Fármacos que liberan óxido nítrico para la maduración cervical y la inducción del trabajo de parto
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006901.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 diciembre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Tony Kelly (TK) completed the initial review of baseline evidence and drafted the text of the original protocol and review. For the purposes of this update Arpita Ghosh (AG) has been the main author and has worked alongside TK and Katherine Lattey (KL). All three authors reviewed all trials and judged suitability and inclusion. All three authors carried out data extraction and resolved any discrepancies by discussion. The final review was drafted by AG, TK and KL.
Declarations of interest
Arpita Ghosh: none known.
Katherine R Lattey: none known.
Anthony J Kelly: none known.
Acknowledgements
Josephine Kavanagh for her input on the protocol for this review.
We are grateful to Luciana Figuera for the translation of the paper Perche 2009 and to Bita Mesgarpour for the translation of the paper (Movahed 2016).
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We thank Anna Cuthbert, Research Assistant, Cochrane Pregnancy and Childbirth, for her help with the 2016 update.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Dec 05 | Nitric oxide donors for cervical ripening and induction of labour | Review | Arpita Ghosh, Katherine R Lattey, Anthony J Kelly | |
| 2011 Jun 15 | Nitric oxide donors for cervical ripening and induction of labour | Review | Anthony J Kelly, Christopher Munson, Lucy Minden | |
| 2008 Jan 23 | Nitric oxide donors for cervical ripening and induction of labour | Protocol | Anthony J Kelly, Josephine Kavanagh | |
Differences between protocol and review
We have re‐structured the comparisons to make the ‘all women’ comparisons sequential and re‐ordered outcomes to put the five primary outcomes first, followed by the secondary outcomes in the order stated in the methods text. A 'Summary of findings' table has been incorporated for this update.
In more recent reviews and updates the following outcomes have been added:
28. neonatal infection;
29. neonatal antibiotics;
30. chorioamnionitis;
31. endometritis;
32. maternal antibiotics.
In addition, in view of the nature of the trials and the intervention studied, we have examined some additional outcomes in this review. These include:
33. additional induction agents required;
34. initiation of cervical ripening to delivery interval (in days).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO
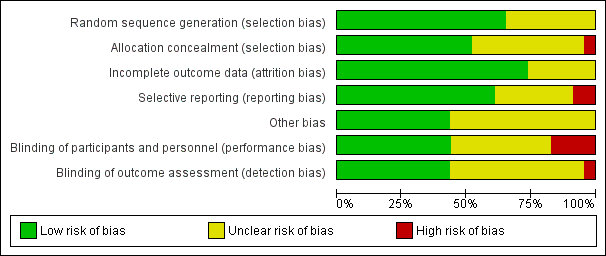
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 2 Uterine hyperstimulation with FHR changes.
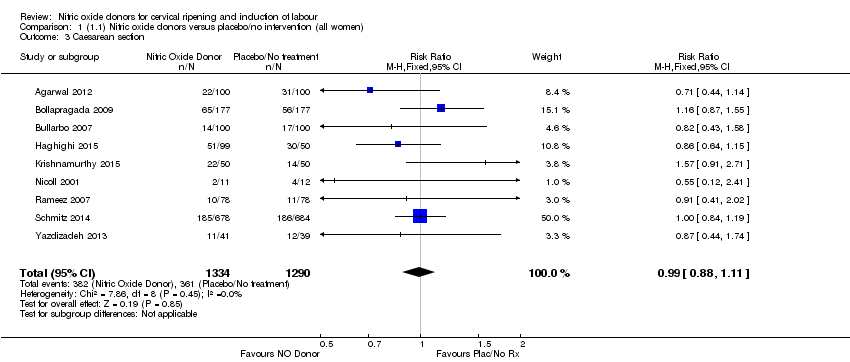
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 3 Caesarean section.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 4 Serious neonatal morbidity/perinatal death.
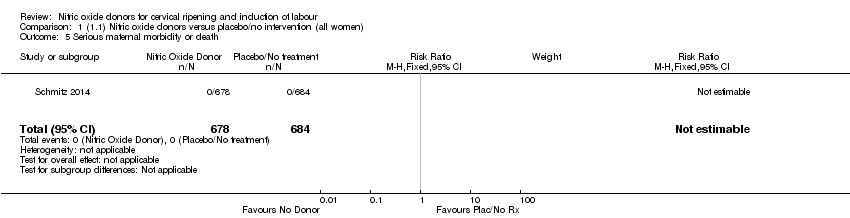
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 5 Serious maternal morbidity or death.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 7 Oxytocin augmentation.
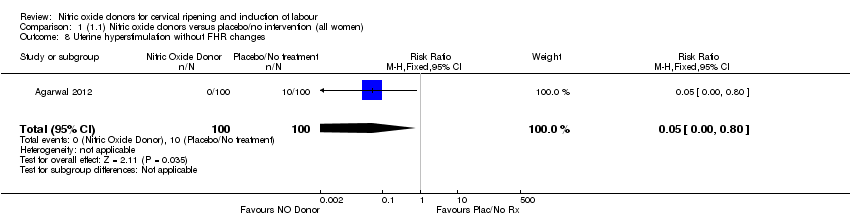
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 8 Uterine hyperstimulation without FHR changes.
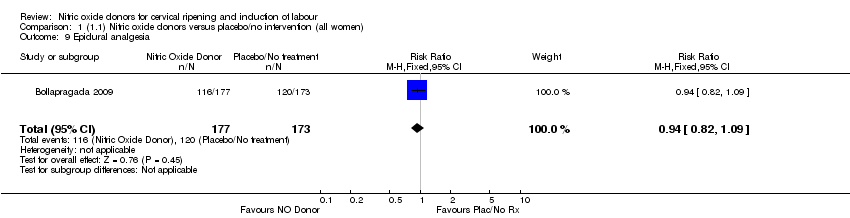
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 9 Epidural analgesia.
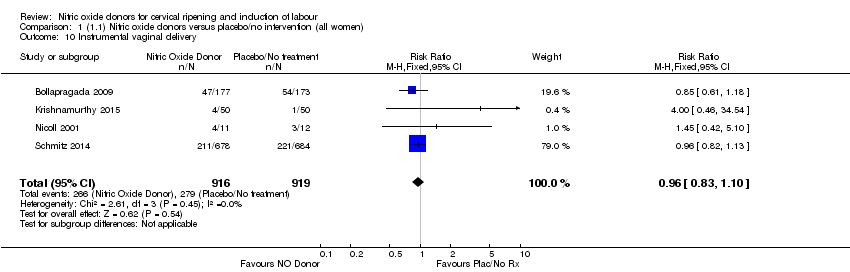
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 10 Instrumental vaginal delivery.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 11 Meconium‐stained liquor.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 12 Apgar score < 7 at 5 minutes.
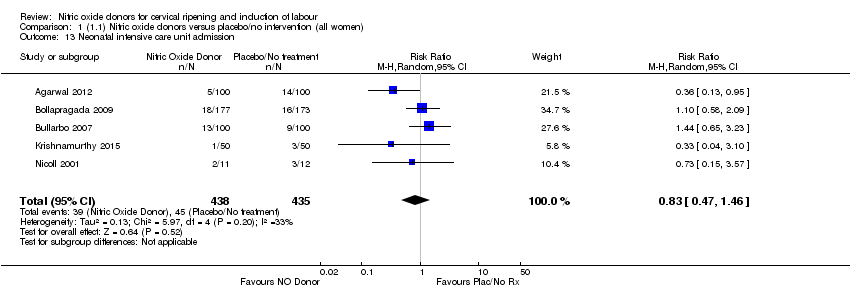
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 13 Neonatal intensive care unit admission.
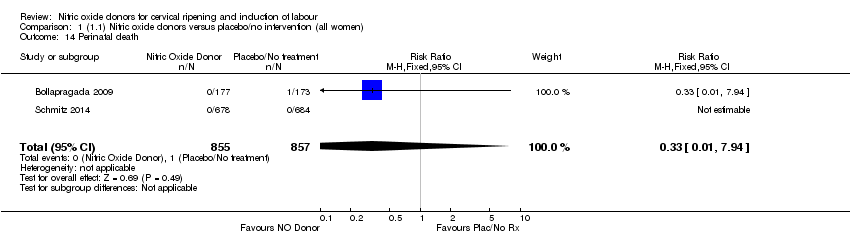
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 14 Perinatal death.
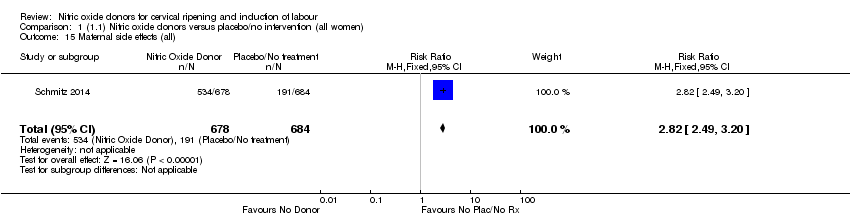
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 15 Maternal side effects (all).

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 16 Maternal side effects (nausea).

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 17 Maternal side effects (headache).

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 18 Maternal side effects (vomiting).

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 19 Maternal side effects (diarrhoea).
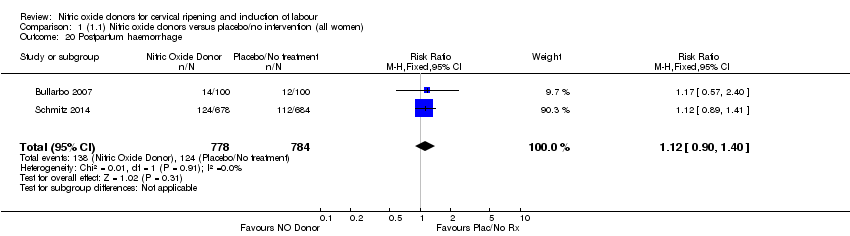
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 20 Postpartum haemorrhage.
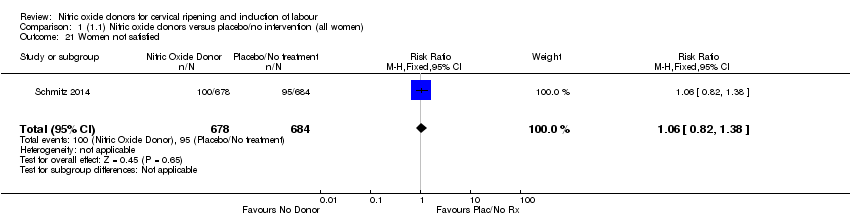
Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 21 Women not satisfied.

Comparison 1 (1.1) Nitric oxide donors versus placebo/no intervention (all women), Outcome 22 Additional induction agents used.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours.
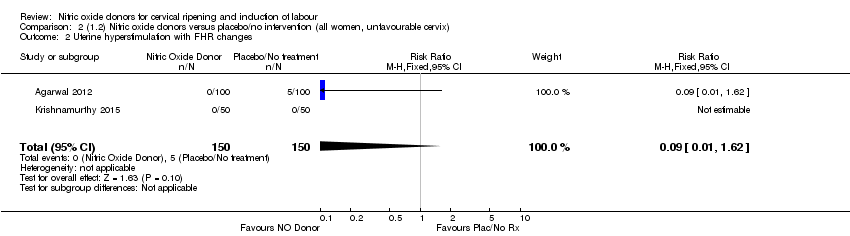
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
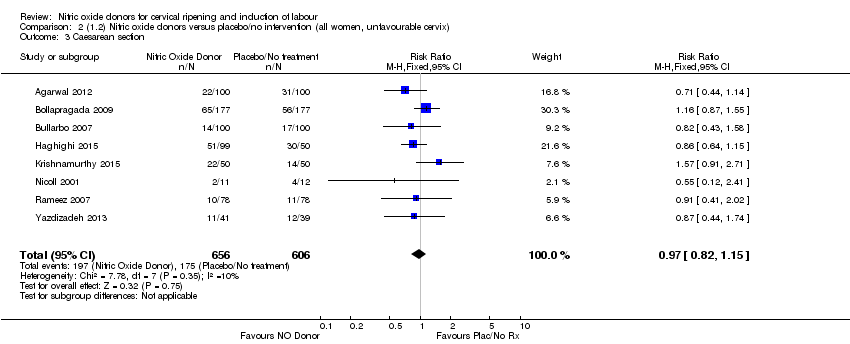
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 3 Caesarean section.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death.
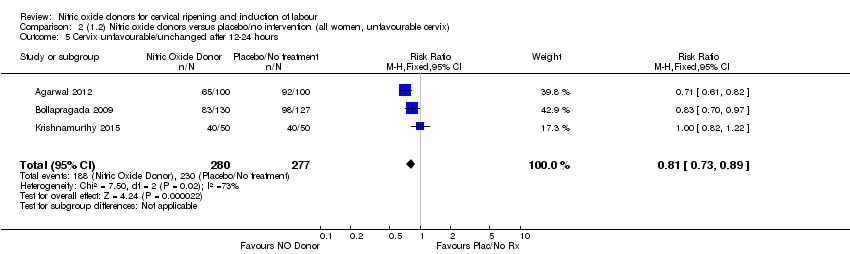
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
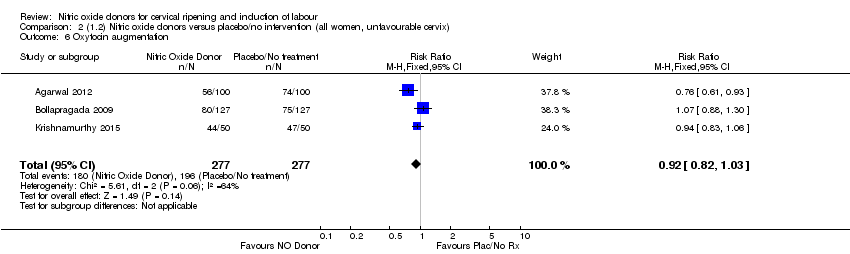
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 6 Oxytocin augmentation.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 7 Uterine hyperstimulation without FHR changes.
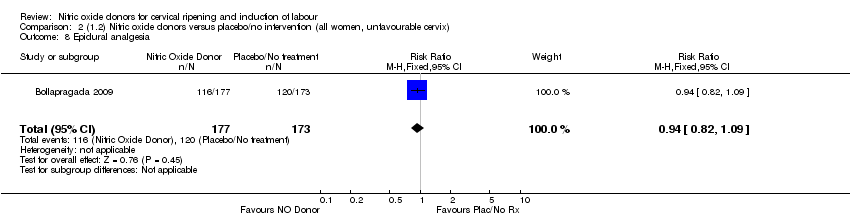
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 8 Epidural analgesia.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 9 Instrumental vaginal delivery.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 10 Meconium‐stained liquor.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 11 Apgar score < 7 at 5 minutes.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 12 Neonatal intensive care unit admission.

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 13 Perinatal death.
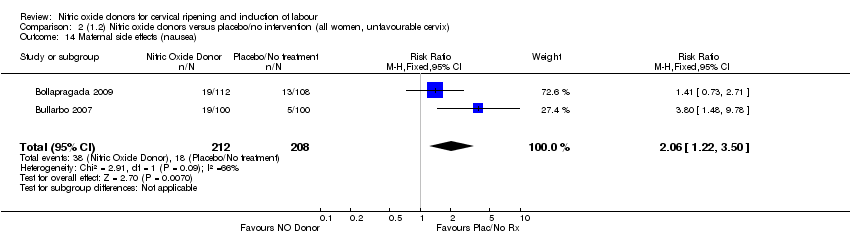
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 14 Maternal side effects (nausea).
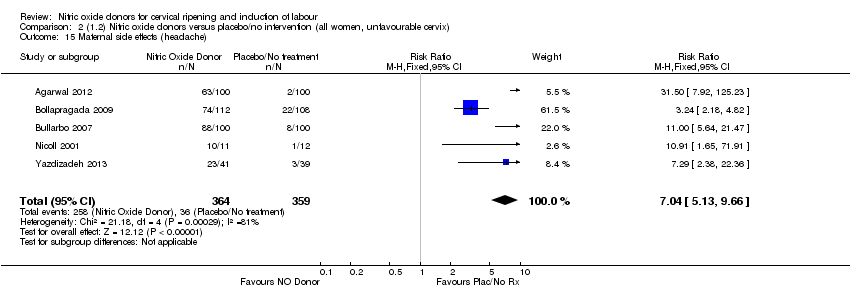
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 15 Maternal side effects (headache).

Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 16 Postpartum haemorrhage.
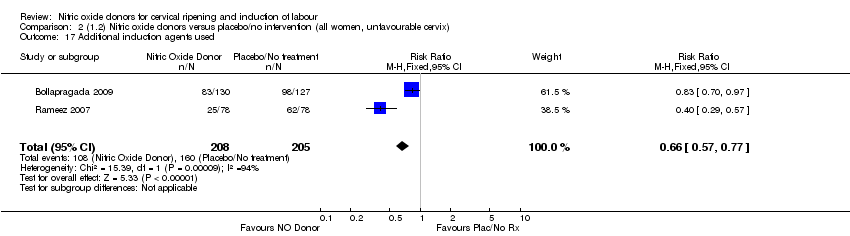
Comparison 2 (1.2) Nitric oxide donors versus placebo/no intervention (all women, unfavourable cervix), Outcome 17 Additional induction agents used.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours.
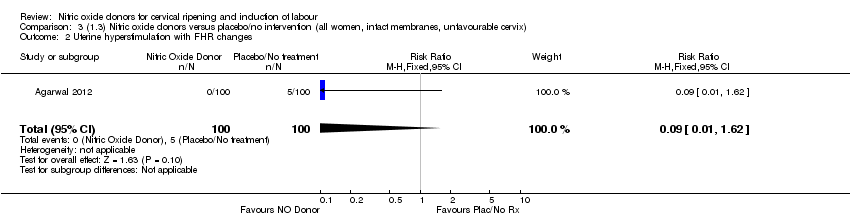
Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 3 Caesarean section.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
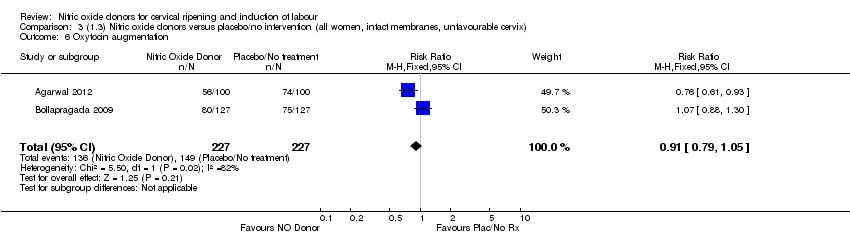
Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 6 Oxytocin augmentation.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 7 Uterine hyperstimulation without FHR changes.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 8 Epidural analgesia.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 9 Instrumental vaginal delivery.
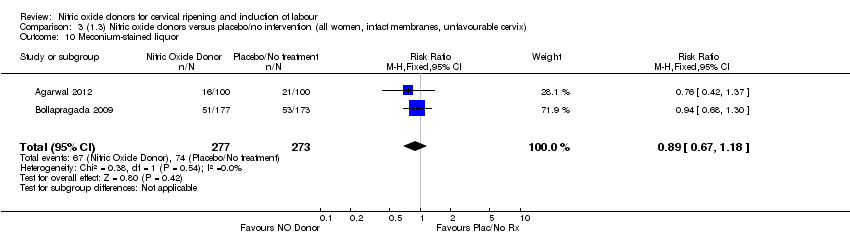
Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 10 Meconium‐stained liquor.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 11 Apgar score < 7 at 5 minutes.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 12 Neonatal intensive care unit admission.

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 13 Perinatal death.
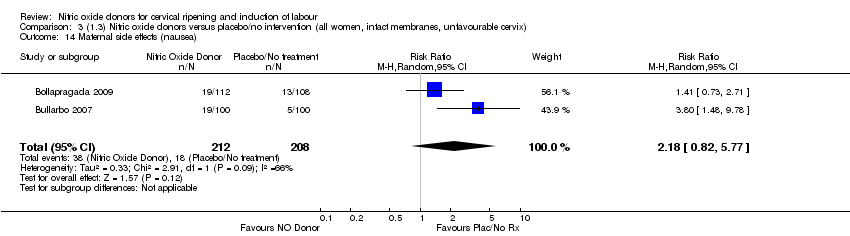
Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 14 Maternal side effects (nausea).

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 15 Maternal side effects (headache).

Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 16 Postpartum haemorrhage.
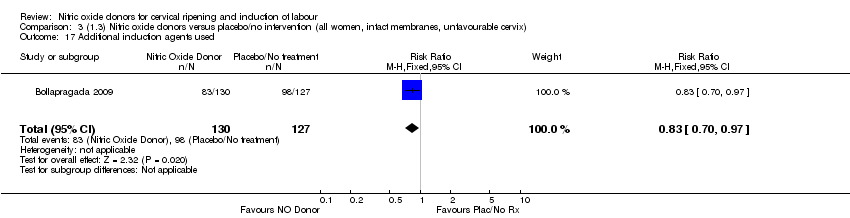
Comparison 3 (1.3) Nitric oxide donors versus placebo/no intervention (all women, intact membranes, unfavourable cervix), Outcome 17 Additional induction agents used.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.
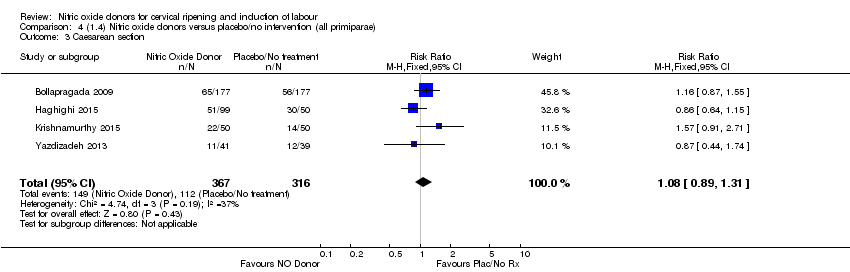
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 3 Caesarean section.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 4 Serious neonatal morbidity/perinatal death.
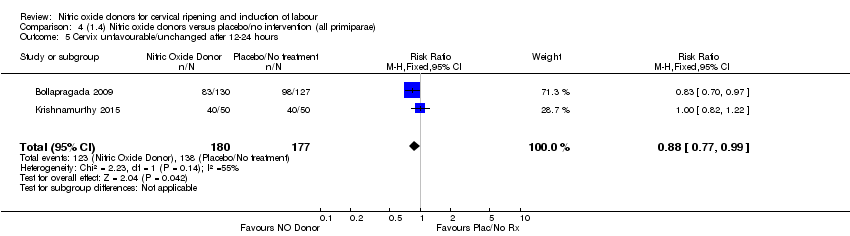
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 6 Oxytocin augmentation.
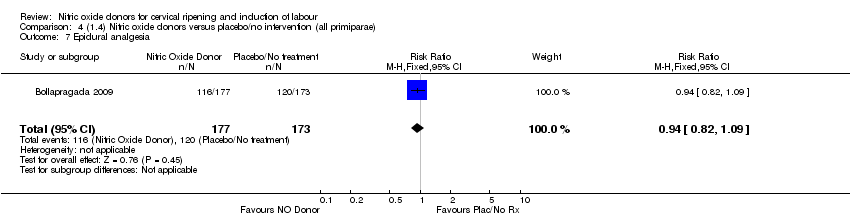
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 7 Epidural analgesia.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 8 Instrumental vaginal delivery.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 9 Meconium‐stained liquor.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 10 Apgar score < 7 at 5 minutes.
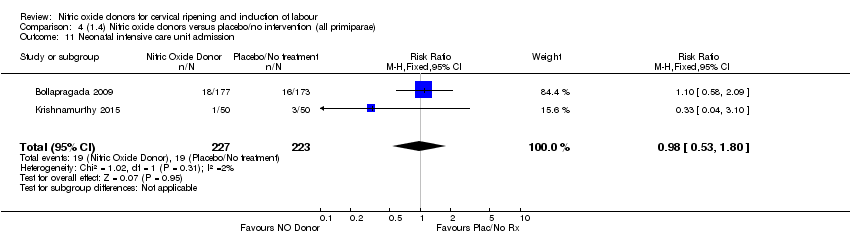
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 11 Neonatal intensive care unit admission.
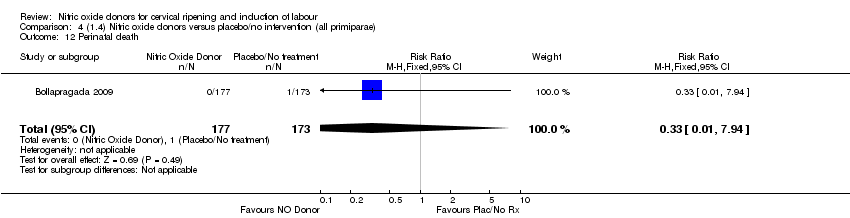
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 12 Perinatal death.

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 13 Maternal side effects (nausea).
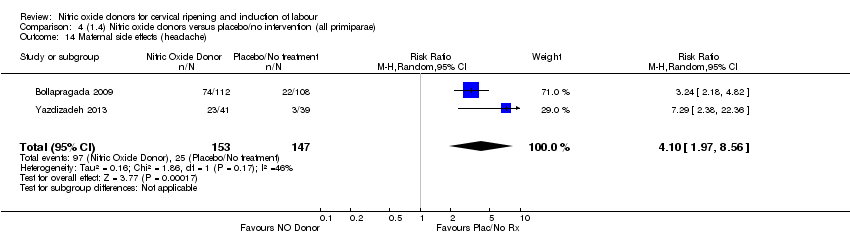
Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 14 Maternal side effects (headache).

Comparison 4 (1.4) Nitric oxide donors versus placebo/no intervention (all primiparae), Outcome 15 Additional induction agents used.
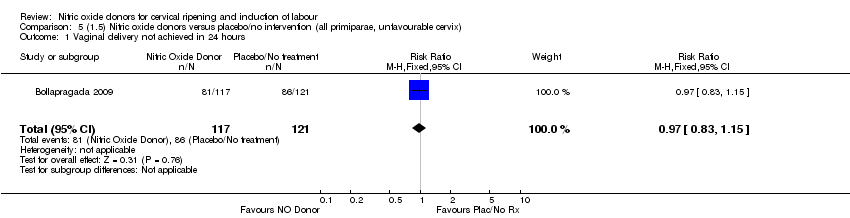
Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
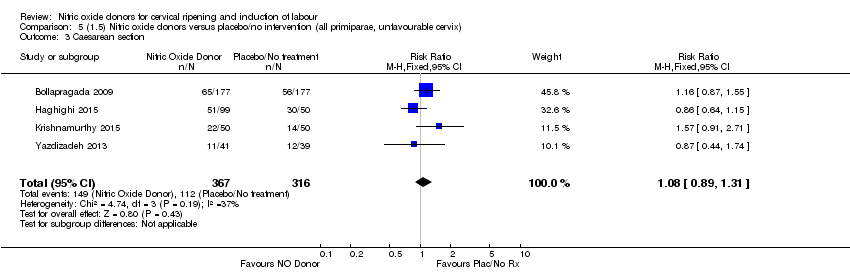
Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 3 Caesarean section.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death.
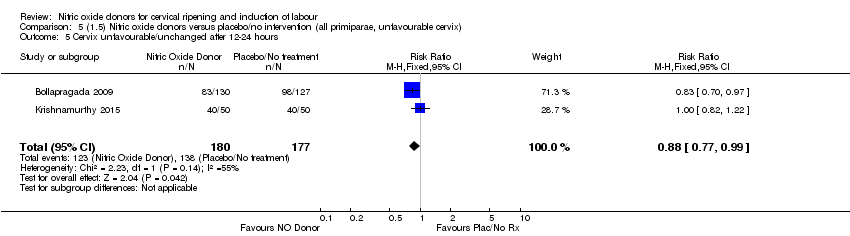
Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 6 Oxytocin augmentation.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 7 Epidural analgesia.
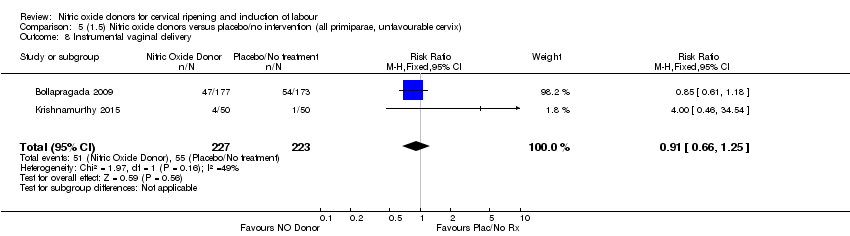
Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 8 Instrumental vaginal delivery.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 9 Meconium‐stained liquor.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 10 Apgar score < 7 at 5 minutes.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 11 Neonatal intensive care unit admission.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 12 Perinatal death.

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 13 Maternal side effects (nausea).

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 14 Maternal side effects (headache).

Comparison 5 (1.5) Nitric oxide donors versus placebo/no intervention (all primiparae, unfavourable cervix), Outcome 15 Additional induction agents used.
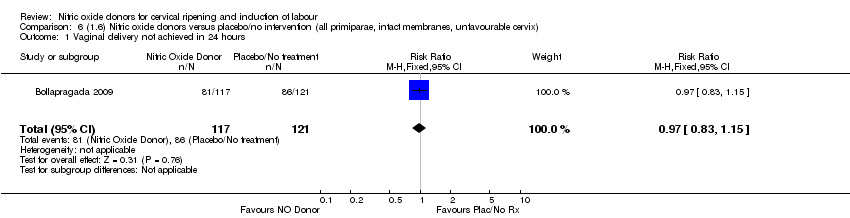
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 2 Caesarean section.
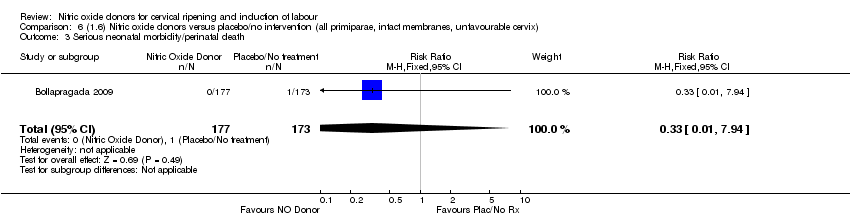
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.
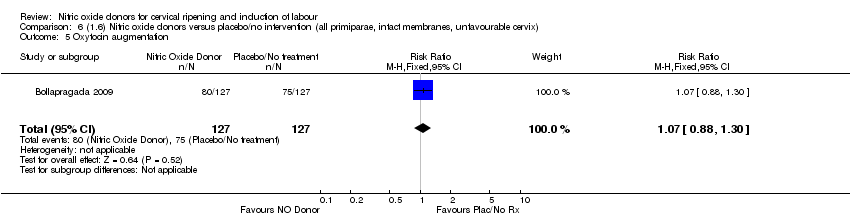
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 5 Oxytocin augmentation.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 6 Epidural analgesia.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 7 Instrumental vaginal delivery.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 8 Meconium‐stained liquor.
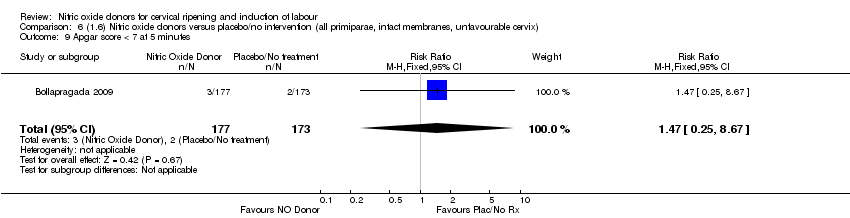
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 9 Apgar score < 7 at 5 minutes.

