Ginkgo biloba para la claudicación intermitente
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: double blind randomised clinical trial. | |
| Participants | Country: Germany. | |
| Interventions | Treatment: 40 mg GbE three times daily. (Coated tablets containing 40 mg GbE. Manufacturer: Intersan, Institut für pharmazeutische und Klinische Forschung GmbH, Ettlingen, Germany) Control: placebo. Duration: 24 weeks. | |
| Outcomes | Walking distance: ACD and ICD by treadmill testing (3 km/h and 10% incline); subjective estimate of pain by a VAS; plethysmography; systolic ankle pressures at rest and after exercise; tolerance. | |
| Notes | In three other articles the clinical course is described of the treatment group after 1 year (Bauer 1986a), 2 years (Bauer 1986b) and 3 years (Bauer 1986c). However, the control group is not mentioned. Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were divided at random into 2 equal groups. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Unclear risk | The placebo group received matching placebo tablets which were taken in the same manner. |
| Methods | Study design: double blind randomised clinical trial. Method of randomisation: EPD number generator. | |
| Participants | Country: Germany. Setting: ambulant patient seeking consultation at a angiological practice. Number randomised: 60 (treatment group: 30, control group: 30). Age: aged between 47 and 82, median treatment group 71 (95% CI 65.2 to 74.8), median control group 68.6 (95% CI 65.5 to 73.2). Sex: 42 males and 18 females. Inclusion criteria: patients with PAD (stage IIb according to Fontaine) angiographically confirmed; stable walking distance in spite of constant walking training; ICD less than 150 metres. Exclusion criteria: age < 18 years; cardiac infarction during the preceding 6 months; NYHA stage III or IV cardiac insufficiency; diastolic pressure > 120 mmHg; renal insufficiency; functional hepatic disorders; respiratory insufficiency or orthopaedic disorder as limiting factor of the walking distance; venous insufficiency stage II (Basle Classification; badly manageable diabetes mellitus; anaemia; hematocrit > 48%; fibrinogen > 500 mg/dl; use of platelet aggregation inhibitor; anti‐inflammatory agents or analgesics; pregnancy or expected insufficient compliance. | |
| Interventions | Treatment: 40 mg Ginkgo biloba special extract GbE 761 three times daily. (40 mg film‐coated tablet containing dry extract from Ginkgo biloba leaves (50:1) 40 mg, adjusted at 9.6 mg Ginkgo flavone glycosides and 2.4 mg terpene lactones (ginkgolides, bilobalide). Manufacturer: Dr. Wilmar Schwabe Arzneimittel / Pharmaceuticals, Karlsruhe, Germany). Control: placebo. | |
| Outcomes | Walking distance: ACD and ICD by treadmill testing (3 km/h and 12% incline); ABI; subjective estimate of pain by a VAS; adverse drug reactions. | |
| Notes | Maximum level trained patients with IC. Funding: not stated. Conflicts of interests: One of the authors is employed by one of the manufacturers Dr. Willmar Schwabe, Karlsruhe, Germany. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned using an EPD random number generator was used for allocation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Low risk | There was an externally indistinguishable placebo and neither the patient nor the examiner or any other person directly involved in the study was able to see which group a patient belonged to. |
| Methods | Study design: double blind randomised clinical trial. Method of randomisation: not stated. | |
| Participants | Country: Germany. Setting: not stated. Number randomised: 41 (treatment group 21, control group 20). Age: mean treatment group 66.4, mean control group 68.2. Sex: treatment group 14 males and 7 females, control group 15 males and 4 females. Inclusion criteria: patients with PAD (stage IIb according to Fontaine) angiographically confirmed. PAD > 6 months present. Exclusion criteria: not stated. | |
| Interventions | Treatment: 80 mg Ginkgo biloba special extract EgB 761 two times daily. (80 mg film‐coated tablet containing dry extract from Ginkgo biloba leaves (35‐67:1) 80 mg, standardised at 19.2 mg Ginkgo flavone glycosides and 4.8 mg terpene lactones (ginkgolides, bilobalide). Control: placebo. Duration: 24 weeks. | |
| Outcomes | Walking distance: ACD and ICD by treadmill testing (3 km/h and 12% incline); tolerance of Ginkgo biloba special extract EgB 761. | |
