Radioterapi adjuvan dan/atau kemoterapi selepas pembedahan untuk karsinosarkoma uterus
Abstract
Background
Uterine carcinosarcomas are uncommon with about 35% not confined to the uterus at diagnosis. The survival of women with advanced uterine carcinosarcoma is poor with a pattern of failure indicating greater likelihood of upper abdominal and distant metastatic recurrence.
Objectives
To evaluate the effectiveness and safety of adjuvant radiotherapy and/or systemic chemotherapy in the management of uterine carcinosarcoma.
Search methods
We searched the Cochrane Gynaecological Cancer Group Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), 2012, Issue 10, MEDLINE and EMBASE up to November 2012. We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria
Randomised controlled trials (RCTs) comparing adjuvant radiotherapy and/or chemotherapy in women with uterine carcinosarcoma.
Data collection and analysis
Two review authors independently abstracted data and assessed risk of bias. Hazard ratios (HRs) for overall survival (OS) and progression‐free survival (PFS) and risk ratios (RRs) comparing adverse events in women who received radiotherapy and/or chemotherapy were pooled in random‐effects meta‐analyses.
Main results
Three trials met the inclusion criteria and these randomised 579 women, of whom all were assessed at the end of the trials. Two trials assessing 373 participants with stage III to IV persistent or recurrent disease, found that women who received combination therapy had a significantly lower risk of death and disease progression than women who received single agent ifosfamide, after adjustment for performance status (HR = 0.75, 95% confidence interval (CI): 0.60 to 0.94 and HR = 0.72, 95% CI: 0.58 to 0.90 for OS and PFS respectively). There was no statistically significant difference in all reported adverse events, with the exception of nausea and vomiting, where significantly more women experienced these ailments in the combination therapy group than the Ifosamide group (RR = 3.53, 95% CI: 1.33 to 9.37).
In one trial there was no statistically significant difference in the risk of death and disease progression in women who received whole body irradiation and chemotherapy, after adjustment for age and FIGO stage (HR = 0.71, 95% CI: 0.48 to 1.05 and HR = 0.79, 95% CI: 0.53 to 1.18 for OS and PFS respectively). There was no statistically significant difference in all reported adverse events, with the exception of haematological and neuropathy morbidities, where significantly less women experienced these morbidities in the whole body irradiation group than the chemotherapy group (RR= 0.02, 95% CI: 0.00 to 0.16) for haematological morbidity and all nine women in the trial experiencing neuropathy morbidity were in the chemotherapy group).
Authors' conclusions
In advanced stage metastatic uterine carcinosarcoma as well as recurrent disease adjuvant combination, chemotherapy with ifosfamide should be considered. Combination chemotherapy with ifosfamide and paclitaxel is associated with lower risk of death compared with ifosfamide alone. In addition, radiotherapy to the abdomen is not associated with improved survival.
PICOs
Ringkasan bahasa mudah
Tambahan kemoterapi dan/atau rawatan radiasi selepas pembedahan dalam karsinosarkoma rahim
Karsinosarkoma uterus (rahim) merupakan kanser yang jarang‐jarang ditemui merangkumi 4.3% daripada semua kanser rahim. Kanser yang nadir ini mempunyai prognosis yang tidak baik; salah satu sebab bagi hasil kelangsungan hidup yang rendah ialah hakikat di mana lebih sepertiga kanser ini (karsinosarkoma) telahpun merebak melampaui rahim pada masa diagnosis.
Rawatan utama ialah pembedahan untuk membuang kanser, bagaimanapun, disebabkan oleh kadar yang tinggi bagi pengulangan kedua‐dua lokal dan berjarak jauh selepas pembedahan, terapi adjuvan yang berkesan diperlukan. Ulasan ini menunjukkan wanita dengan penyakit di peringkat yang tinggi (penyakit berterusan atau berulang, peringkat III‐IV) yang telah menerima kemoterapi kombinasi termasuk ifosfamide mempunyai risiko kematian dan unjuran penyakit yang lebih rendah berbanding mereka yang menerima ifosfamide sahaja, selepas pelarasan ke atas status prestasi.
Di samping itu, radioterapi ke atas abdomen tidak berkaitan dengan penambahbaikan kelangsungan hidup, seperti pengulas temui dalam satu kajian bahawa tiada perbezaan dalam risiko kematian dan unjuran penyakit pada wanita yang menerima penyinaran ke atas seluruh abdomen dan kemoterapi, selepas pelarasan ke atas umur dan peringkat penyakit. Kajian yang lepas telah menunjukkan doxorubicin, walaupun telah diterima penggunaannya dalam rawatan karsinoma uterus, kelihatan tidak begitu aktif.
Kejadian‐kejadian tidak diingini dilaporkan dengan komprehensifnya bagi perbandingan antara terapi kombinasi dan ifosfamide dengan penyinaran keseluruhan tubuh dan kemoterapi. Lebih ramai wanita mengalami kesan sampingan apabila mereka menerima terapi kombinasi berbanding ifosamide semata‐mata, dan kemoterapi berbanding penyinaran keseluruhan badan. Kesan terapi ke atas kualiti kehidupan tidak dilaporkan dalam mana‐mana kajian.
Authors' conclusions
Background
Description of the condition
Uterine carcinosarcomas are uncommon accounting for 4.3% of all cancers of the uterine corpus in Western Populations (El Nashar 2011; Young 1981). The worldwide annual incidence is between 0.5 and 3.3 cases per 100,000 women (Harlow 1986; Brooks 2004). In the UK the incidence of sarcoma is quoted to be 1 per 100,000 women and of these, 87% are carcinosarcomas (Olah 1992). Uterine carcinosarcomas, also called malignant mixed mesodermal tumours (MMT) or malignant mixed mullerian tumours (MMMT) are rare tumours with both malignant epithelial and malignant mesenchymal components. Surveillance Epidemiology and End Results (SEER) programme data also demonstrated that carcinosarcoma was the predominant uterine sarcoma (0.82/100,000) followed by leiomyosarcoma (0.64/100,000) and endometrial stromal sarcoma (0.19/100,000) (Harlow 1986).