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 10 Neonatal intensive care unit admission.
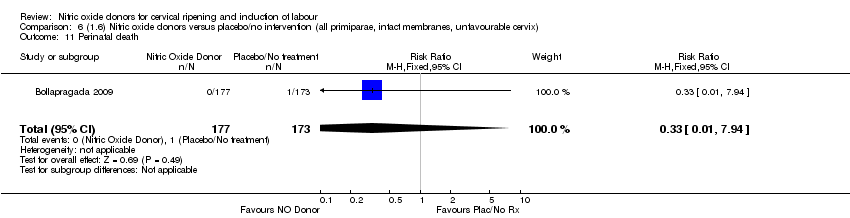
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 11 Perinatal death.
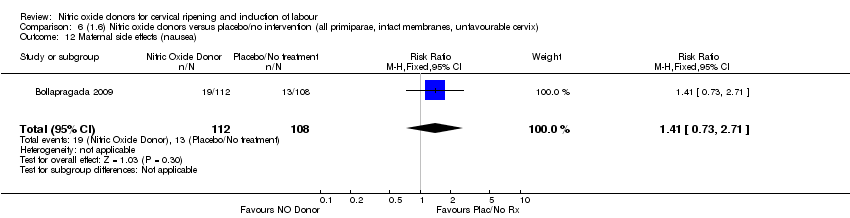
Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 12 Maternal side effects (nausea).

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 13 Maternal side effects (headache).

Comparison 6 (1.6) Nitric oxide donors versus placebo/no intervention (all primiparae, intact membranes, unfavourable cervix), Outcome 14 Additional induction agents used.
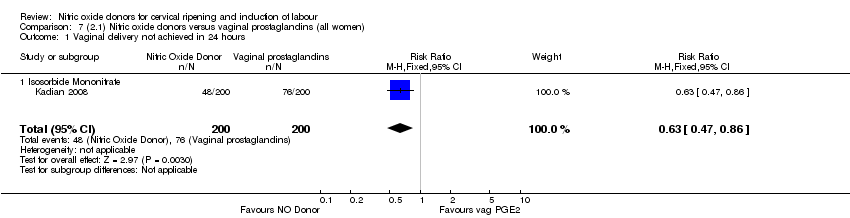
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 1 Vaginal delivery not achieved in 24 hours.
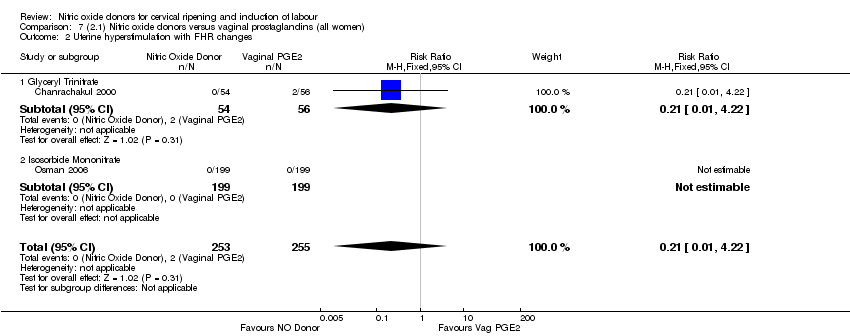
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 2 Uterine hyperstimulation with FHR changes.
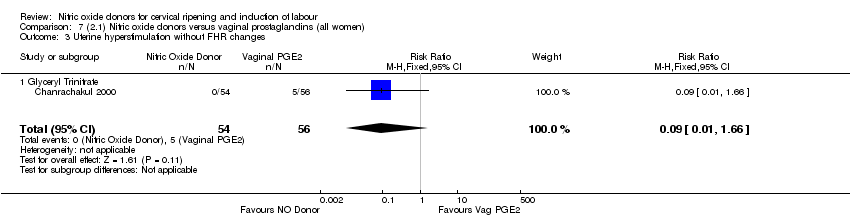
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 3 Uterine hyperstimulation without FHR changes.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 4 Caesarean section.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 5 Instrumental vaginal delivery.
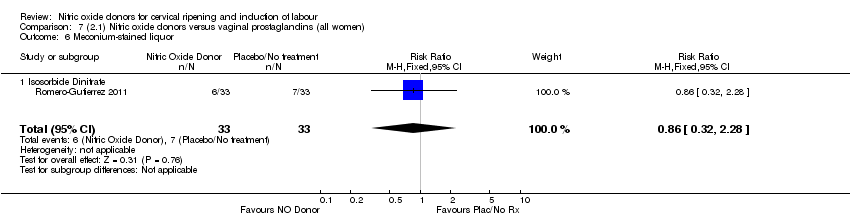
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 6 Meconium‐stained liquor.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 8 Epidural analgesia.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 9 Maternal side effects (nausea).

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 10 Maternal side effects (headache).
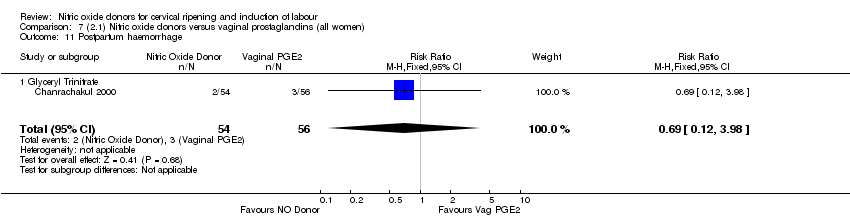
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 11 Postpartum haemorrhage.
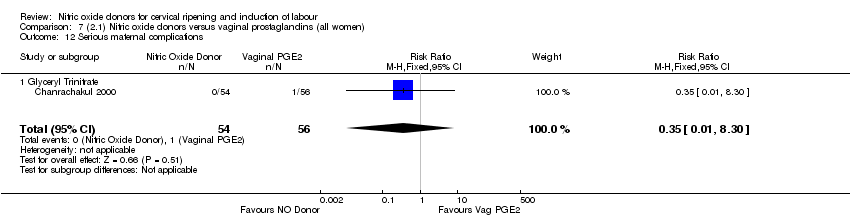
Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 12 Serious maternal complications.

Comparison 7 (2.1) Nitric oxide donors versus vaginal prostaglandins (all women), Outcome 13 Neonatal intensive care unit admission.
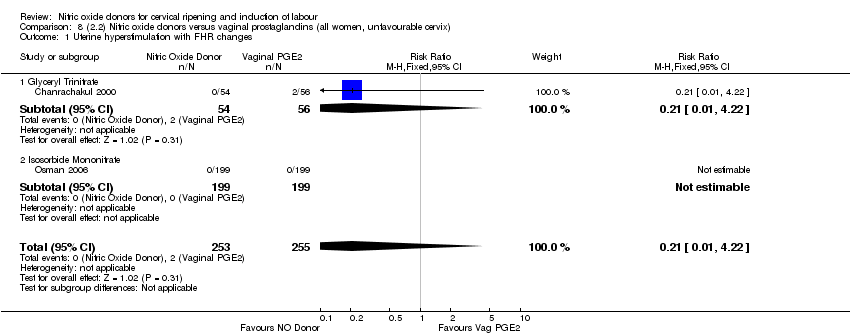
Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.
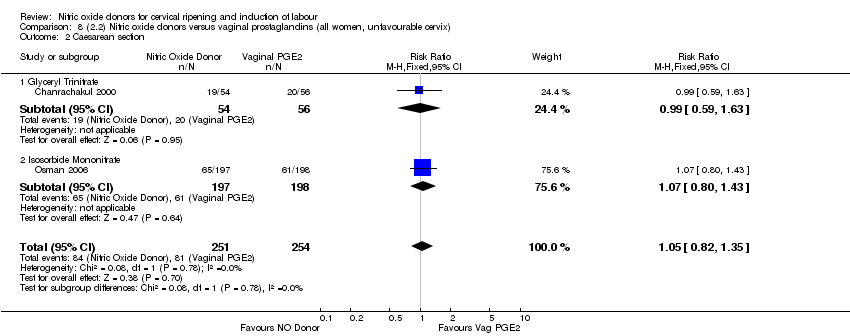
Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 2 Caesarean section.

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 3 Uterine hyperstimulation without FHR changes.

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 4 Epidural analgesia.
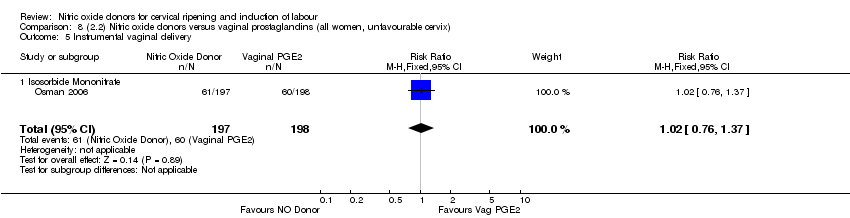
Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 5 Instrumental vaginal delivery.

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 6 Apgar score < 7 at 5 minutes.

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 7 Neonatal intensive care unit admission.
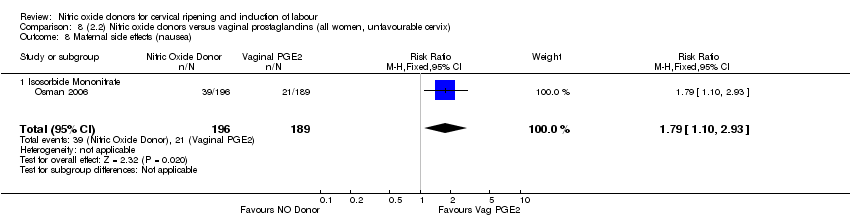
Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 8 Maternal side effects (nausea).

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 9 Maternal side effects (headache).
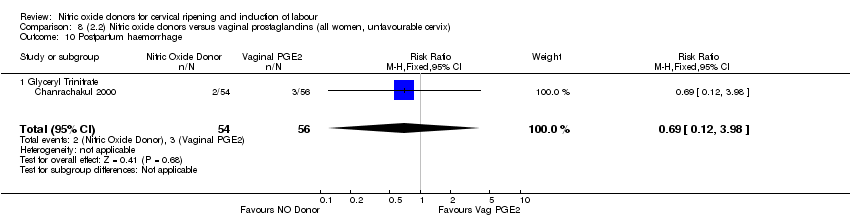
Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 10 Postpartum haemorrhage.

Comparison 8 (2.2) Nitric oxide donors versus vaginal prostaglandins (all women, unfavourable cervix), Outcome 11 Serious maternal complications.
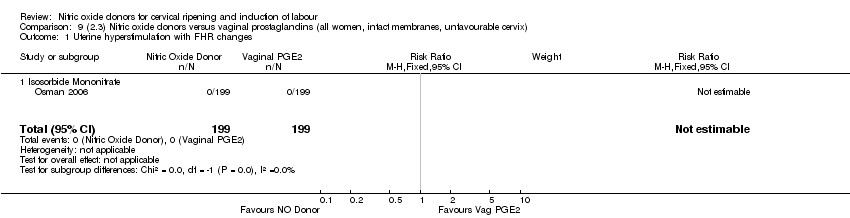
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.
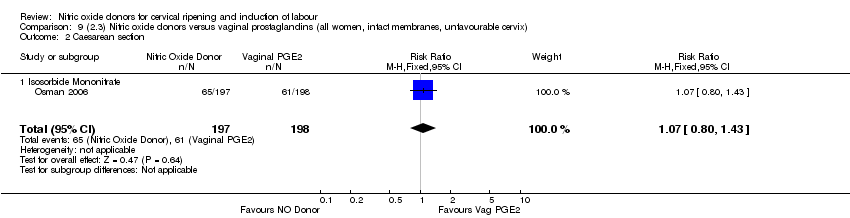
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 2 Caesarean section.

Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 3 Epidural analgesia.

Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 4 Instrumental vaginal delivery.
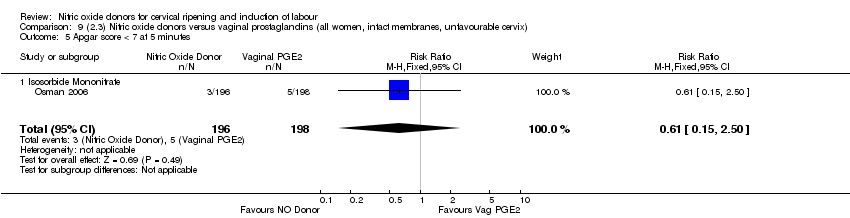
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes.
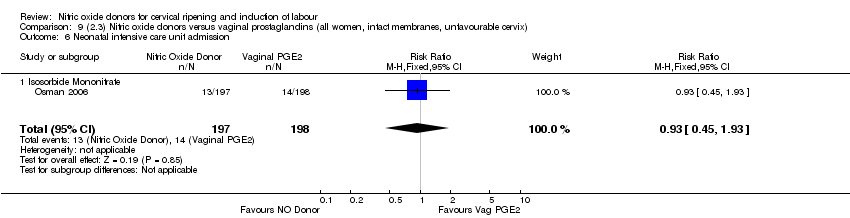
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 6 Neonatal intensive care unit admission.
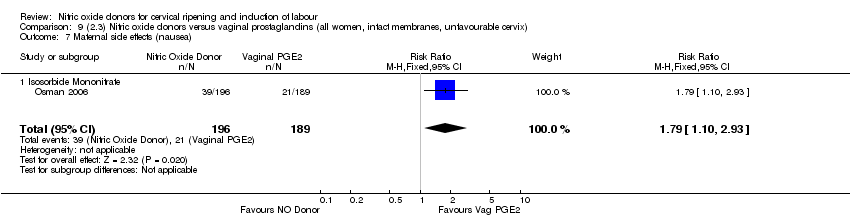
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 7 Maternal side effects (nausea).
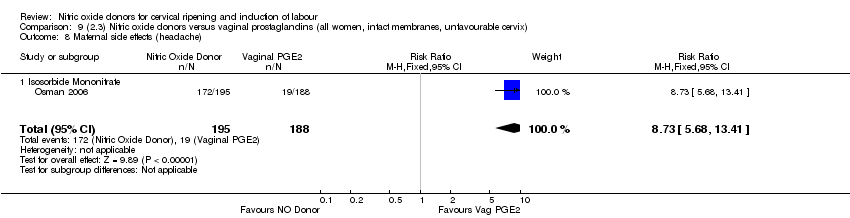
Comparison 9 (2.3) Nitric oxide donors versus vaginal prostaglandins (all women, intact membranes, unfavourable cervix), Outcome 8 Maternal side effects (headache).

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 3 Caesarean section.
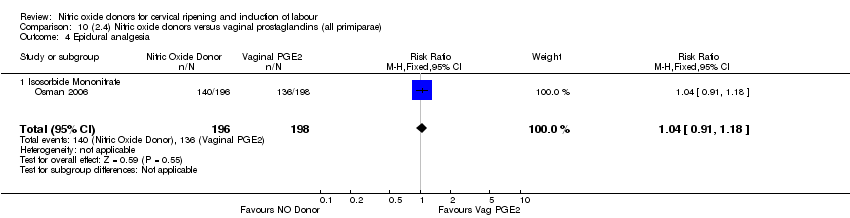
Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 4 Epidural analgesia.

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 5 Instrumental vaginal delivery.

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 6 Apgar score < 7 at 5 minutes.

Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 7 Neonatal intensive care unit admission.
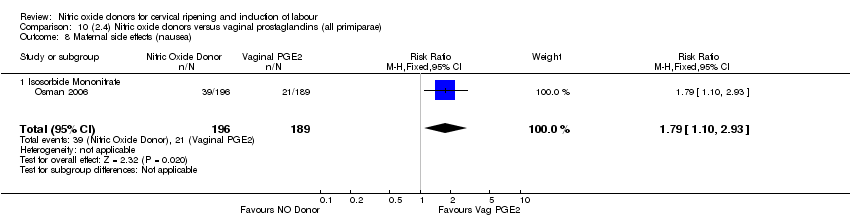
Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 8 Maternal side effects (nausea).
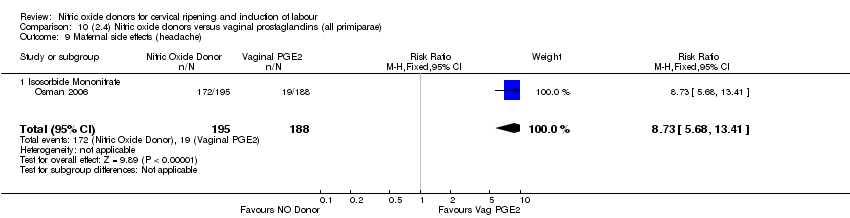
Comparison 10 (2.4) Nitric oxide donors versus vaginal prostaglandins (all primiparae), Outcome 9 Maternal side effects (headache).
Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 2 Caesarean section.

Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 3 Epidural analgesia.
Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 4 Instrumental vaginal delivery.
Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes.
Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 6 Neonatal intensive care unit admission.
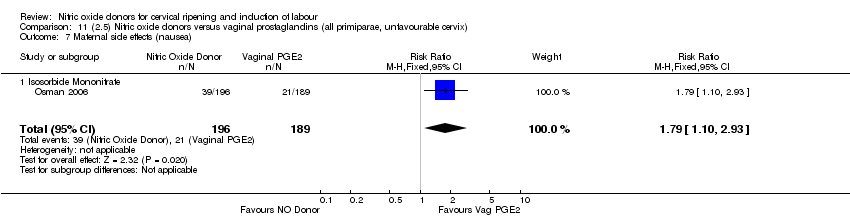
Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 7 Maternal side effects (nausea).

Comparison 11 (2.5) Nitric oxide donors versus vaginal prostaglandins (all primiparae, unfavourable cervix), Outcome 8 Maternal side effects (headache).

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 2 Caesarean section.

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 3 Epidural analgesia.

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 4 Instrumental vaginal delivery.

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes.
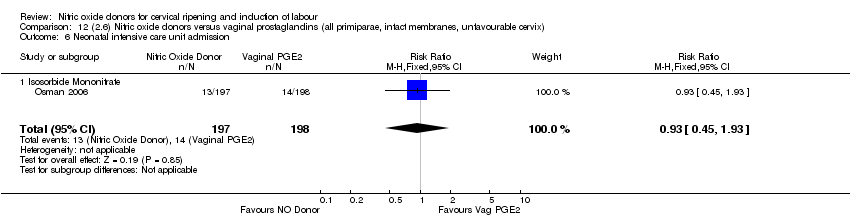
Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 6 Neonatal intensive care unit admission.

Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 7 Maternal side effects (nausea).
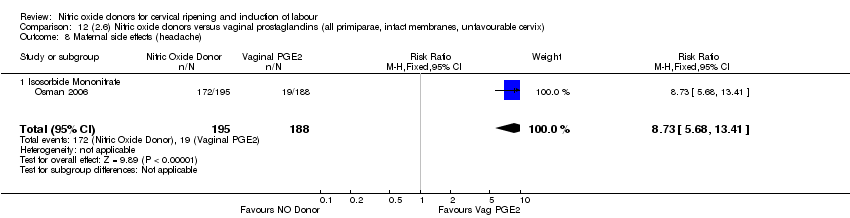
Comparison 12 (2.6) Nitric oxide donors versus vaginal prostaglandins (all primiparae, intact membranes, unfavourable cervix), Outcome 8 Maternal side effects (headache).

Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 1 Vaginal delivery not achieved in 24 hours.
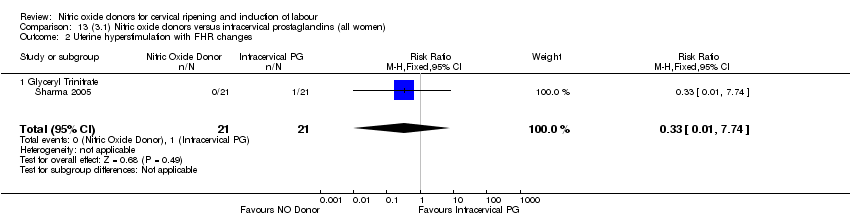
Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 3 Caesarean section.

Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 4 Serious neonatal morbidity/perinatal death.
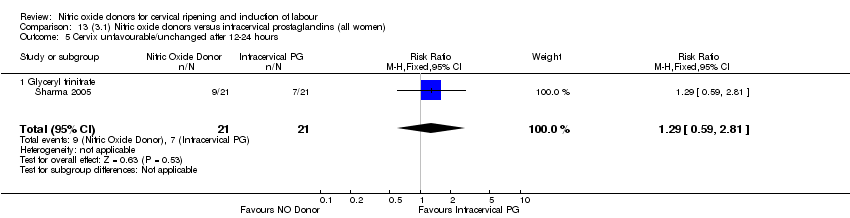
Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
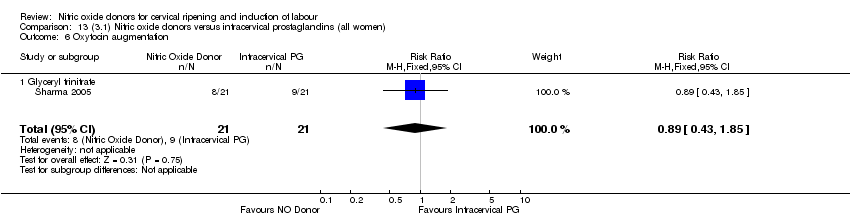
Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 6 Oxytocin augmentation.
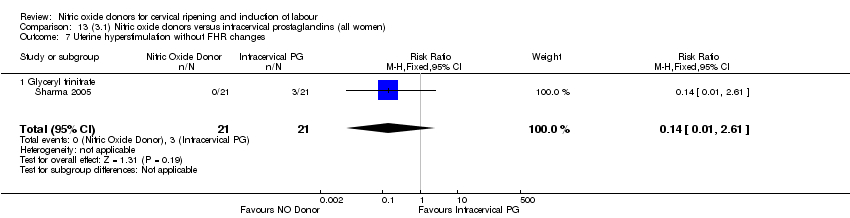
Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 7 Uterine hyperstimulation without FHR changes.
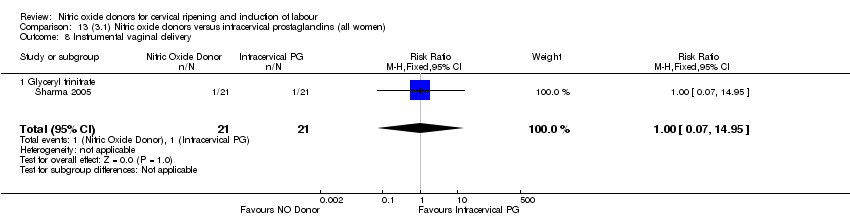
Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 8 Instrumental vaginal delivery.

Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 9 Perinatal death.

Comparison 13 (3.1) Nitric oxide donors versus intracervical prostaglandins (all women), Outcome 10 Maternal side effects (headache).

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
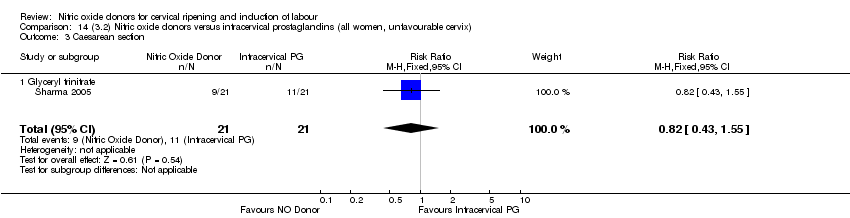
Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 3 Caesarean section.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 4 Serious neonatal morbidity/perinatal death.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 6 Oxytocin augmentation.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 7 Uterine hyperstimulation without FHR changes.

Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 8 Instrumental vaginal delivery.
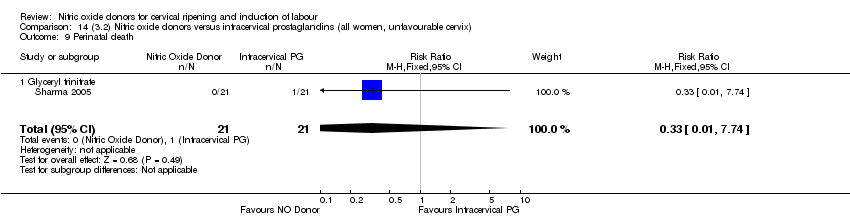
Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 9 Perinatal death.
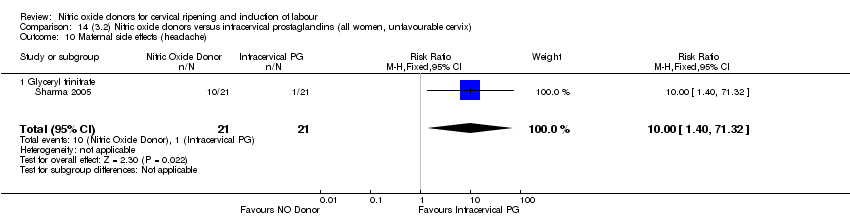
Comparison 14 (3.2) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix), Outcome 10 Maternal side effects (headache).

Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 2 Uterine hyperstimulation with FHR changes.
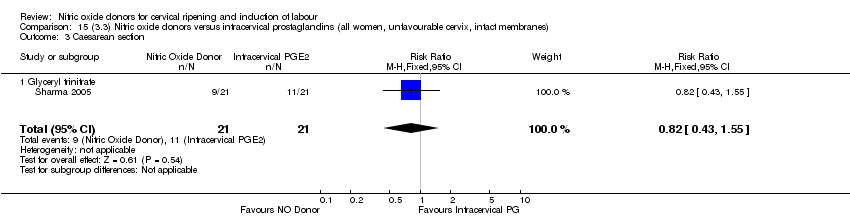
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 3 Caesarean section.

Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 4 Serious neonatal morbidity/perinatal death.

Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
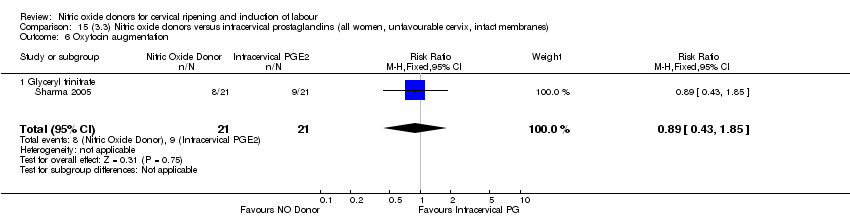
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 6 Oxytocin augmentation.
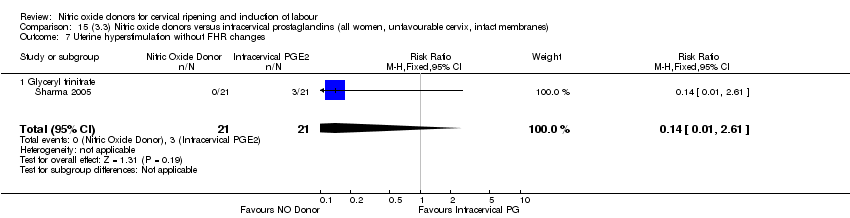
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 7 Uterine hyperstimulation without FHR changes.
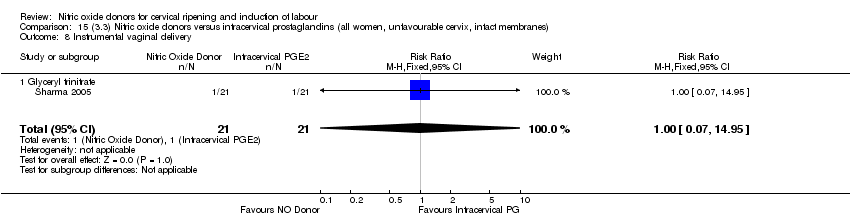
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 8 Instrumental vaginal delivery.
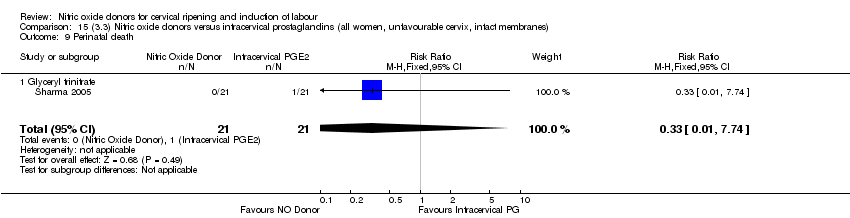
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 9 Perinatal death.
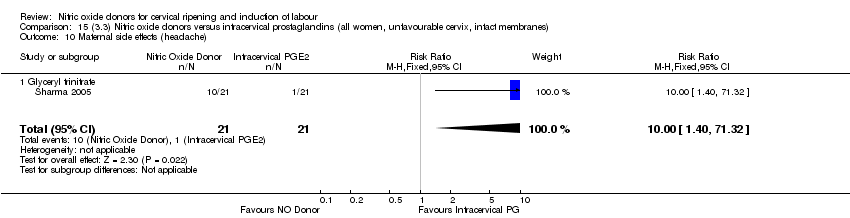
Comparison 15 (3.3) Nitric oxide donors versus intracervical prostaglandins (all women, unfavourable cervix, intact membranes), Outcome 10 Maternal side effects (headache).

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 2 Caesarean section.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 5 Oxytocin augmentation.
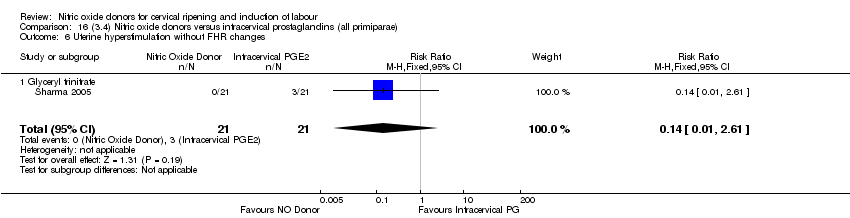
Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 6 Uterine hyperstimulation without FHR changes.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 7 Instrumental vaginal delivery.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 8 Perinatal death.

Comparison 16 (3.4) Nitric oxide donors versus intracervical prostaglandins (all primiparae), Outcome 9 Maternal side effects (headache).

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 2 Caesarean section.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 5 Oxytocin augmentation.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 6 Uterine hyperstimulation without FHR changes.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 7 Instrumental vaginal delivery.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 8 Perinatal death.

Comparison 17 (3.5) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix), Outcome 9 Maternal side effects (headache).

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 1 Uterine hyperstimulation with FHR changes.
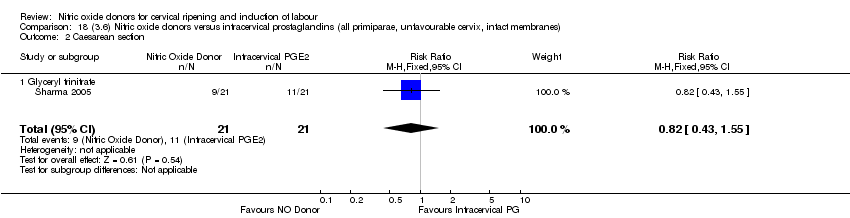
Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 2 Caesarean section.

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.
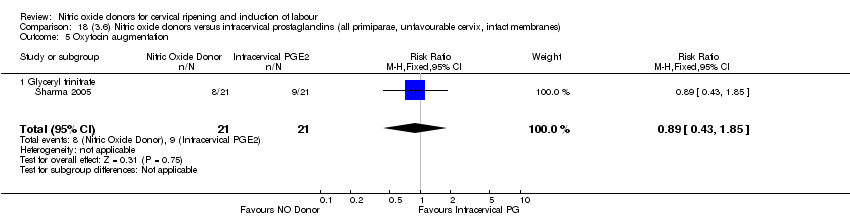
Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 5 Oxytocin augmentation.

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 6 Uterine hyperstimulation without FHR changes.
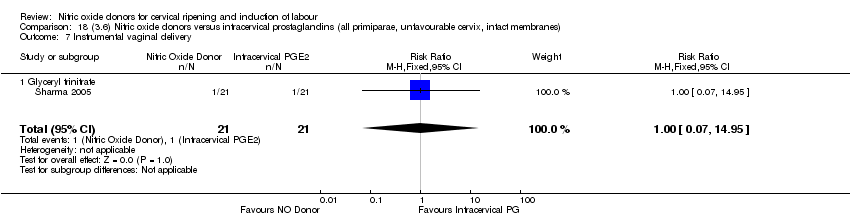
Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 7 Instrumental vaginal delivery.

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 8 Perinatal death.

Comparison 18 (3.6) Nitric oxide donors versus intracervical prostaglandins (all primiparae, unfavourable cervix, intact membranes), Outcome 9 Maternal side effects (headache).

