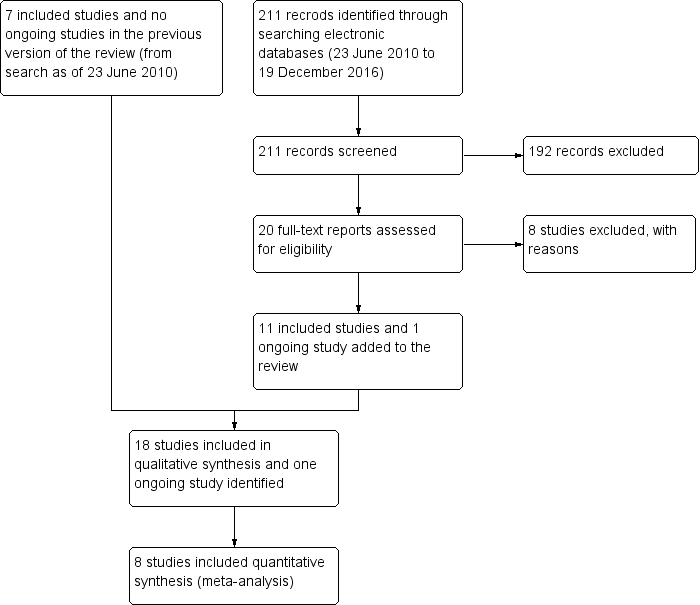
Oclusión puntual para el síndrome de ojo seco
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: parallel randomized controlled trial Unit of analysis: unclear as trial investigators did not report if they had only included one eye in the analysis or took the averaged of both eyes Unit of randomization: participant, both eyes received the same intervention How were missing data handled? No missing data reported Reported power calculation: NR Unusual study design: 22 eyes of age‐ and gender‐matched healthy volunteers were included (tear production and film stability, lacrimal scintigraphy measurements only; no punctal plugs inserted) | |
| Participants | Country: Turkey Number randomized: 24 participants (48 eyes) in total 11 participants (22 eyes) in collagen punctal plugs group 13 participants (26 eyes) in silicone punctal plugs group Exclusions after randomization: none reported Number analyzed: 24 participants (48 eyes) in total 11 participants (22 eyes) in collagen punctal plugs group 13 participants (26 eyes) in silicone punctal plugs group Losses to follow‐up: none reported Overall mean age (SD): NR Age range: NR Sex (%): 21 women (88%) and 3 men (12%) in total; by group not reported Inclusion criteria: participants were diagnosed with aqueous tear deficiency; no previous history of punctal plug insertion. All using artificial tears with no subjective or objective improvement in symptoms Exclusion criteria: NR | |
| Interventions | No mention of artificial tears use Intervention 1: collagen punctal plugs were inserted in the lower punctum Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics Location of manufacturer: Eastsound, WA Intervention 2: silicone punctal plugs were inserted in the lower punctum Punctal plug model: Odyssey‐Parasol Punctal Occluder A14 to 203 Manufacturer of punctal plug: Oasis Medical Location of manufacturer: Memphis, TN Length of follow‐up: Planned: protocol not available | |
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported: tear production (Schirmer I test at 5 minutes performed before and immediately after occlusion); tear film stability (tear break‐up time (TBUT) measured in seconds before and immediately after occlusion); ocular surface staining (Rose Bengal strip before and 3 days after occlusion) Intervals at which outcomes assessed: before treatment, after treatment, and 3 days post‐treatment | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: NR Disclosures of interest: NR Study period: NR Reported subgroup analyses: none reported Investigators did not discuss how they accounted for the correlation between eyes of the same person | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Not described, but given the treatment groups it is not possible to mask participants and people administering interventions. |
| Masking of outcome assessment (detection bias) | Unclear risk | Nuclear medicine specialist (outcome assessor) evaluating lacrimal scintigraphy images was masked to treatment assignment (but not an outcome of this review) Unclear whether other outcome assessors masked |
| Incomplete outcome data (attrition bias) | Unclear risk | No sample size information included in Results section, so it is unclear whether all participants completed follow‐up examinations. Most outcomes assessed immediately after insertion of the punctal plugs (ocular surface staining assessed 3 days after occlusion) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine. Trial registry information and protocol were not available for comparison |
| Other bias | Unclear risk | Insufficient information to determine. Source of funding and conflict of interest not reported |
| Methods | Study design: paired‐eye randomized controlled trial Unit of analysis: eyes; no mention of analysis accounting for correlation between the left and right eye Unit of randomization: each eye of each participant was randomized to a different intervention How were missing data handled? Excluded from analysis Reported power calculation: sample size = 50 participants (25 participants per group); power = 80.7% Unusual study design: none | |
| Participants | Country: Canada Number randomized: 26 participants (52 eyes) in total 26 participants (26 eyes) in collagen punctal plugs group 26 participants (26 eyes) in silicone punctal plugs group Exclusions after randomization: none reported Number analyzed: 25 participants (50 eyes) in total 25 participants (25 eyes) in collagen punctal plugs group 25 participants (25 eyes) in silicone punctal plugs group Losses to follow up: 1 participant (2 eyes) in total 1 participants (1 eyes) in collagen punctal plugs group 1 participants (1 eyes) in silicone punctal plugs group Mean age (SD): 60.05 years (NR) overall 61.7 (17.67) years in the collagen punctal plug group 58.4 (16.18) years in the silicone punctal plug group Age range: NR Sex (%): 21 women (81%) and 5 men (19%) in total 10 women (77%) and 3 men (23%) in the collagen punctal plug group 11 women (85%) and 2 men (15%) in the silicone punctal plug group Inclusion criteria: "moderate to severe subjective dry eye symptoms as per the Canadian Dry Eye Assessment (CDEA), a validated dry eye symptoms questionnaire based on the Ocular Surface Disease Index (OSDI)" (p 239) Exclusion criteria: "dry eye secondary to systemic inflammatory conditions, punctal cautery, punctal stenosis, silicone allergy, and inability to attend multiple follow‐up visits for 6 months" (p 239) | |
| Interventions | Both groups were allowed to use artificial tears throughout the follow up period Intervention 1: collagen punctal plugs were inserted in the lower punctum Punctal plug model: Parasol Manufacturer of punctal plug: Odyssey Medical Location of manufacturer: Memphis, TN, USA Intervention 2: silicone punctal plugs were inserted in the lower punctum Punctal plug model: Super Flex Manufacturer of punctal plug: Eagle Vision Location of manufacturer: Memphis, TN, USA Length of follow‐up: Planned: 6 months Actual: 6 months | |
| Outcomes | Primary outcomes reported: punctal plug retention at 6 months "Retention was characterized by last examined date with the punctal plug in place. For example, if a patient returned for his or her 4‐month visit, and the plug was gone, the plug was recorded as 3 months of retention." (p 239) Secondary outcomes reported: Schirmer I (mm), tear meniscus height as measured at the slit lamp (mm), TBUT (in seconds), inferior fluorescein corneal staining (National Eye Institute (NEI) scale), and average lissamine green conjunctival staining (NEI scale), artificial tear drop frequency Adverse events reported: none reported Intervals at which outcomes assessed: monthly for primary outcome up to 6 months; months 1, 3, and 6 for secondary outcomes | |
| Notes | Trial registry: NCT01947517 (clinicaltrials.gov) Type of study: published full‐text Funding sources: "The authors indicate no funding support" (p 242) Disclosures of interest: "none were reported" (p 242) Study period: September 2013 to May 2014 (from clinicaltrials.gov) *We contacted the authors via email and have received additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was achieved using a mathematical computer‐generated allocation schema based on permuted blocks with blocks of random sizes" (p 239). "Each eye was assigned randomly with equal probability to receive either Super Flex or Parasol brand punctal plugs" (p 239). |
| Allocation concealment (selection bias) | Low risk | From email correspondence with authors: "we had opaque envelopes with assignment once deemed eligible for inclusion." |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | "Participants and all study staff, except an unmasked investigator (A.B.) who inserted punctal plugs, were masked to treatment arms" (p 239). |
| Masking of outcome assessment (detection bias) | Low risk | "Plug retention and all other secondary outcomes were evaluated by 1 examiner masked to the treatment arms (Z.M.)" (p 239). |
| Incomplete outcome data (attrition bias) | Low risk | 2/50 eyes (4%) were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Trial was registered at clinicaltrials.gov and all pre‐specified outcomes in the registry were reported in the full‐text publications. |
| Other bias | Low risk | "All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest and none were reported. The authors indicate no funding support" (p 242). |
| Methods | Study design: parallel randomized controlled trial Unit of analysis: unclear as both eyes of 15 participants could have receive the same or different intervention Unit of randomization: “each eye was treated as independent for the purposes of the study” (p 391) How were missing data handled? NA, no missing data reported Reported power calculation: NR Unusual study design: authors did not perform the appropriate pair‐wise analysis and each eye treated as being independent | |
| Participants | Country: UK (assumed from author's origin) Number randomized: 21 participants (36 eyes) in total NR participant (18 eyes) in silicone punctal plug group NR participants (18 eyes) in acrylic punctal plug group Exclusions after randomization: none reported Number analyzed: 21 participants (36 eyes) in total NR participant (18 eyes) in silicone punctal plug group NR participants (18 eyes) in acrylic punctal plug group Losses to follow‐up: none reported Overall mean age (SD): 60.0 (NR) years in total; by group not reported Age range: 33‐78 years; by group not reported Sex (%): 20 women (95%) and 1 man (5%) in total; by group not reported Inclusion criteria: participants with subjective symptoms consistent with dry eye, tear film break‐up time of < 5 seconds, and ocular surface abnormalities as demonstrated by fluorescein or Rose Bengal staining. All used artificial tears for more than 6 months with no subjective or objective improvement in symptoms. Exclusion criteria: use of punctal plugs within previous 6 months or contact lens use | |
| Interventions | Both groups were allowed to use artificial tears throughout the follow up period Intervention 1: silicone punctal plugs were inserted in the lower punctum Punctal plug model: Soft Plug Manufacturer of punctal plug: Oasis Medical Location of manufacturer: Glendora, CA Intervention 2: acrylic punctal plugs were inserted in the lower punctum Punctal plug model: SmartPlugs Manufacturer of punctal plug: Medennium Location of manufacturer: Irvine, CA Length of follow‐up: Planned: NR 11.27 ± 2.54 weeks in silicone punctal plug group 11.11 ± 2.56 weeks in acrylic punctal plug group | |
| Outcomes | Primary and secondary outcome were not distinguished Outcomes reported: Subjective symptoms: dryness, foreign body sensation, grittiness, stinging, pain, itching and burning; 10 cm visual analog scale; scores added to derive a summary score Tear film stability: TBUT measured in seconds Tear meniscus height: measured using calibrated slit‐lamp in mm, midway between canthi along the lower lid Average of 3 measurements Tear production: Schirmer I test without anesthesia Ocular surface staining: Rose Bengal staining in nasal and temporal cornea and conjunctiva; graded on 0‐3 scale; fluorescein staining in 5 areas of cornea; graded on 0‐3 scale Topical artificial tears used Adverse events reported: NR Intervals at which outcomes assessed: all above outcomes were assessed before and at approximately 11 weeks after occlusion (mean 11.2 weeks; range 8 to 18 weeks) | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: National Health Service Lothian, Edinburgh, UK Disclosures of interest: "the authors state that they have no proprietary interest in the products named in this article" Study period: NR Reported subgroup analyses: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomization; computer generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | Participants were also masked to treatment assignment |
| Masking of outcome assessment (detection bias) | Low risk | Outcome assessments performed by investigators not involved in the treatment administration and not informed of treatment status |
| Incomplete outcome data (attrition bias) | Unclear risk | Sample size information is not included for all outcomes, so it is unclear whether all randomized participants completed follow‐up examinations |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to make comparison. All outcomes reported in the methods section were reported |
| Other bias | Unclear risk | Received government funding (National Health Service Lothian, Edinburgh, UK) |
| Methods | Study design: parallel randomized control trial Unit of analysis: unclear as trial investigators did not report if they had only included one eye in the analysis or took the averaged of both eyes Unit of randomization: participant, both eyes received the same intervention How were missing data handled? NA Reported power calculation: no Unusual study design: the study included 40 participants, 20 of whom were healthy controls. For this review, we will only refer to 20 dry eye participants. Healthy controls were also randomly assigned: "The 40 eyes of the normal subjects (group II) were similarly assigned to group IIA (upper punctal occlusion group, n = 20 eyes) or group IIB (lower punctal occlusion group, n = 20 eyes)." | |
| Participants | Country: China Number randomized: Total: 20 participants (40 eyes) Per group: 10 participants (20 eyes) Exclusions after randomization: none Number analyzed: Total: 20 participants (40 eyes) Per group: 10 participants (20 eyes) Losses to follow‐up: none Mean age ± SD (years): 22.5 ± 2.4 total; by group not reported Sex (%): 16 women (80%) and 4 men (20%); by group not reported Inclusion criteria: subjective symptoms of dry eye, a Schirmer I test result < 5 mm or TBUT < 5 seconds, and evidence of corneal surface damage on fluorescein staining Exclusion criteria: history of atopy; allergic diseases; Stevens‐Johnson syndrome; chemical, thermal, or radiation injury; or any other ocular or systemic disorder or had undergone any ocular surgery or contact lens use that would create an ocular surface problem or dry eye; lacrimal dysfunction, as determined by slit lamp examination and irrigation Equivalence of baseline characteristics: yes | |
| Interventions | No mention of artificial tear use Intervention 1: collagen punctal plugs in the lower puncta (1A) Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics Location of manufacturer: Eastsound, WA Intervention 2: collagen punctal plugs in the upper puncta (1B) Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics Location of manufacturer: Eastsound, WA Length of follow‐up: Planned: protocol not available Actual: 10 days | |
| Outcomes | Primary and secondary outcome not differentiated, as defined in study reports: symptom scoring, upper and lower tear menisci, tear breakup time, corneal fluorescein staining, and Schirmer I test Adverse events reported: "no complication was observed in dry eye patients or control subjects during the period of this study" Intervals at which outcomes assessed: day 1, 4, 7, and 10 | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: Chinese National Science and Technology Development Supporting Program and Zhejiang Provincial Program for the Cultivation of High‐level Innovative Health Talents Disclosures of interest: none Study period: NR Reported subgroup analyses: yes, healthy controls and dry eye groups Did trial investigators need to be contacted? yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed in manuscript |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Not described, but given the treatment groups it is not possible to mask participants or people administering interventions |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to make comparison. All outcomes reported in the Methods section were reported |
| Other bias | Unclear risk | Received government funding (Chinese National Science and Technology Development Supporting Program and Zhejiang Provincial Program for the Cultivation of High‐level Innovative Health Talents) |
| Methods | Study design: parallel randomized controlled trial Unit of analysis: unclear as trial investigators did not report if they had only included one eye in the analysis or took the averaged of both eyes Unit of randomization: participant, both eyes received the same intervention How were missing data handled? NA Reported power calculation: NR Unusual study design: 45 age‐ and gender‐ matched healthy volunteers were included (for comparison with dry eye participant baseline measurements only; no punctal plugs inserted) | |
| Participants | Country: UK Number randomized: 62 participants (121 eyes) in total NR participants (71 eyes) in lower punctum group NR participants (50 eyes) in lower and upper punctum group Exclusions after randomization: none reported Number analyzed: 62 participants (121 eyes) in total NR participants (71 eyes) in lower punctum group NR participants (50 eyes) in lower and upper punctum group Losses to follow‐up: none reported Overall mean age (SD): NR Age range: 24‐87 years Sex (%): 52 women (84%) and 10 men (16%) in total; by group not reported Inclusion criteria: (at least 3 of the 4 had to be met to be eligible): Schirmer score < 10 mm at 5 minutes; TBUT < 10 seconds; Mucin filaments and tear meniscus discontinuity; Rose Bengal score of ≥ 3.5 (corneal epithelium and bulbar conjunctiva) Exclusion criteria: NR | |
| Interventions | No mention of artificial tears use Intervention 1: collagen punctal plugs were inserted in the lower punctum of 71 eyes Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics, Inc Location of manufacturer: NR Intervention 2: collagen punctal plugs were inserted in the lower and upper puncta of 50 eyes Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics, Inc Location of manufacturer: NR Length of follow‐up: | |
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported:
Adverse events reported: Intervals at which outcomes assessed: 5 and 12 days after occlusion | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: Lacrimedics, Inc donated collagen plugs Disclosures of interest: "none of the authors have any other vested interest in Lacrimedics Inc. or their products" Study period: NR Reported subgroup analyses: none reported Each eye treated as independent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed in manuscript |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | Participants were also masked to treatment assignment |
| Masking of outcome assessment (detection bias) | Low risk | Outcome assessors measuring tear parameters were masked |
| Incomplete outcome data (attrition bias) | Low risk | Sample sizes reported in the results were consistent with the number randomized, so no loss to follow‐up or missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine |
| Other bias | Unclear risk | Insufficient information to determine |
| Methods | Study design: unclear if it was a parallel or paired‐eye randomized controlled trial Unit of analysis: eyes; no mention of analysis accounting for correlation between the left and right eye Unit of randomization: unclear if each eye of each participant were randomized to the same or different interventions How were missing data handled? NR Reported power calculation: none reported Unusual study design: no | |
| Participants | Country: China Number randomized: Total: 54 participants (108 eyes) Per group: 27 participants (54 eyes) Exclusions after randomization: none Number analyzed: Total: 54 participants (108 eyes) Per group: 27 participants (54 eyes) Losses to follow‐up: NR Mean age ± SD: 20 ± 6 years; by group not reported Age range: 18‐34 years in total; by group not reported Sex (%): 19 men (35%) and 35 women (65%) in total; by group not reported Inclusion criteria: "patients treated with LASIK in our hospital" (p 1666) Exclusion criteria: NR | |
