Heparina no fraccionada o de bajo peso molecular para la inducción de la remisión en la colitis ulcerosa
Resumen
Antecedentes
Es limitado el número de opciones terapéuticas para los pacientes con colitis ulcerosa (CU). Un mayor riesgo de trombosis en la CU, junto con una observación de que los pacientes con CU tratados con tratamiento anticoagulante para los eventos trombóticos presentaron una mejoría en sus síntomas intestinales, generó ensayos que examinaron el uso de la heparina no fraccionada (HNF) y las heparinas de bajo peso molecular (HBPM) en los pacientes con CU activa.
Objetivos
Examinar los ensayos aleatorizados sobre la eficacia de la heparina no fraccionada (HNF) o las heparinas de bajo peso molecular (HBPM) para la inducción de la remisión en los pacientes con colitis ulcerosa.
Métodos de búsqueda
Se hicieron búsquedas en MEDLINE, EMBASE, CENTRAL y en el registro de ensayos especializados del Grupo Cochrane de EII/TFI hasta junio de 2014. También se realizaron búsquedas en los documentos de revisión sobre la colitis ulcerosa y en las referencias de los documentos identificados, con el fin de identificar ensayos aleatorizados adicionales que estudiaran el uso de HNF o HBPM en pacientes con colitis ulcerosa. Se realizaron búsquedas en los resúmenes de los principales encuentros de gastroenterología para identificar las investigaciones publicadas en forma de resumen.
Criterios de selección
Se incluyeron ensayos controlados aleatorizados que comparaban la HNF o la HBPM con el placebo o un tratamiento de control para la inducción de la remisión en la colitis ulcerosa. Los estudios publicados sólo como resúmenes se incluyeron cuando se pudo establecer contacto con los autores para obtener información adicional.
Obtención y análisis de los datos
Se desarrolló y utilizó un formulario de extracción de datos de los estudios incluidos. Dos autores de la revisión de forma independiente extrajeron los datos. Los desacuerdos se resolvieron por consenso. Se utilizó la herramienta Cochrane de Riesgo de Sesgo para evaluar la calidad de los estudios. Los datos se analizaron por intención de tratar. El resultado principal fue la inducción de la remisión, según la definición de los estudios. Las medidas de resultados secundarios incluían: remisión endoscópica según la definición de los autores; mejoría clínica, histológica o endoscópica según la definición de los autores; aparición de eventos adversos; aparición de hemorragias; y mejoras en la calidad de vida según la medición de un instrumento validado. Se calculó el riesgo relativo (RR) y el intervalo de confianza (IC) del 95% correspondiente para los resultados dicotómicos. Los datos se combinaron para el análisis si evaluaban los mismos tratamientos (HNF o HBPM versus placebo u otro tratamiento). La calidad general de la evidencia que apoya los resultados se evaluó mediante los criterios GRADE.
Resultados principales
Cinco estudios fueron elegibles para su inclusión (329 pacientes). Tres estudios (270 pacientes) compararon la heparina de bajo peso molecular con el placebo, un estudio (34 pacientes) comparó la HBPM además del tratamiento estándar, y un estudio (25 pacientes) comparó la HNF con los corticosteroides. El estudio que comparaba la HNF con los corticosteroides fue calificado como de alto riesgo de sesgo debido a un diseño de cegamiento simple. El estudio que comparó la adición de HBPM a la terapia estándar con la terapia estándar sola se calificó con un alto riesgo de sesgo debido al diseño de etiqueta abierta. Los otros tres estudios fueron calificados como de bajo riesgo de sesgo. Las HBPM administradas por vía subcutánea no mostraron ningún beneficio sobre el placebo para ningún resultado, incluyendo la remisión clínica (muy baja calidad de la evidencia), y la mejoría clínica, endoscópica o histológica. Las HBPM en dosis altas administradas mediante un comprimido de liberación prolongada del colon demostraron beneficios sobre el placebo para la remisión clínica (RR 1,39; IC del 95%: 1,09 a 1,77; P = 0,008; muy baja calidad de la evidencia), la mejoría clínica (RR 1.28; IC del 95%: 1,06 a 1,55; p = 0,01; muy baja calidad de la evidencia), y la mejoría endoscópica (RR 1,21; IC del 95%: 1,00 a 1,47; p = 0,05), pero no la remisión endoscópica o la mejoría histológica. La HBPM no fue beneficiosa cuando se agregó al tratamiento estándar para la remisión clínica, la mejoría clínica, la remisión endoscópica ni la mejoría endoscópica. La HBPM se toleró bien pero no proporcionó un beneficio significativo para la calidad de vida. Un estudio que examinó HNF versus corticosteroides en el tratamiento de la CU grave demostró la inferioridad de la HNF para la mejoría clínica. Una mayor cantidad de pacientes asignados a la HNF presentó hemorragia rectal como un evento adverso.