Uterine carcinosarcomas tend to be aggressive with poor prognosis in comparison to uterine adenocarcinomas (Barwick 1979; Gadducci 2002; Toyoshima 2004). About 35% of carcinosarcomas are not confined to the uterus at diagnosis, and in most reports the median overall survival was about 21 months (Gadducci 2002). The most important prognostic factor is the extent of the tumour at the time of diagnosis; the prognosis being very poor when the tumour has extended beyond the uterus (Sartori 1997). There has been no significant improvement in survival suggested by some reports (Callister 2004; Chi 1997; Le 2001; Sutton 2000).
Carcinosarcomas can be subdivided histologically into homologous and heterologous types and it is important to differentiate the tumours that are monoclonal, that is those derived from a single stem cell, from true mixed cell tumours (Zelmanowicz 1998). This histological distinction (McCluggage 2002) is significant as the natural history of true mixed carcinosarcomas appear to be more aggressive. There is convincing recent evidence that most cases of uterine carcinosarcoma are monoclonal in origin (Szukala 1999; Toyoshima 2004). These data indicate that uterine carcinosarcoma may be metaplastic, with the implication that the sarcomatous components are derived from the carcinomatous elements (McCluggage 2002).
Histological diagnosis and clinical staging (based on findings at surgery) usually follows primary treatment which is surgical. In a prospective multi‐centre Gynecologic Oncology Group (GOG) study of carcinosarcomas 61 of the 301 patients (20%) with clinical Stage I and II disease were reassigned to pathological Stages III and IV on the basis of lymph node metastases. The study also revealed a recurrence rate of 53% for all carcinosarcomas, with 44% for homologous and 63% for heterologous tumours (Major 1993).
Description of the intervention
As with uterine adenocarcinomas, the mainstay of treatment is surgical removal of the tumour (Menczer 2005), however, the high rates of both local and distant relapse after surgery suggests a need for effective adjuvant therapies (Galaal 2009; Sutton 2000). The survival of patients with advanced uterine carcinosarcoma is poor with a pattern of failure indicating a higher likelihood of upper abdominal and distant metastatic recurrence (Galaal 2009; Spanos 1986). These patients are less likely to benefit from local adjuvant therapy and therefore consideration for systemic adjuvant chemotherapy as well as whole body irradiation has been considered in several studies (Chi 1997; Menczer 2005; Ramondetta 2003; Sutton 1989).
How the intervention might work
Several chemotherapeutic agents have been examined as single agent therapy in uterine carcinosarcoma with response rates as follows: 16% to19% with adriamycin (Omura 1983), 32% to 36% with ifosfamide (Sutton 1989; Sutton 2000), 19% with cisplatin (Thigpen 2004), and 18% with paclitaxel (Curtin 2001). Doxorubicin, despite being established in the treatment of uterine carcinoma, does not seem to be highly active in uterine carcinosarcoma (Omura 1983). Combination chemotherapeutic agents have been used in uterine carcinosarcoma with combination therapy appearing to be superior to single‐agent treatment in terms of improvement in progression‐free and overall survival. However, these combination therapies may be associated with increased toxicity (Homesley 2007; Sutton 2000; Van Rijswijk 1994). Whole abdominal irradiation has been investigated in a retrospective study on early staged uterine carcinosarcoma in the adjuvant setting. This study suggested that the addition of whole abdominal irradiation did not improve survival (Chi 1997).
Why it is important to do this review
Carcinosarcoma is a disease with a high recurrence rate (40% to 60%), and a tendency to distant metastasis, therefore, an effective systemic therapy may improve the outcomes of this disease. Several chemotherapeutic agents have been shown to produce objective response rates in patients with advanced carcinosarcoma. In addition, whole abdominal irradiation has been used in the adjuvant setting (Chi 1997; Ramondetta 2003). These treatment modalities may be associated with some costs in terms of toxicity and quality of life (QoL). Therefore, there is a need to assess the effectiveness and safety rigorously.
Objectives
To evaluate the role of adjuvant radiotherapy and/or systemic chemotherapy in the management of uterine carcinosarcoma.
Specifically, we wanted to address the following questions.
-
Is adjuvant systemic chemotherapy more effective than adjuvant radiotherapy?
-
Is adjuvant systemic combination chemotherapy more effective than single agent chemotherapy?
-
Is adjuvant radiotherapy and/or systemic chemotherapy well tolerated?
Methods
Criteria for considering studies for this review
Types of studies
-
Randomised controlled trials (RCTs)
Types of participants
Women of any age with a histological diagnosis of uterine carcinosarcoma of any International Federation of Gynecology and Obstetrics (FIGO) stage (FIGO 2009).
Types of interventions
Intervention
Surgery followed by radiotherapy and/or systemic chemotherapy.
Comparison
Additionally, we considered any direct comparison between:
-
adjuvant radiotherapy or combination chemotherapy;
-
adjuvant single drug chemotherapy versus combination chemotherapy;
-
