皮下注射未分级肝素用于静脉血栓栓塞症的初始治疗
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: open randomised controlled trial Duration of intervention: at least 5 days to INR target Duration of follow‐up: acute phase only Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people with acute DVT Country: Sweden Number of study centres: 3 Setting: hospital Number: 141 (SC UFH group 72; IV UFH group 69) Age mean (range): SC UFH group 64 years (23 to 88); IV UFH group 64 years (20 to 88) Sex (M/F): SC UFH group 47/25; IV UFH group 41/28 Inclusion criteria: clinical signs of acute DVT Exclusion criteria: not stated Diagnostic criteria: phlebography, venous occlusion plethysmography, thermography | |
| Interventions | Intervention (route, total dose/day, frequency): IV UFH bolus dose (sodium heparin) (5000 IU/mL) followed by SC UFH (25000 IU/mL) twice daily aPTT adjusted + warfarin Control (route, total dose/day, frequency): IV UFH bolus dose (sodium heparin) (5000 IU/mL) followed by continuous IV UFH aPTT adjusted + warfarin Treatment before study: NA Titration period: NA | |
| Outcomes | Primary outcome: therapeutic efficacy with repeat imaging Secondary outcomes: bleeding, pulmonary emboli, aPTT, heparin dose | |
| Notes | Stated aim of the study: assess therapeutic effect and number of complications in the two groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) | High risk | No description of blinding provided "Subcutaneous injections were given into the anterior abdominal wall using a 23 gauge needle" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | High risk | 40 participants (out of 141) withdrawn from the study "due to inabilities to achieve these investigations during weekends and holidays, technical reasons or because some patients refused further investigations" 19 participants withdrawn from the subcutaneous group and 21 participants withdrawn from the intravenous group. However, the number of participants withdrawn for each reason is not presented. No deaths were reported as occurring during the course of the study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Low risk | No evidence of other biases |
| Methods | Study design: open randomised aPTT‐controlled trial Duration of intervention: 3 months for SC heparin; until INR target in LMWH and IV heparin Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people with acute DVT Country: Italy (Chieti and Pescara), UK Number of study centres: 3 Setting: SC UFH ‐ outpatient; LMWH ‐ out/inpatient; IV UFH ‐ inpatient Number: 325 randomised, 294 completed the study (SC UFH 99; LMWH 98; IV UFH 97) Age (mean ± SD): SC UFH 54 ± 9 years; LMWH 54 ± 11 years; IV UFH 53 ± 10 years Sex (M/F): SC UFH 52/47; LMWH 54/44; IV UFH 57/40 Inclusion criteria: acute proximal DVT diagnosed by colour duplex ultrasonography Exclusion criteria: 2 or more previous episodes of DVT or PE, current active bleeding, active ulcers, bleeding or coagulation disorder, concurrent PE, treatment for DVT with standard heparin > 48 h, home treatment not possible, neoplasia requiring surgery or chemotherapy in three months, likelihood of low compliance, pregnancy, platelets < 100,000 × 109/L Diagnostic criteria: colour duplex | |
| Interventions | Intervention (route, total dose/day, frequency): SC heparin (12,500 IU twice daily), fixed dose (no oral anticoagulation) administered exclusively at home Control (route, total dose/day, frequency): group 1: LMWH (100 Axa IU/kg twice daily) administered primarily at home + warfarin; group 2: IV bolus (5000 IU) followed by continuous IV UFH aPTT adjusted + warfarin Treatment before study: NA | |
| Outcomes | Outcomes not specified as primary or secondary Outcomes: symptomatic or asymptomatic recurrent DVT or DVT extension at 3 months, bleeding during the administration of the study drug, PE, length of stay in hospital, number of participants treated directly at home without admission | |
| Notes | Stated aim of the study: to compare intravenous standard heparin (in hospital) with oral anticoagulant treatment to LMWH and oral anticoagulant treatment administrated primarily at home, to SC heparin administered at home | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) | High risk | Use of open study design |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes requiring blinding Recurrent VTE at 3 months: data not used – results for symptomatic, asymptomatic and extended VTE not presented separately Recurrent DVT at 3 months: data not used – see recurrent VTE at 3 months PE – excluding PE found at autopsy: data used – "All reported outcome events were reviewed by a central panel including all monitors and, by form evaluation, by five external reviewers unaware of the treatments assigned and the patient's identity" Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: data not used – see recurrent VTE at 3 months Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data not used – unclear how many deaths occurred in each group |
| Incomplete outcome data (attrition bias) | High risk | 31 (out of 325) participants were withdrawn from the study Although the paper states that six participants died during the course of the study – all other withdrawals are unaccounted for |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Unclear risk | Different groups were treated in different locations with groups 1 and 2 receiving different treatments in hospital and group 3 receiving treatment at home |
| Methods | Study design: open randomised controlled trial Duration of intervention: 7 days to INR target Duration of follow‐up: 7 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people with acute DVT Country: UK Number of study centres: 1 Setting: inpatient Age (mean ± SD): SC UFH group 60.49 ± 14.32 years; IV UFH group 58.18 ± 12.66 years Sex (M/F): not specified but describes "well matched for age, sex ..." Inclusion criteria: acute calf DVT diagnosed by venography Exclusion criteria: contra‐indication to heparin, thrombus extension < 5 cm Diagnostic criteria: venography | |
| Interventions | Intervention (route, total dose/day, frequency): SC UFH (calcium heparin), initial dose 40,000 IU/day followed by aPTT‐adjusted dose twice daily + warfarin Control (route, total dose/day, frequency): IV UFH (sodium heparin), initial dose 40,000 IU/day followed by aPTT‐adjusted continuous dose + warfarin Treatment before study: NA | |
| Outcomes | Outcomes not specified as primary or secondary Outcomes: cutaneous haematoma, macroscopic haematuria, major bleeding, DVT extension, new or extended PE, aPTT, heparin level | |
| Notes | Stated aim of the study: to compare the safety and efficacy of IV and SC heparin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised using sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | No description of blinding provided Different methods of heparin administration – intravenous compared to subcutaneous – probably prevented adequate blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used Description of blinding outcome assessors for PE |
| Incomplete outcome data (attrition bias) | High risk | The study states 3 participants (out of 100) were withdrawn from the study – but from which groups is unclear Later in the paper it states that the heparin treatment of 6 participants was halted (2 in SC group and 4 in IV group) However, all participants are included in the final analysis of venographic results without further explanation No deaths were reported as occurring during the course of the study |
| Selective reporting (reporting bias) | Low risk | Study states estimations of platelet count; haemoglobin and hematocrit were made at the beginning, middle and end of the trial period – however – results are only presented for participants with minor bleeds. Nevertheless these were not outcomes of our review and therefore the study was judged to be at low risk of reporting bias. |
| Other bias | Low risk | No evidence of other biases |
| Methods | Study design: open randomised controlled trial Duration of intervention: 10 days Duration of follow‐up: 12 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people with acute DVT Country: Canada Number of study centres: 1 Setting: inpatients Number: 103 SC UFH 51; IV UFH 52 Age mean (range): SC UFH 66.6 years (31 to 96); IV UFH 64.6 (25 to 94) years Sex (M/F): SC UFH 23/28; IV UFH 32/20 Inclusion criteria: acute proximal or calf DVT diagnosed by venography Exclusion criteria: clinically suspected PE, active peptic ulceration, bleeding disorder, no informed consent Diagnostic criteria: venography | |
| Interventions | Intervention (route, total dose/day, frequency): SC UFH (calcium heparin), initial dose 15,000 IU, then twice daily, aPTT adjusted + warfarin Control (route, total dose/day, frequency): IV UFH (calcium heparin), initial dose 5,000 IU, then continuous, aPTT adjusted + warfarin Treatment before study: NA | |
| Outcomes | Primary outcome: PE Secondary outcomes: other lung scan abnormalities, bleeding, leg symptoms, death | |
| Notes | Stated aim of the study: to determine the efficacy and safety of adjusted SC calcium heparin compared with continuous IV calcium heparin as the initial treatment for acute DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Low risk | use of "sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Use of "open" trial design |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used ‐ the scintigrams were interpreted in random order by 2 experienced experimental observers who were blinded to the method of treatment Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – see recurrent VTE at 3 months Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | Low risk | 7 participants (out of 103) were withdrawn from the study – 4 in SC group; 3 in the IV group Reasons for withdrawal were clearly presented: "2 had major bleeding; 1 refused the scan; 1 required surgery and 3 could not have the scans for technical reasons" During follow‐up 10 participants died – none from PE |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
| Methods | Study design: randomised controlled trial Duration of intervention: 10 days Duration of follow‐up: 10 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: French | |
| Participants | Who participated: people with acute DVT and PE Country: France Number of study centres: 1 Setting: inpatient Number: 68 SC UFH 35; SC LMWH 33 (number evaluated: 59 SC UFH 29; SC LMWH 30) Age (mean ± SD) : SC UFH 63.6 ± 16.2 years; SC LMWH 65.6 ± 14.8 years Sex (M/F): 39/29 Inclusion criteria: acute DVT or PE diagnosed with phlebography or perfusion‐ventilation scan Exclusion criteria: over 2 weeks of symptoms, massive PE Diagnostic criteria: phlebography and lung scan | |
| Interventions | Intervention (route, total dose/day, frequency): SC UFH (calcium heparin) 500 IU/kg/day in form of twice daily injections, aPTT adjusted Control (route, total dose/day, frequency): SC LMWH 750 anti‐Xa/kg/day in form of twice daily injections Treatment before study: NA | |