Conclusiones de los autores
La evidencia indica que la HBPM puede ser efectiva para el tratamiento de la CU activa. Cuando se administró mediante comprimidos de liberación prolongada en el colon, la HBPM fue más efectiva que placebo para tratar a los pacientes ambulatorios con enfermedad leve a moderada. Este beneficio se debe confirmar en estudios controlados aleatorizados. Los mismos beneficios no se observaron cuando la HBPM se administró por vía subcutánea a dosis inferiores. No hay evidencia que apoye el uso de HNF para el tratamiento de la CU activa. Un ensayo adicional de HNF en pacientes con enfermedad leve también puede estar justificado. Todo beneficio hallado deberá compararse contra un posible riesgo mayor de hemorragia rectal en los pacientes con CU activa.
PICO
Resumen en términos sencillos
Heparina no fraccionada o de bajo peso molecular para el tratamiento de la colitis ulcerosa activa
Pregunta de la revisión
Se revisó la evidencia sobre los efectos de la heparina no fraccionada o de bajo peso molecular en la inducción de la remisión en pacientes con colitis ulcerosa, con la literatura médica hasta el 17 de junio de 2014.
Antecedentes
¿Qué es la colitis ulcerosa?
La colitis ulcerosa es una enfermedad intestinal inflamatoria crónica caracterizada por episodios recurrentes de la enfermedad activa, que habitualmente afectan al recto o al colon, o a ambos. Los pacientes con enfermedad activa pueden presentar cólicos abdominales, urgencia para defecar y diarrea sanguinolenta. Cuando los síntomas cesan, los pacientes entran en una "fase de remisión" de la colitis ulcerosa.
¿Qué es la heparina?
La heparina es una familia de medicamentos que reducen la capacidad natural del cuerpo para formar coágulos. Las heparinas no fraccionadas y de bajo peso molecular son subtipos de heparinas que actualmente están disponibles para uso clínico. En teoría, la disminución de la formación de coágulos puede ayudar a mejorar los síntomas de la colitis ulcerosa.
Características de los estudios
Los investigadores identificaron cinco estudios que incluyeron un total de 329 pacientes. Tres estudios (270 pacientes) compararon la heparina de bajo peso molecular con un placebo (p.ej., una píldora de azúcar), un estudio (34 pacientes) comparó la heparina de bajo peso molecular además del tratamiento estándar, y un estudio (25 pacientes) comparó la heparina no fraccionada con los esteroides. El estudio que comparaba la heparina no fraccionada con los esteroides y el estudio que comparaba la adición de heparina de bajo peso molecular al tratamiento estándar con la terapia estándar sola se juzgó de baja calidad. Los tres estudios controlados por placebo se consideraron de alta calidad.
Resultados clave
En un pequeño estudio, la heparina no fraccionada fue peor que los esteroides para inducir una mejora clínica (es decir, la reducción de los síntomas) en personas con colitis ulcerosa grave. Además, la hemorragia rectal era más frecuente entre las personas que recibían heparina no fraccionada. En otro pequeño estudio, la heparina de bajo peso molecular utilizada con la terapia estándar no proporcionó ningún beneficio adicional con respecto a la terapia estándar sola en adultos con colitis ulcerosa activa.
La heparina de bajo peso molecular administrada por inyección no mostró ningún beneficio sobre el placebo para ningún resultado, incluyendo la remisión clínica (muy baja calidad de la evidencia), la mejoría clínica y la mejoría endoscópica (es decir, la curación de la inflamación). Una alta dosis de heparina de bajo peso molecular administrada por medio de una tableta de liberación de colon extendida demostró beneficios sobre el placebo para la remisión clínica, la mejora clínica y la mejora endoscópica. Este resultado sugiere que una dosis alta de heparina de bajo peso molecular administrada en cápsulas de liberación prolongada puede ser eficaz para el tratamiento de la colitis ulcerosa activa. Sin embargo, este resultado debe ser verificado en futuros ensayos clínicos.
Authors' conclusions
Summary of findings
| LMWH compared to Placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | LMWH | |||||
| Endoscopic remission | 157 per 10001 | 211 per 1000 | RR 1.34 | 141 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| LMWH + standard therapy compared to Standard therapy for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard therapy | LMWH + standard therapy | |||||
| Clinical remission | 389 per 10001 | 311 per 1000 | RR 0.8 | 34 | ⊕⊝⊝⊝ | |
| Clinical improvement | 778 per 10001 | 879 per 1000 | RR 1.13 | 34 | ⊕⊝⊝⊝ | |
| Endoscopic remission | 389 per 10001 | 311 per 1000 | RR 0.8 | 34 | ⊕⊝⊝⊝ | |
| Endoscopic improvement | 611 per 10001 | 691 per 1000 | RR 1.13 | 34 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| UFH compared to Corticosteroids for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | UFH | |||||
| Clinical improvement | 692 per 10001 | 42 per 1000 | RR 0.06 | 25 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
Background
Ulcerative colitis (UC) is a chronic inflammatory bowel disease with a relatively limited number of treatment options. Traditional therapies include 5‐aminosalicylates and corticosteroids. Azathioprine is effective for maintenance of remission of quiescent disease (Timmer 2012), but its effectiveness for remission induction in patients with active disease is questionable (Ardizzone 2006; Hawthorne 1992; Jewell 1974). Infliximab is effective for induction and maintenance of remission in patients who have failed or not tolerated other therapies (Lawson 2006; Rutgeerts 2005). Cyclosporine may be effective for treating patients with severe disease, but with significant potential for toxicity (Shibolet 2005).