| Notes | Funding: not stated. Conflicts of interests: One of the authors is employed by one of the manufacturers Dr. Willmar Schwabe, Karlsruhe, Germany. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information |
| Methods | Study design: double blind randomised clinical trial. Method of randomisation: not stated. | |
| Participants | Country: Germany. Setting: outpatient setting. Number randomised: 36, 33 were evaluated, treatment group 17, control group 16. Age: mean treatment group 62.8, mean control group 63.3. Sex: treatment group; 12 males and 5 females, control group; 12 males and 4 females. Inclusion criteria: patients with PAD (stage IIb according to Fontaine); ICD at least 50 metres and at most 200 metres. Exclusion criteria: venous complaints; anaemia; decompensated cardiac insufficiency; unstable angina pectoris; coronary heart disease; respiratory insufficiency; non‐controlled hypertension; recent myocardial infarction; badly adjustable diabetes; disease of liver and kidneys; orthopaedic reason for walking restriction. | |
| Interventions | Treatment: standardised Ginkgo biloba extract 761. (The drug extract ratio is 50:1, the standardised content of flavone glycosides is 24% (9.6 mg/40 mg extract), and that of terpene lactones 6% (2.4 mg/40 mg extract) Rökan®) Control: placebo. Duration: 24 weeks. | |
| Outcomes | Walking distance: ACD and ICD by treadmill testing (3 km/h and 10% incline); number of "tip‐toe" stands; systolic ankle pressure in the posterior tibial artery; plasma viscosity and haematocrit; adverse reactions. | |
| Notes | All patients received exercise therapy in combination with participation in this trial. Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Unclear risk | The placebo tablets had an identical appearance. |
| Methods | Study design: double blind randomised clinical trial. Method of randomisation: computerised randomisation program. | |
| Participants | Country: Germany. Setting: not stated. Number randomised: 40, treatment group 22, control group 18. Age: mean treatment group 62.2, mean control group 63.0. Sex: treatment group; 21 males and 1 female, control group; 17 males and 1 female. Inclusion criteria: Patients with PAD (stage IIb according to Fontaine); ICD of 50 to 200 m; age between 40 and 75 years; precisely reproducible pain localization under exercise; pressure in posterior tibial artery > 50 mmHg; ABI < 0.85; Broca deviation < 30 %; abstinence from nicotine. Exclusion criteria: myocardial infarction within half a year before enrolment; heart failure NYHA III or IV; severe chronic venous insufficiency; insufficiently controllable diabetes mellitus; malabsorption; liver disorder: transaminase levels above 3 times the upper limit of the normal range; renal disorder: serum creatinin level above 3.0 mg/dl; gait impairment due to orthopaedic or neurological diseases; respiratory insufficiency limiting walking distance; angina pectoris limiting walking distance; uncontrolled hypertension. | |
| Interventions | Treatment: 120 mg Ginkgo biloba special extract EgB 761 daily. Control: placebo. Duration: 12 weeks. | |
| Outcomes | Walking distance: ACD and ICD by treadmill testing (5 km/h and 10% incline); adverse reactions. | |
| Notes | Conflicts of interests: Internal rapport of Dr. Wilmar Schwabe Arzneimittel/Pharmaceuticals, Karlsruhe, Germany. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised with a computerised randomisation program. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Unclear risk | Tablets of identical appearance, packed and labelled in identical way. |
| Methods | Study design: double blind randomised cross‐over study. Method of randomisation: not stated. | |
| Participants | Country: Denmark. Setting: Not stated. Number randomised: 18. Age: 59 to 82 years (median age 74). Sex: not stated. Inclusion criteria: Stable form of IC more than 6 months; ACD 50 to 500 metres; ABI < 0.85. Exclusion criteria: Chronic obstructive pulmonary disease; diabetes mellitus; artrosis; angina pectoris; cardiac insufficiency. | |
| Interventions | Treatment: 120 mg GbE GB 8 daily. Control: placebo. Duration:6 months, 3 months treatment and 3 months placebo. | |