| Interventions | Intervention 1: collagen punctual occlusion Punctal plug model: A12‐103 Manufacturer of punctal plug: Odyssey Location of manufacturer: NR Intervention 2: artificial tear (dextran and hypromellose eye drops) by Alcon, 1 drop 3 times/day. Length of follow‐up: Planned: protocol not available Actual: 2 weeks after punctual occlusion surgery | |
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported:
Adverse events reported: NR Intervals at which outcomes assessed: NA | |
| Notes | Type of study: published Funding sources: NR Disclosures of interest: NR Study period: June 2009 to September 2009 Reported subgroup analyses: no Do trial investigators need to be contacted? Yes, the investigators need to be contacted for randomization method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The investigator stated "we randomly picked 54 patients (108 eyes) receiving LASIK surgery in our hospital from June to September in 2009 . . . The patients were randomly assigned to 2 groups, 27 individuals (54 eyes) in each group (p 1666)" The trial investigator did not describe how the random sequence were generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described, but given the treatment groups it is not possible to mask participants or people administering interventions. |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Due to the nature of the treatments, participants and personnel cannot be masked for this study, and the results are likely to be influenced by the lack of masking. |
| Masking of outcome assessment (detection bias) | Unclear risk | The investigator stated that "all the tests and surgeries were conducted by the same physician", thus the outcome assessor (i.e., the physician) were aware of the interventions. |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts were not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available and all of the outcomes were reported as prespecified in the Methods section |
| Other bias | Unclear risk | Funding sources and disclosures of interest were not reported. |
| Methods | Study design: parallel randomized controlled trial Unit of analysis: right eye of each participant Unit of randomization: participant; both eyes received the same treatment but only the right eye was analyzed How were missing data handled? NA Reported power calculation: none reported Unusual study design: unclear whether both of eyes had similar values of TBUT at baseline | |
| Participants | Country: Japan Number randomized: 43 participants (43 eyes) in total 19 participants (19 eyes) in upper occlusion group 24 participants (24 eyes) in lower occlusion group Exclusions after randomization: none Number analyzed: 43 participants (43 eyes) in total 19 participants (19 eyes) in upper occlusion group 24 participants (24 eyes) in lower occlusion group Losses to follow‐up: none Mean age ± SD: 55.8 ± 16.1 years; by group not reported Age range: 22‐82 years in total; by group not reported Sex (%): 9 men (21%) and 34 women (79%) in total; by group not reported Inclusion criteria:
Exclusion criteria:
| |
| Interventions | No mention of artificial tears Intervention 1: silicone punctal plugs in the lower puncta Punctal plug model: Super Flex plug Manufacturer of punctal plug: Eagle Vision Location of manufacturer: Memphis, TN Intervention 2: silicone punctal plugs in the upper puncta Punctal plug model: Super Flex plug Manufacturer of punctal plug: Eagle Vision Location of manufacturer: Memphis, TN Length of follow‐up: Planned: protocol not available Actual: 1 month | |
| Outcomes | Primary and secondary outcomes not differentiated, as defined in study reports:
Adverse events reported: no Intervals at which outcomes assessed: 1 month | |
| Notes | Type of study: published full‐text Trial registry: NR Funding sources: NR Disclosures of interest: "The authors have no financial or conflicts of interest to disclose (p 1009)." Study period: NR Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed in manuscript |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Not described, but given the treatment groups it is not possible to mask participants or people administering interventions |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine |
| Other bias | Unclear risk | Source of funding was not reported and authors explicitly stated no conflicts of interest |
| Methods | Study design: paired‐eye randomized controlled trial Unit of analysis: eyes; no mention of analysis accounting for correlation between the left and right eye Unit of randomization: eyes; each eye of each participant were randomized to a different intervention How were missing data handled? NA Reported power calculation: yes, sample size = 32 (64 eyes); P value = 0.05; 50% of the patients indicated that the eye with the implants was better, 25% said the opposite eye was better, and 25% said no difference Unusual study design: authors did not perform the appropriate pair‐wise analysis and each eye treated as independent | |
| Participants | Country: USA (assumed from author's origin) Number randomized: Total: 32 participants (64 eyes) Per group: 32 eyes Exclusions after randomization: none reported Number analyzed: 32 participants (64 eyes); 32 eyes in each group Losses to follow‐up: none reported Overall mean age (SD): participant age was collected but not reported Age range: participant age was collected but not reported Sex (%): NR Inclusion criteria: participants reporting bilateral dry eye symptoms (based on responses to a modified McMonnies' questionnaire) and wearing hydrogel contact lenses were included Exclusion criteria: none reported | |
| Interventions | No mention of artificial tears Intervention 1: collagen intracanalicular plugs were inserted in the upper and lower puncta Punctal plug model: 0.3 mm diameter Manufacturer of punctal plug: Lacrimedics, Inc. Location of manufacturer: Rosemead, CA Intervention 2: sham plug insertion in participants contralateral eye Length of follow‐up: Planned: protocol not available | |
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported: Subjective symptoms: McMonnies symptom questionnaire (modified) Tear meniscus height: measured using video slit‐lamp in mm Tear film stability: TBUT measured in seconds Ocular surface staining: Rose Bengal and fluorescein staining scored 0 (no staining) to 4 (heavy, coalesced staining) Tear lactoferrin immunoassay test: lactoplate tear lactoferrin immunoassay test; left in cul‐de‐sac for 2‐4 minutes; precipitation ring diameter measured in mm 3 days later Intervals at which outcomes assessed: before and at 5 days after occlusion | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: funded by Bausch and Lomb inVision Institute; Eagle Vision, Inc. supplied implants and Lactoplate tests Disclosures of interest: NR Study period: NR Reported subgroup analyses: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed in manuscript. "The patients were randomly assigned to have the implants put into either the right or the left eye but were lead to believe that implants were put in both eyes" (p 238). |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript. "The eye to receive the implants was determined just before insertion by randomization" (p 239). |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | Participants were also masked to treatment assignment. "All of the steps of inserting the implants were performed on both eyes, but an implant was inserted in the upper and lower punctum of one eye only. Therefore, the patients did not know that only one eye received the implants. Because the patients did not know that the puncta of only one eye received the implants and the investigator making the measurements did not know which eye of the patient received the implant, the study was double masked" (p 239). |
| Masking of outcome assessment (detection bias) | Low risk | Outcome assessors were masked. "Because the patients did not know that the puncta of only one eye received the implants and the investigator making the measurements did not know which eye of the patient received the implant, the study was double masked" (p 239). |
| Incomplete outcome data (attrition bias) | Low risk | Sample sizes reported in the results were consistent with the number randomized, so no loss to follow‐up or missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine. Trial registry information and protocol not available for comparison |
| Other bias | Unclear risk | Insufficient information to determine. The source of funding of the study (Bausch and Lomb inVision Institute) was different from the manufacturer (Lacrimedics, Inc.) |
| Methods | Study design: paired‐eye randomized controlled trial Unit of analysis: eyes; no mention of analysis accounting for correlation between the left and right eye Unit of randomization: each eye of each participant was randomized to a different intervention How were missing data handled? Participants lost to follow‐up were excluded from analysis Reported power calculation: NR Unusual study design: authors did not perform the appropriate pair‐wise analysis and each eye treated as independent | |
| Participants | Country: Netherlands (assumed from location of ethics committee) Number randomized: Total: 20 participants (40 eyes) Per group: 20 eyes in each group Exclusions after randomization: none reported Number analyzed: 13 participants (26 eyes); 13 eyes in each group Losses to follow‐up: 7 participants (14 eyes); 7 eyes in each group Overall mean age (SD): NR Age range: NR Sex (%): 17 women (85%) and 3 men (15%) Inclusion criteria: European criteria for the diagnosis of Sjögren's syndrome were used to identify eligible participants: subjective symptom report (ocular and oral symptoms of dryness). Schirmer test, Rose Bengal staining, and TBUT; xerostomia Exclusion criteria: none reported | |
| Interventions | No mention of artificial tears Intervention 1: silicone punctal plugs inserted in the upper and lower puncta Punctal plug model: tapered‐shaft silicone punctal plugs, 0.7 mm diameter Manufacturer of punctal plug: Eagle Vision Location of manufacturer: Memphis, TN Duration of plug occlusion was 6 to 20 weeks If plugs extruded during course of study, larger plug inserted and follow‐up deferred for period of at least 6 weeks Intervention 2: other eye of participant remained unoccluded Length of follow‐up: Planned: protocol not available | |
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported: Subjective discomfort (at least 1 of the following complaints): foreign body sensation, photophobia, stinging, pain, burning, and ocular fatigue Subjective symptoms: abovementioned symptom discomfort complaints scored and scores added to derive a summary score (0 to 10) Tear production: Schirmer test without anesthesia Ocular surface staining: Rose Bengal staining scored according to Van Bijsterveld classification Mucus debris: measured on scale of 0 (no mucus debris) to 3 (mucus threads and filaments) Intervals at which outcomes assessed: before and at least 6 weeks after occlusion | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: NR Disclosures of interest: NR Study period: NR Reported subgroup analyses: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization scheme. "The eye to be occluded was chosen at random using a computer‐generated randomization scheme" (p 148). |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Not described, but given the treatment groups it is not possible to mask participants or people administering interventions |
| Masking of outcome assessment (detection bias) | High risk | The same investigator performed all measurements and was presumably unmasked to treatment assignment |
| Incomplete outcome data (attrition bias) | High risk | 6/20 participants (30%) excluded from the analysis because of spontaneous plug loss and 1 excluded after an inflammatory reaction to plug material |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine. Trial registry information and protocol were not available for comparison |
| Other bias | Unclear risk | Insufficient information to determine. Funding source and disclosure of interest not reported |
| Methods | Study design: parallel randomized controlled trial Unit of analysis: unclear as trial investigators did not report if they had only included one eye in the analysis or took the averaged of both eyes Unit of randomization: participant, both eyes received the same intervention How were missing data handled? NA, no missing data reported Reported power calculation: yes, sample size = 30 participants in each group; "based on the assumption that 80% of the patients subjected to canalicular occlusion would have a successful outcome (reduction in dry eye/conjunctivitis symptoms), in contrast to 30% of those receiving medical treatment alone (artificial tears). It was further assumed that 74 patients would have to be randomized to the 2 treatment groups to allow for dropouts and ensure that 60 patients would complete the study" (p 11) | |
| Participants | Country: Mexico Number randomized: 61 participants (122 eyes) in total 31 participants (31 eyes) in Collagen/silicone plug group 30 participants (30 eyes) in sham group Exclusions after randomization: 1 participant (2 eyes) Number analyzed: 60 participants (120 eyes); 30 participants (60 eyes) in each group Losses to follow‐up: none reported Overall mean age (SD): 49.8 (NR) years in total; by group not reported Age range: 23‐80 years Sex (%): 50 women (82%) and 11 men (18%) in total; by group not reported Inclusion criteria: 18‐80 years old, 2 subjective symptoms of dry eye, ocular surface abnormalities as demonstrated by fluorescein score > 1, conjunctivitis of ≥ 1 month Exclusion criteria: participants were excluded if dry eye attributed to other ocular conditions (see Nava‐Castaneda 2003) or if diagnosed with asthma | |
| Interventions | No mention of artificial tears use Intervention 1: collagen/silicone plug group (experimental): Punctal plug model: NR Manufacturer of punctal plug: Lacrimedics Location of manufacturer: Eastsound, WA Collagen punctal plugs inserted in the upper and lower canaliculi of both eyes. 2 weeks after initial insertion, 1 silicone punctal plug inserted in the upper and 2 collagen plugs inserted in lower canaliculi of both eyes. 4 weeks after initial insertion, permanent plug inserted in lower canaliculi Control: sham plug insertion at same intervals as collagen/silicone plug group. Same procedures as collagen/silicone group, but eyes were not occluded Length of follow‐up: Planned: protocol not available | |
| Outcomes | Primary and secondary outcome were not distinguished. Outcomes reported: Subjective symptoms: visual performance and comfort; assessed using a 10 cm visual analogue scale, very poor vision/very uncomfortable and very good vision/very comfortable at the boundaries of the scale Frequency and severity of dry eye (watery eyes, itching, burning, dryness, fluctuating vision, sandy/foreign body sensation, light sensitivity) and conjunctival (discharge and redness) symptoms; frequency scored 0 (never) to 5 (continually, every hour of the day) and severity 0 (no symptom) to 3 (severe); frequency and severity score for each symptom multiplied and scores summed to derive summary score. Ocular surface staining: fluorescein staining scored 0 (absent) to 4 (severe) Topical artificial tears used: frequency scored 0 (never) to 5 (continually, every hour of the day) Visual acuity Intervals at which outcomes assessed: before and at 1 hour and 2, 4, 8 weeks after initial occlusion | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: Lacrimedics, Inc Disclosures of interest: none reported Study period: NR Reported subgroup analyses: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme. |
| Allocation concealment (selection bias) | Low risk | Random assignments prepared by Statistical Committee and placed in sealed envelopes numbered 1‐74; randomization list maintained by Statistical Committee |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | Participants masked to treatment assignment. "Patients subjected to the sham procedure were treated identically to the collagen/silicone plug implantation group (i.e., the punctum was dilated and the canaliculus probed), except that a plug was not actually inserted" (p 11). |
| Masking of outcome assessment (detection bias) | Low risk | Outcome assessors masked to treatment assignment. "Subsequent patient evaluations were performed by one of the initial evaluators who were kept uninformed of the patient's treatment status" (p 11). |
| Incomplete outcome data (attrition bias) | Low risk | 1 randomized participant was found to be ineligible, but was not excluded from the analysis. 1/61 participants (2%) discontinued treatment and was excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine although the investigators list the following protocol deviations in the publication: 61 patients instead of 60 enrolled, 1 patient was ineligible, and covariate analyses were not performed |
| Other bias | Unclear risk | Device manufacturer is the funding source |
| Methods | Study design: parallel randomized controlled trial Unit of analysis: unclear as trial investigators did not report if they had only included one eye in the analysis or took the averaged of both eyes Unit of randomization: participant, both eyes received the same intervention How were missing data handled? Excluded from analysis Reported power calculation: NR Unusual study design: no | |
| Participants | Country: China Number randomized: 28 participants (56 eyes) in total 12 participants (24 eyes) in punctal plug group 16 participants (32 eyes) in artificial tears group Exclusions after randomization: none reported Number analyzed: 28 participants (56 eyes) in total 12 participants (24 eyes) in punctal plug group 16 participants (32 eyes) in artificial tears group Losses to follow‐up: none reported Mean age (SD): 31.75 (NR) years 31.4 (15.1) years in punctal plug group 32.1 (12.8) years in artificial tears group Age range: 22‐67 Sex (%): 18 women (64%) and 10 men (36%) in total; by group not reported Inclusion criteria: "The entry criteria for the patients were that they were diagnosed with dry eyes at our ophthalmology clinic and had no evidence of ocular diseases other than those associated with dry eye changes, such as superficial punctate keratopathy (SPK)" (p 20) Exclusion criteria: none listed | |
| Interventions | Intervention 1: acrylic punctal plug Punctal plug model: SmartPLUG500 Manufacturer of punctal plug: Medenium Location of manufacturer: Irvine, CA Control: artificial tear solution (Zhuhai Yisheng, Guangdong, China) containing a carbomer gel and basic fibroblast growth factor Length of follow‐up: Planned: 2 weeks Actual: 2 weeks, extended to 4 weeks for corneal fluorescein staining | |
| Outcomes | Primary and secondary outcomes not differentiated Outcomes, as defined by study:
Adverse events reported: punctate epithelial keratopathy Intervals at which outcomes assessed: baseline, 2 weeks | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: "seed fund (no. 79495‐01) and Linhu fund (no. 79495‐02) of Peking University Hospital" Disclosures of interest: reported no conflicts of interest Enrollement period: May 2009 to October 2009 Reported subgroup analyses: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors did not report how the random sequence was generated. "They were randomly assigned into artificial tears group and punctual plugs group" (p 20) |
| Allocation concealment (selection bias) | Unclear risk | Authors did not report how allocation was concealed. "They were randomly assigned into artificial tears group and punctual plugs group" (p 20) |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Due to the nature of the treatments, participants and personnel cannot be masked for this study, and the results are likely to be influenced by the lack of masking |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Only 2/42 lost to follow‐up, even across groups. However, reason for lost to follow‐up was not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and trial registry number not reported |