surgery alone or best supportive care.
Types of outcome measures
Primary outcomes
Overall survival (OS): Survival until death from all causes. Survival was assessed from the time when women were enrolled in the study.
Secondary outcomes
-
Progression‐free survival (PFS).
-
Quality of life (QoL), measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication.
-
Grades 3 and 4 chemotherapeutic and radiotherapeutic toxicity, classified according to CTCAE 2006, was extracted and grouped as:
-
-
haematological (leucopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage);
-
gastrointestinal (nausea, vomiting, anorexia, diarrhoea, liver, proctitis);
-
genitourinary;
-
skin (stomatitis, mucositis, alopecia, allergy);
-
neurological (peripheral and central);
-
pulmonary.
-
Search methods for identification of studies
Papers in all languages were sought and translations carried out when necessary.
Electronic searches
See: Cochrane Gynaecological Cancer Group methods used in reviews.
The following electronic databases were searched.
-
The Cochrane Gynaecological Cancer Collaborative Review Group's Trial Register
-
Cochrane Central Register of Controlled Trials (CENTRAL), 2012, Issue 10
-
MEDLINE
-
EMBASE
The MEDLINE, EMBASE and CENTRAL search strategies based on terms related to the review topic are presented in Appendix 1, Appendix 2 and Appendix 3 respectively.
Databases were searched from January 1966 until November 2012.
All relevant articles found were identified on PubMed and using the 'related articles' feature; a further search was carried out for newly published articles.
Searching other resources
Unpublished and Grey literature
Metaregister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov, www.cancer.gov/clinicaltrials, NHMRC Clinical Trials Register and the UKCCCR Register of Cancer Trials were searched for ongoing trials.
Handsearching
The citation list of relevant publications, abstracts of scientific meetings and list of included studies were checked through handsearching and experts in the field contacted to identify further reports trials. Reports of conferences were handsearched in the following sources.
-
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologist)
-
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society )
-
British Journal of Cancer
-
British Cancer Research Meeting
-
Annual Meeting of the International Gynecologic Cancer Society
-
Annual Meeting of the American Society of Gynecologic Oncologist
-
British Gynaecological Cancer Society (BGCS)
-
Annual Meeting of European Society of Medical Oncology (ESMO)
-
Annual Meeting of the American Society of Clinical Oncology (ASCO)
-
European Society of Gynecological Cancer (ESGO)
Reference lists
The reference lists of all relevant trials obtained by this search were handsearched for further trials.
Correspondence
Authors of relevant trials were contacted to ask if they knew of further data which may or may not have been published.
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic searching were downloaded to the reference management database Endnote, duplicates were removed and the remaining references were independently examined by three review authors (KG, KG1, EH). These three review authors screened titles and abstracts of references identified from the search and eliminated articles that were obviously not relevant to the search question. When all authors determined that the trial was not eligible for inclusion no further action was taken. When any of the authors determined that the article may have been eligible for inclusion, the full text article was obtained. Each review author then independently determined if these trials were eligible for inclusion. Disagreements about inclusions were resolved by discussion. Further information was sought from the study authors when papers contained insufficient information to make a decision about eligibility. No attempt was made to blind review authors to article authors or journals.
Data extraction and management
For included trials, data were abstracted as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Data were independently extracted by two review authors and included the following details.
-
Author, year of publication and journal citation (including language)
-
Country
-
Setting
-
Inclusion and exclusion criteria
-
Study design, methodology
-
Study population (participant characteristics, age, stage and postoperative residual disease)
-
Number of participants in each arm of the trial
-
Total number of intervention groups
-
Uterine carcinosarcoma details (FIGO stage, histology, tumour grade)
-
Type of intervention (chemotherapy agents and radiotherapy, dosage and timing of administration relative to surgery)
-
Length of follow‐up
-
Withdrawals from treatment protocol
-
Number of participants who experienced delays in treatment or received all, part, or none of the proposed treatment
-
Risk of bias in study (see below)
-
Outcomes: OS, PFS, QoL and adverse events
-
for each outcome: outcome definition
-
unit of measurement (if relevant)
-
for scales: upper and lower limits, and whether high or low score is good
-
results: Number of participants allocated to each intervention group
-
for each outcome of interest: Sample size; Missing participants
-
Data on outcomes were extracted as below.
-
For time‐to‐event (Overall and progression‐free survival) data, we extracted the log of the hazard ratio [log(HR)] and its standard error from trial reports. If these were not reported, we attempted to estimate them from other reported statistics using the methods of Parmer 1998.
-