| Outcomes | Walking distance: ACD and ICD by treadmill testing (individual speed (2.5 to 4 km/h) and incline (8 to 16% incline); ABI; subjective estimate of pain by a VAS; cognitive functions: concentration and short term memory. | |
| Notes | Only the first study period (3 months) before the cross‐over is considered. Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information. |
| Methods | Study design: double blind randomised clinical trial. Method of randomisation: computerised randomisation program. | |
| Participants | Country: USA. Setting: patients recruited from the local community through advertisement. Number randomised: 62, treatment group 31, control group 31. Age: mean treatment group 69, mean control group 70. Sex: treatment group 13 males and 18 females, control group 24 males and 7 females. Inclusion criteria: Patients with a resting ABI < 0.90; ACD between 1 and 10 minutes; drop in ABI after exercise of at least 25%. (For diabetics all ABI values were allowed). Exclusion criteria: major surgery; chronic disease in the last three months; use of pentoxifylline, carnitine, arginine, prostacycline, dietary antioxidant supplements, supplements containing Ginkgo biloba. | |
| Interventions | Treatment: 300 mg standardised Ginkgo biloba extract 761 daily (180 mg with breakfast and 120 mg with dinner). (60 mg tablet standardised at 24% Ginkgo flavone glycosides and 6% terpene lactones. Manufacturer: Dr. Wilmar Schwabe Arzneimittel/Pharmaceuticals, Karlsruhe, Germany). Control: placebo. Duration: 4 months. | |
| Outcomes | ABI; walking distance: ACD and ICD by treadmill testing (3.2 km/h and 10% incline); flow‐mediated vasodilatation of the brachial artery; antibodies to epitopes of oxidized LDL; walking impairment questionnaire; QoL: MOS SF‐36 questionnaire. | |
| Notes | Funding: NIH grant R01 AT002004. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by generating random numbers assigned to treatment and control and assigning each new participant the next number in sequence. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Low risk | Subjects, study staff, and laboratory technicians were blinded to treatment assignments until the conclusions of the trial. |
| Methods | Study design: double blind randomised clinical trial. Method of randomisation: not stated | |
| Participants | Country: France. Setting: patients recruited from the outpatient clinic. Number randomised: 20. Age: not stated. Sex: not stated. Inclusion criteria: patients between the ages of 35 and 75 years suffering from IC (stage II according to Fontaine); diagnosed for more than 1 year; stable for three months; ACD on treadmill (3.2 km/h and 10% incline) > 100 metres and < 500 metres; transcutaneous partial pressure of oxygen in rest > 40 mmHg. Exclusion criteria: Patients with stage III and IV arterial disease; recent acute arterial occlusion; any form of peripheral circulatory insufficiency due to causes other than atherosclerosis; planned surgical revascularisation; decompensated heart failure; coronary insufficiency; poorly controlled hypertension; locomotor handicap. | |
| Interventions | Treatment: 160 mg EGb 761 two times daily. Control: placebo. Duration: 4 weeks. | |
| Outcomes | Transcutaneous partial pressure of oxygen in rest; transcutaneous partial pressure of oxygen after exercise; functional impairment by VAS. | |
| Notes | Although walking distances were not reported, functional impairment as measured by a VAS was used for the analysis. Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by random number generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information. |
| Methods | Study design: double blind randomised clinical trial. Method of randomisation: not stated. | |
| Participants | Country: France. Setting: outpatient setting. Number randomised: 18, treatment group 9, control group 9. Age: treatment group 60.8, control group 66.2. Sex: treatment group 7 males and 2 females, control group 8 males and 1 female. Inclusion criteria: Patients with PAD (stage IIb according to Fontaine); age 30 to 75 years; occlusive lesions of deep femoral arteries verified by arteriography; stable condition. Exclusion criteria: non co‐operative patient; significant psychiatric disorder; physical condition that does not allow exercise tests to be performed; any other ailment that could influence the course of the PAD or interfere with the assessment of treatment effects (e.g. Parkinson's disease, tumours); treatment of the same type as the product studied; treatment that could interfere with the assessment of treatment effects. | |