| Other bias | Low risk | Non‐industry funding and reported no conflict of interest |
| Methods | Study design: parallel randomized controlled trial Unit of analysis: right eye of each participant Unit of randomization: participant as only the right eye of each participant was randomized to intervention or control How were missing data handled? Excluded from analysis Reported power calculation: NR Unusual study design: no | |
| Participants | Country: China Number randomized: 42 participants (42 eyes) in total 22 participants (22 eyes) in punctal plug group 20 participants (20 eyes) in artificial tears group Exclusions after randomization: none reported Number analyzed: 40 participants (40 eyes) in total 21 participants (21 eyes) in punctal plug group 19 participants (19 eyes) in artificial tears group Losses to follow‐up: 2 participants (2 eyes) in total 1 participant (1 eye) in punctal plug group 1 participant (1 eye) in artificial tears group Mean age (SD): overall NR 35.2 (16.5) years in punctal plug group 34.6 (1.3) years in artificial tears group Age range: 22‐67 years Sex (%): 36 women (90%) and 4 men (10%) in total 19 women (90.5%) and 2 men (9.5%) in the intervention group 17 women (89.5%) and 2 men (10.5%) in the observation group Inclusion criteria: "patients with dry eye who sought for treatment in our ophthalmology clinic from March to October in 2010, diagnosed with primary Sjögren's syndrome …The entry criteria for the patients were that they were diagnosed with dry eyes at our ophthalmology clinic and had no evidence of ocular diseases other than those associated with dry eye changes, such as superficial punctuate keratopathy (SPK)" (p 2544). Exclusion criteria: none specified | |
| Interventions | Intervention 1: acrylic punctal plug punctal plug model: SmartPLUG500 Manufacturer of punctal plug: Medenium Location of manufacturer: Irvine, CA Control: artificial tears (Zhuhai Yisheng, Guangdong, China) containing a carbomer gel and bFGF Length of follow‐up:3 months Planned: 3 months Actual: 3 months | |
| Outcomes | Primary and secondary outcomes not differentiated Outcomes, defined by the study:
Adverse events reported: NR Intervals at which outcomes assessed: baseline, 3 months | |
| Notes | Trial registry: not reported Type of study: published full‐text Funding sources: Seed Fund (No. 79495‐01) and Linhu fund (no. 79495) of Peking University Third Hospital Disclosures of interest: no conflicts of interest to report Enrollement period: March 2010 to October 2010 Reported subgroup analyses: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomly assigned into artificial tears group and punctual plugs group using a computer‐generated random number table" (p 2544). |
| Allocation concealment (selection bias) | Low risk | "Written allocation assignments were sealed in individual opaque envelopes marked only with study identification numbers" (p 2544). |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | "All para‐clinical examinations and analyses were performed by the same experienced technician and the same clinical staff who both were masked to the type of treatment" (p 2544). However given the nature of the interventions, participants and personnel could not be masked. |
| Masking of outcome assessment (detection bias) | Low risk | "All para‐clinical examinations and analyses were performed by the same experienced technician and the same clinical staff who both were masked to the type of treatment" (p 2544). |
| Incomplete outcome data (attrition bias) | Low risk | "Two patients did not complete the follow‐up period and were excluded from the analysis" (p 2545). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and trial registry number not reported |
| Other bias | Low risk | Non‐industry funding and reported no conflict of interest |
| Methods | Study design: parallel randomized controlled trial Unit of analysis: participant; trial investigators reported the average of both eyes of each participant Unit of randomization: participant; both eyes received the same intervention How were missing data handled? Excluded from analysis Reported power calculation: NR Unusual study design: no | |
| Participants | Country: Austria Number randomized: 30 participants (60 eyes) in total 15 participants (30 eyes) in silicone punctual plugs group 15 participants (30 eyes) in intracanalicular SmartPlugs group Exclusions after randomization: none reported Number analyzed: 30 participants (57 eyes) in total 15 participants (27 eyes) in silicone punctual plugs group 15 participants (30 eyes) in intracanalicular SmartPlugs group Losses to follow‐up: none reported Mean age (SD): overall NR; by group not reported Age range: NR Sex (%): NR Inclusion criteria: "moderate to severe dry eye syndrome as described by the DEWS report 2007… typical dry eye symptoms, reduced tear break–up time of less than 5 seconds with either a Schirmer test without local anesthesia below 5 mm/ 5 min, or a vital staining score of the cornea (fluorescein) and conjunctiva (Rose Bengal) according to van Bijsterveld (> 3)" (p 522) Exclusion criteria: "Sjogren's syndrome, eyelid or blinking problems, contact lens use and previous punctal plug use" (p 522) | |
| Interventions | Both groups used artificial tears as needed Intervention 1: silicone punctual plugs Punctal plug model: silicone punctual plugs Manufacturer of punctal plug: FCI Opthalmics Location of manufacturer: Issy‐les‐Moulineaux Cedex, France Intervention 2: intracanalicular SmartPlugs Punctal plug model: punctual plugs Manufacturer of punctal plug: Medenium Location of manufacturer: Irvine, CA Length of follow‐up: Planned: 3 months Actual: 3 months | |
| Outcomes | Primary and secondary outcomes not differentiated Outcomes, as defined by study:
Intervals at which outcomes assessed: baseline, 3 months | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: "no funding source to declare" (p 524) Disclosures of interest: no conflicts of interest Enrollement period: unclear Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The 30 patients were randomized into two groups … using the next available number from a set of block randomized computer numbers" (p 522). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | Participants were masked to treatment group. "Patients were not informed into which arm of the trial they had been allocated" (p 522). |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up was differential across groups. "All 30 patients completed the study, but in three eyes of group I (collared silicone plugs) spontaneous loss of the plug was noticed at the follow‐up visit" (p 522) "Eyes with a spontaneous lost punctual plug at follow up visit were excluded" (p 522). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and trial registry number not reported |
| Other bias | Unclear risk | Non‐industry funding and reported no conflict of interest |
| Methods | Study design: parallel group randomized controlled trial Unit of analysis: participant; trial investigators reported the average of both eyes of each participant Unit of randomization: participant, both eyes received the same intervention How were missing data handled? Excluded from analyses Reported power calculation: no Unusual study design: "for Schirmer testing and rose bengal staining, data were collected from both eyes and averaged before statistical analysis" | |
| Participants | Country: USA Number randomized: 32 participants (64 eyes) in total 11 participants (22 eyes) in punctal plug group 11 participants (22 eyes) in cyclosporine group 10 participants (20 eyes) in cyclosporine + punctal plugs Exclusions after randomization: none Number analyzed: 30 participants (60 eyes) in total 10 participants (20 eyes) in each group Losses to follow‐up: 2 participants Mean age: 52.1 years in total; by group not reported Age range: 38‐63 years; by group not reported Sex (%): 25 women (83.3%) and 5 men (16.7%); by group not reported Inclusion criteria: "(1) chronic symptoms of burning, sandy, or scratchiness in both eyes; (2) daily need for multiple applications of artificial tears; and (3) rose bengal staining of grade 2 or higher (scale described below)." (p 391) Exclusion criteria:
| |
| Interventions | All groups were allowed to use artificial tears throughout the follow up period Intervention 1: cyclosporine ophthalmic emulsion 0.05% (RESTASIS; Allergan, Irvine, CA) eye drops to both eyes twice daily Intervention 2: bilateral collagen punctal plugs in the lower lids only Punctal plug model: PARASOL (Punctal Occluder) Manufacturer of punctal plug: Odyssey Medical Location of manufacturer: Memphis, TN Intervention 3: bilateral collagen punctal plugs in the lower lids + cyclosporine eye drops to both eyes twice daily Length of follow‐up: Planned: protocol not available Actual: 6 months | |
| Outcomes | Primary and secondary outcome not differentiated, as defined in study reports: "Schirmer scores without anaesthesia, corneal and conjunctival rose bengal staining, and artificial tear use" Adverse events reported: 2 participants withdrew: 1 due to discomfort of plugs and 1 due to burning caused by cyclosporine Intervals at which outcomes assessed: 1, 3, and 6 months after occlusion | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: Allergan Disclosures of interest: "Dr. Roberts is a consultant for Allergan. The authors state that they have no proprietary interest in the products named in this article" (p 391) Study period: October 2003 to January 2005 Reported subgroup analyses: none reported Do trial investigators need to be contacted? Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[A] computer‐generated randomization schedule" (p 391). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias and detection bias) | High risk | "Medication was dispensed open‐label" (p 391). |
| Masking of outcome assessment (detection bias) | High risk | "Medication was dispensed open‐label" (p 391). |
| Incomplete outcome data (attrition bias) | High risk | Participants who with drew were replaced and data from withdrawals not included in analyses |
| Selective reporting (reporting bias) | Unclear risk | Trial registry record not reported; we were not able to compare the reported outcomes with the trial registry record |
| Other bias | High risk | Industry funded study |
| Methods | Study design: paired‐eye randomized controlled trial Unit of analysis: eyes; used paired t‐tests to account for correlation between left and right eyes of each participant Unit of randomization: each eye of each participant was randomized to a different intervention How were missing data handled? Excluded from analysis Reported power calculation: no Unusual study design: used correct matched analysis | |
| Participants | Country: USA Number randomized: Total: 35 participants (70 eyes) Per group: 35 eyes per group Exclusions after randomization: 7 participants (14 eyes) Number analyzed: Total: 28 participants (56 eyes) Per group: 28 eyes Losses to follow‐up: 1 participant (2 eyes) Mean age (SD): NR Age range: 21‐69 years in total; by group not reported Sex (%): 26 women (74%) and 9 men (26%) in total; by group not reported Inclusion criteria: "1. Dry eye based on the response of at least 'sometimes' on at least 2 of the 3 questions concerning dryness, lens awareness, and cloudy vision on the recruitment questionnaire (see Table 2). 2. At least one of the following objective signs: grade 1 or greater vital staining; prelens tear film break‐up time of less than 15 seconds; tear meniscus height of less than 0.5 mm on slitlamp examination; grade 2 or more tear debris on a 0 to 4 scale. 3. Bilateral involvement of the above criteria. 4. Ability to understand and complete subjective scales daily. 5. Wearing contact lenses that are of equal age, type, and material. 6. Must be able to wear both contact lenses at least 20 hours/week." Exclusion criteria: "1. Dry eye attributed to poor lid apposition or blinking mechanism. 2. Contact lens surface abnormalities (deposits), which cause distortion of a reflected grid pattern. 3. Clinically apparent nasolacrimal occlusion. 4. Patients who take certain hormones (birth control pills, menopausal replacement therapy) who cannot maintain current dosage throughout the study duration. 5. Ocular infection, including active corneal ulcers, keratitis, or conjunctivitis. 6. Use of any topical agents in the eye other than artificial tears, saline, or rewetting drops at the time of study entry. 7. Under 18 yr of age. 8. Known pregnancy." | |
| Interventions | Intervention 1: silicone punctal plugs in the lower and upper puncta Punctal plug model: Herrick Lacrimal Plugs Manufacturer of punctal plug: Lacrimedics Location of manufacturer: Rialto, CA (upper and lower) – 1 eye 1st 4 weeks; plugs + re‐wetting drops 5th week Intervention 2: sham treatment No plugs (sham) ‐ fellow eye – 1st 4 weeks; re‐wetting drops only 5th week Length of follow‐up: Planned: protocol not available Actual: 5 weeks | |
| Outcomes | Primary and secondary outcome not differentiated, and were defined in study reports: tear film break‐up time, lens water content, vital staining, bulbar conjunctiva with fluorescein, Rose Bengal, patient questionnaires Adverse events reported: yes, 3 participants reported epiphora and plugs removed Intervals at which outcomes assessed: day 0, 7, 28, 35 | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: Vistaken; Dr. Slusser. "Lacrimedics, Inc. (Rialto, CA) donated the lacrimal plugs and supportive equipment used in this study. Allergan, Inc. (Irvine, CA) provided the non‐preserved rewetting drops (p 337)." Disclosures of interest: NR Study period: March to June; year of study period not reported Reported subgroup analyses: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed in manuscript |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Low risk | "To provide a placebo control, the fellow eye was carefully manipulated in a similar fashion without actual insertion of any plugs. The patient was masked as much as possible to avoid visualizing the plugs during the insertion procedure." |
| Masking of outcome assessment (detection bias) | Low risk | "Outcome assessors masked as independent investigator inserted the plugs and performed sham insertion for those receiving no plugs" |
| Incomplete outcome data (attrition bias) | High risk | 7/35 participants (20%) were excluded after randomization |
| Selective reporting (reporting bias) | Unclear risk | Trial registry or protocol was not reported, hence we were not able to check if all outcomes in the protocol were reported in the full‐text publication |
| Other bias | High risk | Received industry funding |
| Methods | Study design: parallel randomized controlled trial Unit of analysis: participant; (both eyes of each participant; mean outcome of left and right eyes reported respectively) Unit of randomization: participant; both eyes received the same intervention How were missing data handled? NR Reported power calculation: power = 90%; sample size = 25 participants; assuming 90% response rate in the group receiving pilocarpine and a 30% response rate in the group receiving artificial tears Unusual study design: participants with dry eye due to Sjögren's syndrome | |
| Participants | Country: Greece (assumed from author's origin) Number randomized: 85 participants (170 eyes) in total 28 participants (NR eyes) in collagen plugs group 29 participants (NR eyes) in oral pilocarpine group 28 participants in artificial tears only group Exclusions after randomization: 1 participant (2 eyes) in collagen plugs group Number analyzed: NR Losses to follow‐up: 1 participant (2 eyes) in collagen plugs group Mean age (SD): 57.8 (12.9) years in collagen plugs group 59.9 (9.9) years in oral pilocarpine group 57.0 (11.5) years in artificial tears only group Age range: NR Sex (%): 85 women (100%) and 0 men in total Inclusion criteria: European criteria for the diagnosis of Sjögren's syndrome were used to identify eligible participants Exclusion criteria: none reported | |
| Interventions | All groups used artificial tears Intervention 1: collagen plugs inserted in the lower puncta of both eyes for 7 days then permanent collagen plugs for the duration of the trial + artificial tears Punctal plug model: Collagen Plugs Manufacturer of punctal plug: Lacrimedics Inc. Location of manufacturer: NR Intervention 2: oral pilocarpine (5 mg twice a day) + artificial tears Intervention 3: artificial tears only Length of follow‐up: Planned: protocol not available | |
| Outcomes | Primary outcome, as defined in study reports: assessed with a dry eye questionnaire Subjective symptoms: 100 mm visual analogue scale; score defined as improvement of > 55 mm in symptoms over course of study Tear production: Schirmer I test without anesthesia Ocular surface staining: Rose Bengal staining Tear film stability: TBUT Fluorophotometer method Imprint test (conjunctival impression cytology): improvement defined as increase in cytoplasm/nucleus ratio (epithelial cells) and goblet cells Intervals at which outcomes assessed: every week for the first month, then every month after up to 12 weeks | |
| Notes | Trial registry: Not reported (NR) Type of study: published full‐text Funding sources: NR Disclosures of interest: NR Study period: NR Reported subgroup analyses: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme. "Patients were randomized according to a computer generated schedule" (p 1204) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed in manuscript |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | It is difficult to mask participants and personnel as the interventions may be visible during examination. Eye examination, ocular surface staining, aqueous tear production, and imprint test performed by masked investigators |
| Masking of outcome assessment (detection bias) | Low risk | Eye examination, ocular surface staining, aqueous tear production, and imprint test performed by masked investigators. "Eye examination, Schirmer‐I test, and rose bengal staining were all performed by another investigator (GK), who was unaware of the treatment allocation. Furthermore, the imprint test was completed by another investigator (CAP) who was also unaware of the treatment arms and the results of Schirmer‐I and rose bengal tests" (p 1204) |
| Incomplete outcome data (attrition bias) | Unclear risk | 1/85 participants (1%) lost to follow‐up and 1/85 participants (1%) discontinued due to local infection; not clear if these participants were excluded from the analyses |
| Selective reporting (reporting bias) | Unclear risk | Trial registry information and protocol were not available for comparison |
| Other bias | Unclear risk | Source of funding and conflict of interest not reported |
| Methods | Study design: quasi‐randomized trial – divided into 2 groups on the basis of their ID numbers; the plug (even numbers) and non‐plug (odd numbers) groups Unit of analysis: unclear as both eyes of 10 participants receive the same intervention, but the trial investigators did not report how they analyzed them Unit of randomization: participant; assuming ID numbers were assigned to each participant, both eyes of 10 participants receive the same intervention How were missing data handled? Excluded from analysis Reported power calculation: none Unusual study design: participants' patient ID number as used to assigned them to each group | |