There is an increased risk of thrombosis in patients with ulcerative colitis (Bernstein 2001). This may be due to an association with underlying coagulopathies (Hudson 1996; Souto 1995). Microthrombosis may also play a role in the pathogenesis of ulcerative colitis (Dhillon 1992). The finding of an improvement in bowel symptoms in ulcerative colitis patients treated with unfractionated heparin for acute thrombotic events led to hypotheses about the role of thrombosis in the pathogenesis of ulcerative colitis (Zavgorodnii 1982). Subsequently, the potential role of unfractionated heparin and low molecular weight heparins in the treatment of patients with active ulcerative colitis was investigated. However, if a benefit were to be found, it would need to be balanced against the potential for worsening of rectal bleeding in patients with active ulcerative colitis treated with anticoagulants. This systematic review is an update of a previously published Cochrane review (Chande 2008; Chande 2010).
Objectives
To review randomized trials examining the efficacy of unfractionated heparin (UFH) or low molecular weight heparins (LMWH) for remission induction in patients with ulcerative colitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials comparing UFH or LMWH with placebo or an active comparator were considered for inclusion. Studies published as abstracts only were included if the authors could be contacted for further information.
Types of participants
Adult patients with active ulcerative colitis defined by a combination of clinical, radiographic, endoscopic and histological criteria were included.
Types of interventions
UFH or LMWH given by any route.
Types of outcome measures
The primary outcome measure was the number of patients achieving clinical remission and off steroids as defined by the studies and expressed as a percentage of the number of patients randomized (intention‐to‐treat analysis). Secondary outcomes measures included:
a) Endoscopic remission as defined by the authors;
b) Clinical, histological or endoscopic improvement as defined by the authors;
c) The occurrence of adverse events;
d) The occurrence of bleeding; and
e) Improvements in quality of life as measured by a validated instrument.
Search methods for identification of studies
See: Appendix 1 for search strategy.
The MEDLINE (PUBMED), and EMBASE databases, The Cochrane Central Register of Controlled Trials, the Cochrane IBD/FBD group specialized trials register, review papers on ulcerative colitis, and references from identified papers were searched up to June 17, 2014 in an effort to identify all randomized trials studying UFH or LMWH use in patients with ulcerative colitis. Abstracts from major gastroenterological meetings were searched to identify research published in abstract form only.
Data collection and analysis
Study selection
Each author independently reviewed potentially relevant trials to determine their eligibility for inclusion based on the criteria identified above. Studies published in abstract form only were included if the authors could be contacted for further information.
Data collection
A data extraction form was developed and used to extract data from included studies. At least 2 authors independently extracted data. Any disagreements were resolved by consensus.
Statistical analysis
Data were analyzed using Review Manager (RevMan 5.3.5). Data were analyzed on an intention‐to‐treat basis, and treated dichotomously. In cross‐over studies, only data from the first arm were included. The primary endpoint was induction of remission, as defined by the studies. Data were combined for analysis if they assessed the same treatments (UFH or LMWH versus placebo or other therapy). If a comparison was only assessed in a single trial, P‐values were derived using the chi‐square test. If the comparison was assessed in more than one trial, summary test statistics were derived using the Peto risk ratio and 95% confidence intervals (95% CI). The presence of heterogeneity among studies was assessed using the chi‐square test (a P value of 0.10 was regarded as statistically significant). If statistically significant heterogeneity was identified the risk ratio and 95% CI were calculated using a random effects model.
Quality assessment
All authors independently assessed the risk of bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Factors assessed included:
-
sequence generation (i.e. was the allocation sequence adequately generated?);
-
allocation sequence concealment (i.e. was allocation adequately concealed?);
-
blinding (i.e. was knowledge of the allocated intervention adequately prevented during the study?);
-
incomplete outcome data (i.e. were incomplete outcome data adequately addressed?);
-
selective outcome reporting (i.e. are reports of the study free of suggestion of selective outcome reporting?); and
-
other potential sources of bias (i.e. was the study apparently free of other problems that could put it at a high risk of bias?).
A judgement of 'Yes' indicates low risk of bias, 'No' indicates high risk of bias, and 'Unclear' indicates unclear or unknown risk of bias. Disagreements were resolved by consensus. Study authors were contacted when insufficient information was provided to determine risk of bias. The overall quality of the evidence supporting the outcomes was evaluated using the GRADE criteria.
Results
Description of studies
The June 2014 literature identified a total of 35 full‐text articles (See Figure 1). Of these, nine manuscripts representing five distinct trials met the inclusion criteria and were included in the review (Bloom 2004; Celasco 2010; de Bievre 2007; Panes 2000; Zezos 2006). The June 2014 literature search did not identify any new studies for inclusion in the updated review.

Study flow diagram.
