Tratamiento de la neuropatía cubital del codo
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register (CRS) search strategy
#1 MeSH DESCRIPTOR Ulnar Neuropathies Explode All [REFERENCE] [STANDARD]
#2 "ulnar neuropath*" or "ulnar nerve" or "compression syndrome*" [REFERENCE] [STANDARD]
#3 #1 or #2 [REFERENCE] [STANDARD]
#4 elbow [REFERENCE] [STANDARD]
#5 #3 and #4 [REFERENCE] [STANDARD]
#6 "cubital tunnel syndrome" [REFERENCE] [STANDARD]
#7 #5 or #6 [REFERENCE] [STANDARD]
#8 (#5 or #6) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. CENTRAL search strategy
#1 elbow
#2 ulnar next neuropath*
#3 ulnar next nerve*
#4 nerve next compression
#5 #2 or #3 or #4
#6 #1 and #5
#7 cubital next tunnel
#8 #6 or #7
Appendix 3. MEDLINE (OvidSP) search strategy
Ovid MEDLINE(R) 1946 to May Week 3 2016
Database: Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 randomized controlled trial.pt. (417272)
2 controlled clinical trial.pt. (90753)
3 randomized.ab. (354957)
4 placebo.ab. (172532)
5 drug therapy.fs. (1860309)
6 randomly.ab. (254384)
7 trial.ab. (367092)
8 groups.ab. (1586892)
9 or/1‐8 (3789565)
10 exp animals/ not humans.sh. (4247320)
11 9 not 10 (3266063)
12 Ulnar Neuropathies/ or ulnar neuropath$.tw. or Ulnar Nerve Compression Syndromes/ or Ulnar Nerve/ or ulnar nerve$.tw. (9958)
13 Elbow/ or elbow$.tw. (27662)
14 12 and 13 (2081)
15 Cubital Tunnel Syndrome/ or (cubital tunnel adj5 syndrome$).tw. (667)
16 14 or 15 (2452)
17 11 and 16 (265)
18 remove duplicates from 17 (264)
Appendix 4. Embase (OvidSP) search strategy
Database: Embase <1980 to 2016 Week 22>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 crossover‐procedure.sh. (47128)
2 double‐blind procedure.sh. (128476)
3 single‐blind procedure.sh. (22118)
4 randomized controlled trial.sh. (401880)
5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1252828)
6 trial.ti. (199320)
7 or/1‐6 (1402288)
8 (animal/ or nonhuman/ or animal experiment/) and human/ (1483388)
9 animal/ or nonanimal/ or animal experiment/ (3570735)
10 9 not 8 (2954815)
11 7 not 10 (1290837)
12 limit 11 to embase (1064995)
13 Cubital Tunnel Syndrome/ or (Cubital Tunnel adj5 Syndrome).tw. (2023)
14 ulnar neuropath$.tw. or ulnar nerve/ or ulnar nerve.tw. or nerve compression/ (21186)
15 elbow.tw. or elbow/ (33336)
16 14 and 15 (2860)
17 13 or 16 (4114)
18 12 and 17 (138)
19 remove duplicates from 18 (137)
Appendix 5. AMED (OvidSP) search strategy
Database: AMED (Allied and Complementary Medicine) <1985 to May 2016>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Randomized controlled trials/ (1780)
2 Random allocation/ (313)
3 Double blind method/ (585)
4 Single‐Blind Method/ (75)
5 exp Clinical Trials/ (3543)
6 (clin$ adj25 trial$).tw. (6397)
7 ((singl$ or doubl$ or treb$ or trip$) adj25 (blind$ or mask$ or dummy)).tw. (2664)
8 placebos/ (571)
9 placebo$.tw. (2887)
10 random$.tw. (15869)
11 research design/ (1847)
12 Prospective Studies/ (898)
13 meta analysis/ (175)
14 (meta?analys$ or systematic review$).tw. (2852)
15 control$.tw. (32646)
16 (multicenter or multicentre).tw. (916)
17 ((study or studies or design$) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment$)).tw. (11738)
18 or/12‐17 (41971)