For dichotomous outcomes (e.g. adverse events), we extracted the number of patients in each group who experienced the outcome of interest and the number of patients assessed at endpoint, in order to estimate a risk ratio (RR).
-
The scales, grades and sites of acute toxicity information were extracted from the trials.The toxicity scales used varied from trial to trial and reporting of toxicity was otherwise inconsistent. Also, the type and severity of side effects will depend on the drugs being used in the individual trials. However, there was some commonality in the types of toxicity documented, namely: nausea and vomiting; diarrhoea/other gastrointestinal, leucopenia, thrombocytopenia, anaemia, alopecia, fever/infection, neurological toxicity and renal/GU and in the scales of toxicity used. By analysing the common types, it is possible to get an indication of the relative levels of serious toxicity associated with chemotherapy regimens and radiation therapy.
-
Both unadjusted and adjusted statistics were extracted, if reported.
Where possible, all data extracted were those relevant to an intention‐to‐treat (ITT) analysis, in which participants were analysed in groups to which they were assigned.
The time points at which outcomes were collected and reported were noted.
Data were abstracted independently by two review authors (KG, KG1) onto a data abstraction form specially designed for the review. Differences between review authors were resolved by discussion or by appeal to a third review author (AB) when necessary. Where appropriate, authors were contacted for further information and data were updated.
Assessment of risk of bias in included studies
The risk of bias in included RCTs was assessed using The Cochrane Collaboration's tool and the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included an assessment of:
-
sequence generation;
-
allocation concealment;
-
blinding (of participants, healthcare providers and outcome assessors);
-
incomplete outcome data: we coded a satisfactory level of loss to follow‐up for each outcome as:
-
'Yes', if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
-
'No', if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms;
-
'Unclear' if loss to follow‐up was not reported;
-
-
selective reporting of outcomes;
-
other possible sources of bias.
The 'Risk of bias' tool was applied independently by two review authors (KG1, KG) and differences resolved by discussion or by appeal to a third review author (AB). Results are presented in the 'Risk of bias' table and also in both a 'Risk of bias' graph and a 'Risk of bias' summary section. Results of meta‐analyses were interpreted in light of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment.
-
For time‐to‐event data, we used the hazard ratio (HR), when possible.
-
For dichotomous outcomes, we used the risk ratio (RR).
Dealing with missing data
We did not impute missing outcome data; if only imputed data were reported, we contacted trial authors to request data on the outcomes only among participants who were assessed.
Assessment of heterogeneity
Heterogeneity between trials was assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003; Higgins 2011), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, the possible reasons for this were investigated and reported.
Data synthesis
If sufficient, clinically similar studies were available, their results were pooled in meta‐analyses. Adjusted summary statistics were used where available, otherwise, unadjusted results were used.
-
For time‐to‐event data, HRs were pooled using the generic inverse variance facility of RevMan 5.
-
For any dichotomous outcomes, the RR was calculated for each trial and these were then pooled.
Random‐effects models with inverse variance weighting were used for all meta‐analyses (DerSimonian 1986).
Results
Description of studies
see Characteristics of included studies; Characteristics of excluded studies
Results of the search
The original search strategy identified 445 references in MEDLINE, 745 in EMBASE and 10 in CENTRAL. When the search results were merged into Endnote and duplicates removed, 895 unique references remained. The title and abstract screening of these references identified 15 trials as potentially eligible for this review. The full text screening of these 15 references excluded 12 for the reasons described in the table Characteristics of excluded studies. The remaining three randomised controlled trials (RCTs) Homesley 2007; Sutton 2000; Wolfson 2007) met our inclusion criteria and are described in the table Characteristics of included studies.
Searches of the grey literature identified one additional relevant ongoing trial (EORTC‐55874).
The updated search from May 2010 to October 2012 identified another 77 references in MEDLINE, 348 in EMBASE and no additional references in CENTRAL, but did not identify any new eligible trials.
Included studies
The three included trials (Homesley 2007; Sutton 2000; Wolfson 2007) randomised 579 women, of whom all (100%) were assessed at the end of the trials. Two trials (Homesley 2007; Sutton 2000) reported the comparison of combination chemotherapy versus single agent chemotherapy in the adjuvant setting for advanced or recurrent uterine carcinosarcoma. Both these trials used ifosfamide as a single agent and in the comparison arm in combination with other chemotherapeutic agents that showed activity in uterine carcinosarcoma, paclitaxel, cisplatin.
The other trial (Wolfson 2007) randomised previously untreated patients with stages I, II, III, and IV primary carcinosarcomas of the uterus or cervix to whole abdominal irradiation (WAI) and combination chemotherapy with cisplatin and Ifosfamide with mesna (CIM).
Design
Phase III RCT.
Participants