| Participants | Country: Japan Number randomized: 18 participants (28 eyes) in total 9 participants (13 eyes) in silicone punctal plugs group 9 participants (15 eyes) in non‐plug group Exclusions after randomization: 2 participants (NR eyes) in total 2 participants (NR eyes) in silicone punctal plugs group 0 participants (0 eyes) in non‐plug group Number analyzed: 18 participants (28 eyes) in total 9 participants (13 eyes) in silicone punctal plugs group 9 participants (15 eyes) in non‐plug group Losses to follow‐up: none Mean age (SD): 32.32 (7.69) years in total* 35.67 (10.74) years in silicone punctal plugs group 30.89 (3.89) years in non‐plug group Age range: (20‐56) years overall Sex (%): 16 women (89%) and 2 men (11%) in total 8 women (89%) and 1 men (11%) in silicone punctal plugs group 8 women (89%) and 1 men (11%) in non‐plug group Inclusion criteria: "patients who underwent LASIK. All eyes fulfilled the Japanese dry‐eye criteria and had not responded to conventional treatment with artificial tears by 1 month postsurgery (p 208)." Exclusion criteria: none reported | |
| Interventions | Both groups used artificial tears Intervention 1: silicone punctal plugs in upper and lower puncta + artificial tears Punctal plug model: Eagle plug Manufacturer of punctal plug: EagleVison Location of manufacturer: Memphis, TN Intervention 2: observation + artificial tears Length of follow‐up: Planned: protocol not available Actual: 3 months | |
| Outcomes | Outcomes not identified as primary or secondary. As defined in study reports: subjective symptoms and satisfaction, corneal sensitivity, tear function and ocular surface (Schimer value, TBUT, and fluorescein score), and visual performance (uncorrected and best‐corrected visual acuity (UCVA and BCVA), manifest refraction, and functional visual acuity (FVA)) Adverse events reported: yes, excessive lacrimation and plugs that were lost were reinserted Intervals at which outcomes assessed: 1 and 3 months | |
| Notes | Trial registry: NR Type of study: published full‐text Funding sources: NR Disclosures of interest: NR Study period: January 2008 to March 2009 Reported subgroup analyses: none reported *We contacted the authors via email and have received additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi randomized study – assigned based on patient ID number (odd/even) |
| Allocation concealment (selection bias) | High risk | "These candidates were divided into two groups on the basis of their ID numbers; the plug (even numbers) and non‐plug (odd numbers) groups" (p 209) "They were randomly divided into a plug and a non‐plug group" (p 208) |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Given the treatment groups it is not possible to mask participants or people administering interventions. From email correspondence with authors: "The participant and those assessing the outcome were not blinded." |
| Masking of outcome assessment (detection bias) | High risk | From email correspondence with authors: "The participant and the those assessing the outcome were not blinded." |
| Incomplete outcome data (attrition bias) | Low risk | "2 patients lost plug(s) from either eye during the follow‐up and had new plug(s) re‐inserted but were excluded from the study" (p 208) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for comparison |
| Other bias | Unclear risk | From email correspondence with authors: "Source of funding and conflict of interest not reported. There was no financial support for the trial. We did not use a clinical trial registry for this study.The protocol is only in the patients and methods in the manuscript. I will attach the paragraphs bellow (quoted from full‐text)." |
| Methods | Study design: parallel randomized controlled trial Unit of analysis: unclear as both eyes of 10 participants could have receive the same or different intervention Unit of randomization: unclear How were missing data handled? NA, no missing data reported Reported power calculation: NR Unusual study design: for 10 participants, both eyes were included, but for the remaining 70 participants only 1 eye was included | |
| Participants | Country: China (assumed from author's affiliations) Number randomized: 80 participants (90 eyes) in total 40 participants (46 eyes) in punctal plug group 40 participants (44 eyes) in artificial tears group Exclusions after randomization: none reported Number analyzed: 80 participants (90 eyes) in total 40 participants (46 eyes) in punctal plug group 40 participants (44 eyes) in artificial tears group Losses to follow‐up: none reported Mean age (SD): 35.21 (NR) overall 35.28 (5.58) years in the punctal plug group 35.13 (6.25) years in the artificial tears group Age range: 22 to 46 years in the punctal plug group 21 to 48 years in the artificial tears group Sex (%): 31 women (39%) and 49 men (61%)in total 15 women (38%) and 25 men (62%) in the punctal plug group 16 women (40%) and 24 men (60%) in the artificial tears group Inclusion criteria: time on viewing a monitor ≥ 5 hours per day; itching, foreign body sensation, tearing, redness, and photophobia; aqueous tear production < 10 mm/5 min, TBUT< 10 s; artificial tears use > 3 times/d Exclusion criteria: not meeting inclusion criteria; corneal, conjunctiva, iris disorder; pregnant; diabetes | |
| Interventions | Intervention 1: Thermal Memory hydrophobic acrylic polymer rigid rod punctal plug Punctal plug model: NR Manufacturer of punctal plug: NR Location of manufacturer: NR Control: 1g/L sodium hyaluronate eye drops; 1 drop/time; 4‐6 times /day Length of follow‐up: Planned: NR Actual: 3 months | |
| Outcomes | Primary and secondary outcomes not differentiated: Outcomes reported: tear secretion test, tear film break‐up time, scores of dry eye symptoms Adverse events reported: inflammation: 12 in control group and 10 in intervention group. Plug prolapses: 1 in control group and 2 in intervention group Intervals at which outcomes assessed: baseline and 3 months | |
| Notes | Trial registry: none reported Type of study: published full‐text Funding sources: NR Disclosures of interest: NR Enrollement period: March 2013 to March 2015 Reported subgroup analyses: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated using SAS 9.2 program |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias and detection bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No participants were reported to have been lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Published protocol or trial registry number were not available for comparison |
| Other bias | Unclear risk | Source of funding and conflict of interest were not reported |
NA: not applicable; NR: not reported; SD: standard deviation; TBUT: tear break‐up time.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong intervention; botulinum neurotoxin type A | |
| Wrong intervention; 0.05 mL of hypromellose | |
| No diagnosis of dry eye syndrome | |
| Synthetic punctal plugs | |
| Not a randomized or quasi‐randomized controlled trial | |
| Not a randomized or quasi‐randomized controlled trial | |
| Not a randomized or quasi‐randomized controlled trial | |
| No relevant comparisons | |
| Wrong comparison. Trial registration number: JPRN‐UMIN000011574 | |
| No relevant comparisons | |
| Not a randomized or quasi‐randomized controlled trial | |
| Not a randomized or quasi‐randomized controlled trial | |
| No relevant outcome data | |
| Not a randomized or quasi‐randomized controlled trial | |
| No relevant comparisons | |
| Not a randomized or quasi‐randomized controlled trial | |
| Not a randomized or quasi‐randomized controlled trial | |
| Conference proceeding abstract; study met inclusion criteria, but data were not presented by relevant treatment groups; investigators were unable to provide further details | |
| Not a randomized or quasi‐randomized controlled trial | |
| Conference proceeding abstract; study met inclusion criteria, but no data presented in abstract; unable to locate contact information for sole investigator | |
| Not a randomized or quasi‐randomized controlled trial. Authors could not be contacted |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Treatment of dry eye with absorbable punctual plug (VisiPlug) in a randomized, observer‐blind and parallel study |
| Methods | Randomized controlled trial |
| Participants | Inclusion criteria: "1. Voluntarily participated in this clinical study and signed an informed consent 2. Male or female, aged 18 to 70 years old 3. Patients with dry eye signs and symptoms, consistent with either criterion as follows:
4. Willing to follow the requirements of this study; 5. Subjects did not participate in other clinical trials during the last 4 weeks; 6. Subjects did not use any topical medications other than artifical tears, or have used these medications, but withdrew them more than 2 weeks before; 7. The daily life vision of included subjects eye should be equal to or more than 0.1." Exclusion criteria: "1. Subjects with inflammation and infection in lacrimal system; 2. Subjects with nasolacrimal duct blocking or stenosis; 3. Subjects with severe conjunctivochalasis; 4. Allergy to any ingredient of the test materials; 5. Clinically diagnosed as fungal, bacterial or viral keratitis/conjunctivitis in active stage; 6. Co‐existence with other conjunctiva, cornea and iris lesions; 7. Patients with severe primary disease such as severe heart, brain and blood vessels, liver, kidney and hematopoietic systems disease; 8. Patients who received intraocular surgery or with intraocular trauma in the last 6 months; 9. Postmenopausal women with hormone replacement therapy; 10. Patients received permanent punctal occlusion or absorbable punctal occlusion in the last 6 months; 11. Patients who cannot stop wearing contact lenses during the trial; 12. Patients who cannot obey the required treatments and follow‐ups during the trial." |
| Interventions | Intervention 1: both upper and lower punctal occlusion Intervention 2: lower punctal occlusion Intervention 3: upper punctal occlusion |
| Outcomes | Primary outcomes*:
Secondary outcomes**:
* Primary outcomes will be measured at week 4 after treatment ** Secondary outcomes will be measured at week 1, week 4 or week 12 after treatment |
| Starting date | NR |
| Contact information | Lan Gong ([email protected]) Eye & ENT Hospital of Fudan University, 83 Fenyang Road, Shanghai 200031 |
| Notes | Trial registration number: ChiCTR‐IPR‐16007760 (registered at Chinese Clinical Trial Registry) Source of funding: Eye & ENT Hospital of Fudan University Accessed on 9 January 2017 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement at 1 month Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1 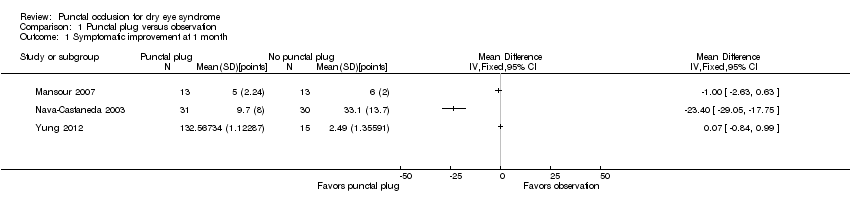 Comparison 1 Punctal plug versus observation, Outcome 1 Symptomatic improvement at 1 month. | ||||
| 2 Symptomatic improvement (long‐term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Punctal plug versus observation, Outcome 2 Symptomatic improvement (long‐term). | ||||
| 3 Ocular surface staining at 2 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Punctal plug versus observation, Outcome 3 Ocular surface staining at 2 weeks. | ||||
| 4 Ocular surface staining at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Punctal plug versus observation, Outcome 4 Ocular surface staining at 1 month. | ||||
| 5 Ocular surface staining (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Punctal plug versus observation, Outcome 5 Ocular surface staining (long‐term). | ||||
| 6 Tear film stability at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6 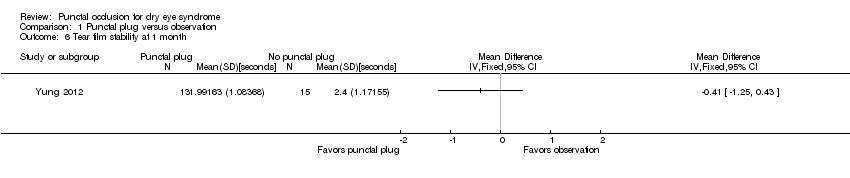 Comparison 1 Punctal plug versus observation, Outcome 6 Tear film stability at 1 month. | ||||
| 7 Tear film stability (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7 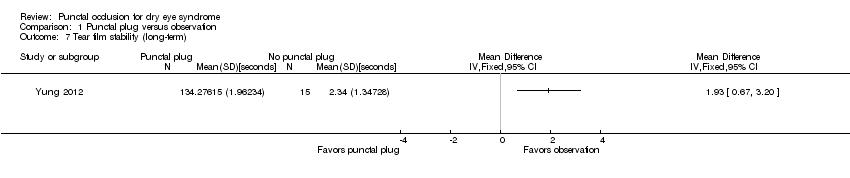 Comparison 1 Punctal plug versus observation, Outcome 7 Tear film stability (long‐term). | ||||
| 8 Artificial tear use at 2 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Punctal plug versus observation, Outcome 8 Artificial tear use at 2 weeks. | ||||
| 9 Artificial tear use at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9 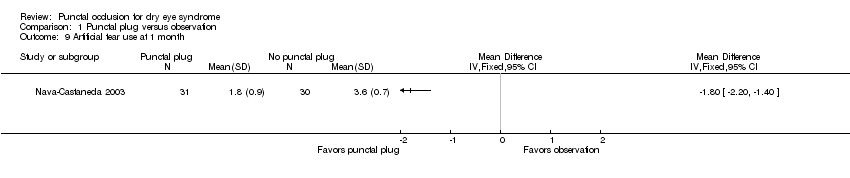 Comparison 1 Punctal plug versus observation, Outcome 9 Artificial tear use at 1 month. | ||||
| 10 Artificial tear use (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Punctal plug versus observation, Outcome 10 Artificial tear use (long‐term). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ocular surface staining at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Punctal plugs versus cyclosporine, Outcome 1 Ocular surface staining at 1 month. | ||||
| 2 Ocular surface staining at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2 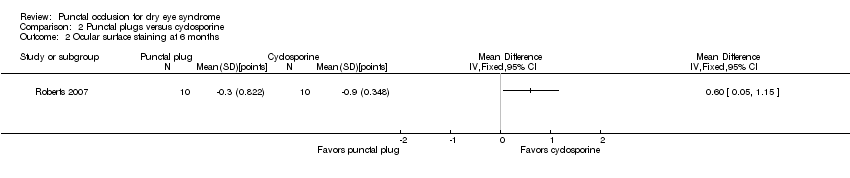 Comparison 2 Punctal plugs versus cyclosporine, Outcome 2 Ocular surface staining at 6 months. | ||||
| 3 Aqueous tear production at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3 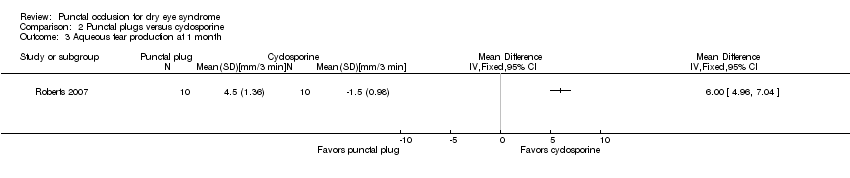 Comparison 2 Punctal plugs versus cyclosporine, Outcome 3 Aqueous tear production at 1 month. | ||||
| 4 Aqueous tear production at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Punctal plugs versus cyclosporine, Outcome 4 Aqueous tear production at 6 months. | ||||
| 5 Artificial tear use at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5 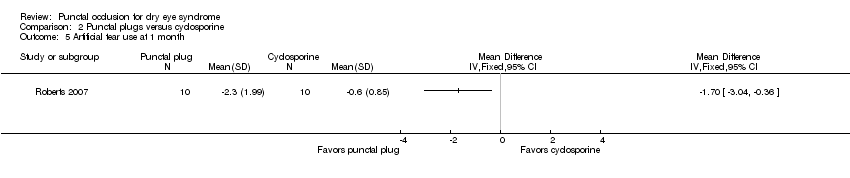 Comparison 2 Punctal plugs versus cyclosporine, Outcome 5 Artificial tear use at 1 month. | ||||
| 6 Artificial tear use at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Punctal plugs versus cyclosporine, Outcome 6 Artificial tear use at 6 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Punctal plugs versus oral pilocarpine, Outcome 1 Symptomatic improvement at 3 months. | ||||
| 2 Ocular surface staining Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Punctal plugs versus oral pilocarpine, Outcome 2 Ocular surface staining. | ||||
| 2.1 Right eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Left eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Aqueous tear production Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Punctal plugs versus oral pilocarpine, Outcome 3 Aqueous tear production. | ||||
| 3.1 Right eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Left eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement at 2 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Punctal plugs versus artificial tears, Outcome 1 Symptomatic improvement at 2 weeks. | ||||
| 2 Symptomatic improvement at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2 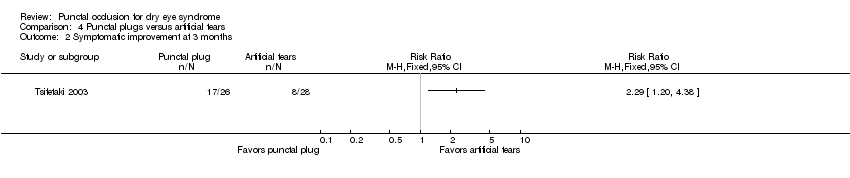 Comparison 4 Punctal plugs versus artificial tears, Outcome 2 Symptomatic improvement at 3 months. | ||||
| 3 Symptomatic improvement at 3 months Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐4.20 [‐5.87, ‐2.53] |
| Analysis 4.3 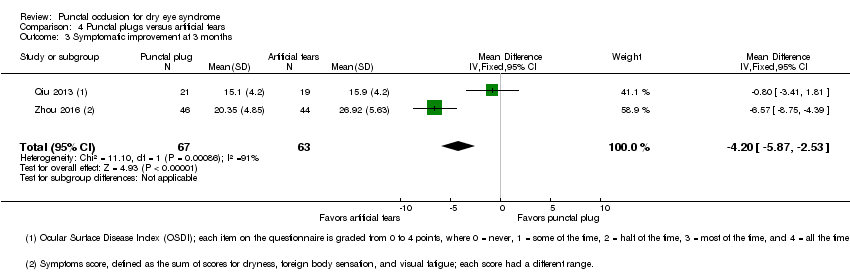 Comparison 4 Punctal plugs versus artificial tears, Outcome 3 Symptomatic improvement at 3 months. | ||||
| 4 Ocular surface staining at 2 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Punctal plugs versus artificial tears, Outcome 4 Ocular surface staining at 2 weeks. | ||||
| 5 Ocular surface staining at 3 months (Rose Bengal) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.5  Comparison 4 Punctal plugs versus artificial tears, Outcome 5 Ocular surface staining at 3 months (Rose Bengal). | ||||
| 5.1 Right eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Left eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Ocular surface staining at 3 months (fluorescein) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.6  Comparison 4 Punctal plugs versus artificial tears, Outcome 6 Ocular surface staining at 3 months (fluorescein). | ||||
| 7 Aqueous tear production at 2 weeks Show forest plot | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [‐1.05, 2.71] |
| Analysis 4.7 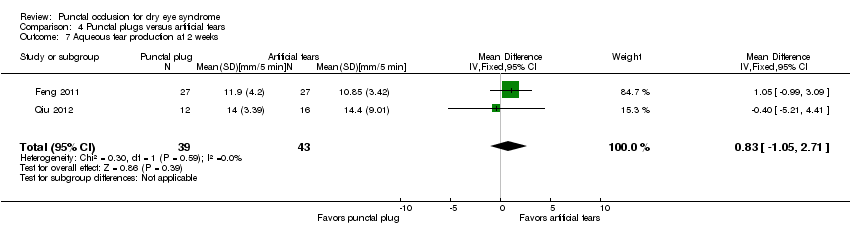 Comparison 4 Punctal plugs versus artificial tears, Outcome 7 Aqueous tear production at 2 weeks. | ||||
| 8 Aqueous tear production at 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.8 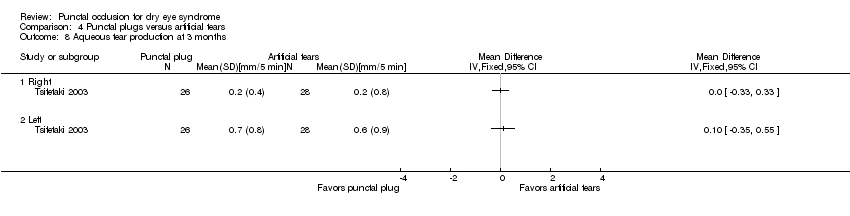 Comparison 4 Punctal plugs versus artificial tears, Outcome 8 Aqueous tear production at 3 months. | ||||
| 8.1 Right | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Left | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Aqueous tear production at 3 months Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 2.16 [1.41, 2.90] |
| Analysis 4.9  Comparison 4 Punctal plugs versus artificial tears, Outcome 9 Aqueous tear production at 3 months. | ||||
| 10 Tear film stability at 2 weeks Show forest plot | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.57, 1.09] |
| Analysis 4.10  Comparison 4 Punctal plugs versus artificial tears, Outcome 10 Tear film stability at 2 weeks. | ||||
| 11 Tear film stability at 3 months Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 1.02 [0.60, 1.44] |
| Analysis 4.11 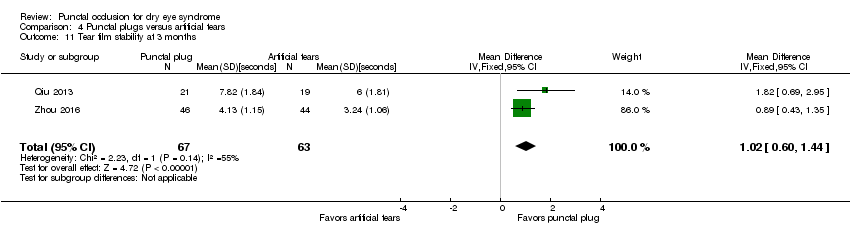 Comparison 4 Punctal plugs versus artificial tears, Outcome 11 Tear film stability at 3 months. | ||||
| 12 Punctate epithelial keratopathy Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.12  Comparison 4 Punctal plugs versus artificial tears, Outcome 12 Punctate epithelial keratopathy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement at 1 month Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Punctal plugs in the upper versus lower puncta, Outcome 1 Symptomatic improvement at 1 month. | ||||
| 2 Aqueous tear production at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Punctal plugs in the upper versus lower puncta, Outcome 2 Aqueous tear production at 1 month. | ||||
| 3 Tear film stability at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3 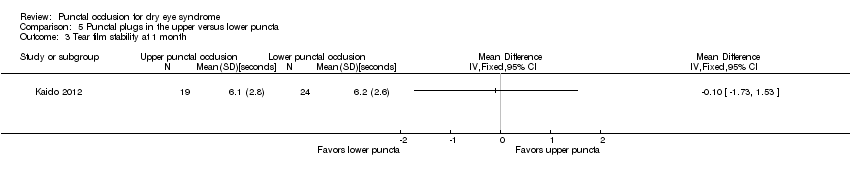 Comparison 5 Punctal plugs in the upper versus lower puncta, Outcome 3 Tear film stability at 1 month. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement at 11 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 1 Symptomatic improvement at 11 weeks. | ||||
| 2 Ocular surface staining at 11 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 2 Ocular surface staining at 11 weeks. | ||||
| 2.1 Rose Bengal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Fluorescein | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Aqueous tear production at 11 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 3 Aqueous tear production at 11 weeks. | ||||
| 4 Tear film stability at 11 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.4  Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 4 Tear film stability at 11 weeks. | ||||
| 5 Artificial tear use at 11 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.5  Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 5 Artificial tear use at 11 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 1 Symptomatic improvement (long‐term). | ||||
| 2 Ocular surface staining (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.2 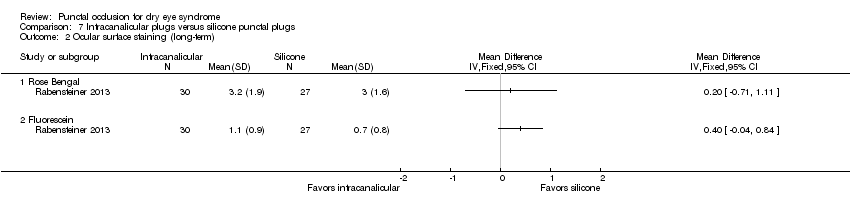 Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 2 Ocular surface staining (long‐term). | ||||
| 2.1 Rose Bengal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Fluorescein | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Aqueous tear production (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 3 Aqueous tear production (long‐term). | ||||
| 3.1 Schirmer test I without anesthesia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Tear film stability (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.4 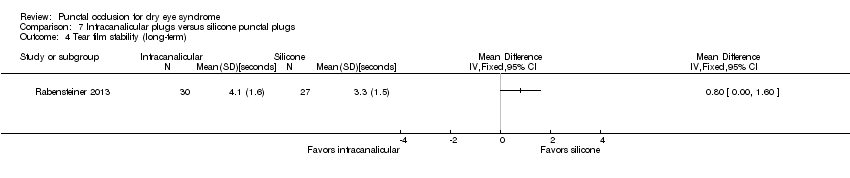 Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 4 Tear film stability (long‐term). | ||||
| 5 Artificial tear use (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.5 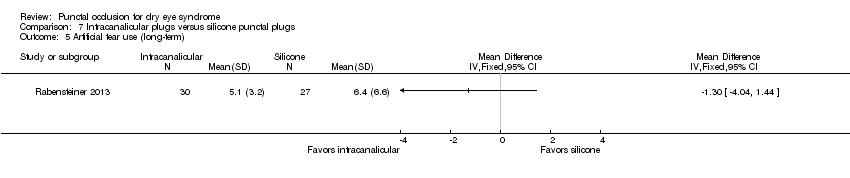 Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 5 Artificial tear use (long‐term). | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Punctal plug versus observation, Outcome 1 Symptomatic improvement at 1 month.