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, bleeding | |
| Notes | Stated aim of the study: to assess the efficacy and safety of CY222 for the treatment of DVT compared with SC heparin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) | High risk | No description of blinding provided and although both treatments were administered subcutaneously, the authors state that in the CY222 group participants received a fixed dose of (750 U anti‐Xa IC/kg/24 h) whist in the unfractionated heparin group dosage was adjusted to maintain partial thromboplastin time, making it unlikely participant and personnel were adequately blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes requiring blinding Recurrent VTE at 3 months: NA Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: NA Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: NA VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | High risk | 9 participants (out of 68) were withdrawn from the study In the CY22 group 3 participants withdrew (cardiac insufficiency, migration of Greenfield filter) In the SC group 6 participants withdrew (3 retroperitoneal haematoma; 3 recurrent PE) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
| Methods | Study design: double‐blind randomised controlled trial Duration of intervention: 7 days Duration of follow‐up: 7 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people with acute DVT Country: Norway Number of study centres: 1 Setting: inpatients Number: 56 (SC UFH 27; SC LMWH 29) Age (mean ± SD): SC UFH 60 ± 15.8 years; SC LMWH 61 ± 15.3 years Sex (M/F): 33/23 (SC UFH 17/10; SC LMWH 16/13) Inclusion criteria: acute DVT below the groin diagnosed by phlebography, with symptoms for fewer than 14 days Exclusion criteria: PE, pregnancy, history of cerebral haemorrhage, surgery in previous 6 days, diastolic BP > 115 mmHg, retinal haemorrhage, impaired renal function, impaired PT Diagnostic criteria: phlebography | |
| Interventions | Intervention (route, total dose/day, frequency): IV continuous infusion UFH for 24 hours, followed by SC UFH 10,000‐15,000 IU twice daily, anti‐Xa adjusted + warfarin Control (route, total dose/day, frequency): IV continuous infusion UFH for 24 hours, followed by SC LMWH 5000‐7500 IU twice daily, anti‐Xa adjusted + warfarin Treatment before study: NA | |
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, new PE, bleeding, leg pain, death, haemoglobin, platelets | |
| Notes | Stated aim of the study: to compare subcutaneous heparin and LMWH for the treatment of DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | High risk | "the vials [of low molecular weight or unfractionated heparin] had been randomised in advance and numbered consecutively, the number of patient admission determining the number of vial used" It is possible personnel had access to the order of the vials |
| Blinding of participants and personnel (performance bias) | Unclear risk | Paper states only "double blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding of outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | Low risk | 3 participants (out of 56) were withdrawn from the trial – 2 from the LMWH group; 1 from UFH group Reasons for withdrawals are clearly presented: Reversal of DVT diagnosis; incorrect injection of ordinary heparin and suspected cerebral haemorrhage No deaths were reported as occurring during the course of the study |
| Selective reporting (reporting bias) | High risk | Study presents results for leg pain "leg pain disappeared somewhat quicker in patients receiving LH"; however, pain measures were not presented as an outcome in the Methods section In addition, the paper states that "there was no drop in platelet count or haemoglobin concentration"; however, how these parameters were measured is also unreported in the Methods section |
| Other bias | Low risk | No significant evidence of other biases; however, one patient was included twice (once in each group) and one patient transferred to the UFH group and so was not included in the final analysis This could potentially be considered an as‐treated analysis, and as such it may have potentially introduced selection bias; however, as only one patient was affected the potential risk of bias was considered small and was deemed unlikely to have significantly affected the results of the study |
| Methods | Study design: double‐blind randomised controlled trial Duration of intervention: 10 days Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people with acute DVT Country: Canada Number of study centres: 1 Setting: inpatients Number: 115 Age (< 60 years / > 60 years): SC UFH 10/4; 7 IV UFH 11/47 Sex (M/F): SC UFH 27/30; IV UFH 28/30 Inclusion criteria: acute proximal (± calf) DVT diagnosed by venography Exclusion criteria: active bleeding, contraindication to heparin, already on heparin, no outpatient follow‐up available Diagnostic criteria: venography | |
| Interventions | Intervention (route, total dose/day, frequency): IV UFH 5000 IU bolus followed by SC UFH 15000 twice daily, aPTT adjusted + warfarin Control (route, total dose/day, frequency): IV UFH 5000 IU bolus followed by continuous IV UFH aPTT adjusted + warfarin Treatment before study: NA | |
| Outcomes | Outcomes not specified as primary or secondary Outcomes: recurrent DVT, PE, bleeding, aPTT, death | |
| Notes | Stated aim of the study: to compare continuous IV heparin to intermittent SC heparin for the initial treatment of proximal DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer generated prescribed randomised arrangement was used to assign patients" |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) | Low risk | "those to receive continuous IV heparin were ... started on a continuous IV infusion … and placebo SC injections" "those to receive SC heparin were given SC heparin injections … and IV placebo infusions" "to prevent un‐blinding … masked pre‐labelled syringes and IV packs were used" "to prevent un‐blinding on the basis of knowledge of heparin clearance … all dose adjustments and anticoagulant monitoring … were [done at a] daily mid interval measurement" |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used ‐ "[d]iagnostic tests were interpreted independently and without knowledge of the results of the other tests or the patient's clinical state or the treatment group to which the patient had been assigned" Recurrent DVT at 3 months: data used – see recurrent VTE at 3 months PE – excluding PE found at autopsy: data used – See recurrent VTE at 3 months Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | Low risk | 0 participants (out of 115) were withdrawn from the study "[A]ll patients were followed during primary therapy and for three months during long term therapy and none were lost to follow up" 6 participants died in the subcutaneous group, 2 from VTE‐related causes; 3 participants died in the intravenous group, none from VTE‐related causes |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Low risk | No evidence of other biases |
| Methods | Study design: open‐label, adjudicator‐blinded randomised controlled trial Duration of intervention: 5 days to INR target Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people with acute DVT or PE Country: Canada and New Zealand Number of study centres: 6 Setting: inpatients and outpatients Number: 708 (SC UFH 355; SC LMWH 353) Age (mean ± SD): SC UFH 60 ± 17 years; SC LMWH 60 ± 16 years Sex (M/F): SC UFH 182/173; SC LMWH 206/147 Inclusion criteria: 18 years or older with newly diagnosed DVT of the legs or PE diagnosed by compression ultrasonography or by venography, and by a high probability ventilation‐perfusion lung scan, by non diagnostic findings on lung scan accompanied by diagnostic findings for DVT, or by computed tomographic angiography Exclusion criteria: contraindication to subcutaneous therapy such as shock or major surgery in the past 48 hours, active bleeding, a life expectancy of less than 3 months, previous acute treatment for venous thromboembolism for more than 48 hours, receiving long‐term anticoagulant therapy, contraindication to heparin or to radiographic contrast, creatinine level of greater than 200 µmol/L (2.3 mg/dL), pregnant, enrolled in a competing study, unable to have follow‐up assessments because of geographic inaccessibility Diagnostic criteria: compression ultrasonography or venography, and high probability ventilation‐perfusion lung scan, non‐diagnostic findings on lung scan accompanied by diagnostic findings for deep vein thrombosis, or computed tomographic angiography Type of VTE: 571 DVT/174 PE | |
| Interventions | Intervention (route, total dose/day, frequency): unmonitored SC UFH, initial 333 IU/kg followed by 250 IU/kg twice daily + warfarin Control (route, total dose/day, frequency): SC LMWH 100 IU/kg twice daily + warfarin Treatment before study: NA | |
| Outcomes | Primary outcomes: the primary analysis for efficacy was the absolute difference in the proportion of eligible participants who had recurrent venous thromboembolism at 3 months. The primary analysis for safety was the absolute difference in the proportion of participants who received at least 1 dose of study drug who had an episode of major bleeding within 10 days of randomisation Secondary outcomes: recurrent VTE at 10 days, major or minor bleeding, death, aPTT | |
| Notes | Stated aim of the study: to determine if fixed‐dose, weight‐adjusted, subcutaneous unfractionated heparin is as effective and safe as low molecular‐weight heparin for treatment of venous thromboembolism | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was computer generated with block sizes of 2 or 4" |
| Allocation concealment (selection bias) | Low risk | "[C]linical centres telephone an automated centralised system" |
| Blinding of participants and personnel (performance bias) | High risk | Use of "open‐label" study design |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used ‐ all outcome events and deaths were classified by a central adjudication committee whose members were unaware of treatment assignment Recurrent DVT at 3 months: data used – see recurrent VTE at 3 months PE – excluding PE found at autopsy: data used – see recurrent VTE at 3 months Incidence of heparin‐induced thrombocytopenia: data used – see recurrent VTE at 3 months Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | Low risk | 11 participants (out of 708) were withdrawn from the study – 10 in the UFH group; 1 in the LMWH group Reasons for withdrawals were clearly reported and the asymmetry in the withdrawals did not appear to be caused by the different treatment methods: UFH – 4 participants were receiving long‐term anticoagulant therapy; 3 diagnosis of VTE were reversed; 1 randomisation error; 1 withdrawal of consent and 1 withdrawal by physician LMWH – 1 withdrawal of consent During follow‐up there were 18 deaths in the UFH group (1 from bleeding) and 22 deaths in the LMWH group (3 from PE and 1 from bleeding) |