19 Cubital Tunnel Syndrome/ or (Cubital Tunnel adj5 Syndrome).tw. (11)
20 ulnar neuropath$.mp. or ulnar nerve/ or ulnar nerve.tw. or nerve compression syndromes/ (381)
21 elbow.tw. or elbow/ (1959)
22 20 and 21 (65)
23 19 or 22 (71)
24 18 and 23 (15)
25 remove duplicates from 24 (15)
Appendix 6. LILACS search strategy
(((MH:C10.668.829.500.850$ or "ulnar neuropathy" or "ulnar neuropathies" or "neuropatias cubitale" or "neuropatias ulnares" or "ulnar nerve" or "nervo ulnar") and (elbow or elbows or codo or cotovelo)) or ("cubital tunnel syndrome" or "sindrome del tunel cubital" or "sindrome do tunel ulnar")) and ((PT:"Randomized Controlled Trial" or "Randomized Controlled trial" or "Ensayo Clínico Controlado Aleatorio" or "Ensaio Clínico Controlado Aleatório" or PT:"Controlled Clinical Trial" or "Ensayo Clínico Controlado" or "Ensaio Clínico Controlado" or "Random allocation" or "Distribución Aleatoria" or "Distribuição Aleatória" or randon$ or Randomized or randomly or "double blind" or "duplo‐cego" or "duplo‐cego" or "single blind" or "simples‐cego" or "simples cego" or placebo$ or trial or groups) AND NOT (B01.050$ AND NOT (humans or humanos or humanos)))
Appendix 7. EBSCOhost CINAHL search strategy
Monday, June 06, 2016 11:01:44 AM
S30 S28 AND S29 8
S29 EM 20141014‐ 577,335
S28 S18 and S27 74
S27 S25 and S26 312
S26 MH elbow or ti elbow or ab elbow 3,585
S25 S19 or S20 or S21 or S22 or S23 or S24 2,330
S24 nerve compression n5 syndrome* 1,487
S23 (MH "Nerve Compression Syndromes") 1,401
S22 cubital tunnel n5 syndrome* 119
S21 ulnar neuropath* 279
S20 ti (ulnar nerve*) or ab (ulnar nerve*) 298
S19 (MH "Ulnar Nerve") 809
S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 836,818
S17 ABAB design* 91
S16 TI random* or AB random* 173,912
S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 345,985
S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 125,186
S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 46,385
S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 26,841
S11 PT ("clinical trial" or "systematic review") 132,020
S10 (MH "Factorial Design") 972
S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 282,840
S8 (MH "Meta Analysis") 24,634
S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 49
S6 (MH "Quasi‐Experimental Studies") 7,850
S5 (MH "Placebos") 9,729
S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 33,490
S3 (MH "Clinical Trials+") 198,849
S2 (MH "Crossover Design") 13,769
S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 72,718
Appendix 8. PEDro search strategy
Simple search: "cubital tunnel syndrome", "ulnar neuropathy"
Advanced search
Abstract and Title: "cubital tunnel syndrome" OR "ulnar neuropathy elbow"
Appendix 9. ClinicalTrials.gov search strategy
Simple search: "ulnar nerve"
Appendix 10. World Health Organization International Clinical Trials Registry Platform search strategy
Simple search: "ulnar nerve"

A flow diagram illustrating the study selection process.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Clinical and neurophysiological effect of simple decompression versus transposition, outcome: 1.1 Proportion of participants with clinical improvement in function compared to baseline.
