Intervenciones para la prevención de la recurrencia posoperatoria de la enfermedad de Crohn
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Open label prospective randomised controlled trial | |
| Participants | 140 patients randomised following small intestinal resection or stricturoplasty | |
| Interventions | Azathioprine 2mg/kg/day or Mesalamine 3g/day for 24 months | |
| Outcomes | Clinical or surgical recurrence at 24 months | |
| Notes | Jadad score 2 Inadequate description of use of placebo controls. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | High risk | Open label |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Randomised double blind placebo controlled trial | |
| Participants | 87 patients randomised following curative ileal or ileo‐cecal reection | |
| Interventions | Mesalamine (Pentasa®) 3g/day or placebo for 12 months | |
| Outcomes | Clinical recurrence and severe endoscopic recurrence at 12 months | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Prospective multi‐center randomised placebo controlledtrial | |
| Participants | 206 patients randomised following ileal or ileo‐colonic resection | |
| Interventions | Eudragit‐S‐coated mesalamine (Asacol®) 4g/day or mesalamine 2.4g/day with placebo for 12 months | |
| Outcomes | Clinical (CDAI>150) and endoscopic recurrence at 12 months | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Multicenter randomised placebo controlled trial | |
| Participants | 30 patients randomised in a 2 to 1 ratio | |
| Interventions | Synbiotic 2000 or placebo daily for up to 24 months | |
| Outcomes | Clinical and endoscopic recurrence | |
| Notes | Jadad score 3. Early termination of recruitment. Incomplete reporting of results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | High risk | Early termination of recruitment. Incomplete reporting of results |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Multicenter randomised double blind placebo controlled trial | |
| Participants | 65 patients randomised following first ileal or ileocolonic resection | |
| Interventions | Tenovil (IL‐10) 4 or 8 microg/kg or placebo for 3 months | |
| Outcomes | Clinical, endoscopic and histologic recurrence at 3 months | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Multicenter randomised placebo controlled trial | |
| Participants | 81 patients randomised following ileal or ileocolonic resection | |
| Interventions | Azathioprine 100mgs (<60kgs) or 150mgs (>60kgs) or placebo for 12 months with metronidazole 750mg/day for 3 months (both arms) | |
| Outcomes | Clinical (CDAI>250) and endoscopic recurrence (Rutgeerts score>/=2) at 3 and 12 months | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Multi‐center randomised placebo controlled trial | |
| Participants | 232 patients randomised following either radical or non‐radical resection for Crohn's disease | |
| Interventions | Sulphasalazine 3g/day or placebo for 3 years | |
| Outcomes | Recurrence of Crohn's disease proven by either radiology, endoscopy or further surgery | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | High cumulative drop‐out rate but withdrawals and drop‐outs well described |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | randomised multi‐center double‐blind placebo controlled trial | |
| Participants | 83 patients randomised following ileal, ileo‐colonic or colonic resection for Crohn's disease | |
| Interventions | Budesonide 3mg/day or placebo for 12 months | |
| Outcomes | Clinical, endoscopic and histologic recurrence at 12 months | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Double‐blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | randomised placebo controlled trial | |
| Participants | 48 patients with Crohn's disease who underwent intestinal resection | |
| Interventions | Eudragid‐L‐coated 5‐aminosalicylic acid 1.5g/day or placebo for 12 months | |
| Outcomes | Clinical and endoscopic recurrence | |
| Notes | Jadad score 2 Abstract only available for review Only preliminary results only are reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Unclear risk | Not described |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The abstract includes all expected outcomes |
| Methods | Multi‐center randomised double‐blind placebo controlled trial | |
| Participants | 126 patients randomised following curative ileal/colonic resection | |
| Interventions | Eudragit‐L mesalamine (Claversal®) 3g/day or placebo for 12 weeks | |
| Outcomes | Endoscopic recurrence at 12 weeks | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was carried out with a permutation table at each center |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Multi‐center randomised double‐blind double dummy placebo controlled trial | |
| Participants | 131 patients randomised following ileocolic resection and anastomosis | |
| Interventions | 6‐mercaptopurine 50mg/day or mesalamine 3g/day or placebo for 24 months | |
| Outcomes | Clinical, endoscopic and radiologic recurrence at 24 months | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Unclear risk | Manuscript reports percentages without absolute numbers associated |
| Methods | randomised, multi‐center, double‐blind, placebo controlled trial | |
| Participants | 130 patients randomised following ileocolonic resection for Crohn's disease | |
| Interventions | Budesonide (controlled ileal release) 6mg/day or placebo for 12 months | |
| Outcomes | Endoscopic recurrence and CDAI scores to 12 months. | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Double dummy, double blind, randomised placebo controlled trial | |
| Participants | 79 patients with randomised within two weeks of intestinal resection for Crohn's disease | |
| Interventions | Azathioprine 2‐2.5mg/kg or 5‐ASA (Salofalk®, Eudragit‐L mesalamine) 4g daily | |
| Outcomes | Clinical and endoscopic recurrence. Withdrawal due to clinical relapse or adverse drug reaction. | |
| Notes | Jadad score 2. Trial stopped early due to inadequate sample size Unpublished data from Peyrin‐Birloulet, 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Unclear risk | Trial stopped early due to inadequate sample size |
| Methods | randomised, multi‐center, double‐blind, placebo controlled trial | |
| Participants | 324 patients randomised following intestinal resection for Crohn's disease | |
| Interventions | Mesalamine (Pentasa®) 4g/day or placebo for 18 months | |
| Outcomes | Clinical or endoscopic recurrence at 18 months | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Multi‐center randomised double‐blind placebo controlled trial | |
| Participants | 120 patients randomised following ileocolonic resection | |
| Interventions | VSL#3 or placebo for 3 months | |
| Outcomes | Endoscopic recurrence at 3 months | |
| Notes | Jadad score 2 Results only published in abstract form to date | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | High risk | No description of dropouts or withdrawals |
| Free of selective reporting? | Unclear risk | Pre‐specified outcomes were reported but some other post‐hoc outcomes are reported too |
| Methods | Multi‐center randomised double‐blind placebo controlled trial | |
| Participants | 98 patients randomised following CD resection | |
| Interventions | LA1 twice daily or placebo for 6 months | |
| Outcomes | Clinical and endoscopic recurrence at 6 months | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | randomised, multi‐center, double‐blind, placebo controlled trial | |
| Participants | 163 patients randomised following intestinal resection for Crohn's disease | |
| Interventions | Eudragit‐L mesalamine 3g/day or placebo for up to 72 months | |
| Outcomes | Primary outcome ‐ symptomatic (clinical recurrence) recurrence within follow up | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Prospective comparative study | |
| Participants | 39 patients following ileal or ileocecal resection | |
| Interventions | Azathioprine 50mg/day or Mesalamine 3g/day for 24 months | |
| Outcomes | Clinical or morphologic (radiologic/endoscopic) or serologic recurrence up to 24 months | |
| Notes | Randomization and placebo control unclear. Jadad score 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Unclear risk | Not described |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Unclear risk | Not described |
| Methods | randomised, double ‐blind, placebo controlled trial | |
| Participants | 45 patients randomised following resection for Crohn's | |
| Interventions | Lactobacillus GG (6 billion CFU) or placebo for 52 weeks | |
| Outcomes | Clinical or endoscopic recurrence at 52 weeks | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Single centre randomised, double‐blind, placebo controlled trial | |
| Participants | 24 adult patient undergoing resection and anastomosis for ileal or ileo‐colonic Crohn's disease | |
| Interventions | Infliximab infusion (5mg/kg) or placebo at 0,2 and 6 weeks and subsequently at 8 week intervals for 54 weeks | |
| Outcomes | Endoscopic recurrence (primary outcome, Rutgeerts score I2 or greater) and clinical (CDAI>200), histologic and biochemical activity scores (secondary outcomes) at 1 year | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Patients were randomised in blocks |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | randomised, double ‐blind, placebo controlled trial | |
| Participants | 60 patients randomised undergoing first ileocolonic resection for ileal Crohn's | |
| Interventions | Metronidazole 20mg/kg/day or placebo for 3 months | |
| Outcomes | Clinical (symptomatic), endoscopic or histologic recurrence in follow up to 3 years | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | randomised, double ‐blind, placebo controlled trial | |
| Participants | 80 patients randomised undergoing ileocolonic resection for ileal or ileocolonic Crohn's | |
| Interventions | Ornidazole 1g/day or placebo for 54 weeks | |
| Outcomes | Clinical (symptomatic with CDAI>250), endoscopic or histologic recurrence at 3 months and 1 year | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
| Methods | Multi‐center randomised double‐blind placebo controlled trial | |
| Participants | 70 patients randomised following ileocecal resection | |
| Interventions | LA1 or placebo for 3 months | |
| Outcomes | Clinical, endoscopic and histologic recurrence at 3 months | |
| Notes | Jadad score 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Centralized randomisation |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Double blind |
| Incomplete outcome data addressed? | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Free of selective reporting? | Low risk | The published report includes all expected outcomes |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Treatment, not prevention study | |
| Not a randomised trial | |
| Non‐medical intervention | |
| Treatment, not prevention study | |
| Not a randomised trial | |
| Not a randomised trial | |
| Absence of placebo control. No blinding to treatment allocation possible. | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Non‐medical intervention | |
| Absence of placebo control. No blinding to treatment allocation possible. | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Treatment, not prevention study | |
| Treatment, not prevention study | |
| Not a randomised trial | |
| Not a randomised trial | |
| Non‐medical intervention | |
| Not a randomised trial | |
| Duplicate | |
| Duplicate | |
| Duplicate | |
| Duplicate | |
| Non‐medical intervention | |
| Treatment, not prevention study | |
| Not a randomised trial | |
| Treatment, not prevention study | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Non‐medical intervention | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Duplicate | |
| Non‐medical intervention | |
| Not a randomised trial | |
| Treatment, not prevention study | |
| Treatment, not prevention study | |
| Not a randomised trial | |
| Treatment, not prevention study | |
| Not a randomised trial | |
| Treatment, not prevention study | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Treatment, not prevention study | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Treatment, not prevention study | |
| Non‐randomised intervention | |
| Non‐randomised intervention | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial | |
| Non‐medical intervention | |
| Not a randomised trial | |
| Not a randomised trial | |
| Not a randomised trial |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Recurrence Show forest plot | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.59, 3.36] |
| Analysis 1.1  Comparison 1 Probiotics vs Placebo, Outcome 1 Clinical Recurrence. | ||||
| 2 Any Endoscopic Recurrence Show forest plot | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.29] |
| Analysis 1.2  Comparison 1 Probiotics vs Placebo, Outcome 2 Any Endoscopic Recurrence. | ||||
| 3 Severe Endoscopic Recurrence Show forest plot | 4 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.58, 1.58] |
| Analysis 1.3  Comparison 1 Probiotics vs Placebo, Outcome 3 Severe Endoscopic Recurrence. | ||||
| 4 Patient Withdrawal Show forest plot | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.65, 1.75] |
| Analysis 1.4  Comparison 1 Probiotics vs Placebo, Outcome 4 Patient Withdrawal. | ||||
| 5 Serious Adverse Events Show forest plot | 3 | 213 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.35, 1.52] |
| Analysis 1.5  Comparison 1 Probiotics vs Placebo, Outcome 5 Serious Adverse Events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Recurrence (12 months) Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.09, 0.57] |
| Analysis 2.1  Comparison 2 Nitroimidazole vs Placebo, Outcome 1 Clinical Recurrence (12 months). | ||||
| 2 Severe (score >/=2) Endoscopic Recurrence (3months) Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.26, 0.74] |
| Analysis 2.2  Comparison 2 Nitroimidazole vs Placebo, Outcome 2 Severe (score >/=2) Endoscopic Recurrence (3months). | ||||
| 3 Patient Withdrawal Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [1.37, 6.58] |
| Analysis 2.3  Comparison 2 Nitroimidazole vs Placebo, Outcome 3 Patient Withdrawal. | ||||
| 4 Serious Adverse Events Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.54, 3.70] |
| Analysis 2.4  Comparison 2 Nitroimidazole vs Placebo, Outcome 4 Serious Adverse Events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Recurrence (at study completion) Show forest plot | 4 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.62, 0.94] |
| Analysis 3.1  Comparison 3 5‐ASA vs Placebo, Outcome 1 Clinical Recurrence (at study completion). | ||||
| 2 Any Endoscopic Recurrence (at study completion) Show forest plot | 4 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.76, 1.13] |
| Analysis 3.2  Comparison 3 5‐ASA vs Placebo, Outcome 2 Any Endoscopic Recurrence (at study completion). | ||||
| 3 Severe (score>/=3) Endoscopic Recurrence (at study completion) Show forest plot | 3 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.29, 0.84] |
| Analysis 3.3  Comparison 3 5‐ASA vs Placebo, Outcome 3 Severe (score>/=3) Endoscopic Recurrence (at study completion). | ||||
| 4 Patient Withdrawal Show forest plot | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.89, 1.39] |
| Analysis 3.4  Comparison 3 5‐ASA vs Placebo, Outcome 4 Patient Withdrawal. | ||||
| 5 Serious Adverse Events Show forest plot | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.62, 1.66] |
| Analysis 3.5  Comparison 3 5‐ASA vs Placebo, Outcome 5 Serious Adverse Events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
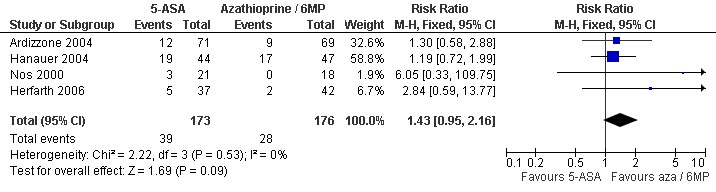
| 1 Clinical Recurrence within 12 months Show forest plot | 4 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.95, 2.16] |
| Analysis 4.1  Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 1 Clinical Recurrence within 12 months. | ||||
| 2 Clinical recurrence within 24 months Show forest plot | 3 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.95, 1.81] |
| Analysis 4.2  Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 2 Clinical recurrence within 24 months. | ||||
| 3 Any endoscopic recurrence (score >/=1) within 12 months Show forest plot | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.03, 2.06] |
| Analysis 4.3  Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 3 Any endoscopic recurrence (score >/=1) within 12 months. | ||||
| 4 Severe endoscopic recurrence (score >/=2) within 12 months Show forest plot | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.94, 2.29] |
| Analysis 4.4  Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 4 Severe endoscopic recurrence (score >/=2) within 12 months. | ||||
| 5 Very Severe endoscopic recurrence (score >/=3) within 12 months Show forest plot | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.63, 3.79] |
| Analysis 4.5  Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 5 Very Severe endoscopic recurrence (score >/=3) within 12 months. | ||||
| 6 Patient Withdrawal Show forest plot | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.26] |
| Analysis 4.6  Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 6 Patient Withdrawal. | ||||
| 7 Serious Adverse Events Show forest plot | 4 | 347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.30, 0.89] |
| Analysis 4.7  Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 7 Serious Adverse Events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Recurrence at 12 months Show forest plot | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.92] |
| Analysis 5.1  Comparison 5 Azathioprine/6MP vs Placebo, Outcome 1 Clinical Recurrence at 12 months. | ||||
| 2 Severe Endoscopic Recurrence (score>/=2) at 12 months Show forest plot | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.92] |
| Analysis 5.2  Comparison 5 Azathioprine/6MP vs Placebo, Outcome 2 Severe Endoscopic Recurrence (score>/=2) at 12 months. | ||||
| 3 Patient Withdrawal Show forest plot | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.51, 1.00] |
| Analysis 5.3  Comparison 5 Azathioprine/6MP vs Placebo, Outcome 3 Patient Withdrawal. | ||||
| 4 Serious Adverse Events Show forest plot | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.71, 3.66] |
| Analysis 5.4  Comparison 5 Azathioprine/6MP vs Placebo, Outcome 4 Serious Adverse Events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Recurrence at 12 months Show forest plot | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.92] |
| Analysis 6.1  Comparison 6 Azathioprine/6MP versus Other Intervention (5ASA or placebo), Outcome 1 Clinical Recurrence at 12 months. | ||||
| 2 Severe Endoscopic Recurrence (score>/=2) at 12 months Show forest plot | 3 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.49, 0.93] |
| Analysis 6.2  Comparison 6 Azathioprine/6MP versus Other Intervention (5ASA or placebo), Outcome 2 Severe Endoscopic Recurrence (score>/=2) at 12 months. | ||||
| 3 Patient Withdrawal Show forest plot | 4 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.69, 1.26] |
| Analysis 6.3  Comparison 6 Azathioprine/6MP versus Other Intervention (5ASA or placebo), Outcome 3 Patient Withdrawal. | ||||
| 4 Serious Adverse Events Show forest plot | 5 | 468 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.16, 3.02] |
| Analysis 6.4  Comparison 6 Azathioprine/6MP versus Other Intervention (5ASA or placebo), Outcome 4 Serious Adverse Events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severe Endoscopic Recurrence (12 months) Show forest plot | 2 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.49] |
| Analysis 7.1  Comparison 7 Budesonide vs Placebo, Outcome 1 Severe Endoscopic Recurrence (12 months). | ||||
| 2 Patient Withdrawal Show forest plot | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.80, 1.86] |
| Analysis 7.2  Comparison 7 Budesonide vs Placebo, Outcome 2 Patient Withdrawal. | ||||
| 3 Serious Adverse Events Show forest plot | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.37, 2.78] |
| Analysis 7.3  Comparison 7 Budesonide vs Placebo, Outcome 3 Serious Adverse Events. | ||||