Comparison 1 Punctal plug versus observation, Outcome 2 Symptomatic improvement (long‐term).

Comparison 1 Punctal plug versus observation, Outcome 3 Ocular surface staining at 2 weeks.

Comparison 1 Punctal plug versus observation, Outcome 4 Ocular surface staining at 1 month.
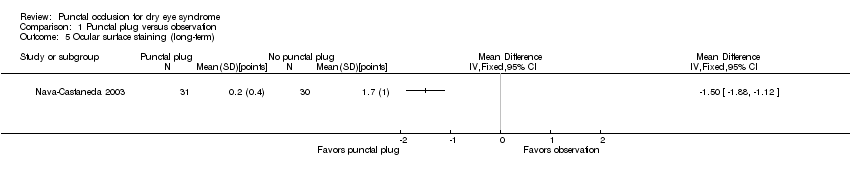
Comparison 1 Punctal plug versus observation, Outcome 5 Ocular surface staining (long‐term).
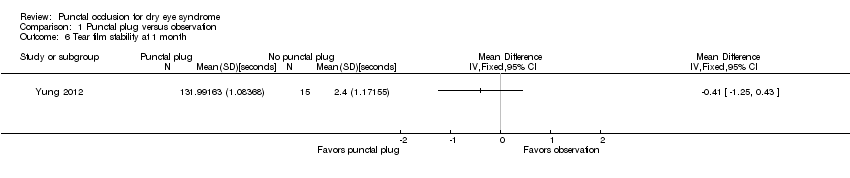
Comparison 1 Punctal plug versus observation, Outcome 6 Tear film stability at 1 month.