| Interventions | Treatment: 160 mg Ginkgo biloba special extract EgB 761 daily. Control: placebo. Duration: 6 months. | |
| Outcomes | Walking distance: ACD and ICD by treadmill testing (3 km/h and 5% incline); adverse reactions. | |
| Notes | Conflicts of interests: Internal rapport of Dr. Wilmar Schwabe Arzneimittel/Pharmaceuticals, Karlsruhe, Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Unclear risk | The intervention and the placebo were presented the same way. |
| Methods | Study design: double blind randomised clinical trial. Method of randomisation: assigned at random. | |
| Participants | Country: Germany. Setting: multicenter study. Number randomised: 109, treatment group 52, control group 59. Age: mean treatment group 62.8, mean control group 61.2. Sex: treatment group 29 males and 23 females, control group 34 males and 23 females. Inclusion criteria: Patients with PAD (stage IIb according to Fontaine) angiographically confirmed; older than 18 years; IC for more than 6 months; ICD < 150 metres. Exclusion criteria: severe impairment of heart, liver and/or kidney; walking limitation due to respiratory insufficiency or orthopaedic disorder; poorly controlled diabetes; pathologically altered hemorrheology; drugs for the same indication as the test substance; use of platelet aggregation inhibitors; anti‐inflammatories; or analgesics. | |
| Interventions | Treatment: 40 mg standardised Egb 761 three times daily. (1 film‐coated tablet contains 40 mg special extract EGb 761 from Ginkgo biloba leaves (35‐67:1), standardised to 9.6 mg (24%) of ginkgo flavone glycosieds and 2.4 (6%) to terpene lactones. Manufacturer: Dr. Wilmar Schwabe Arzneimittel/Pharmaceuticals, Karlsruhe, Germany). Control: placebo. Duration: 24 weeks. | |
| Outcomes | Walking distance: ACD and ICD by treadmill testing (3 km/h and 12% incline); ABI; subjective assessment of therapeutic outcome using VAS; tolerance. | |
| Notes | During the course of thee study, participation in a walking exercise program was offered for all patients. Funding: Not stated. Conflicts of interests: One of the authors is employed by one of the manufacturers Dr. Willmar Schwabe, Karlsruhe, Germany. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were assigned at random. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information. |
| Methods | Study design: double blind randomised cross‐over study. Method of randomisation: assigned at random. | |
| Participants | Country: Germany. Setting: not stated. Number randomised: 29, treatment group 13, control group 13. Age: mean age males 69.0, mean age females 64.4. Sex: 11 males and 18 females. Inclusion criteria: Patients with PAD (stage IIb according to Fontaine) in both legs. Exclusion criteria:not stated. | |
| Interventions | Treatment: 40 mg standardised Egb 761 two times daily 2 tablets. (Rökan®). Control: placebo. Duration: two times 6 week cross‐over study. | |
| Outcomes | Walking distance: ACD and ICD assessed by walking with 2 steps per second; Ratschow‐test; maximal time of toe‐standing; temperature of the skin; laboratory parameters. | |
| Notes | Only the first study period (6 weeks) before the cross‐over is considered. Funding: Not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned to the trial according to a random code van 1 to 30 in a consecutive way. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information. |
| Methods | Study design: double blind randomised clinical trial. Method of randomisation: not stated. | |
| Participants | Country: Germany. Setting: not stated. Number randomised: 205, treatment group 105, control group 100. Age: mean treatment group 64.3, mean control group 67.2. Sex: treatment group 80 males and 25 females, control group 77 males and 23 females. Inclusion criteria: Patients with PAD (stage IIb according to Fontaine). Exclusion criteria: not stated. | |
| Interventions | Treatment: 40 mg standardised GbE three times daily. (1 film‐coated tablet contains 40 mg dry extract from Ginkgo biloba leaves (50:1), standardised to 9.6 mg of ginkgo flavone glycosieds and 2.4 to terpene lactones. (ginkgolides, bilobalide). Manufacturer: not stated). Control: placebo. Duration: 24 weeks. | |