| Selective reporting (reporting bias) | Low risk | Protocol available ‐ no evidence of selective reporting |
| Other bias | Low risk | No significant evidence of other biases ‐ 5 participants who did not receive the study drug were not included in the final analysis of either safety or efficacy – something which could be considered an 'as‐treated' analysis that potentially introduced selection bias; however, the number of participants affected was considered too small to have had a significant impact on the results |
| Methods | Study design: randomised controlled trial Duration of intervention: 7 days Duration of follow‐up: 6 weeks Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: French | |
| Participants | Who participated: people with acute DVT of the lower limb Country: Switzerland Number of study centres: 1 Setting: inpatients Number: 48 (SC UFH 23; IV UFH 25) Age: not stated Sex (M/F): SC UFH 18/5; IV UFH 13/12) Inclusion criteria: DVT of lower limbs diagnosed by phlebography or colour duplex US, with symptoms < 1 week Exclusion criteria: none stated Diagnostic criteria: phlebography or colour duplex ultrasound | |
| Interventions | Intervention (route, total dose/day, frequency): IV bolus UFH (sodium heparin) 5000 IU, followed by SC UFH 15,000U/day twice daily (aPTT adjusted) Control (route, total dose/day, frequency): IV bolus UFH (sodium heparin) 5000 IU followed by IV continuous UFH (aPTT adjusted) Treatment before study: NA | |
| Outcomes | Outcomes not specified as primary or secondary Primary Outcomes: symptoms duration, DVT extension, PE, aPTT | |
| Notes | Stated aim of the study: to compare subcutaneous heparin and intravenous heparin for the treatment of deep vein thrombosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Drawing of lots" |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) | High risk | No description of blinding provided Different methods of heparin administration – intravenous compared to subcutaneous – probably prevented adequate blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | High risk | Only 24 participants (out of 48) received a second phlebograph: reasons for this loss are not clearly presented in the article |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Low risk | No evidence of other biases |
| Methods | Study design: international, multicentre, centrally randomised, open, parallel‐group study with blinded adjudication Duration of intervention: 90 ± 5 days Duration of follow‐up: NA Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people aged ≥ 75 years with creatinine clearance (CrCl) ≤ 60 mL/min or people aged ≥ 70 years with a CrCl of ≤ 30 mL/min (calculated using the Cockcroft–Gault formula) and with an acute, objectively confirmed (by compression ultrasonography or venography) lower limb DVT which required treatment Countries: Belgium; France; Germany; Spain; Serbia; Croatia; Romania and Poland Number of study centres: 8 Setting: inpatients at the time of randomisation; however, participants could be followed on a daily basis in or out of hospital after this point Number: 539 Age (< 60 years/ > 60 years): SC UFH 0/270; tinzaparin 0/269 Sex (M/F): SC UFH 102/168; tinzaparin 92/177 Inclusion criteria: objectively confirmed symptomatic proximal or distal DVT (or objectively confirmed asymptomatic DVT if proximal and associated with a PE) and provision of written informed consent Exclusion criteria: received treatment doses of heparins or thrombolytic agents within the previous 4 weeks (excluding the last 36 h) prior to randomisation; received oral anticoagulation within the preceding week; planned use of high doses of acetylsalicylic acid (ASA) (> 300 mg/day) or a non‐steroidal anti‐inflammatory drug (NSAID); requirement for thrombolytic therapy; end stage renal disease requiring dialysis; hepatic insufficiency (INR ≥ 1.5); bacterial endocarditis; planned epidural or spinal anaesthesia; planned surgery or recent surgery (within 2 weeks); thrombocytopenia (< 100 x 109/L); severe uncontrolled hypertension, overt bleeding and recent stroke Diagnostic criteria: compression ultrasonography or venography | |
| Interventions | Intervention (route, total dose/day, frequency): tinzaparin (SC, 175 IU/kg, once daily) Control (route, total dose/day, frequency): UFH (IV, 50 IU/kg bolus followed by SC, 400–600 IU/kg, twice daily which was then adjusted by APTT according to local practice) Treatment before study: NA | |
| Outcomes | Primary outcomes: clinically relevant bleedings (CRBs) by day 90 ± 5 Secondary outcomes: occurrence of symptomatic recurrent VTE prior to day 90 ± 5 and major and minor bleedings prior to day 90 ± 5 Tertiary outcomes: CRBs during the SC treatment phase, death from any cause prior to day 90 ± 5 and heparin‐induced thrombocytopenia | |
| Notes | Stated aim of the study: to compare the safety profile of full weight‐based unadjusted‐dose tinzaparin (Innohep, LEO Pharma, Ballerup, Denmark) vs activated partial thromboplastin time (APTT)‐adjusted UFH as initial treatment of elderly participants with impaired renal function and acute DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment assignment was pre‐planned according to a computer generated randomisation sequence" |
| Allocation concealment (selection bias) | Low risk | "Central telephone randomisation" However, the paper also states: "No allocation concealment mechanism was attempted as the study was open. But care was taken to ensure that outcome assessors and data analysts were kept blinded to the allocation" This statement appears to be in contradiction with the description of central telephone randomisation and so it was assumed that in this context 'allocation concealment' referred to the blinding of participants and personnel, as an open study design does not preclude adequate allocation concealment ‐ this assumption was also more consistent with the reference to the blinding of outcome assessors |
| Blinding of participants and personnel (performance bias) | High risk | Use of an "open" study design |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used ‐ care was taken to ensure that outcome assessors and data analysts were kept blinded to the allocation Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – see recurrent VTE at 3 months Incidence of heparin‐induced thrombocytopenia: data used – see recurrent VTE at 3 months Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | Low risk | 5 participants (out of 539) were withdrawn from the study for reasons that were clearly presented: 2 from the tinzaparin group as "no treatment [was] taken" 3 from the unfractionated heparin group 2 because of a withdrawal of consent and 1 because "no treatment [was] taken" During the course of the study 48 participants died: 31 participants from the tinzaparin group and 17 from the unfractionated heparin group The large imbalance in mortality between the treatment groups has been addressed by the authors and appears to have been caused by an increased prevalence of specific risk factors in the tinzaparin group including presence of infectious disease; ongoing malignancy; cardiac insufficiency; stratum of renal impairment and leg paralysis, which all correlated significantly with mortality Only 4 deaths could be directly attributed to the heparin treatment 3 in the tinzaparin group – 2 from bleeding and 1 from pulmonary embolism 1 in the unfractionated heparin group also from pulmonary embolism |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Low risk | No significant evidence of other biases ‐ 3 participants transferred from the unfractionated heparin to the tinzaparin group and were included in the tinzaparin group for the analysis of adverse effects – something which constitutes an 'as treated' analysis and as such potentially introduced selection bias; however, the number of participants affected was considered too small to significantly affect the results |
| Methods | Study design: open randomised controlled trial Duration of intervention: 7 days Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: Polish | |
| Participants | Who participated: people with acute proximal or calf DVT (with or without PE) Country: Poland Number of study centres: 5 Setting: inpatients Number: 94 (SC UFH 48; IV UFH 46) Age (mean ± SD): SC UFH 53.6 ± 13.1 years; IV UFH 50.5 ± 16.9 years Sex (M/F): SC UFH 23/25; IV UFH 24/22 Inclusion criteria: calf or proximal DVT diagnosed by phlebography, age 20 to 79 years Exclusion criteria: PE necessitating thrombolysis, gastric or duodenal ulcer Diagnostic criteria: phlebography Type of VTE: DVT | |
| Interventions | Intervention (route, total dose/day, frequency): bolus IV UFH (sodium heparin) 5000 IU, followed by SC UFH 500 IU/kg/day twice daily, aPTT adjusted + sintron (after 7 days) Control (route, total dose/day, frequency): bolus IV UFH (sodium heparin) 5000 IU, followed by continuous IV UFH aPTT adjusted + sintron (after 7 days) Treatment before study: NA | |
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, aPTT, platelets, PE, bleeding, death | |
| Notes | Stated aim of the study: to compare efficacy and safety of SC heparin versus IV heparin for DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Low risk | Use of "sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | No description of blinding provided Different methods of heparin administration – intravenous compared to subcutaneous – probably prevented adequate blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | Low risk | 2 participants (out of 94) were withdrawn from the study Reasons for withdrawals are clearly presented: Intravenous group – 1 patient died following a pulmonary embolism Subcutaneous group – 1 patient was withdrawn because of bleeding Inclusion of these participants into calculations does not change the results and they participants are correctly included in the analysis of bleeding and thrombotic complications |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Other bias | Low risk | No evidence of other biases |
| Methods | Study design: open, stratified randomised controlled trial with blind evaluation of phlebographic results Duration of intervention: 10 days Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people with acute proximal or calf DVT Country: Poland Number of study centres: 6 Setting: inpatients Number: 149 (SC UFH 75 (3 excluded from analysis); SC LMWH 74) Age (mean ± SD): SC UFH 47.8 ±15.4 years; SC LMWH 49.1 ± 15.4 years Sex (M/F): SC UFH 42/30; SC LMWH 39/35 Inclusion criteria: calf or proximal DVT diagnosed by phlebography, symptoms shorter than 10 days Exclusion criteria: clinically suspected PE, phlegmasia caerulea dolens, treatment with anticoagulation prior to enrolment, VTE in previous 2 years, surgery or trauma in recent 3 days, contraindication to heparin, pregnancy, ATIII deficiency Diagnostic criteria: phlebography (blind evaluation of phlebographic results) | |