Three randomized, blinded studies published as abstracts only were excluded after attempts to contact the authors for further details about methodology and results were not successful (Korzenik 1999; Korzenik 2003; Torkvist 2001). Ang 2000 enrolled patients with ulcerative colitis and Crohn's disease. This study was excluded because separate results were not provided for patients with ulcerative colitis. Seven papers were excluded for being case series (Gaffney 1991; Gaffney 1995; Miyake 1998; Zavgorodnii 1982; Zhernakova 1984) or reports (Thorsteinsson 2008; Ikehata 2003; Matsuzaki 2001, Di Fabio 2009). The remaining studies were excluded as they were non‐randomized, open label studies (Ashizuka 2006; Brazier 1996; Cui 1999; Dotan 2001; Evans 1997; Folwaczny 1995; Folwaczny 1997; Folwaczny 1999;Pastorelli 2008; Saibeni 2006; Torkvist 1998; Torkvist 1999; Vrij 2001; See characteristics of excluded studies table).
LMWH versus placebo
Bloom 2004
This randomized, double‐blind trial included 100 patients (48 male, 52 female) with active ulcerative colitis. Active disease was defined by a clinical assessment of disease flare‐up and a minimum score of three on a combined score of bowel frequency, rectal bleeding, and procto‐sigmoidoscopy as defined in the paper. A histology score was added where available to give a baseline colitis activity score. The patients were randomized to subcutaneous tinzaparin 175 anti‐Xa IU/kg/day for two weeks, followed by subcutaneous tinzaparin 4500 anti‐Xa IU/day sc for four weeks (n = 48) or placebo (n = 52) subcutaneous injection daily for six weeks. Concurrent treatment with stable doses of salicylates or without improvement from increasing doses of salicylates was allowed, but no other therapies for ulcerative colitis were permitted. Patients underwent a sigmoidoscopy with biopsies within four days prior to randomization as well as within two to seven days after the end of the 42 days of treatment. The primary outcome measure was the degree of clinical improvement based on a change in the colitis activity score at the end of therapy compared to the baseline score. Complete response was defined as a colitis activity score of zero whereas a partial response was defined as a reduction of three points or more. Other outcome measures included numbers and percentages of patients in each category of activity score, quality of life scores using the Inflammatory Bowel Disease Questionnaire (IBDQ) (completed by patients in English speaking countries only), compliance with therapy, and adverse events.
Celasco 2010
This double‐blind, randomized trial included 141 patients (73 male, 68 female) with mild to moderately active left‐sided ulcerative colitis (defined by authors as a CAI ≥5 and ≤ 12) receiving stable‐dose oral aminosalicylate therapy. Patients were randomized to receive either an extended‐release 210 mg parnaparin sodium (87‐91 anti‐Xa U/mg) tablet that targets the colon or an identical placebo tablet every day for eight weeks while continuing their existing aminosalicylate regimens. Each patient underwent a colonoscopy, histologic evaluation and clinical assessment using the Clinical Activity Index (Rachmilewitz 1989) (CAI) no earlier than two weeks prior to entry. Clinical response was also measured at two, four and eight weeks with a second colonoscopy and histologic evaluation performed at the end of treatment. The primary outcome measure was clinical remission (CAI <4) after eight weeks of treatment. Secondary outcome measures included clinical improvement (decrease in CAI by ≥ 2), endoscopic healing and improvement, histologic improvement, bleeding, and adverse events.
de Bievre 2007
This double‐blind, randomized trial included 29 patients (16 male, 13 female) with mild to moderately active ulcerative colitis (diagnosis based on Lennard‐Jones criteria) and a Truelove classification severity score of 4 to 14 were included. The patients were randomized to reviparin 3436 IU (n = 15) or placebo (n = 14) subcutaneous injections twice daily for up to eight weeks. All patients were concurrently treated with comparable stable doses of salicylates. Patients underwent a sigmoidoscopy with biopsies at baseline and after eight weeks, and were seen regularly during the eight week study period. A Clinical Symptom Grading and Colitis Activity Index (Lichtiger 1994) was calculated at each visit (Lichtiger 1994). If there was no improvement after four weeks or a clinical regression at any study visit the treatment was discontinued and corticosteroid therapy was initiated. The primary outcome measure was clinical improvement at the end of eight weeks where improvement was defined as a reduction in CAI by six points compared to baseline. Secondary outcomes included endoscopic and histologic improvements, and quality of life based on the IBDQ.
Vrij 2007 describes the same cohort of patients as de Bievre 2007. The main focus of this paper was whether clinical improvement with LMWH therapy was associated with reduced thrombin generation. Other hematological factors were also measured. Other outcome measures reported in this patient included the number of patients with clinical improvement after eight weeks, endoscopic and histologic improvement, and IBDQ results.