One hundred and seventy‐nine women with histologically‐confirmed stage III or IV, persistent or recurrent uterine carcinosarcoma not amenable to curative intent by other means. Median age for both arms was 64 years.
Interventions
Ninety‐one women were randomised to arm one (single agent ifosfamide),18% had stage III, 31% stage IV and 52% had recurrent/persistent disease.
Eighty‐eight women were randomised to arm two (ifosfamide + paclitaxel),18% had stage III, 29% had stage IV disease and 52% had recurrent/persistent disease. Because of the reported toxicity when a five‐day schedule of ifosfamide was used (Sutton 2000), a three‐day schedule was used in this trial in both arms.
The trial reported 150 (84%) deaths and 162 (91%) disease recurrences.
Design
Phase III RCT.
Participants
One hundred and ninety‐four women with histologically‐confirmed advanced or recurrent carcinosarcoma no longer amenable to control by surgery and/or radiotherapy. All patients had to have measurable disease that could be defined in at least two dimensions by palpation or imaging. Median age for arm one was 67 years (range 32 to 84), 66 years for arm two (range 35 to 83). The two intervention arms were balanced for age, grade, and Gynecologic Oncology Group (GOG) performance status.
Intervention
One hundred and two women were in arm one (ifosfamide) and 92 women in arm two (ifosfamide + cisplatin). Each patient received eight cycles of therapy unless there was disease progression or toxicity. The dose of the combination regimen was reduced by 20% early in this trial (day one) because of toxicity.
The trial reported 175 (90%) deaths and 182 (94%) disease recurrences.
Design
Phase III RCT.
Participants
Two hundred and six women with previously untreated stages I, II, III, and IV primary carcinosarcomas of the uterus or cervix (without any demonstrable parenchymal hepatic involvement or extra‐abdominal distant disease). Forty‐three per cent of patients randomised to the whole abdominal irradiation (WAI) arm had stage III, and 46% in the CIM arm had stage III.
Median age for WAI was 68 years and 65 years for CIM arm.
Interventions
One hundred and five women were randomised to the WAI arm and 101 to the CIM arm. Forty‐three per cent of patients randomised to the WAI arm had stage III, and 46% in the CIM arm had stage III.
The trial reported 122 (59%) deaths and 132 (64%) disease recurrences.
Outcomes reported
All three trials reported overall and recurrence‐free survival and used appropriate statistical techniques (hazard ratios (HRs) to correctly allow for censoring). Prognostic factors were adjusted for in the analysis of survival outcomes in each trial.
The HR in the trial of Homesley 2007 was adjusted for performance status.
The HR in the trial of Sutton 2000 was adjusted for performance status.
The HR in the trial of Wolfson 2007 was adjusted for age and FIGO stage.
For the distribution of these factors at baseline for each trial by treatment arm see the table Characteristics of included studies.
Grades 3 and 4 severe adverse events (haematological, gastrointestinal, genitourinary, skin, neurological, pulmonary) were reported in all trials.
Excluded studies
We excluded 12 references after obtaining the full text (see Characteristics of excluded studies). Nine references (Currie 1996; Curtin 2001; Fowler 2002; Miller 2005; Powell 2010; Ramondetta 2003; Resnik 1995; Sutton 1989; Asbury 1998) reported the results of non‐comparative phase II trials; two references (Perez 1979; Toyoshima 2004) were retrospective studies and one reference (Sutton 2005) was a study that did not have a concurrent control group.
Risk of bias in included studies
Two trials (Homesley 2007; Wolfson 2007) were at low risk of bias: they satisfied four of the criteria that we used to assess risk of bias. The trial of (Sutton 2000) was at high risk of bias as it only satisfied two criteria ‐ see Figure 1, Figure 2.
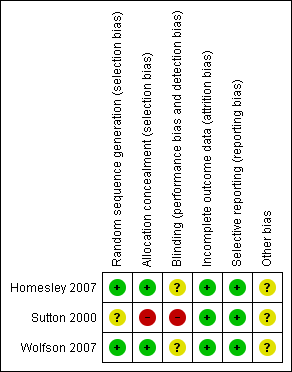
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Two trials (Homesley 2007; Wolfson 2007) reported the method of generation of the sequence of random numbers used to allocate women to treatment arms and concealment of this allocation sequence from patients and healthcare professionals involved in the trial. In the trial of (Sutton 2000) it was unclear whether the method of assigning women to treatment groups was carried out using an adequate method of sequence generation and there was no attempt to conceal the allocation. None of the trials reported whether the patients, healthcare professionals or outcome assessors were blinded. It was highly likely that all trials reported all the outcomes that they assessed, but it was not clear whether any other bias may have been present. At least 96% of women who were enrolled were assessed at endpoint in all three trials.
Effects of interventions
All meta‐analyses pooled data from two of the included trials (Homesley 2007; Sutton 2000), comparing ifosfamide and combination therapy. The trial of Wolfson 2007 compared whole body irradiation and chemotherapy in single trial analyses.
Meta‐analyses of survival are based on hazard ratios (HRs) that were adjusted for important prognostic variables.
For dichotomous outcomes, we were unable to estimate a risk ratio (RR) for comparison of whole body irradiation and chemotherapy if one or both treatment groups experienced no events, as in the trial of Wolfson 2007 for hepatic and neuropathy adverse event outcomes and in the Sutton 2000 trial for cardiovascular adverse events.
1. Combination therapy versus ifosfamide
Two trials (Homesley 2007; Sutton 2000)
Overall survival
(See Analysis 1.1)
