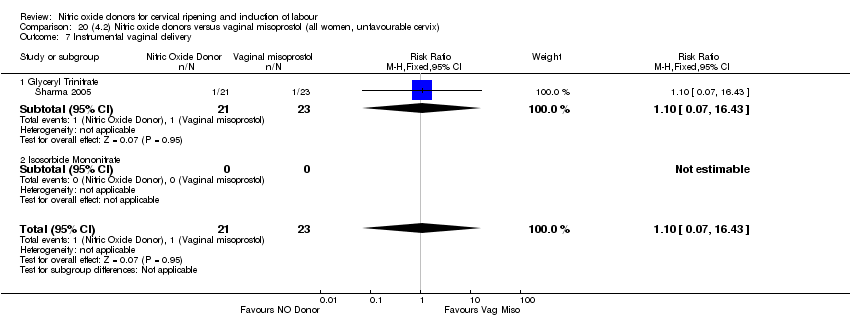 Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 7 Instrumental vaginal delivery. | ||||
| 7.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Apgar score < 7 at 5 minutes Show forest plot | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.46] |
| Analysis 20.8 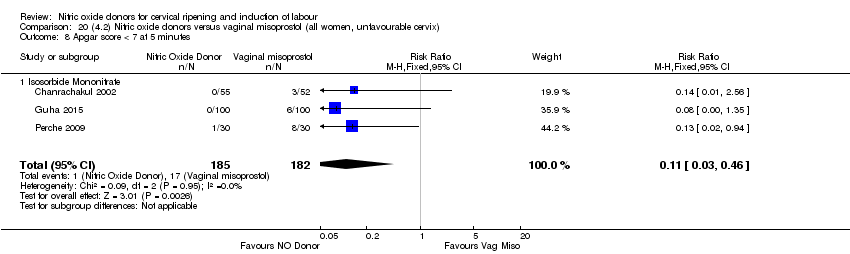 Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 8 Apgar score < 7 at 5 minutes. | ||||
| 8.1 Isosorbide Mononitrate | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.46] |
| 9 Neonatal intensive care unit admission Show forest plot | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.07, 0.53] |
| Analysis 20.9  Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 9 Neonatal intensive care unit admission. | ||||
| 9.1 Isosorbide Mononitrate | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.07, 0.53] |
| 10 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 20.10  Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 10 Perinatal death. | ||||
| 10.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal side effects (nausea) Show forest plot | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.69] |
| Analysis 20.11  Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 11 Maternal side effects (nausea). | ||||
| 11.1 Isosorbide Mononitrate | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.69] |
| 12 Maternal side effects (headache) Show forest plot | 3 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.01 [3.11, 26.06] |
| Analysis 20.12 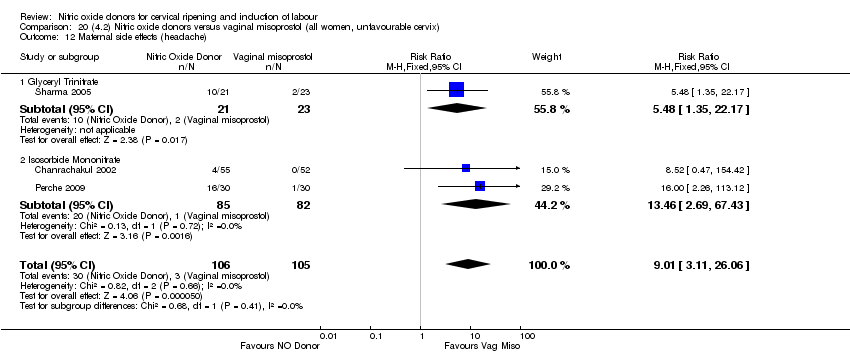 Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 12 Maternal side effects (headache). | ||||
| 12.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 12.2 Isosorbide Mononitrate | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.46 [2.69, 67.43] |
| 13 Postpartum haemorrhage Show forest plot | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.61] |
| Analysis 20.13 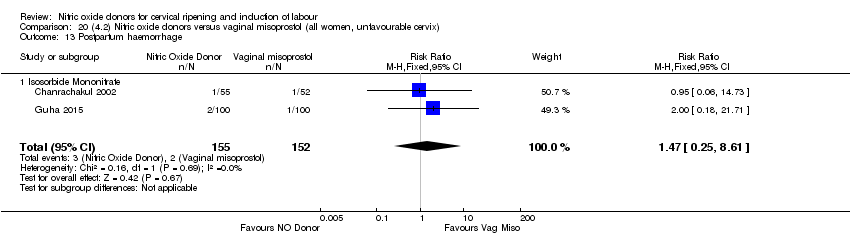 Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 13 Postpartum haemorrhage. | ||||
| 13.1 Isosorbide Mononitrate | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| Analysis 21.1 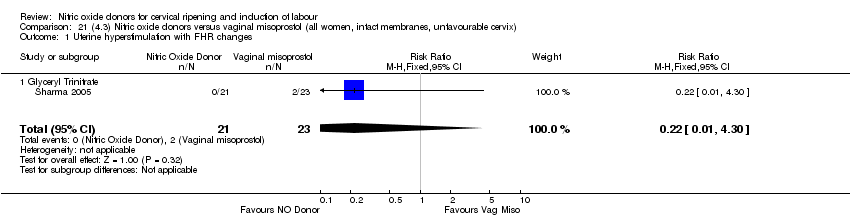 Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes. | ||||
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2 Caesarean section Show forest plot | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.14] |
| Analysis 21.2 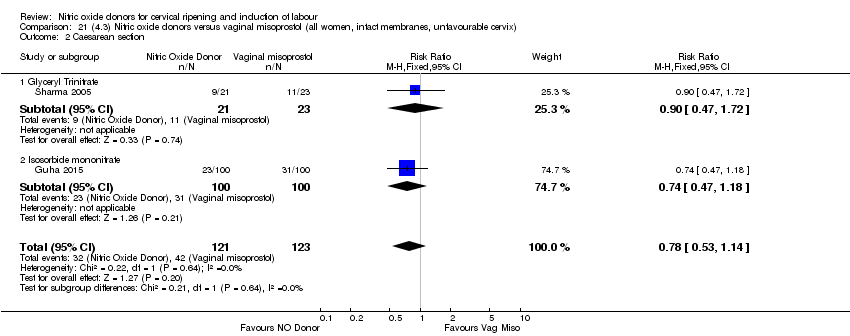 Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 2 Caesarean section. | ||||
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.18] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 21.3 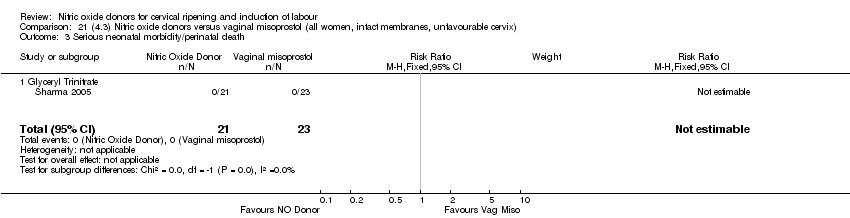 Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death. | ||||
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| Analysis 21.4  Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5 Oxytocin augmentation Show forest plot | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [2.29, 4.33] |
| Analysis 21.5 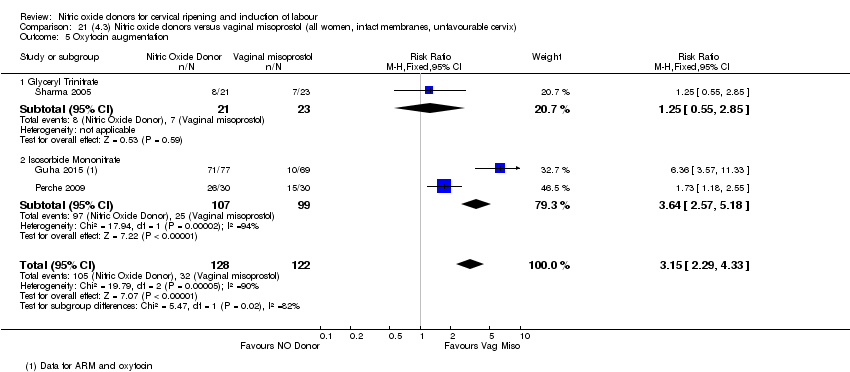 Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 5 Oxytocin augmentation. | ||||
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide Mononitrate | 2 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [2.57, 5.18] |
| 6 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| Analysis 21.6  Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 6 Instrumental vaginal delivery. | ||||
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.54] |
| Analysis 21.7  Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes. | ||||
| 7.1 Isosorbide Mononitrate | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.54] |
| 8 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 21.8  Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 8 Perinatal death. | ||||
| 8.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal side effects (nausea) Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.31] |
| Analysis 21.9  Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 9 Maternal side effects (nausea). | ||||
| 9.1 Isosorbide Mononitrate | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.31] |
| 10 Maternal side effects (headache) Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.09 [2.90, 28.47] |
| Analysis 21.10  Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (headache). | ||||
| 10.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 10.2 Isosorbide Mononitrate | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.0 [2.26, 113.12] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Analysis 21.11 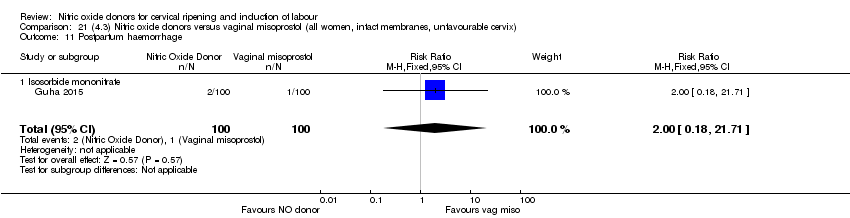 Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 11 Postpartum haemorrhage. | ||||
| 11.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| Analysis 22.1  Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 1 Vaginal delivery not achieved in 24 hours. | ||||
| 1.1 Isosorbide Mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| Analysis 22.2 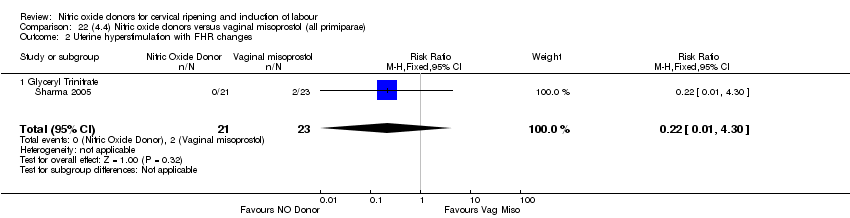 Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 2 Uterine hyperstimulation with FHR changes. | ||||
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 3 Caesarean section Show forest plot | 3 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.21] |
| Analysis 22.3 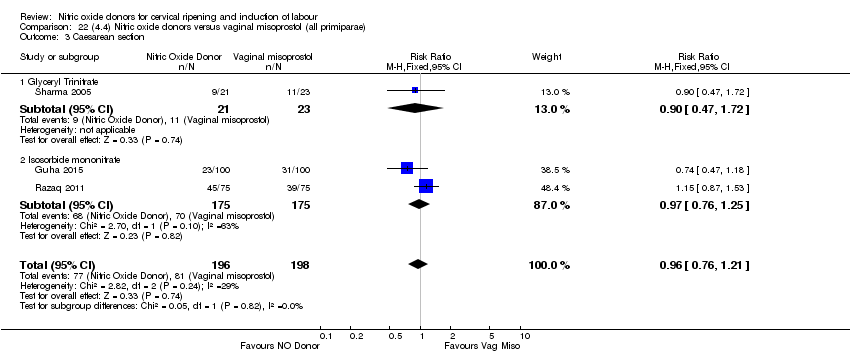 Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 3 Caesarean section. | ||||
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 3.2 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.25] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 22.4  Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 4 Serious neonatal morbidity/perinatal death. | ||||
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Isosorbide Mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| Analysis 22.5  Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 6 Oxytocin augmentation Show forest plot | 3 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [2.27, 4.71] |
| Analysis 22.6 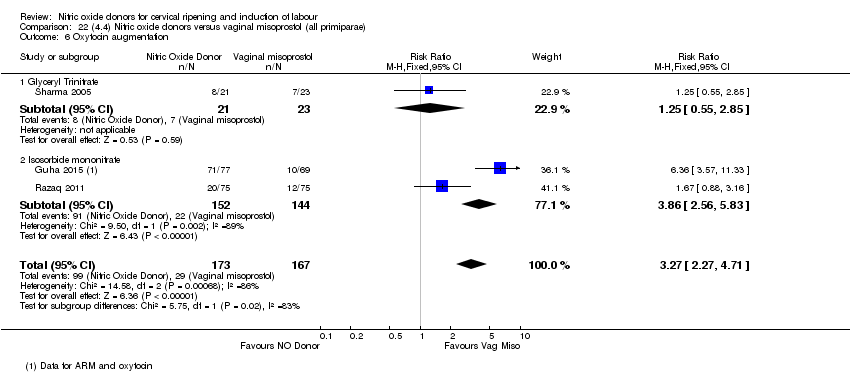 Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 6 Oxytocin augmentation. | ||||
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 6.2 Isosorbide mononitrate | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [2.56, 5.83] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| Analysis 22.7  Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 7 Instrumental vaginal delivery. | ||||
| 7.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 8 Apgar score < 7 at 5 minutes Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.44] |
| Analysis 22.8  Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 8 Apgar score < 7 at 5 minutes. | ||||
| 8.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.44] |
| 9 Neonatal intensive care unit admission Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.06, 0.42] |
| Analysis 22.9 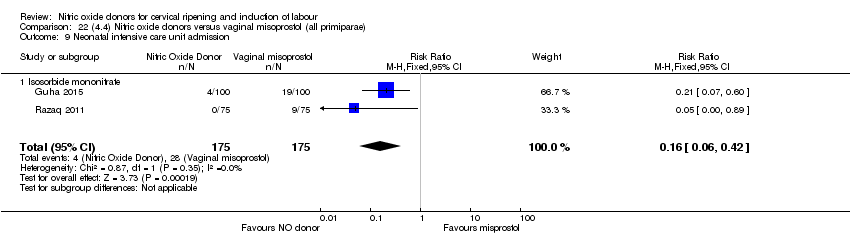 Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 9 Neonatal intensive care unit admission. | ||||
| 9.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.06, 0.42] |
| 10 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 22.10  Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 10 Perinatal death. | ||||
| 10.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal side effects (nausea) Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.89] |
| Analysis 22.11 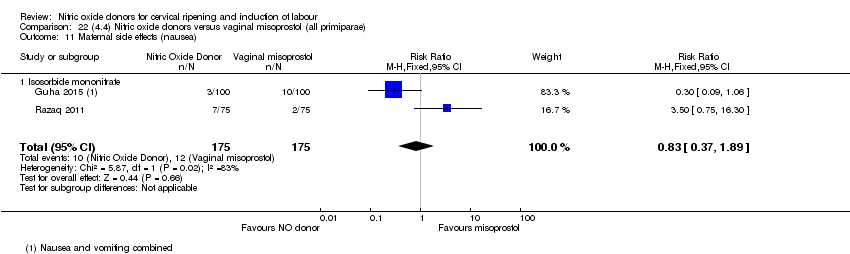 Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 11 Maternal side effects (nausea). | ||||
| 11.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.89] |
| 12 Maternal side effects (headache) Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| Analysis 22.12  Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 12 Maternal side effects (headache). | ||||
| 12.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 13 Postpartum haemorrhage Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.50, 3.33] |
| Analysis 22.13  Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 13 Postpartum haemorrhage. | ||||
| 13.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.50, 3.33] |
| 14 Additional induction agents required Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| Analysis 22.14  Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 14 Additional induction agents required. | ||||
| 14.1 Isosorbide mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| Analysis 23.1  Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes. | ||||
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2 Caesarean section Show forest plot | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.14] |
| Analysis 23.2  Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 2 Caesarean section. | ||||
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide monotrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.18] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 23.3  Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death. | ||||
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| Analysis 23.4  Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5 Oxytocin augmentation Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [2.77, 6.93] |
| Analysis 23.5  Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 5 Oxytocin augmentation. | ||||
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide mononitrate | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.36 [3.57, 11.33] |
| 6 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| Analysis 23.6  Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 6 Instrumental vaginal delivery. | ||||
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| Analysis 23.7  Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes. | ||||
| 7.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| Analysis 23.8 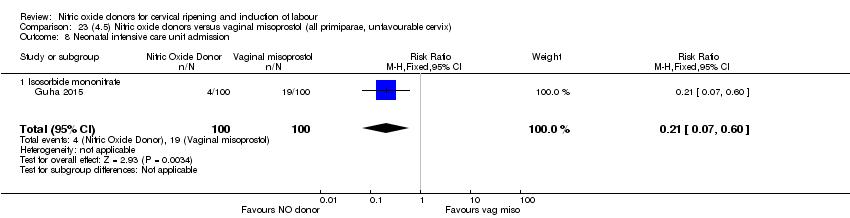 Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission. | ||||
| 8.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| 9 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 23.9  Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 9 Perinatal death. | ||||
| 9.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal side effects (nausea) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| Analysis 23.10  Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 10 Maternal side effects (nausea). | ||||
| 10.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| 11 Maternal side effects (headache) Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| Analysis 23.11  Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 11 Maternal side effects (headache). | ||||
| 11.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 12 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Analysis 23.12  Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 12 Postpartum haemorrhage. | ||||
| 12.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| Analysis 24.1  Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes. | ||||
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2 Caesarean section Show forest plot | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.14] |
| Analysis 24.2  Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 2 Caesarean section. | ||||
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.18] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 24.3  Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death. | ||||
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| Analysis 24.4  Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours. | ||||
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5 Oxytocin augmentation Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [2.77, 6.93] |
| Analysis 24.5  Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 5 Oxytocin augmentation. | ||||
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide mononitrate | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.36 [3.57, 11.33] |
| 6 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| Analysis 24.6 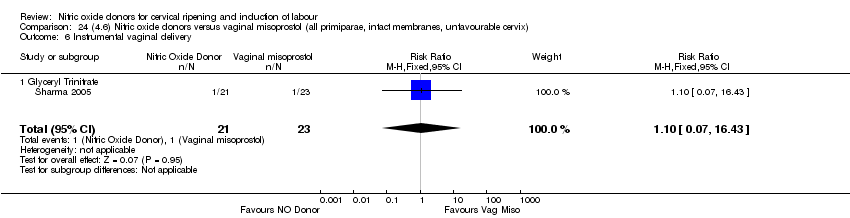 Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 6 Instrumental vaginal delivery. | ||||
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| Analysis 24.7  Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes. | ||||
| 7.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| Analysis 24.8 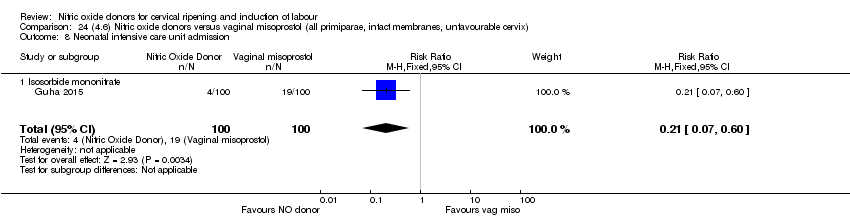 Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission. | ||||
| 8.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| 9 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 24.9  Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 9 Perinatal death. | ||||
| 9.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal side effects (nausea) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| Analysis 24.10  Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (nausea). | ||||
| 10.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| 11 Maternal side effects (headache) Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| Analysis 24.11  Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 11 Maternal side effects (headache). | ||||
| 11.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 12 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Analysis 24.12 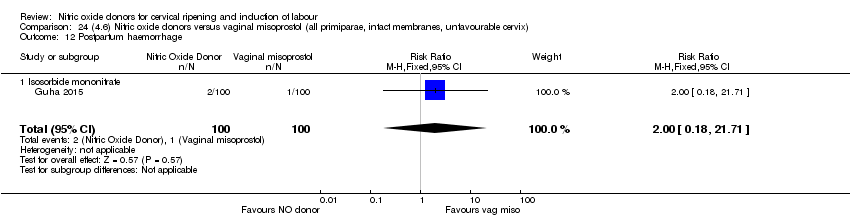 Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 12 Postpartum haemorrhage. | ||||
| 12.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 25.1  Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 1 Caesarean section. | ||||
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| Analysis 25.2 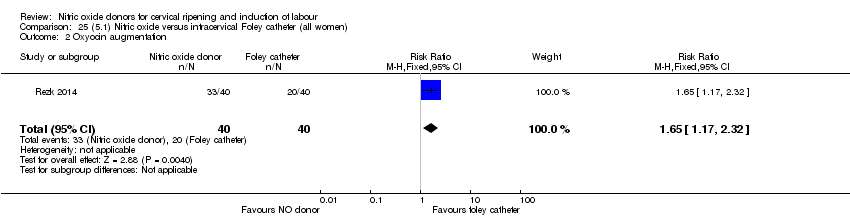 Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 2 Oxyocin augmentation. | ||||