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 2 Uterine hyperstimulation with FHR changes.
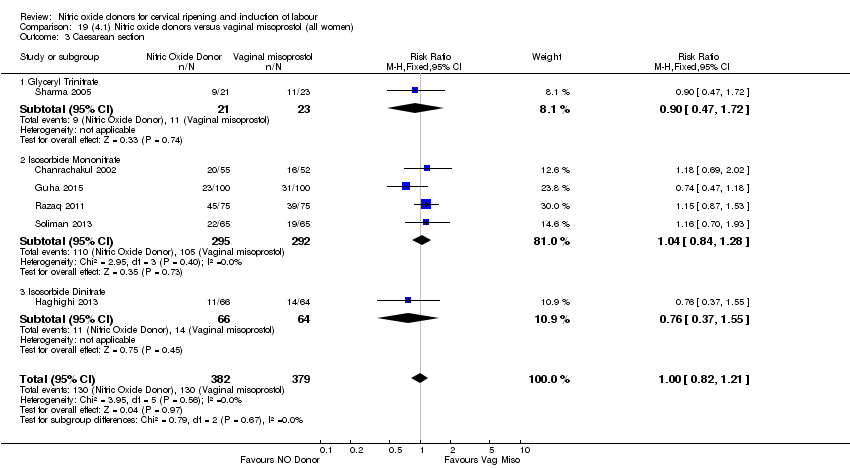
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 3 Caesarean section.
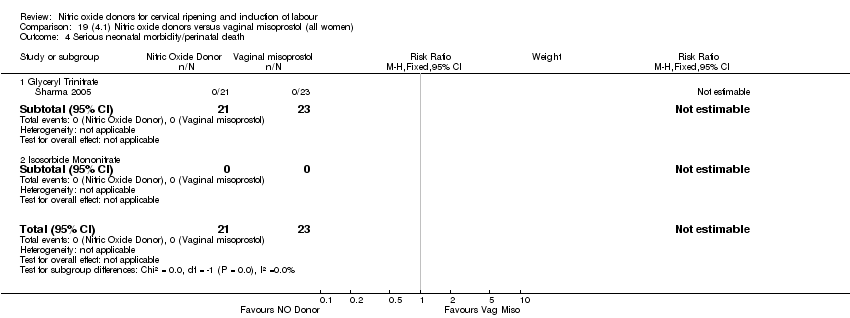
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 4 Serious neonatal morbidity/perinatal death.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
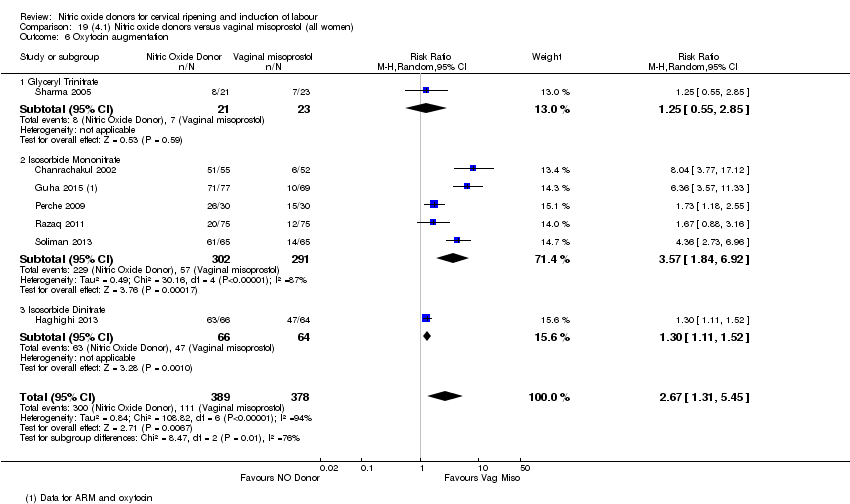
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 6 Oxytocin augmentation.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 7 Uterine hyperstimulation without FHR changes.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 8 Epidural analgesia.
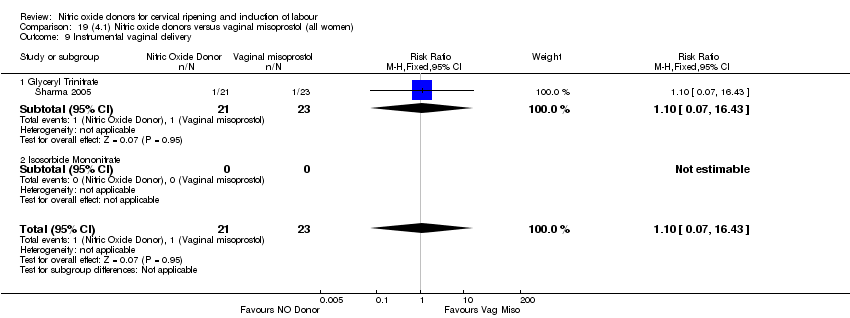
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 9 Instrumental vaginal delivery.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 10 Meconium‐stained liquor.
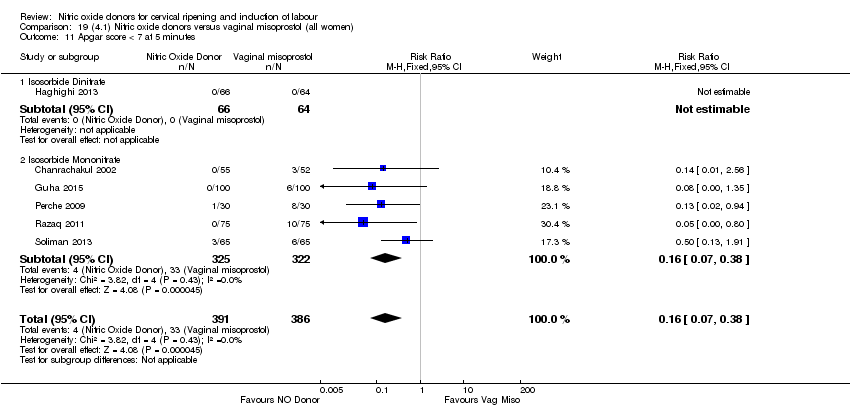
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 11 Apgar score < 7 at 5 minutes.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 12 Neonatal intensive care unit admission.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 13 Perinatal death.

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 14 Maternal side effects (nausea).

Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 15 Maternal side effects (headache).
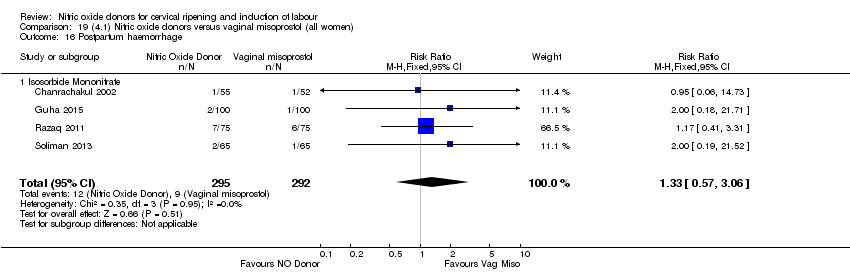
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 16 Postpartum haemorrhage.
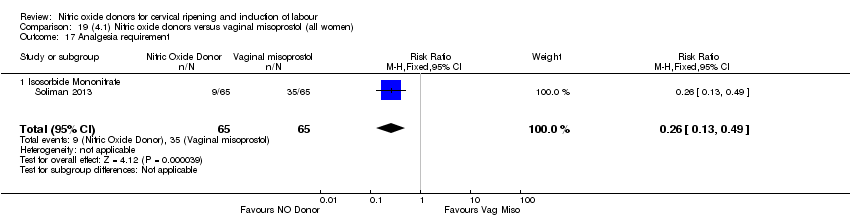
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 17 Analgesia requirement.
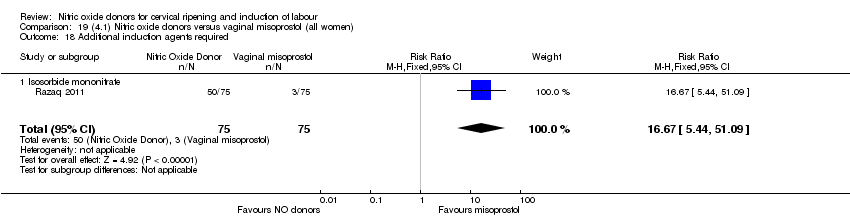
Comparison 19 (4.1) Nitric oxide donors versus vaginal misoprostol (all women), Outcome 18 Additional induction agents required.
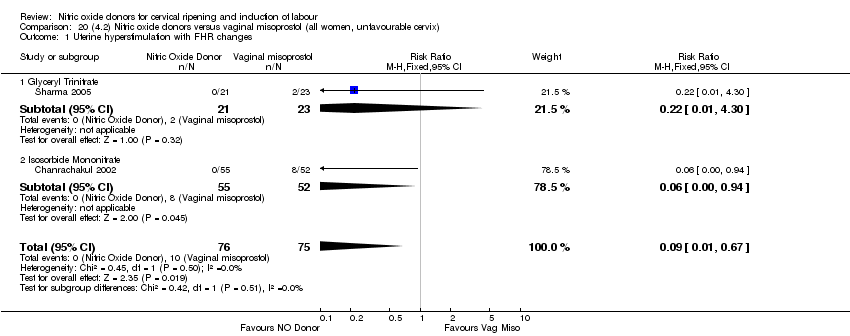
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 2 Caesarean section.
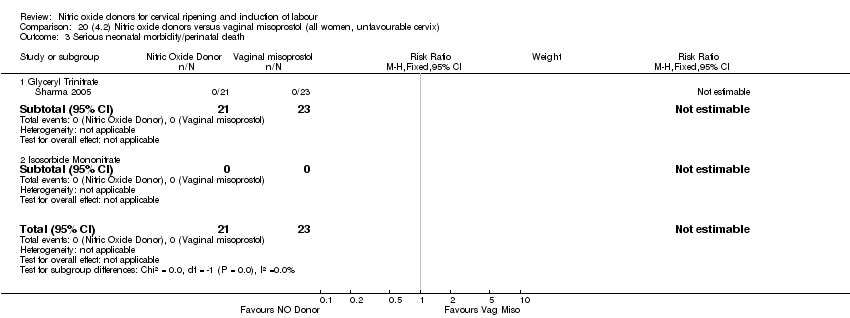
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.
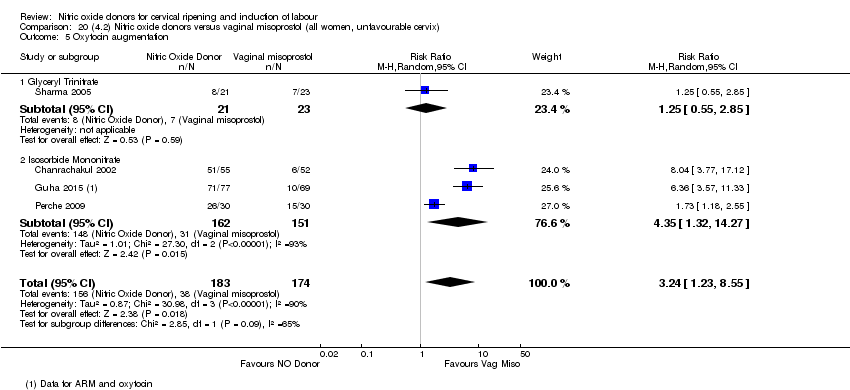
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 5 Oxytocin augmentation.

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 6 Uterine hyperstimulation without FHR changes.

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 7 Instrumental vaginal delivery.

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 8 Apgar score < 7 at 5 minutes.
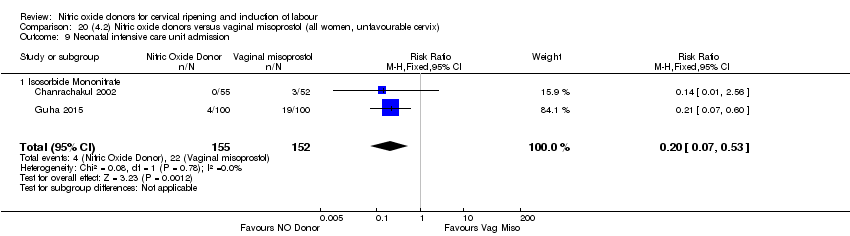
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 9 Neonatal intensive care unit admission.
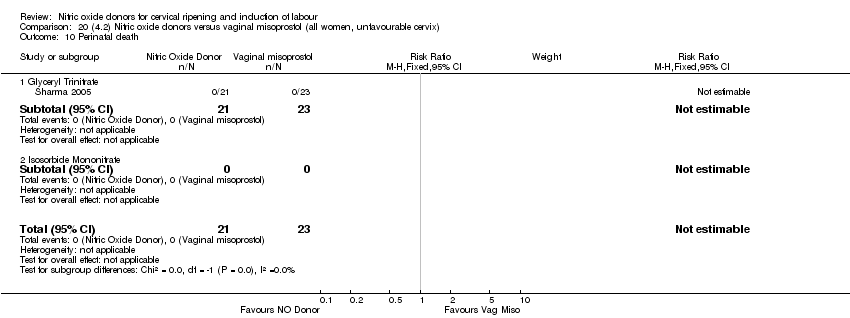
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 10 Perinatal death.
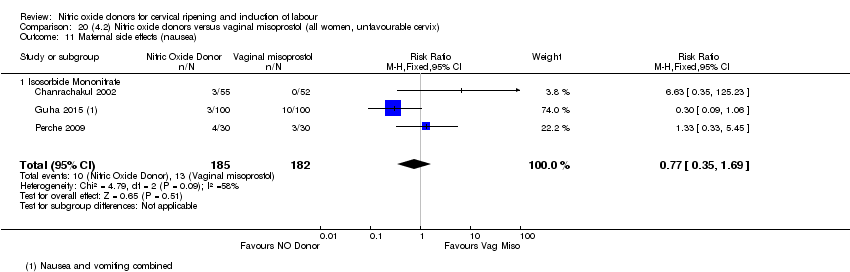
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 11 Maternal side effects (nausea).
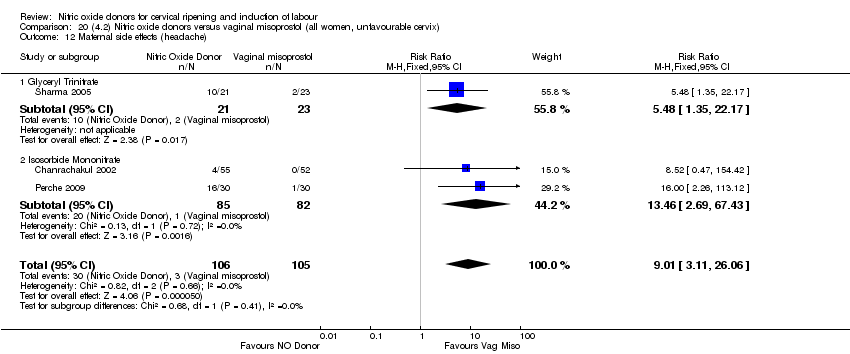
Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 12 Maternal side effects (headache).

Comparison 20 (4.2) Nitric oxide donors versus vaginal misoprostol (all women, unfavourable cervix), Outcome 13 Postpartum haemorrhage.

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 2 Caesarean section.
Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.
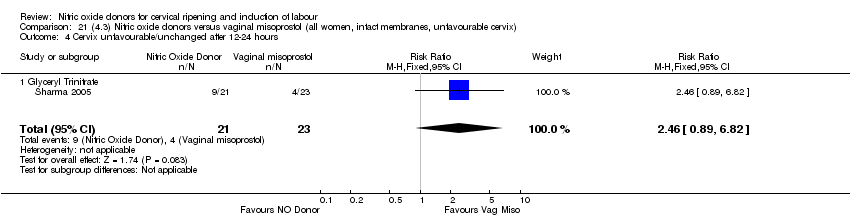
Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 5 Oxytocin augmentation.
Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 6 Instrumental vaginal delivery.

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.
Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 8 Perinatal death.

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 9 Maternal side effects (nausea).

Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (headache).
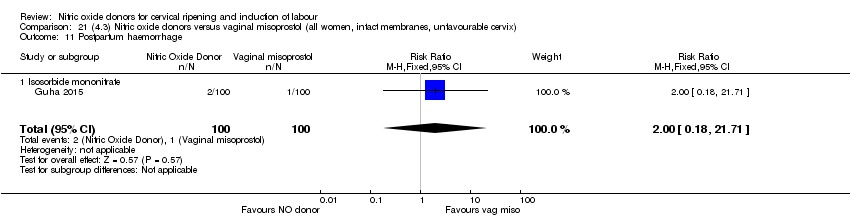
Comparison 21 (4.3) Nitric oxide donors versus vaginal misoprostol (all women, intact membranes, unfavourable cervix), Outcome 11 Postpartum haemorrhage.
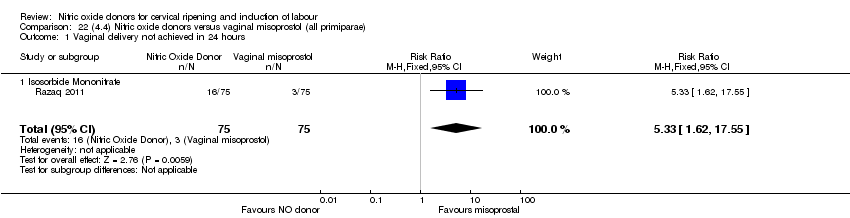
Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 1 Vaginal delivery not achieved in 24 hours.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 3 Caesarean section.
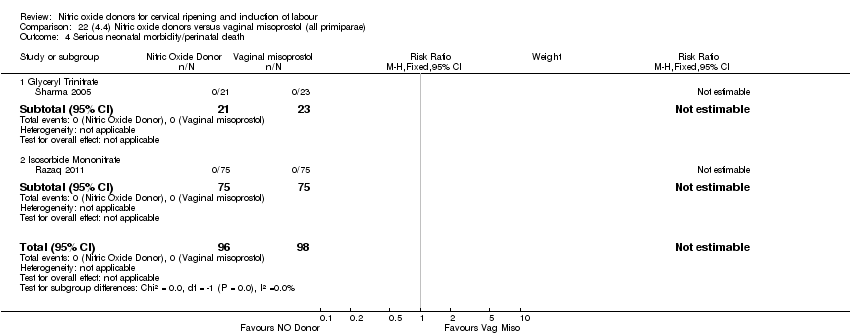
Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 4 Serious neonatal morbidity/perinatal death.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 6 Oxytocin augmentation.
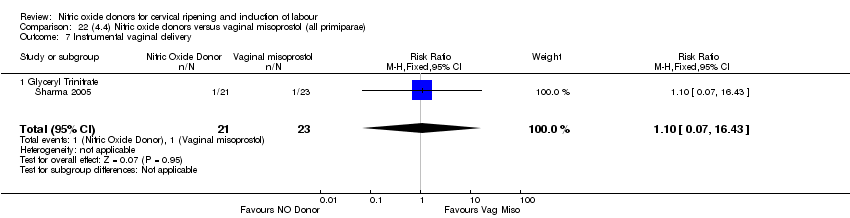
Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 7 Instrumental vaginal delivery.
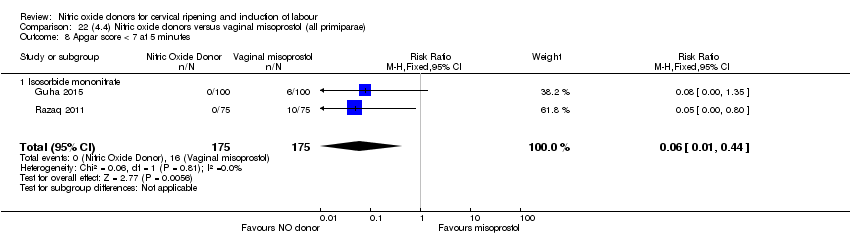
Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 8 Apgar score < 7 at 5 minutes.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 9 Neonatal intensive care unit admission.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 10 Perinatal death.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 11 Maternal side effects (nausea).

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 12 Maternal side effects (headache).

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 13 Postpartum haemorrhage.

Comparison 22 (4.4) Nitric oxide donors versus vaginal misoprostol (all primiparae), Outcome 14 Additional induction agents required.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 2 Caesarean section.
Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 5 Oxytocin augmentation.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 6 Instrumental vaginal delivery.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.
Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 9 Perinatal death.

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 10 Maternal side effects (nausea).

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 11 Maternal side effects (headache).

Comparison 23 (4.5) Nitric oxide donors versus vaginal misoprostol (all primiparae, unfavourable cervix), Outcome 12 Postpartum haemorrhage.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 2 Caesarean section.
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 3 Serious neonatal morbidity/perinatal death.
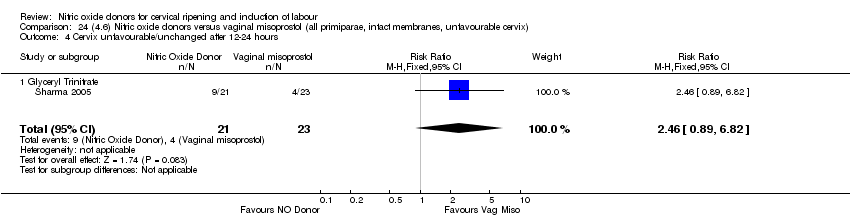
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 5 Oxytocin augmentation.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 6 Instrumental vaginal delivery.
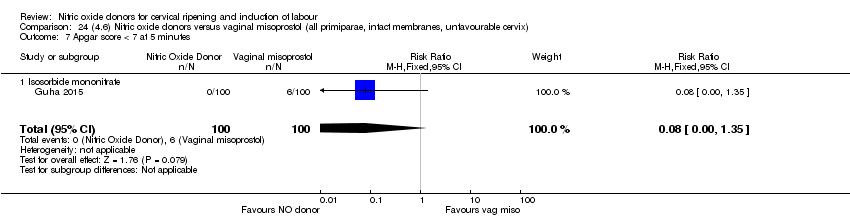
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.
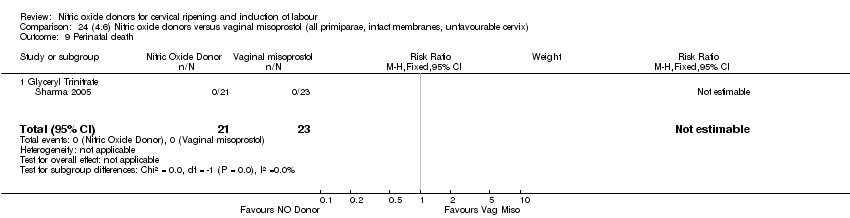
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 9 Perinatal death.

Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (nausea).
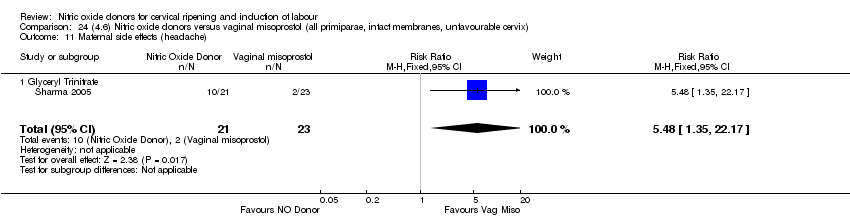
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 11 Maternal side effects (headache).
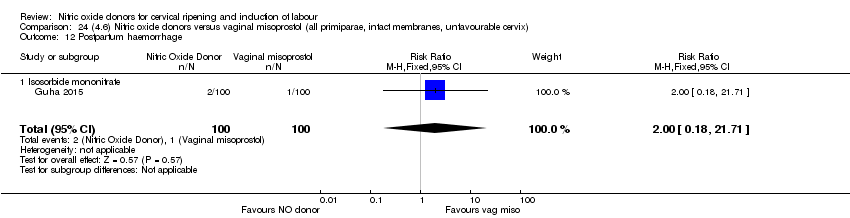
Comparison 24 (4.6) Nitric oxide donors versus vaginal misoprostol (all primiparae, intact membranes, unfavourable cervix), Outcome 12 Postpartum haemorrhage.
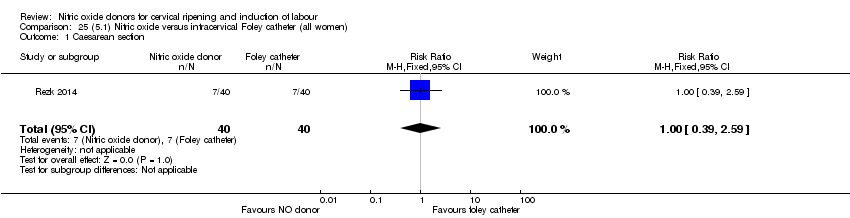
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 1 Caesarean section.
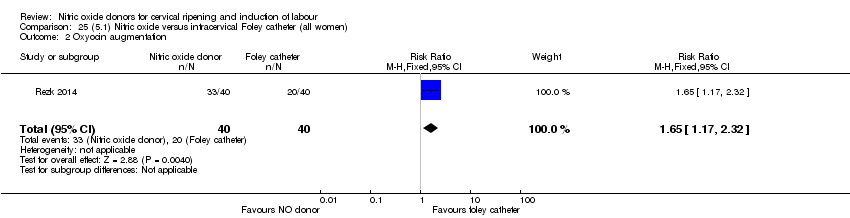
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 2 Oxyocin augmentation.
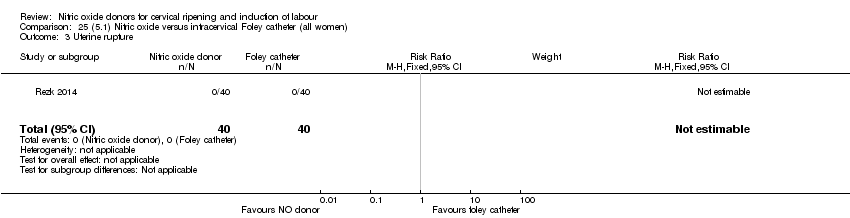
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 3 Uterine rupture.

Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 4 Epidural analgesia.
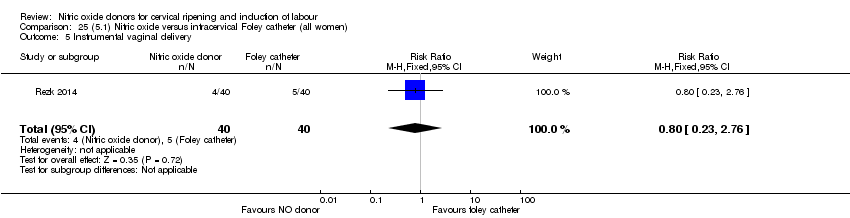
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 5 Instrumental vaginal delivery.
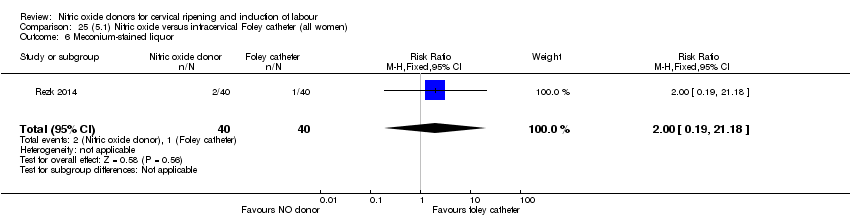
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 6 Meconium‐stained liquor.

Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 7 Apgar score < 7 at 5 minutes.
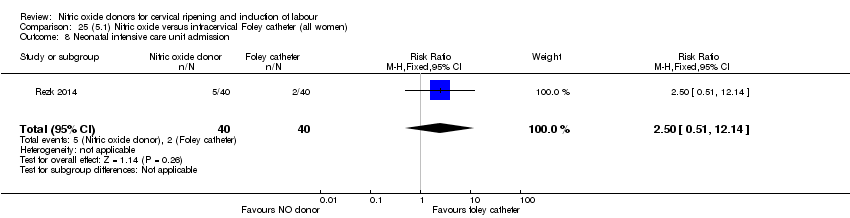
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 8 Neonatal intensive care unit admission.
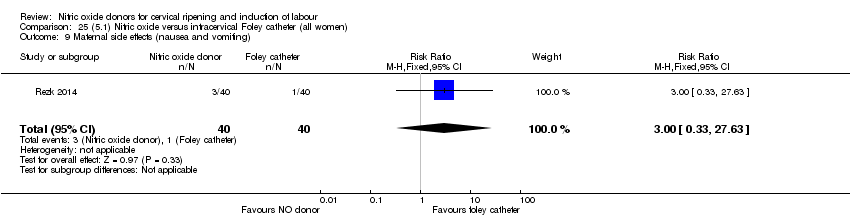
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 9 Maternal side effects (nausea and vomiting).

Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 10 Maternal side effects (headache).

Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 11 Postpartum haemorrhage.
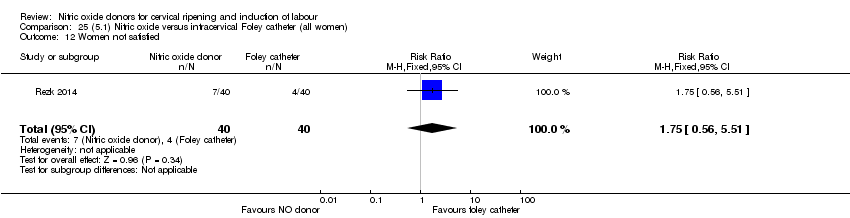
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 12 Women not satisfied.
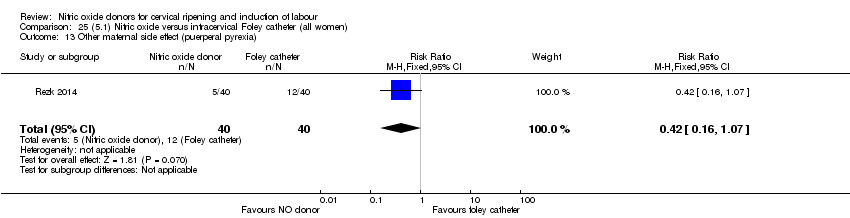
Comparison 25 (5.1) Nitric oxide versus intracervical Foley catheter (all women), Outcome 13 Other maternal side effect (puerperal pyrexia).
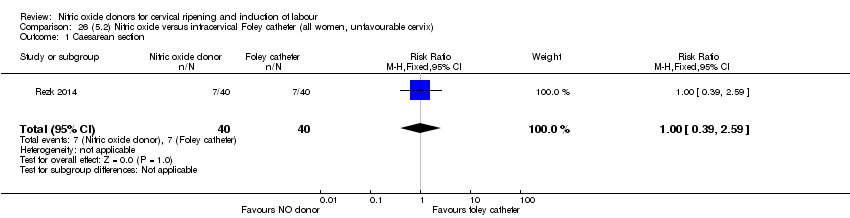
Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 1 Caesarean section.
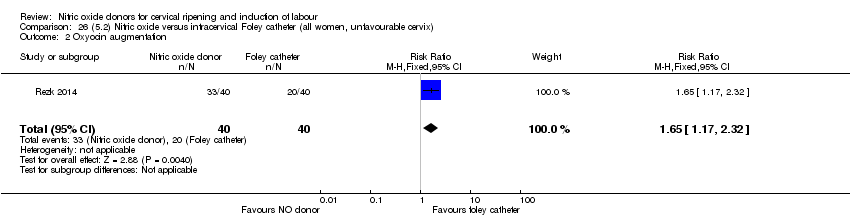
Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 2 Oxyocin augmentation.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 3 Uterine rupture.
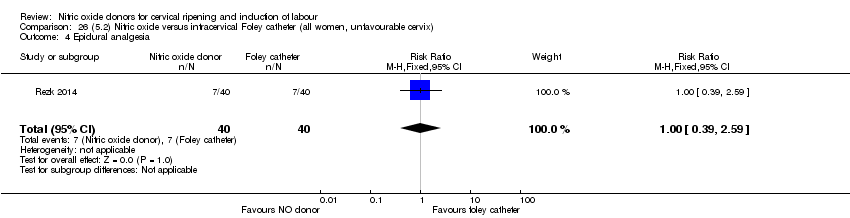
Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 4 Epidural analgesia.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 5 Instrumental vaginal delivery.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 6 Meconium‐stained liquor.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting).