LMWH in addition to standard therapy
Zezos 2006
This randomized non‐blinded trial included 34 patients (22 male, 12 female) with active ulcerative colitis (including 6 patients hospitalized at time of enrollment). Patients were randomized to two groups. The standard therapy group received corticosteroids and aminosalicylates (n = 18). The heparin therapy group received similar standard therapy plus subcutaneous enoxaparin 100 anti‐Xa IU/kg daily for 12 weeks (n = 16). For all patients the corticosteroids were initially administered either intravenously (prednisolone 50 to 75 mg/day) or orally (methylprednisolone 32 to 48 mg/day) depending on disease severity. After initial response, IV corticosteroids were switched to oral, and oral corticosteroids were tapered in a dose‐reduction schedule over 12 weeks. Aminosalicylates were given in steady doses (2 to 4 g/day) throughout the study. Patients underwent a colonoscopy at baseline, and were seen at regular intervals for clinical and biochemical assessments during the 12 week study period. Another colonoscopy was performed at the end of 12 weeks. Disease activity was assessed clinically with the Simple Clinical Colitis Activity Index (SCCAI) at each study visit. Endoscopic disease severity was assessed with a modified endoscopic grading system (scored 0 to 18). Histologic disease activity was scored from 0 to 12 based on four features (ulcers, erosion, crypt abscess, cryptitis). The primary outcome measures were the percentages of patients with complete responses (coexisting clinical and endoscopic remission, with SCCAI score < 2 and endoscopic score < 3) and partial responses (> 50% reduction of SCCAI score and a reduction in endoscopic score by at least 1 grade) after 12 weeks of therapy in the "heparin therapy group" compared to the "standard therapy group". Other outcome measures included compliance and tolerability to therapy, withdrawals, adverse events, change in clinical, endoscopic, and histological disease activities and laboratory parameters of inflammation and coagulation.
UFH versus corticosteroids
Panes 2000
This single‐blind, randomized trial included 25 patients (12 male, 13 female) with moderate to severely active ulcerative colitis requiring hospitalization, with a Seo activity index of at least 150, and a sigmoidoscopic score of at least 2 on a 0 to 3 scale. Patients were stratified into moderate disease (Seo index 150 to 220) and severe disease (Seo index > 220). Patients were randomized to a continuous infusion of UFH (n = 12; adjusted to achieve a prolongation of activated partial thromboplastin time (APTT) of 1.5 to 2 times control)) or a single daily dose of 6‐methylprednisolone 0.75 mg/kg/day (moderate disease) or 1 mg/kg/day (severe disease) plus a placebo infusion (n = 13). Physician assessors were blinded to the assigned therapies. Patients with significant improvement (decrease in Seo index > 70 points) or remission (Seo index < 100) were discharged from hospital on 12500 U of UFH twice daily or a tapering dose of steroids until week 12 of the study. Patients were seen regularly during hospital admission and after discharge. A sigmoidoscopy was performed at baseline, and at weeks four and eight. The primary outcome measure was clinical improvement (decrease in Seo index > 70 points) at day 10. Patients not achieving this outcome or with a clinical worsening (increase in Seo index > 70 points) at any point were considered treatment failures and withdrawn from the study. Secondary outcome measures included amount of rectal bleeding and changes in inflammatory markers on serial serology.
Risk of bias in included studies
LMWH versus placebo
After obtaining further clarification from the authors (Bloom 2004; de Bievre 2007; Celasco 2010), the overall risk of bias for the placebo‐controlled trials was determined to be low (See Figure 2). The Bloom 2004 and Celasco 2010 studies were industry funded which is a potential source of bias. However, this potential source of bias probably does not have too much impact on the Bloom 2004 study as the results were negative, showing no benefit for LMWH.
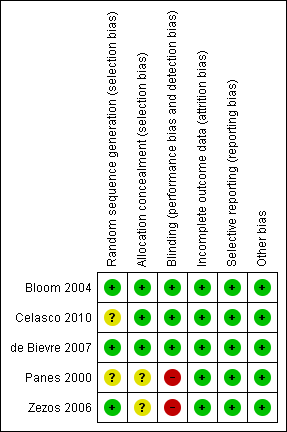
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
LMWH in addition to standard therapy
After obtaining further information from the authors (Zezos 2006), the risk of bias was determined to be high for two key domains (allocation concealment and blinding). The study was non‐blinded but adequate methods were used for randomization. Although there is a higher risk of bias the overall interpretation of the results is not affected, as the study demonstrated no benefit for LMWH for the treatment of active ulcerative colitis (See Figure 2).
UFH versus corticosteroids
No further details regarding methodology were obtained from Panes 2000. The risk of bias was determined to be unclear for two key domains (sequence generation and allocation concealment). Nonetheless, randomization was likely to be adequate in this study. The risk of bias for blinding was determined to be high because patients were not blinded. However, the study was single blind (physicians assessing the clinical state of the patients were blind). Despite the higher risk of bias in three key domains, the overall interpretation of the results is not affected, as the study demonstrated no benefit for UFH compared to corticosteroids which are of known efficacy (See Figure 2).
Effects of interventions
See: Summary of findings for the main comparison LMWH compared to Placebo for induction of remission in ulcerative colitis; Summary of findings 2 LMWH + standard therapy compared to Standard therapy for induction of remission in ulcerative colitis; Summary of findings 3 UFH compared to Corticosteroids for induction of remission in ulcerative colitis
LMWH versus placebo
Clinical remission
In Bloom 2004 4/48 patients (8.3%) assigned to LMWH and 4/52 patients (7.7%) assigned to placebo achieved clinical remission during the study period (RR 1.08; 95% CI 0.29 to 4.09; P = 0.91). In Celasco 2010, 55/71 (77.5%) patients assigned to LMWH achieved clinical remission after 8 weeks of treatment compared to 39/70 patients (55.7%) in the placebo group (RR 1.39; 95% CI 1.09 to 1.77; P = 0.008). The studies were not pooled for analysis because the parenteral LMWH administered in Bloom 2004 and the non‐parenteral LMWH administered in Celasco 2010 exhibit different therapeutic profiles, locally and systemically.
Endoscopic remission
In Celasco 2010, 15/71 (21.1%) patients assigned to LMWH achieved endoscopic remission after 8 weeks of treatment compared to 11/70 (15.7%) patients in the placebo group (RR 1.34; 95% CI 0.66 to 2.72; P = 0.41).
Clinical improvement
In Bloom 2004 15/48 patients (31.3%) assigned to LMWH and 20/52 patients (38.5%) assigned to placebo improved clinically over the study period (RR 0.81, 95% CI 0.47 to 1.40 , P = 0.45) during the study period. Over the study duration, the mean colitis activity score changed from 7.6 to 6.84 (‐10.0%) in the LMWH group and from 7.4 to 6.44 (‐13.0%) in the placebo group. In Celasco 2010, 61/71 (85.9%) patients assigned to LMWH and 47/70 (67.1%) patients assigned to the placebo improved clinically (RR 1.28 ; 95% CI 1.06 to 1.55 ; P = 0.01). The mean CAI decreased from 6.22 to 2.02 in LMWH patients compared to a decrease from 6.64 to 2.96 in placebo patients over the treatment period. In de Bievre 2007 12/15 patients (80%) assigned to LMWH and 11/14 patients (78.6%) assigned to placebo clinically improved over the study period (RR 1.02; 95% CI 0.70 to 1.48; P = 0.92). The mean CAI decreased from 9.87 at baseline to 5 at week 8 in the LMWH group and from 9.14 to 6 in the placebo group. The mean Colitis Symptom Grading decreased from 8.33 at baseline to 4 in the LMWH group and from 6.36 to 4 in the placebo group. The definitions of clinical improvement in the Bloom 2004, Celasco 2010, and de Bievre 2007 studies were not sufficiently similar to pool data for meta‐analysis.
Endoscopic improvement
In Celasco 2010, 59/71 (83.1%) patients in the LMWH group and 48/70 (68.6%) patients in the placebo group improved endoscopically over the study duration (RR 1.21 ; 95%CI 1.00 to 1.47; P = 0.05). The mean Endoscopic Index decreased from 5.93 to 2.47 in the LMWH group and from 6.24 to 3.44 in the placebo group. In de Bievre 2007 9/15 patients (60.0%) assigned to LMWH and 8/14 patients (57.1%) assigned to placebo improved endoscopically over the study period (RR 1.05 ; 95% CI 0.57 to 1.94 ; P = 0.88). The mean Endoscopic Grading System decreased from 9.64 at baseline to 7.36 after 8 weeks in the LMWH group and from 9.46 to 7.63 in the placebo group. The grading scales used in these two studies were not sufficiently similar to justify pooling the data for analysis.
Endoscopic Remission
In Celasco 2010, 15/71 (21.1%) patients in the LMWH group and 11/70 (15.7%) patients in the placebo group achieved endoscopic remission over the study duration (RR 1.34; 95% CI 0.66 to 2.72; P = 0.41). This outcome was not reported in de Bievre 2007.
Histological improvement
In Celasco 2010, 33/71 (46.5%) patients in the LMWH group and 32/70 (45.7%) patients in the placebo group improved histologically over the study duration (RR 1.02 ; 95% CI 0.71 to 1.45; p = 0.93). The mean Histological Score decreased from 4.83 to 2.64 in the LMWH group and from 5.28 to 3.39 in the placebo group. In de Bievre 2007 10/15 patients (66.7%) assigned to LMWH and 7/14 patients (50%) assigned to placebo improved histologically over the study period (RR 1.33; 95% CI 0.71 to 2.51; P = 0.37). The mean Histological Grading System decreased from 2.93 at baseline to 2.00 after 8 weeks in the LMWH group and from 3.93 to 3.11 in the placebo group. The grading scales used in these two studies were not sufficiently similar to justify pooling the data for analysis.
Adverse events
In Bloom 2004 0/48 patients (0%) assigned to LMWH and 1/52 patients (1.9%) assigned to placebo developed significant rectal hemorrhage. Other adverse events considered severe in the LMWH group included one patient with colitis not otherwise specified and two patients with aggravated ulcerative colitis; in the placebo group one patient had aggravated ulcerative colitis and one developed nausea. Overall 29/48 patients (60.4%) in the LMWH group and 28/52 patients (53.8%) in the placebo group reported at least one adverse event. No serious adverse events were reported in either the LMWH or placebo groups in de Bievre 2007, including no reports of rectal hemorrhage. In Celasco 2010, 22/71 (31.0%) patients in the LMWH group and 23/70 (32.9%) patients in the placebo group experienced at least one adverse event during the study although none were serious. Bloody stools indicative of rectal bleeding were absent in 48/71 (67.6%) patients in the LMWH group and 35/70 (50.0%) patients in the placebo groups at 8 weeks compared to 2/71 (28.2%) and 3/70 (42.9%) at week 0, respectively.
Quality of life
Bloom 2004 reported the subscores of the different domains of the IBDQ, and found no significant differences in any domain between the LMWH and placebo groups from the start to the end of treatment. In de Bievre 2007, there were no significant differences in the mean IBDQ score in the LMWH versus placebo group at baseline (LMWH 132.1, placebo 141.2, P = 0.57) or at the end of treatment (LMWH 162.1, placebo 173.1, P = 0.71).
LMWH in addition to standard therapy
Clinical remission
In Zezos 2006 5/16 patients (31.3%) assigned to LMWH in addition to standard therapy with corticosteroids and aminosalicylates and 7/18 patients (38.9%) assigned to standard therapy alone achieved clinical remission during the study period (RR 0.80; 95% CI 0.32 to 2.04 ; P = 0.64).
Clinical improvement
In Zezos 2006 14/16 patients (87.5%) assigned to LMWH in addition to standard therapy with corticosteroids and aminosalicylates and 14/18 patients (77.8%) assigned to standard therapy alone improved clinically during the study period (RR 1.13 ; 95% CI 0.83 to 1.53 ; P = 0.46).
Endoscopic remission
In Zezos 2006 5/16 patients (31.3%) assigned to LMWH in addition to standard therapy with corticosteroids and aminosalicylates and 7/18 patients (38.9%) assigned to standard therapy achieved endoscopic remission during the study period (RR 0.80 ; 95% CI 0.32 to 2.04; P = 0.64).
Endoscopic improvement
In Zezos 2006 11/16 patients (68.9%) assigned to LMWH in addition to standard therapy with corticosteroids and aminosalicylates and 11/18 patients (61.1%) assigned to standard therapy alone improved endoscopically during the study period (RR 1.13 ; 95% CI 0.69 to 1.85 ; P = 0.64).
Histological improvement
This outcome was not reported in Zezos 2006.
Adverse events
In Zezos 2006, no severe adverse events, including no significant rectal bleeding complications, occurred in patients in either the LMWH + standard therapy group nor in the standard therapy group.
Quality of life
This outcome was not reported in Zezos 2006.
UFH versus corticosteroids
Clinical remission
This outcome was not reported in Panes 2000.
Endoscopic remission
This outcome was not reported in Panes 2000.
Clinical improvement
In Panes 2000 0/12 patients (0%) assigned to UFH and 9/13 patients (69.2%) assigned to corticosteroids improved clinically over the study period (RR 0.06; 95% CI 0 to 0.88; P = 0.04).
Endoscopic improvement
This outcome was not reported in Panes 2000.
Histological improvement
This outcome was not reported in Panes 2000.
Adverse events
In Panes 2000, three patients assigned to UFH were withdrawn from the study due to an increase in rectal bleeding. In 2 of these patients, the bleeding was controlled after stopping the UFH, but the third patient required urgent surgery. No other adverse events occurred in this group. One patient in the corticosteroid group required urgent surgery following the development of toxic megacolon. No other severe adverse events were reported.
Quality of life
This outcome was not reported in Panes 2000.
Discussion
There are a limited number of effective treatments for remission induction in active UC. An increased risk of thrombosis in patients with UC and reports suggesting an improvement in UC symptoms while on anticoagulant therapy led to randomized controlled studies of heparin and LMWH in active UC. However, an increased risk of rectal bleeding due to anticoagulant therapy would need to be considered as a potential complication of treatment, and balanced against any therapeutic benefit.
There are four manuscripts reporting three distinct randomized, placebo‐controlled double blind studies evaluating LMWH in the treatment of outpatients with mild‐moderate active UC (Bloom 2004; Celasco 2010; de Bievre 2007; Vrij 2007). Two of these studies showed no benefit for adjuvant LMWH therapy over placebo in a variety of outcome measures, including clinical remission, clinical improvement, endoscopic improvement, histological improvement, or quality of life (Bloom 2004; de Bievre 2007). However, the results of the Celasco 2010 showed statistically significant benefit for LMWH over placebo in achieving clinical remission and improvement, endoscopic improvement, and reduced rectal bleeding when administered by extended colon ‐release tablets.
The benefit of LMWH described by Celasco 2010 may be due to the investigators' decision to study a LMWH administered via extended colon‐release tablets rather than through the conventional subcutaneous route. Oral LMWH is absorbed in the gastrointestinal tract , has enhanced efficacy in targeting GI lesions, and exerts a weaker systemic antithrombotic effect (Lever 2002). Taken together, these factors allowed Celasco 2010 to administer a much higher dose of LMWH with a decreased risk of bleeding (18,000‐19,000 anti‐Xa IU versus 4500 anti‐Xa IU Bloom 2004 versus 3436 anti‐Xa IU de Bievre 2007) and deliver the drug directly to the diseased colon rather than have the drug systemically distributed to unaffected tissues.
The two studies with negative findings enrolled relatively small numbers of patients, and may have lacked the power to detect a statistically significant difference between LMWH and placebo. However, the large difference in effect size between these studies weakens this hypothesis. Lastly, but of note, five of the eight Celasco 2010 investigators worked as consultants or employees for the firm that provided all study funding and developed the extended colon‐release technology. Despite being a well‐designed trial with thorough outcome reporting and analysis, the heavy degree of industry involvement is a potential source of bias which weakens the conclusions one can draw from the study. Empirical research has demonstrated that industry funded studies are more likely to be associated with positive results for the products made by the company funding the research (Bhandari 2004; Lexchin 2003).
No benefit was found with the addition of LMWH to standard therapy in Zezos 2006, in which participants and evaluators were not blinded to treatment allocation. However, LMWH was well‐tolerated in all of these studies, without any reports of significant rectal bleeding in those patients assigned to LMWH. Two other placebo‐controlled studies published as abstracts only also demonstrated no benefit for LMWH over placebo (Korzenik 2003; Torkvist 2001).
Only one of the included studies evaluated UFH monotherapy for active ulcerative colitis (Panes 2000). This study included inpatients with severe disease, and corticosteroids were used as the comparator. The UFH‐treated patients had a significantly lower rate of clinical improvement than those who received steroids (0% versus 69%). Increased rectal bleeding also occurred in 3 of 12 patients given UFH. Although this study was limited by its single‐blind design, in that only the physician examiners were blinded to treatment allocation, the results strongly suggest inferiority of UFH compared to corticosteroids in this patient population. Furthermore, this study shows that UFH used as monotherapy does not provide any benefit for the treatment of moderate or severe exacerbations of ulcerative colitis. Whether or not UFH would be effective for treating patients with milder disease is not known. A single randomized, placebo‐controlled trial testing UFH in this setting has been published in abstract form only (Korzenik 1999), but the final results have not been published.

Study flow diagram.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 LMWH versus placebo, Outcome 1 Clinical remission.

Comparison 1 LMWH versus placebo, Outcome 2 Clinical improvement.

Comparison 1 LMWH versus placebo, Outcome 3 Endoscopic improvement.

Comparison 1 LMWH versus placebo, Outcome 4 Endoscopic remission.
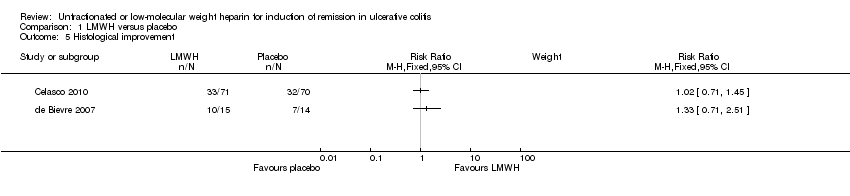
Comparison 1 LMWH versus placebo, Outcome 5 Histological improvement.

Comparison 2 LMWH + standard therapy versus standard therapy, Outcome 1 Clinical remission.

Comparison 2 LMWH + standard therapy versus standard therapy, Outcome 2 Clinical improvement.
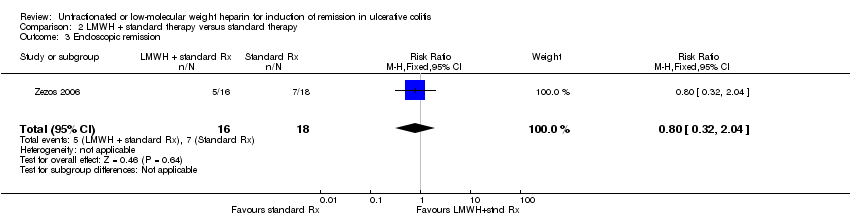
Comparison 2 LMWH + standard therapy versus standard therapy, Outcome 3 Endoscopic remission.

Comparison 2 LMWH + standard therapy versus standard therapy, Outcome 4 Endoscopic improvement.

Comparison 3 UFH versus corticosteroids, Outcome 1 Clinical improvement.
| LMWH compared to Placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | LMWH | |||||
| Endoscopic remission | 157 per 10001 | 211 per 1000 | RR 1.34 | 141 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| LMWH + standard therapy compared to Standard therapy for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard therapy | LMWH + standard therapy | |||||
| Clinical remission | 389 per 10001 | 311 per 1000 | RR 0.8 | 34 | ⊕⊝⊝⊝ | |
| Clinical improvement | 778 per 10001 | 879 per 1000 | RR 1.13 | 34 | ⊕⊝⊝⊝ | |
| Endoscopic remission | 389 per 10001 | 311 per 1000 | RR 0.8 | 34 | ⊕⊝⊝⊝ | |
| Endoscopic improvement | 611 per 10001 | 691 per 1000 | RR 1.13 | 34 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| UFH compared to Corticosteroids for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | UFH | |||||
| Clinical improvement | 692 per 10001 | 42 per 1000 | RR 0.06 | 25 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical remission Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Clinical improvement Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Endoscopic improvement Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Endoscopic remission Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.66, 2.72] |
| 5 Histological improvement Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical remission Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.04] |
| 2 Clinical improvement Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.83, 1.53] |
| 3 Endoscopic remission Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.04] |
| 4 Endoscopic improvement Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.69, 1.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical improvement Show forest plot | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.88] |