| Outcomes | Walking distance: ICD by treadmill testing (3 km/h and 12% incline); tolerance. | |
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information. |
| Methods | Study design: double blind randomised clinical trial. Method of randomisation: randomly allocated. | |
| Participants | Country: United Kingdom. Setting: not stated. Number randomised: 49, treatment group 25, control group 24. Age: not stated. Sex: not stated. Inclusion criteria: Patients with PAD (stage IIb according to Fontaine) affecting the iliac or femoral arteries, involving predominantly one leg. Exclusion criteria: ICD > 300 metres; alternating side of pain; poorly controlled diabetes; significant concomitant illness. | |
| Interventions | Treatment: Ginkgo biloba extract (Tanakan®). Control: placebo. Duration: 24 weeks. | |
| Outcomes | Walking distance: ICD by treadmill testing (4 km/h and 10 degrees (= 17.6% incline)); recovery times; systolic ankle pressure at rest and after exercise; tolerance. | |
| Notes | Funding: The work was supported and assisted by Ipsen International (one of the manufacturers). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) | Unclear risk | Randomisation to Ginkgo biloba or placebo was made in a double blind manner. |
| Methods | Study design: double blind randomised clinical trial. Method of randomisation: randomly allocated. | |
| Participants | Country: Australia. Setting: not stated. Number randomised: 22, treatment group 11, control group 11. Age: mean treatment group 71.6, mean control group 71.2. Sex: treatment group 9 males and 2 females, control group 11 males. Inclusion criteria: Patients with stable IC for at least 6 months; 50‐80 years of age; ABI at rest < 0.90 and after exercise < 0.80. Exclusion criteria: resting ischaemic pain; ulceration; gangrene; unable to walk on treadmill with 3.2 km/h, exercise capacity limited by angina; congestive heart failure; chronic obstructive pulmonary disease or arthritis. | |
| Interventions | Treatment: 240 mg standardised GbE 761 daily. (The Ginkgo biloba tablets were standardized to 26.7% ginkgo flavone glycosides and 6.7% terpenoids (ginkgolides or bilobalide). Manufacturer: not stated). Control: placebo. Duration: 12 weeks. | |
| Outcomes | Walking distance: ACD and ICD by treadmill testing (3.2 km/h and 0% incline); ABI in rest and after exercise; plasma viscosity; whole blood viscosity; peak VO2; walking economy. | |
| Notes | Only the first 12 weeks of the study in which Ginkgo biloba treatment was compared with placebo is considered. The second part was a 12‐week supervised treadmill walking programme, while the subjects continued taking the same dosage of Ginkgo biloba or placebo. Funding: The Ginkgo biloba and placebo tablets were kindly donated by Mayne Health Consumer Products, Australia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were randomly allocated following a randomisation code. The code was created by a computerised program. |
| Allocation concealment (selection bias) | Low risk | The code was administered by a third party who was not involved in the study. |
| Blinding (performance bias and detection bias) | Low risk | The code was revealed to the subject and the researcher after the database of the study was completed. |
ABI: ankle brachial index
ACD: absolute claudication distance
GbE: Ginkgo biloba extract
ICD: initial claudication distance
LDL: low density lipoprotein
PAD: peripheral arterial disease
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Separate data for patients diagnosed with PAD stage II according to Fontaine were not available. | |
| This study compares Ginkgo biloba extract with dextran in patients with PAD stage IV according to Fontaine. | |
| Ginkgo biloba extract is compared with buflomedil. | |
| (According to Fontaine), diagnosed for more than 1 year. | |
| Separate data for patients diagnosed with PAD stage II according to Fontaine were not available. | |
| Separate data for patients diagnosed with PAD stage II according to Fontaine were not available. | |
| Separate data for patients diagnosed with PAD stage II according to Fontaine were not available. | |
| Ginkgo biloba extract is compared with naftidrofuryl in patients with PAD stage III and IV according to Fontaine. | |
| Primary aim of this study was to compare diabetic and non‐diabetic PAD patients. | |
| This study compares the combination of Ginkgo biloba extract, magnesium and Levo‐arginine with acetylsalicyl acid. | |
| Double blind randomised cross‐over study evaluating the short term effect (60 minutes) of Ginkgo biloba extract versus placebo in patients with PAD stage II according to Fontaine. | |
| This study only included patients diagnosed with PAD stage III according to Fontaine. | |
| This study compares two dosages of Ginkgo biloba extract without a placebo group. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Absolute claudication distance (expressed as kilocalories) at the end of the study Show forest plot | 11 | 477 | Mean Difference (IV, Random, 95% CI) | 3.57 [‐0.10, 7.23] |
| Analysis 1.1  Comparison 1 Ginkgo biloba versus placebo, Outcome 1 Absolute claudication distance (expressed as kilocalories) at the end of the study. | ||||
| 2 Absolute claudication distance (expressed as kilocalories) after 24 weeks Show forest plot | 6 | 321 | Mean Difference (IV, Random, 95% CI) | 4.72 [2.27, 7.16] |
| Analysis 1.2  Comparison 1 Ginkgo biloba versus placebo, Outcome 2 Absolute claudication distance (expressed as kilocalories) after 24 weeks. | ||||
| 3 Absolute claudication distance (expressed as kilocalories) after 12 to 16 weeks Show forest plot | 8 | 339 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐2.63, 5.36] |
| Analysis 1.3 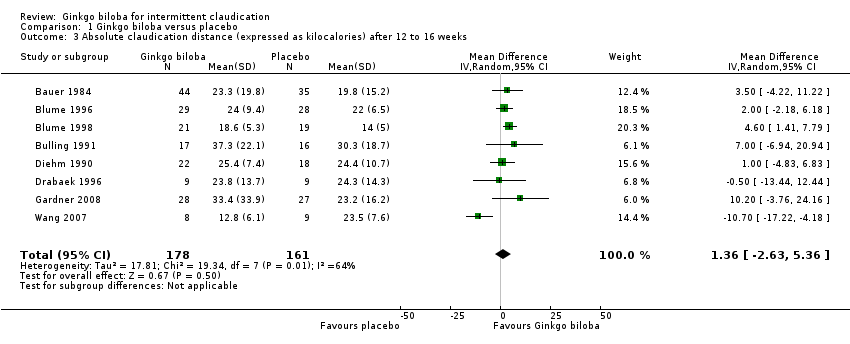 Comparison 1 Ginkgo biloba versus placebo, Outcome 3 Absolute claudication distance (expressed as kilocalories) after 12 to 16 weeks. | ||||
| 4 Absolute claudication distance (expressed as kilocalories) after 6 to 8 weeks Show forest plot | 5 | 236 | Mean Difference (IV, Random, 95% CI) | 2.19 [‐0.62, 4.99] |
| Analysis 1.4 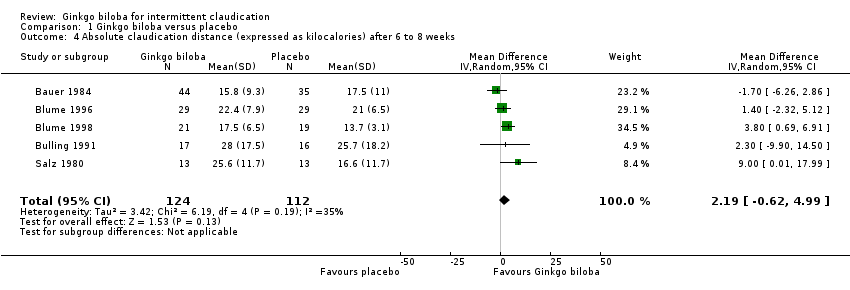 Comparison 1 Ginkgo biloba versus placebo, Outcome 4 Absolute claudication distance (expressed as kilocalories) after 6 to 8 weeks. | ||||
| 5 Initial claudication distance (expressed as kilocalories) at the end of the study Show forest plot | 13 | 723 | Mean Difference (IV, Random, 95% CI) | 1.84 [‐0.92, 4.61] |
| Analysis 1.5  Comparison 1 Ginkgo biloba versus placebo, Outcome 5 Initial claudication distance (expressed as kilocalories) at the end of the study. | ||||
| 6 Initial claudication distance (expressed as kilocalories) after 24 weeks Show forest plot | 8 | 564 | Mean Difference (IV, Random, 95% CI) | 2.15 [‐2.06, 6.36] |
| Analysis 1.6 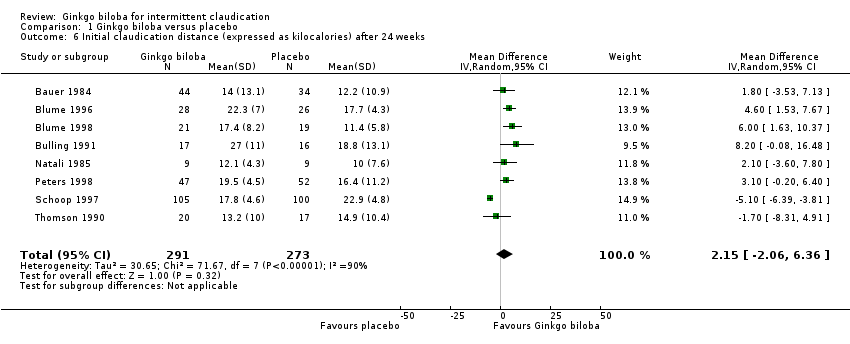 Comparison 1 Ginkgo biloba versus placebo, Outcome 6 Initial claudication distance (expressed as kilocalories) after 24 weeks. | ||||
| 7 Initial claudication distance (expressed as kilocalories) after 12 to 16 weeks Show forest plot | 9 | 441 | Mean Difference (IV, Random, 95% CI) | 1.54 [‐0.04, 3.12] |
| Analysis 1.7  Comparison 1 Ginkgo biloba versus placebo, Outcome 7 Initial claudication distance (expressed as kilocalories) after 12 to 16 weeks. | ||||
| 8 Initial claudication distance (expresses as kilocalories) after 6 to 8 weeks Show forest plot | 6 | 335 | Mean Difference (IV, Random, 95% CI) | 2.72 [0.75, 4.69] |
| Analysis 1.8 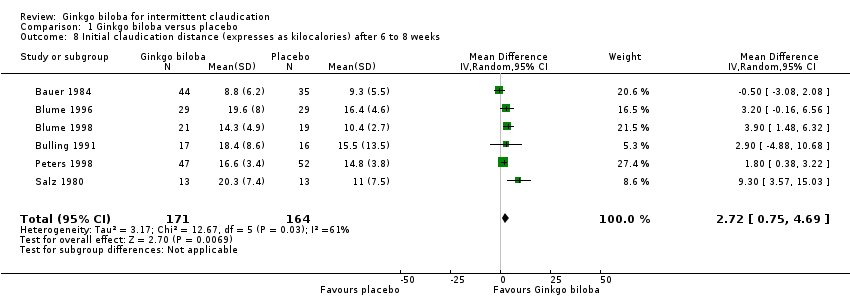 Comparison 1 Ginkgo biloba versus placebo, Outcome 8 Initial claudication distance (expresses as kilocalories) after 6 to 8 weeks. | ||||
| 9 Ankle brachial index and ankle pressure at the end of study Show forest plot | 6 | 274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.58, 0.15] |
| Analysis 1.9 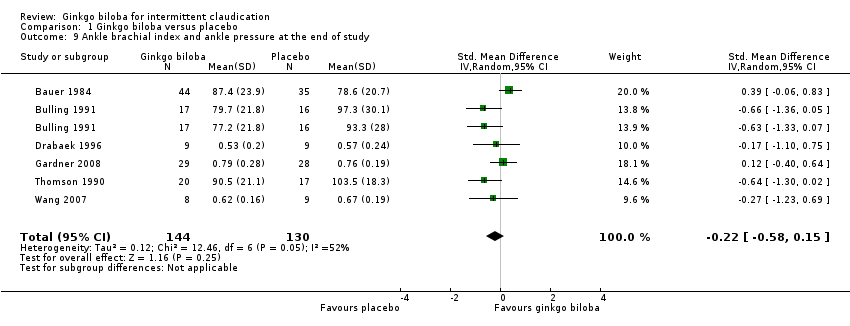 Comparison 1 Ginkgo biloba versus placebo, Outcome 9 Ankle brachial index and ankle pressure at the end of study. | ||||
| 10 Quality of life (expressed as a Visual Analoque Scale for complaints) Show forest plot | 5 | 208 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.94, 1.70] |
| Analysis 1.10 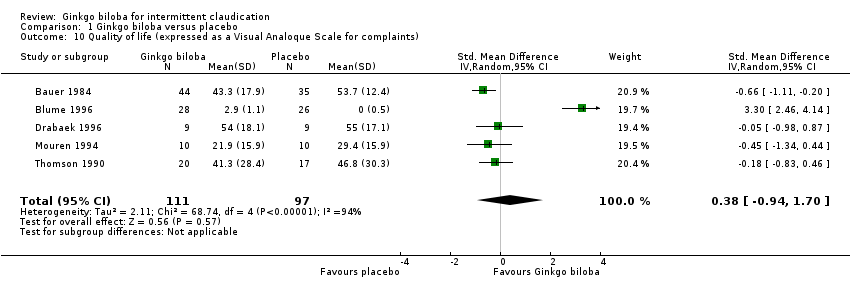 Comparison 1 Ginkgo biloba versus placebo, Outcome 10 Quality of life (expressed as a Visual Analoque Scale for complaints). | ||||
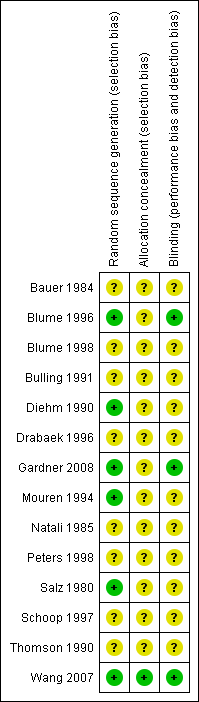
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
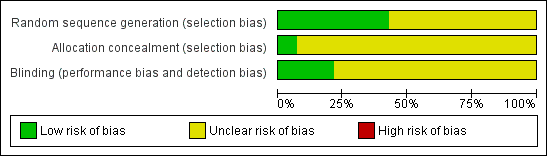
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
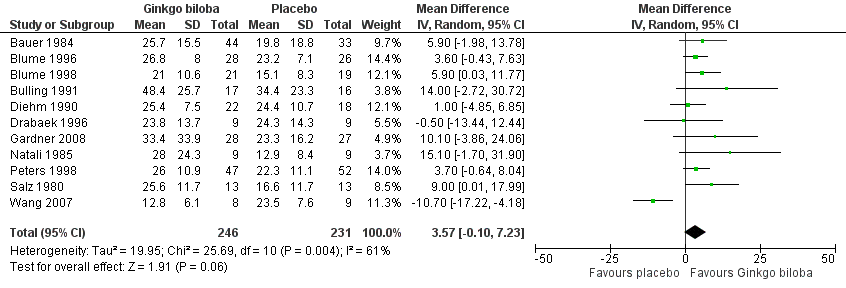
Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.1 Absolute claudication distance (expressed as kilocalories) at the end of the study.