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 25.3  Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 3 Uterine rupture. | ||||
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 25.4  Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 4 Epidural analgesia. | ||||
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| Analysis 25.5  Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 5 Instrumental vaginal delivery. | ||||
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| Analysis 25.6  Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 6 Meconium‐stained liquor. | ||||
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| Analysis 25.7  Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 7 Apgar score < 7 at 5 minutes. | ||||
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| Analysis 25.8  Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 8 Neonatal intensive care unit admission. | ||||
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| Analysis 25.9 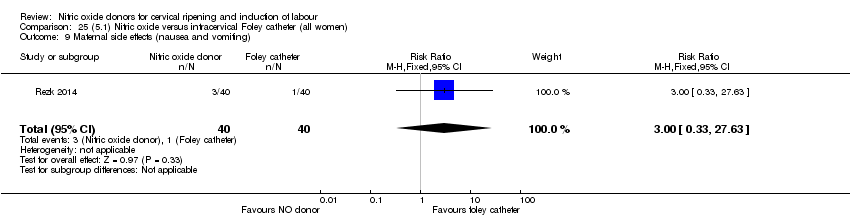 Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 9 Maternal side effects (nausea and vomiting). | ||||
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| Analysis 25.10  Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 10 Maternal side effects (headache). | ||||
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| Analysis 25.11 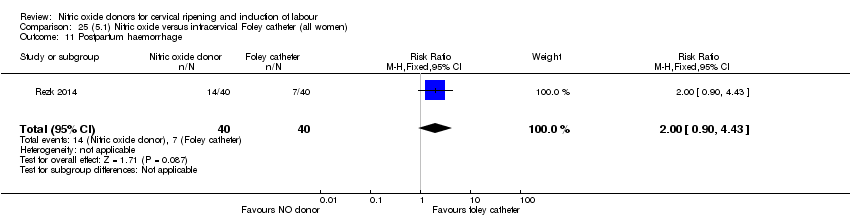 Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 11 Postpartum haemorrhage. | ||||
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| Analysis 25.12  Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 12 Women not satisfied. | ||||
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Analysis 25.13 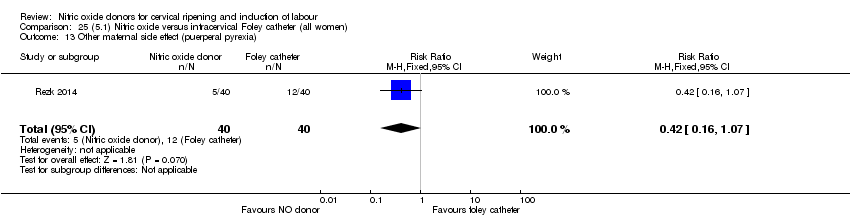 Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 13 Other maternal side effect (puerperal pyrexia). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 26.1  Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 1 Caesarean section. | ||||
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| Analysis 26.2  Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 2 Oxyocin augmentation. | ||||
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 26.3  Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 3 Uterine rupture. | ||||
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 26.4 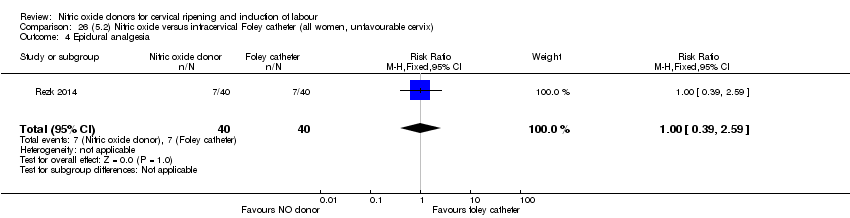 Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 4 Epidural analgesia. | ||||
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| Analysis 26.5 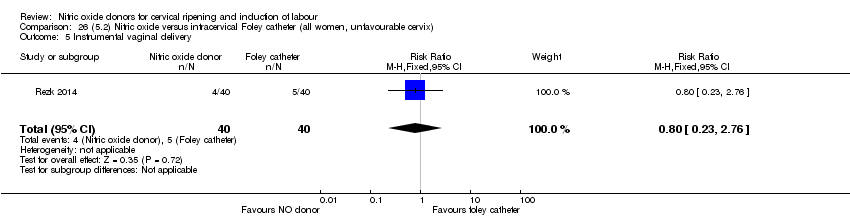 Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 5 Instrumental vaginal delivery. | ||||
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| Analysis 26.6  Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 6 Meconium‐stained liquor. | ||||
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| Analysis 26.7  Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes. | ||||
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| Analysis 26.8  Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission. | ||||
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| Analysis 26.9 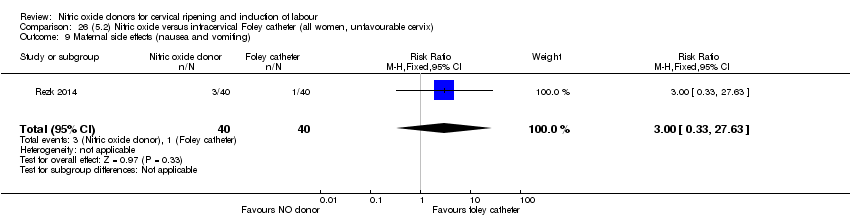 Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting). | ||||
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| Analysis 26.10  Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 10 Maternal side effects (headache). | ||||
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| Analysis 26.11 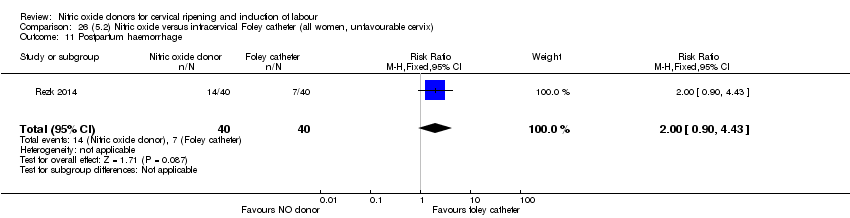 Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 11 Postpartum haemorrhage. | ||||
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| Analysis 26.12 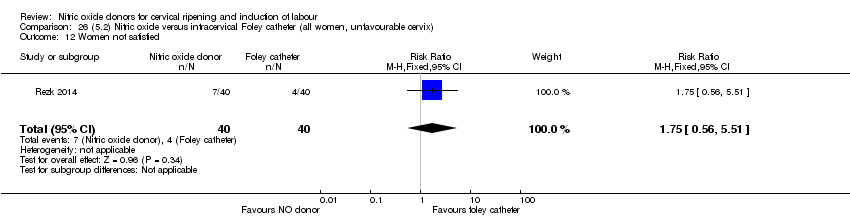 Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 12 Women not satisfied. | ||||
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Analysis 26.13 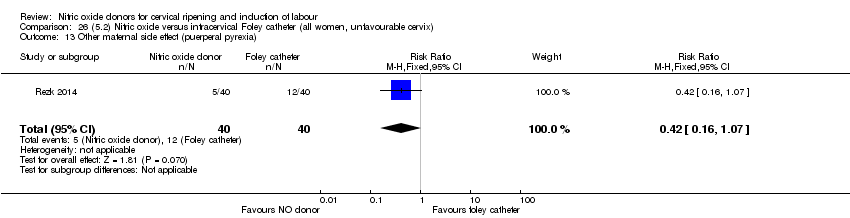 Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 27.1  Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 1 Caesarean section. | ||||
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| Analysis 27.2 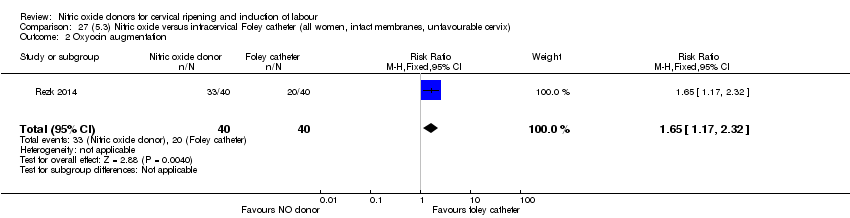 Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 2 Oxyocin augmentation. | ||||
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 27.3  Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 3 Uterine rupture. | ||||
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 27.4  Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 4 Epidural analgesia. | ||||
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| Analysis 27.5  Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 5 Instrumental vaginal delivery. | ||||
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| Analysis 27.6  Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 6 Meconium‐stained liquor. | ||||
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| Analysis 27.7  Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes. | ||||
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| Analysis 27.8  Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission. | ||||
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| Analysis 27.9 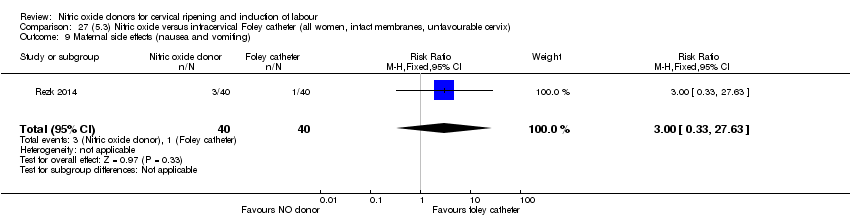 Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting). | ||||
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| Analysis 27.10  Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (headache). | ||||
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| Analysis 27.11  Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 11 Postpartum haemorrhage. | ||||
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| Analysis 27.12  Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 12 Women not satisfied. | ||||
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Analysis 27.13  Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 28.1  Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 1 Caesarean section. | ||||
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| Analysis 28.2 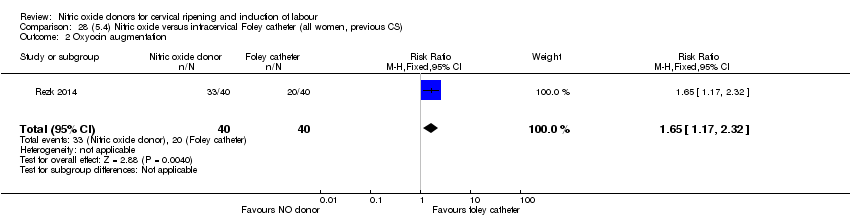 Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 2 Oxyocin augmentation. | ||||
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 28.3  Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 3 Uterine rupture. | ||||
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 28.4 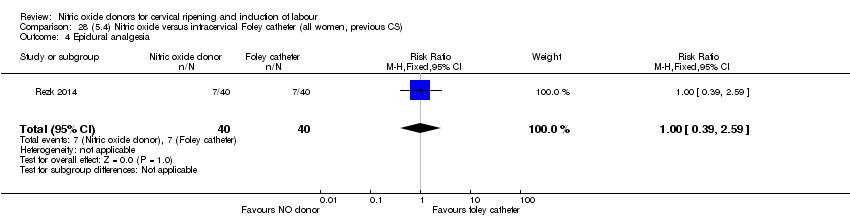 Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 4 Epidural analgesia. | ||||
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| Analysis 28.5  Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 5 Instrumental vaginal delivery. | ||||
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| Analysis 28.6 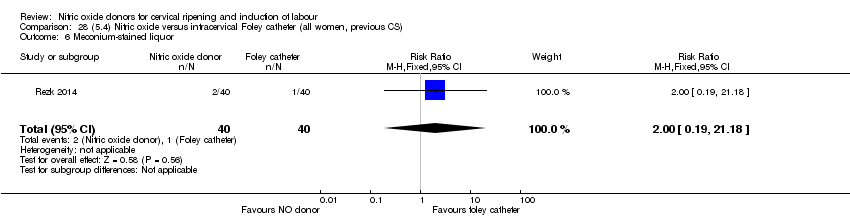 Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 6 Meconium‐stained liquor. | ||||
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| Analysis 28.7  Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 7 Apgar score < 7 at 5 minutes. | ||||
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| Analysis 28.8 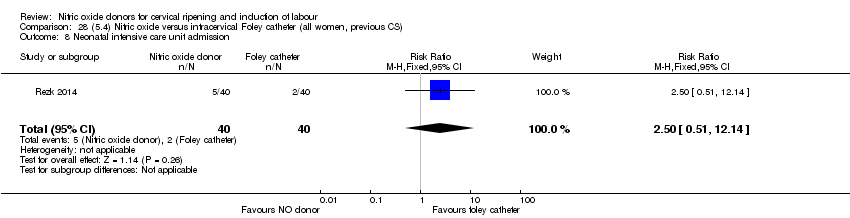 Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 8 Neonatal intensive care unit admission. | ||||
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| Analysis 28.9 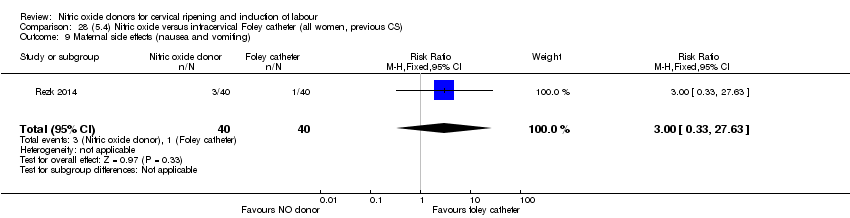 Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 9 Maternal side effects (nausea and vomiting). | ||||
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| Analysis 28.10 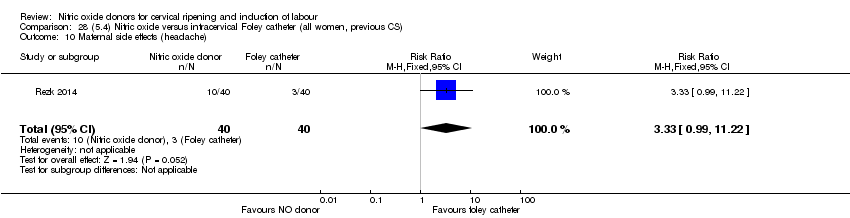 Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 10 Maternal side effects (headache). | ||||
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| Analysis 28.11 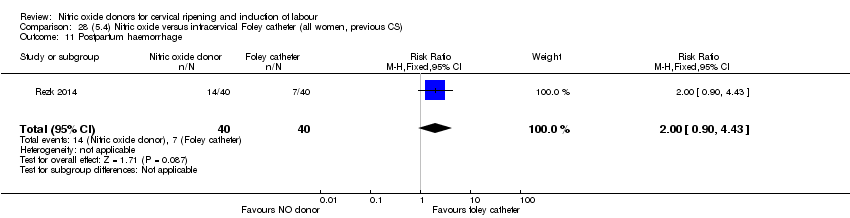 Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 11 Postpartum haemorrhage. | ||||
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| Analysis 28.12 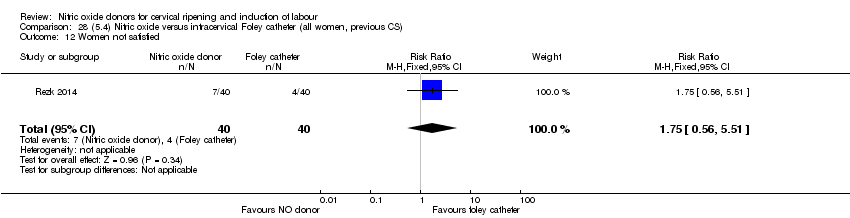 Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 12 Women not satisfied. | ||||
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Analysis 28.13 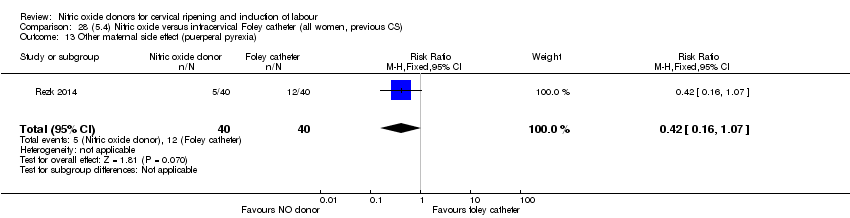 Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 13 Other maternal side effect (puerperal pyrexia). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 29.1 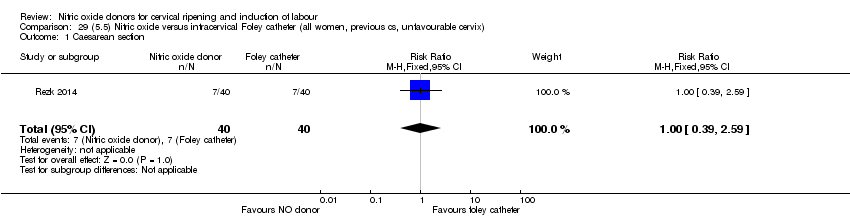 Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 1 Caesarean section. | ||||
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| Analysis 29.2  Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 2 Oxyocin augmentation. | ||||
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 29.3 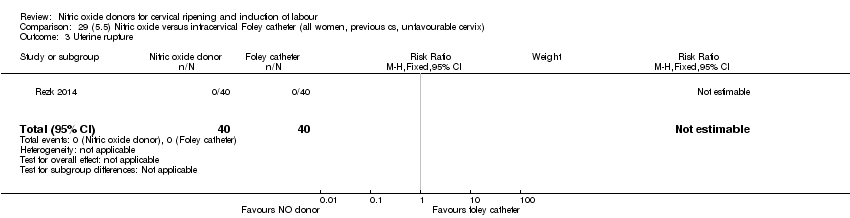 Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 3 Uterine rupture. | ||||
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 29.4  Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 4 Epidural analgesia. | ||||
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| Analysis 29.5  Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 5 Instrumental vaginal delivery. | ||||
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| Analysis 29.6  Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 6 Meconium‐stained liquor. | ||||
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| Analysis 29.7  Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes. | ||||
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| Analysis 29.8  Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission. | ||||
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| Analysis 29.9 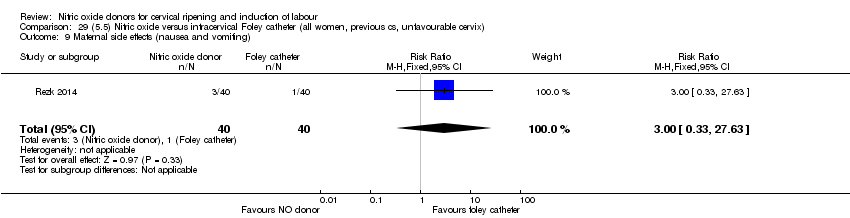 Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting). | ||||
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| Analysis 29.10  Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 10 Maternal side effects (headache). | ||||
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| Analysis 29.11 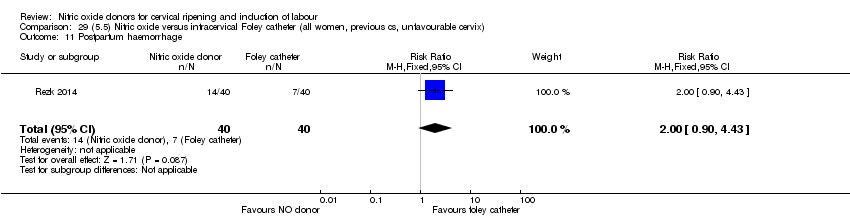 Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 11 Postpartum haemorrhage. | ||||
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| Analysis 29.12  Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 12 Women not satisfied. | ||||
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Analysis 29.13  Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 30.1  Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 1 Caesarean section. | ||||
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| Analysis 30.2 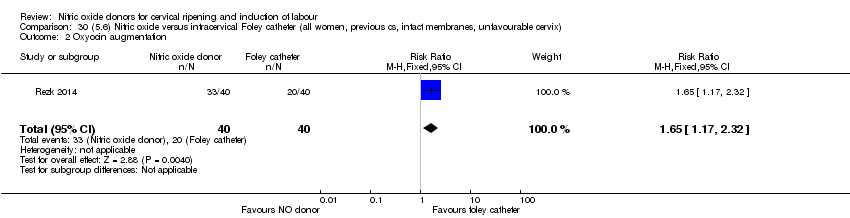 Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 2 Oxyocin augmentation. | ||||
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 30.3 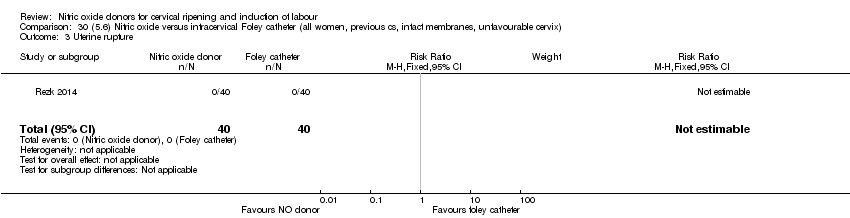 Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 3 Uterine rupture. | ||||
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| Analysis 30.4  Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 4 Epidural analgesia. | ||||
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| Analysis 30.5  Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 5 Instrumental vaginal delivery. | ||||
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| Analysis 30.6 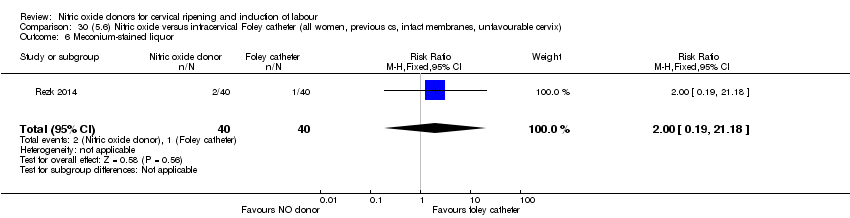 Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 6 Meconium‐stained liquor. | ||||
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| Analysis 30.7  Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes. | ||||
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| Analysis 30.8  Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission. | ||||
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| Analysis 30.9  Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting). | ||||
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| Analysis 30.10  Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (headache). | ||||
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| Analysis 30.11  Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 11 Postpartum haemorrhage. | ||||
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| Analysis 30.12  Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 12 Women not satisfied. | ||||
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Analysis 30.13 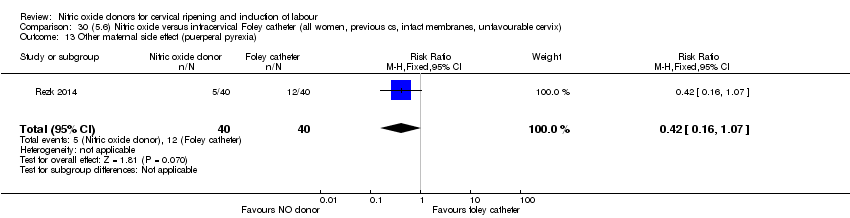 Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia). | ||||
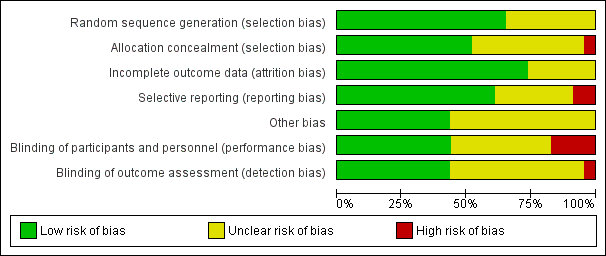
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 2 Uterine hyperstimulation with FHR changes.
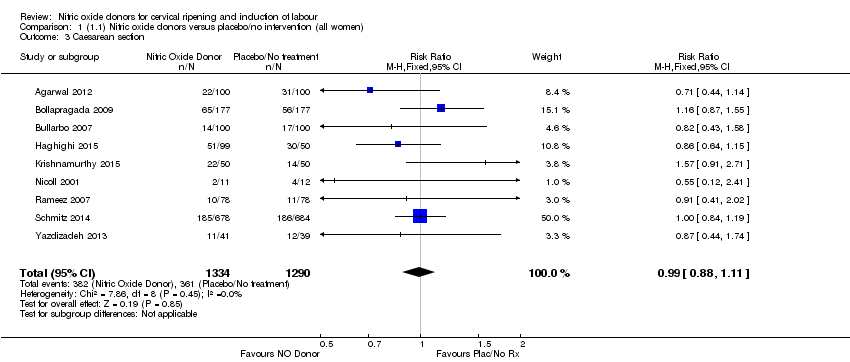
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 3 Caesarean section.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 4 Serious neonatal morbidity/perinatal death.
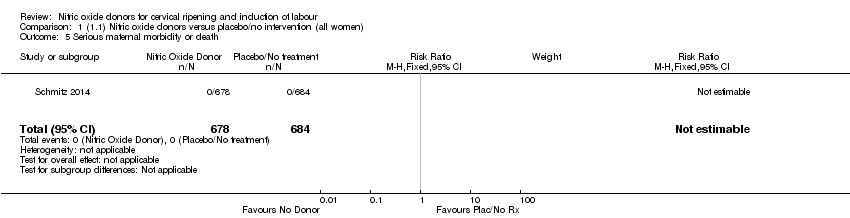
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 5 Serious maternal morbidity or death.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 7 Oxytocin augmentation.
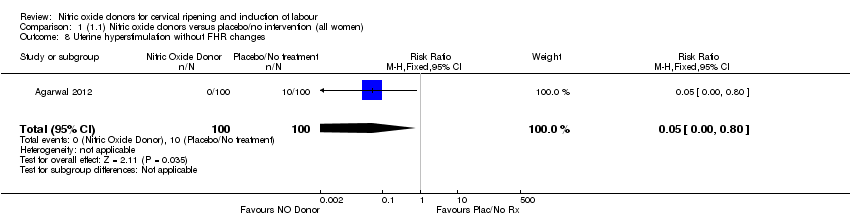
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 8 Uterine hyperstimulation without FHR changes.
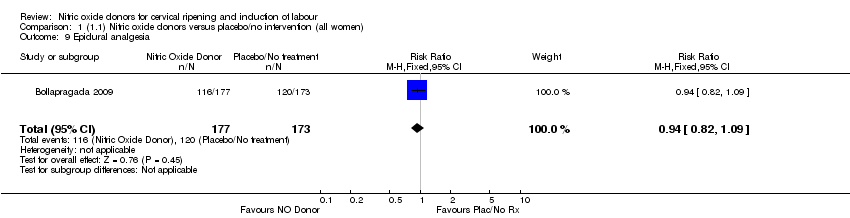
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 9 Epidural analgesia.
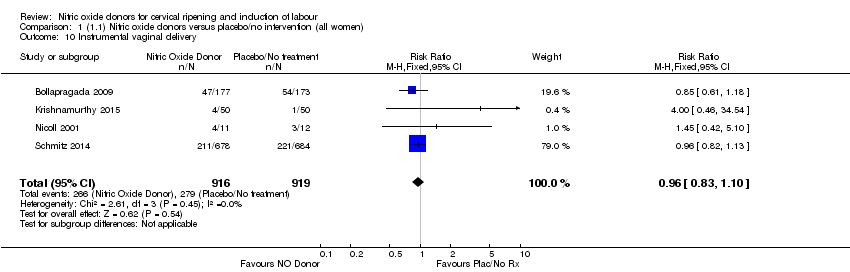
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 10 Instrumental vaginal delivery.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 11 Meconium‐stained liquor.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 12 Apgar score < 7 at 5 minutes.
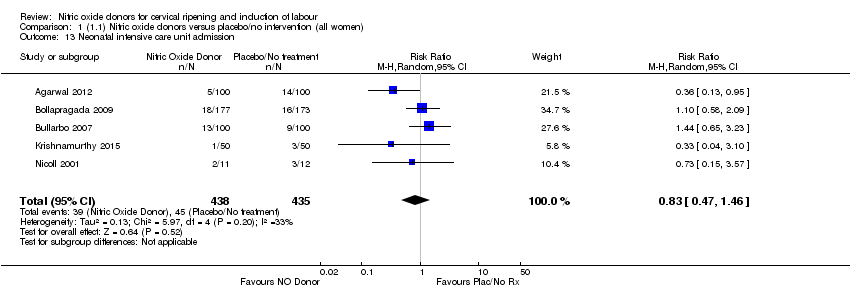
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 13 Neonatal intensive care unit admission.
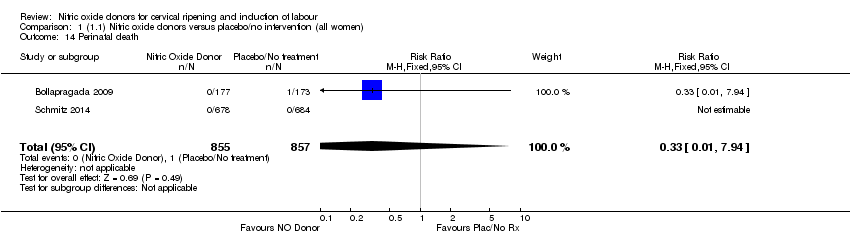
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 14 Perinatal death.
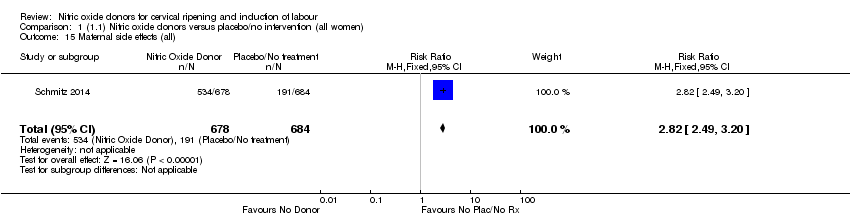
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 15 Maternal side effects (all).