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 10 Maternal side effects (headache).

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 11 Postpartum haemorrhage.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 12 Women not satisfied.

Comparison 26 (5.2) Nitric oxide versus intracervical Foley catheter (all women, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia).

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 1 Caesarean section.
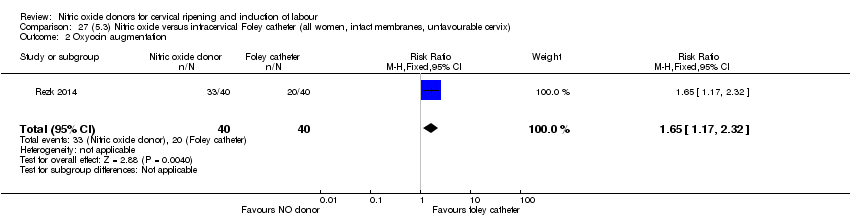
Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 2 Oxyocin augmentation.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 3 Uterine rupture.
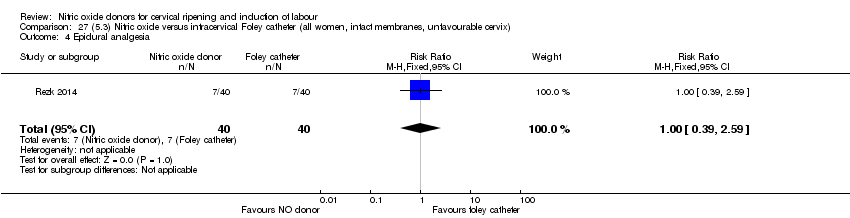
Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 4 Epidural analgesia.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 5 Instrumental vaginal delivery.
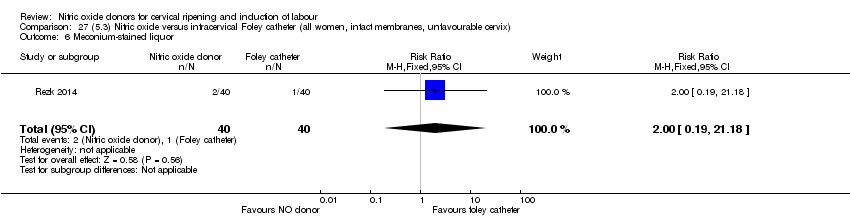
Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 6 Meconium‐stained liquor.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting).

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (headache).

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 11 Postpartum haemorrhage.
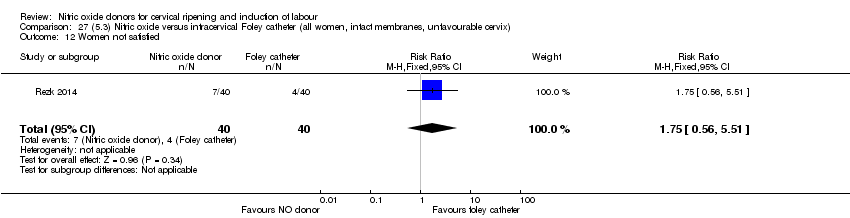
Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 12 Women not satisfied.

Comparison 27 (5.3) Nitric oxide versus intracervical Foley catheter (all women, intact membranes, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia).
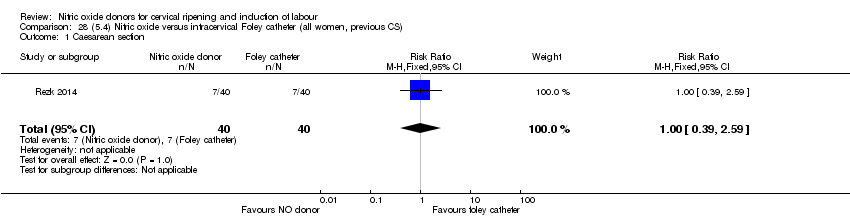
Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 1 Caesarean section.
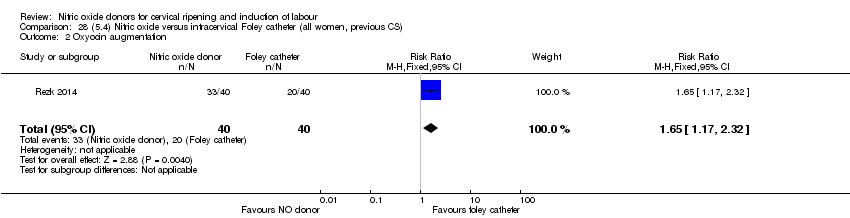
Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 2 Oxyocin augmentation.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 3 Uterine rupture.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 4 Epidural analgesia.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 5 Instrumental vaginal delivery.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 6 Meconium‐stained liquor.
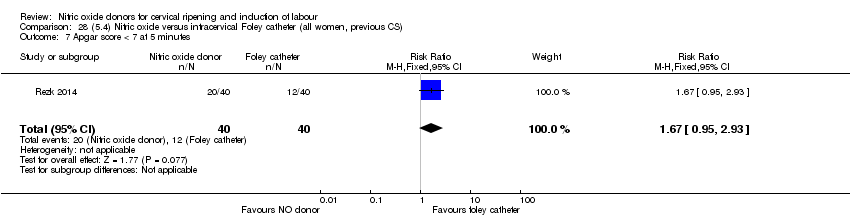
Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 7 Apgar score < 7 at 5 minutes.
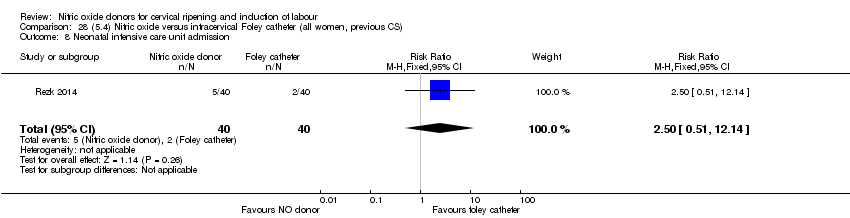
Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 8 Neonatal intensive care unit admission.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 9 Maternal side effects (nausea and vomiting).

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 10 Maternal side effects (headache).

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 11 Postpartum haemorrhage.

Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 12 Women not satisfied.
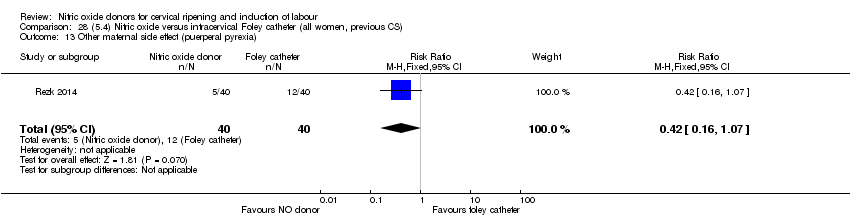
Comparison 28 (5.4) Nitric oxide versus intracervical Foley catheter (all women, previous CS), Outcome 13 Other maternal side effect (puerperal pyrexia).

Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 1 Caesarean section.
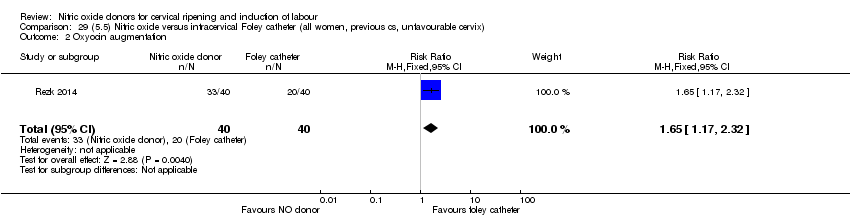
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 2 Oxyocin augmentation.
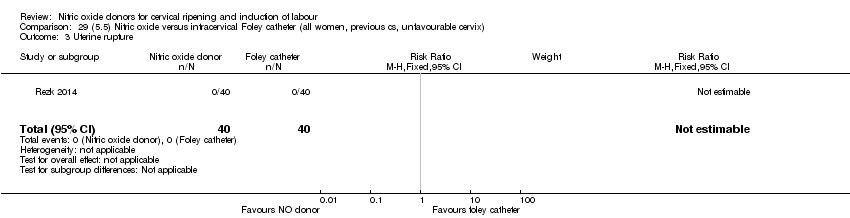
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 3 Uterine rupture.

Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 4 Epidural analgesia.
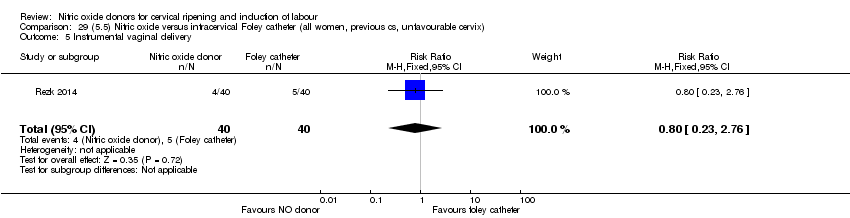
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 5 Instrumental vaginal delivery.
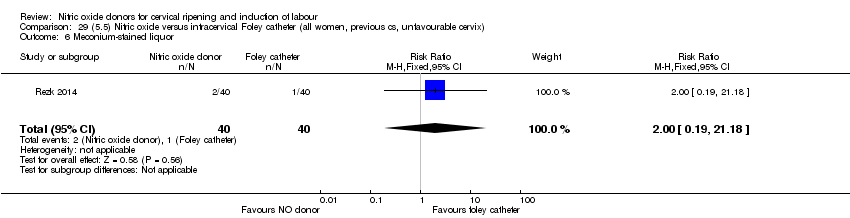
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 6 Meconium‐stained liquor.
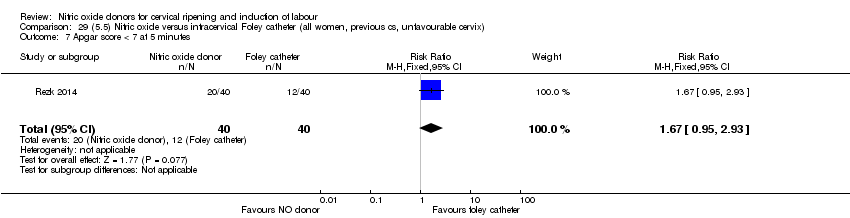
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.
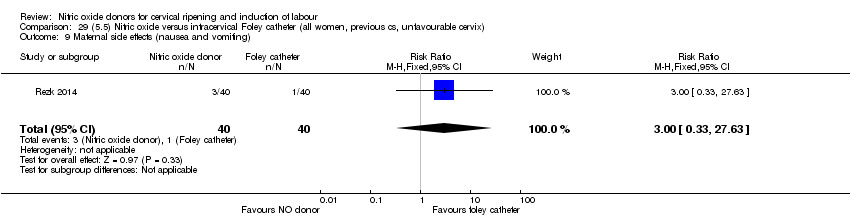
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting).
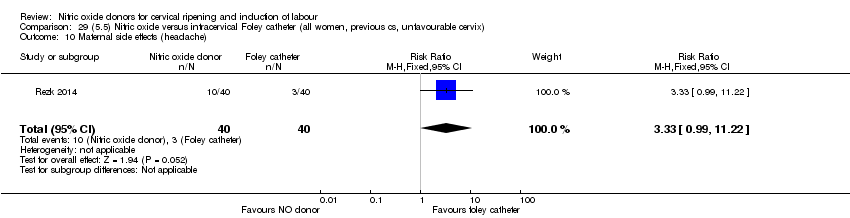
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 10 Maternal side effects (headache).

Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 11 Postpartum haemorrhage.

Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 12 Women not satisfied.
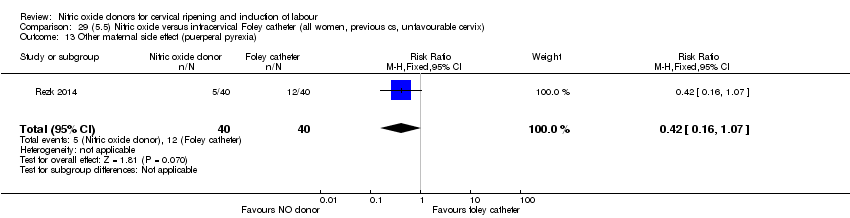
Comparison 29 (5.5) Nitric oxide versus intracervical Foley catheter (all women, previous cs, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia).

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 1 Caesarean section.

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 2 Oxyocin augmentation.
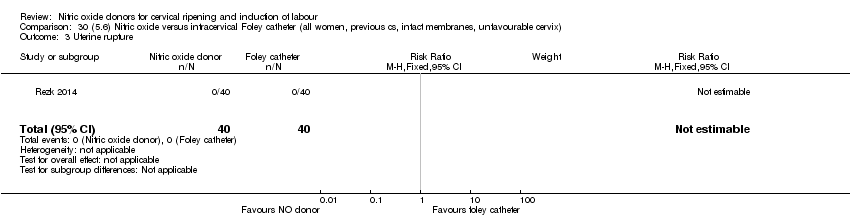
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 3 Uterine rupture.
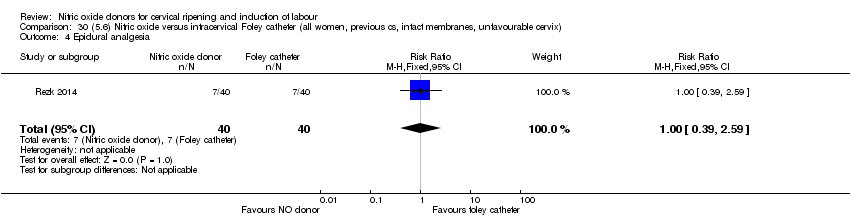
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 4 Epidural analgesia.

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 5 Instrumental vaginal delivery.

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 6 Meconium‐stained liquor.
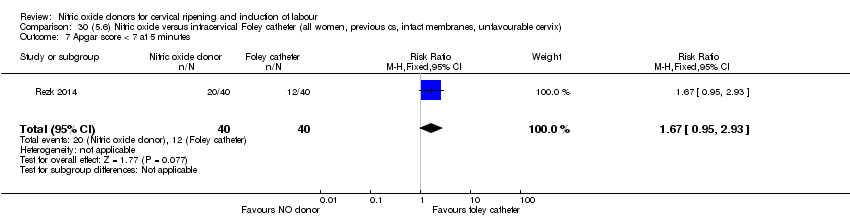
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 7 Apgar score < 7 at 5 minutes.
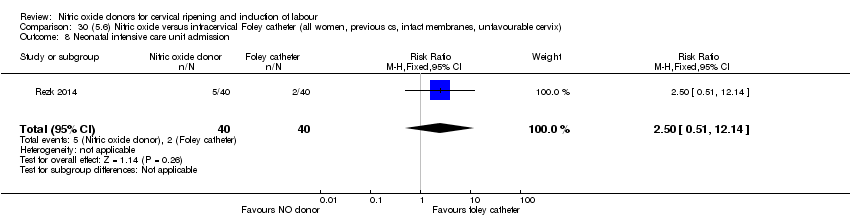
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 8 Neonatal intensive care unit admission.

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 9 Maternal side effects (nausea and vomiting).

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 10 Maternal side effects (headache).
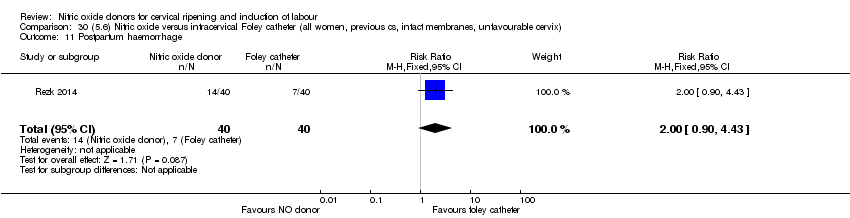
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 11 Postpartum haemorrhage.

Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 12 Women not satisfied.
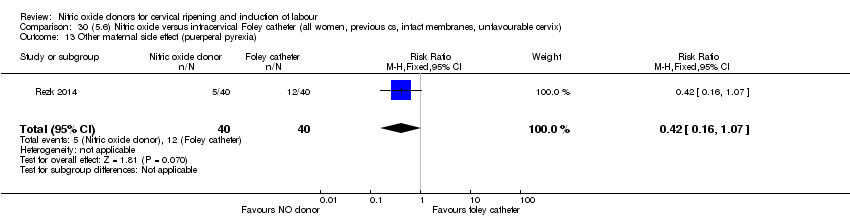
Comparison 30 (5.6) Nitric oxide versus intracervical Foley catheter (all women, previous cs, intact membranes, unfavourable cervix), Outcome 13 Other maternal side effect (puerperal pyrexia).
| Nitric oxide donors for cervical ripening and induction of labour | ||||||
| Patient or population: pregnant women undergoing cervical ripening and induction of labour | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo/no intervention (all women) | Risk with (1.1) Nitric oxide donors | |||||
| Vaginal delivery not achieved in 24 hours | Study population | RR 0.97 | 238 | ⊕⊕⊝⊝ | ||
| 711 per 1000 | 689 per 1000 | |||||
| Uterine hyperstimulation with FHR changes | Study population | RR 0.09 | 300 | ⊕⊕⊝⊝ | ||
| 33 per 1000 | 3 per 1000 | |||||
| Caesarean section | Study population | RR 0.99 | 2624 | ⊕⊕⊕⊝ | ||
| 280 per 1000 | 277 per 1000 | |||||
| Serious neonatal morbidity/perinatal death | Study population | RR 1.61 | 1712 | ⊕⊕⊝⊝ | ||
| 1 per 1000 | 2 per 1000 | |||||
| Serious maternal morbidity or death | Study population | not estimable | 1362 | There were no events for this outcome. | ||
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only one study with few events, small sample size and wide confidence interval. 2 High risk of bias for allocation concealment and blinding. 3 Only two studies with few or no events, small sample size and wide confidence interval. 4 High risk of bias for allocation concealment, blinding and selective outcome reporting. 5 Confidence intervals do not overlap (opposite directions of effect) and I2 = 48%. 6 Only two studies with few events and wide confidence intervals. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.62] |
| 3 Caesarean section Show forest plot | 9 | 2624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.11] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 2 | 1712 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.08, 33.26] |
| 5 Serious maternal morbidity or death Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 4 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.67, 0.90] |
| 6.1 Standard release | 4 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.69, 0.94] |
| 6.2 Slow release | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.82] |
| 7 Oxytocin augmentation Show forest plot | 4 | 1916 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 8 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.80] |
| 9 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 10 Instrumental vaginal delivery Show forest plot | 4 | 1835 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.10] |
| 11 Meconium‐stained liquor Show forest plot | 3 | 699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.14] |
| 12 Apgar score < 7 at 5 minutes Show forest plot | 5 | 2212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.54, 2.07] |
| 13 Neonatal intensive care unit admission Show forest plot | 5 | 873 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.47, 1.46] |
| 14 Perinatal death Show forest plot | 2 | 1712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 15 Maternal side effects (all) Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [2.49, 3.20] |
| 16 Maternal side effects (nausea) Show forest plot | 3 | 1782 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.47, 4.05] |
| 17 Maternal side effects (headache) Show forest plot | 6 | 2085 | Risk Ratio (M‐H, Random, 95% CI) | 6.59 [3.97, 10.95] |
| 18 Maternal side effects (vomiting) Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.54, 3.81] |
| 19 Maternal side effects (diarrhoea) Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.95, 2.19] |
| 20 Postpartum haemorrhage Show forest plot | 2 | 1562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.90, 1.40] |
| 21 Women not satisfied Show forest plot | 1 | 1362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.82, 1.38] |
| 22 Additional induction agents used Show forest plot | 5 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.88] |
| 22.1 Standard release | 5 | 2077 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.58, 0.92] |
| 22.2 Slow release | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.62] |
| 3 Caesarean section Show forest plot | 8 | 1262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 3 | 557 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.73, 0.89] |
| 6 Oxytocin augmentation Show forest plot | 3 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.82, 1.03] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.80] |
| 8 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 9 Instrumental vaginal delivery Show forest plot | 3 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.28] |
| 10 Meconium‐stained liquor Show forest plot | 3 | 699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.14] |
| 11 Apgar score < 7 at 5 minutes Show forest plot | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.42, 2.98] |
| 12 Neonatal intensive care unit admission Show forest plot | 5 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.30] |
| 13 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 14 Maternal side effects (nausea) Show forest plot | 2 | 420 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.22, 3.50] |
| 15 Maternal side effects (headache) Show forest plot | 5 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.04 [5.13, 9.66] |
| 16 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.57, 2.40] |
| 17 Additional induction agents used Show forest plot | 2 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.57, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.62] |
| 3 Caesarean section Show forest plot | 3 | 754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.22] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.61, 0.85] |
| 6 Oxytocin augmentation Show forest plot | 2 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.05] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.80] |
| 8 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 9 Instrumental vaginal delivery Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 10 Meconium‐stained liquor Show forest plot | 2 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.67, 1.18] |
| 11 Apgar score < 7 at 5 minutes Show forest plot | 3 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.27, 2.59] |
| 12 Neonatal intensive care unit admission Show forest plot | 3 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.42, 1.84] |
| 13 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 14 Maternal side effects (nausea) Show forest plot | 2 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.82, 5.77] |
| 15 Maternal side effects (headache) Show forest plot | 3 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 9.27 [2.47, 34.73] |
| 16 Postpartum haemorrhage Show forest plot | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.55, 2.07] |
| 17 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section Show forest plot | 4 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.31] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 0.99] |
| 6 Oxytocin augmentation Show forest plot | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 7 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 8 Instrumental vaginal delivery Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.25] |
| 9 Meconium‐stained liquor Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.22] |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.50, 7.77] |
| 11 Neonatal intensive care unit admission Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.53, 1.80] |
| 12 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 13 Maternal side effects (nausea) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.73, 2.71] |
| 14 Maternal side effects (headache) Show forest plot | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 4.10 [1.97, 8.56] |
| 15 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section Show forest plot | 4 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.31] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 0.99] |
| 6 Oxytocin augmentation Show forest plot | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 7 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 8 Instrumental vaginal delivery Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.25] |
| 9 Meconium‐stained liquor Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.22] |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.50, 7.77] |
| 11 Neonatal intensive care unit admission Show forest plot | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.53, 1.80] |
| 12 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 13 Maternal side effects (nausea) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.73, 2.71] |
| 14 Maternal side effects (headache) Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.73 [2.56, 5.43] |
| 15 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Caesarean section Show forest plot | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.55] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.42, 1.44] |
| 5 Oxytocin augmentation Show forest plot | 1 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.30] |
| 6 Epidural analgesia Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 8 Meconium‐stained liquor Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.30] |
| 9 Apgar score < 7 at 5 minutes Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.67] |
| 10 Neonatal intensive care unit admission Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.58, 2.09] |
| 11 Perinatal death Show forest plot | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 12 Maternal side effects (nausea) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.73, 2.71] |
| 13 Maternal side effects (headache) Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [2.18, 4.82] |
| 14 Additional induction agents used Show forest plot | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 1.1 Isosorbide Mononitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 2.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 2.2 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| 3.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| 4 Caesarean section Show forest plot | 3 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| 4.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.59, 1.63] |
| 4.2 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 4.3 Isosorbide Dinitrate | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.06] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.32, 2.28] |
| 6.1 Isosorbide Dinitrate | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.32, 2.28] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 2 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 1.98] |
| 7.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 7.2 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 8 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 8.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 9 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 9.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 10 Maternal side effects (headache) Show forest plot | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.79 [5.75, 13.45] |
| 10.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.4 [0.65, 201.32] |
| 10.2 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 11.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 12 Serious maternal complications Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 12.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 13 Neonatal intensive care unit admission Show forest plot | 3 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.78] |
| 13.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 13.2 Isosorbide Dinitrate | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 1.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 1.2 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 2 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.82, 1.35] |
| 2.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.59, 1.63] |
| 2.2 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| 3.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.66] |
| 4 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 2 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 1.98] |
| 6.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 6.2 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 7 Neonatal intensive care unit admission Show forest plot | 2 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.78] |
| 7.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 7.2 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 8 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 9 Maternal side effects (headache) Show forest plot | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.79 [5.75, 13.45] |
| 9.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.4 [0.65, 201.32] |
| 9.2 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 10 Postpartum haemorrhage Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 10.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 11 Serious maternal complications Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 11.1 Glyceryl Trinitrate | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 2.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 3.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 4.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 5.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 6 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 6.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 7 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 7.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 8.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 1.1 Isosorbide Mononitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section Show forest plot | 2 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| 3.1 Isosorbide Mononitrate | 2 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| 4 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 6.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 7 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 7.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 8 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 9.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 2.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 3.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 4.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 5.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 6 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 6.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 7 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 7.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 8.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Isosorbide Mononitrate | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 2.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
| 3 Epidural analgesia Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 3.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 4 Instrumental vaginal delivery Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 4.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 5.1 Isosorbide Mononitrate | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.50] |
| 6 Neonatal intensive care unit admission Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 6.1 Isosorbide Mononitrate | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 7 Maternal side effects (nausea) Show forest plot | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 7.1 Isosorbide Mononitrate | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.10, 2.93] |
| 8 Maternal side effects (headache) Show forest plot | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| 8.1 Isosorbide Mononitrate | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [5.68, 13.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 1.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3 Caesarean section Show forest plot | 2 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.90] |
| 3.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.38, 0.89] |
| 3.2 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 6 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 9 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 10.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 1.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 6 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 9 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 10.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 1.1 Isosorbide dinitrate | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 6 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 9 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 10.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 1.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 2.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 1.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 2.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 1.1 Glyceryl Trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 2 Caesarean section Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 2.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.55] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 3.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 4.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.81] |
| 5 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 5.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.85] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 6.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.61] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 7.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 8 Perinatal death Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 8.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 9 Maternal side effects (headache) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| 9.1 Glyceryl trinitrate | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.40, 71.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| 1.1 Isosorbide mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 3 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.37] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2.2 Isosorbide Mononitrate | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.40] |
| 3 Caesarean section Show forest plot | 6 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.82, 1.21] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 3.2 Isosorbide Mononitrate | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.28] |
| 3.3 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.55] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [2.07, 5.66] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5.2 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [2.12, 6.75] |
| 6 Oxytocin augmentation Show forest plot | 7 | 767 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [1.31, 5.45] |
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.85] |
| 6.2 Isosorbide Mononitrate | 5 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 3.57 [1.84, 6.92] |
| 6.3 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.11, 1.52] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.32] |
| 7.1 Isosorbide Mononitrate | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.34] |
| 7.2 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.96] |
| 8 Epidural analgesia Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.31] |
| 8.1 Isosorbide Mononitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.31] |
| 9 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 9.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 9.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Meconium‐stained liquor Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.65] |
| 10.1 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.56] |
| 10.2 Isosorbide mononitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.15, 0.84] |
| 11 Apgar score < 7 at 5 minutes Show forest plot | 6 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.07, 0.38] |
| 11.1 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Isosorbide Mononitrate | 5 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.07, 0.38] |
| 12 Neonatal intensive care unit admission Show forest plot | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.43] |
| 12.1 Isosorbide Mononitrate | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.43] |
| 13 Perinatal death Show forest plot | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Isosorbide Mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal side effects (nausea) Show forest plot | 5 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.63, 2.17] |
| 14.1 Isosorbide Mononitrate | 5 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.63, 2.17] |
| 15 Maternal side effects (headache) Show forest plot | 4 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.98 [4.05, 29.73] |
| 15.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 15.2 Isosorbide Mononitrate | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.46 [2.69, 67.43] |
| 15.3 Isosorbide Dinitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 24.25 [1.47, 401.26] |
| 16 Postpartum haemorrhage Show forest plot | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.57, 3.06] |
| 16.1 Isosorbide Mononitrate | 4 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.57, 3.06] |
| 17 Analgesia requirement Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.49] |
| 17.1 Isosorbide Mononitrate | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.49] |
| 18 Additional induction agents required Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| 18.1 Isosorbide mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.67] |
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 1.2 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.94] |
| 2 Caesarean section Show forest plot | 3 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.66, 1.22] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide Mononitrate | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.27] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [2.07, 5.66] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 4.2 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [2.12, 6.75] |
| 5 Oxytocin augmentation Show forest plot | 4 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [1.23, 8.55] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide Mononitrate | 3 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 4.35 [1.32, 14.27] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.75] |
| 6.1 Isosorbide Mononitrate | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.75] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Apgar score < 7 at 5 minutes Show forest plot | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.46] |
| 8.1 Isosorbide Mononitrate | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.46] |
| 9 Neonatal intensive care unit admission Show forest plot | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.07, 0.53] |
| 9.1 Isosorbide Mononitrate | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.07, 0.53] |
| 10 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Isosorbide Mononitrate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal side effects (nausea) Show forest plot | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.69] |
| 11.1 Isosorbide Mononitrate | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.69] |
| 12 Maternal side effects (headache) Show forest plot | 3 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.01 [3.11, 26.06] |
| 12.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 12.2 Isosorbide Mononitrate | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.46 [2.69, 67.43] |
| 13 Postpartum haemorrhage Show forest plot | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.61] |
| 13.1 Isosorbide Mononitrate | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2 Caesarean section Show forest plot | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.14] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.18] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5 Oxytocin augmentation Show forest plot | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [2.29, 4.33] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide Mononitrate | 2 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [2.57, 5.18] |
| 6 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.54] |
| 7.1 Isosorbide Mononitrate | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.54] |
| 8 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal side effects (nausea) Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.31] |
| 9.1 Isosorbide Mononitrate | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.31] |
| 10 Maternal side effects (headache) Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.09 [2.90, 28.47] |
| 10.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 10.2 Isosorbide Mononitrate | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.0 [2.26, 113.12] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 11.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved in 24 hours Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| 1.1 Isosorbide Mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.62, 17.55] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 3 Caesarean section Show forest plot | 3 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.21] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 3.2 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.25] |
| 4 Serious neonatal morbidity/perinatal death Show forest plot | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Isosorbide Mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 6 Oxytocin augmentation Show forest plot | 3 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [2.27, 4.71] |
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 6.2 Isosorbide mononitrate | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [2.56, 5.83] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 8 Apgar score < 7 at 5 minutes Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.44] |
| 8.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.44] |
| 9 Neonatal intensive care unit admission Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.06, 0.42] |
| 9.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.06, 0.42] |
| 10 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal side effects (nausea) Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.89] |
| 11.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.89] |
| 12 Maternal side effects (headache) Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 12.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 13 Postpartum haemorrhage Show forest plot | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.50, 3.33] |
| 13.1 Isosorbide mononitrate | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.50, 3.33] |
| 14 Additional induction agents required Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| 14.1 Isosorbide mononitrate | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.67 [5.44, 51.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2 Caesarean section Show forest plot | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.14] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide monotrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.18] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5 Oxytocin augmentation Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [2.77, 6.93] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide mononitrate | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.36 [3.57, 11.33] |
| 6 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 7.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| 8.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| 9 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal side effects (nausea) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| 10.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| 11 Maternal side effects (headache) Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 11.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 12 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 12.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 1.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 2 Caesarean section Show forest plot | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.14] |
| 2.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 2.2 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.18] |
| 3 Serious neonatal morbidity/perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 4.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [0.89, 6.82] |
| 5 Oxytocin augmentation Show forest plot | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [2.77, 6.93] |
| 5.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.55, 2.85] |
| 5.2 Isosorbide mononitrate | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.36 [3.57, 11.33] |
| 6 Instrumental vaginal delivery Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 6.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.43] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 7.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| 8.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.60] |
| 9 Perinatal death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal side effects (nausea) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| 10.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.09, 1.06] |
| 11 Maternal side effects (headache) Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 11.1 Glyceryl Trinitrate | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [1.35, 22.17] |
| 12 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 12.1 Isosorbide mononitrate | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 2 Oxyocin augmentation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 3 Uterine rupture Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 9 Maternal side effects (nausea and vomiting) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 10 Maternal side effects (headache) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 12 Women not satisfied Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
| 13 Other maternal side effect (puerperal pyrexia) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |