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 3 Probiotics vs Placebo, outcome: 3.1 Clinical Recurrence.

Forest plot of comparison: 3 Probiotics vs Placebo, outcome: 3.3 Severe endoscopic recurrence.
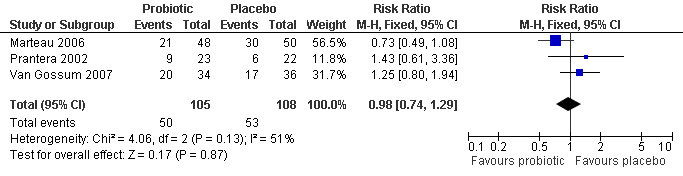
Forest plot of comparison: 3 Probiotics vs Placebo, outcome: 3.2 Any endoscopic recurrence.

Forest plot of comparison: 5 Nitroimidazole vs Placebo, outcome: 5.1 Severe Endoscopic Recurrence (3months).
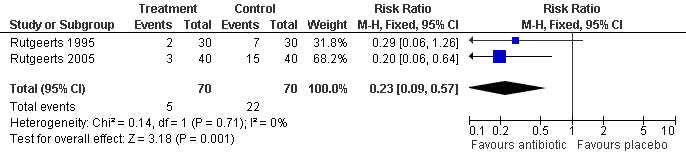
Forest plot of comparison: 5 Nitroimidazole vs Placebo, outcome: 5.2 Clinical Recurrence (1 year).

Forest plot of comparison: 2 Nitroimidazole vs Placebo, outcome: 2.3 Patient Withdrawal.
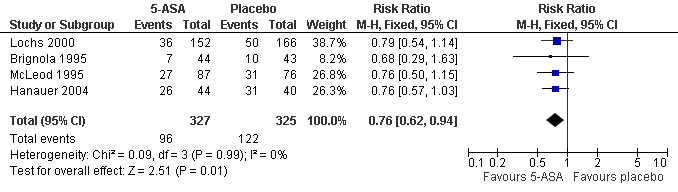
Forest plot of comparison: 1 5‐ASA vs Placebo, outcome: 1.1 Clinical Recurrence within 12 months.
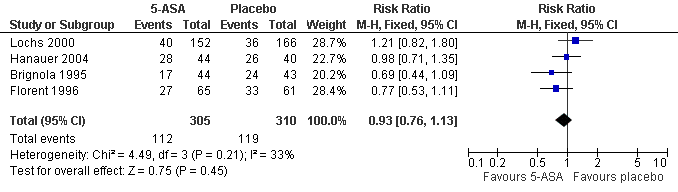
Forest plot of comparison: 1 5‐ASA vs Placebo, outcome: 1.2 Any endoscopic recurrence within 12 months.

Forest plot of comparison: 1 5‐ASA vs Placebo, outcome: 1.3 Severe (score>/=3) endoscopic recurrence within 12 months.
Forest plot of comparison: 2 5‐ASA vs Azathioprine/6MP, outcome: 2.1 Clinical Recurrence within 12 months.

Forest plot of comparison: 2 5‐ASA vs Azathioprine/6MP, outcome: 2.2 Any endoscopic recurrence within 12 months.

Forest plot of comparison: 2 5‐ASA vs Azathioprine/6MP, outcome: 2.3 Severe (>/=3 score) within 12 months.

Forest plot of comparison: 5 Azathioprine/6MP vs placebo, outcome: 5.1 Clinical recurrence at 12 months.

Forest plot of comparison: 5 Azathioprine/6MP vs placebo, outcome: 5.2 Severe endoscopic recurrence (score>/=2) at 12 months.
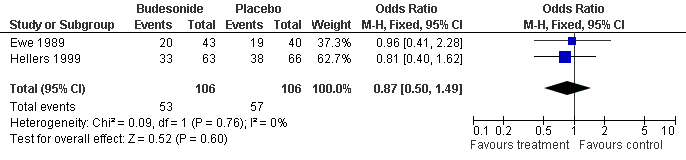
Forest plot of comparison: 4 Budesonide vs Placebo, outcome: 4.1 Severe Endoscopic Recurrence (12 months).

Forest plot of comparison: 6 Azathioprine/6MP versus other interventions (5ASA or placebo), outcome: 6.1 Clinical recurrence at 12 months.

Forest plot of comparison: 6 Azathioprine/6MP versus other interventions (5ASA or placebo), outcome: 6.2 Severe endoscopic recurrence (score>/=2) at 12 months.

Comparison 1 Probiotics vs Placebo, Outcome 1 Clinical Recurrence.