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 16 Maternal side effects (nausea).

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 17 Maternal side effects (headache).

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 18 Maternal side effects (vomiting).

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 19 Maternal side effects (diarrhoea).
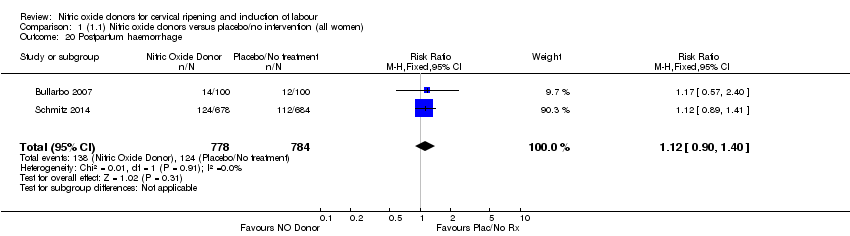
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 20 Postpartum haemorrhage.
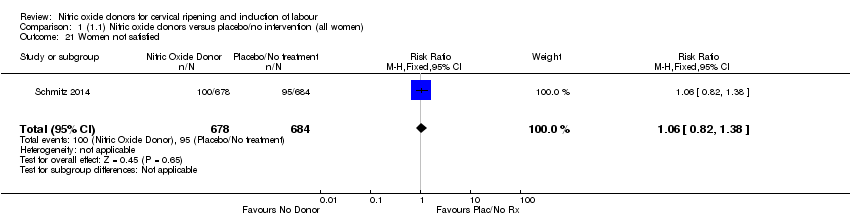
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 21 Women not satisfied.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 22 Additional induction agents used.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours.
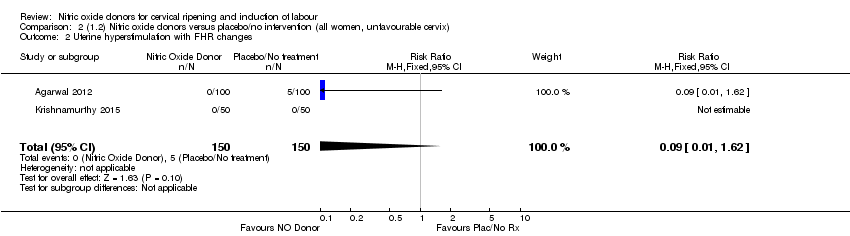
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
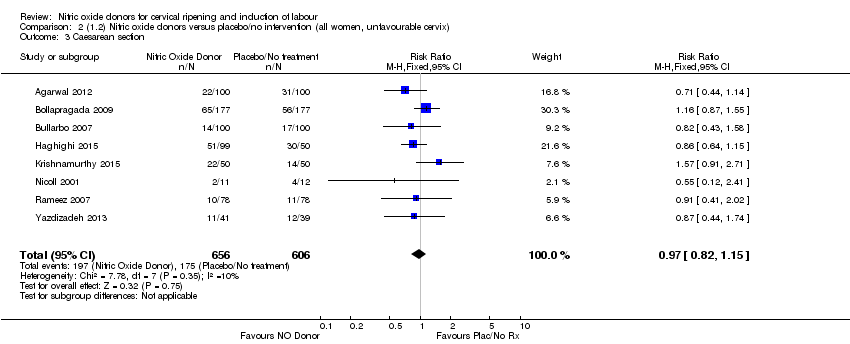
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 3 Caesarean section.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death.
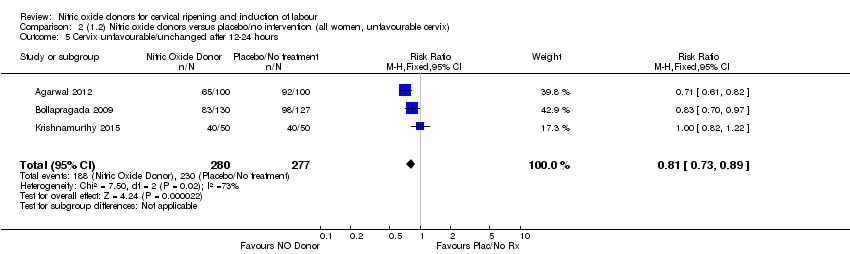
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
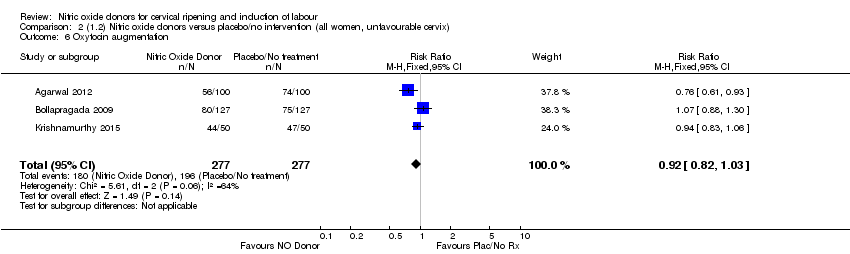
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 6 Oxytocin augmentation.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 7 Uterine hyperstimulation without FHR changes.
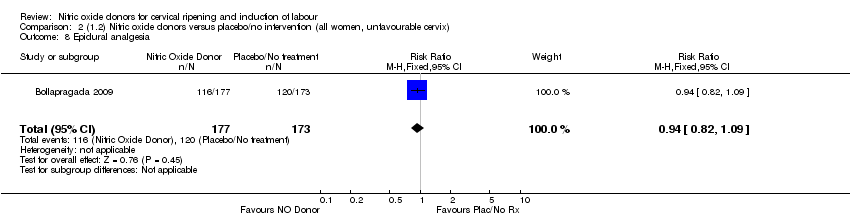
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 8 Epidural analgesia.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 9 Instrumental vaginal delivery.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 10 Meconium‐stained liquor.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 11 Apgar score < 7 at 5 minutes.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 12 Neonatal intensive care unit admission.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 13 Perinatal death.
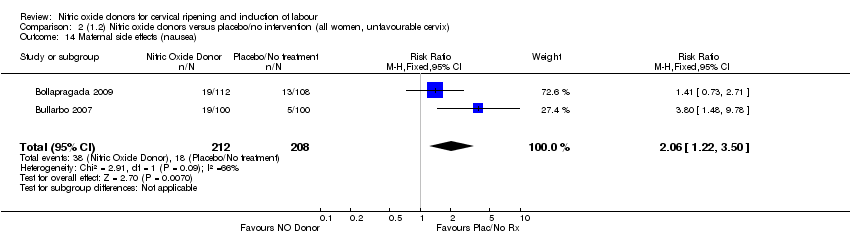
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 14 Maternal side effects (nausea).
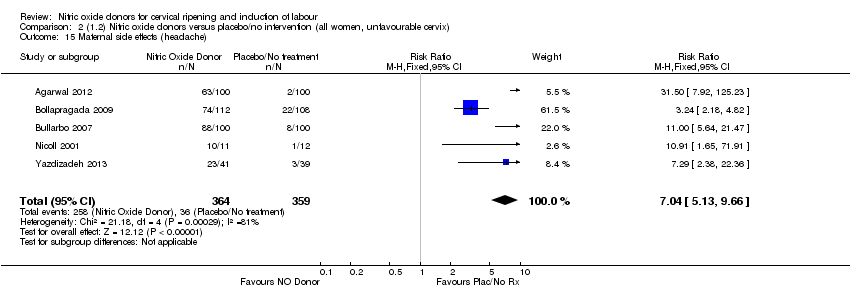
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 15 Maternal side effects (headache).

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 16 Postpartum haemorrhage.
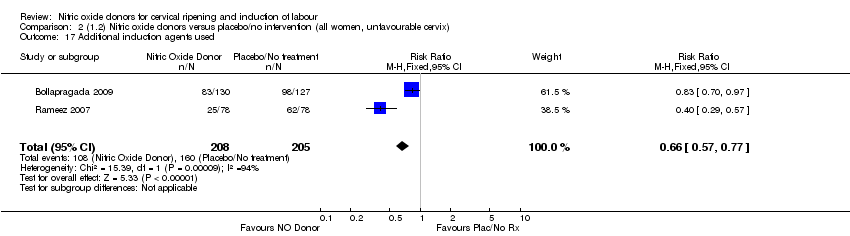
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 17 Additional induction agents used.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours.
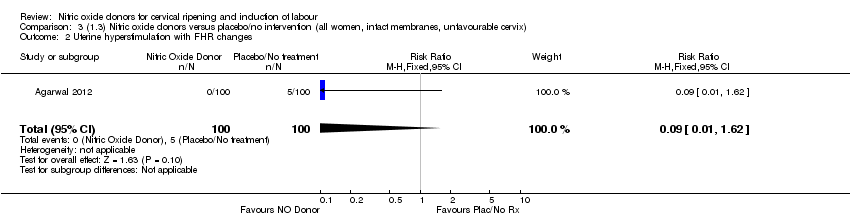
Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 3 Caesarean section.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
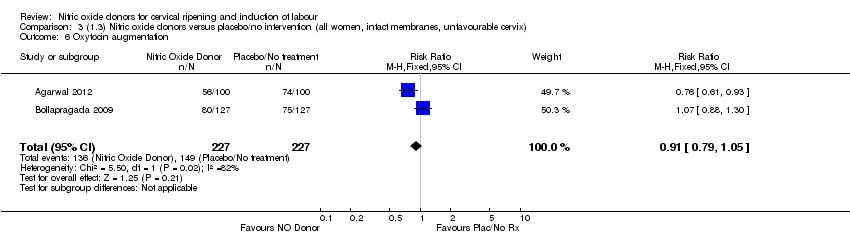
Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 6 Oxytocin augmentation.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 7 Uterine hyperstimulation without FHR changes.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 8 Epidural analgesia.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 9 Instrumental vaginal delivery.
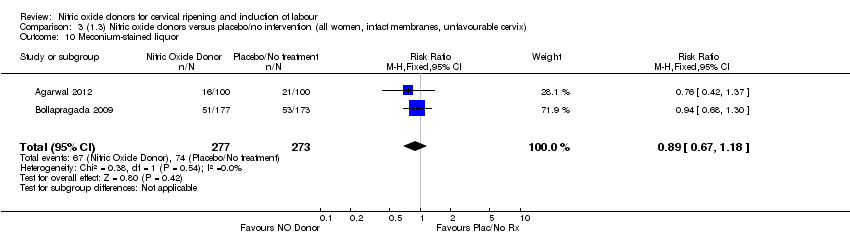
Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 10 Meconium‐stained liquor.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 11 Apgar score < 7 at 5 minutes.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 12 Neonatal intensive care unit admission.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 13 Perinatal death.
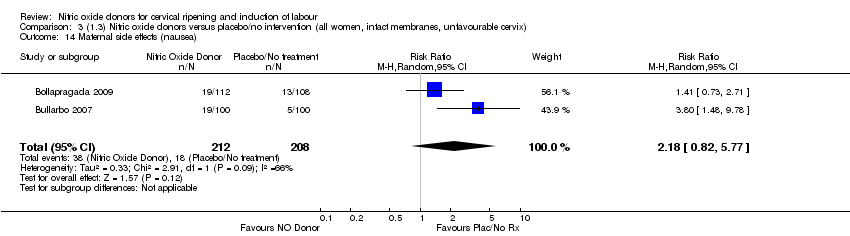
Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 14 Maternal side effects (nausea).