| Interventions | Intervention (route, total dose/day, frequency): bolus IV UFH 5000 IU, followed by SC UFH 250 IU/kg twice daily, aPTT adjusted + sintron Control (route, total dose/day, frequency): SC LMWH 225 IU/kg twice daily, fixed dose + sintron Treatment before study: NA | |
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, recurrent DVT, PE, bleeding, death | |
| Notes | Stated aim of the study: to determine the efficacy and safety of subcutaneous LMWH compared with SC UFH as the initial treatment of DVT of the lower limbs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Low risk | Use of "sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Use of "open" study design |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used – Outcome assessors blinded for assessment of recurrent DVT "pre and post‐treatment phlebograms were assessed blindly" ‐ but no description of blinding of assessors for PE is provided Recurrent DVT at 3 months: data used – see recurrent VTE at 3 months PE – excluding PE found at autopsy: data used – see recurrent VTE at 3 months Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | Low risk | 3 participants (out of 149) were withdrawn from the trial Reasons for withdrawals are clearly presented: UFH group ‐ 1 patient had a recent history of DVT; 1 patient was diagnosed with antithrombin III deficiency and 1 patient developed major bleeding and was withdrawn from the study; however, their results did appear in the final analysis During follow‐up 1 patient from the UFH group died from renal failure |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
| Methods | Study design: open, randomised controlled trial Duration of intervention: to INR target Duration of follow‐up: 7 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people with acute proximal DVT Country: Slovenia Number of study centres: 1 Setting: inpatients Number: 59 (SC UFH 28; SC LMWH 31) Age (mean ± SD): SC UFH 68 ± 13 years; SC LMWH 69 ±14 years Sex (M/F): SC UFH 15/13; SC LMWH 17/14 Inclusion criteria: proximal DVT diagnosed by ultrasound duplex Exclusion criteria: anticoagulant treatment with heparin or coumarins in the period of 10 days before admission, clinically significant pulmonary embolism or pregnancy Diagnostic criteria: ultrasound duplex | |
| Interventions | Intervention (route, total dose/day, frequency): bolus IV UFH, followed by SC UFH twice daily or TID, aPTT adjusted + warfarin Control (route, total dose/day, frequency): SC LMWH 200 IU/kg 4 times daily + warfarin Treatment before study: NA | |
| Outcomes | Outcomes not specified as primary or secondary Outcomes: major bleeding, death, aPTT, haemostatic markers (F1+2, TAT, D‐dimer) | |
| Notes | Stated aim of the study: to compare these markers in the acute phase of DVT during treatment either with subcutaneous aPTT‐adjusted UFH or with weight‐adjusted LMWH in order to estimate control of haemostatic system activation during both regimens | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random but no description of randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) | High risk | No description of blinding provided Different numbers of injections at different times – probably prevented adequate blinding UFH – 1 bolus of heparin given intravenously followed by 2‐3 subcutaneous injections daily LWMH – 1 subcutaneous injection daily |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes requiring blinding Recurrent VTE at 3 months: NA Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: NA Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | High risk | Many of the 59 participants were withdrawn from the study; however, exact numbers withdrawn and from which group they were withdrawn are not presented in the paper Reasons for withdrawal are also not clearly identified – the paper does state that 2 participants died and other participants were withdrawn when INR > 2 for 2 days; however, if all participants were withdrawn for this reason is unclear |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
| Methods | Study design: open randomised controlled trial Duration of intervention: 7 days Duration of follow‐up: 7 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people with acute DVT Country: Italy Number of study centres: 1 Setting: inpatients Number: 271(SC UFH 138; IV UFH 133) Age mean (range): SC UFH 63.4 (16 to 87) years; IV UFH 60.9 (11 to 86) years Sex (M/F): SC UFH 83/55; IV UFH 72/61 Inclusion criteria: acute DVT diagnosed with strain‐gauge plethysmography or venography Exclusion criteria: bleeding disorder, abnormal results in haemostatic function screening tests, active peptic disease, on heparin treatment + acenocoumarol Diagnostic criteria: plethysmography or venography in diagnosis not concluded | |
| Interventions | Intervention (route, total dose/day, frequency): SC UFH (calcium heparin) 250 U/kg twice daily + acenocoumarol Control (route, total dose/day, frequency): IV UFH (sodium heparin bolus) followed by continuous IV UFH 500 U/Kg/day + acenocoumarol Treatment before study: NA | |
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, PE, death, bleeding | |
| Notes | Stated aim of the study: to compare IV and SC heparin for acute DVT in a large population study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned by computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) | High risk | No description of blinding provided Different methods of heparin administration – intravenous compared to subcutaneous – probably prevented adequate blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | High risk | Number of participants (out of 271) who were withdrawn from the study is not presented The study states that 23 participants were reported as not undergoing strain gauge plethysmography (SGP) but which group they came from is omitted as is weather any other participants were withdrawn – as only a subset of participants (251) underwent SGP – is unclear 4 participants in the SC group died (1 from PE and 1 from cerebral haemorrhage; 2 participants died in the intravenous group 1 from PE and 1 from pulmonary haemorrhage |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
| Methods | Study design: open randomised controlled trial Duration of intervention: 5 days to INR Duration of follow‐up: 3 months Run‐in period: NA Intention‐to‐treat analysis: yes Language of publication: English | |
| Participants | Who participated: people with acute VTE (DVT + PE) Number of study centres: 19 Setting: inpatients Number: 720 (SC UFH 360; SC LMWH 360) Age (mean ± SD): SC UFH 65.7 ± 15.6 years; SC LMWH 67.0 ± 14.8 years Sex M/F: SC UFH 158/202; SC LMWH 167/193 Inclusion criteria: people with DVT of the lower extremities and/or PE were eligible for the study, provided that the suspicion was objectively confirmed Exclusion criteria: age less than 18 years, pregnancy, contraindications to anticoagulant treatment, full‐dose anticoagulant treatment (either heparin or oral anticoagulants) for more than 24 h, haemodynamic instability, previous (less than 1 year earlier) episode of VTE, life expectancy less than 3 months, poor compliance, and geographic inaccessibility for follow‐up Diagnostic criteria: a positive result of at least 1 of the following tests was accepted for inclusion: ascending phlebography, compression ultrasound of the proximal vein system, echo colour Doppler scan of the calf vein system in the case of clinical suspicion of DVT, ventilation‐perfusion scanning, spiral computed tomographic scanning, and pulmonary angiography in the case of clinical suspicion of PE. In the presence of abnormal results of an ultrasound test of the lower extremities, the diagnosis of PE was also accepted if a perfusion lung scan was compatible with a high probability of PE when compared with the chest x‐ray Type of VTE: 601 DVT/119 PE | |
| Interventions | Intervention (route, total dose/day, frequency): IV bolus UFH (calcium heparin) 4000‐5000 IU followed by SC UFH twice daily, aPTT adjusted + warfarin Control (route, total dose/day, frequency): SC LMWH 85 U/kg twice daily + warfarin Treatment before study: NA | |
| Outcomes | Primary outcome: recurrent VTE at 3 month follow‐up Secondary outcomes: recurrent VTE during heparin treatment, bleeding during heparin treatment, death | |
| Notes | Stated aim of the study: to assess the value of UFH or LMWH for treating the full spectrum of patients with VTE, including recurrent VTE and PE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation … was performed with a computer algorithm" |
| Allocation concealment (selection bias) | Low risk | Use of a "24‐hour telephone service that recorded patient information before disclosure of the treatment assigned" |
| Blinding of participants and personnel (performance bias) | High risk | Use of an open study design |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data used Minor bleeding: data used VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | Low risk | 0 participants (out of 720) were withdrawn from the study "[N]o patients were lost to follow up" "We … ensured follow up was complete for all randomised patients" During follow‐up 24 participants died: In the UFH group 12 participants died (3 from PE and 1 from haemorrhage); in the LMWH group 12 participants died (4 from PE) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other biases |
| Methods | Study design: open randomised controlled trial Duration of intervention: 14 days Duration of follow‐up: 14 days Run‐in period: NA Intention‐to‐treat analysis: no Language of publication: English | |
| Participants | Who participated: people with acute lower limb DVT Country: UK Number of study centres: 5 Setting: inpatients Number: 100 (SC UFH 50; IV continuous UFH 50) Age (mean ± SD): SC UFH M 61 ± 11 years, F 63 ± 16 years; IV continuous UFH M 60 ± 14 years, F 63 ±15 years Sex (M/F): SC UFH 25/25; IV continuous UFH 28/22 Inclusion criteria: people with DVT of the legs (calf + proximal), phlebography proven, with a thrombus > 5 cm Exclusion criteria: PE or occlusive thrombus Diagnostic criteria: phlebography | |
| Interventions | Intervention (route, total dose/day, frequency): SC UFH (calcium heparin) 250 U/kg, aPTT adjusted + warfarin Control (route, total dose/day, frequency): IV continuous UFH (sodium heparin) aPTT adjusted + warfarin Treatment before study: NA | |
| Outcomes | Outcomes not specified as primary or secondary Outcomes: DVT extension, injection site pain, PE, haemoglobin, platelets, aPTT | |
| Notes | Stated aim of the study: to compare the efficacy and safety of SC versus IV heparin for leg DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[T]he randomisation code was drafted using a standard random number table" |
