Heparina no fraccionada o de bajo peso molecular para la inducción de la remisión en la colitis ulcerosa
Appendices
Appendix 1. Search strategies
MEDLINE
1. ulcerative colitis.mp. or exp ulcerative colitis/
2. (proctocolitis or proctosigmoiditis or rectocolitis or rectosigmoiditis or proctitis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
3. 1 or 2
4. heparin.mp. or exp heparin/
5. (unfractionated heparin or low molecular weight heparin).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
6. 4 or 5
7. 3 and 6
EMBASE
1. ulcerative colitis.mp. or exp ulcerative colitis/
2. (proctocolitis or proctosigmoiditis or rectocolitis or rectosigmoiditis or proctitis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
3. 1 or 2
4. heparin.mp. or exp heparin/
5. (unfractionated heparin or low molecular weight heparin).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
6. 4 or 5
7. 3 and 6
CENTRAL
#1. (ulcerat* and colitis) or proctocolitis or proctosigmoiditis or rectocolitis or rectosigmoiditis or proctitis
#2. heparin
#3. unfractionated heparin
#4. low molecular weight heparin
#5. #2 or #3 or #4
#6. #1 and #5
SR‐IBD
1. heparin*

Study flow diagram.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 LMWH versus placebo, Outcome 1 Clinical remission.

Comparison 1 LMWH versus placebo, Outcome 2 Clinical improvement.

Comparison 1 LMWH versus placebo, Outcome 3 Endoscopic improvement.

Comparison 1 LMWH versus placebo, Outcome 4 Endoscopic remission.
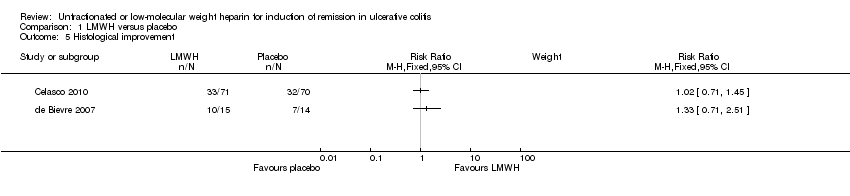
Comparison 1 LMWH versus placebo, Outcome 5 Histological improvement.

Comparison 2 LMWH + standard therapy versus standard therapy, Outcome 1 Clinical remission.

Comparison 2 LMWH + standard therapy versus standard therapy, Outcome 2 Clinical improvement.
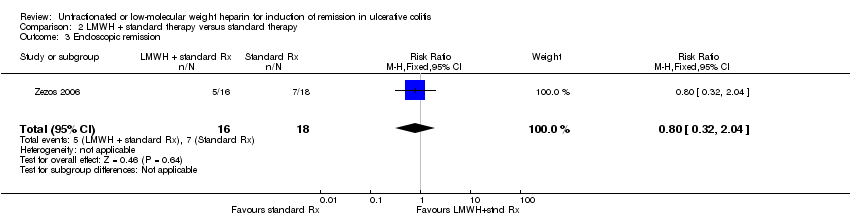
Comparison 2 LMWH + standard therapy versus standard therapy, Outcome 3 Endoscopic remission.

Comparison 2 LMWH + standard therapy versus standard therapy, Outcome 4 Endoscopic improvement.

Comparison 3 UFH versus corticosteroids, Outcome 1 Clinical improvement.
| LMWH compared to Placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | LMWH | |||||
| Endoscopic remission | 157 per 10001 | 211 per 1000 | RR 1.34 | 141 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| LMWH + standard therapy compared to Standard therapy for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard therapy | LMWH + standard therapy | |||||
| Clinical remission | 389 per 10001 | 311 per 1000 | RR 0.8 | 34 | ⊕⊝⊝⊝ | |
| Clinical improvement | 778 per 10001 | 879 per 1000 | RR 1.13 | 34 | ⊕⊝⊝⊝ | |
| Endoscopic remission | 389 per 10001 | 311 per 1000 | RR 0.8 | 34 | ⊕⊝⊝⊝ | |
| Endoscopic improvement | 611 per 10001 | 691 per 1000 | RR 1.13 | 34 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| UFH compared to Corticosteroids for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | UFH | |||||
| Clinical improvement | 692 per 10001 | 42 per 1000 | RR 0.06 | 25 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical remission Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Clinical improvement Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Endoscopic improvement Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Endoscopic remission Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.66, 2.72] |
| 5 Histological improvement Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical remission Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.04] |
| 2 Clinical improvement Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.83, 1.53] |
| 3 Endoscopic remission Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.04] |
| 4 Endoscopic improvement Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.69, 1.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical improvement Show forest plot | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.88] |