Comparison 1 Punctal plug versus observation, Outcome 7 Tear film stability (long‐term).
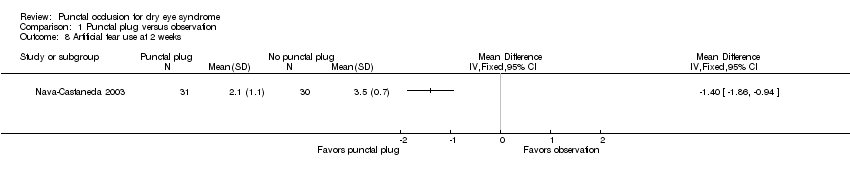
Comparison 1 Punctal plug versus observation, Outcome 8 Artificial tear use at 2 weeks.

Comparison 1 Punctal plug versus observation, Outcome 9 Artificial tear use at 1 month.

Comparison 1 Punctal plug versus observation, Outcome 10 Artificial tear use (long‐term).

Comparison 2 Punctal plugs versus cyclosporine, Outcome 1 Ocular surface staining at 1 month.
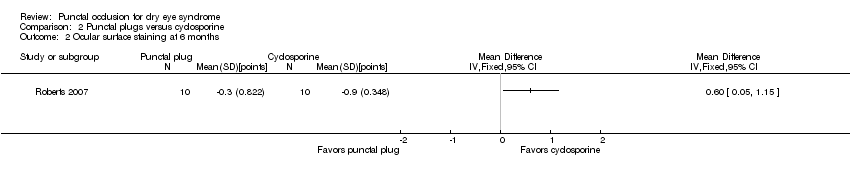
Comparison 2 Punctal plugs versus cyclosporine, Outcome 2 Ocular surface staining at 6 months.

Comparison 2 Punctal plugs versus cyclosporine, Outcome 3 Aqueous tear production at 1 month.

Comparison 2 Punctal plugs versus cyclosporine, Outcome 4 Aqueous tear production at 6 months.

Comparison 2 Punctal plugs versus cyclosporine, Outcome 5 Artificial tear use at 1 month.

Comparison 2 Punctal plugs versus cyclosporine, Outcome 6 Artificial tear use at 6 months.

Comparison 3 Punctal plugs versus oral pilocarpine, Outcome 1 Symptomatic improvement at 3 months.
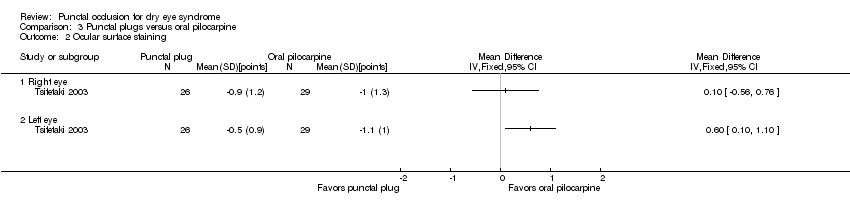
Comparison 3 Punctal plugs versus oral pilocarpine, Outcome 2 Ocular surface staining.

Comparison 3 Punctal plugs versus oral pilocarpine, Outcome 3 Aqueous tear production.
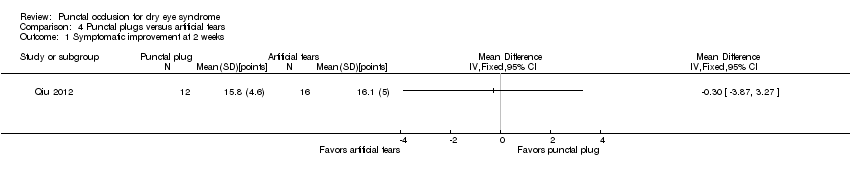
Comparison 4 Punctal plugs versus artificial tears, Outcome 1 Symptomatic improvement at 2 weeks.
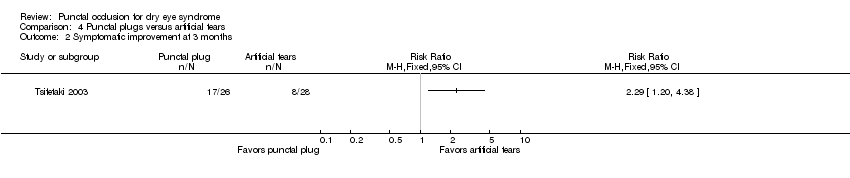
Comparison 4 Punctal plugs versus artificial tears, Outcome 2 Symptomatic improvement at 3 months.
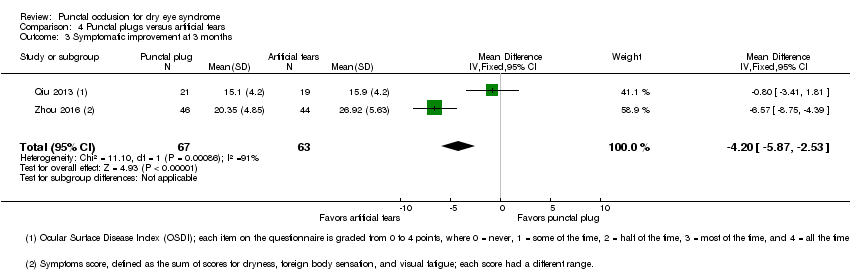
Comparison 4 Punctal plugs versus artificial tears, Outcome 3 Symptomatic improvement at 3 months.

Comparison 4 Punctal plugs versus artificial tears, Outcome 4 Ocular surface staining at 2 weeks.

Comparison 4 Punctal plugs versus artificial tears, Outcome 5 Ocular surface staining at 3 months (Rose Bengal).

Comparison 4 Punctal plugs versus artificial tears, Outcome 6 Ocular surface staining at 3 months (fluorescein).
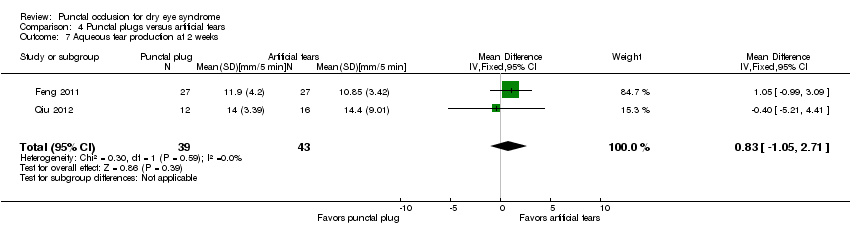
Comparison 4 Punctal plugs versus artificial tears, Outcome 7 Aqueous tear production at 2 weeks.
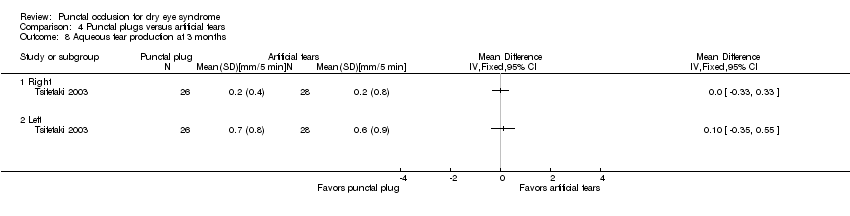
Comparison 4 Punctal plugs versus artificial tears, Outcome 8 Aqueous tear production at 3 months.

Comparison 4 Punctal plugs versus artificial tears, Outcome 9 Aqueous tear production at 3 months.

Comparison 4 Punctal plugs versus artificial tears, Outcome 10 Tear film stability at 2 weeks.

Comparison 4 Punctal plugs versus artificial tears, Outcome 11 Tear film stability at 3 months.

Comparison 4 Punctal plugs versus artificial tears, Outcome 12 Punctate epithelial keratopathy.

Comparison 5 Punctal plugs in the upper versus lower puncta, Outcome 1 Symptomatic improvement at 1 month.

Comparison 5 Punctal plugs in the upper versus lower puncta, Outcome 2 Aqueous tear production at 1 month.

Comparison 5 Punctal plugs in the upper versus lower puncta, Outcome 3 Tear film stability at 1 month.
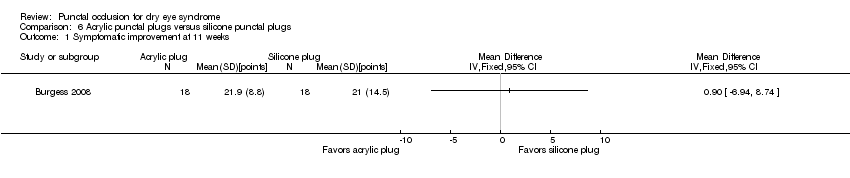
Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 1 Symptomatic improvement at 11 weeks.

Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 2 Ocular surface staining at 11 weeks.
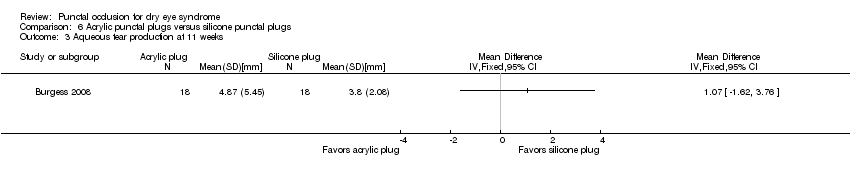
Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 3 Aqueous tear production at 11 weeks.
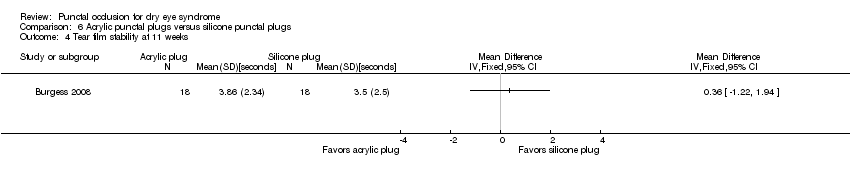
Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 4 Tear film stability at 11 weeks.
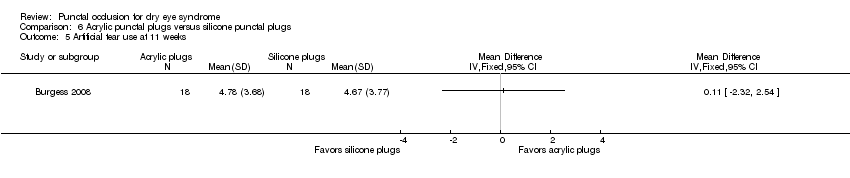
Comparison 6 Acrylic punctal plugs versus silicone punctal plugs, Outcome 5 Artificial tear use at 11 weeks.
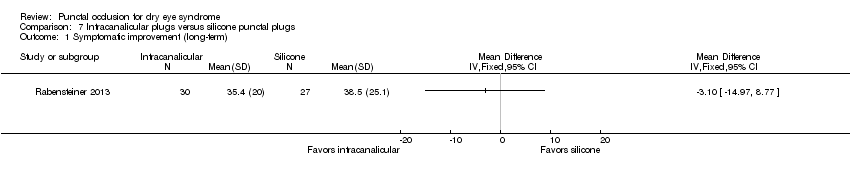
Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 1 Symptomatic improvement (long‐term).
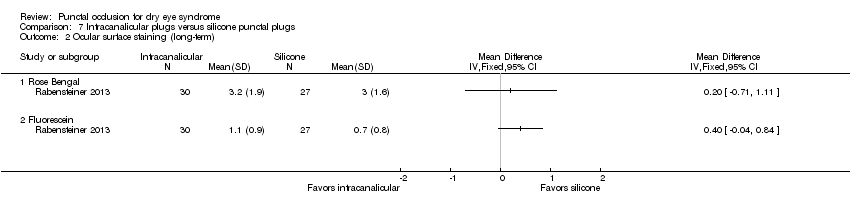
Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 2 Ocular surface staining (long‐term).
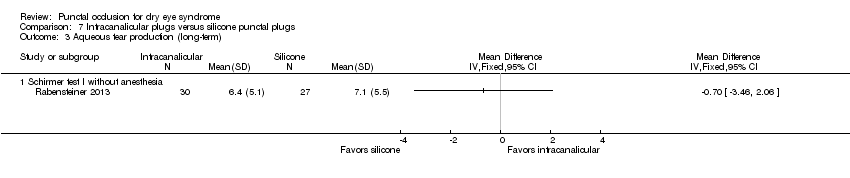
Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 3 Aqueous tear production (long‐term).

Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 4 Tear film stability (long‐term).

Comparison 7 Intracanalicular plugs versus silicone punctal plugs, Outcome 5 Artificial tear use (long‐term).
| Punctal plugs compared with no punctal plugs for dry eye syndrome | ||||||
| Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: silicone or collagen punctal plugs Comparison: no punctal plugs (observation or sham treatment) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No punctal plugs | Punctal plugs | |||||
| Symptomatic improvement Follow‐up: long‐term* A lower score favors punctal plugs | See comments | See comments | — | 89 (2 RCT) | ⊕⊝⊝⊝ | Mansour 2007 reported little or no difference in Ocular and Oral Symptoms score, which ranged from 0 to 10 points (MD ‐0.46 points, 95% CI ‐1.24 to 0.32; eyes = 26). Nava‐Castaneda 2003 reported a slight decrease in symptom score, assumed to range from 0 to 105 points, but it does not seem to be clinically important (MD ‐2.62 points, 95% CI ‐3.32 to ‐1.93; eyes = 61). Yung 2012 reported little or no difference in dry eye symptom score, ranging from 0 to 3 points (MD ‐0.75 points, 95% CI ‐1.53 to 0.02; eyes = 28) |
| Ocular surface staining Follow‐up: long‐term* A higher value is less advantageous Both Rose Bengal and fluorescein staining scores ranged from 0 to 4, where 0 represented no staining and 4 represented heavy staining | The mean fluorescein staining score was 1.70 | MD 1.50 lower than observation group (1.88 to 1.12 lower (better) than observation group) | — | 61 (1 RCT) | ⊕⊕⊝⊝ | — |
| Aqueous tear production Follow‐up: long‐term* A higher value is more advantageous | See comments | See comments | — | 28 (1 RCT) | ⊕⊕⊝⊝ | Yung 2012 did not provide quantitative data, but reported that "Schirmer values tended to increase in the plug group after plug insertion; however, the changes did not reach significance" |
| Tear film stability Follow‐up: 6 months A higher value is more advantageous | The mean tear film stability was 2.34 seconds | MD 1.93 seconds longer than observation group (0.67 to 3.20 seconds longer (better) than observation group) | — | 28 (1 RCT) | ⊕⊕⊝⊝ | — |
| Artificial tear use Follow‐up: long‐term* Fewer applications favors punctal plugs | The mean number of applications was 3.6 applications | MD 2.70 fewer applications than observation group (3.11 to 2.29 fewer applications than observation group) | — | 61 (1 RCT) | ⊕⊕⊝⊝ | — |
| Adverse events Follow‐up: end of study | See comments | See comments | — | 146 | ⊕⊝⊝⊝ | Slusser 1998: all adverse events occurred in the punctal plug group, reported 23/28 participants had epiphora, 3/28 participants reported itching in area of plug placement, 1/28 participants had tenderness and swelling of lids with mucous discharge. Spontaneous plug loss occurred in 6/20 eyes with silicone punctal plugs in the Mansour 2007. One or 31 participants receiving collagen and silicone punctal plugs experienced epiphora in the Nava‐Castaneda 2003 study. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for methodological heterogeneity. cDowngraded one level for imprecision as indirectness of evidence because the confidence interval was either wide or clinically not important. dDowngraded one level for high risk of attrition bias. eDowngraded two levels for high risk of performance, detection, and attrition bias. fDowngraded two levels for attrition bias. gDowngraded one level for sparse and inconsistent data, particularly with respect to epiphora. *We defined long‐term follow‐up as between two months and one year. | ||||||
| Punctal plugs compared with cyclosporine for dry eye syndrome | |||||
| Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: punctal plugs Comparison: cyclosporine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| Cyclosporine | Punctal plugs | ||||
| Symptomatic improvement | Study investigators did not report this outcome at 2 weeks, 1 month, or long‐term. | ||||
| Ocular surface staining Follow‐up: 6 months Any value greater than zero is abnormal Range: 0 to 4 points; 0 = no staining, 1 = staining of the nasal conjunctiva only, 2 = staining of both the nasal and temporal conjunctiva, 3 = peripheral corneal staining, 4 = central corneal staining | See comments | See comments | 20 (1 RCT) | ⊕⊝⊝⊝ | The study investigators of Roberts 2007 stated: "there was greater improvement in conjunctival staining with cyclosporine or the combination than with plugs alone." It was unclear whether Rose Bengal or fluorescein staining was used. Also, the study investigators did not specify the time point and so we assumed that their statement applies for 1, 3, and 6 months follow‐up. |
| Aqueous tear production Follow‐up: 6 months A higher value is more advantageous | The mean change in aqueous tear production was 1.5 mm/3 min lower than baseline | MD 0.80 mm/3 min higher than cyclosporine group (0.74 lower (better) to 2.34 higher (worse) than cyclosporine group) | 20 | ⊕⊝⊝⊝ | The study investigators of Roberts 2007 stated: "There was a greater increase in Schirmer score with plugs, either alone or in combination with cyclosporine." The study investigators did not specify the for which time point and so we assumed that their statement applies for 1, 3, and 6 months follow‐up. |
| Tear film stability Follow‐up: 6 months | Study investigators did not report this outcome at 2 weeks, 1 month, or long‐term. | ||||
| Artificial tear use Follow‐up: 6 months range: 1‐5 applications Fewer applications favors punctal plugs | The mean change in number of applications from baseline was 3.2 more applications than baseline | MD 1.10 applications more than baseline (0.04 fewer to 2.24 more applications than cyclosporine group) | 20 | ⊕⊝⊝⊝ | The study investigators of Roberts 2007 stated: "decreased frequency of artificial tears was greatest for combination therapy and least for punctal plugs." The study investigators did not specify the for which time point and so we assumed that their statement applies for 1, 3, and 6 months follow‐up. |
| Adverse events Follow‐up: end of study | See comments | See comments | 22 | ⊕⊝⊝⊝ | Roberts 2007 reported 1/11 participants experienced plug displacement in the plug group, while 1/11 participants experienced a burning sensation in the cyclosporine group. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level for imprecision of results (wide confidence intervals). bDowngraded two levels for high risk of detection, performance, attrition, and other bias. *We defined long‐term follow‐up as between two months and one year. | |||||
| Punctal plugs compared with oral pilocarpine for dry eye syndrome | ||||||
| Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: punctal plugs Comparison: oral pilocarpine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral pilocarpine | Punctal plugs | |||||
| Symptomatic improvement Follow‐up: 3 months The study investigators defined improvements in subjective ocular symptoms as an improvement of >55 mm for responses to the eye questionnaire on a 100 mm visual analog scale. A RR less than one favors punctal plugs. | 897 per 1000 | 619 per 1000 (439 to 852) | RR 0.69 (0.49 to 0.95) | 55 | ⊕⊝⊝⊝ | ‐ |
| Ocular surface staining Follow‐up: 3 months A higher value is less advantageous Range: van Bijsterveld schema, which is on a scale of 0 to 9 points | The mean change in Rose Bengal staining score was 1.00 lower (better) than baseline in the right eye | MD 0.10 higher (worse) than oral pilocarpine group (0.56 lower (better) to 0.76 higher (worse) than oral pilocarpine group) | — | 55 | ⊕⊝⊝⊝ | — |
| The mean change in Rose Bengal staining score was 1.10 lower (better) than baseline in the left eye | MD 0.60 higher (worse) than oral pilocarpine group (0.10 to 1.10 higher (worse) than oral pilocarpine group) | — | 55 | ⊕⊝⊝⊝ | — | |
| Aqueous tear production Follow‐up: 3 months A higher value more advantageous | The mean change in aqueous tear production was 0.30 mm/5 min higher (better) than baseline in the right eye | MD 0.10 mm/5 min lower (worse) than oral pilocarpine group (0.53 mm/5 min lower (worse) to 0.33 mm/5 min higher (better) than oral pilocarpine group) | — | 55 | ⊕⊝⊝⊝ | — |
| The mean change in aqueous tear production was 1.2 mm/5 min higher (better) than baseline in the left eye | MD 0.50 mm/5 min lower (worse) than oral pilocarpine group (1.06 mm/5 min lower (worse) to 0.06 mm/5 min higher (better) than oral pilocarpine group) | — | 55 | — | ||
| Tear film stability | Study investigators did not report this outcome. | |||||
| Artificial tear use | Study investigators did not report this outcome. | |||||
| Adverse events Follow‐up: 3 months | See comments | See comments | — | 55 | ⊕⊝⊝⊝ | Tsifetaki 2003 reported: "commonly reported adverse events were headache, increased sweating,nausea, and vomiting in the pilocarpine group, while 1 patient in the inferior puncta occlusion group had blepharitis and was withdrawn from the study." pg 1204 |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for high risk of performance and detection bias as the participant and outcome assessors were not masked to the treatment groups and the self‐reported symptomatic improvement might be biased. *We defined long‐term follow‐up as between two months and one year. | ||||||
| Punctal plugs compared with artificial tears for dry eye syndrome | ||||||
| Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: punctal plugs Comparison: artificial tears | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Artificial tears | Punctal plugs | |||||
| Symptomatic improvement Follow‐up: 3 months The study investigators defined improvements in subjective ocular symptoms as an improvement of >55 mm for responses to the eye questionnaire on a 100 mm visual analog scale. A RR greater than one favors punctal plugs. Both studies used different symptomatic improvement score; one study used the Ocular Surface Disease Index that ranged from 0 points = never to 4 points = all the time. The second study use the sum of scores for dryness, foreign body sensation, and visual fatigue; each score had a different range, but a higher score corresponded to more symptoms. | 286 per 1000 | 654 per 1000 (343 to 1000) | RR 2.29 (1.2 to 4.38) | 54 (1 RCT) | ⊕⊝⊝⊝ | — |
| The mean symptomatic improvement score ranged from 15.9 to 26.92 | SMD 0.88 lower (better) than artificial tears group (1.24 to 0.51 lower (better) than artificial tears group) | — | 130 (2 RCTs) | — | ||
| Ocular surface staining Follow‐up: 3 months Range: 0 = absent; 1 = trace; 2 = mild; 3 = moderate; 4 = severe A higher value is worse | The mean change in Rose Bengal staining score was 1.0 point lower (better) than baseline in the right eye | MD 0.10 points higher (worse) than artificial tears group (0.56 lower (better) to 0.76 higher (worse) than artificial tears group) | — | 55 | ⊕⊕⊝⊝ | — |
| The mean change in Rose Bengal staining score was 1.1 lower (better) than baseline in the left eye | MD 0.60 points higher (worse) than artificial tears group (0.10 to 1.10 higher (worse) than artificial tears group) | — | 55 | — | ||
| Aqueous tear production Follow‐up: long‐term* A higher value is more advantageous | The mean change in aqueous tear production was 0.2 mm/5 min higher (better) than baseline in the right eye | MD 0.00 mm/5 min higher (better) than artificial tears group (0.33 mm/5 min lower (worst) to 0.33 mm/5 min higher (better) than artificial tears group) | — | 54 | ⊕⊕⊝⊝ | — |
| The mean change in aqueous tear production was 0.6 mm/5 min higher (better) than baseline in the left eye | MD 0.10 mm/5 min higher (better) than artificial tears group (0.35 mm/5 min lower (worst) to 0.55 mm/5 min higher (better) than artificial tears group) | — | 54 | — | ||
| The mean aqueous tear production ranged from 4.89 to 8.95 mm/ 5 min | MD 2.16 mm/ 5 min higher (better) (1.41 to 2.90 mm/ 5 min higher (better) than artificial tears group) | — | 130 (2 RCTs) | — | ||
| Tear film stability Follow‐up: long‐term* A higher value is more advantageous | The mean tear film stability ranged from 3.24 to 6 seconds | MD 1.02 seconds longer (better) than artificial tears group (0.60 to 1.44 seconds longer (better) than artificial tears group) | — | 130 | ⊕⊕⊕⊝ | — |
| Artificial tear use | Outcome not relevant to this comparison | |||||
| Adverse events (punctate epithelial keratopathy) Follow‐up: end of study | 375 per 1000 | 499 per 1000 (214 to 1000) | RR 1.33 (0.57 to 3.12) | 54 | ⊕⊕⊝⊝ | Tsifetaki 2003 reported: "four patients had mild headache, of whom three also presented with nausea, vomiting, and sweating" (p 1205) and "one patient in the inferior puncta occlusion group had blepharitis and was withdrawn from the study" (p 1204) |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for high risk of performance and detection bias as participants and outcome assessors were unmasked to the assigned treatment group and might influence the self‐reported symptomatic improvement. *We defined long‐term follow‐up as between two months and one year. | ||||||
| Punctal plugs occluded in the upper puncta compared with the lower puncta for dry eye syndrome | ||||||
| Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: punctal plugs occluded in the upper puncta Comparison: punctal plugs occluded in the lower puncta | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Lower puncta | Upper puncta | |||||
| Symptomatic improvement Follow‐up: long‐term* | Study investigators did not report this outcome. | |||||
| Ocular surface staining Follow‐up: long‐term* | Study investigators did not report this outcome. | |||||
| Aqueous tear production Follow‐up: long‐term* | Study investigators did not report this outcome. | |||||
| Tear film stability Follow‐up: long‐term* | Study investigators did not report this outcome. | |||||
| Artificial tear use | Study investigators did not report this outcome. | |||||
| Adverse events | See comments | See comments | See comments | 40 | ⊕⊝⊝⊝ | Chen 2010 reported "no complication was observed in dry eye patients or control subjects during the period of this study." It is unclear which complications were collected. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for potential bias as there was unclear risk of selection, performance, detection, attrition, and reporting bias. *We defined long‐term follow‐up as between two months and one year. | ||||||
| Acrylic punctal plugs compared with silicone punctal plugs for dry eye syndrome | ||||||
| Patient or population: mostly women with dry eye syndrome Settings: eye clinics Intervention: acrylic punctal plugs Comparison: silicone punctal plugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Silicone punctal plugs | Acrylic punctal plugs | |||||
| Symptomatic improvement Follow‐up: 11 weeks Range: 0 to 70 points A visual analog scales 10 cm in length for seven symptoms: dryness, grittiness, foreign body sensation, pain, stinging, burning, and itching was used. A lower score favors acrylic punctal plugs | The mean symptomatic improvement score was 21.9 points | MD 0.90 points higher than silicone punctal plug group (6.94 points lower than silicone punctal plug group to 8.74 points higher than silicone punctal plug group) | — | 36 | ⊕⊝⊝⊝ | — |
| Ocular surface staining Follow‐up: 11 weeks A higher value is less advantageous Range: 0 to 3 points | The mean fluorescein staining score was 1.63 points | MD 0.43 points higher (worst) than silicone punctal plug group (1.61 lower (better) than silicone punctal plug group to 2.47 higher (worse) than silicone punctal plug group) | — | 36 | ⊕⊝⊝⊝ | — |
| The mean Rose Bengal staining score was 0.55 points | MD 0.45 points higher (worst) than silicone punctal plug group (0.09 lower (better) than silicone punctal plug group to 0.99 higher (worst) than silicone punctal plug group) | — | — | |||
| Aqueous tear production Follow‐up: 11 weeks A higher value is more advantageous | The mean aqueous tear production was 3.8 mm/5 min | MD 1.07 mm/5 min higher than silicone punctal plug group (1.62 lower than silicone punctal plug group to 3.76 higher than silicone punctal plug group) | — | 36 | ⊕⊝⊝⊝ | Authors did not report the time interval in which the Schirmer's test 1 without anesthesia was performed. We assumed it was done over 5 minutes. |
| Tear film stability Follow‐up: 11 weeks A higher value is more advantageous | The mean tear film stability was 3.5 seconds | MD 0.36 seconds longer than silicone punctal plug group (1.22 seconds shorter than silicone punctal plug group to 1.94 longer than silicone punctal plug group) | — | 36 | ⊕⊝⊝⊝ | — |
| Artificial tear use Follow‐up: 11 weeks Range: 1‐5 applications Fewer applications favors punctal plugs | The mean number of applications was 4.67 applications | MD 0.11 more applications than silicone punctal plug group (2.32 fewer applications than silicone punctal plug group to 2.54 more applications than silicone punctal plug group) | — | 36 | ⊕⊝⊝⊝ | — |
| Adverse events Follow‐up: 11 weeks | See comments | See comments | — | 36 | ⊕⊝⊝⊝ | 1 acrylic punctal plug participant experienced epiphora, 1 silicone punctal plug participant experienced intermittent ocular irritation, and 2 silicone and 1 acrylic punctal plug participants experienced temporary foreign body sensation. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for imprecision of results (wide confidence intervals). bDowngraded one level for risk of bias as selection, attrition and reporting bias were judged to be unclear. *We defined long‐term follow‐up as between two months and one year. | ||||||
| Intracanalicular punctal plugs compared with silicone punctal plugs for dry eye syndrome | ||||||
| Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: intracanalicular punctal plugs Comparison: silicone punctal plugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Silicone punctal plugs | Intracanalicular punctal plugs | |||||
| Symptomatic improvement Follow‐up: 3 months Subjective dry eye symptoms for each eye was reported; investigators measured soreness, scratching, grittiness, dryness and/or burning using a 100 mm visual analog scale (VAS; 0 mm = no symptoms, 100 mm = maximum intensity) | The mean symptomatic improvement was 38.5 points | The mean difference in symptomatic improvement was 3.10 points lower (14.97 lower to 8.77 higher) | — | 57 | ⊕⊝⊝⊝ | — |
| Ocular surface staining Follow‐up: 3 months A higher value is less advantageous Range: 0 to 3 points; 0 = no staining and 3 = most intense staining | The mean Rose Bengal staining score was 3.0 | MD 0.20 higher than observation group (0.71 lower to 1.11 higher than silicone punctal plugs group) | — | 57 | ⊕⊝⊝⊝ | — |
| The mean fluorescein staining score was 0.7 points | MD 0.40 points higher than observation group (0.04 lower to 0.84 higher than silicone punctal plugs group) | — | 57 | — | ||
| Aqueous tear production Follow‐up: 3 months | Study investigators did not report this outcome. | |||||
| Tear film stability Follow‐up: 3 months | Study investigators did not report this outcome. | |||||
| Artificial tear use Follow‐up: 3 months Fewer applications favors intracanalicular plugs | The mean artificial tear use was 6.4 | MD 1.30 fewer applications (4.04 fewer to 1.44 more applications) | — | 57 | ⊕⊝⊝⊝ | — |
| Adverse events | Study investigators did not report on adverse events. | |||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for imprecision of results (wide confidence intervals). *We defined long‐term follow‐up as between two months and one year. | ||||||
| Collagen punctal plugs compared with silicone punctal plugs for dry eye syndrome | ||||||
| Patient or population: adults with dry eye syndrome Settings: eye clinics Intervention: collagen punctal plugs Comparison: silicone punctal plugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Silicone punctal plugs | Collagen punctal plugs | |||||
| Symptomatic improvement Follow‐up: 3 months The Canadian Dry Eye Assessment range from 0 to 48 points; where less than 5 points was normal, 5 to 15 points was mild, 20 to 25 points was moderate, 30 to 48 points was severe. | The mean symptomatic improvement score was 0.25 points | MD 0.81 higher than silicone punctal group (2.94 lower to 4.56 higher than silicone punctal group) | — | 50 (1 RCT) | ⊕⊝⊝⊝ | — |
| Ocular surface staining Follow‐up: 3 months Range: 0 to 15 points A higher value is less advantageous | The mean fluorescein stain score was 2.00 | MD 0.76 lower than silicone punctal group (18.5 lower to 17.0 higher than silicone punctal group) | — | 50 (1 RCT) | ⊕⊝⊝⊝ | — |
| Aqueous tear production Follow‐up: 3 months A higher value is more advantageous | The mean aqueous tear production was 16.89 mm/5 min | MD 0.67 mm/5 min higher than silicone punctal group (17.28 lower to 18.62 higher than silicone punctal group) | — | 50 (1 RCT) | ⊕⊝⊝⊝ | Authors did not report the time interval in which the Schirmer's test 1 without anesthesia was performed. We assumed it was done over 5 minutes. |
| Tear film stability Follow‐up: 3 months A higher value is more advantageous | The mean tear film stability was 4.67 seconds | MD 0.21 seconds higher than silicone punctal group | — | 50 (1 RCT) | ⊕⊝⊝⊝ | — |
| Artificial tear use Follow‐up: 3 months Fewer applications favors collagen punctal plugs | The mean number of artificial tear applications was 1.34 applications | MD 0.06 fewer applications (0.23 fewer to 0.12 more applications than silicone punctal group) | — | 50 (1 RCT) | ⊕⊕⊝⊝ | — |
| Adverse events Follow‐up: end of study | See comments | See comments | See comments | 98 (2 RCT) | ⊕⊝⊝⊝ | Both studies reported that none of the participants developed adverse events or complications related to punctal plugs. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for imprecision of results as the confidence interval was wide and clinically not important. bDowngraded one level for risk of bias as risk of bias as we judged selection, performance, detection, attrition, and reporting bias to be unclear. *We defined long‐term follow‐up as between two months and one year. | ||||||
| 1. Punctal plugs versus observation. | ||
| Collagen intracanalicular plugs were inserted in the upper and lower puncta | Sham treatment | |
| Silicone punctal plugs | No occlusion | |
| Collagen plus silicone punctal plugs | Sham treatment | |
| Bilateral collagen punctal plugs in the lower lids + cyclosporine eye drops to both eyes twice daily | Cyclosporine ophthalmic emulsion 0.05% | |
| Silicone punctal plugs in the upper and lower puncta | Sham treatment | |
| Silicone punctal plugs | Observation | |
| 2. Punctal plugs versus cyclosporine. | ||
| Bilateral collagen punctal plugs in the lower lids only | Cyclosporine ophthalmic emulsion 0.05% | |
| 3. Punctal plugs versus oral pilocarpine. | ||
| Collagen punctal plugs | Oral pilocarpine | |
| 4. Punctal plugs versus artificial tears. | ||
| Collagen punctal plugs | Artificial tears | |
| Acrylic punctal plugs | Artificial tears | |
| Acrylic punctal plugs | Artificial tears | |
| Collagen punctal plugs | Artificial tears | |
| Thermal Memory hydrophobic acrylic polymer rigid rod punctal plug | Artificial tears | |
| 5. Punctal plugs in the lower puncta versus the upper puncta. | ||
| Collagen punctal plugs in the lower puncta | Collagen punctal plugs in the upper puncta | |
| Collagen punctal plugs in the lower puncta | Collagen punctal plugs in the lower and upper puncta | |
| Silcone punctal plugs in the lower puncta | Silcone punctal plugs in the upper puncta | |
| 6. Acrylic punctal plugs versus silicone punctal plugs. | ||
| Acrylic punctal plugs | Silicone punctal plugs | |
| 7. Intracanalicular plugs versus Silicone punctal plugs. | ||
| Intracanicular | Silicone punctal plugs | |
| 8. Collagen punctal plugs versus silicone punctal plugs. | ||
| Collagen punctal plugs | Silicone punctal plugs | |
| Collagen punctal plugs were inserted in the lower punctum | Silicone punctal plugs were inserted in the lower punctum | |
| Excluded comparisons | ||
| Artificial tears | Oral pilocarpine | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement at 1 month Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Symptomatic improvement (long‐term) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Ocular surface staining at 2 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Ocular surface staining at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Ocular surface staining (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Tear film stability at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Tear film stability (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Artificial tear use at 2 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Artificial tear use at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Artificial tear use (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ocular surface staining at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Ocular surface staining at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Aqueous tear production at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Aqueous tear production at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Artificial tear use at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Artificial tear use at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Ocular surface staining Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Right eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Left eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Aqueous tear production Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Right eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Left eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement at 2 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Symptomatic improvement at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Symptomatic improvement at 3 months Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐4.20 [‐5.87, ‐2.53] |
| 4 Ocular surface staining at 2 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Ocular surface staining at 3 months (Rose Bengal) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Right eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Left eye | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Ocular surface staining at 3 months (fluorescein) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Aqueous tear production at 2 weeks Show forest plot | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [‐1.05, 2.71] |
| 8 Aqueous tear production at 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Right | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Left | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Aqueous tear production at 3 months Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 2.16 [1.41, 2.90] |
| 10 Tear film stability at 2 weeks Show forest plot | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.57, 1.09] |
| 11 Tear film stability at 3 months Show forest plot | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 1.02 [0.60, 1.44] |
| 12 Punctate epithelial keratopathy Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement at 1 month Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Aqueous tear production at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Tear film stability at 1 month Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement at 11 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Ocular surface staining at 11 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Rose Bengal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Fluorescein | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Aqueous tear production at 11 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Tear film stability at 11 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Artificial tear use at 11 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic improvement (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Ocular surface staining (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Rose Bengal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Fluorescein | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Aqueous tear production (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Schirmer test I without anesthesia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Tear film stability (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Artificial tear use (long‐term) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |