Tratamiento de la neuropatía cubital del codo
Resumen
Antecedentes
La neuropatía cubital del codo (NCC) es la segunda neuropatía por atrapamiento más común después del síndrome del túnel carpiano. Aunque el tratamiento puede ser conservador o quirúrgico, aún sigue siendo discutible cuál es la mejor opción. Ésta es una actualización de una revisión publicada por primera vez en 2010 y actualizada previamente en 2012.
Objetivos
Determinar la efectividad y la seguridad del tratamiento conservador y quirúrgico en la neuropatía cubital del codo (NCC). Se programó evaluar:
‐ si el tratamiento quirúrgico es efectivo para reducir los síntomas y signos y aumentar la función nerviosa;
‐ si el tratamiento conservador es efectivo para reducir los síntomas y signos y aumentar la función nerviosa;
‐ si es posible identificar el mejor tratamiento según la evaluación clínica, neurofisiológica o por imagenología del nervio.
Métodos de búsqueda
El 31 de mayo de 2016, se hicieron búsquedas en el registro especializado del Grupo Cochrane Neuromuscular (Cochrane Neuromuscular Group), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, Embase, AMED, CINAHL Plus y en LILACS. También se hicieron búsquedas en PEDro (14 octubre 2016), y en los artículos citados en revisiones relevantes. El 4 de julio de 2016, se realizaron búsquedas de ensayos en curso y no publicados en los registros de ensayos.
Criterios de selección
La revisión incluyó solo ensayos clínicos controlados aleatorios (ECA) o cuasialeatorios que evaluaron pacientes con síntomas clínicos que indicaban la presencia de NCC. Se incluyeron ensayos que evaluaron todas las formas de tratamientos quirúrgicos y conservadores. Se consideraron los estudios con respecto al tratamiento de la NCC con o sin pruebas neurofisiológicas de atrapamiento.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, examinaron los títulos y los resúmenes de las referencias recuperadas mediante las búsquedas y seleccionaron todos los estudios potencialmente relevantes. Los autores de la revisión, de forma independiente, extrajeron los datos de los ensayos incluidos y evaluaron la calidad de los ensayos. Se estableció contacto con los investigadores de los ensayos para solicitarles información faltante.
Resultados principales
Se identificaron nueve ECA (587 participantes) para la inclusión en la revisión, de los cuales tres se encontraron en esta actualización. La generación de la secuencia no fue insuficiente en un estudio y no se describió en dos. Se realizaron dos metanálisis para evaluar los resultados clínicos (3 ensayos, 261 participantes) y los resultados neurofisiológicos (2 ensayos, 101 participantes) de la descompresión simple versus la descompresión con transposición submuscular o subcutánea; cuatro ensayos examinaron esta comparación.
No se encontró ninguna diferencia entre la descompresión simple y la transposición del nervio cubital para la mejoría clínico (cociente de riesgos [CR] 0,93; intervalo de confianza [IC] del 95%: 0,80 a 1,08; pruebas de calidad moderada) y la mejoría neurofisiológica (diferencia de medias [en m/s] 1,47; IC del 95%: ‐0,94 a 3,87). El número de pacientes con mejoría clínica fue de 91 de cada 131 en el grupo de descompresión simple y 97 de cada 130 en el grupo de transposición. La transposición mostró un número mayor de infecciones de la herida (CR 0,32; IC del 95%: 0,12 a 0,85; pruebas de calidad moderada).
En un ensayo (47 participantes), los autores compararon la epicondilectomía medial con la transposición anterior y no encontraron diferencias en los resultados clínicos ni neurofisiológicos.
En un ensayo (48 participantes), los investigadores compararon la transposición subcutánea con la transposición submuscular y no encontraron diferencias en los resultados clínicos.
En un ensayo (54 participantes para 56 nervios tratados), los autores no encontraron diferencias entre la descompresión endoscópica y abierta en cuanto a la mejoría de la función clínica.
Un ensayo (51 participantes) evaluó el tratamiento conservador en la NCC clínicamente leve o moderada. A partir de pruebas de baja calidad, los autores del ensayo encontraron que la información sobre evitar posiciones o movimientos prolongados fue efectiva en cuanto a la mejoría del malestar subjetivo. Las férulas nocturnas y los ejercicios de deslizamiento del nervio además de la información no resultaron en una mayor mejoría.
Un ensayo (55 participantes) evaluó la efectividad de la inyección de corticosteroides y no encontró diferencias versus placebo en cuanto a la mejoría de los síntomas a tres meses de seguimiento.
Conclusiones de los autores
Se encontraron sólo dos estudios de tratamiento de la neuropatía cubital que usaron un tratamiento conservador como comparador. Las pruebas disponibles sobre el tratamiento comparativo no son suficientes para apoyar un metanálisis de tratamientos múltiples que identifique el mejor tratamiento para la NCC idiopática sobre la base de las características clínicas, neurofisiológicas y de imagenología. No se sabe cuándo tratar a un paciente con esta afección de manera conservadora o quirúrgica. Las pruebas de calidad moderada indican que la descompresión simple y la descompresión con transposición son igualmente efectivas en la NCC idiopática, incluso cuando el deterioro nervioso es severo. La descompresión con transposición se asocia con más infecciones de la herida superficiales y profundas que la descompresión simple, también sobre la base de pruebas de calidad moderada. Fue más probable que los pacientes sometidos a cirugía endoscópica presentaran un hematoma. Las pruebas de un ECA pequeño sobre tratamiento conservador mostraron que, en los casos leves, la información sobre los movimientos o las posiciones a evitar puede reducir el malestar subjetivo.
PICO
Resumen en términos sencillos
Tratamiento de la neuropatía cubital del codo (NCC)
Pregunta de la revisión
¿Cuáles son los efectos de los tratamientos para la neuropatía cubital del codo (NCC)?
Antecedentes
La neuropatía cubital del codo es el segundo tipo más común de afección en que un nervio es atrapado o comprimido (la más común afecta la muñeca). El nervio cubital se desplaza por debajo de la región lateral del codo. Este nervio es importante para el movimiento y el sentido del tacto en la mano del lado del dedo meñique. Los síntomas de la NCC incluyen sensación de hormigueo en el cuarto y quinto dedo por la noche, dolor en el codo y un cambio en el sentido del tacto si se mantiene doblado el codo durante mucho tiempo. Cuando la NCC es severa, pueden debilitarse algunos músculos de la mano. El diagnóstico se basa en los signos y síntomas de la enfermedad, así como en pruebas neurofisiológicas. El tratamiento de la NCC puede ser quirúrgico o no quirúrgico (p.ej. férulas, fisioterapia y rehabilitación). Sigue sin poder precisarse la mejor manera de tratar la NCC.
Características de los estudios
Se encontraron dos ensayos controlados aleatorios (ECA) sobre el tratamiento no quirúrgico. Un ECA comparó a tres grupos de pacientes con NCC leve o moderada (51 pacientes en total). Los tres grupos recibieron instrucciones escritas para evitar movimientos o posiciones que provocaban los síntomas. El segundo grupo tuvo la misma información con férulas del codo por la noche durante tres meses. El tercer grupo tuvo la misma información con ejercicios de deslizamiento del nervio. El otro estudio no quirúrgico (55 pacientes) comparó una inyección de corticosteroides con una inyección simulada.
Siete ECA compararon diferentes métodos quirúrgicos:
• descompresión simple o transposición del nervio (transposición submuscular o subcutánea) (cuatro ensayos, 327 participantes);
• epicondilectomía medial o transposición anterior (un ensayo, 47 participantes);
• transposición subcutánea anterior o transposición submuscular anterior (un ensayo, 48 participantes);
• cirugía mínimamente invasiva o cirugía a cielo abierto (1 ensayo, 54 participantes con 56 nervios atrapados).
Resultados clave y calidad de las pruebas
La información escrita sola fue efectiva en la mejoría de las actividades en el trabajo y en el alivio del dolor por la noche, igual que cuando los pacientes también usaron las férulas o hicieron los ejercicios.
Los investigadores no encontraron pruebas de que la inyección de corticosteroides fue efectiva para mejorar los síntomas de la NCC.
Se pudieron combinar los resultados de tres ensayos que compararon dos técnicas quirúrgicas: la descompresión simple y la transposición del nervio cubital (subcutánea o submuscular). No se encontró ninguna diferencia importante en las puntuaciones de los síntomas entre las técnicas a los seis a 12 meses. La descompresión con transposición puede dar lugar a más infecciones de la herida superficiales y profundas. Los investigadores no encontraron diferencias clínicas entre las técnicas quirúrgicas de los otros ensayos quirúrgicos. Fue más probable que los pacientes sometidos a cirugía endoscópica presentaran un hematoma (una colección anormal de sangre) después de la intervención quirúrgica.
Las pruebas fueron insuficientes como para elegir el mejor tratamiento para la NCC. Sin embargo, se halló que, en los casos leves, la información sobre los movimientos y las posiciones a evitar puede reducir el malestar. Además, los resultados combinados de tres ensayos quirúrgicos aportaron pruebas de calidad moderada de que la descompresión simple y la descompresión con transposición pueden tener la misma efectividad, pero la descompresión con transposición puede dar lugar a más infecciones de la herida superficiales y profundas.
Las pruebas están actualizadas hasta el 31 de mayo de 2016.
Authors' conclusions
Summary of findings
| Simple decompression versus transposition for ulnar neuropathy at the elbow | ||||||
| Patient or population: people with ulnar neuropathy at the elbow | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Transposition | Simple decompression | |||||
| Proportion of participants with clinical improvement in function compared to baseline | 746 per 10001 | 694 per 1000 | RR 0.93 | 261 | ⊕⊕⊕⊝ | |
| Subgroup: proportion of participants with clinical improvement in function compared to baseline ‐ simple decompression versus subcutaneous transposition | 730 per 10001 | 672 per 1000 | RR 0.92 | 147 | ⊕⊕⊕⊝ | |
| Subgroup: proportion of participants with clinical improvement in function compared to baseline ‐ simple decompression versus submuscular transposition | 768 per 10001 | 730 per 1000 | RR 0.95 | 114 | ⊕⊕⊕⊝ | |
| Adverse events: proportion of participants with deep/superficial wound infections | 115 per 10001 | 37 per 1000 | RR 0.32 | 261 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The assumed risk was considered as the median of the risks in the control groups across studies. We did not consider the mean, since the number of studies was low, and the median is the best measure of central tendency in this case. | ||||||
Background
Description of the condition
Ulnar neuropathy at the elbow (UNE) is the second most common entrapment neuropathy after carpal tunnel syndrome. Its mean annual crude incidence is 24.7 cases per 100,000 person‐years (Mondelli 2005). One of the sites of entrapment is the cubital tunnel. The tunnel is defined by Osbourne's ligament, the medial collateral ligament of the elbow, the elbow joint capsule, and the olecranon (Palmer 2010). The clinical picture is typically characterised by paraesthesias involving the fourth and fifth finger, pain at the elbow, sensory symptoms with prolonged flexion of the elbow, and in severe cases motor deficit of the ulnar innervated hand muscles (Dellon 1989). Diagnosis is based on signs, symptoms, and electrodiagnostic studies (Robertson 2005). Imaging, particularly ultrasound, in Beekman 2004, and magnetic resonance imaging (MRI), in Bordalo 2004, is gaining more attention as a sensitive diagnostic tool. Provocative clinical tests are not reliable or useful in the diagnosis of UNE (Beekman 2009). Electrodiagnostic examination is necessary to confirm the diagnosis, quantify the severity, and identify the exact site of ulnar nerve compression (AAEM 1999; Padua 2001).
Description of the intervention
Treatment of UNE may be conservative (splint device, physical therapy, rehabilitation) or surgical (Bartels 2005a; Bartels 2005b; Biggs 2006). The goal of conservative treatment is to eliminate or reduce the frequency of external compression on the nerve (Dellon 1993; Robertson 2005). Regarding surgical therapy, many procedures are employed for the treatment of cubital tunnel syndrome, including simple decompression, anterior transposition (subcutaneous, submuscular, and intramuscular), and medial epicondylectomy (Eaton 1980; Kleinman 1989; Kuschner 1996; Tsai 1999; Robertson 2005).
Why it is important to do this review
The basis for choosing a surgical technique must relate to the pathophysiology of the compression of the ulnar nerve at the elbow, an understanding of the aetiology of the ulnar nerve compression in a particular case, and the potential drawbacks of the various operative procedures. Despite the different options for treating UNE, optimal management remains controversial. This review was first published in 2010 and previously updated in 2012.
Objectives
To determine the effectiveness and safety of conservative and surgical treatment in ulnar neuropathy at the elbow (UNE). We intended to test whether:
-
surgical treatment is effective in reducing symptoms and signs and in increasing nerve function;
-
conservative treatment is effective in reducing symptoms and signs and in increasing nerve function;
-
it is possible to identify the best treatment on the basis of clinical, neurophysiological, or nerve imaging assessment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials (RCTs) using truly random or quasi‐random allocation of treatment. We would consider prospective, consecutive series of more than 10 participants where outcomes were collected by an observer other than the operating surgeon in the Discussion. We did not consider single‐case reports.
Types of participants
People with clinical symptoms suggesting the presence of UNE with or without neurophysiological evidence of entrapment.
Types of interventions
All forms of surgical and conservative treatments.
Types of outcome measures
Primary outcomes
We defined the primary outcome measure as clinically relevant improvement in function compared to baseline. We assessed function with whatever scale was used by the trial authors, with a preference for validated scales such as the Disability of the Arm, Shoulder and Hand questionnaire, in Hudak 1996, or the UNE questionnaire (Mondelli 2006). When self administered scales were used, we would have evaluated if statistically significant changes were reported regarding the main scores in the questionnaires. We dichotomised the primary outcome measure into improvement or no improvement, regardless of the differences between the tools used. If a study evaluated more than one functional outcome measure, a better score on at least one of the functional outcome measures was enough to be considered as an improvement.
Secondary outcomes
-
Change in neurological impairment measured by:
-
the strength of ulnar nerve innervated muscles with the Medical Research Council (MRC) sum score (BMRC 1976);
-
the presence and extent of sensory deficit measured with whatever instrument was used by the authors, but with a preference for cotton wool or Semmes‐Weinstein filaments (Bell‐Krotoski 1987).
-
-
Change from baseline of the motor nerve conduction velocity across the elbow.
-
Change from baseline in the nerve diameter at the elbow, evaluated by ultrasound or MRI.
-
Change in quality of life.
-
Adverse events.
We evaluated primary and secondary outcomes at a short follow‐up (one to six months) and at a long follow‐up (between six months and two years).
Search methods for identification of studies
Electronic searches
On 31 May 2016, we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (in the Cochrane Register of Studies Online (CRSO)), MEDLINE (January 1966 to May 2016), Embase (January 1980 to May 2016), AMED (Allied and Complementary Medicine) (January 1985 to May 2016), CINAHL (Cumulative Index to Nursing and Allied Health Literature) Plus (January 1937 to May 2016), and LILACS (Latin American and Caribbean Health Science Information database) (January 1982 to May 2016). On 14 October 2016, we searched PEDro (Physiotherapy Evidence Database) (January 1980 to October 2016). We applied no limitations as to language.
The detailed search strategies are in the appendices: Cochrane Neuromuscular Specialised Register (Appendix 1), CENTRAL (Appendix 2), MEDLINE (Appendix 3), Embase (Appendix 4), AMED (Appendix 5), LILACS (Appendix 6), CINAHL Plus (Appendix 7), PEDro (Appendix 8), ClinicalTrials.gov (Appendix 9), and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (Appendix 10).
Searching other resources
We searched the references of relevant trials identified by the search strategy and in June 2015 contacted authors of identified papers to determine whether other published or unpublished trials were available.
We also searched the following trials registers.
-
ClinicalTrials.gov (clinicaltrials.gov) (4 July 2016)
-
WHO ICTRP (who.int/ictrp/en/) (4 July 2016)
Data collection and analysis
Selection of studies
Two review authors independently reviewed titles and abstracts of the references retrieved from the searches and selected all potentially relevant studies. We compared the results of our literature search to the review articles found using the previously mentioned databases. Furthermore, when information from one paper was re‐published by the same author in a larger investigation, or written in English, we considered only the most recent article. We obtained copies of these articles, and the same review authors independently reviewed them against the inclusion criteria of the review. The review authors then independently extracted data from included trials and assessed risk of bias with a data extraction form specifically designed for this purpose.
Data extraction and management
We extracted the following data.
Study methods
-
Design (e.g. randomised or quasi‐randomised, cohort study, case‐control study)
-
Randomisation method (including list generation)
-
Method of allocation concealment
-
Blinding method
-
Stratification factors
Participants
-
Inclusion and exclusion criteria
-
Number (total, per group)
-
Age distribution
-
Associated morbidities
-
Treatments
-
Pre‐treatment quality of life and functional status, as measured by validated scales
Intervention and control
-
Type of therapy
-
Type of control
-
Details of control treatment including duration of non‐operative treatment
-
Concomitant treatments
Follow‐up data
-
Duration of follow‐up
-
Loss to follow‐up
Outcome data
-
British Medical Research Council (BMRC) scale
-
Presence of sensory deficits (evaluated with cotton wool or Semmes‐Weinstein filaments)
-
Self administered scales including questionnaires assessing regional function and symptoms (Disability of the Arm, Shoulder and Hand questionnaire; UNE questionnaire; visual analogue scale (VAS) (Sriwatanakul 1983), and quality of life measures (such as the 36‐Item Short Form Health Survey (SF‐36)) (Ware 1992)
-
Neurophysiology
-
Ultrasound
-
MRI
We considered the BMRC scale, presence of sensory deficits, and a regional self administered questionnaire the main outcome measures.
Analysis data
-
Methods of analysis (intention‐to‐treat or per‐protocol analysis)
-
Comparability of groups at baseline (age, gender, clinical impairment, neurophysiological impairment, associated diseases)
-
Statistical techniques
Other data
-
Date
-
Location
-
Conflicts of interest
-
Funding
-
Single or multicentre
The first review author entered data into the Cochrane statistical software Review Manager 5, and the second review author checked the data entry.
At this update the review included information on trial funding and conflicts of interest.
Assessment of risk of bias in included studies
We evaluated the risk of bias in the trials by scoring the following items and reported our assessments in the 'Risk of bias' tables:
-
sequence generation;
-
allocation concealment;
-
blinding of participants;
-
blinding of outcome assessors;
-
incomplete outcome data;
-
selective outcome reporting;
-
other risk of bias.
We assessed the included studies under each domain and judged the risk of bias as 'high', 'low', or 'unclear'. We used unclear when the report included insufficient information to make a judgement or when the risk of bias was uncertain (Higgins 2011).
Measures of treatment effect
We used risk ratio (RR) estimations with 95% confidence intervals (CI) for binary outcomes and mean difference (MD) estimations with 95% CI for continuous outcomes. All analyses included all participants in the treatment groups to which they were allocated.
If we had collected data from case‐control studies, we would have considered using odds ratios and 95% CI.
Dealing with missing data
In the first instance, we contacted study authors to supply data missing from included studies. We assessed missing data and dropouts or attrition for each included study, and assessed and discussed the extent to which the missing data could have altered the results or conclusions of the review. If less than 70% of participants allocated to treatments provided data at the end of the trial for a particular outcome, we would have discarded those data as we would have considered them to be too prone to bias.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important participant factors between trials (age, gender, clinical impairment, neurophysiological impairment, and associated diseases) and trial factors (randomisation concealment, blinding of outcome assessment, losses to follow‐up, treatment type, and co‐interventions). We assessed statistical heterogeneity by examining the I2 statistic, a quantity which approximately describes the proportion of variation in point estimates that is due to heterogeneity rather than sampling error. In addition, we planned to employ a Chi2 test to determine the strength of the evidence that heterogeneity was genuine.
Assessment of reporting biases
In order to detect potential publication bias, we would plot RRs and 95% CIs against standard errors in each study (funnel plots). Asymmetry in such plots could be due to publication bias, but could also be due to a relationship between trial size and effect size. In the event of finding a relationship, we planned to examine the clinical diversity of the studies (Egger 1997).
Data synthesis
Where the interventions were the same, or similar enough, we carried out a meta‐analysis (DerSimonian 1986). We undertook statistical analysis using Review Manager 5 (RevMan 2014). We planned to synthesise results in a meta‐analysis.
'Summary of findings' table
We included a 'Summary of findings' table for comparisons for which information from more than one study was available and assessed the quality of the body of evidence using the GRADE approach for the following outcomes:
-
proportion of participants with clinically relevant improvement in function compared to baseline at 6 to 12 months (showing subgroups);
-
change in quality of life;
-
adverse events.
We downgraded the quality of the evidence for each outcome using the five GRADE considerations: limitations in design or implementation of studies suggesting a high risk of bias; indirectness of evidence; unexplained heterogeneity; imprecision; and high probability of publication bias (Schünemann 2011). RCTs start with a high quality assessment, which may be downgraded to moderate, low, or very low if these considerations are present to a serious degree.
Subgroup analysis and investigation of heterogeneity
If possible, we would have conducted subgroup analysis for the following groups.
-
Two age groups: (≤ 45 years old, > 45 years old).
-
Two groups with different electrophysiological abnormalities, namely:
-
participants with pathological motor conduction velocity across the elbow and no other neurophysiological abnormality;
-
participants with concomitant pathological motor conduction velocity across the elbow and one of the following criteria:
-
denervation signs in the ulnar innervated muscle of the hand; or
-
reduction of amplitude of sensory response in the fifth digit‐wrist segment.
-
-
Sensitivity analysis
If possible, we would have conducted sensitivity analyses to assess the impact of study quality. This would have included separate analyses of RCTs and quasi‐RCTs.
Adverse events
Since randomised studies rarely deal with adverse events adequately because the numbers are small and follow‐up too short, we discussed adverse events (infections, worsening of symptoms) taking into account the non‐randomised literature.
Cost‐benefit analyses
We considered cost‐effectiveness of interventions in the Discussion, where relevant data were available.
Results
Description of studies
Results of the search
The previous version of this review included six studies. In 2016 we identified 253 new papers from database searches as potentially relevant and after we reviewed these, a total of nine RCTs (587 participants) met the inclusion criteria for the review. The following list reports the number of papers identified in each database by the new current strategies and the number newly identified at this update.
-
MEDLINE: 264 (60 new papers)
-
Embase: 137 (23 new papers)
-
CENTRAL: 78 (31 new papers)
-
Cochrane Neuromuscular Specialised Register: 31 (9 new papers)
-
AMED: 15 (1 new paper)
-
LILACS: 3 (1 new paper)
-
CINAHL Plus: 74 (8 new papers)
-
PEDro: 5 (2 new papers)
-
ClinicalTrials.gov: 86
-
WHO ICTRP: 32
Figure 1 illustrates the study selection process.

A flow diagram illustrating the study selection process.
Included studies
See Characteristics of included studies.
To evaluate the clinical outcome we included three studies in the meta‐analysis: Bartels 2005 (152 participants; 5 lost to follow‐up), Gervasio 2005 (70 participants), and Biggs 2006 (44 participants). The three studies compared simple decompression with transposition of the ulnar nerve. In two studies the latter was a submuscular transposition (Gervasio 2005; Biggs 2006), and in one study, it was anterior subcutaneous transposition (Bartels 2005). A total of 131 participants were treated by simple decompression, and a total of 130 participants were treated by transposition of the nerve (submuscular or subcutaneous). In all three studies the participants had clinical and electrophysiological evidence of ulnar nerve impairment.
We performed two different evaluations to assess the effectiveness of surgery. In the first, we compared simple decompression with subcutaneous transposition (Bartels 2005), and in the second analysis, we compared simple decompression with submuscular transposition (Gervasio 2005; Biggs 2006).
Bartels 2005 and Biggs 2006 used clinical scores to assess changes after surgical treatment. Gervasio 2005 used a clinical score and a neurophysiological evaluation. The clinical outcome measures in the three studies varied. Bartels 2005 used a clinical scale that included a combination of historical and physical findings (evaluation of sensation and muscular strength); Biggs 2006 used the McGowan score and Louisiana State University Medical Center (LSUMC) score, both of which graded the sensory and muscular deficits; and Gervasio 2005 evaluated the clinical picture preoperatively by Dellon’s classification and postoperatively by the Bishop score. Dellon’s staging system includes the assessment of sensory and motor function and the response to provocative tests. The Bishop score assesses objective and subjective parameters: grip strength, sensory measurement of static two‐point discrimination, severity of residual symptoms, subjective improvement compared with the preoperative period, and preoperative and postoperative work status.
Bartels 2005 performed a clinical follow‐up at one year after surgery; Biggs 2006 at six weeks and six months after surgery; and Gervasio 2005 at six months after surgery (clinical and neurophysiological assessment). At the six‐month follow‐up, Gervasio 2005 performed a neurophysiological assessment in all the participants in both groups. These investigators also performed a second clinical follow‐up (the mean duration of the second follow‐up was 47 months for the simple decompression group and 46.9 months for the anterior submuscular transposition group).
We also performed a meta‐analysis of neurophysiological outcome, including two papers (Gervasio 2005; Nabhan 2005).
Nabhan 2005 (66 participants) compared two surgical procedures: subcutaneous anterior transposition and decompression without transposition. Thirty‐two participants underwent simple nerve decompression, and 34 had subcutaneous transposition. All participants had clinical and electrophysiological evidence of ulnar neuropathy. The main outcome measure was motor conduction velocity across the elbow, although the trialists also assessed muscular strength of ulnar innervated muscles pre‐ and postoperatively. All the participants in both groups underwent neurophysiological assessment. The investigators performed follow‐up examinations at three months and nine months after surgery.
We included five other studies in the review but not in the meta‐analyses (Geutjens 1996; Svernlov 2009; Zarezadeh 2012; Schmidt 2014; vanVeen 2015).
Geutjens 1996 (47 participants) compared medial epicondylectomy with anterior transposition in people with clinical and electrophysiological evidence of ulnar neuropathy. The authors measured the clinical outcome by:
-
the MRC scale;
-
evaluation of sensation by light touch and static two‐point discrimination; and
-
assessment of pain in the hand by a non‐validated five‐item scale.
The neurophysiological outcome was measured by ulnar motor nerve conduction velocity in all the participants in both groups. All the evaluations were performed before treatment and at 12 months. Participant satisfaction was assessed by a non‐validated tool.
Svernlov 2009 (51 participants) compared three groups treated conservatively with:
-
night splinting for three months and "written information of the anatomy of the ulnar nerve, an explanation of the probable pathomechanics and the regimen regarding the avoidance of movements and positions provoking the symptoms";
-
nerve gliding exercises and the same written information;
-
written information only.
All the participants had clinically mild or moderate UNE, classified with the Dellon’s staging system. All participants underwent electrophysiological assessment preoperatively, but only 12 participants had abnormal findings. The clinical outcome measures were: (1) evaluation of fifth‐digit and grip strength, measured by two different dynamometers; and (2) the VAS. Electrophysiological outcomes were ulnar motor and sensory nerve conduction studies and electromyography. Participants assessed their symptoms according to the Canadian Occupational Performance Measure (COPM). The COPM is a 10‐point scale that measures the person’s own opinion of his or her ability to perform occupational activities and satisfaction with performance. The investigators evaluated the participants in the three groups before the treatment and at six months.
Zarezadeh 2012 (48 participants) compared anterior subcutaneous transposition with anterior submuscular transposition in participants with clinical and electrophysiological evidence of ulnar neuropathy. The authors measured the clinical outcome by:
-
MRC scale;
-
subjective evaluation of muscle atrophy;
-
Yale Sensory Scale; and
-
VAS.
The authors measured the neurophysiological outcome by ulnar motor nerve conduction velocity in all participants but did not report the findings. All the evaluations were performed before treatment and at 12 months. The clinical outcome was assessed by a total score based on the results of the four outcome measures; however, no information was provided on the score generation process.
Schmidt 2014 (54 participants with 56 ulnar entrapments) compared endoscopic surgery with open decompression in people with clinical, neurophysiological, and ultrasonographic findings of ulnar neuropathy. The clinical outcome was measured by a modified Bishop scale, and the neurophysiological outcome by ulnar motor nerve conduction velocity. The trialists performed clinical and neurophysiological evaluations before surgery, during an early follow‐up (a mean of 16 weeks), and a long‐term follow‐up (a mean of 16.8 months).
vanVeen 2015 (63 participants enrolled, 55 participants analysed) compared an ultrasound‐guided injection of a 1 mL injection containing 40 mg methylprednisolone acetate and 10 mg lidocaine hydrochloride with a placebo injection. The trialists measured the clinical outcome by two subjective scales evaluating change in symptoms and severity of symptoms. Moreover, the trial authors evaluated the neurophysiological outcome by ulnar motor nerve conduction velocity and the utrasonographic outcome by measuring the ulnar nerve cross‐section in a segment of 4 cm across the medial epicondyle.
Excluded studies
See Characteristics of excluded studies.
We excluded two studies from the review (Chen 2006; Zhong 2011). Chen 2006 compared two groups of participants. In the first group, participants "were immobilized with the plaster slab for an external fixation for 3 weeks" after operation; in the second group, they "began an immediate range of motion on the 2nd day after operation". It seems that both groups were comprised of participants who underwent ulnar neurolysis or nerve anterior transposition. We excluded the study because the authors did not compare different therapeutic approaches, but rather two management approaches after surgery. From the translation it was difficult to evaluate the quality of the study. Zhong 2011 compared two groups of participants treated surgically. Participants in the first group were treated with subcutaneous transposition, and in the second group with submuscular transposition. The main outcome measures were the cross‐sectional area (CSA) of the ulnar nerve at the elbow and neurophysiological parameters. The authors concluded that submuscular transposition produced greater improvement than subcutaneous transposition in severe cases. The preoperative values of CSA and of neurophysiological parameters were identical for the two compared groups in the report (even the same decimal values); since this is statistically improbable, we had serious concerns about the methodological quality of the work and therefore excluded it.
Risk of bias in included studies
Figure 2 summarises the risk of bias in the included RCTs.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation was adequate in five studies (Bartels 2005; Nabhan 2005; Biggs 2006; Zarezadeh 2012; vanVeen 2015). Three studies did not clearly describe the method of sequence generation (Geutjens 1996; Svernlov 2009; Schmidt 2014). In one study the method of randomisation was inadequate (Gervasio 2005).
The allocation concealment method was described and adequate in three studies (Geutjens 1996; Nabhan 2005; Svernlov 2009). In six studies the method of allocation concealment was not described (Bartels 2005; Gervasio 2005; Biggs 2006; Zarezadeh 2012; Schmidt 2014; vanVeen 2015); we concluded that no allocation concealment procedure was used in these studies.
Blinding
In six studies the authors did not describe if the participants were blinded (Geutjens 1996; Bartels 2005; Gervasio 2005; Nabhan 2005; Biggs 2006; Zarezadeh 2012); in two studies the participants were blinded (Schmidt 2014; vanVeen 2015); and in one study the participants were not blinded (Svernlov 2009).
In five studies the examiner was blinded (Geutjens 1996; Gervasio 2005; Svernlov 2009; Schmidt 2014; vanVeen 2015). In one study only a subgroup of 30 participants was evaluated by an independent examiner (Bartels 2005). In two studies the authors did not specify whether the examiner was blinded (Nabhan 2005; Biggs 2006). In one study the authors specified that the neurophysiological evaluation was blinded, but no information was reported about the assessment of clinical outcomes (Zarezadeh 2012).
Incomplete outcome data
In three studies no participants were lost to follow‐up (Gervasio 2005; Nabhan 2005; Zarezadeh 2012). In the remaining six studies a low number of participants were lost to follow‐up: five participants (3.3%) in Bartels 2005, three (6.4%) in Biggs 2006, nine (4.7%) in Geutjens 1996, 13 (6.6%) in Svernlov 2009, three (5.5%) in Schmidt 2014, and five (5%) in vanVeen 2015. In the study evaluating conservative treatments (Svernlov 2009), six participants were dropouts because they underwent surgical decompression.
Selective reporting
It was unclear whether two studies were free of selective outcome reporting (Bartels 2005; Gervasio 2005). In Bartels 2005, the methods listed two validated self report questionnaires (McGill Pain Questionnaire‐Dutch language version (MPQ‐DLV), SF‐36), but provided no statistical information on the questionnaires in the results. The authors simply reported that the improvement in the MPQ‐DLV and SF‐36 scores did not differ for participants treated with simple decompression or anterior subcutaneous transposition. In Gervasio 2005, trial authors performed the pre‐ and postoperative evaluations using two different staging systems: preoperatively the Dellon scale, and postoperatively the Bishop rating system. No information on the Dellon scale was available for the follow‐up evaluation. In another study, the authors declared that all participants were neurophysiologically evaluated but reported no data (Zarezadeh 2012).
Six studies were free of selective outcome reporting (Geutjens 1996; Nabhan 2005; Biggs 2006; Svernlov 2009; Schmidt 2014; vanVeen 2015).
Other potential sources of bias
In all nine trials the authors did not specify whether the study was designed to be a non‐inferiority or a superiority trial (Geutjens 1996; Bartels 2005; Gervasio 2005; Nabhan 2005; Biggs 2006; Svernlov 2009; Zarezadeh 2012; Schmidt 2014; vanVeen 2015), and in eight trials the authors did not calculate sample size (Geutjens 1996; Bartels 2005; Gervasio 2005; Nabhan 2005; Biggs 2006; Svernlov 2009; Zarezadeh 2012; Schmidt 2014). In two studies, the clinical outcome measures used may have low sensitivity (Geutjens 1996; Nabhan 2005). One study only included people with severe neuropathy (Gervasio 2005).
Since none of the trials were at an overall high risk of bias, we did not conduct a sensitivity analysis.
Effects of interventions
Surgery: simple decompression versus transposition
Bartels 2005, Biggs 2006, Gervasio 2005, and Nabhan 2005.
Primary outcome: proportion of participants with a clinically relevant improvement in function compared to baseline
Reported in Bartels 2005, Biggs 2006, and Gervasio 2005. See summary of findings Table for the main comparison.
We found clinical improvement in 70% of participants treated with simple decompression and 75% of those treated with transposition in the period from 6 to 12 months after surgery. We found no significant difference in postoperative clinical improvement between simple decompression and transposition (subcutaneous or submuscular) of the ulnar nerve (RR 0.93, 95% CI 0.80 to 1.08; n = 261) (Analysis 1.1). Figure 3 shows the forest plot for the studies in the meta‐analysis. We observed no significant difference in clinical outcome between simple decompression and subcutaneous transposition (RR 0.92, 95% CI 0.74 to 1.14; n = 147) or between simple decompression and submuscular transposition (RR 0.95, 95% CI 0.77 to 1.17; n = 114) (Analysis 1.1; Figure 3).
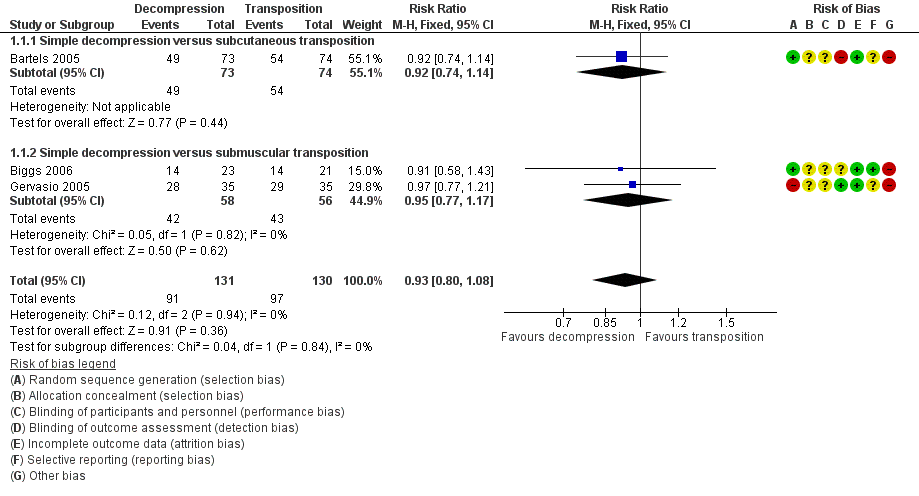
Forest plot of comparison: 1 Clinical and neurophysiological effect of simple decompression versus transposition, outcome: 1.1 Proportion of participants with clinical improvement in function compared to baseline.
Secondary outcomes
Change in neurological impairment
Reported in Nabhan 2005.
Nabhan 2005 found a slight improvement in the mean value of the MRC sum scale (measuring specifically strength in ulnar intrinsic muscles) (BMRC 1976), and in the mean value of a non‐validated sensory scale after simple decompression (pre‐surgery MRC 4.0 ± 1.0, postsurgery MRC 4.5 ± 0.7) and after decompression with anterior subcutaneous transposition (pre‐surgery MRC 3.8 ± 1.0, postsurgery MRC 4.3 ± 0.6). No difference was found between the two procedures.
Change from baseline of the motor nerve conduction velocity across the elbow
Reported in Gervasio 2005 and Nabhan 2005.
We found a statistically significant improvement in motor nerve conduction velocity after simple decompression and after transposition at the six months' follow‐up (Gervasio 2005; Nabhan 2005). We observed no difference in postoperative motor nerve conduction velocity (m/s) between the two procedures (MD 1.47, 95% CI ‐0.94 to 3.87; n = 101) (Analysis 1.2). Figure 4 shows the forest plot for this outcome.

Forest plot of comparison: 1 Clinical and neurophysiological effect of simple decompression versus transposition, outcome: 1.2 Postoperative motor nerve conduction velocity.
For the Gervasio 2005 study, we only included in the meta‐analysis the participants who preoperatively had motor responses at the neurophysiological assessment (17 out of 35 participants in the simple decompression group and 18 out of 35 participants in the transposition group). The motor response, preoperatively absent in 18 participants in the simple decompression group, reappeared postoperatively in eight participants, while in the transposition group, it was absent in 17 participants and reappeared in six. Among the 30 participants with preoperative absence of sensory response in the simple decompression group, the sensory response reappeared in 16 participants. Among the 29 participants with absence of sensory response in the transposition group, the sensory response reappeared in 14 participants.
The available data did not allow a subgroup analysis between participants with pathological motor conduction velocity across the elbow only and participants who also had denervation signs or reduction of amplitude of sensory responses, or both, or between age groups.
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
No data were available on change from baseline in the ulnar nerve diameter at the elbow.
Change in quality of life
No data were available for the outcome change in quality of life.
Adverse events
Reported in Bartels 2005, Gervasio 2005, and Biggs 2006.
Decompression with transposition was associated with a higher number of deep and superficial wound infections on meta‐analysis of data from three trials (RR 0.32, 95% CI 0.12 to 0.85; n = 261; Analysis 1.3) (4 deep infections and 11 superficial infections in the transposition group, 0 deep infections and 5 superficial infections in the simple decompression group). Bartels and colleagues also found a higher scar area sensory loss in the transposition group (14 cases) than in the simple decompression group (2 cases). Other adverse events affected small numbers of participants.
Surgery: medial epicondylectomy versus anterior transposition
Studied in Geutjens 1996.
Primary outcome: proportion of participants with a clinically relevant improvement in function compared to baseline
Clinical improvement in function was not evaluated.
Secondary outcomes
Change in neurological impairment
In Geutjens 1996, the authors found no difference between medial epicondylectomy (n = 25) and anterior transposition (n = 22) in modifying muscular strength and sensory deficits after 12 months. After surgery, the number of participants with muscular deficits at the MRC grading was 13 in the medial epicondylectomy group and 10 in the anterior transposition group (P = 0.119). We were unable to report the grade 2 comparison as the data in the report were unclear. The mean (± standard deviation (SD)) two‐point discrimination scores were 6.8 ± 2.8 in the medial epicondylectomy group and 9.2 ± 3.3 in the anterior transposition groups(P = 0.711).
Change from baseline of the motor nerve conduction velocity across the elbow
Medial epicondylectomy and anterior transposition had similar efficacy on neurophysiological outcomes (postoperative motor nerve conduction velocity was 32.6 ± 7.55 in the medial epicondylectomy group and 34.0 ± 8.01 in the anterior transposition group, P = 0.772)
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
Not measured.
Change in quality of life
Not measured.
Adverse events
Postoperative pain in the hand occurred with anterior transposition. The mean (0‐to‐5 scale) pain score (± SD) after medial epicondylectomy (n = 25) was 0.0 (± 0) compared to 0.45 ± 0.86 after anterior transposition (n = 22) (P = 0.029).
Surgery: submuscular transposition versus anterior subcutaneous transposition
Studied in Zarezadeh 2012.
Primary outcome: proportion of participants with a clinically relevant improvement in function compared to baseline
Clinical improvement in function was not evaluated.
Secondary outcomes
Change in neurological impairment
In Zarezadeh 2012, the authors found no difference between submuscular transposition (n = 24) and anterior subcutaneous transposition (n = 24) in improving sensory and strength deficits after 12 months. After surgery, in the submuscular group, 0% of participants had absence of sensation, 50% had decreased or abnormal sensation, and 50% had normal sensation; in the subcutaneous group, 0% of participants had absence of sensation, 54.2% had decreased or abnormal sensation, and 45.8% had normal sensation (P = 1.0). No participant in either group had severe strength deficits; 37.5% of participants in the submuscular group and 29.2% in the subcutaneous group had moderate deficits; and 62.5% in the submuscular group and 70.8% in the subcutaneous group had slight or no deficit. Submuscular transposition was associated with a greater pain reduction (after surgery, in the submuscular group, 0% of participants had severe pain, 12.5% had slight pain, and 87.5% had no pain; in the subcutaneous group, 0% of participants had severe pain, 66.7% had slight pain, and 33.3% had no pain; P = 0.0004).
Change from baseline of the motor nerve conduction velocity across the elbow
The authors declare that neurophysiological studies were performed before and after treatment, but no data on the change from baseline of the motor conduction velocity across the elbow are available.
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
Not measured.
Change in quality of life
Not measured.
Adverse events
No adverse event was reported.
Surgery: endoscopic versus open decompression
Studied in Schmidt 2014.
Primary outcome: proportion of participants with a clinically relevant improvement in function compared to baseline
The authors found no difference between the endoscopic group (n = 29 nerves) and the open decompression group (n = 27 nerves) in improving clinical function measured by Bishop score (early (mean 16 weeks) follow‐up, P = 1.00; long‐term (mean 16.8 months) follow‐up, P = 0.47). In the endoscopic group at early follow‐up, the clinical outcome, measured by the modified Bishop score, was poor in 2 arms, fair in 1, good in 11, and excellent in 15 arms. At long‐term follow‐up, the outcome was poor in 4 arms, fair in 1, good in 2, and excellent in 22 arms. In the open decompression group, at early follow‐up the clinical outcome was poor in 3 arms, fair in 1, good in 10, and excellent in 13 arms. At long‐term follow‐up, the outcome was poor in 5 arms, fair in 0, good in 3, and excellent in 19 arms.
Secondary outcomes
Change in neurological impairment
In Schmidt 2014, the trial authors found no difference between the two procedures in improving pain at the sulcus or in the supplemented area of the nerve both in early follow‐up (P = 0.84) and late follow‐up (P = 0.84). In the endoscopic group, the postoperative value of numeric analogue scale (NAS) was 0.97 in the early follow‐up and 0.64 in the long‐term follow‐up. In the open decompression group, the NAS score was 0.85 in the early follow‐up and 0.79 in the long‐term follow‐up. Two‐point discrimination was assessed, but no data comparing intervention groups were available.
Change from baseline of the motor nerve conduction velocity across the elbow
At long‐term follow‐up, after a mean of 13.8 months, the authors found no difference between the procedures in improving electrophysiological findings (in the endoscopic group 21 cases out of 27 improved, and in the open group 22 out of 26 cases improved; P = 0.62).
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
Not measured.
Change in quality of life
Not measured.
Adverse events
A significantly higher rate of postoperative haematoma occurred in the endoscopic group (7/29 (24.14%) of arms in the endoscopic group and 1/27 (3.7%) of arms in the open group, P = 0.05). No difference was found in the rate of disturbance of wound healing (3/29 (10.34%) of arms in the endoscopic group and 1/27 (3.7%) of arms in the open group, P = 0.61).
Conservative treatment: information provision versus information provision and nerve gliding exercises or versus information provision and night splinting
Assessed in one study (Svernlov 2009).
Primary outcome: proportion of participants with a clinically relevant improvement in function compared to baseline
In clinically mild or moderate UNE, night splinting plus information on the movements and positions provoking the symptoms (n = 26), nerve gliding exercises plus information (n = 23), and just information (n = 21) determined an improvement of occupational activity at six‐month follow‐up (P = 0.0001, P = 0.0003, and P = 0.039, respectively). Night splinting for three months and nerve gliding exercises did not provide further improvement in occupational activities and nocturnal pain at six months when compared with just information. Nerve gliding exercises and information alone improved satisfaction and diurnal pain (P = 0.0001 for both treatments), while night splinting did not.
Secondary outcomes
Change in neurological impairment
Conservative treatments (night splinting plus information to avoid movements or positions provoking the symptoms, nerve gliding exercises plus information, and information alone) did not improve muscular strength (Svernlov 2009).
Change from baseline of the motor nerve conduction velocity across the elbow
Before treatment, 12 out of 51 participants had impaired nerve conduction velocity over the elbow segment. At six months' follow‐up, 58% of these participants had normal conduction velocity.
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
Not measured.
Change in quality of life
Not measured.
Adverse events
No adverse event was reported.
Conservative treatment: corticosteroid injection versus placebo
Studied in vanVeen 2015.
Primary outcome: proportion of participants with a clinically relevant improvement in symptoms
The authors found no difference between the corticosteroid group (n = 30 participants) and the placebo group (n = 25 participants) in improving symptoms at three months' follow‐up (P = 0.871). In the corticosteroid group, 9 out of 30 participants (30%) had a favourable outcome, and 21 participants (70%) had an unfavourable outcome. In the placebo group, 7 out of 25 participants (28%) had a favourable outcome, and 18 participants (72%) had an unfavourable outcome.
Secondary outcomes
Change in neurological impairment
In vanVeen 2015, at baseline and at follow‐up the authors found no difference between the two groups regarding the severity of symptoms and the neurological examination findings. The authors reported data only at the baseline; no data are given at follow‐up. At baseline in the corticosteroid group, 30 participants (100%) had sensory symptoms, and 5 participants (17%) had atrophy; the mean MRC score was 19.7 (range 18 to 20). In the placebo group, 25 participants (100%) had sensory symptoms and 5 participants (20%) had atrophy; the mean MRC score was 19.6 (range 15 to 20).
Change from baseline of the motor nerve conduction velocity across the elbow
The trial authors found no difference between the groups in improving electrophysiological findings at follow‐up. The mean motor nerve conduction velocity across the elbow at follow‐up was 48.3 m/s in the corticosteroid group (n = 26) and 50.3 m/s in the placebo group (n = 23) (these velocities were 45.1 m/s and 46.2 m/s at inclusion). The paper does not report SD, preventing calculation of a MD and 95% CI for the change.
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
The nerve cross‐sectional area changed significantly (P = 0.043) in the corticosteroid group (n = 26), decreasing from 11.9 mm2 to 10.9 mm2. In the placebo group (n = 23), the cross‐sectional area was unchanged (13.2 mm2 at baseline and at follow‐up). Without measures of variability, we were unable to calculate a MD and 95% CI.
Change in quality of life
Not measured.
Adverse events
In the corticosteroid group, four participants experienced adverse events: one participant reported swelling at the injection site, one had pain at the injection site, one had a swollen hand, and one had depigmentation at the injection site. In the placebo group, one participant reported pain at the injection site.
Discussion
Summary of main results
Participants who underwent surgical procedures in the included studies had, in the majority of cases, improved symptoms and nerve function, but there were no studies comparing surgical treatment to conservative management to support this in a controlled trial environment.
The available evidence suggests that simple decompression and decompression with transposition are equally effective in the treatment of the clinical and neurophysiological impairment of the ulnar nerve. Transposition is associated with a greater possibility of deep wound infections. In 2005, Bartels and colleagues performed a cost analysis in the Netherlands and found that the total median costs per patient were EUR 1124 for simple decompression and EUR 2730 for anterior subcutaneous transposition. This difference was mainly due to the costs related to sick leave, which was shorter for simple decompression (Bartels 2005b). In 2012, Song and colleagues performed a cost analysis and compared simple decompression, anterior subcutaneous transposition, anterior submuscular transposition, and medial epicondylectomy (Song 2012). They found that simple decompression yielded incremental cost‐effectiveness ratios of less than USD 2027 per quality‐adjusted life‐year, and as a result was the most cost‐effective treatment. Endoscopic and open decompression are equally effective in improving clinical function, but a significantly higher rate of postoperative haematoma occurred with the endoscopic approach.
In clinically mild or moderate UNE, instructions to avoid movements or positions provoking the symptoms were sufficient to improve subjective discomfort, but the quality of the evidence was very poor (some bias, small numbers, electrophysiologically unconfirmed UNE in most cases, dropouts). Corticosteroid injection does not improve symptoms of UNE.
Overall completeness and applicability of evidence
The available evidence is insufficient to identify the best treatment based on clinical, neurophysiological, and imaging characteristics. We did not think there was enough evidence to justify a multiple‐treatment meta‐analysis. Only two RCTs were available on the effectiveness of conservative treatments, and in one of these studies only 24% of participants had neurophysiological evidence of UNE. No RCT compared a surgically treated UNE group and an untreated or conservatively treated group. Currently, the most common practice is to treat patients with mild symptoms and without muscular weakness conservatively, while surgery is reserved for cases that do not show benefit after conservative treatments or with severe neurological symptoms and signs (persistent paraesthesia, objective motor weakness, or muscular atrophy). Our meta‐analysis suggests that simple decompression and decompression with transposition are equally effective in people with severe UNE, but simple decompression is associated with a lower rate of complications (wound infections and scar area sensory loss) than decompression with transposition. Not all studies measured adverse events.
No evidence was available on the effects of surgery on quality of life and imaging characteristics of the ulnar nerve at the elbow.
Quality of the evidence
None of the studies identified and included in our meta‐analysis was at an overall high risk of bias. All were small. All the studies were RCTs, which permitted evaluation of a group of participants with clinical and electrophysiological evidence of ulnar nerve impairment who were sufficiently representative of the UNE population. All degrees of severity of nerve impairment were considered, and the number of participants was high (261 participants for the clinical outcomes and 101 participants for the neurophysiological outcome). The follow‐up rate was very high (only eight participants were lost to follow‐up). The method used to generate the allocation sequence was adequate in three of the four RCTs included in the meta‐analyses.
We observed no significant heterogeneity among studies, which allowed good precision of the estimated intervention effect. Some methodological problems must be highlighted. The four most important methodological weaknesses were:
-
an unblinded observer in one study (in two studies it was unclear whether the examiner was blinded);
-
inadequate sequence generation and unclear allocation concealment method in one study;
-
unclear allocation concealment method in two studies; and
-
no clear definition of the hypothesis being tested in all the studies.
Moreover, in all seven trials the authors neither specified whether the study was designed to be a non‐inferiority or a superiority trial, nor did they calculate sample size.
Potential biases in the review process
We believe the present review has a low likelihood of language or location bias. Indeed, we searched in different databases without any language limitations. We obtained translations of articles written in languages other than English. We cannot exclude publication bias due to a higher rate of publication among studies with statistically significant effects, or time lag bias due to research findings published after our analysis of the literature.
Agreements and disagreements with other studies or reviews
In a previous review on the surgical treatment of UNE (Zlowodzki 2007), the authors identified the same RCTs that we included in our meta‐analysis up to that point. However, the authors used a different statistical approach. Zlowodzki and colleagues analysed the clinical scores as continuous variables and, because different scoring systems were used in each study, they applied a conversion of effect sizes (standardised mean difference). In accordance with our protocol, we dichotomised the primary outcome measure into improvement or no improvement, regardless of differences between the tools used. Despite the difference in statistical evaluation, Zlowodzki and colleagues concluded that simple decompression and decompression with transposition are equally effective. In their review, two different and not entirely comparable surgical techniques (submuscular and subcutaneous transposition) were considered together in the meta‐analysis. We also performed a meta‐analysis comparing submuscular transposition and simple decompression, and found no difference between the procedures. Macadam and colleagues used a similar approach in their review, but the they also introduced non‐randomised trials into the meta‐analysis, with a higher likelihood of selection bias (Macadam 2008).

A flow diagram illustrating the study selection process.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Clinical and neurophysiological effect of simple decompression versus transposition, outcome: 1.1 Proportion of participants with clinical improvement in function compared to baseline.
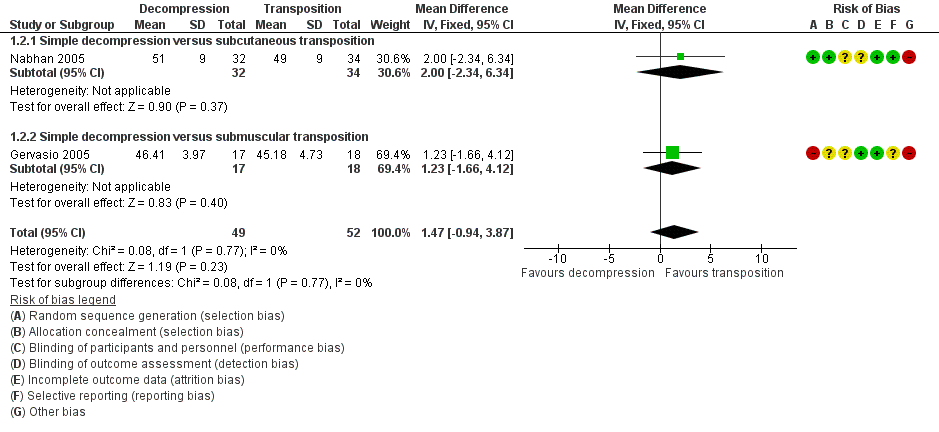
Forest plot of comparison: 1 Clinical and neurophysiological effect of simple decompression versus transposition, outcome: 1.2 Postoperative motor nerve conduction velocity.
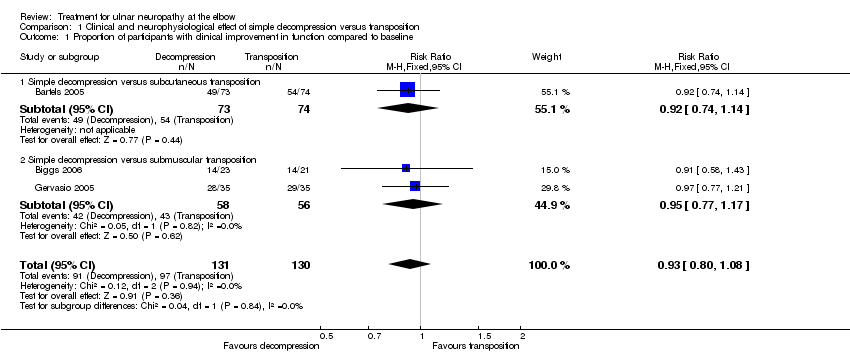
Comparison 1 Clinical and neurophysiological effect of simple decompression versus transposition, Outcome 1 Proportion of participants with clinical improvement in function compared to baseline.

Comparison 1 Clinical and neurophysiological effect of simple decompression versus transposition, Outcome 2 Postoperative motor nerve conduction velocity.

Comparison 1 Clinical and neurophysiological effect of simple decompression versus transposition, Outcome 3 Proportion of participants with deep/superficial wound infections.
| Simple decompression versus transposition for ulnar neuropathy at the elbow | ||||||
| Patient or population: people with ulnar neuropathy at the elbow | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Transposition | Simple decompression | |||||
| Proportion of participants with clinical improvement in function compared to baseline | 746 per 10001 | 694 per 1000 | RR 0.93 | 261 | ⊕⊕⊕⊝ | |
| Subgroup: proportion of participants with clinical improvement in function compared to baseline ‐ simple decompression versus subcutaneous transposition | 730 per 10001 | 672 per 1000 | RR 0.92 | 147 | ⊕⊕⊕⊝ | |
| Subgroup: proportion of participants with clinical improvement in function compared to baseline ‐ simple decompression versus submuscular transposition | 768 per 10001 | 730 per 1000 | RR 0.95 | 114 | ⊕⊕⊕⊝ | |
| Adverse events: proportion of participants with deep/superficial wound infections | 115 per 10001 | 37 per 1000 | RR 0.32 | 261 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The assumed risk was considered as the median of the risks in the control groups across studies. We did not consider the mean, since the number of studies was low, and the median is the best measure of central tendency in this case. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with clinical improvement in function compared to baseline Show forest plot | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.08] |
| 1.1 Simple decompression versus subcutaneous transposition | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.74, 1.14] |
| 1.2 Simple decompression versus submuscular transposition | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.17] |
| 2 Postoperative motor nerve conduction velocity Show forest plot | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | 1.47 [‐0.94, 3.87] |
| 2.1 Simple decompression versus subcutaneous transposition | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐2.34, 6.34] |
| 2.2 Simple decompression versus submuscular transposition | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [‐1.66, 4.12] |
| 3 Proportion of participants with deep/superficial wound infections Show forest plot | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.85] |