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 15 Maternal side effects (headache).

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 16 Postpartum haemorrhage.
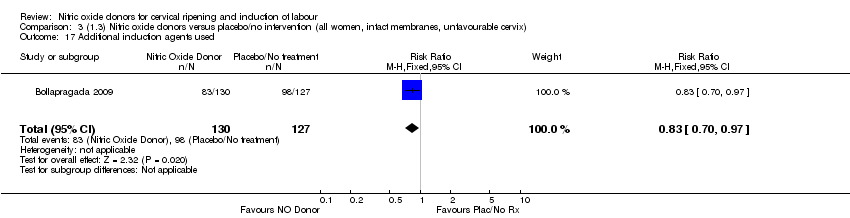
Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 17 Additional induction agents used.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.
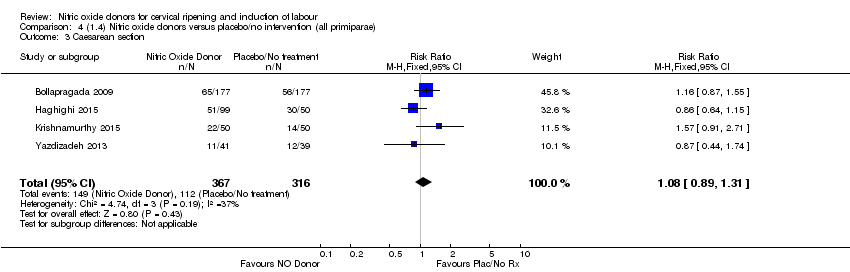
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 3 Caesarean section.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 4 Serious neonatal morbidity/perinatal death.
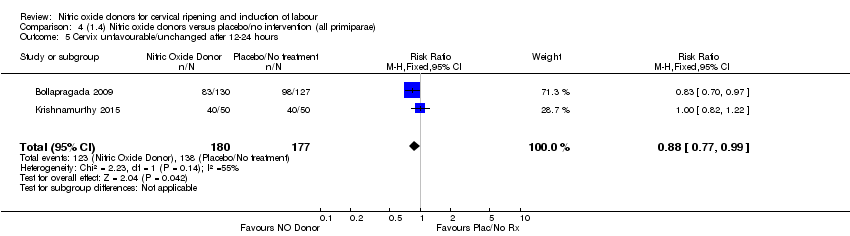
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 6 Oxytocin augmentation.
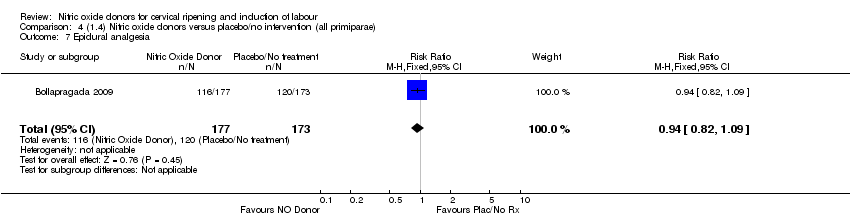
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 7 Epidural analgesia.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 8 Instrumental vaginal delivery.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 9 Meconium‐stained liquor.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 10 Apgar score < 7 at 5 minutes.
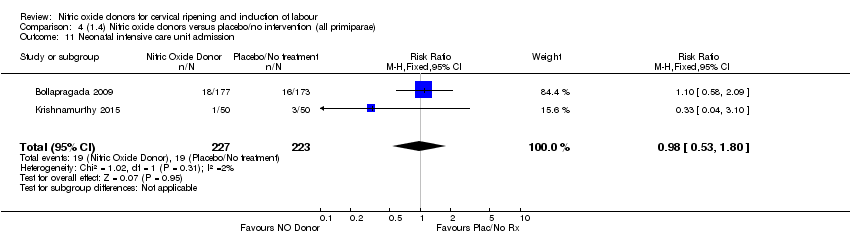
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 11 Neonatal intensive care unit admission.
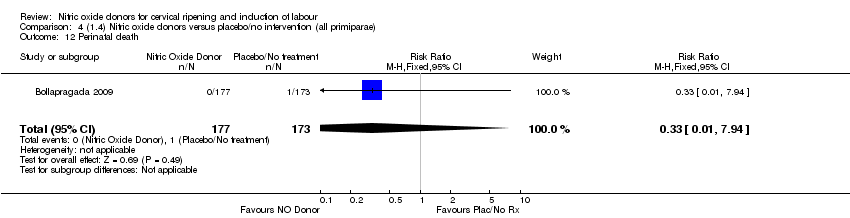
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 12 Perinatal death.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 13 Maternal side effects (nausea).
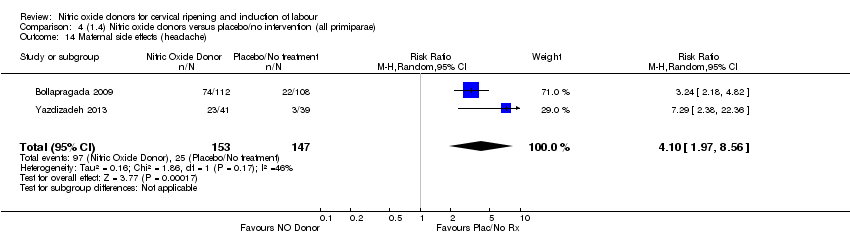
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 14 Maternal side effects (headache).

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 15 Additional induction agents used.
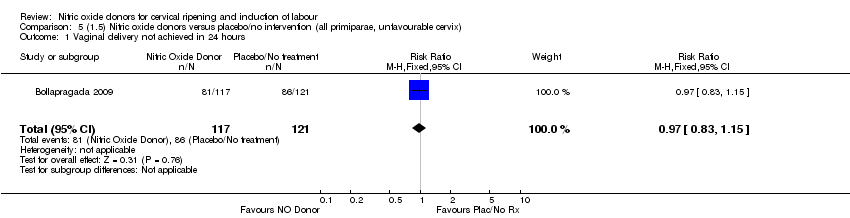
Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
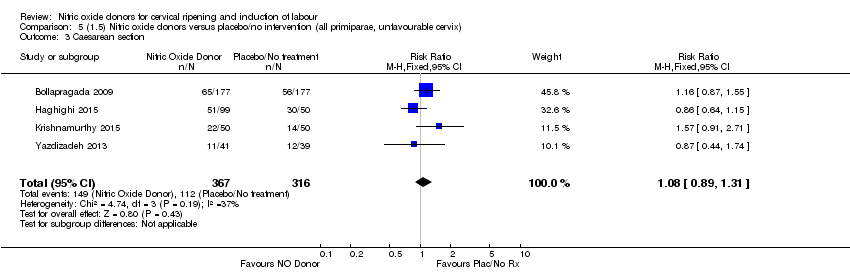
Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 3 Caesarean section.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death.
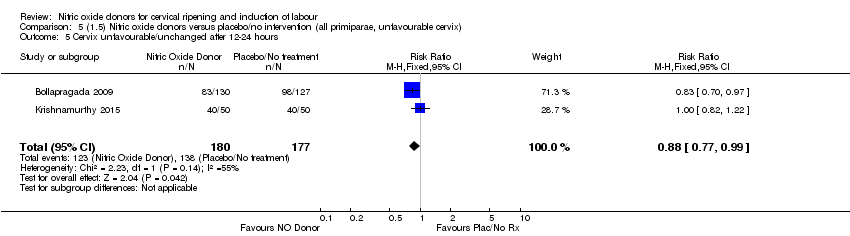
Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 6 Oxytocin augmentation.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 7 Epidural analgesia.
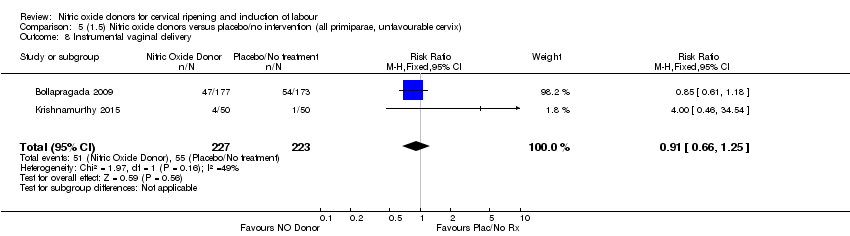
Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 8 Instrumental vaginal delivery.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 9 Meconium‐stained liquor.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 10 Apgar score < 7 at 5 minutes.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 11 Neonatal intensive care unit admission.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 12 Perinatal death.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 13 Maternal side effects (nausea).

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 14 Maternal side effects (headache).

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 15 Additional induction agents used.
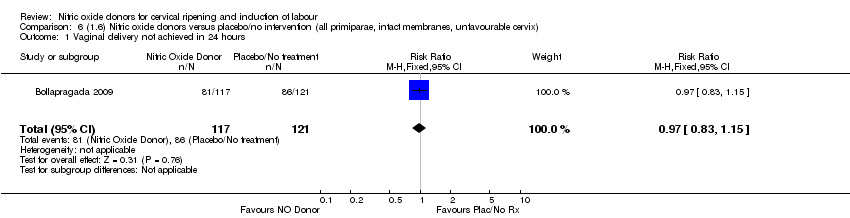
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 2 Caesarean section.
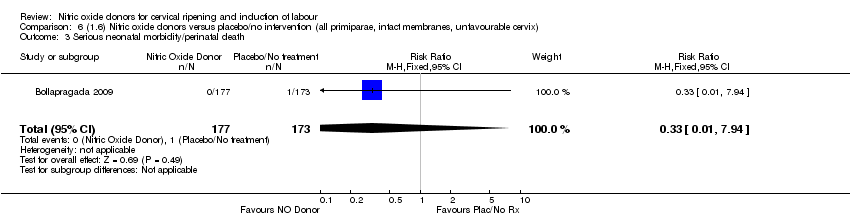
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.
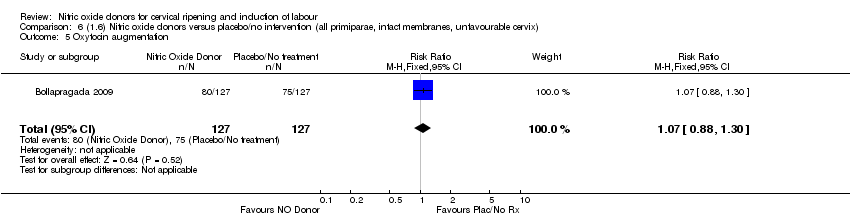
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 5 Oxytocin augmentation.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 6 Epidural analgesia.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 7 Instrumental vaginal delivery.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 8 Meconium‐stained liquor.
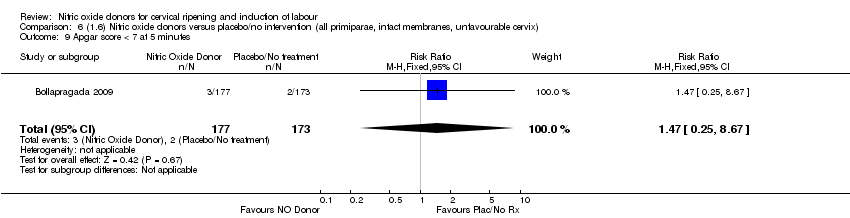
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 9 Apgar score < 7 at 5 minutes.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 10 Neonatal intensive care unit admission.
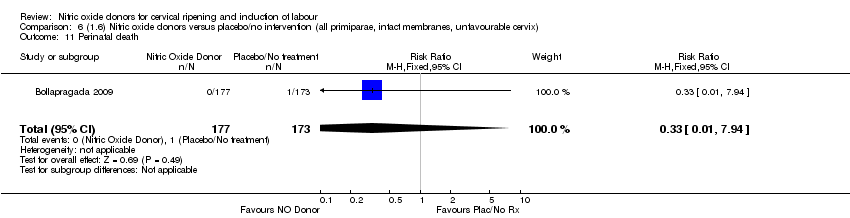
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 11 Perinatal death.
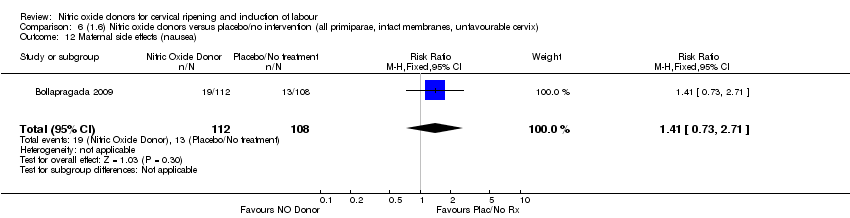
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 12 Maternal side effects (nausea).

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 13 Maternal side effects (headache).

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 14 Additional induction agents used.
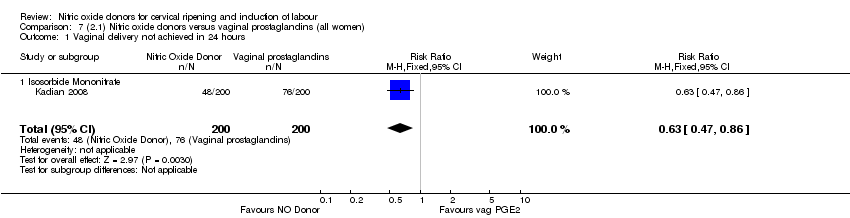
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 1 Vaginal delivery not achieved in 24 hours.
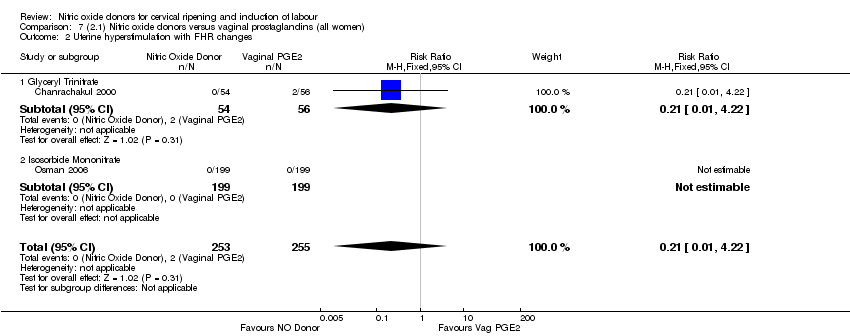
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 2 Uterine hyperstimulation with FHR changes.
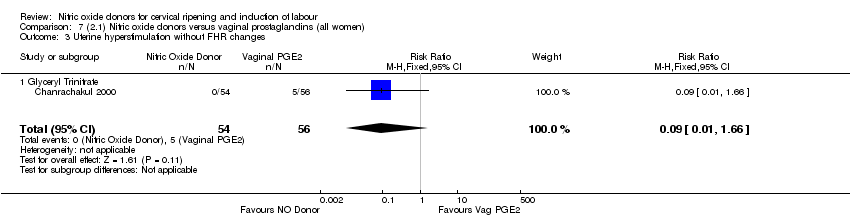
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 3 Uterine hyperstimulation without FHR changes.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 4 Caesarean section.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 5 Instrumental vaginal delivery.
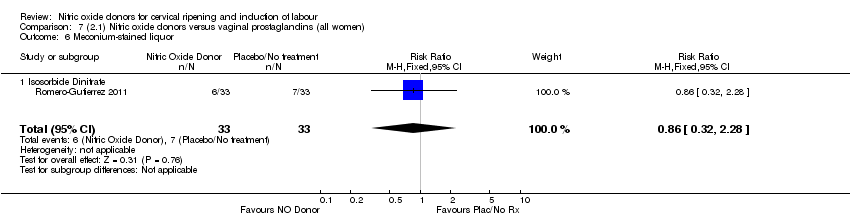
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 6 Meconium‐stained liquor.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 8 Epidural analgesia.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 9 Maternal side effects (nausea).

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 10 Maternal side effects (headache).
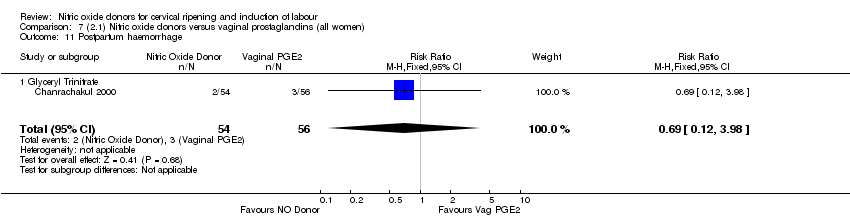
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 11 Postpartum haemorrhage.
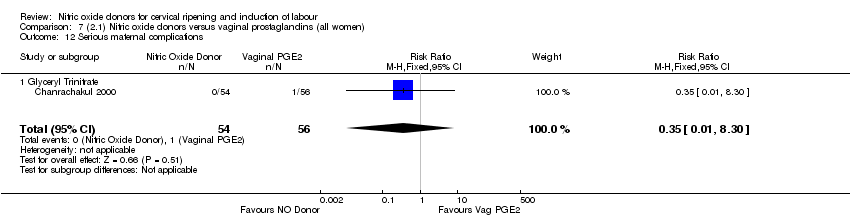
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 12 Serious maternal complications.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 13 Neonatal intensive care unit admission.
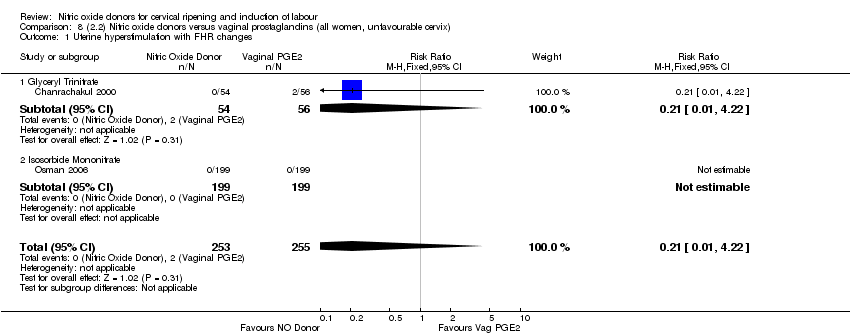
Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.
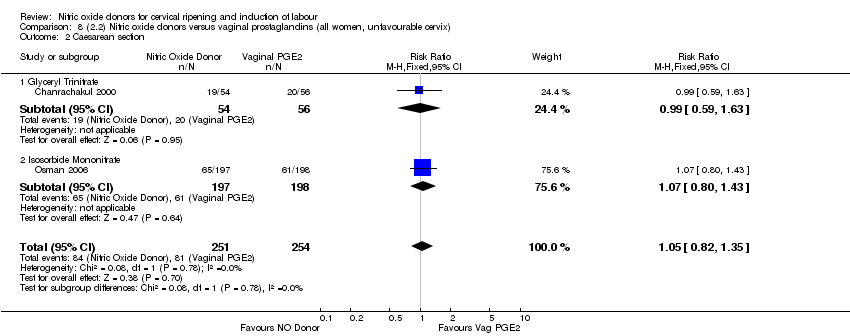
Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 2 Caesarean section.

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 3 Uterine hyperstimulation without FHR changes.