Comparison 1 Probiotics vs Placebo, Outcome 2 Any Endoscopic Recurrence.
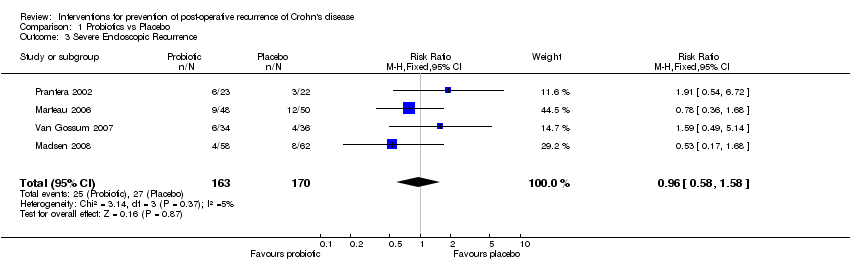
Comparison 1 Probiotics vs Placebo, Outcome 3 Severe Endoscopic Recurrence.

Comparison 1 Probiotics vs Placebo, Outcome 4 Patient Withdrawal.
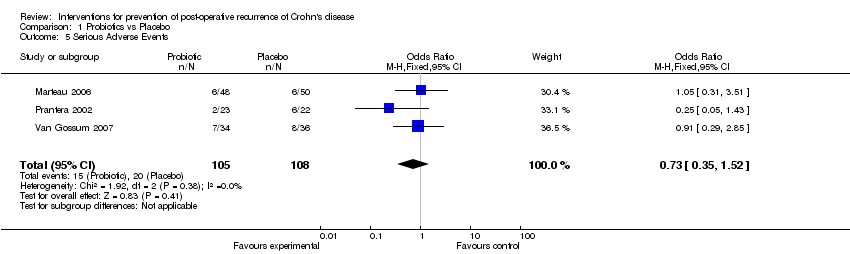
Comparison 1 Probiotics vs Placebo, Outcome 5 Serious Adverse Events.

Comparison 2 Nitroimidazole vs Placebo, Outcome 1 Clinical Recurrence (12 months).
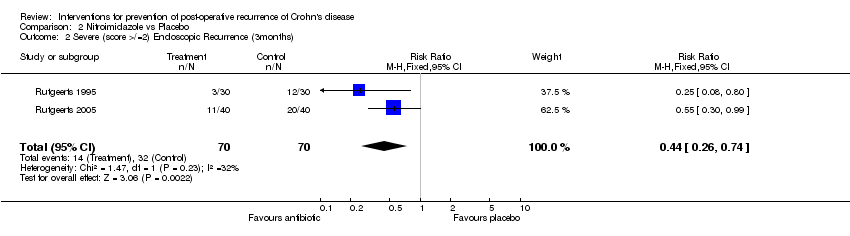
Comparison 2 Nitroimidazole vs Placebo, Outcome 2 Severe (score >/=2) Endoscopic Recurrence (3months).

Comparison 2 Nitroimidazole vs Placebo, Outcome 3 Patient Withdrawal.

Comparison 2 Nitroimidazole vs Placebo, Outcome 4 Serious Adverse Events.

Comparison 3 5‐ASA vs Placebo, Outcome 1 Clinical Recurrence (at study completion).
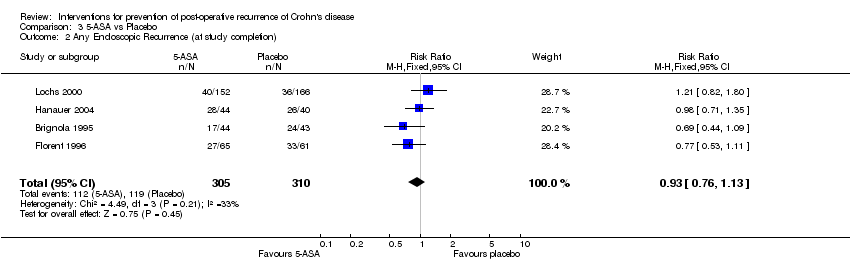
Comparison 3 5‐ASA vs Placebo, Outcome 2 Any Endoscopic Recurrence (at study completion).

Comparison 3 5‐ASA vs Placebo, Outcome 3 Severe (score>/=3) Endoscopic Recurrence (at study completion).
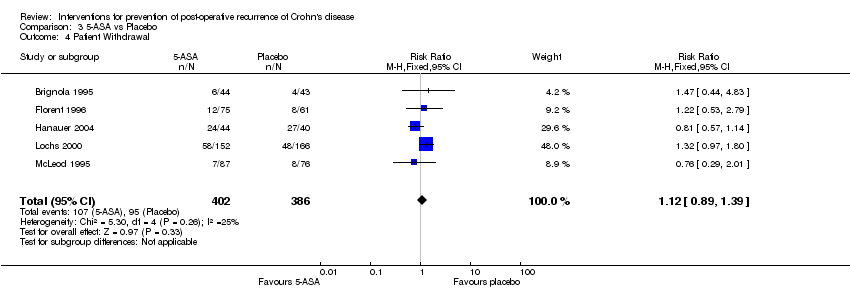
Comparison 3 5‐ASA vs Placebo, Outcome 4 Patient Withdrawal.
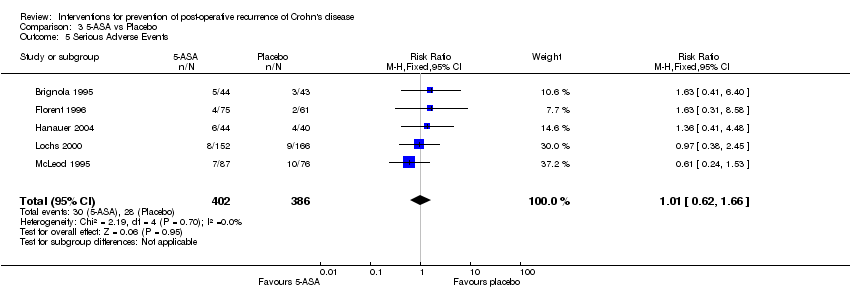
Comparison 3 5‐ASA vs Placebo, Outcome 5 Serious Adverse Events.

Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 1 Clinical Recurrence within 12 months.

Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 2 Clinical recurrence within 24 months.

Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 3 Any endoscopic recurrence (score >/=1) within 12 months.

Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 4 Severe endoscopic recurrence (score >/=2) within 12 months.

Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 5 Very Severe endoscopic recurrence (score >/=3) within 12 months.

Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 6 Patient Withdrawal.

Comparison 4 5‐ASA vs Azathioprine/6MP, Outcome 7 Serious Adverse Events.

Comparison 5 Azathioprine/6MP vs Placebo, Outcome 1 Clinical Recurrence at 12 months.

Comparison 5 Azathioprine/6MP vs Placebo, Outcome 2 Severe Endoscopic Recurrence (score>/=2) at 12 months.
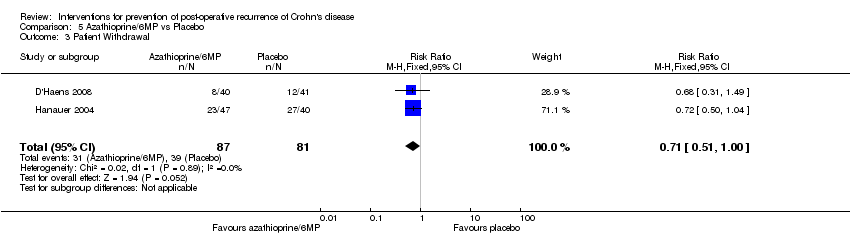
Comparison 5 Azathioprine/6MP vs Placebo, Outcome 3 Patient Withdrawal.

Comparison 5 Azathioprine/6MP vs Placebo, Outcome 4 Serious Adverse Events.
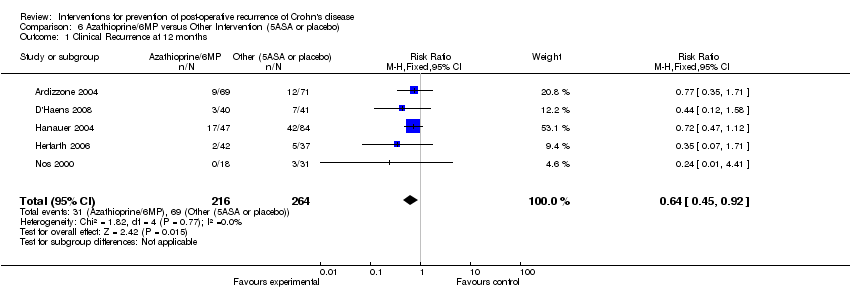
Comparison 6 Azathioprine/6MP versus Other Intervention (5ASA or placebo), Outcome 1 Clinical Recurrence at 12 months.
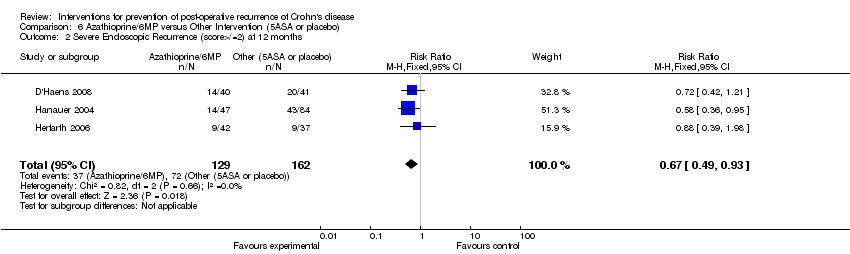
Comparison 6 Azathioprine/6MP versus Other Intervention (5ASA or placebo), Outcome 2 Severe Endoscopic Recurrence (score>/=2) at 12 months.

Comparison 6 Azathioprine/6MP versus Other Intervention (5ASA or placebo), Outcome 3 Patient Withdrawal.

Comparison 6 Azathioprine/6MP versus Other Intervention (5ASA or placebo), Outcome 4 Serious Adverse Events.
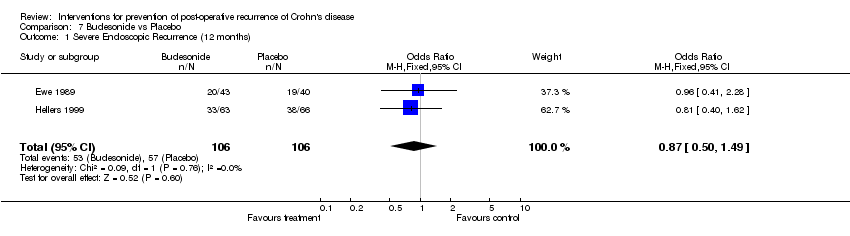
Comparison 7 Budesonide vs Placebo, Outcome 1 Severe Endoscopic Recurrence (12 months).

Comparison 7 Budesonide vs Placebo, Outcome 2 Patient Withdrawal.

Comparison 7 Budesonide vs Placebo, Outcome 3 Serious Adverse Events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Recurrence Show forest plot | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.59, 3.36] |
| 2 Any Endoscopic Recurrence Show forest plot | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.29] |
| 3 Severe Endoscopic Recurrence Show forest plot | 4 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.58, 1.58] |
| 4 Patient Withdrawal Show forest plot | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.65, 1.75] |
| 5 Serious Adverse Events Show forest plot | 3 | 213 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.35, 1.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Recurrence (12 months) Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.09, 0.57] |
| 2 Severe (score >/=2) Endoscopic Recurrence (3months) Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.26, 0.74] |
| 3 Patient Withdrawal Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [1.37, 6.58] |
| 4 Serious Adverse Events Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.54, 3.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Recurrence (at study completion) Show forest plot | 4 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.62, 0.94] |
| 2 Any Endoscopic Recurrence (at study completion) Show forest plot | 4 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.76, 1.13] |
| 3 Severe (score>/=3) Endoscopic Recurrence (at study completion) Show forest plot | 3 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.29, 0.84] |
| 4 Patient Withdrawal Show forest plot | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.89, 1.39] |
| 5 Serious Adverse Events Show forest plot | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.62, 1.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Recurrence within 12 months Show forest plot | 4 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.95, 2.16] |
| 2 Clinical recurrence within 24 months Show forest plot | 3 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.95, 1.81] |
| 3 Any endoscopic recurrence (score >/=1) within 12 months Show forest plot | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.03, 2.06] |
| 4 Severe endoscopic recurrence (score >/=2) within 12 months Show forest plot | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.94, 2.29] |
| 5 Very Severe endoscopic recurrence (score >/=3) within 12 months Show forest plot | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.63, 3.79] |
| 6 Patient Withdrawal Show forest plot | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.26] |
| 7 Serious Adverse Events Show forest plot | 4 | 347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.30, 0.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Recurrence at 12 months Show forest plot | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.92] |
| 2 Severe Endoscopic Recurrence (score>/=2) at 12 months Show forest plot | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.92] |
| 3 Patient Withdrawal Show forest plot | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.51, 1.00] |
| 4 Serious Adverse Events Show forest plot | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.71, 3.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Recurrence at 12 months Show forest plot | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.92] |
| 2 Severe Endoscopic Recurrence (score>/=2) at 12 months Show forest plot | 3 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.49, 0.93] |
| 3 Patient Withdrawal Show forest plot | 4 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.69, 1.26] |
| 4 Serious Adverse Events Show forest plot | 5 | 468 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.16, 3.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severe Endoscopic Recurrence (12 months) Show forest plot | 2 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.49] |
| 2 Patient Withdrawal Show forest plot | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.80, 1.86] |
| 3 Serious Adverse Events Show forest plot | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.37, 2.78] |