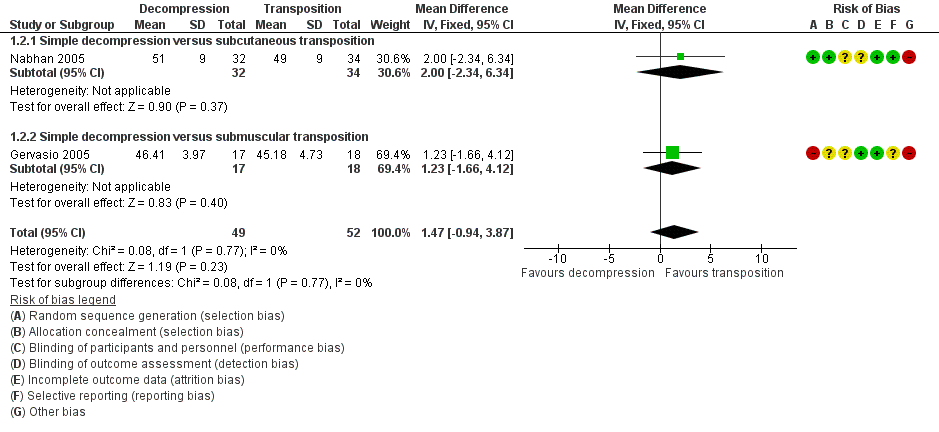
Forest plot of comparison: 1 Clinical and neurophysiological effect of simple decompression versus transposition, outcome: 1.2 Postoperative motor nerve conduction velocity.
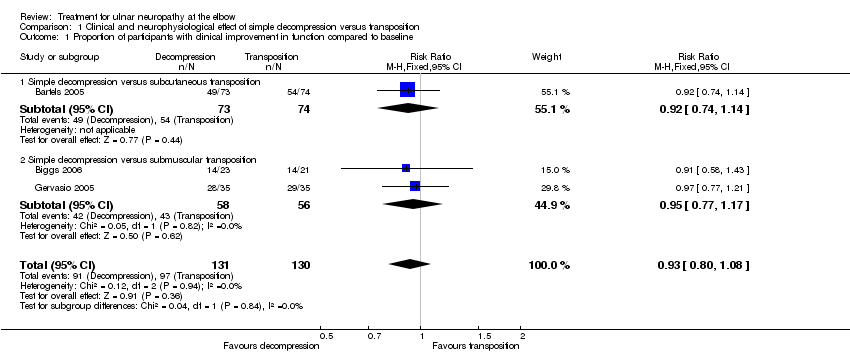
Comparison 1 Clinical and neurophysiological effect of simple decompression versus transposition, Outcome 1 Proportion of participants with clinical improvement in function compared to baseline.

Comparison 1 Clinical and neurophysiological effect of simple decompression versus transposition, Outcome 2 Postoperative motor nerve conduction velocity.

Comparison 1 Clinical and neurophysiological effect of simple decompression versus transposition, Outcome 3 Proportion of participants with deep/superficial wound infections.
| Simple decompression versus transposition for ulnar neuropathy at the elbow | ||||||
| Patient or population: people with ulnar neuropathy at the elbow | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Transposition | Simple decompression | |||||
| Proportion of participants with clinical improvement in function compared to baseline | 746 per 10001 | 694 per 1000 | RR 0.93 | 261 | ⊕⊕⊕⊝ | |
| Subgroup: proportion of participants with clinical improvement in function compared to baseline ‐ simple decompression versus subcutaneous transposition | 730 per 10001 | 672 per 1000 | RR 0.92 | 147 | ⊕⊕⊕⊝ | |
| Subgroup: proportion of participants with clinical improvement in function compared to baseline ‐ simple decompression versus submuscular transposition | 768 per 10001 | 730 per 1000 | RR 0.95 | 114 | ⊕⊕⊕⊝ | |
| Adverse events: proportion of participants with deep/superficial wound infections | 115 per 10001 | 37 per 1000 | RR 0.32 | 261 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The assumed risk was considered as the median of the risks in the control groups across studies. We did not consider the mean, since the number of studies was low, and the median is the best measure of central tendency in this case. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with clinical improvement in function compared to baseline Show forest plot | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.08] |
| 1.1 Simple decompression versus subcutaneous transposition | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.74, 1.14] |
| 1.2 Simple decompression versus submuscular transposition | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.17] |
| 2 Postoperative motor nerve conduction velocity Show forest plot | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | 1.47 [‐0.94, 3.87] |
| 2.1 Simple decompression versus subcutaneous transposition | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐2.34, 6.34] |
| 2.2 Simple decompression versus submuscular transposition | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [‐1.66, 4.12] |
| 3 Proportion of participants with deep/superficial wound infections Show forest plot | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.85] |