Meta‐analysis of two trials, assessing 373 participants, found that women who received combination therapy had a significantly lower risk of death than women who received ifosfamide, after adjustment for performance status (HR = 0.75, 95% confidence interval (CI) 0.60 to 0.94). The percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) is not important (I2 = 0%).
Progression‐free survival
(See Analysis 1.2)
Meta‐analysis of two trials, assessing 373 participants, found that women who received combination therapy had a significantly lower risk of disease progression than women who received ifosfamide, after adjustment for performance status (HR= 0.72, 95% CI 0.58 to 0.90). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance is not important (I2 = 0%).
Adverse events
Severe nausea/vomiting
(See Analysis 1.3)
Meta‐analysis of two trials, assessing 365 participants, found that women who received combination therapy had a significantly higher risk of severe nausea or vomiting than women who received ifosfamide (risk ratio) (RR) = 3.53, 95% CI 1.33 to 9.37). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance is not important (I2 = 0%).
Diarrhoea and other gastrointestinal morbidities
(See Analysis 1.4)
Meta‐analysis of two trials, assessing 365 participants, showed no statistically significant difference in the risk of diarrhoea and other gastrointestinal morbidities in women who received combination therapy and those who received ifosfamide (RR 1.51, 95% CI 0.31 to 7.52). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent moderate heterogeneity (I2 = 58%).
Haematological morbidities
(See Analysis 1.5)
Meta‐analysis of two trials, assessing 365 participants, showed no statistically significant difference in the risk of haematological morbidity in women who received combination therapy and those who received ifosfamide (RR = 1.56, 95% CI 0.84 to 2.90). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent substantial heterogeneity (I2 = 78%).
Genitourinary morbidities
(See Analysis 1.6)
Meta‐analysis of two trials, assessing 365 participants, showed no statistically significant difference in the risk of genitourinary morbidity in women who received combination therapy and those who received ifosfamide (RR = 1.68, 95% CI 0.54 to 5.18). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance is not important (I2 = 0%).
Cardiovascular morbidities
(See Analysis 1.7)
Two trials (Homesley 2007; Sutton 2000), found no statistically significant difference in the risk of cardiovascular morbidity in women who received combination therapy and those who received ifosfamide (RR = 0.63, 95% CI: 0.13 to 3.11). In the trial of Homesley 2007 and the Sutton 2000 trial reported only three cases of cardiovascular morbidity, which were of woman in the Ifosamide group).
Hepatic morbidities
(See Analysis 1.8)
One trial reported on hepatic toxicity (Homesley 2007); it reported that the single agent Ifosfamide was associated with less hepatic toxicity. However, there was no statistically significant difference in the risk of hepatic morbidity in women who received combination therapy and those who received ifosfamide (RR = 2.05, 95% CI 0.73 to 5.74).
Neuropathy
(See Analysis 1.9)
Meta‐analysis of two trials, assessing 365 participants, found that women who received combination therapy had a significantly higher risk of neuropathy than women who received ifosfamide (RR = 1.59, 95% CI 0.99 to 2.55). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance is not important (I2 = 0%).
2. Whole body irradiation versus combination chemotherapy
One trial (Wolfson 2007)
Overall survival
(see Analysis 2.1)
There was no statistically significant difference in the risk of death in women who received whole body irradiation and chemotherapy, after adjustment for age and FIGO stage (HR = 0.71, 95% CI: 0.48 to 1.05).
Progression‐free survival
(see Analysis 2.2)
There was no statistically significant difference in the risk of disease progression in women who received whole body irradiation and chemotherapy, after adjustment for age and FIGO stage (HR = 0.79, 95% CI: 0.53 to 1.18).
Adverse events
Gastrointestinal morbidities
(see Analysis 2.3)
There was no statistically significant difference in the risk of gastrointestinal morbidity in women who received whole body irradiation and chemotherapy (RR = 0.92, 95% CI: 0.41 to 2.06).
Haematological morbidities
(see Analysis 2.4)
Women who received whole body irradiation after surgery for treatment of uterine carcinosarcoma were associated with significantly less chance of haematological morbidity compared with those who received chemotherapy (RR = 0.02, 95% CI: 0.00 to 0.16).
Genitourinary morbidities
(see Analysis 2.5)
There was no statistically significant difference in the risk of genitourinary morbidity in women who received whole body irradiation and chemotherapy (RR = 0.30, 95% CI: 0.09 to 1.07).
Cardiovascular morbidities
(see Analysis 2.6)
There was no statistically significant difference in the risk of cardiovascular morbidity in women who received whole body irradiation and chemotherapy (RR = 0.25, 95% CI: 0.03 to 2.22).
Hepatic morbidities
There was no statistically significant difference in the risk of hepatic morbidity in women who received whole body irradiation and chemotherapy. The trial reported only two cases of hepatic morbidity in women who were in the whole body irradiation group.
Neuropathy
Women who received whole body irradiation after surgery for treatment of uterine carcinosarcoma were associated with significantly less chance of neuropathy compared to those who received chemotherapy. The trial reported nine cases of neuropathy morbidity, all of women in the chemotherapy group.
Discussion
Summary of main results