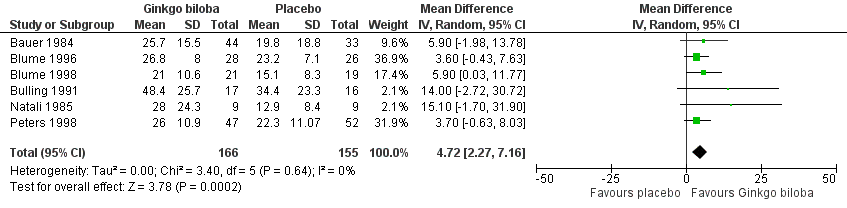
Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.2 Absolute claudication distance (expressed as kilocalories) after 24 weeks.
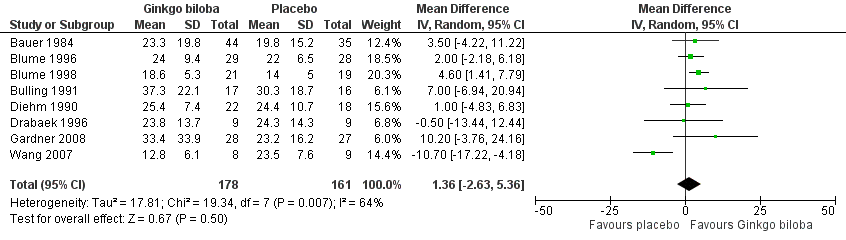
Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.3 Absolute claudication distance (expressed as kilocalories) after 12 to 16 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.4 Absolute claudication distance (expressed as kilocalories) after 6 to 8 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.5 Initial claudication distance (expressed as kilocalories) at the end of the study.
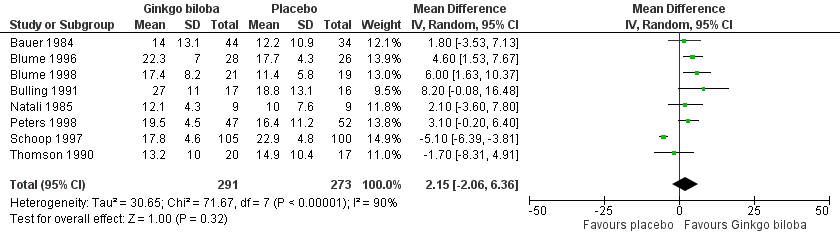
Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.6 Initial claudication distance (expressed as kilocalories) after 24 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.7 Initial claudication distance (expressed as kilocalories) after 12 to 16 weeks.
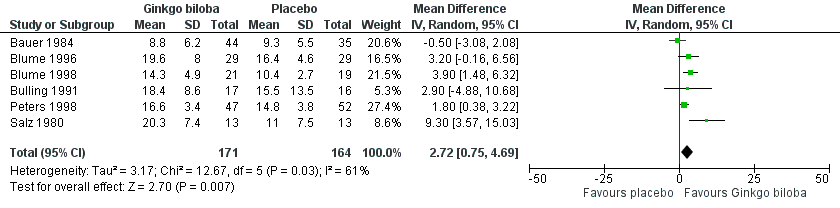
Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.8 Initial claudication distance (expresses as kilocalories) after 6 to 8 weeks.
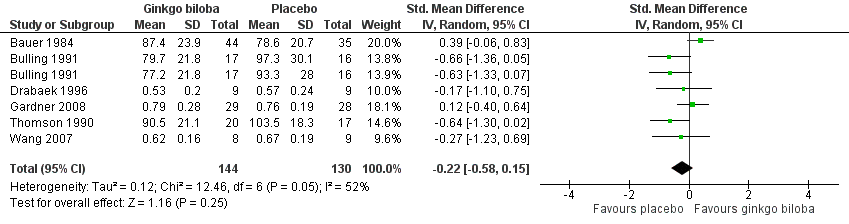
Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.9 Ankle brachial index and ankle pressure at the end of study.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.10 Quality of life (expressed as a Visual Analogue Scale for complaints).

Funnel plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.1 Absolute claudication distance (expressed as kilocalories) at the end of the study. On the horizontal axis the effect estimate of Ginkgo biloba versus placebo on the absolute claudication distance at the end of the study is presented as the mean difference. The vertical axis presents the standard error of the intervention effect on a reversed scale.

Funnel plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.2 Absolute claudication distance (expressed as kilocalories) after 24 weeks. On the horizontal axis the effect estimate of Ginkgo biloba versus placebo on the absolute claudication distance after 24 weeks is presented as the mean difference. The vertical axis presents the standard error of the intervention effect on a reversed scale.