| Allocation concealment (selection bias) | Low risk | "[P]atient allocations were taken from sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | No description of blinding provided Different methods of heparin administration – intravenous compared to subcutaneous – probably prevented adequate blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes requiring blinding Recurrent VTE at 3 months: data used – no description of blinding outcome assessors Recurrent DVT at 3 months: NA PE – excluding PE found at autopsy: data used – no description of blinding outcome assessors Incidence of heparin‐induced thrombocytopenia: NA Incidence of asymptomatic recurrent VTE at 3 months: NA Quality of life: NA Outcomes not requiring blinding Major bleeding: data not used ‐ not meeting ISTH definition Minor bleeding: data not used ‐ not meeting definition of minor bleeding VTE‐related mortality: data used All‐cause mortality: data used |
| Incomplete outcome data (attrition bias) | Low risk | 4 participants (out of 100) were withdrawn from the study, reasons for withdrawals are clearly presented: Intravenous group ‐ 3 participants were excluded due to "technically unsatisfactory" phlebograms Subcutaneous group ‐ 1 patient died during the course of the study |
| Selective reporting (reporting bias) | Low risk | The paper states that haemoglobin concentration; packed red cell count and platelet count were estimated on days 1,7,14 but no results are presented for these measurements. Nevertheless these were not outcomes of our review and therefore the study was judged to be at low risk of reporting bias |
| Other bias | Low risk | No evidence of other biases |
aPTT: activated partial thromboplastin time;AT: antithrombin;BP: blood pressure; DVT: deep vein thrombosis;INR: international normalised ratio;ISTH: International Society on Thrombosis and Haemostasis; IU: international units; IV: intravenous; LMWH: low molecular weight heparin;NA: not applicable; PE: pulmonary embolism; SC: subcutaneous; UFH: unfractionated heparin;US: ultrasound; VTE: venous thromboembolism.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| RCT comparing continuous versus intermittent intravenous heparin administration in people diagnosed with DVT | |
| RCT comparing continuous versus intermittent Intravenous heparin administration in people with PE | |
| RCT comparing subcutaneous heparin and dextran for the prophylaxis of VTE | |
| RCT comparing subcutaneous LMWH versus subcutaneous UFH for the prophylaxis of VTE | |
| RCT comparing intravenous UFH versus intravenous LMWH in people diagnosed with DVT | |
| RCT of people diagnosed with superficial vein thrombosis | |
| RCT comparing long‐term treatment of people with VTE | |
| RCT comparing intravenous UFH versus LMWH in people diagnosed with PE | |
| RCT comparing UFH versus LMWH plus thrombolytic treatment in people diagnosed with PE | |
| RCT comparing intravenous UFH versus intravenous LMWH in people diagnosed with DVT | |
| RCT comparing intravenous UFH versus LMWH in people diagnosed with PE | |
| RCT comparing intravenous UFH versus LMWH in people diagnosed with cancer‐associated DVT | |
| RCT comparing LMWH versus VKA in people diagnosed with DVT | |
| RCT comparing UFH versus LMWH plus thrombolytic treatment in people diagnosed with PE | |
| RCT comparing LMWH only in cancer‐related VTE | |
| RCT comparing LMWH only in cancer‐related DVT |
DVT: deep vein thrombosis; LMWH: low molecular weight heparin; PE: pulmonary embolism; RCT: randomised controlled trial; UFH: unfractionated heparin; VKA: vitamin K antagonist; VTE: venous thromboembolism.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic recurrent VTE at 3 months Show forest plot | 8 | 965 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.89, 3.10] |
| Analysis 1.1  Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 1 Symptomatic recurrent VTE at 3 months. | ||||
| 1.1 DVT with/without PE | 8 | 965 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.89, 3.10] |
| 2 Symptomatic recurrent DVT at 3 months Show forest plot | 1 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.64, 17.06] |
| Analysis 1.2  Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 2 Symptomatic recurrent DVT at 3 months. | ||||
| 2.1 DVT with/without PE | 1 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.64, 17.06] |
| 3 PE at 3 months Show forest plot | 9 | 1161 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.73, 2.84] |
| Analysis 1.3 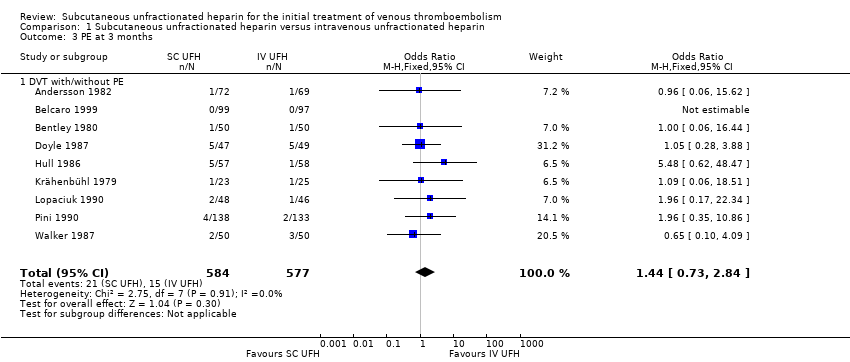 Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 3 PE at 3 months. | ||||
| 3.1 DVT with/without PE | 9 | 1161 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.73, 2.84] |
| 4 VTE‐related mortality at 3 months Show forest plot | 9 | 1168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.88] |
| Analysis 1.4  Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 4 VTE‐related mortality at 3 months. | ||||
| 4.1 DVT with/without PE | 9 | 1168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.88] |
| 5 Major bleeding Show forest plot | 4 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.97] |
| Analysis 1.5  Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 5 Major bleeding. | ||||
| 5.1 DVT with/without PE | 4 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.97] |
| 6 All‐cause mortality Show forest plot | 8 | 972 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.67, 4.51] |
| Analysis 1.6 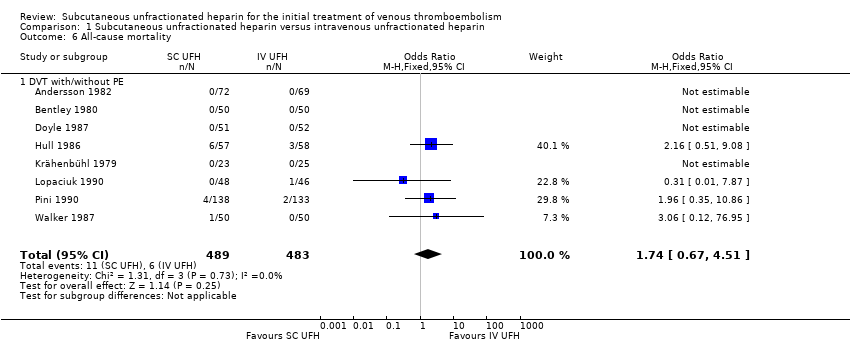 Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 6 All‐cause mortality. | ||||
| 6.1 DVT with/without PE | 8 | 972 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.67, 4.51] |
| 7 Treatment related morbidity ‐ minor bleeding Show forest plot | 5 | 779 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.20] |
| Analysis 1.7 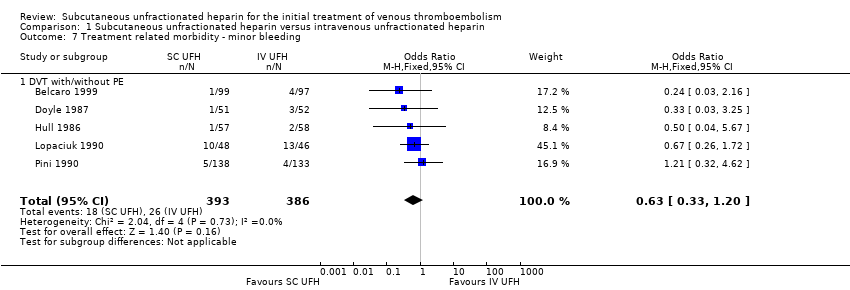 Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 7 Treatment related morbidity ‐ minor bleeding. | ||||
| 7.1 DVT with/without PE | 5 | 779 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic recurrent VTE at 3 months Show forest plot | 5 | 2156 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.63, 1.63] |
| Analysis 2.1  Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 1 Symptomatic recurrent VTE at 3 months. | ||||
| 1.1 DVT with/without PE | 3 | 1954 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.57, 1.56] |
| 1.2 DVT without PE | 2 | 202 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.38, 11.84] |
| 2 Symptomatic recurrent DVT at 3 months Show forest plot | 3 | 1566 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.73, 2.63] |
| Analysis 2.2 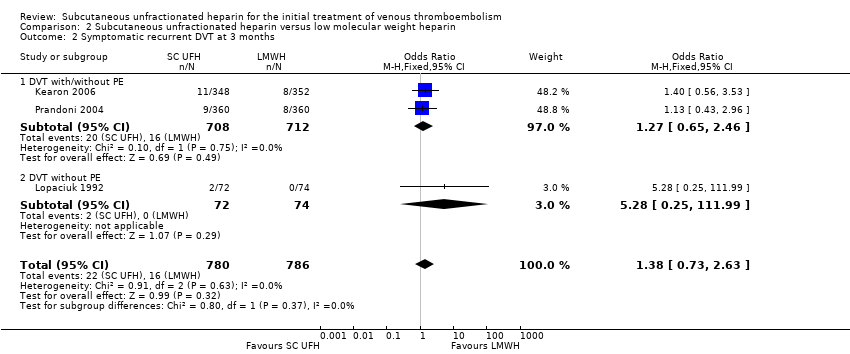 Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 2 Symptomatic recurrent DVT at 3 months. | ||||
| 2.1 DVT with/without PE | 2 | 1420 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.65, 2.46] |
| 2.2 DVT without PE | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.25, 111.99] |
| 3 PE at 3 months Show forest plot | 5 | 1819 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.36, 1.96] |
| Analysis 2.3  Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 3 PE at 3 months. | ||||
| 3.1 DVT with/without PE | 2 | 1420 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.31, 2.04] |
| 3.2 DVT without PE | 3 | 399 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.14, 7.63] |
| 4 VTE‐related mortality at 3 months Show forest plot | 8 | 2469 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.67] |
| Analysis 2.4  Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 4 VTE‐related mortality at 3 months. | ||||
| 4.1 DVT with/without PE | 4 | 2016 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.67] |
| 4.2 DVT without PE | 4 | 453 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Major bleeding Show forest plot | 5 | 2300 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.43, 1.20] |
| Analysis 2.5 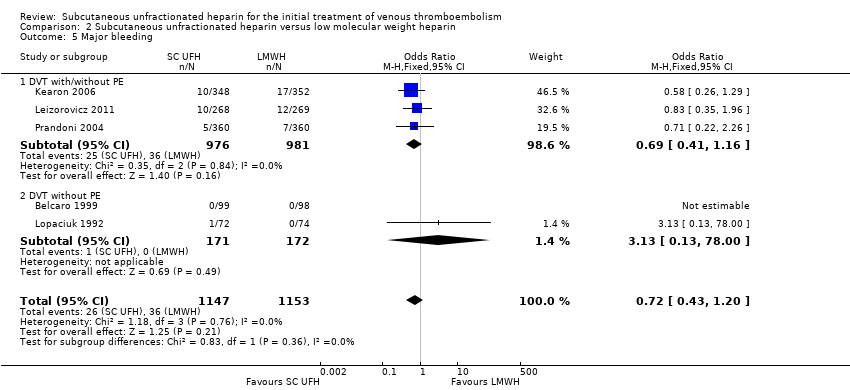 Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 5 Major bleeding. | ||||
| 5.1 DVT with/without PE | 3 | 1957 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.41, 1.16] |
| 5.2 DVT without PE | 2 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 78.00] |
| 6 All‐cause mortality Show forest plot | 7 | 2272 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.50, 1.07] |
| Analysis 2.6  Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 6 All‐cause mortality. | ||||
| 6.1 DVT with/without PE | 4 | 2016 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.48, 1.05] |
| 6.2 DVT without PE | 3 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.22, 13.26] |
| 7 Treatment related morbidity ‐ minor bleeding Show forest plot | 5 | 2300 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.71, 1.37] |
| Analysis 2.7 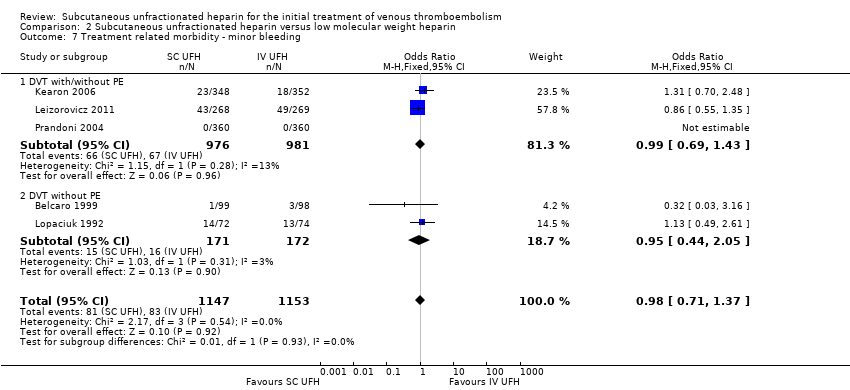 Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 7 Treatment related morbidity ‐ minor bleeding. | ||||
| 7.1 DVT with/without PE | 3 | 1957 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.43] |
| 7.2 DVT without PE | 2 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.44, 2.05] |
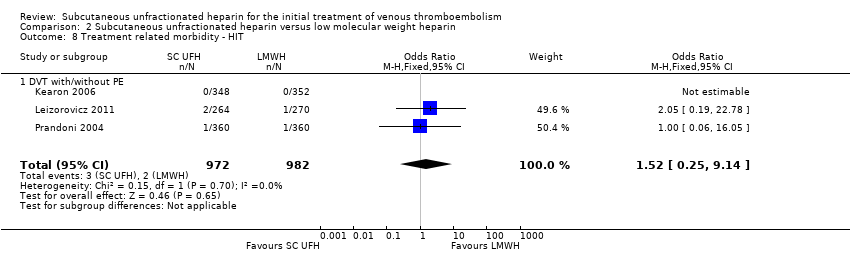
| 8 Treatment related morbidity ‐ HIT Show forest plot | 3 | 1954 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.25, 9.14] |
| Analysis 2.8  Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 8 Treatment related morbidity ‐ HIT. | ||||
| 8.1 DVT with/without PE | 3 | 1954 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.25, 9.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
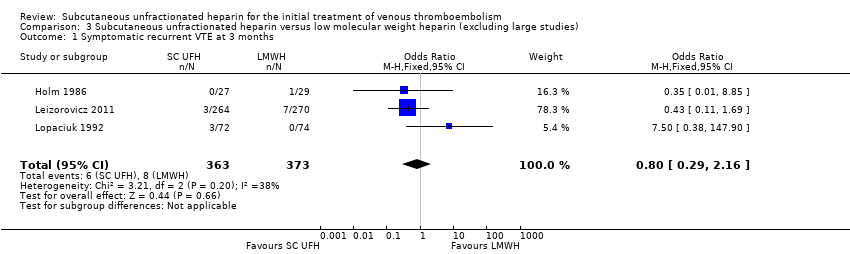
| 1 Symptomatic recurrent VTE at 3 months Show forest plot | 3 | 736 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.29, 2.16] |
| Analysis 3.1  Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 1 Symptomatic recurrent VTE at 3 months. | ||||
| 2 Symptomatic recurrent DVT at 3 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 2 Symptomatic recurrent DVT at 3 months. | ||||
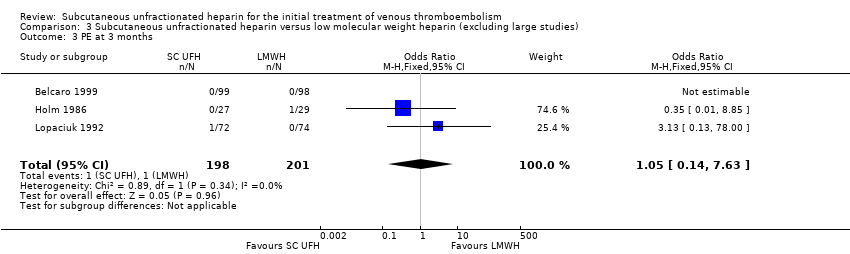
| 3 PE at 3 months Show forest plot | 3 | 399 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.14, 7.63] |
| Analysis 3.3  Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 3 PE at 3 months. | ||||
| 4 VTE‐related mortality at 3 months Show forest plot | 6 | 1049 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.13] |
| Analysis 3.4 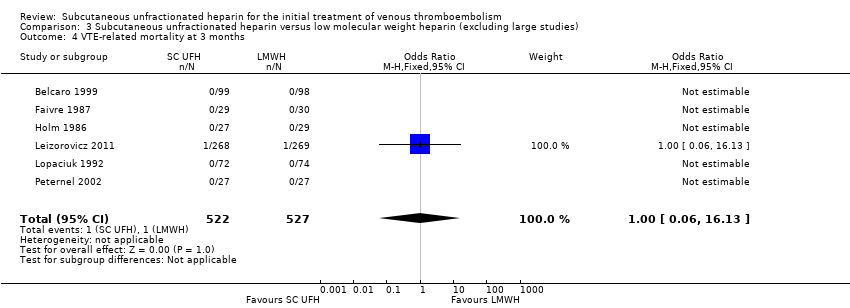 Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 4 VTE‐related mortality at 3 months. | ||||
| 5 Major bleeding Show forest plot | 3 | 880 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.41, 2.09] |
| Analysis 3.5  Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 5 Major bleeding. | ||||
| 6 All‐cause mortality Show forest plot | 5 | 852 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.03] |
| Analysis 3.6  Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 6 All‐cause mortality. | ||||
| 7 Treatment‐related morbidity Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.7  Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 7 Treatment‐related morbidity. | ||||
| 7.1 Minor bleeding | 3 | 880 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.60, 1.30] |
| 7.2 Heparin‐induced thrombocytopenia | 1 | 534 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.19, 22.78] |

Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
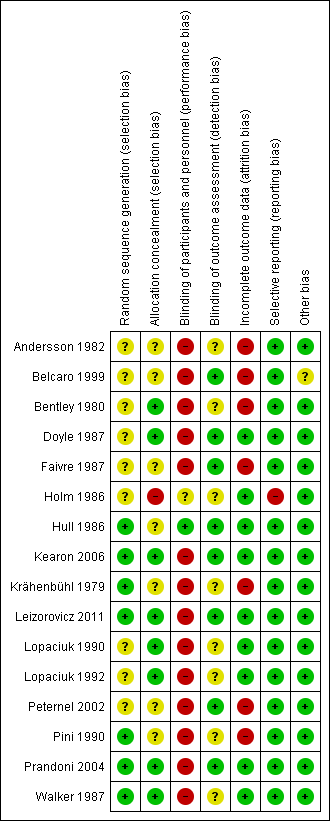
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 1 Symptomatic recurrent VTE at 3 months.

Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 2 Symptomatic recurrent DVT at 3 months.
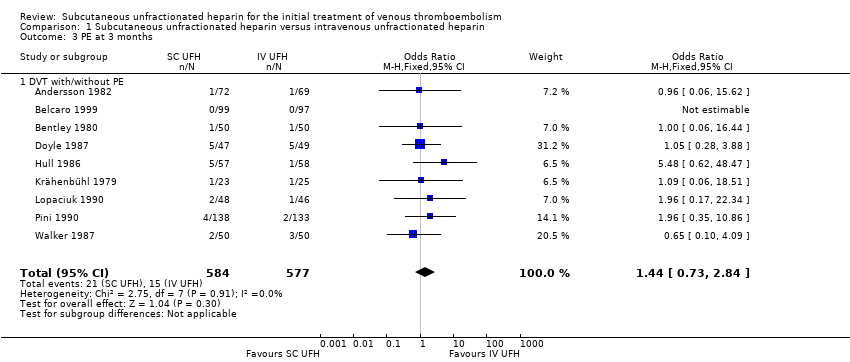
Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 3 PE at 3 months.

Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 4 VTE‐related mortality at 3 months.

Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 5 Major bleeding.

Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 6 All‐cause mortality.
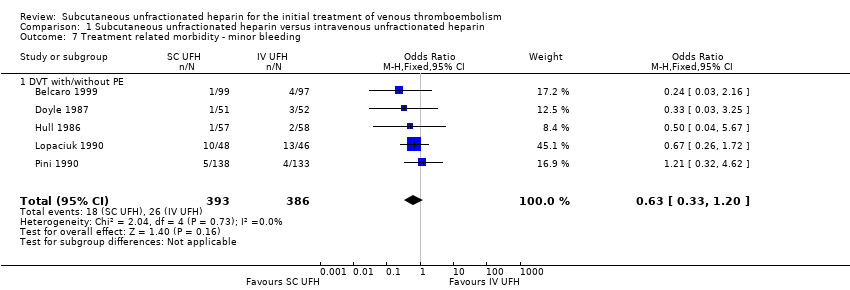
Comparison 1 Subcutaneous unfractionated heparin versus intravenous unfractionated heparin, Outcome 7 Treatment related morbidity ‐ minor bleeding.

Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 1 Symptomatic recurrent VTE at 3 months.

Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 2 Symptomatic recurrent DVT at 3 months.
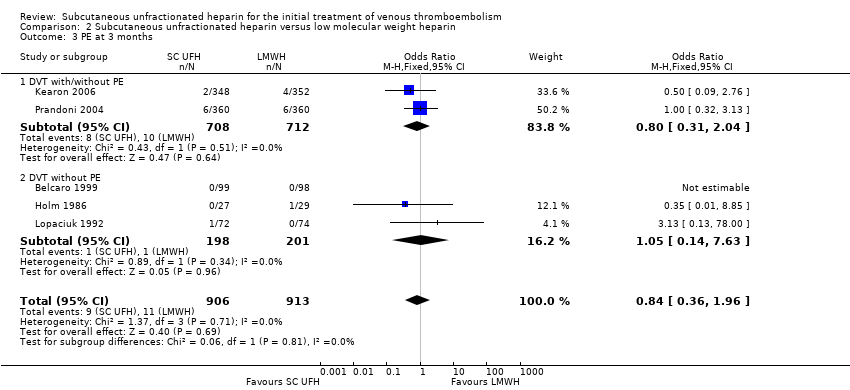
Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 3 PE at 3 months.

Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 4 VTE‐related mortality at 3 months.

Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 5 Major bleeding.
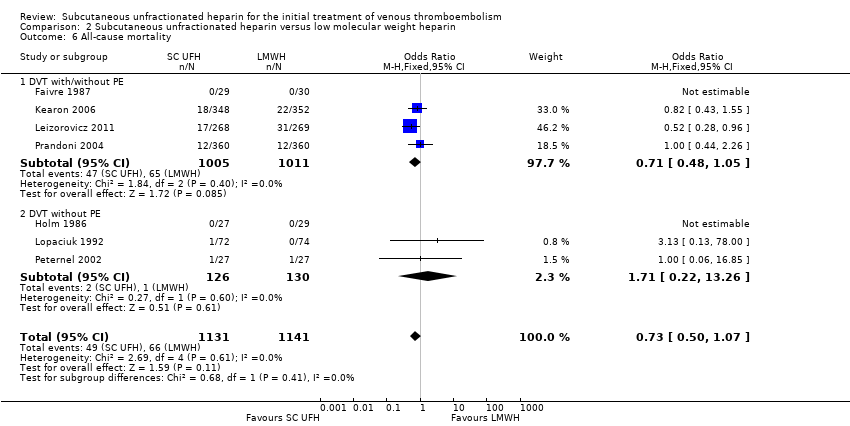
Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 6 All‐cause mortality.

Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 7 Treatment related morbidity ‐ minor bleeding.
Comparison 2 Subcutaneous unfractionated heparin versus low molecular weight heparin, Outcome 8 Treatment related morbidity ‐ HIT.
Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 1 Symptomatic recurrent VTE at 3 months.

Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 2 Symptomatic recurrent DVT at 3 months.
Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 3 PE at 3 months.

Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 4 VTE‐related mortality at 3 months.

Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 5 Major bleeding.

Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 6 All‐cause mortality.

Comparison 3 Subcutaneous unfractionated heparin versus low molecular weight heparin (excluding large studies), Outcome 7 Treatment‐related morbidity.
| Subcutaneous unfractionated heparin compared to intravenous unfractionated heparin for the initial treatment of venous thromboembolism | |||||
| Patient or population: people aged ≥ 18 years with a diagnosis of new or recurrent VTE | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Risk with intravenous unfractionated heparin | Risk with subcutaneous unfractionated heparin | ||||
| Symptomatic recurrent VTE at 3 months | Study population | OR 1.66 | 965 | ⊕⊕⊝⊝ | |
| 35 per 1000 | 57 per 1000 | ||||
| Symptomatic recurrent DVT at 3 months | Study population | OR 3.29 | 115 | ⊕⊕⊝⊝ | |
| 34 per 1000 | 105 per 1000 | ||||
| PE at 3 months | Study population | OR 1.44 | 1161 | ⊕⊕⊝⊝ | |
| 26 per 1000 | 37 per 1000 | ||||
| VTE‐related mortality at 3 months | Study population | OR 0.98 | 1168 | ⊕⊕⊝⊝ | |
| 3 per 1000 | 3 per 1000 | ||||
| Major bleedingd (7 days ‐ 12 months) | Study population | OR 0.91 | 583 | ⊕⊕⊝⊝ | |
| 48 per 1000 | 44 per 1000 | ||||
| All‐cause mortality (5 days to 12 months) | Study population | OR 1.74 | 972 | ⊕⊕⊝⊝ | |
| 12 per 1000 | 21 per 1000 | ||||
| Asymptomatic VTE at 3 months | No study measured this outcome | ||||
| *The basis for the assumed risk was the average risk in the intravenous unfractionated heparin group (i.e. the number of participants with events divided by total number of participants of the intravenous heparin group included in the meta‐analysis). The risk in the subcutaneous unfractionated heparin group (and its 95% confidence interval) is based on the assumed risk in the intravenous unfractionated heparin group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded the quality of the evidence to low due to a high risk of performance bias in eight studies (Andersson 1982; Belcaro 1999; Bentley 1980; Doyle 1987; Krähenbühl 1979; Lopaciuk 1990; Pini 1990; Walker 1987), plus a high risk of attrition bias in five studies (Andersson 1982; Belcaro 1999; Bentley 1980; Krähenbühl 1979; Pini 1990). We also downgraded for imprecision, as reflected by the wide confidence intervals. | |||||
| Subcutaneous unfractionated heparin compared to low molecular weight heparin for the initial treatment of venous thromboembolism | |||||
| Patient or population: people aged ≥ 18 years with a diagnosis of new or recurrent VTE | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Risk with low molecular weight heparin | Risk with subcutaneous unfractionated heparin | ||||
| Symptomatic recurrent VTE at 3 months | Study population | OR 1.01 | 2156 | ⊕⊕⊝⊝ | |
| 31 per 1000 | 32 per 1000 | ||||
| Symptomatic recurrent DVT at 3 months | Study population | OR 1.38 | 1566 | ⊕⊕⊝⊝ | |
| 20 per 1000 | 28 per 1000 | ||||
| PE at 3 months | Study population | OR 0.84 | 1819 | ⊕⊕⊝⊝ | |
| 12 per 1000 | 10 per 1000 | ||||
| VTE‐related mortality at 3 months | Study population | OR 0.53 | 2469 | ⊕⊕⊝⊝ | |
| 6 per 1000 | 3 per 1000 | ||||
| Major bleedingd (3 months) | Study population | OR 0.72 | 2300 | ⊕⊕⊝⊝ | |
| 31 per 1000 | 23 per 1000 | ||||
| All‐cause mortality (7 days ‐ 3 months) | Study population | OR 0.73 | 2272 | ⊕⊕⊝⊝ | |
| 58 per 1000 | 43 per 1000 | ||||
| Asymptomatic VTE at 3 months | No study measured this outcome | ||||
| *The basis for the assumed risk was the average risk in the low molecular weight heparin group (i.e. the number of participants with events divided by total number of participants of the low molecular weight heparin group included in the meta‐analysis). The risk in the subcutaneous unfractionated heparin group (and its 95% confidence interval) is based on the assumed risk in the low molecular weight heparin group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded the quality of the evidence to low due to high risk of selection and reporting bias in one study (Holm 1986), plus a high risk of performance bias in four studies (Kearon 2006; Leizorovicz 2011; Lopaciuk 1992; Prandoni 2004). We also downgraded for imprecision, as reflected by the wide confidence intervals. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic recurrent VTE at 3 months Show forest plot | 8 | 965 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.89, 3.10] |
| 1.1 DVT with/without PE | 8 | 965 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.89, 3.10] |
| 2 Symptomatic recurrent DVT at 3 months Show forest plot | 1 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.64, 17.06] |
| 2.1 DVT with/without PE | 1 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.64, 17.06] |
| 3 PE at 3 months Show forest plot | 9 | 1161 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.73, 2.84] |
| 3.1 DVT with/without PE | 9 | 1161 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.73, 2.84] |
| 4 VTE‐related mortality at 3 months Show forest plot | 9 | 1168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.88] |
| 4.1 DVT with/without PE | 9 | 1168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.88] |
| 5 Major bleeding Show forest plot | 4 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.97] |
| 5.1 DVT with/without PE | 4 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.97] |
| 6 All‐cause mortality Show forest plot | 8 | 972 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.67, 4.51] |
| 6.1 DVT with/without PE | 8 | 972 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.67, 4.51] |
| 7 Treatment related morbidity ‐ minor bleeding Show forest plot | 5 | 779 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.20] |
| 7.1 DVT with/without PE | 5 | 779 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic recurrent VTE at 3 months Show forest plot | 5 | 2156 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.63, 1.63] |
| 1.1 DVT with/without PE | 3 | 1954 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.57, 1.56] |
| 1.2 DVT without PE | 2 | 202 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.38, 11.84] |
| 2 Symptomatic recurrent DVT at 3 months Show forest plot | 3 | 1566 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.73, 2.63] |
| 2.1 DVT with/without PE | 2 | 1420 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.65, 2.46] |
| 2.2 DVT without PE | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.25, 111.99] |
| 3 PE at 3 months Show forest plot | 5 | 1819 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.36, 1.96] |
| 3.1 DVT with/without PE | 2 | 1420 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.31, 2.04] |
| 3.2 DVT without PE | 3 | 399 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.14, 7.63] |
| 4 VTE‐related mortality at 3 months Show forest plot | 8 | 2469 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.67] |
| 4.1 DVT with/without PE | 4 | 2016 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.67] |
| 4.2 DVT without PE | 4 | 453 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Major bleeding Show forest plot | 5 | 2300 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.43, 1.20] |
| 5.1 DVT with/without PE | 3 | 1957 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.41, 1.16] |
| 5.2 DVT without PE | 2 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 78.00] |
| 6 All‐cause mortality Show forest plot | 7 | 2272 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.50, 1.07] |
| 6.1 DVT with/without PE | 4 | 2016 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.48, 1.05] |
| 6.2 DVT without PE | 3 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.22, 13.26] |
| 7 Treatment related morbidity ‐ minor bleeding Show forest plot | 5 | 2300 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.71, 1.37] |
| 7.1 DVT with/without PE | 3 | 1957 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.43] |
| 7.2 DVT without PE | 2 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.44, 2.05] |
| 8 Treatment related morbidity ‐ HIT Show forest plot | 3 | 1954 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.25, 9.14] |
| 8.1 DVT with/without PE | 3 | 1954 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.25, 9.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic recurrent VTE at 3 months Show forest plot | 3 | 736 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.29, 2.16] |
| 2 Symptomatic recurrent DVT at 3 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 PE at 3 months Show forest plot | 3 | 399 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.14, 7.63] |
| 4 VTE‐related mortality at 3 months Show forest plot | 6 | 1049 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.13] |
| 5 Major bleeding Show forest plot | 3 | 880 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.41, 2.09] |
| 6 All‐cause mortality Show forest plot | 5 | 852 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.03] |
| 7 Treatment‐related morbidity Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Minor bleeding | 3 | 880 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.60, 1.30] |
| 7.2 Heparin‐induced thrombocytopenia | 1 | 534 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.19, 22.78] |