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 4 Epidural analgesia.
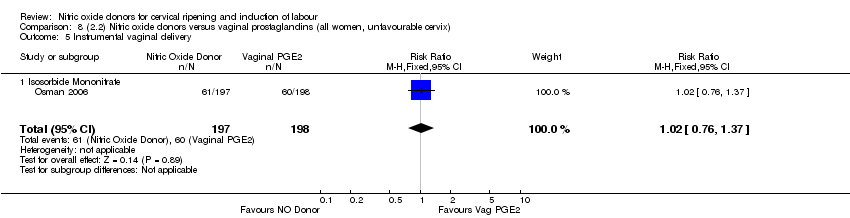
Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 5 Instrumental vaginal delivery.

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 6 Apgar score < 7 at 5 minutes.

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 7 Neonatal intensive care unit admission.
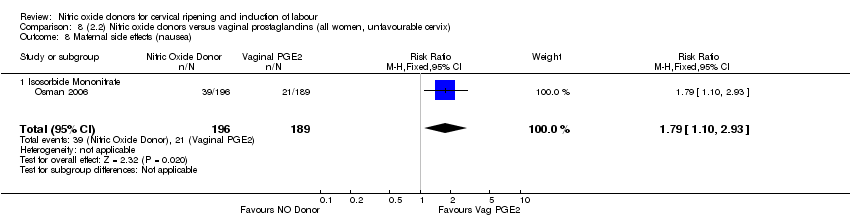
Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 8 Maternal side effects (nausea).

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 9 Maternal side effects (headache).
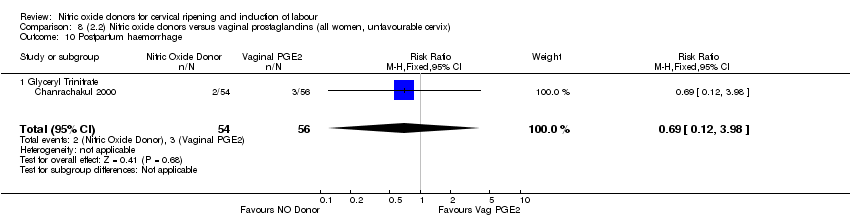
Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 10 Postpartum haemorrhage.

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 11 Serious maternal complications.
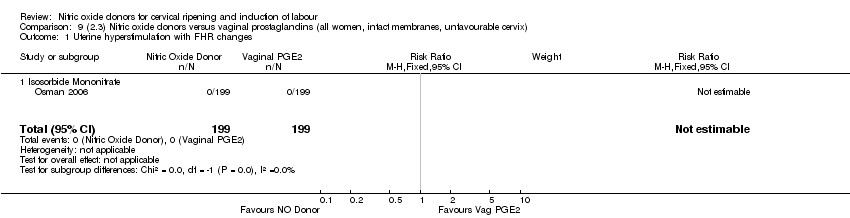
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.
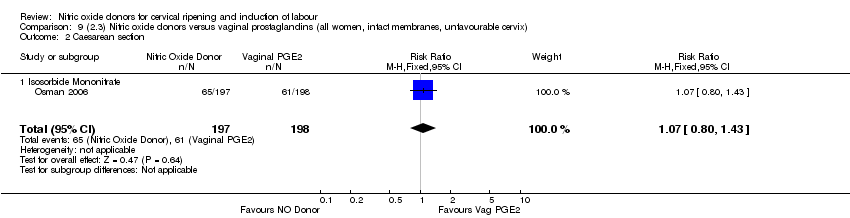
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 2 Caesarean section.

Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 3 Epidural analgesia.

Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 4 Instrumental vaginal delivery.
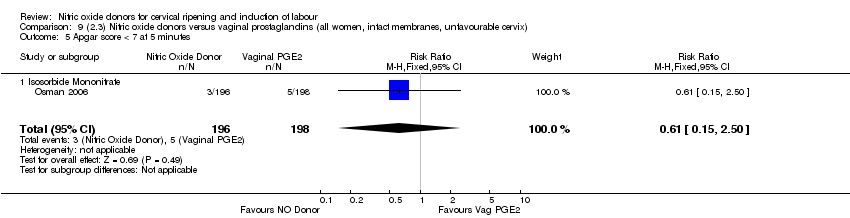
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes.
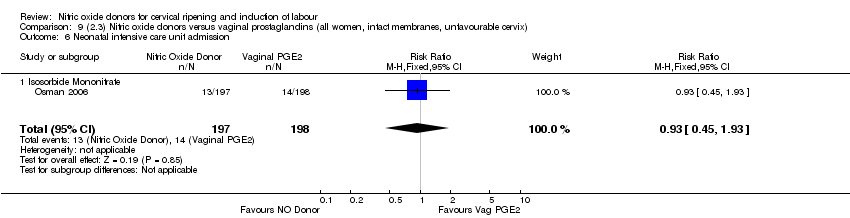
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 6 Neonatal intensive care unit admission.
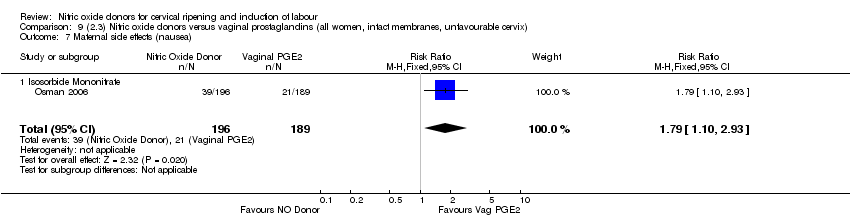
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 7 Maternal side effects (nausea).
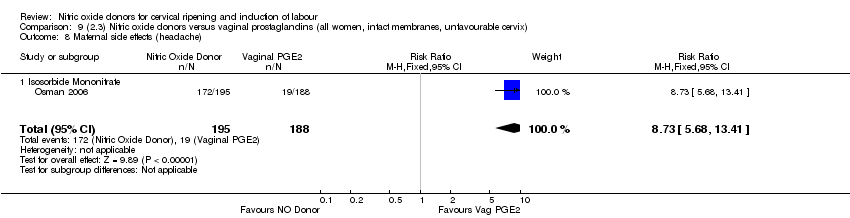
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 8 Maternal side effects (headache).

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 3 Caesarean section.
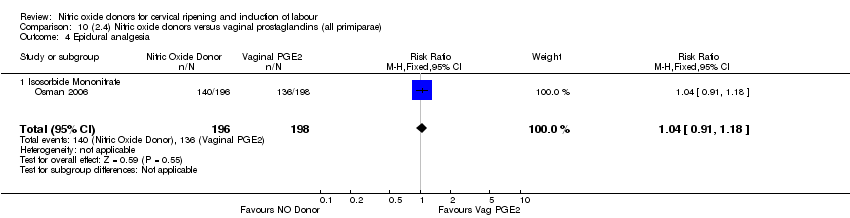
Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 4 Epidural analgesia.

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 5 Instrumental vaginal delivery.

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 6 Apgar score < 7 at 5 minutes.

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 7 Neonatal intensive care unit admission.
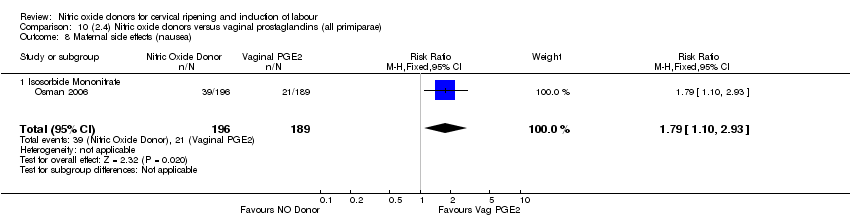
Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 8 Maternal side effects (nausea).
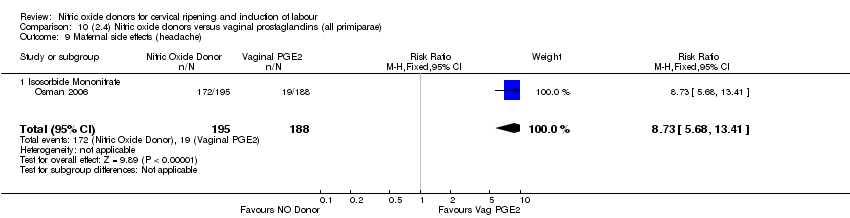
Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 9 Maternal side effects (headache).
Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 2 Caesarean section.

Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 3 Epidural analgesia.
Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 4 Instrumental vaginal delivery.
Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes.
Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 6 Neonatal intensive care unit admission.
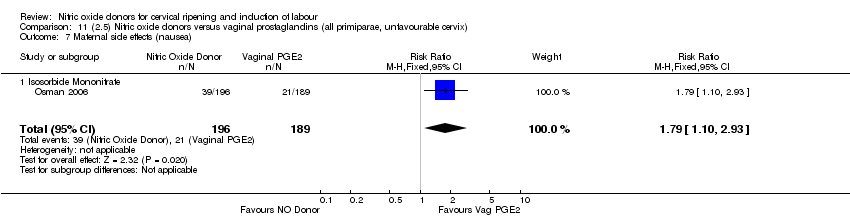
Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 7 Maternal side effects (nausea).

Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 8 Maternal side effects (headache).

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 2 Caesarean section.

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 3 Epidural analgesia.

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 4 Instrumental vaginal delivery.

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes.
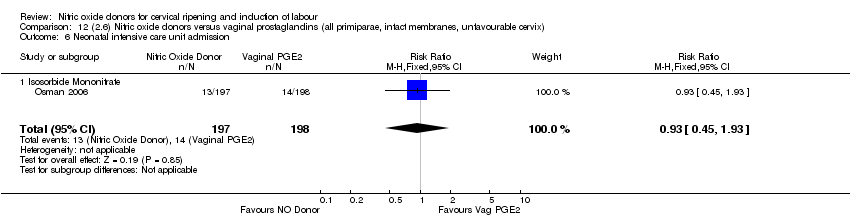
Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 6 Neonatal intensive care unit admission.

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 7 Maternal side effects (nausea).
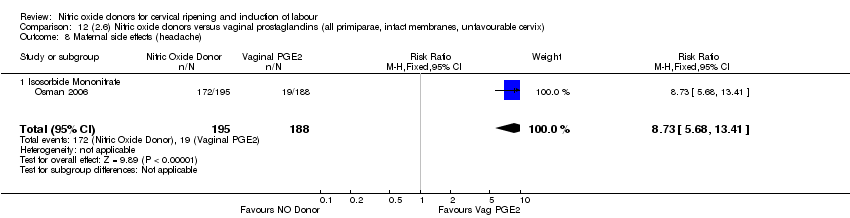
Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 8 Maternal side effects (headache).

Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 1 Vaginal delivery not achieved in 24 hours.
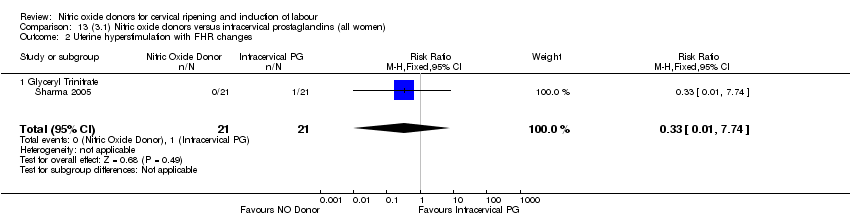
Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 3 Caesarean section.

Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 4 Serious neonatal morbidity/perinatal death.
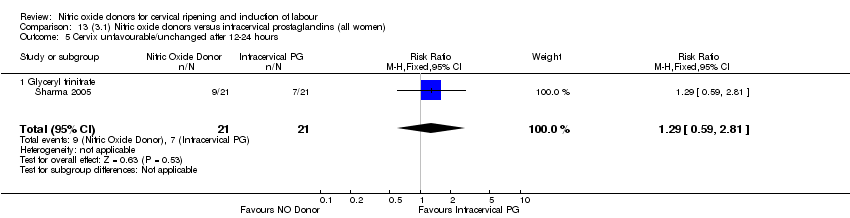
Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
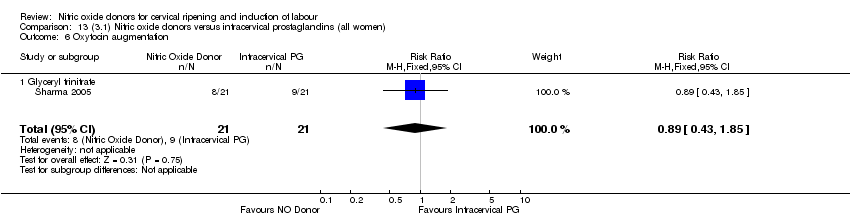
Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 6 Oxytocin augmentation.
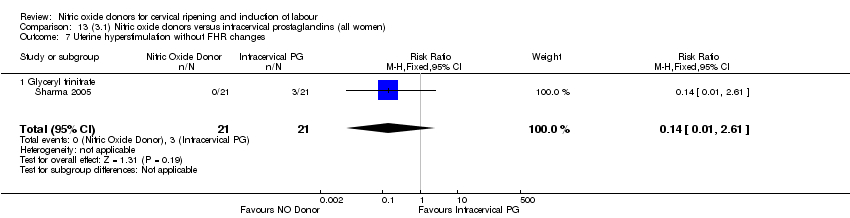
Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 7 Uterine hyperstimulation without FHR changes.
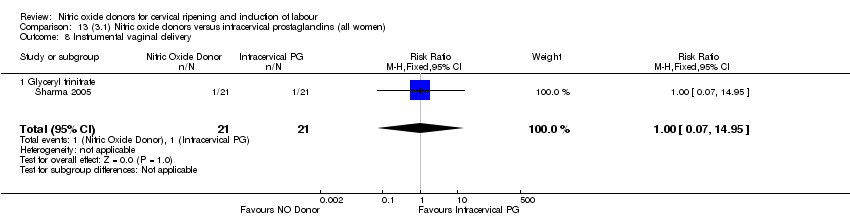
Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 8 Instrumental vaginal delivery.

Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 9 Perinatal death.

Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 10 Maternal side effects (headache).

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
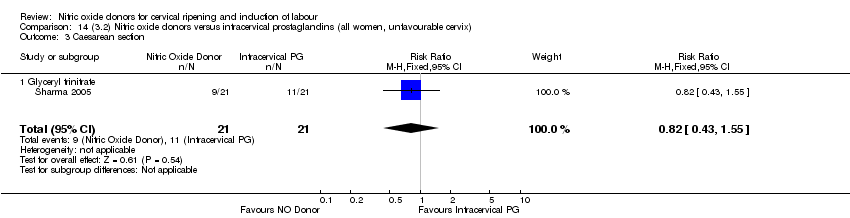
Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 3 Caesarean section.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 6 Oxytocin augmentation.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 7 Uterine hyperstimulation without FHR changes.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 8 Instrumental vaginal delivery.
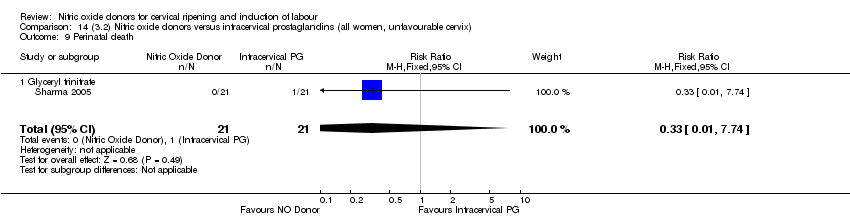
Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 9 Perinatal death.
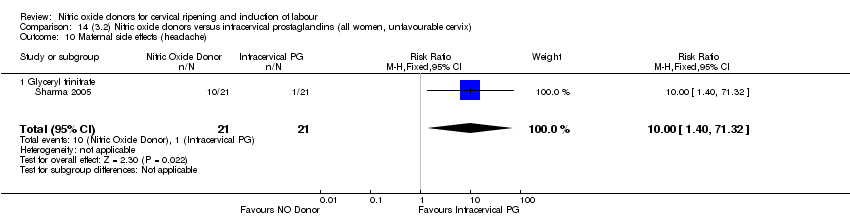
Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 10 Maternal side effects (headache).

Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 2 Uterine hyperstimulation with FHR changes.
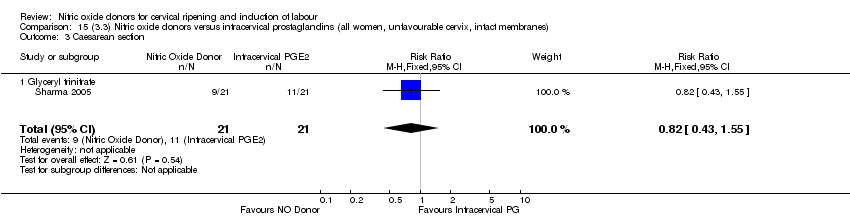
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 3 Caesarean section.

Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 4 Serious neonatal morbidity/perinatal death.

Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
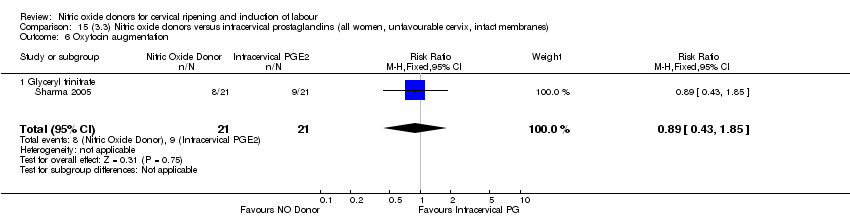
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 6 Oxytocin augmentation.
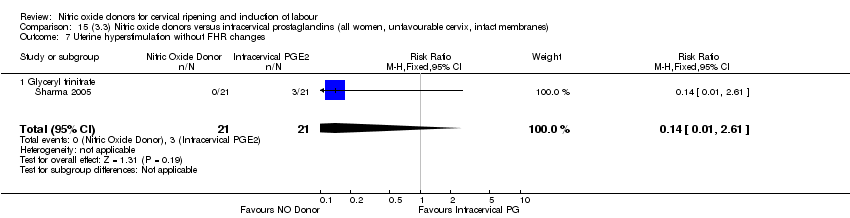
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 7 Uterine hyperstimulation without FHR changes.
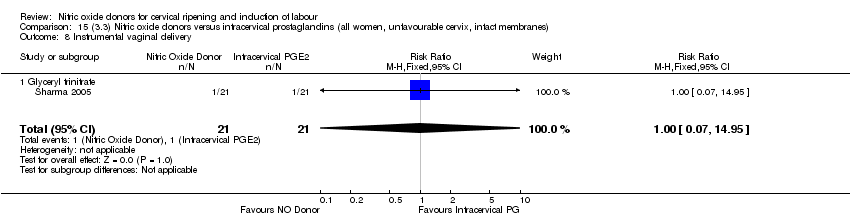
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 8 Instrumental vaginal delivery.
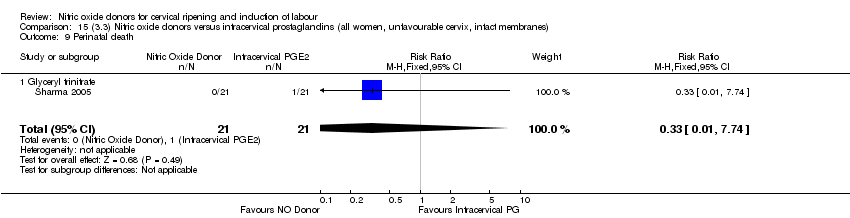
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 9 Perinatal death.
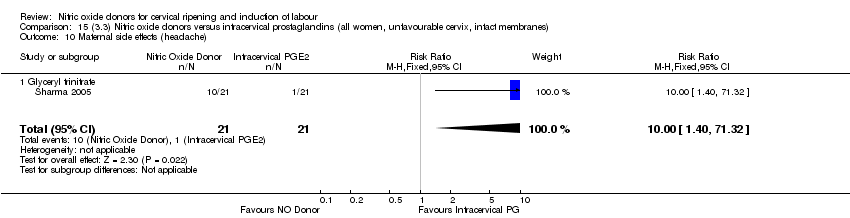
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 10 Maternal side effects (headache).