Comparison 1 Ginkgo biloba versus placebo, Outcome 1 Absolute claudication distance (expressed as kilocalories) at the end of the study.

Comparison 1 Ginkgo biloba versus placebo, Outcome 2 Absolute claudication distance (expressed as kilocalories) after 24 weeks.

Comparison 1 Ginkgo biloba versus placebo, Outcome 3 Absolute claudication distance (expressed as kilocalories) after 12 to 16 weeks.
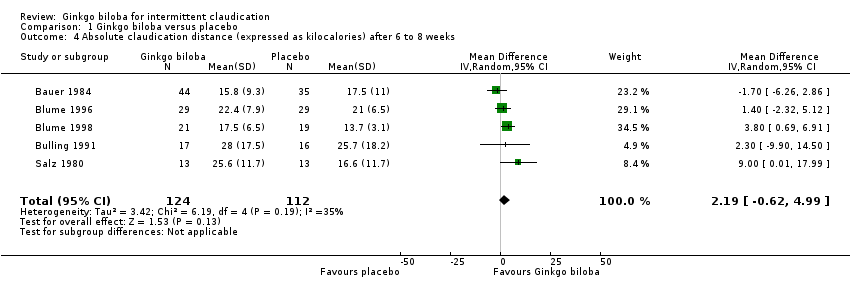
Comparison 1 Ginkgo biloba versus placebo, Outcome 4 Absolute claudication distance (expressed as kilocalories) after 6 to 8 weeks.

Comparison 1 Ginkgo biloba versus placebo, Outcome 5 Initial claudication distance (expressed as kilocalories) at the end of the study.

Comparison 1 Ginkgo biloba versus placebo, Outcome 6 Initial claudication distance (expressed as kilocalories) after 24 weeks.

Comparison 1 Ginkgo biloba versus placebo, Outcome 7 Initial claudication distance (expressed as kilocalories) after 12 to 16 weeks.
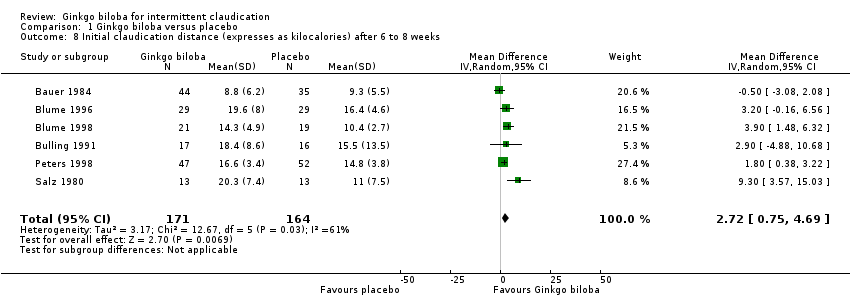
Comparison 1 Ginkgo biloba versus placebo, Outcome 8 Initial claudication distance (expresses as kilocalories) after 6 to 8 weeks.

Comparison 1 Ginkgo biloba versus placebo, Outcome 9 Ankle brachial index and ankle pressure at the end of study.

Comparison 1 Ginkgo biloba versus placebo, Outcome 10 Quality of life (expressed as a Visual Analoque Scale for complaints).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Absolute claudication distance (expressed as kilocalories) at the end of the study Show forest plot | 11 | 477 | Mean Difference (IV, Random, 95% CI) | 3.57 [‐0.10, 7.23] |
| 2 Absolute claudication distance (expressed as kilocalories) after 24 weeks Show forest plot | 6 | 321 | Mean Difference (IV, Random, 95% CI) | 4.72 [2.27, 7.16] |
| 3 Absolute claudication distance (expressed as kilocalories) after 12 to 16 weeks Show forest plot | 8 | 339 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐2.63, 5.36] |
| 4 Absolute claudication distance (expressed as kilocalories) after 6 to 8 weeks Show forest plot | 5 | 236 | Mean Difference (IV, Random, 95% CI) | 2.19 [‐0.62, 4.99] |
| 5 Initial claudication distance (expressed as kilocalories) at the end of the study Show forest plot | 13 | 723 | Mean Difference (IV, Random, 95% CI) | 1.84 [‐0.92, 4.61] |
| 6 Initial claudication distance (expressed as kilocalories) after 24 weeks Show forest plot | 8 | 564 | Mean Difference (IV, Random, 95% CI) | 2.15 [‐2.06, 6.36] |
| 7 Initial claudication distance (expressed as kilocalories) after 12 to 16 weeks Show forest plot | 9 | 441 | Mean Difference (IV, Random, 95% CI) | 1.54 [‐0.04, 3.12] |
| 8 Initial claudication distance (expresses as kilocalories) after 6 to 8 weeks Show forest plot | 6 | 335 | Mean Difference (IV, Random, 95% CI) | 2.72 [0.75, 4.69] |
| 9 Ankle brachial index and ankle pressure at the end of study Show forest plot | 6 | 274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.58, 0.15] |
| 10 Quality of life (expressed as a Visual Analoque Scale for complaints) Show forest plot | 5 | 208 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.94, 1.70] |