We found three trials, enrolling 579 women, that met our inclusion criteria. Two of these trials (Homesley 2007; Sutton 2000) compared combination chemotherapy and ifosfamide alone, whereas the other trial (Wolfson 2007), compared whole body irradiation and combination chemotherapy in women with uterine carcinosarcoma.
When we combined the findings from the two similar trials, adjusted for important prognostic factors, we found that the risk of death and disease progression was lower among women who received combination therapy than among women who received ifosfamide alone (HR = 0.75, 95% CI 0.60 to 0.94 and HR = 0.72, 95% CI 0.58 to 0.90 for overall and progression‐free survival respectively). Risk of adverse events was not significantly different for most outcomes but the rate of nausea and vomiting was higher in women who received combination therapy (RR = 3.53, 95% CI 1.33, 9.37).
The other trial (Wolfson 2007) found no significant difference between whole abdominal irradiation and combination chemotherapy in terms of overall and progression‐free survival, but did seem to suggest that in general whole body irradiation was associated with less morbidity than combination chemotherapy, where haematological and neuropathic morbidities were significantly lower in women who received whole body irradiation compared to those who received combination chemotherapy (RR= 0.02, 95% CI 0.00 to 0.16 for haematological morbidity and all nine women in the trial experiencing neuropathy morbidity were in the chemotherapy group).
The trials had many strengths. They gave HRs that correctly allowed for censoring and they provided information about adverse events. Both trials recruited a satisfactory number of participants and a reasonably large number of events were observed in the two survival outcomes, but the number of women with adverse events was generally low so lacked statistical power to detect a difference.
Overall completeness and applicability of evidence
To date, two RCTs have compared the effect of ifosfamide with combination of ifosfamide and other chemotherapeutic agents. These trials suggested that combination chemotherapy may be better than single agent therapy in the treatment of advanced staged and recurrent uterine carcinosarcoma. The combination of ifosfamide and paclitaxel was associated with significant improvement in overall and progression‐free survival. The evidence from a single RCT suggested no benefit of whole abdominal irradiation over combination chemotherapy.
We were unable to report on Quality‐of‐life (QoL) as none of the included trials had QoL assessments as a component of the trials. Treatment‐related morbidity very often degrades the quality of the time that patients live, which is especially important after the completion of treatment for advanced cancer where patients have poor prognosis and will want to enjoy a comfortable standard of living during their final months.
Quality of the evidence
The amount of available evidence does allow robust conclusions for the comparison of combination therapy and ifosfamide, as there is consistency and commonality in the results, but the comparison of whole body irradiation is restricted to single trial analyses.
The reporting of the methodological quality of the trials showed that two trials (Homesley 2007; Wolfson 2007) were at low risk of bias while the trial of Sutton 2000 was at high risk of bias as it only satisfied two of the criteria used to assess risk of bias.
All three trials reported a hazard ratio (HR), which is the best statistic to summarise the difference in risk in two treatment groups over the duration of a trial, when there is "censoring" i.e. the time to death (or disease progression) is unknown for some women as they were still alive (or disease‐free) at the end of the trial.
The two similar trials (Homesley 2007; Sutton 2000) gave consistent evidence about all outcomes as individual trials were robust to the findings of the meta‐analyses, although in some instances point estimates were not necessarily similar. For survival outcomes, there is evidence that combination therapy delays death and disease progression compared with single agent ifosfamide, but we are not sure how safe combination therapy is as there were relatively low numbers of adverse events and it was associated with significantly more grades three and four nausea and vomiting. A substantial number of women experienced disease progression and death, which helps to ensure high quality evidence.
Potential biases in the review process
A comprehensive search was performed, including a thorough search of the grey literature and all studies were sifted and data independently extracted by three review authors. We restricted the included studies to RCTs as they provide the strongest level of evidence available. Hence we have attempted to reduce bias in the review process.
The greatest threat to the validity of the review is likely to be the possibility of publication bias i.e. studies that did not find the treatment to have been effective may not have been published. We were unable to assess this possibility as we found only three included trials.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the first systematic review on the effects of adjuvant treatments for uterine carcinosarcoma. There are large number of trials other than RCTs studying adjuvant chemotherapeutic agents at present. These phase II trials, observational studies or non‐controlled trials were limited by small numbers and methodological limitations. There are several non systematic reviews on uterine sarcomas and/or uterine carcinosarcoma mainly addressing clinicopathological and prognostic factors (Arrastia 1997).