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 2 Caesarean section.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 5 Oxytocin augmentation.
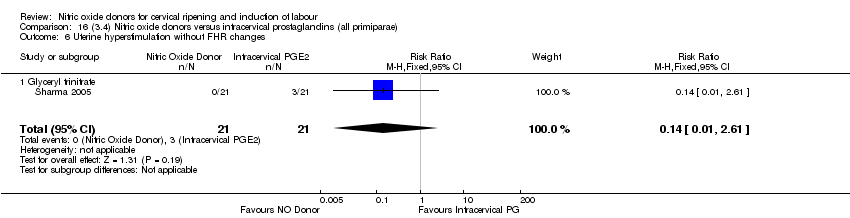
Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 6 Uterine hyperstimulation without FHR changes.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 7 Instrumental vaginal delivery.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 8 Perinatal death.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 9 Maternal side effects (headache).

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 2 Caesarean section.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 5 Oxytocin augmentation.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 6 Uterine hyperstimulation without FHR changes.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 7 Instrumental vaginal delivery.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 8 Perinatal death.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 9 Maternal side effects (headache).

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 1 Uterine hyperstimulation with FHR changes.
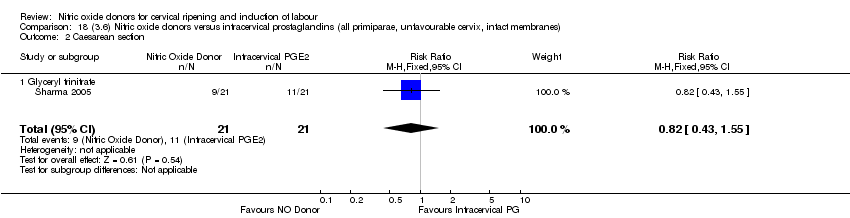
Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 2 Caesarean section.

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.
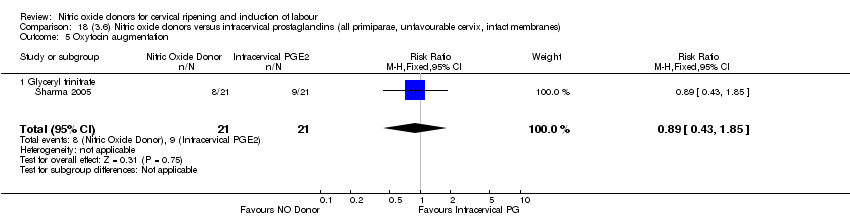
Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 5 Oxytocin augmentation.

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 6 Uterine hyperstimulation without FHR changes.
Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 7 Instrumental vaginal delivery.

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 8 Perinatal death.

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 9 Maternal side effects (headache).

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 2 Uterine hyperstimulation with FHR changes.
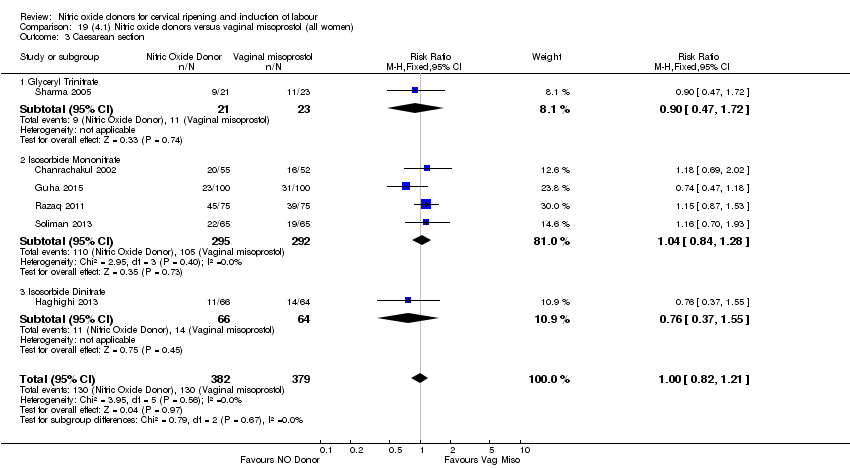
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 3 Caesarean section.
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 4 Serious neonatal morbidity/perinatal death.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
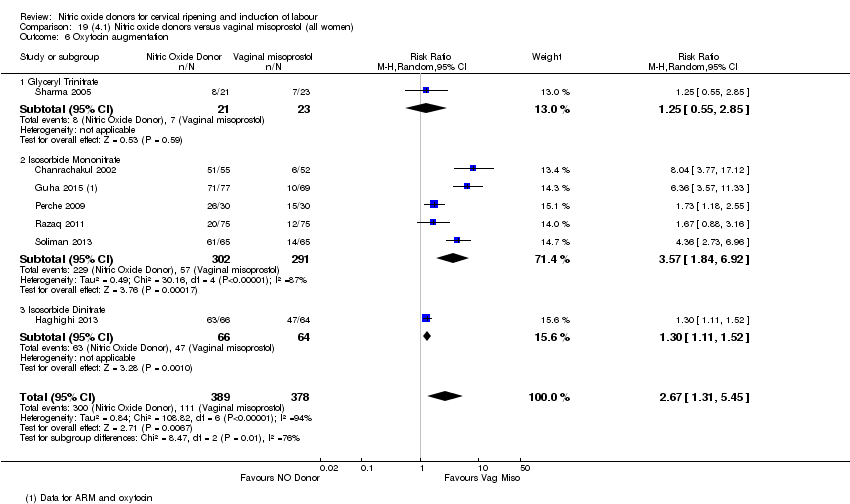
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 6 Oxytocin augmentation.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 7 Uterine hyperstimulation without FHR changes.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 8 Epidural analgesia.
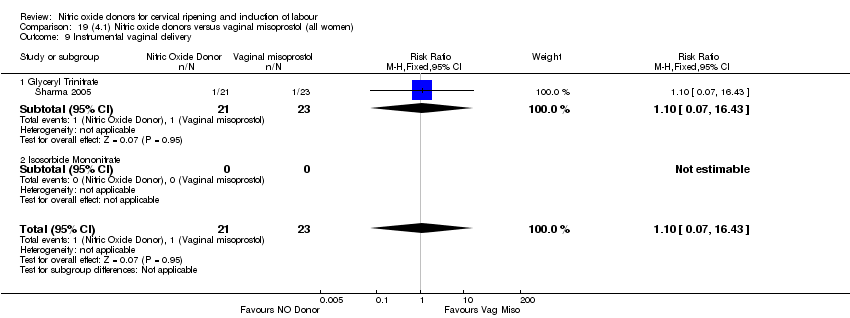
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 9 Instrumental vaginal delivery.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 10 Meconium‐stained liquor.
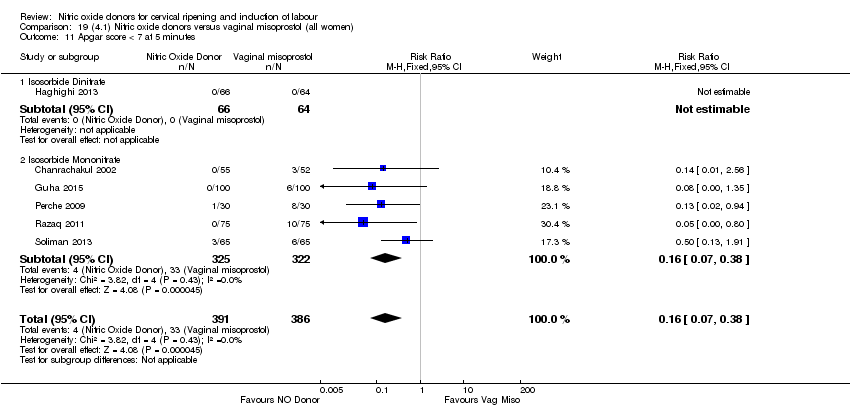
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 11 Apgar score < 7 at 5 minutes.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 12 Neonatal intensive care unit admission.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 13 Perinatal death.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 14 Maternal side effects (nausea).

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 15 Maternal side effects (headache).
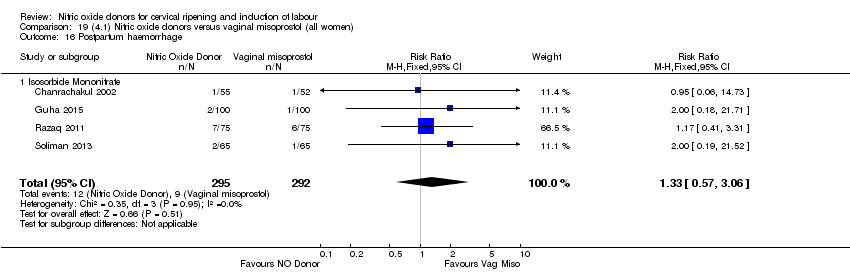
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 16 Postpartum haemorrhage.
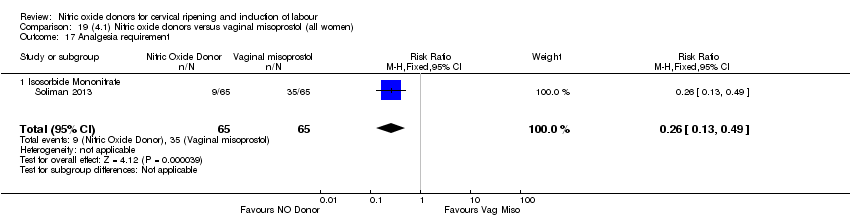
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 17 Analgesia requirement.
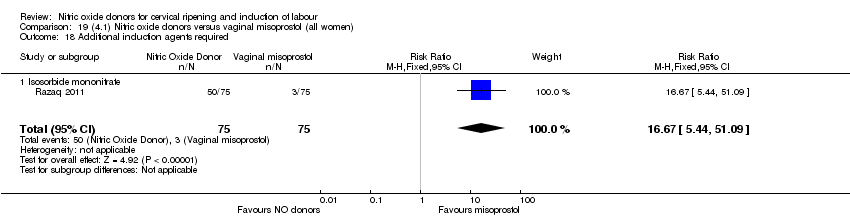
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 18 Additional induction agents required.
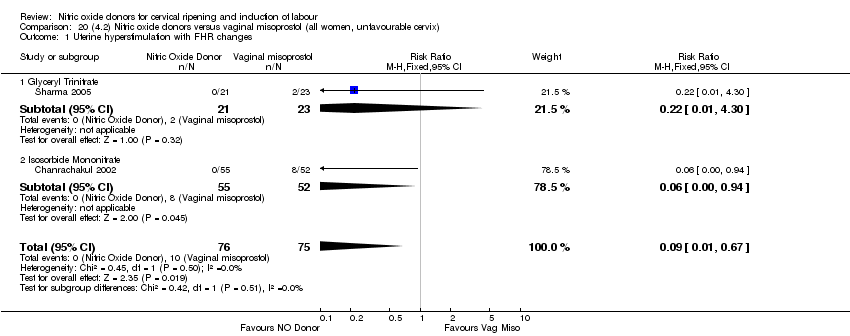
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 2 Caesarean section.
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.
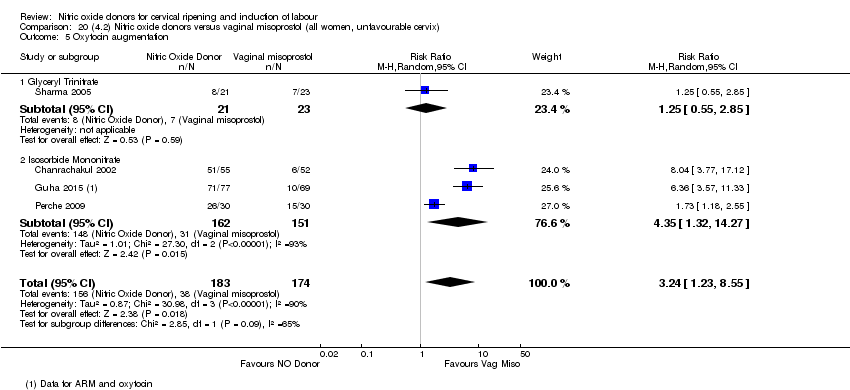
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 5 Oxytocin augmentation.

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 6 Uterine hyperstimulation without FHR changes.

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 7 Instrumental vaginal delivery.

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 8 Apgar score < 7 at 5 minutes.
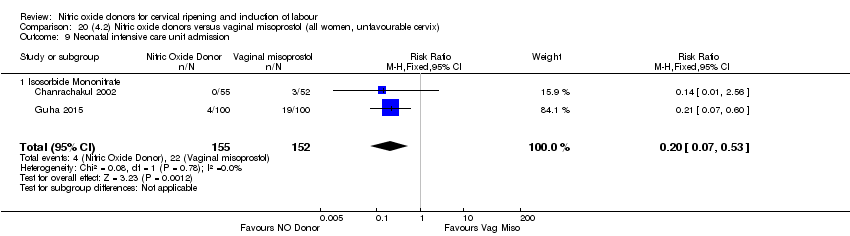
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 9 Neonatal intensive care unit admission.
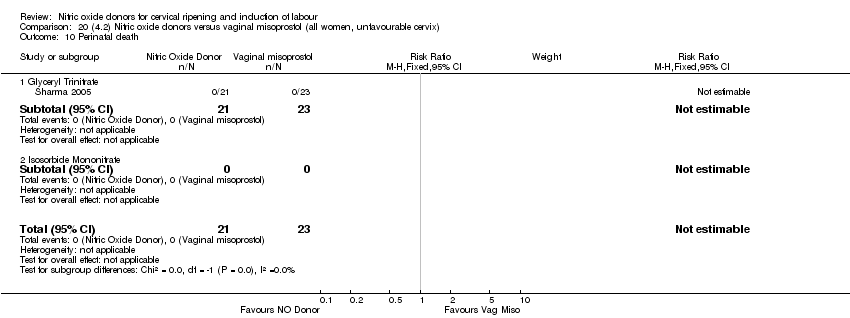
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 10 Perinatal death.
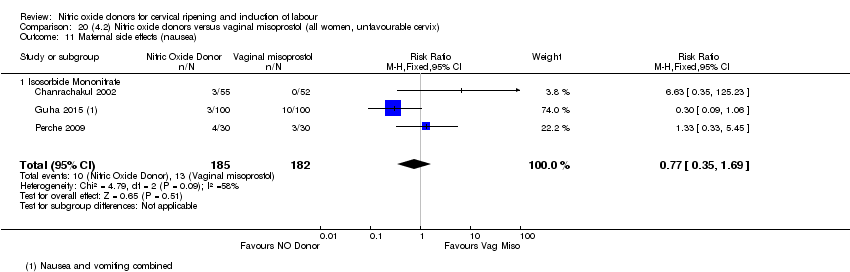
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 11 Maternal side effects (nausea).
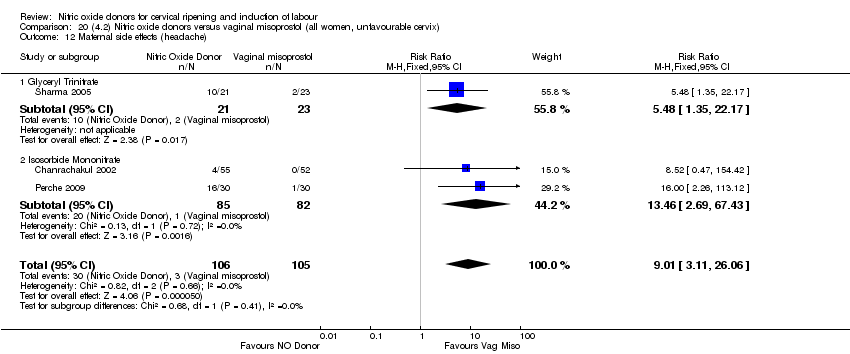
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 12 Maternal side effects (headache).

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 13 Postpartum haemorrhage.

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 2 Caesarean section.
Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.
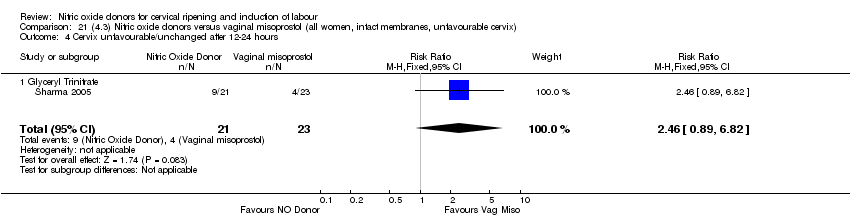
Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 5 Oxytocin augmentation.
Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 6 Instrumental vaginal delivery.

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.
Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 8 Perinatal death.

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 9 Maternal side effects (nausea).

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (headache).
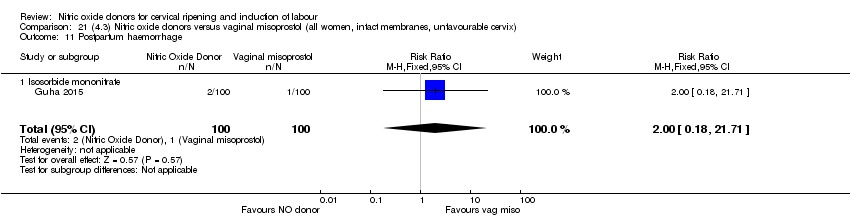
Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 11 Postpartum haemorrhage.
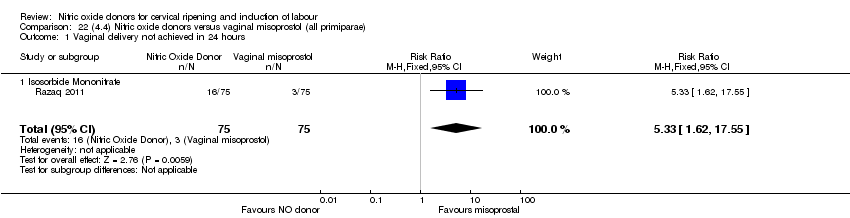
Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 3 Caesarean section.
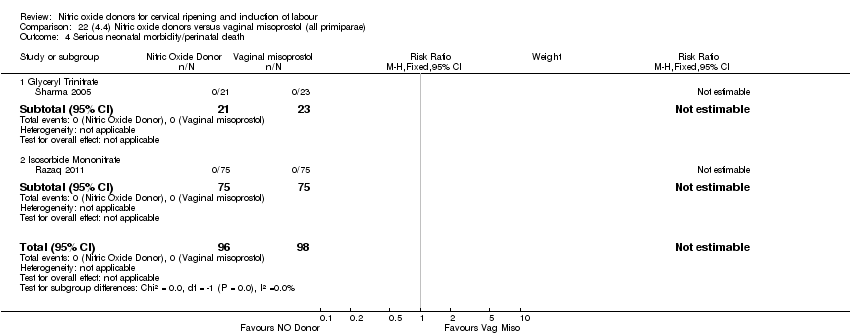
Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 4 Serious neonatal morbidity/perinatal death.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 6 Oxytocin augmentation.
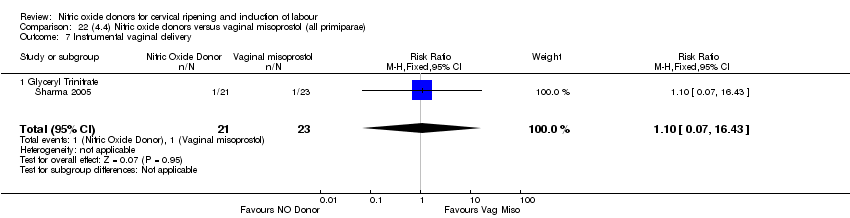
Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 7 Instrumental vaginal delivery.
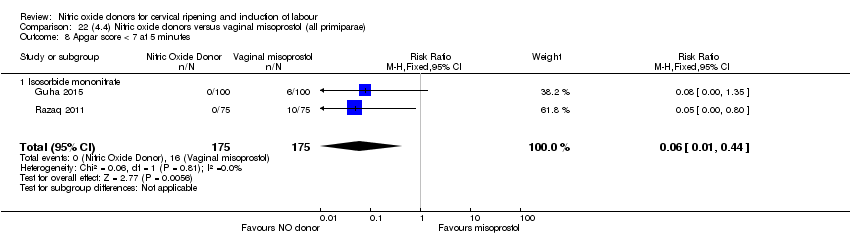
Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 8 Apgar score < 7 at 5 minutes.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 9 Neonatal intensive care unit admission.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 10 Perinatal death.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 11 Maternal side effects (nausea).

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 12 Maternal side effects (headache).

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 13 Postpartum haemorrhage.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 14 Additional induction agents required.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 2 Caesarean section.
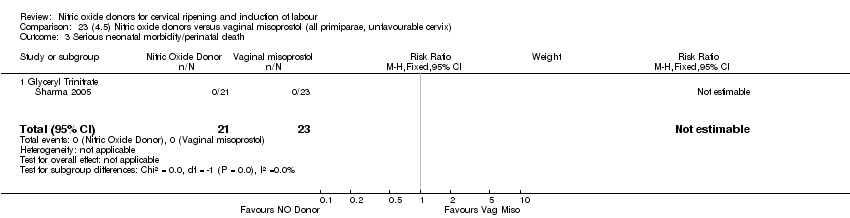
Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 5 Oxytocin augmentation.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 6 Instrumental vaginal delivery.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.
Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 9 Perinatal death.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 10 Maternal side effects (nausea).

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 11 Maternal side effects (headache).

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 12 Postpartum haemorrhage.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 2 Caesarean section.
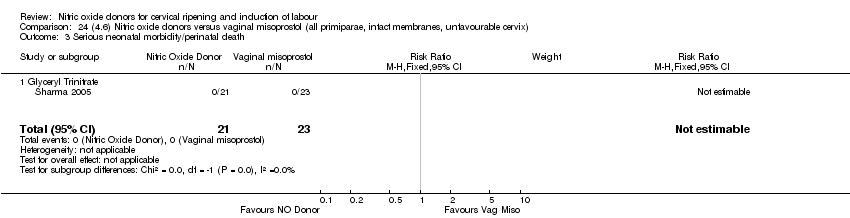
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.
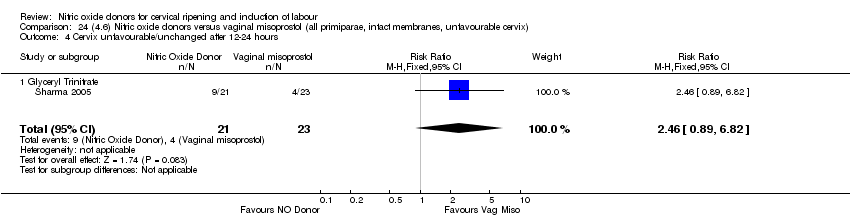
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 5 Oxytocin augmentation.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 6 Instrumental vaginal delivery.
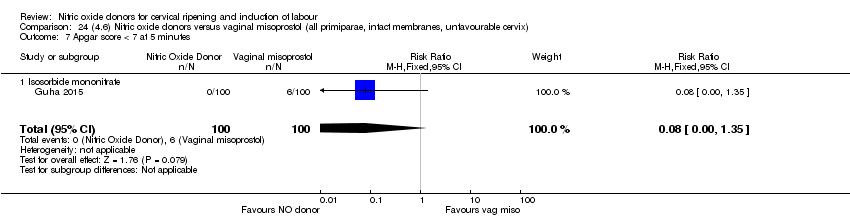
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.
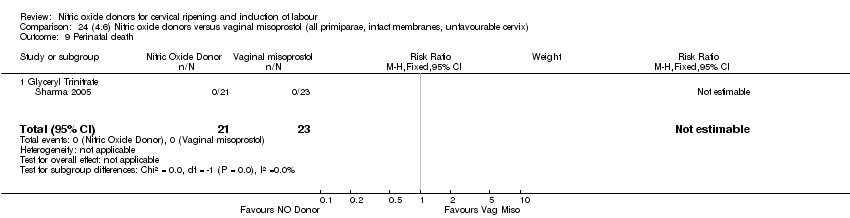
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 9 Perinatal death.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (nausea).
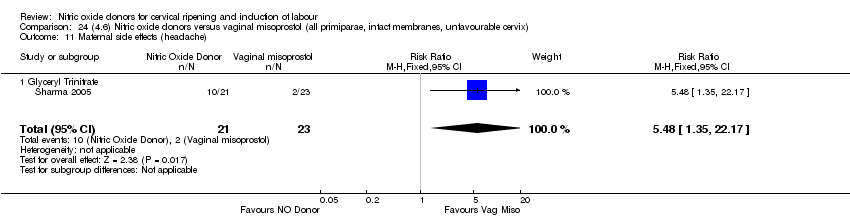
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 11 Maternal side effects (headache).
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 12 Postpartum haemorrhage.
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 1 Caesarean section.
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 2 Oxyocin augmentation.
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 3 Uterine rupture.

Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 4 Epidural analgesia.
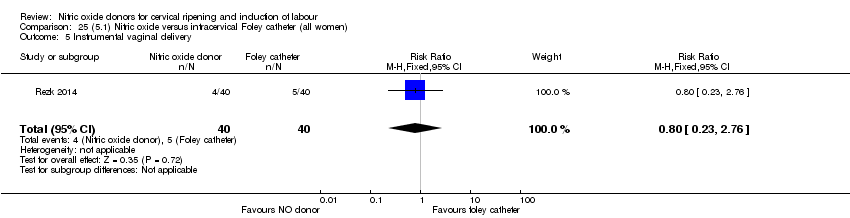
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 5 Instrumental vaginal delivery.
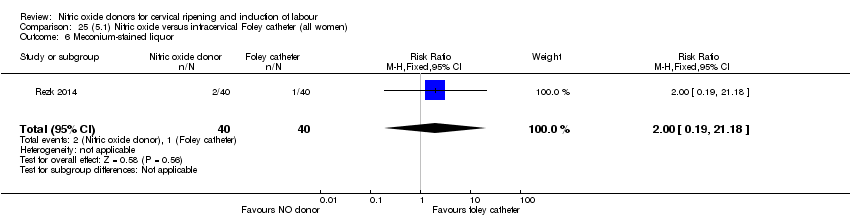
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 6 Meconium‐stained liquor.

Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 7 Apgar score < 7 at 5 minutes.
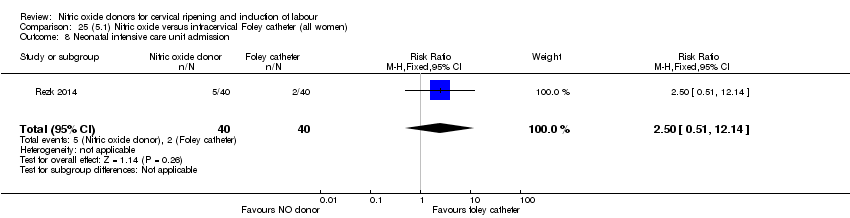
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 8 Neonatal intensive care unit admission.
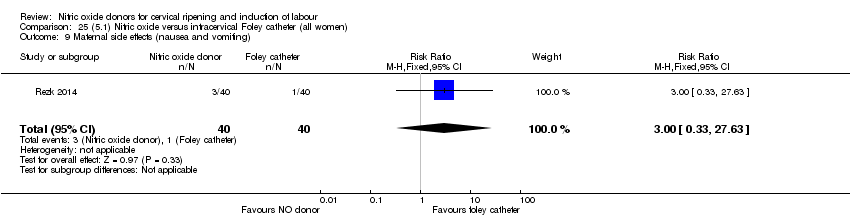
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 9 Maternal side effects (nausea and vomiting).

Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 10 Maternal side effects (headache).

Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 11 Postpartum haemorrhage.
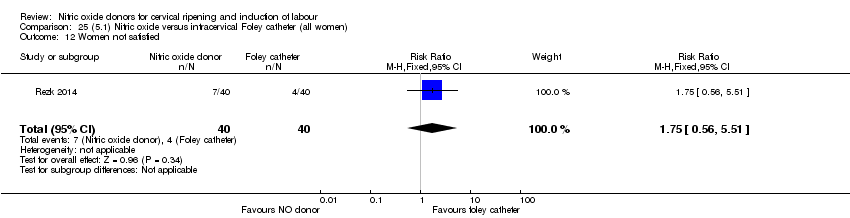
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 12 Women not satisfied.
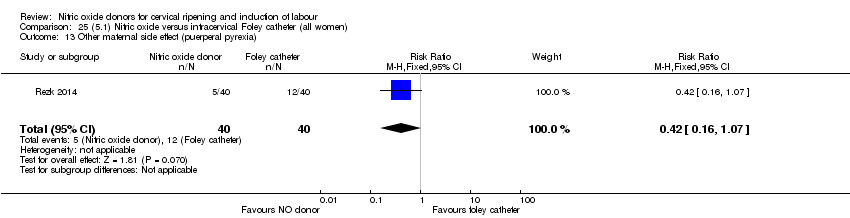
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 13 Other maternal side effect (puerperal pyrexia).
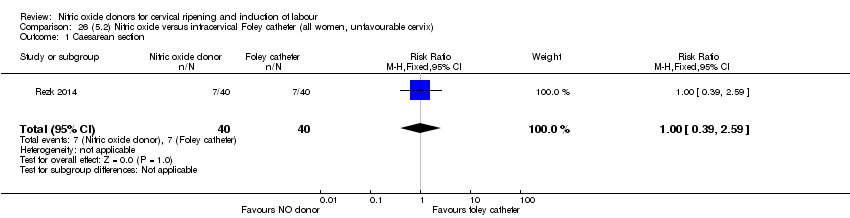
Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 1 Caesarean section.
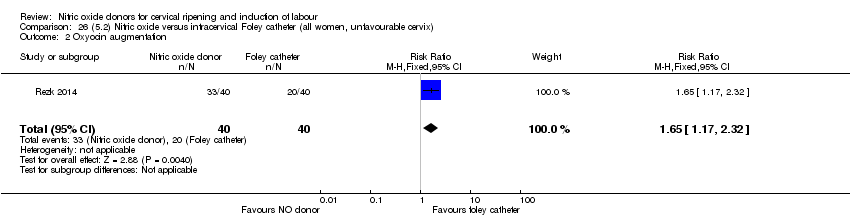
Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 2 Oxyocin augmentation.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 3 Uterine rupture.
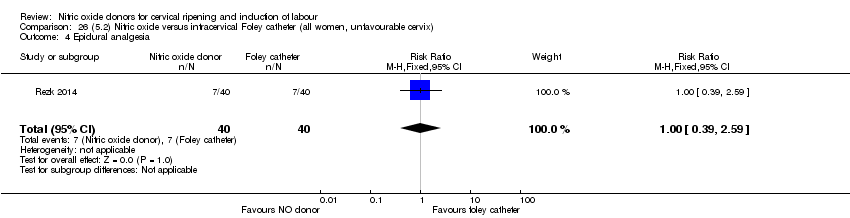
Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 4 Epidural analgesia.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 5 Instrumental vaginal delivery.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 6 Meconium‐stained liquor.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting).

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 10 Maternal side effects (headache).

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 11 Postpartum haemorrhage.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 12 Women not satisfied.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia).

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 1 Caesarean section.
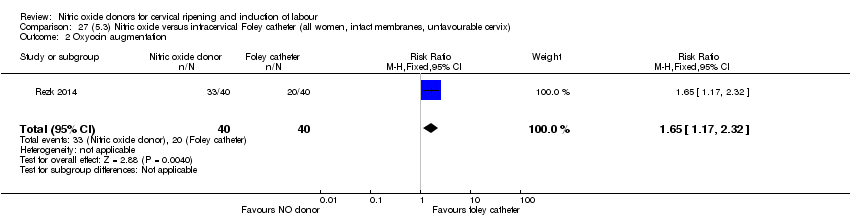
Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 2 Oxyocin augmentation.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 3 Uterine rupture.
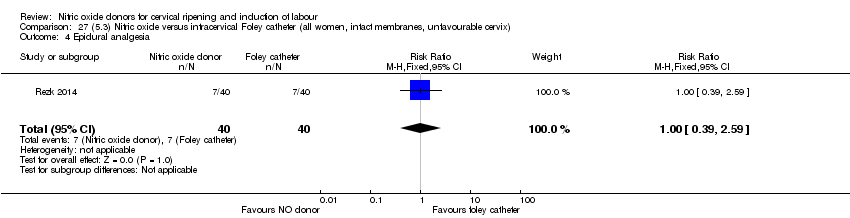
Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 4 Epidural analgesia.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 5 Instrumental vaginal delivery.
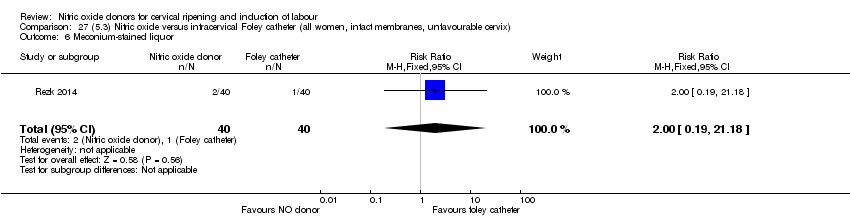
Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 6 Meconium‐stained liquor.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting).

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (headache).

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 11 Postpartum haemorrhage.
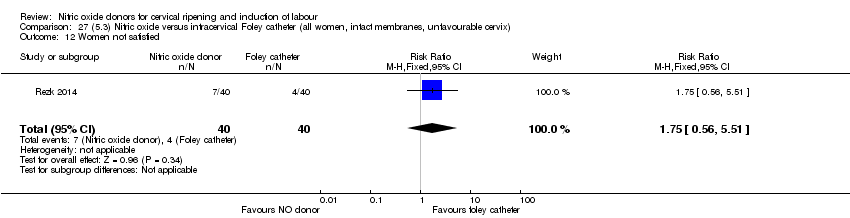
Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 12 Women not satisfied.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia).
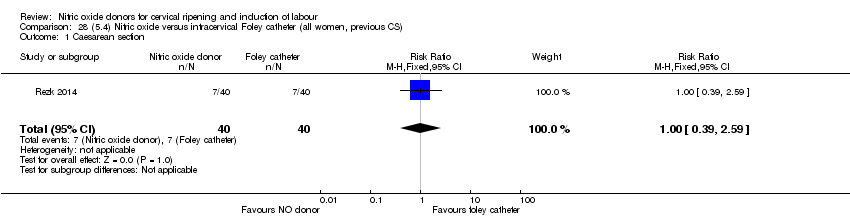
Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 1 Caesarean section.
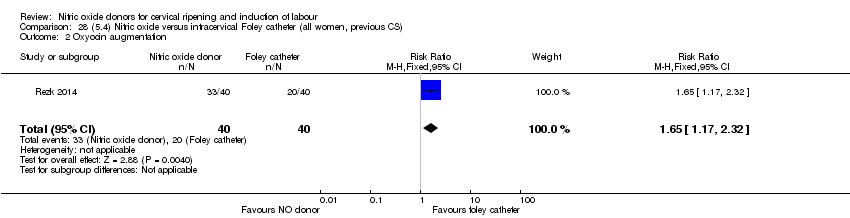
Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 2 Oxyocin augmentation.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 3 Uterine rupture.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 4 Epidural analgesia.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 5 Instrumental vaginal delivery.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 6 Meconium‐stained liquor.
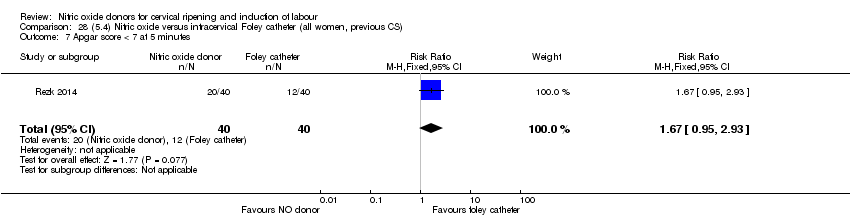
Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 7 Apgar score < 7 at 5 minutes.
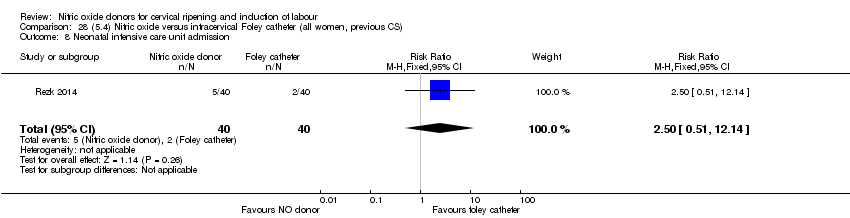
Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 8 Neonatal intensive care unit admission.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 9 Maternal side effects (nausea and vomiting).

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 10 Maternal side effects (headache).

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 11 Postpartum haemorrhage.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 12 Women not satisfied.
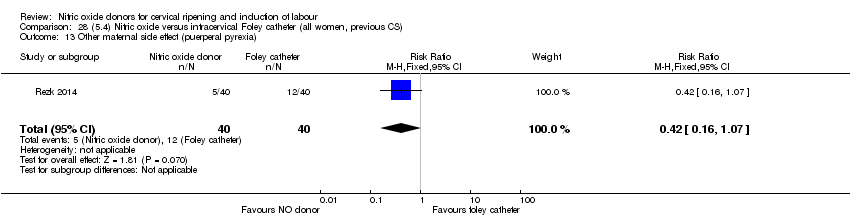
Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 13 Other maternal side effect (puerperal pyrexia).

Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 1 Caesarean section.
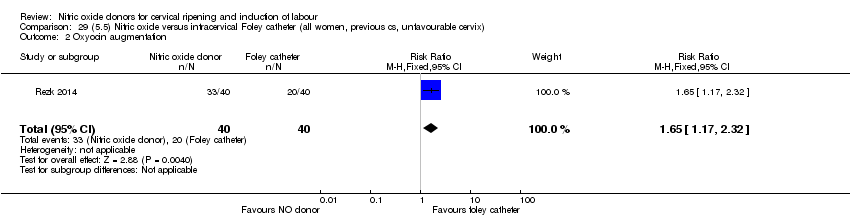
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 2 Oxyocin augmentation.
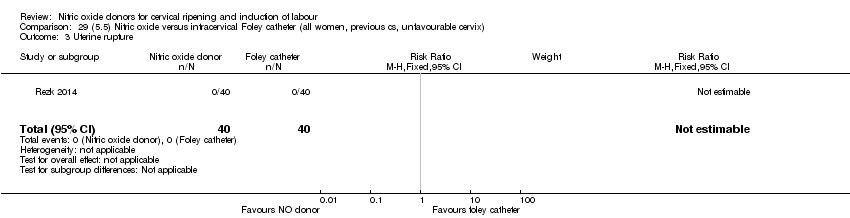
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 3 Uterine rupture.

Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 4 Epidural analgesia.
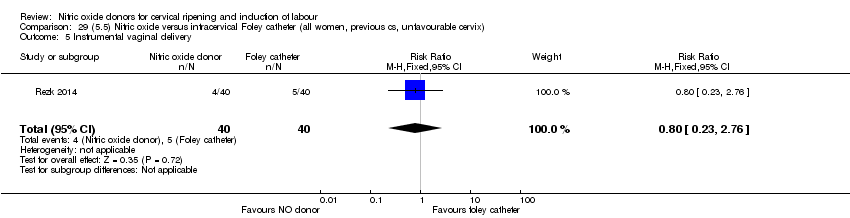
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 5 Instrumental vaginal delivery.
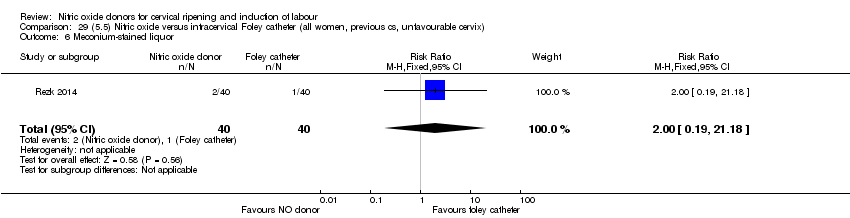
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 6 Meconium‐stained liquor.
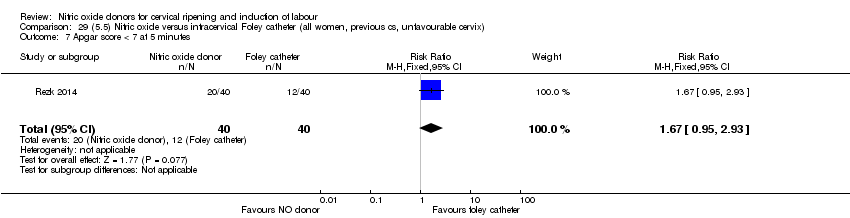
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.
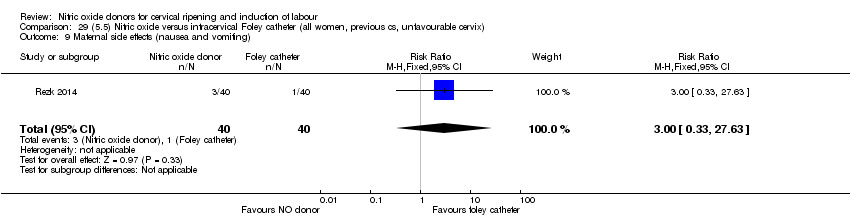
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting).
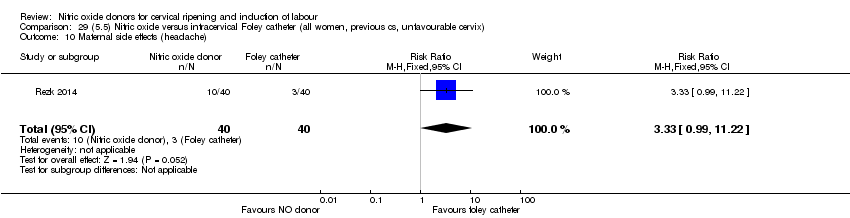
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 10 Maternal side effects (headache).

Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 11 Postpartum haemorrhage.

Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 12 Women not satisfied.
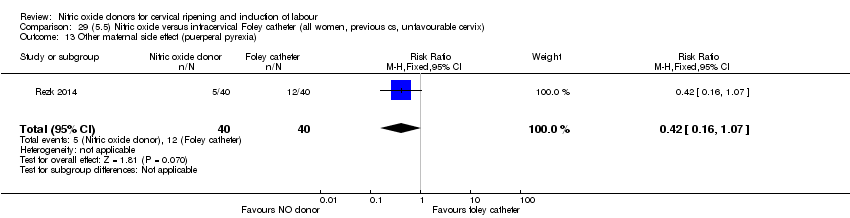
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia).

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 1 Caesarean section.

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 2 Oxyocin augmentation.
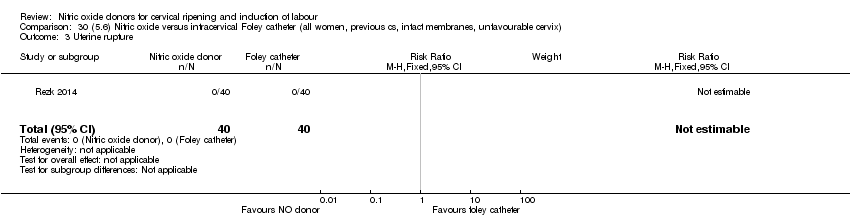
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 3 Uterine rupture.
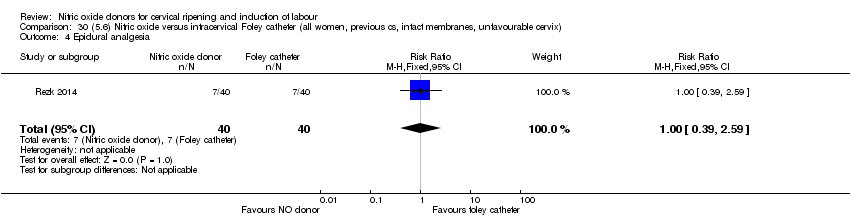
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 4 Epidural analgesia.

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 5 Instrumental vaginal delivery.

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 6 Meconium‐stained liquor.
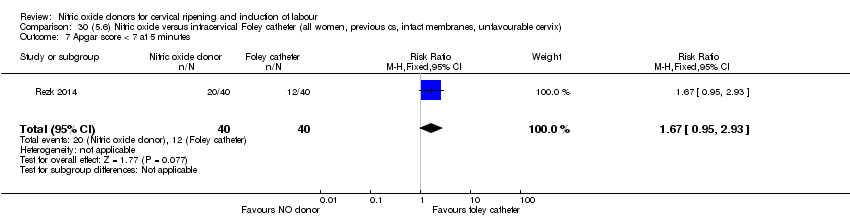
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.
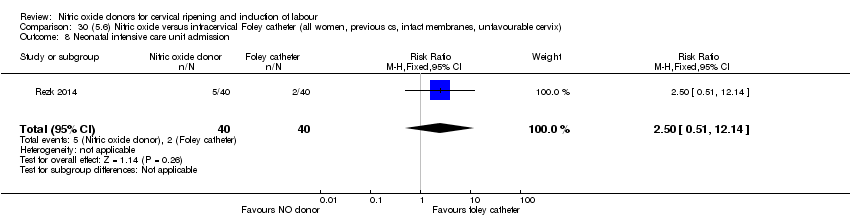
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting).

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (headache).
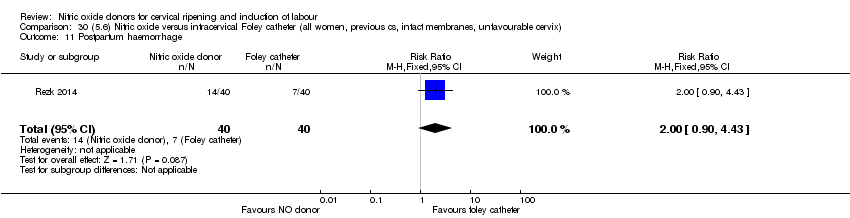
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 11 Postpartum haemorrhage.

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 12 Women not satisfied.
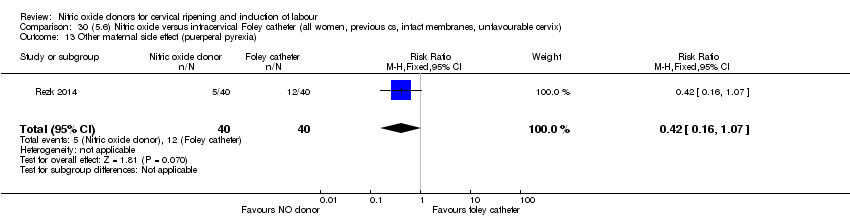
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia).
| Nitric oxide donors for cervical ripening and induction of labour | ||||||
| Patient or population: pregnant women undergoing cervical ripening and induction of labour | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo/no intervention (all women) | Risk with (1.1) Nitric oxide donors | |||||
| Vaginal delivery not achieved in 24 hours | Study population | RR 0.97 | 238 | ⊕⊕⊝⊝ | ||
| 711 per 1000 | 689 per 1000 | |||||
| Uterine hyperstimulation with FHR changes | Study population | RR 0.09 | 300 | ⊕⊕⊝⊝ | ||
| 33 per 1000 | 3 per 1000 | |||||
| Caesarean section | Study population | RR 0.99 | 2624 | ⊕⊕⊕⊝ | ||
| 280 per 1000 | 277 per 1000 | |||||
| Serious neonatal morbidity/perinatal death | Study population | RR 1.61 | 1712 | ⊕⊕⊝⊝ | ||
| 1 per 1000 | 2 per 1000 | |||||
| Serious maternal morbidity or death | Study population | not estimable | 1362 | There were no events for this outcome. | ||
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only one study with few events, small sample size and wide confidence interval. 2 High risk of bias for allocation concealment and blinding. 3 Only two studies with few or no events, small sample size and wide confidence interval. 4 High risk of bias for allocation concealment, blinding and selective outcome reporting. 5 Confidence intervals do not overlap (opposite directions of effect) and I2 = 48%. 6 Only two studies with few events and wide confidence intervals. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.62] |
| 3 Caesarean section Show forest plot | 9 | 2624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.11] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 2 | 1712 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.08, 33.26] |
| 5 Serious maternal morbidity or death Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 4 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.67, 0.90] |
| 6.1 Standard release | 4 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.69, 0.94] |
| 6.2 Slow release | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.82] |
| 7 Oxytocin augmentation Show forest plot | 4 | 1916 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 8 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.80] |
| 9 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 10 Instrumental vaginal delivery Show forest plot | 4 | 1835 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.10] |
| 11 Meconium‐stained liquor Show forest plot | 3 | 699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.14] |
| 12 Apgar score < 7 at 5 minutes Show forest plot | 5 | 2212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.54, 2.07] |
| 13 Neonatal intensive care unit admission Show forest plot | 5 | 873 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.47, 1.46] |
| 14 Perinatal death Show forest plot | 2 | 1712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 15 Maternal side effects (all) Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [2.49, 3.20] |
| 16 Maternal side effects (nausea) Show forest plot | 3 | 1782 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.47, 4.05] |
| 17 Maternal side effects (headache) Show forest plot | 6 | 2085 | Risk Ratio (M‐H, Random, 95% CI) | 6.59 [3.97, 10.95] |
| 18 Maternal side effects (vomiting) Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.54, 3.81] |
| 19 Maternal side effects (diarrhoea) Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.95, 2.19] |
| 20 Postpartum haemorrhage Show forest plot | 2 | 1562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.90, 1.40] |
| 21 Women not satisfied Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.82, 1.38] |
| 22 Additional induction agents used Show forest plot | 5 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.88] |
| 22.1 Standard release | 5 | 2077 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.58, 0.92] |
| 22.2 Slow release | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.62] |
| 3 Caesarean section Show forest plot | 8 | 1262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 3 | 557 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.73, 0.89] |
| 6 Oxytocin augmentation Show forest plot | 3 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.82, 1.03] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.80] |
| 8 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 9 Instrumental vaginal delivery Show forest plot | 3 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.28] |
| 10 Meconium‐stained liquor Show forest plot | 3 | 699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.14] |
| 11 Apgar score < 7 at 5 minutes Show forest plot | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.42, 2.98] |
| 12 Neonatal intensive care unit admission Show forest plot | 5 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.30] |
| 13 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 14 Maternal side effects (nausea) Show forest plot | 2 | 420 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.22, 3.50] |
| 15 Maternal side effects (headache) Show forest plot | 5 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.04 [5.13, 9.66] |
| 16 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.57, 2.40] |
| 17 Additional induction agents used Show forest plot | 2 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.57, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.62] |
| 3 Caesarean section Show forest plot | 3 | 754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.22] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.61, 0.85] |
| 6 Oxytocin augmentation Show forest plot | 2 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.05] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.80] |
| 8 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 9 Instrumental vaginal delivery Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 10 Meconium‐stained liquor Show forest plot | 2 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.67, 1.18] |
| 11 Apgar score < 7 at 5 minutes Show forest plot | 3 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.27, 2.59] |
| 12 Neonatal intensive care unit admission Show forest plot | 3 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.42, 1.84] |
| 13 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 14 Maternal side effects (nausea) Show forest plot | 2 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.82, 5.77] |
| 15 Maternal side effects (headache) Show forest plot | 3 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 9.27 [2.47, 34.73] |
| 16 Postpartum haemorrhage Show forest plot | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.55, 2.07] |
| 17 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section Show forest plot | 4 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.31] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 0.99] |
| 6 Oxytocin augmentation Show forest plot | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 7 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 8 Instrumental vaginal delivery Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.25] |
| 9 Meconium‐stained liquor Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.22] |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.50, 7.77] |
| 11 Neonatal intensive care unit admission Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.53, 1.80] |
| 12 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 13 Maternal side effects (nausea) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.73, 2.71] |
| 14 Maternal side effects (headache) Show forest plot | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 4.10 [1.97, 8.56] |
| 15 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section Show forest plot | 4 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.31] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 0.99] |
| 6 Oxytocin augmentation Show forest plot | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 7 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 8 Instrumental vaginal delivery Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.25] |
| 9 Meconium‐stained liquor Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.22] |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.50, 7.77] |
| 11 Neonatal intensive care unit admission Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.53, 1.80] |
| 12 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 13 Maternal side effects (nausea) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.73, 2.71] |
| 14 Maternal side effects (headache) Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.73 [2.56, 5.43] |
| 15 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Caesarean section Show forest plot | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.55] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.42, 1.44] |
| 5 Oxytocin augmentation Show forest plot | 1 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.30] |
| 6 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 8 Meconium‐stained liquor Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.30] |
| 9 Apgar score < 7 at 5 minutes Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.67] |
| 10 Neonatal intensive care unit admission Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.58, 2.09] |
| 11 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 12 Maternal side effects (nausea) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.73, 2.71] |
| 13 Maternal side effects (headache) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [2.18, 4.82] |
| 14 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 1.1 Isosorbide Mononitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 2.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 2.2 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| 3.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| 4 Caesarean section Show forest plot | 3 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| 4.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.59, 1.63] |
| 4.2 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 4.3 Isosorbide Dinitrate | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.06] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.32, 2.28] |
| 6.1 Isosorbide Dinitrate | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.32, 2.28] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 2 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 1.98] |
| 7.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 7.2 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 8 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 8.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 9 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 9.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 10 Maternal side effects (headache) Show forest plot | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.79 [5.75, 13.45] |
| 10.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.4 [0.65, 201.32] |
| 10.2 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 11.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 12 Serious maternal complications Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 12.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 13 Neonatal intensive care unit admission Show forest plot | 3 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.78] |
| 13.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 13.2 Isosorbide Dinitrate | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 1.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 1.2 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 2 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.82, 1.35] |
| 2.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.59, 1.63] |
| 2.2 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| 3.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| 4 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 2 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 1.98] |
| 6.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 6.2 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 7 Neonatal intensive care unit admission Show forest plot | 2 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.78] |
| 7.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 7.2 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 8 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 9 Maternal side effects (headache) Show forest plot | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.79 [5.75, 13.45] |
| 9.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.4 [0.65, 201.32] |
| 9.2 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 10 Postpartum haemorrhage Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 10.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 11 Serious maternal complications Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 11.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 2.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 3.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 4.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 5.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 6 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 6.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 7 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 7.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 8.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 1.1 Isosorbide Mononitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section Show forest plot | 2 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| 3.1 Isosorbide Mononitrate | 2 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| 4 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 6.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 7 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 7.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 8 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 9.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 2.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 3.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 4.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 5.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 6 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 6.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 7 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 7.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 8.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 2.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 3.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 4.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 5.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 6 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 6.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 7 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 7.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 8.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 1.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3 Caesarean section Show forest plot | 2 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.90] |
| 3.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.38, 0.89] |
| 3.2 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 6 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 9 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 10.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 1.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 6 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 9 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 10.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 1.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 6 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 9 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 10.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 1.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 2.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 1.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 2.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 1.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 2.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| 1.1 Isosorbide mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 3 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.37] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2.2 Isosorbide Mononitrate | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.40] |
| 3 Caesarean section Show forest plot | 6 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.82, 1.21] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 3.2 Isosorbide Mononitrate | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.28] |
| 3.3 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [2.07, 5.66] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5.2 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [2.12, 6.75] |
| 6 Oxytocin augmentation Show forest plot | 7 | 767 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [1.31, 5.45] |
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.85] |
| 6.2 Isosorbide Mononitrate | 5 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 3.57 [1.84, 6.92] |
| 6.3 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.11, 1.52] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.32] |
| 7.1 Isosorbide Mononitrate | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.34] |
| 7.2 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.96] |
| 8 Epidural analgesia Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.31] |
| 8.1 Isosorbide Mononitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.31] |
| 9 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 9.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 9.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Meconium‐stained liquor Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.65] |
| 10.1 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.56] |
| 10.2 Isosorbide mononitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.15, 0.84] |
| 11 Apgar score < 7 at 5 minutes Show forest plot | 6 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.07, 0.38] |
| 11.1 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Isosorbide Mononitrate | 5 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.07, 0.38] |
| 12 Neonatal intensive care unit admission Show forest plot | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.43] |
| 12.1 Isosorbide Mononitrate | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.43] |
| 13 Perinatal death Show forest plot | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Isosorbide Mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal side effects (nausea) Show forest plot | 5 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.63, 2.17] |
| 14.1 Isosorbide Mononitrate | 5 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.63, 2.17] |
| 15 Maternal side effects (headache) Show forest plot | 4 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.98 [4.05, 29.73] |
| 15.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 15.2 Isosorbide Mononitrate | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.46 [2.69, 67.43] |
| 15.3 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 24.25 [1.47, 401.26] |
| 16 Postpartum haemorrhage Show forest plot | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.57, 3.06] |
| 16.1 Isosorbide Mononitrate | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.57, 3.06] |
| 17 Analgesia requirement Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.49] |
| 17.1 Isosorbide Mononitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.49] |
| 18 Additional induction agents required Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| 18.1 Isosorbide mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.67] |
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 1.2 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.94] |
| 2 Caesarean section Show forest plot | 3 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.66, 1.22] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide Mononitrate | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.27] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [2.07, 5.66] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 4.2 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [2.12, 6.75] |
| 5 Oxytocin augmentation Show forest plot | 4 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [1.23, 8.55] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide Mononitrate | 3 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 4.35 [1.32, 14.27] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.75] |
| 6.1 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.75] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Apgar score < 7 at 5 minutes Show forest plot | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.46] |
| 8.1 Isosorbide Mononitrate | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.46] |
| 9 Neonatal intensive care unit admission Show forest plot | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.07, 0.53] |
| 9.1 Isosorbide Mononitrate | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.07, 0.53] |
| 10 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal side effects (nausea) Show forest plot | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.69] |
| 11.1 Isosorbide Mononitrate | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.69] |
| 12 Maternal side effects (headache) Show forest plot | 3 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.01 [3.11, 26.06] |
| 12.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 12.2 Isosorbide Mononitrate | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.46 [2.69, 67.43] |
| 13 Postpartum haemorrhage Show forest plot | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.61] |
| 13.1 Isosorbide Mononitrate | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2 Caesarean section Show forest plot | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.14] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.18] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5 Oxytocin augmentation Show forest plot | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [2.29, 4.33] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide Mononitrate | 2 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [2.57, 5.18] |
| 6 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.54] |
| 7.1 Isosorbide Mononitrate | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.54] |
| 8 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal side effects (nausea) Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.31] |
| 9.1 Isosorbide Mononitrate | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.31] |
| 10 Maternal side effects (headache) Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.09 [2.90, 28.47] |
| 10.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 10.2 Isosorbide Mononitrate | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.0 [2.26, 113.12] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 11.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| 1.1 Isosorbide Mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 3 Caesarean section Show forest plot | 3 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.21] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 3.2 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.25] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Isosorbide Mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 6 Oxytocin augmentation Show forest plot | 3 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [2.27, 4.71] |
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 6.2 Isosorbide mononitrate | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [2.56, 5.83] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 8 Apgar score < 7 at 5 minutes Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.44] |
| 8.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.44] |
| 9 Neonatal intensive care unit admission Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.06, 0.42] |
| 9.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.06, 0.42] |
| 10 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal side effects (nausea) Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.89] |
| 11.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.89] |
| 12 Maternal side effects (headache) Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 12.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 13 Postpartum haemorrhage Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.50, 3.33] |
| 13.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.50, 3.33] |
| 14 Additional induction agents required Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| 14.1 Isosorbide mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2 Caesarean section Show forest plot | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.14] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide monotrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.18] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5 Oxytocin augmentation Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [2.77, 6.93] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide mononitrate | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.36 [3.57, 11.33] |
| 6 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 7.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| 8.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| 9 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal side effects (nausea) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| 10.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| 11 Maternal side effects (headache) Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 11.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 12 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 12.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2 Caesarean section Show forest plot | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.14] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.18] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5 Oxytocin augmentation Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [2.77, 6.93] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide mononitrate | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.36 [3.57, 11.33] |
| 6 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 7.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| 8.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| 9 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal side effects (nausea) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| 10.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| 11 Maternal side effects (headache) Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 11.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 12 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 12.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |