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Comparison 1 Combination therapy versus Ifosfamide, Outcome 1 Overall survival.

Comparison 1 Combination therapy versus Ifosfamide, Outcome 2 Progression‐free survival.

Comparison 1 Combination therapy versus Ifosfamide, Outcome 3 G3‐4 Nausea/vomiting.

Comparison 1 Combination therapy versus Ifosfamide, Outcome 4 Diarrhoea and other GI.

Comparison 1 Combination therapy versus Ifosfamide, Outcome 5 Haematological.

Comparison 1 Combination therapy versus Ifosfamide, Outcome 6 Genitourinary.

Comparison 1 Combination therapy versus Ifosfamide, Outcome 7 Cardiovascular.

Comparison 1 Combination therapy versus Ifosfamide, Outcome 8 Hepatic.

Comparison 1 Combination therapy versus Ifosfamide, Outcome 9 Neuropathy.

Comparison 2 Whole body irradiation versus chemotherapy, Outcome 1 Overall survival.

Comparison 2 Whole body irradiation versus chemotherapy, Outcome 2 Progression‐free survival.

Comparison 2 Whole body irradiation versus chemotherapy, Outcome 3 G3‐4 Gastrointestinal.

Comparison 2 Whole body irradiation versus chemotherapy, Outcome 4 Haematological.

Comparison 2 Whole body irradiation versus chemotherapy, Outcome 5 Genitourinary.

Comparison 2 Whole body irradiation versus chemotherapy, Outcome 6 Cardiovascular.

Comparison 2 Whole body irradiation versus chemotherapy, Outcome 7 Hepatic.

Comparison 2 Whole body irradiation versus chemotherapy, Outcome 8 Neuropathy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 2 | 373 | Hazard Ratio (Random, 95% CI) | 0.75 [0.60, 0.94] |
| 2 Progression‐free survival Show forest plot | 2 | 373 | Hazard Ratio (Random, 95% CI) | 0.72 [0.58, 0.90] |
| 3 G3‐4 Nausea/vomiting Show forest plot | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 3.53 [1.33, 9.37] |
| 4 Diarrhoea and other GI Show forest plot | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.31, 7.52] |
| 5 Haematological Show forest plot | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 1.56 [0.84, 2.90] |
| 6 Genitourinary Show forest plot | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 1.68 [0.54, 5.18] |
| 7 Cardiovascular Show forest plot | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.13, 3.11] |
| 8 Hepatic Show forest plot | 1 | 174 | Risk Ratio (IV, Random, 95% CI) | 2.05 [0.73, 5.74] |
| 9 Neuropathy Show forest plot | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 1.59 [0.99, 2.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2 Progression‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3 G3‐4 Gastrointestinal Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4 Haematological Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5 Genitourinary Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6 Cardiovascular Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7 Hepatic Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8 Neuropathy Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |

