Uklanjanje ili postepeno smanjenje doze inhibitora kalcineurina kod primatelja presađenog bubrega
Abstract
Background
Calcineurin inhibitors (CNI) can reduce acute transplant rejection and immediate graft loss but are associated with significant adverse effects such as hypertension and nephrotoxicity which may contribute to chronic rejection. CNI toxicity has led to numerous studies investigating CNI withdrawal and tapering strategies. Despite this, uncertainty remains about minimisation or withdrawal of CNI.
Objectives
This review aimed to look at the benefits and harms of CNI tapering or withdrawal in terms of graft function and loss, incidence of acute rejection episodes, treatment‐related side effects (hypertension, hyperlipidaemia) and death.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register to 11 October 2016 through contact with the Information Specialist using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE, and EMBASE; handsearching conference proceedings; and searching the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
All randomised controlled trials (RCTs) where drug regimens containing CNI were compared to alternative drug regimens (CNI withdrawal, tapering or low dose) in the post‐transplant period were included, without age or dosage restriction.
Data collection and analysis
Two authors independently assessed studies for eligibility, risk of bias, and extracted data. Results were expressed as risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI).
Main results
We included 83 studies that involved 16,156 participants. Most were open‐label studies; less than 30% of studies reported randomisation method and allocation concealment. Studies were analysed as intent‐to‐treat in 60% and all pre‐specified outcomes were reported in 54 studies. The attrition and reporting bias were unclear in the remainder of the studies as factors used to judge bias were reported inconsistently. We also noted that 50% (47 studies) of studies were funded by the pharmaceutical industry.
We classified studies into four groups: CNI withdrawal or avoidance with or without substitution with mammalian target of rapamycin inhibitors (mTOR‐I); and low dose CNI with or without mTOR‐I. The withdrawal groups were further stratified as avoidance and withdrawal subgroups for major outcomes.
CNI withdrawal may lead to rejection (RR 2.54, 95% CI 1.56 to 4.12; moderate certainty evidence), may make little or no difference to death (RR 1.09, 95% CI 0.96 to 1.24; moderate certainty), and probably slightly reduces graft loss (RR 0.85, 95% CI 0.74 to 0.98; low quality evidence). Hypertension was probably reduced in the CNI withdrawal group (RR 0.82, 95% CI 0.71 to 0.95; low certainty), while CNI withdrawal may make little or no difference to malignancy (RR 1.10, 95% CI 0.93 to 1.30; low certainty), and probably makes little or no difference to cytomegalovirus (CMV) (RR 0.87, 95% CI 0.52 to 1.45; low certainty)
CNI avoidance may result in increased acute rejection (RR 2.16, 95% CI 0.85 to 5.49; low certainty) but little or no difference in graft loss (RR 0.96, 95% CI 0.79 to 1.16; low certainty). Late CNI withdrawal increased acute rejection (RR 3.21, 95% CI 1.59 to 6.48; moderate certainty) but probably reduced graft loss (RR 0.84, 95% CI 0.72 to 0.97, low certainty).
Results were similar when CNI avoidance or withdrawal was combined with the introduction of mTOR‐I; acute rejection was probably increased (RR 1.43; 95% CI 1.15 to 1.78; moderate certainty) and there was probably little or no difference in death (RR 0.96; 95% CI 0.69 to 1.36, moderate certainty). mTOR‐I substitution may make little or no difference to graft loss (RR 0.94, 95% CI 0.75 to 1.19; low certainty), probably makes little of no difference to hypertension (RR 0.86, 95% CI 0.64 to 1.15; moderate), and probably reduced the risk of cytomegalovirus (CMV) (RR 0.60, 95% CI 0.44 to 0.82; moderate certainty) and malignancy (RR 0.69, 95% CI 0.47 to 1.00; low certainty). Lymphoceles were increased with mTOR‐I substitution (RR 1.45, 95% CI 0.95 to 2.21; low certainty).
Low dose CNI combined with mTOR‐I probably increased glomerular filtration rate (GFR) (MD 6.24 mL/min, 95% CI 3.28 to 9.119; moderate certainty), reduced graft loss (RR 0.75, 95% CI 0.55 to 1.02; moderate certainty), and made little or no difference to acute rejection (RR 1.13 ; 95% CI 0.91 to 1.40; moderate certainty). Hypertension was decreased (RR 0.98, 95% CI 0.80 to 1.20; low certainty) as was CMV (RR 0.41, 95% CI 0.16 to 1.06; low certainty). Low dose CNI plus mTOR‐I makes probably makes little of no difference to malignancy (RR 1.22, 95% CI 0.42 to 3.53; low certainty) and may make little of no difference to death (RR 1.16, 95% CI 0.71 to 1.90; moderate certainty).
Authors' conclusions
CNI avoidance increased acute rejection and CNI withdrawal increases acute rejection but reduced graft loss at least over the short‐term. Low dose CNI with induction regimens reduced acute rejection and graft loss with no major adverse events, also in the short‐term. The use of mTOR‐I reduced CMV infections but increased the risk of acute rejection. These conclusions must be tempered by the lack of long‐term data in most of the studies, particularly with regards to chronic antibody‐mediated rejection, and the suboptimal methodological quality of the included studies.
PICOs
Laički sažetak
Uklanjanje ili postepeno smanjenje doze inhibitora kalcineurina kod primatelja presađenog bubrega
Istraživačko pitanje
Inhibitori kalcineurina (ciklosporin i takrolimus) su važan dio terapije za potiskivanje imunološkog sustava kako bi se spriječilo odbacivanje presađenog bubrega. No, inhibitori kalcineurina mogu uzrokovati povišeni tlak i ožiljkavanje bubrega što može dovesti do pogoršanja rizika za srčani i moždani udar kao i do gubitka presađenog organa.
Postoje poprečni podaci učinka uklanjanja ovih lijekova kod primatelja presađenog organa; neke studije sugeriraju poboljšanu bubrežnu funkciju dok druge ističu umjereni rizik odbacivanja presađenog organa. Zbog ove nesigurnosti, istraživala se korisnost i štetnost uklanjanja ili postepenog smanjenja inhibitora kalcineurina kod primatelja presađenog bubrega kako bi se saznao najbolji pristup.
Kako je provedeno istraživanje?
Uključene su 83 studije s ukupno 16,000 sudionika u ovaj sustavni pregledni rad. Analizirane su studije koje su uspoređivale protokole sa standardnim dozama CNI i one koje su potpuno uklonile, postepeno smanjivale ili koristile niske doze CNI u post‐transplantacijskom periodu.
Rezultati istraživanja
Iako je uklanjanje inhibitora kalcineurina u kratkoročnom periodu rezultiralo većim brojem odbacivanja presađenog organa, nije bilo jasne razlike u zatajenju presađenog organa, smrti, razvoju raka ili infekcije. Zamjena inhibitora kalcineurina s drugom skupinom lijekova koji se nazivaju inhibitori mTOR‐a, nije značajnije utjecalo na ishode istraživanja osim na smanjenje broja infekcija citomegalovirusom (CMV). Manje doze inhibitora kalcineurina su bile povezane s manjim brojem odbacivanja i gubitaka organa u prvih 5 godina nakon presađivanja.
Zaključci
Nisu jasni dugoročni učinci potpunog uklanjanja ili smanjenja doze inhibitora kalcineurina, a mTOR inhibitori smanjuju učestalost CMV infekcija s većim rizikom akutnog odbacivanja presađenog organa. Trenutno nemamo na raspolaganju dovoljan broj studija s dugoročnim praćenjem kako bi se jasnije odredila bolja terapija za primatelje presađenog bubrega.
Authors' conclusions
Summary of findings
| CNI withdrawal versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard dose CNI | Risk with CNI withdrawal | ||||
| Death | Study population | RR 1.09 | 2010 (14 ) | ⊕⊕⊕⊝ | |
| 225 per 1,000 | 245 per 1,000 | ||||
| Acute rejection | Study population | RR 2.54 | 1666 (15) | ⊕⊕⊕⊝ | |
| 137 per 1,000 | 348 per 1,000 | ||||
| GFR | The mean GFR in the intervention group was 3.56 mL/min more (1.13 less to 8.25 more) than the control group | ‐ | 910 (8) | ⊕⊕⊝⊝ | |
| Graft loss | Study population | RR 0.85 | 2090 (16) | ⊕⊕⊝⊝ | |
| 236 per 1,000 | 201 per 1,000 | ||||
| Adverse events: hypertension | Study population | RR 0.82 | 950 (5 ) | ⊕⊕⊝⊝ | |
| 555 per 1,000 | 455 per 1,000 | ||||
| Adverse events: CMV infection | Study population | RR 0.87 | 608 (7) | ⊕⊕⊝⊝ | |
| 98 per 1,000 | 86 per 1,000 | ||||
| Adverse events: malignancy | Study population | RR 1.10 | 1079 (6) | ⊕⊕⊝⊝ | |
| 257 per 1,000 | 282 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 despite different follow up times, heterogeneity not noted on analysis 2 Most studies were ITT analysis, some small studies did not specify randomisation and allocation concealment 3 Larger studies closer to pooled estimate on funnel plot 4 Some studies were small with large confidence intervals, CI fails to exclude benefit or harm 5 Heterogeneity low when biopsy‐proven rejections were analysed in subgroup 6 Smaller studies not distributed around point estimate 7 Significant heterogeneity noted despite separating time periods of reporting GFR 8 Only few studies reported GFR with possible attrition bias 9 2 large studies had more than 2 comparison groups 10 Very few studies reported the outcome 11 Symmetric distribution studies around estimate of effect 12 2 studies with high event rates skew the effect | |||||
| Low dose CNI versus standard dose CNI for kidney transplant recipients | ||||||
| Patient or population: kidney transplant recipients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with standard dose CNI | Risk with low dose CNI | |||||
| Death | Study population | RR 0.79 | 3462 (15) | ⊕⊕⊕⊝ | ||
| 23 per 1,000 | 19 per 1,000 | |||||
| Acute rejection | Study population | RR 0.87 | 3757 (19) | ⊕⊕⊕⊝ | ||
| 183 per 1,000 | 159 per 1,000 | |||||
| GFR | The mean GFR in the intervention group was 4.1 mL/min more (2.07 more to 6.12 more) than the control group | ‐ | 2623 (13) | ⊕⊕⊕⊝ | ||
| Graft loss | Study population | RR 0.75 | 3286 (15) | ⊕⊕⊕⊝ | Sensitivity analysis after excluding 1 study which also involved steroid withdrawal; significant reduction in graft loss in the low dose regimen | |
| 58 per 1,000 | 44 per 1,000 | |||||
| Adverse events: hypertension | Study population | RR 0.84 | 1877 (5) | ⊕⊕⊝⊝ | ||
| 218 per 1,000 | 184 per 1,000 | |||||
| Adverse events: CMV infection | Study population | RR 1.23 | 1948 (6) | ⊕⊕⊕⊝ | ||
| 101 per 1,000 | 124 per 1,000 | |||||
| Adverse events: malignancy | Study population | RR 0.90 | 1637 (5) | ⊕⊕⊝⊝ | ||
| 15 per 1,000 | 14 per 1,000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies with ITT analysis, randomisation procedure and allocation concealment not clear from most publications 2 Minimal heterogeneity noted on analysis 3 Several small studies with wide confidence intervals 4 Despite studies with or without induction, sensitivity analysis made no difference to outcome 5 Heterogeneity noted only between subgroups 6 Only 2/15 studies had more than 2 comparison groups 7 Industry sponsored 8 1/6 studies did not report some outcomes due to high dropout 9 Only 5 studies reported the outcome and had wide CI 10 Few studies reported the outcome | ||||||
| CNI withdrawal + mTORi versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard dose CNI | Risk with CNI withdrawal + mTOR | ||||
| Death | Study population | RR 0.99 | 5427 (23) | ⊕⊕⊕⊝ | |
| 26 per 1,000 | 26 per 1,000 | ||||
| Acute rejection | Study population | RR 1.43 | 5903 (30) | ⊕⊕⊕⊝ | |
| 134 per 1,000 | 191 per 1,000 | ||||
| Graft loss | Study population | RR 0.94 | 5446 (25) | ⊕⊕⊝⊝ | |
| 53 per 1,000 | 50 per 1,000 | ||||
| Adverse events: hypertension | Study population | RR 0.86 | 2207 (7) | ⊕⊕⊝⊝ | |
| 218 per 1,000 | 187 per 1,000 | ||||
| Adverse events: CMV Infection | Study population | RR 0.60 | 2503 (13) | ⊕⊕⊕⊝ | |
| 150 per 1,000 | 90 per 1,000 | ||||
| Adverse events: malignancy | Study population | RR 0.69 | 3699 (14) | ⊕⊕⊝⊝ | |
| 54 per 1,000 | 38 per 1,000 | ||||
| Adverse events: lymphocele | Study population | RR 1.45 | 1926 (8) | ⊕⊕⊝⊝ | |
| 100 per 1,000 | 144 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Randomisation method and allocation concealment performed in most studies 2 No significant heterogeneity noted in analysis 3 Only 2 studies had more than 2 comparison arms 4 Many studies with small events and wide CI 5 Significant heterogeneity in studies in biopsy‐proven acute rejection 6 Funnel plot skewed 7 Significant heterogeneity noted 8 Few studies reported this outcome 9 Moderate heterogeneity but follow‐up times are variable 10 Not all studies reported the outcome 11 Heterogeneity is not significant when 1 long‐term study was excluded | |||||
| Low dose CNI + mTORi versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard dose CNI | Risk with low dose CNI + mTORi | ||||
| Death | Study population | RR 1.16 | 2750 (11) | ⊕⊕⊕⊝ | |
| 22 per 1,000 | 26 per 1,000 | ||||
| Acute rejection | Study population | RR 1.13 | 3300 (16) | ⊕⊕⊕⊝ | |
| 132 per 1,000 | 149 per 1,000 | ||||
| GFR | The mean GFR in the intervention group was 6.24 mL/min more (3.28 more to 9.19 more) than the control group | ‐ | 1749 (11) | ⊕⊕⊕⊝ | |
| Graft loss | Study population | RR 0.67 | 3304 (16) | ⊕⊕⊕⊝ | |
| 38 per 1,000 | 25 per 1,000 | ||||
| Adverse events: hypertension | Study population | RR 0.98 | 1421 (5) | ⊕⊕⊝⊝ | |
| 203 per 1,000 | 199 per 1,000 | ||||
| Adverse events: CMV infection | Study population | RR 0.41 | 1250 (5) | ⊕⊕⊝⊝ | |
| 105 per 1,000 | 43 per 1,000 | ||||
| Adverse events: malignancy | Study population | RR 1.22 | 1074 (5) | ⊕⊕⊝⊝ | |
| 11 per 1,000 | 14 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Randomisation and allocation process not clear in some studies 2 No significant heterogeneity 3 Only 2 of the studies had more than 2 comparisons 4 Some small studies with wide CI 5 Substantial heterogeneity noted due to recording at different time periods 6 Small number of events and some small studies with wide CI 7 Only few studies reported this outcome 8 95% CI fails to exclude benefit or harm 9 Heterogeneity present but when abstract only studies are removed, heterogeneity is zero | |||||
| Subgroup analysis: CNI avoidance and late withdrawal versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard dose CNI | Risk with CNI avoidance and withdrawal | ||||
| Acute rejection: avoidance | Study population | RR 2.16 | 238 (3) | ⊕⊕⊝⊝ | |
| 344 per 1,000 | 744 per 1,000 | ||||
| Acute rejection: late withdrawal | Study population | RR 3.21 | 1428 (12) | ⊕⊕⊕⊝ | |
| 102 per 1,000 | 328 per 1,000 | ||||
| GFR: avoidance | The mean GFR for avoidance studies in the intervention group was 2.22 mL/min lower (14.84 less to 10.4 more) than the control group | ‐ | 242 (3) | ⊕⊝⊝⊝ | |
| GFR: late withdrawal | The mean GFR for late withdrawal studies in the intervention group was 5.54 mL/min more (1.66 more to 9.43 more) than the control group | ‐ | 668 (5) | ⊕⊕⊝⊝ | |
| Graft loss: avoidance | Study population | RR 0.96 | 566 (4) | ⊕⊕⊝⊝ | |
| 355 per 1,000 | 341 per 1,000 | ||||
| Graft loss: late withdrawal | Study population | RR 0.84 | 1831 (13) | ⊕⊕⊕⊝ | |
| 260 per 1,000 | 219 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 3 small studies with one study including a non‐randomised arm 2 Significant heterogeneity 3 Several small studies with wide confidence intervals 4 Small numbers to make a judgement of difference 5 Skewed funnel plot 6 Substantial heterogeneity 7 2/4 are small studies with wide CI 8 4 studies included with one study with high event rate 9 No heterogeneity identified on analysis 10 Larger studies are not industry sponsored | |||||
| Subgroup analysis: CNI avoidance and late withdrawal + mTORi versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard dose CNI | Risk with CNI avoidance and withdrawal + mTORi | ||||
| Acute rejection: avoidance | Study population | RR 1.27 | 1844 (11) | ⊕⊕⊕⊝ | |
| 234 per 1,000 | 297 per 1,000 | ||||
| Acute rejection: late withdrawal | Study population | RR 1.90 | 3636 (17) | ⊕⊕⊕⊝ | |
| 65 per 1,000 | 124 per 1,000 | ||||
| GFR: avoidance | The mean GFR for avoidance studies in the intervention group was 6.45 mL/min higher (1.33 higher to 11.58 higher) than the control group | ‐ | 1748 (9) | ⊕⊕⊝⊝ | |
| GFR: late withdrawal | The mean GFR for late withdrawal studies in the intervention group was MD 4.55 higher | ‐ | 2679 (14) | ⊕⊕⊝⊝ | |
| Graft loss: avoidance | Study population | RR 1.03 | 1420 (8) | ⊕⊕⊕⊝ | |
| 74 per 1,000 | 76 per 1,000 | ||||
| Graft loss: late withdrawal | Study population | RR 0.92 | 4026 (17) | ⊕⊕⊕⊝ | |
| 46 per 1,000 | 42 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Several smaller studies with wide CI 2 Significant heterogeneity | |||||
Background
Description of the condition
Standard immunosuppressive protocols to prevent acute graft rejection in kidney transplantation involve three major groups of drugs ‐ calcineurin inhibitor(s) (CNI), antimetabolites and steroids. CNI have been an important part of primary immunosuppression therapy together with adjunctive agents such as mycophenolate mofetil (MMF), azathioprine (AZA) and steroids in kidney transplant recipients (Hariharan 2000).
CNI inhibit the calcium‐dependent enzyme serine phosphatase calcineurin. This process prevents the dephosphorylation of nuclear factors of activated T lymphocytes (NFAT), which is essential for translocation into the nucleus leading to reduced activation of cytokine genes for interleukin‐2 (IL2) production. Cyclosporin (CsA) and tacrolimus (TAC) are CNI used for kidney transplant recipients (Melk 2003).
Description of the intervention
CNI have dramatically reduced the incidence of acute transplant rejection and decreased early graft loss (Ahsan 2001). However, CNI have been associated with significant adverse effects such as nephrotoxicity (Bennett 1996) causing decreased glomerular filtration rate (GFR), hypertension, hyperlipidaemia and a significant contribution to chronic allograft nephropathy. These effects could lead to subsequent graft loss and contribute directly or indirectly to patient morbidity and mortality by affecting the cardiovascular risk factors (Kasiske 1996). The immunological causes of graft loss have to be however considered. The potential risks of CNI use should be balanced against the risks of acute rejection and chronic antibody‐mediated rejection, especially in patients with a high immunological risk.
How the intervention might work
The significant toxicity profile of CNI have prompted many studies investigating CNI withdrawal and tapering strategies. However, some highlighted an increase in acute rejection following withdrawal (Abramowicz 2002) and others showed no effect on graft survival and a short term improvement in creatinine values (Gonwa 2002).
Why it is important to do this review
Despite the large number of studies conducted, uncertainty remains about tapering or withdrawing CNI. These strategies must be balanced with the significant benefits conferred by CNI in preventing early graft rejection. In the absence of a clear clinical consensus, this review aimed to assess the benefits and harms of CNI withdrawal or tapering for kidney transplant recipients.
Objectives
This review aimed to look at the benefits and harms of CNI tapering or withdrawal in terms of graft function and loss, incidence of acute rejection episodes, treatment‐related side effects (hypertension, hyperlipidaemia) and death.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) where standard dose CNI regimens were compared with CNI withdrawal or tapering for kidney transplant recipients were included. The first period of randomised cross‐over studies were also included.
Types of participants
Inclusion criteria
Patients with end‐stage kidney disease (ESKD), irrespective of age or gender, who received a first or subsequent cadaveric or living donor kidney transplant and received CNI (CsA or TAC) as the primary immunosuppression, were included.
Exclusion criteria
Recipients who received another solid organ in addition to a kidney transplant (e.g. pancreas) were excluded.
Types of interventions
-
Transplant recipients who received CNI (CsA or TAC) as the primary immunosuppression which was subsequently tapered or withdrawn completely were included.
-
All studies where tapering or withdrawal was compared with controls were included irrespective of the duration of treatment prior to the intervention. In cases of significant heterogeneity, subgroup analysis was performed.
-
All definitions of tapering mentioned in the studies were included irrespective of the duration of tapering; sensitivity analysis was used to differentiate between the tapering groups.
-
Studies that defined low dose either by exposure to CsA and TAC calculated using 12‐hour post‐dose nadir (trough; C0) blood levels, or studies which employed fixed doses (mg/kg) were included.
Specific comparisons were made between:
-
Standard dose CNI versus CNI withdrawal
-
Low dose CNI versus standard dose CNI
-
CNI withdrawal with conversion to mammalian target of rapamycin inhibitor (mTOR‐I) versus standard dose CNI
-
Low dose CNI with conversion to mTOR‐I versus normal dose CNI.
In case of significant heterogeneity among interventions, subgroup analysis was carried out in:
-
Duration of tapering or withdrawal
-
AZA and MMF groups.
Types of outcome measures
-
Graft loss (censored and not censored for death)
-
All‐cause mortality
-
Acute rejection episodes: both clinical and biopsy‐proven acute rejection (BPAR) were included
-
Graft kidney function at six months and at one, two and five years measured by serum creatinine (SCr), calculated GFR or creatinine clearance (CrCl)
-
Treatment‐related side effects (e.g. hyperlipidaemia, hypertension)
-
Rates of malignancy
-
Incidence of infections.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 11 October 2016 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from the following sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials CENTRAL
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected kidney journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
-
Reference lists of review articles, relevant studies and clinical practice guidelines.
-
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that were relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews that included relevant data or information on trials were retained initially. The same two authors independently assessed retrieved abstracts, and if necessary, the full text of studies which satisfied the inclusion criteria. Studies reported in non‐English language journals were translated before assessment. Discrepancies were resolved by discussion with a third author.
Data extraction and management
Data extraction was carried out independently by the same authors using standard data extraction forms. Where more than one publication of one study existed, reports were grouped together and the most recent or most complete data set were used. Any discrepancies between published versions were highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Results for dichotomous outcomes (e.g. incidence of acute rejections, graft loss, death) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. blood pressure, SCr, GFR), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales were used.
Dealing with missing data
Further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review.
Assessment of heterogeneity
Heterogeneity was analysed using a Cochran Q test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). In case of significant heterogeneity, subgroup analysis was considered.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity (e.g. interventions and study quality). Heterogeneity among participants could be related to age and renal pathology. Heterogeneity in treatments could be related to prior agent(s) used, the agent (CsA/TAC) and duration of therapy prior to withdrawal or tapering. Adverse effects are tabulated and assessed with descriptive techniques, as they are likely to be different for the various agents used.
Sensitivity analysis
Sensitivity analysis was used to differentiate between tapering groups.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
-
Death
-
Graft loss
-
Acute rejection
-
GFR
-
Adverse events (e.g. hypertension, CMV infection, malignancy).
Results
Description of studies
Results of the search
Our search identified 2398 records. After title and abstract review we excluded 1605 records. The remaining 793 records were for 159 studies. We included only studies that compared standard dose CNI with tapering or withdrawal with or without mTOR‐I substitution which resulted in 83 studies (583 reports) being included in the analyses. We excluded 72 studies (202 records). Four studies (8 records) are ongoing (David‐Neto 2014; ERIC Study 2010; ISRCTN63298320; TRANSFORM Study 2013) and will be assessed in a future update of this review. See Figure 1.
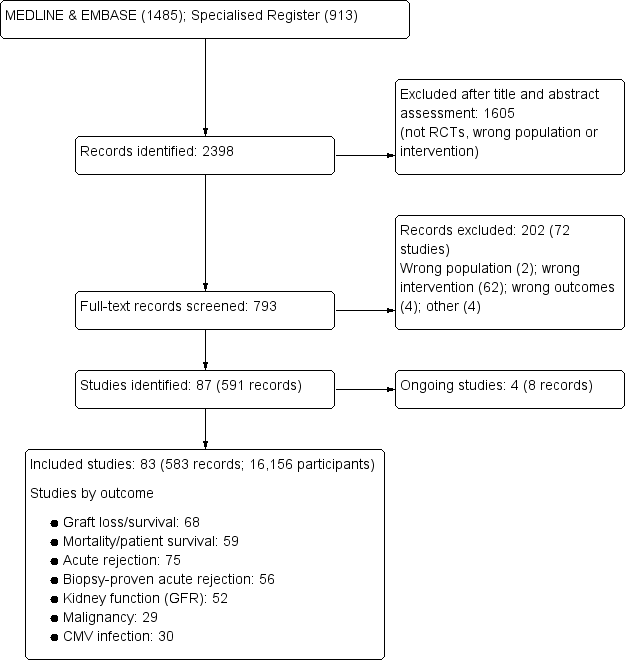
Flow chart showing number of studies identified
Included studies
See Characteristics of included studies.
The 83 studies included 16,156 randomised participants. Of these, 13 studies were available only in abstract form (2345 participants) (Alsina 1987; Bertoni 2007; Cockfield 2002; El‐Agroudy 2014; Heering 1993; HERAKLES Study 2012; Holm 2008; Kreis 2003; MODIFY Study 2012; Pacheco‐Silva 2013; Qazi 2014; Rossini 2007; Salvadori 2007).
CNI withdrawal or avoidance versus standard dose CNI regimens
We found 17 studies (81 reports, 1939 participants) that compared CNI withdrawal or avoidance with standard dose CNI regimens; four studies compared avoidance with standard dose CNI regimens (Asberg 2006; Garcia 2007; Grimbert 2002; Kosch 2003a), and one study with three arms and compared avoidance and withdrawal with standard dose CNI (Hall 1988). The remainder compared CNI withdrawal with standard dose CNI regimen.
Garcia 2007 and CTOT‐09 Study 2015 investigated TAC; two studies involved patients on either CsA or TAC (Pascual 2008; Suwelack 2002), and the remainder were CsA‐based studies (Abramowicz 2002; Asberg 2006; Dudley 2005; Grimbert 2002; Hall 1988; Hazzan 2005; Heering 1993; Hollander 1995; Isoniemi 1990; Kosch 2003a; MacPhee 1998; Pedersen 1991; Smak Gregoor 1999).
Standard versus low dose CNI
We included 18 studies (89 reports, 2904 participants) that compared standard dose CNI with low dose CNI. Of these, 15 were CsA‐based studies (Alsina 1987; Andres 2009; Baczkowska 2003; Budde 2007; Cai 2014; Chadban 2013; Cibrik 2007; de Sevaux 2001; DICAM Study 2010, Fangmann 2010; Ferguson 2006; Kreis 2003; Pascual 2003; REFERENCE Study 2006; Salvadori 2007); two investigated TAC (Chan 2012; MODIFY Study 2012); and OPTICEPT Study 2009 included either TAC or CsA. Of these, 12 studies involved introduction of low dose CNI regimen early in the post‐transplant period and six introduced low dose CNI later in the post‐transplant period (Cibrik 2007; DICAM Study 2010; Kreis 2003; MODIFY Study 2012; Pascual 2003; REFERENCE Study 2006).
Standard dose CNI versus CNI withdrawal or avoidance with mTOR‐I substitution
There were 29 studies (252 reports, 5012 participants) that compared standard dose CNI with CNI withdrawal or avoidance combined with mTOR‐I substitution (APOLLO Study 2015; Bansal 2013; Barsoum 2007; CALFREE Study 2010; CENTRAL Study 2012; CERTITEM Study 2015; Chhabra 2013; CONCEPT Study 2009; CONVERT Trial 2009; El‐Agroudy 2014; Flechner‐318 Study 2002; Grinyo 2004; Holm 2008; Martinez‐Mier 2006; Nafar 2012; ORION Study 2011; Pacheco‐Silva 2013; Pontrelli 2008; Rivelli 2015; RMR Study 2001; Rossini 2007; Schaefer 2006; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; Stallone 2003; Stallone 2004; Stegall 2003; Watson 2005; ZEUS Study 2011). Of these, nine compared CNI avoidance with mTOR‐I substitution versus conventional CNI regimen (CENTRAL Study 2012; Nafar 2012; Stegall 2003; Schaefer 2006; Barsoum 2007; CALFREE Study 2010; Flechner‐318 Study 2002; Martinez‐Mier 2006,SMART TX Study 2010). The rest looked at delayed CNI withdrawal with mTOR‐I substitution.
We included only five studies that investigated everolimus (APOLLO Study 2015; CENTRAL Study 2012; CERTITEM Study 2015; Pacheco‐Silva 2013; ZEUS Study 2011); the remainder investigated sirolimus. The CNI studied were:
-
TAC (eight studies: Chhabra 2013; El‐Agroudy 2014; Grinyo 2004; ORION Study 2011; Pacheco‐Silva 2013; Rivelli 2015; Schaefer 2006; Stegall 2003)
-
CsA (13 studies: Barsoum 2007; CALFREE Study 2010; CERTITEM Study 2015; CONCEPT Study 2009; Flechner‐318 Study 2002; Holm 2008; Martinez‐Mier 2006; CENTRAL Study 2012; Nafar 2012; RMR Study 2001; SMART TX Study 2010; Stallone 2003; ZEUS Study 2011)
-
TAC or CsA (seven studies: APOLLO Study 2015; Bansal 2013; CONVERT Trial 2009; Holm 2008; Spare‐the‐Nephron Study 2011; Rossini 2007; Stallone 2004; Watson 2005).
Standard dose CNI versus low dose CNI and mTOR‐I
We identified 14 studies (80 reports, 3110 participants) that compared standard dose CNI with combination of low dose CNI and mTOR‐I; (Bechstein‐193 2013; Bertoni 2007; Bertoni 2011; Chan 2008; Cockfield 2002; Muhlbacher 2014; Nashan 2004; Oh 2012; Paoletti 2012; Qazi 2014; Russ 2003; Takahashi 2013a; Tedesco‐Silva 2010; Velosa‐212 Study 2001). Interventions were administered immediately post‐transplant in all studies.
There were nine studies that investigated everolimus as the mTOR‐I (Bertoni 2007; Bertoni 2011; Chan 2008; Nashan 2004; Oh 2012; Paoletti 2012; Qazi 2014; Takahashi 2013a; Tedesco‐Silva 2010); the remainder looked at sirolimus. TAC (CNI) was studied in five studies (Bechstein‐193 2013; Chan 2008; Cockfield 2002; Qazi 2014; Russ 2003) and the rest of the studies used CsA.
Low versus normal dose CNI with or without mTOR‐I (mixed studies)
Five studies (83 reports, 3191 participants) had more than two arms and compared low dose versus normal dose CNI with or without mTOR‐I (ASCERTAIN Study 2011; CAESAR Study 2007; HERAKLES Study 2012; MECANO Study 2009; SYMPHONY Study 2007). Each were split to form two studies comparing low dose or withdrawal with or without mTOR‐I.
Reporting of outcomes was variable, and definitions of outcomes were unclear in most studies. Acute rejection episodes were reported as biopsy proven (56 studies) or unspecified/mixed (19 studies). Most reported graft loss or failure (68 studies) and GFR (52 studies). Methods used to determine GFR varied: 15 studies applied the Nankivell formula; 17 used Cockcroft‐Gault; 12 used MDRD; six used nuclear GFR (iothalamate or Cr EDTA); and four did not state the method used. CMV infection rates were reported in 30 studies and malignancy rates were reported in 29 studies.
Excluded studies
We excluded 72 studies following full text assessment: two studies included populations that did not match our inclusion criteria; 62 investigated interventions that were not relevant to this review; four measured outcomes not relevant to this review; two were incomplete studies that stopped early; one was only published as an abstract 35 years ago; and one study converted patients from TAC to sirolimus, however 40% we converted back to TAC. See Characteristics of excluded studies.
This review excluded studies involving Belatacept as the intervention assessed efficacy of the new biologic agent rather than CNI withdrawal. The Belatacept studies has been analysed and published recently (Mason 2014).
Risk of bias in included studies
Study methodology reporting was incomplete in most studies. Randomisation methods and allocation concealment were clearly described in fewer than 50% of studies. Most were open‐label studies. Intention‐to‐treat (ITT) analysis was either not reported or did not contain adequate information in 20% of studies to assess reporting bias. Seven studies did not report all possible outcomes due to early termination. Details are summarised below and in Figure 2.
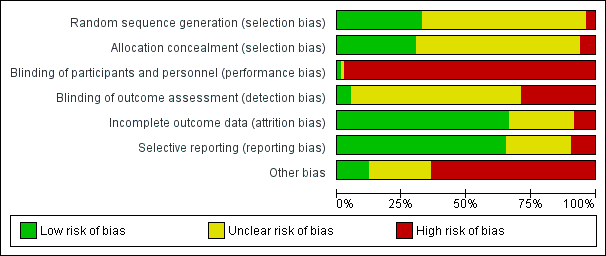
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Random sequence generation
Randomisation methods were reported in detail in 27 studies (APOLLO Study 2015; ASCERTAIN Study 2011; Bansal 2013; CAESAR Study 2007; Cai 2014; CENTRAL Study 2012; Chan 2012; Cibrik 2007; CONCEPT Study 2009; CONVERT Trial 2009; DICAM Study 2010; Dudley 2005; Fangmann 2010; Flechner‐318 Study 2002; Grinyo 2004; Hall 1988; MacPhee 1998; MECANO Study 2009; Paoletti 2012; REFERENCE Study 2006; Rivelli 2015; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; SYMPHONY Study 2007; Takahashi 2013a; Watson 2005; ZEUS Study 2011). Three studies were judged to be at high risk of bias; Pedersen 1991 randomised alternate participants to intervention and control groups, and Garcia 2007 and Schaefer 2006 included a third non‐randomised arm to the studies. The remaining 53 studies did not report randomisation methods.
Allocation concealment
Methods of allocation concealment were adequate in 25 studies (Abramowicz 2002; APOLLO Study 2015; Bansal 2013; CAESAR Study 2007; CENTRAL Study 2012; Chan 2008; Cibrik 2007; CONVERT Trial 2009; de Sevaux 2001; DICAM Study 2010; Dudley 2005; Fangmann 2010; Hall 1988; Isoniemi 1990; MacPhee 1998; MECANO Study 2009; Paoletti 2012; REFERENCE Study 2006; Smak Gregoor 1999; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; SYMPHONY Study 2007; Tedesco‐Silva 2010; Watson 2005; ZEUS Study 2011). Five studies were judged to be at high risk of bias (Barsoum 2007; Garcia 2007; Grinyo 2004; OPTICEPT Study 2009; Schaefer 2006) and the method of allocation concealment was not reported or unclear in 53 studies.
Blinding
Almost all studies were open‐label. Ferguson 2006 reported blinding of investigators and participants in one part of the study, and four studies (Cibrik 2007; DICAM Study 2010; Oh 2012; Rivelli 2015) reported blinding of outcome investigators.
Incomplete outcome data
Outcome data was reported or analysed as Intention‐ to‐treat in (ITT) in 55 studies (Abramowicz 2002; Andres 2009; APOLLO Study 2015; ASCERTAIN Study 2011; Bansal 2013; Barsoum 2007; Bertoni 2011; Budde 2007; CAESAR Study 2007; Cai 2014; CALFREE Study 2010; CENTRAL Study 2012; Chadban 2013; Chan 2008; Chhabra 2013; Cibrik 2007; CONCEPT Study 2009; CTOT‐09 Study 2015; de Sevaux 2001; DICAM Study 2010; El‐Agroudy 2014; Fangmann 2010; Ferguson 2006; Flechner‐318 Study 2002; Garcia 2007; Grimbert 2002; Hall 1988; Hazzan 2005; HERAKLES Study 2012; Hollander 1995; Isoniemi 1990; Kosch 2003a; MacPhee 1998; Martinez‐Mier 2006; MODIFY Study 2012; Oh 2012; Paoletti 2012; Pascual 2003; Pontrelli 2008; Qazi 2014; REFERENCE Study 2006; Rivelli 2015; RMR Study 2001; Salvadori 2007; Smak Gregoor 1999; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; Stallone 2003; Stegall 2003; Suwelack 2002; SYMPHONY Study 2007; Takahashi 2013a; Tedesco‐Silva 2010; Velosa‐212 Study 2001; Watson 2005; ZEUS Study 2011).
There was missing outcome data in seven studies (CENTRAL Study 2012; Cockfield 2002; Holm 2008; Heering 1993; Muhlbacher 2014; OPTICEPT Study 2009).
Attrition bias was judged to be unclear for the remaining 21 studies.
Selective reporting
There were 54 studies that reported prespecified outcomes (Abramowicz 2002; Andres 2009; APOLLO Study 2015; ASCERTAIN Study 2011; Bansal 2013; Barsoum 2007; Bertoni 2007; Bertoni 2011; Budde 2007; CAESAR Study 2007; Cai 2014; CENTRAL Study 2012; Chadban 2013; Chan 2008; Chan 2012; Chhabra 2013; Cibrik 2007; CONCEPT Study 2009; CONVERT Trial 2009; de Sevaux 2001; DICAM Study 2010; Dudley 2005; Fangmann 2010; Ferguson 2006; Flechner‐318 Study 2002; Garcia 2007; Grinyo 2004; Hall 1988; HERAKLES Study 2012; Isoniemi 1990; Kosch 2003a; MacPhee 1998; MODIFY Study 2012; Nashan 2004; Oh 2012; Pacheco‐Silva 2013; Pascual 2003; Pascual 2008; Qazi 2014; Pontrelli 2008; RMR Study 2001; Russ 2003; Salvadori 2007; Smak Gregoor 1999; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; Stallone 2003; Stallone 2004; Pontrelli 2008; Suwelack 2002; SYMPHONY Study 2007; Takahashi 2013a; Tedesco‐Silva 2010; Watson 2005; ZEUS Study 2011).
Eight studies were judged to be at high risk of reporting bias. Three studies did not report all possible outcomes due to early termination (CTOT‐09 Study 2015; MECANO Study 2009; ORION Study 2011). Cockfield 2002 and CERTITEM Study 2015 did not report all prespecified outcomes. Full‐text publications had not been identified for three studies 10 years after the abstracts were first published (Holm 2008; Rossini 2007; Salvadori 2007).
Twenty four studies had insufficient information to ascertain reporting bias.
Other potential sources of bias
Of the 83 included studies, 49 received pharmaceutical industry funding, which is a potential source for bias (Abramowicz 2002; Andres 2009; APOLLO Study 2015; Asberg 2006; ASCERTAIN Study 2011; Bansal 2013; Bechstein‐193 2013; Budde 2007; CAESAR Study 2007; Cai 2014; CALFREE Study 2010; CENTRAL Study 2012; CERTITEM Study 2015; Chadban 2013; Chan 2008; Chan 2012; Chhabra 2013; Cibrik 2007; CONCEPT Study 2009; CONVERT Trial 2009; de Sevaux 2001; Dudley 2005; Ferguson 2006; Flechner‐318 Study 2002; Grinyo 2004; Hall 1988; MECANO Study 2009; Muhlbacher 2014; Nashan 2004; Oh 2012; OPTICEPT Study 2009; ORION Study 2011; Pascual 2003; Pascual 2008; Qazi 2014; REFERENCE Study 2006; RMR Study 2001; Russ 2003; Smak Gregoor 1999; SMART TX Study 2010; Spare‐the‐Nephron Study 2011; Stegall 2003; Suwelack 2002; SYMPHONY Study 2007; Takahashi 2013a; Tedesco‐Silva 2010; Velosa‐212 Study 2001; Watson 2005; ZEUS Study 2011).
In two studies, one study arm was terminated due to increased rates of acute rejection (MECANO Study 2009; ORION Study 2011) and in Heering 1993 and CTOT‐09 Study 2015 the studies were stopped due to increased acute rejections in the CNI withdrawal group.
Garcia 2007 included a third group of non‐randomised patients after the interim analysis of randomised patients.
Only preliminary data were reported in Cockfield 2002 and Muhlbacher 2014.
There was a high drop‐out rate in four studies (Grinyo 2004; OPTICEPT Study 2009, Stegall 2003, Tedesco‐Silva 2010) which resulted in protocol amendment in Grinyo 2004.
Effects of interventions
See: Summary of findings for the main comparison Calcineurin inhibitor (CNI) withdrawal versus standard dose CNI for kidney transplant recipients; Summary of findings 2 Low dose calcineurin inhibitors (CNI) versus to standard dose CNI for kidney transplant recipients; Summary of findings 3 Calcineurin inhibitor (CNI) withdrawal + mammalian target of rapamycin inhibitor (mTORi) versus standard dose CNI for kidney transplant recipients; Summary of findings 4 Low dose CNI calcineurin inhibitor (CNI) + mammalian target of rapamycin inhibitor (mTORi) versus standard dose CNI for kidney transplant recipients; Summary of findings 5 Calcineurin inhibitor (CNI) avoidance and late CNI withdrawal versus standard dose CNI; Summary of findings 6 Calcineurin inhibitor (CNI) avoidance and late withdrawal with mammalian target of rapamycin inhibitor (mTORi) versus standard dose CNI
CNI withdrawal (avoidance or late withdrawal) versus standard dose CNI
There was little or no difference in patient death between CNI withdrawal and standard dose CNI regimens (Analysis 1.1 (14 studies 2010 participants): RR 1.09, 95% CI 0.96 to 1.24; I2 = 0%; moderate certainty evidence).
Acute rejection episodes were higher with CNI withdrawal whether diagnosed by biopsy or clinically (Analysis 1.2 (15 studies, 1666 participants): RR 2.54, 95% CI 1.56 to 4.12; I2 = 70%; moderate certainty). However GFR increased (Analysis 1.3 (8 studies, 910 participants): MD 3.56 mL/min, 95% CI ‐1.25 to 8.25; I2 = 66%; low certainty) and graft loss decreased (Analysis 1.4 (16 studies, 2090 participants): RR 0.85, 95% CI 0.74 to 0.98; I2 = 0%; low certainty) with CNI withdrawal.
There was 18% reduction in hypertension noted with CNI withdrawal (Analysis 1.6.1 (5 studies, 950 participants): RR 0.82, 95% CI 0.71 to 0.95; I2 = 36%; low certainty). There was no differences in incidences of hyperlipidaemia (Analysis 1.6.2 (3 studies, 562 participants): RR 0.88, 95% CI 0.63 to 1.21; I2 = 2%), CMV infection (Analysis 1.6.3 (7 studies, 608 participants): RR 0.87, 95% CI 0.52 to 1.45; I2 = 0%; low certainty), diabetes mellitus (Analysis 1.6.4 (6 studies, 810 participants): RR 0.85, 95% CI 0.94 to 1.42; I2 = 0%), malignancy (Analysis 1.6.5 (6 studies, 1079 participants): RR 1.10, 95% CI 0.936 to 1.30; I2 = 0%; low certainty), or total infections (Analysis 1.6.6 (6 studies, 724 participants): RR 0.96, 95% CI 0.61 to 1.51; I2 = 46%) between the groups.
Subgroup analyses
CNI avoidance versus standard dose CNI
There was more acute rejection episodes in CNI avoidance compared with standard dose CNI (Analysis 1.7.1 (3 studies, 238 participants): RR 2.16, 95% CI 0.85 to 5.49; I2 = 84%, low certainity). However, there was no difference in death (Analysis 1.1.1 (4 studies, 566 participants): RR 1.11, 95% CI 0.94 to 1.32; I2 = 0%), GFR (Analysis 1.8.1 (3 studies, 242 participant): MD ‐2.22 mL/min, 95% CI ‐14.84 to 10.40; I2 = 84%, very low certainity), and graft loss (Analysis 1.9.1 (4 studies, 566 participants): RR 0.96, 95% CI 0.79 to 1.16; I2 = 0%, low certainity).
Late withdrawal versus standard dose CNI
Analysis of late withdrawal studies indicated that there was no difference in death (Analysis 1.1.2 (10 studies, 1444 participants): RR 1.06, 95% CI 0.88 to 1.29; I2 = 0%), however acute rejection episodes were higher in CNI withdrawal group (Analysis 1.7.2 (12 studies, 1428 participants): RR 3.21, 95% CI 1.59 to 6.48; I2 = 66%, moderate certainity). GFR was higher (Analysis 1.8.2 (5 studies, 668 participants): MD 5.54 mL/min, 95% CI 1.66 to 9.43; I2 = 29%, low certainity) and there was less graft loss (Analysis 1.9.2 (13 studies, 1848 participants): RR 0.84, 95% CI 0.72 to 0.97; I2 = 0%, low certainity) in the CNI withdrawal group.
Type of antimetabolite (MMF/MPA or AZA)
Subgroup analysis on antimetabolites found a higher acute rejection episodes associated with CNI withdrawal compared with standard dose CNI in the MMF/MPA studies (Analysis 2.1.1 (10 studies, 1110 participants): RR 3.51, 95% CI 1.79 to 6.88; I2 = 65%) but not in AZA studies (Analysis 2.1.2 (5 studies, 556 participants): RR 1.81, 95% CI 0.78 to 4.19; I2 = 72%).
Type of CNI (CsA or TAC)
When classified by CNI type, acute rejection episodes increased in the withdrawal arm of CsA studies (Analysis 3.1.1 (11 studies, 1500 participants): RR 2.13, 95% CI 1.31 to 3.48; I2 = 71%), TAC (Analysis 3.1.2 (2 studies, 88 participants): RR 5.65, 95% CI 1.96 to 16.27; I2 = 0%), and in studies that investigated either CsA or TAC (Analysis 3.1.3 (2 studies, 78 participants): RR 9.00, 95% CI 0.52 to 156.9) compared with standard dose CNI.
Sensitivity analyses
On sensitivity analyses stratified for steroid‐free regimens the effects were not different from steroid regimens for death, acute rejection and GFR. When stratified for time of follow‐up, the reduction in graft loss observed in the CNI withdrawal group was not significant when the long‐term studies were excluded in the analysis (RR 1.07, 95% CI 0.72 to 1.57; forest plot not shown).
Low dose CNI versus standard dose CNI
There was little or no difference in patient death between low dose and standard dose CNI regimens (Analysis 4.1 (15 studies, 3462 participants): RR 0.79, 95% CI 0.50 to 1.27; I2 = 0%; moderate certainty).
There was a lower incidence of acute rejection (Analysis 4.2 (19 studies, 3757 participants): RR 0.87, 95% CI 0.76 to 1.00; I2 = 0%; moderate certainty) and graft loss (Analysis 4.4 (15 studies, 3286 participants): RR 0.75, 95% CI 0.55 to 1.02; I2 = 0%; moderate certainty) in the low dose CNI group.
Patients treated with low dose CNI had higher GFR (Analysis 4.3 (13 studies, 2623 participants): MD 4.10, 95% CI 2.07 to 6.12; I2 = 16%; moderate certainty). Low dose CNI regimen probably slightly lowers SCr (Analysis 4.5 (6 studies, 742 participants): MD ‐4.28 µmol/L, 95% CI ‐14.65 to 6.10; I2 = 37%; low certainty).
Hypertension was probably reduced (Analysis 4.7.1 (5 studies, 1877 participants): RR 0.84, 95% CI 0.70 to 1.00; I2 = 0%; low certainty) in the low dose CNI group. There was no difference in hyperlipidaemia (Analysis 4.7.2 (3 studies, 1443 participants): RR 1.04, 95% CI 0.90 to 1.19; I2 = 12%), CMV infection (Analysis 4.7.3 (6 studies, 1948 participants): RR 1.23, 95% CI 0.94 to 1.62; I2 = 10%; moderate certainty), diabetes mellitus (Analysis 4.7.4 (5 studies, 1292 participants): RR 0.82, 95% CI 0.50 to 1.34; I2 = 53%), malignancy (Analysis 4.7.5 (5 studies, 1637 participants): RR 0.90, 95% CI 0.41 to 1.97; I2 = 0%; low certainty), and total infections (Analysis 4.7.6 (9 studies, 1437 participants): RR 0.95, 95% CI 0.84 to 1.07; I2 = 0%).
Subgroup analyses
Low dose CNI immediately post‐transplant versus standard dose CNI
For studies which compared low dose CNI immediately post‐transplant with standard dose CNI regimens, there were less acute rejection episodes (Analysis 4.8.1 (12 studies, 2209 participants): RR 0.82, 95% CI 0.67 to 1.00; I2 = 0%) and graft loss (Analysis 4.10.1 (11 studies, 2800 participants): RR 0.75, 95% CI 0.55 to 1.03; I2 = 0%), and GFR improved (Analysis 4.9.1 (9 studies, 2200 participants): MD 3.09 mL/min, 95% CI 0.95 to 5.23; I2 = 4%) with the low dose regimen.
Late intervention with low dose CNI versus standard dose CNI
For studies which compared late intervention with low dose CNI, there was no difference acute rejection (Analysis 4.8.2 (6 studies, 759 participants): RR 1.05, 95% CI 0.61 to 1.81; I2 = 21%) or graft loss (Analysis 4.10.2 (3 studies, 306 participants): RR 0.95, 95% CI 0.12 to 7.56; I2 = 0%) however GFR was higher (Analysis 4.9.2 (3 studies, 243 participants): MD 8.81 mL/min, 95% CI 3.79 to 13.83; I2 = 0%).
Type of CNI (CsA or TAC)
When studies were classified on the type of CNI, there was less acute rejection in the low dose CsA (Analysis 5.1.1 (16 studies, 2906 participants): RR 0.87, 95% CI 0.76 to 1.01; I2 = 0%) compared to standard dose CsA but the results were not significant for low dose TAC (Analysis 5.1.2 (2 studies, 371 participants): RR 1.53, 95% CI 0.61 to 3.83; I2 = 0%) and for studies which used either CsA or TAC (Analysis 5.1.3 (1 study, 480 participants): RR 0.64, 95% CI 0.34 to 1.19).
Sensitivity analysis
When stratified for steroid‐free regimens, the reduction in graft loss was significant when the study using a steroid‐free regimen was excluded from the analysis (RR 0.72, 95% CI 0.52 to 0.98; forest plot not shown).
When stratified for induction treatment with IL2RA or anti‐lymphocyte serum or globulin, the incidence of acute rejection was similar between the groups (12 studies: RR 0.84, 95% CI 0.66 to 1.07; forest plot not shown).
CNI withdrawal (avoidance or withdrawal) with mTOR‐I substitution versus standard dose CNI
There was little or no difference in death (Analysis 6.1 (23 studies, 5427 participants): RR 0.96, 95% CI 0.68 to 1.36; I2 = 0%; moderate certainty) and graft loss (Analysis 6.4 (25 studies, 5446 participants): RR 0.94, 95% CI 0.75 to 1.19; I2 = 0%; low certainty) between the CNI withdrawal with mTOR‐I and standard dose CNI regimens.
There was an increase in acute rejection episodes (Analysis 6.2 (30 studies, 5903 participants): RR 1.43, 95% CI 1.15 to 1.78; I2 = 52%; moderate certainty) in the mTOR‐I group. Patients in the CNI withdrawal with mTOR‐I group had a higher GFR compared to standard dose CNI regimen (Analysis 6.3 (23 studies, 4427 participants): MD 5.29, 95% CI 2.08 to 8.51; I2 = 90%). SCr was lower at one year in the CNI withdrawal with mTOR‐I group (Analysis 6.5 (12 studies, 1702 participants): MD ‐17.10 µmol/L, 95% CI ‐26.95 to ‐7.25; I2 = 76%).
CNI withdrawal with mTOR‐I group had a higher incidence of hyperlipidaemia (Analysis 6.7.2 (13 studies 3494 participants): RR 1.76, 95% CI 1.40 to 2.20; I2 = 49%). There was little or no difference in hypertension Analysis 6.7.1 (7 studies, 2207 participants): RR 0.86, 95% CI 0.64 to 1.15; I2 = 79%), diabetes mellitus (Analysis 6.7.4 (11 studies, 2833 participants): RR 1.27, 95% CI 0.97 to 1.66; I2 = 0%), and infections (Analysis 6.7.6 (9 studies, 1624 participants): RR 0.99, 95% CI 0.92 to 1.07; I2 = 0%) between the two groups. There was a reduction in malignancy (Analysis 6.7.5 (14 studies, 3699 participants): RR 0.69, 95% CI 0.47 to 1.00; I2 = 19%; low certainty) and CMV infection (Analysis 6.7.3 (13 studies, 2503 participants): RR 0.60, 95% CI 0.44 to 0.82; I2 = 43%; moderate certainty) in the mTOR‐I group compared to those treated with standard dose CNI regimen. There was an increase in lymphoceles in the CNI withdrawal, mTOR‐I group (Analysis 6.7.7 (8 studies, 1926 participants): RR 1.45, 95% CI 0.95, 2.21; I2 = 56%; low certainty).
Subgroup analysis
CNI avoidance with mTOR‐I substitution versus standard dose CNI
There was an increase acute rejection episodes (Analysis 6.8.1 (11 studies, 1844 participants): RR 1.27, 95% CI 0.98 to 1.65; I2 = 31%), while GFR was better (Analysis 6.9.1 (9 studies, 1748 participants): MD 6.45 mL/min, 95% CI 1.33 to 11.58; I2 = 86%) in the CNI avoidance with mTOR‐I regimen. Graft loss (Analysis 6.10.1 (8 studies, 1420 participants): RR 1.03, 95% CI 0.72 to 1.48; I2 = 0%) was similar in the two groups.
Late CNI withdrawal with mTOR‐I substitution versus standard dose CNI
Acute rejection episodes were higher in the late CNI withdrawal with mTOR‐I substitution group (Analysis 6.8.2 (17 studies, 3636 participants): RR 1.90, 95% CI 1.44 to 2.51; I2 = 23%). GFR was not significantly higher (Analysis 6.9.2 (14 studies, 2679 participants): MD 4.55 mL/min, 95% CI 0.26 to 8.85; I2 = 92%) and there was no difference in graft loss (Analysis 6.10.2 (17 studies, 4026 participants): RR 0.92, 95% CI 0.65 to 1.30; I2 = 13%) in the late CNI withdrawal with mTOR‐I group.
Type of CNI (CsA or TAC)
There were more acute rejection episodes in the late CNI withdrawal with mTOR‐I group compared to standard dose CsA (Analysis 7.1.1 (18 studies, 5903 participants): RR 1.42, 95% CI 1.15 to 1.76; I2 = 37%) and standard dose TAC (Analysis 7.1.2 (7 studies, 753 participants): RR 2.23, 95% CI 1.43 to 3.49; I2 = 15%), however in studies which used either CsA or TAC (Analysis 7.1.3 (5 studies, 1687 participants): RR 0.97, 95% CI 0.40 to 2.33; I2 = 64%) there were no differences in acute rejection episodes.
Sensitivity analyses
On sensitivity analyses stratified for steroid‐free regimens the effects were not different from steroid regimens for death, acute rejection, and GFR.
Low dose CNI with mTOR‐I versus standard dose CNI
There was little or no difference in patient deaths (Analysis 8.1 (11 studies, 2750 participants): RR 1.16, 95% CI 0.71 to 1.90; I2 = 0%; moderate certainty), acute rejection episodes (Analysis 8.2 (16 studies, 3300 participants): RR 1.13, 95% CI 0.91 to 1.40; I2 = 22%; moderate certainty), and graft loss (Analysis 8.4 (16 studies, 3304 participants): RR 0.67, 95% CI 0.45 to 1.01; I2 = 0%; moderate certainty) when low dose CNI with mTOR‐I was compared to standard dose CNI.
Patients treated with low dose CNI in combination with mTOR‐I had a higher GFR compared with standard dose CNI regimens (Analysis 8.3 (11 studies, 1749 participants): MD 6.24 mL/min, 95% CI 3.28 to 9.19; I2 = 56%; moderate certainty), and a lower SCr at one year (Analysis 8.5 (6 studies, 1320 participants): MD ‐14.14 µmol/L, 95% CI ‐22.55 to ‐5.72; I2 = 17%).
Hypertension (Analysis 8.7.1 (5 studies, 1421 participants): RR 0.98, 95% CI 0.80 to 1.20; I2 = 0%, low certainity), hyperlipidaemia (Analysis 8.7.2 (8 studies, 1793 participants): RR 1.07, 95% CI 0.89 to 1.28; I2 = 30%), and diabetes mellitus (Analysis 8.7.4 (5 studies, 686 participants): RR 1.36, 95% CI 0.81 to 2.27; I2 = 0%) were noted to be similar in patients treated with either low dose CNI in combination with mTOR‐I or standard dose CNI regimens. There was no reduction in malignancy in the low CNI in combination with mTOR‐I group compared to those treated with standard dose CNI regimens (Analysis 8.7.5 (5 studies, 1074 participants): RR 1.22, 95% CI 0.42 to 3.52; I2 = 0%, low certainity). There was little or no difference in total Infections (Analysis 8.7.6 (5 studies, 1271 participants): RR 0.95, 95% CI 0.83 to 1.08; I2 = 28%) and CMV infection (Analysis 8.7.3 (5 studies, 1250 participants): RR 0.41, 95% CI 0.16 to 1.06; I2 = 74%; low certainty) between the two groups.
Subgroup analysis
CNI and mTOR‐I combination with standard dose CNI regimen in the immediate post‐transplant period
GFR was higher in the low dose CNI with mTOR‐I group (Analysis 8.9.1 (10 studies, 1537 participants): MD 6.91 mL/min, 95% CI 3.86 to 9.96; I2 = 53%), however acute rejection (Analysis 8.10.1 (14 studies, 2736 participants): RR 1.09, 95% CI 0.86 to 1.39; I2 = 27%) and graft loss (Analysis 8.8.1 (14 studies, 2736 participants): RR 0.75, 95% CI 0.48 to 1.18; I2 = 0%) were similar in the two groups.
Late introduction of low dose CNI regimen with mTOR‐I substitution
Incidence of acute rejection was higher in the low dose CNI with mTOR‐I group (Analysis 8.10.2 (2 studies, 564 participants): RR 1.38, 95% CI 0.82 to 2.31; I2 = 0%), there was no difference in graft loss (Analysis 8.8.2 (2 studies, 568 participants): RR 0.40, 95% CI 0.15 to 1.04; I2 = 0%) and one study reported no difference in GFR in the late withdrawal group (Analysis 8.9.2 (1 study, 212 participants): MD 0.58 mL/min, 95% CI ‐5.00 to 6.16).
Type of CNI (CsA or TAC)
There was no difference in acute rejection in the low dose CsA with mTOR‐I compared to standard dose CsA (Analysis 9.1.1 (11 studies, 2232 participants): RR 0.97, 95% CI 0.78 to 1.22; I2 = 7%), however acute rejection was higher when low dose TAC with mTOR‐I was compared to standard dose TAC (Analysis 9.1.2 (5 studies, 1068 participants): RR 1.58, 95% CI 1.16 to 2.13; I2 = 0%).
Sensitivity analysis
On sensitivity analyses stratified for steroid free regimens the effects were not different from steroid regimens for death, acute rejection, or GFR.
Discussion
Summary of main results
This review describes CNI withdrawal or tapering classified according to: CNI withdrawal, low dose CNI, CNI withdrawal with mTOR‐I substitution and low dose CNI with mTOR‐I compared to standard dose CNI regimens. The four groups were further stratified into CNI avoidance and withdrawal studies for major outcomes.
In the CNI withdrawal comparison with standard regimens, there was an increase in both clinical acute rejection and BPAR. GFR was higher in the withdrawal group especially over longer time periods. Death, diabetes mellitus, hyperlipidaemia, total and CMV infections were not significantly different between the groups. Standard dose CNI regimens were more likely to be associated with hypertension when compared to CNI withdrawal patients. Graft loss was lower in the CNI withdrawal group; however, when stratified for avoidance studies, there was no difference in graft loss between the groups. These protocols (late withdrawal or avoidance) resulted in an increase in acute rejection with no clear benefit in terms of reduced graft loss. There was also no difference in the type of CNI (TAC or CsA) used or steroid‐free regimens in causing acute rejection. The beneficial effects of CNI withdrawal in reducing graft loss were lost when studies with long‐term outcomes were excluded.
In the low dose CNI comparison with standard dose regimens, there was a reduction in acute rejection, however when studies which administered induction treatment (IL2RA or anti‐lymphocyte serum or globulin) were excluded from the analysis, acute rejection was similar in the low dose CNI and standard dose CNI regimens, both in the immediate and late introduction groups. GFR was higher in the low dose CNI group at both one and five years. There were no significant differences in death, diabetes mellitus, hyperlipidaemia, and CMV infection between the groups. Low dose CNI regimens had a marginal reduction in hypertension and total infections. Graft loss was reduced in the low dose CNI regimen, however when stratified for early and late intervention (taper), the effect was limited to the early intervention studies.
In the CNI avoidance or tapering with mTOR‐I substitution compared to standard dose CNI regimens, there was no difference in death between the two groups. The mTOR‐I substitution regimen however had more acute rejections (clinical and biopsy‐proven) and had more hyperlipidaemia. CMV infection and malignancy were significantly lower in the mTOR‐I substitution group. GFR was higher in the CNI avoidance with mTOR‐I subgroup but not in the late intervention subgroup. There was no difference in other outcomes when stratified for early or late intervention. Overall these protocols (avoidance or tapering) showed no major change compared to CNI alone except for the increase in acute rejection when compared with either CNI (CsA or TAC). The major benefit of mTOR‐I substitution is seen in the reduction in malignancies and CMV infections over time.
When low dose CNI was combined with mTOR‐I and compared to standard dose CNI regimens, there were no differences in death, graft loss or acute rejection. Adverse events including malignancy were not significantly different between the groups. GFR and SCr at one year favoured the low dose CNI with mTOR‐I regimen. However when stratified for early and late intervention there was increased acute rejection in the low dose CNI with mTOR‐I regimens.
This review investigated a large number of studies comparing different CNI regimens. Many studies and reports were published in multiple journals at various time points and were presented as abstracts at scientific meetings without acknowledging previous publications. The same studies were also published under different authors and this review combined these reports under a single study and reported outcomes systematically. The methodology was robust and the studies were also assessed for study quality and heterogeneity explored by subgroup and stratified analysis. The review classified interventions into four groups which reduced multiple comparisons due to several different regimens.
Overall completeness and applicability of evidence
Short time scales of most studies restrict the external validity of this review. Moving away from CNI may have multiple adverse long‐term effects that will not be measured by these studies. The studies also do not mention of antibody‐mediated rejection and pretransplant donor specific antibodies which could impact on short‐ and long‐term graft survival. Removal of CNI may remove one long‐term problem (CNI toxicity) but potentially cause worsening of other immunological issues which may in turn limit the duration of the graft. Low dose CNI seem the best option and mTOR‐I benefits appear to be limited to a reduction in the risks of malignancy and CMV infection, though these benefits are uncertain and are not the case when combined with CNI.
Quality of the evidence
The overall quality of the evidence was poor, with unclear risks of bias due to poor reporting (Figure 2); only 30% reported randomisation method and allocation concealment. Almost all studies were open‐label however for study outcomes such as death and graft loss they were not downgraded on GRADE assessment. Studies were analysed as intent to treat in 60% and all pre specified outcomes were reported in 54 studies. Almost half the studies received pharmaceutical funding which were classified as a high risk of bias.
The studies also used variable outcome measures and induction immunosuppression regimens. There is also variability in dosing, drug monitoring and time intervals of reporting outcomes. Most studies did not indicate baseline SCr or GFR to assess for changes due to the intervention. The follow‐up duration in majority of the included studies was between six months and three years which is a major limitation for concluding long‐term outcomes such as patient and graft survival.
Potential biases in the review process
There are multiple limitations of this review. The quality of data reporting was variable in terms of outcome and adverse effects. Most studies did not indicate the baseline creatinine or GFR to assess for changes due to the intervention. The standard deviation or confidence intervals were not noted when recording outcomes such as GFR and creatinine. Adverse effects were prevalent rather than incident cases which may affect outcomes such as diabetes mellitus, hyperlipidaemia and hypertension. The number of patients affected by individual outcomes were not indicated but mentioned as being significant with or without P values. Outcome reporting was not defined in cases of CMV, hypertension, hyperlipidaemia (total or low‐density lipoprotein) or diabetes mellitus. Different studies used different targets for CNI monitoring and also used either trough (C0) or two hour (C2) levels; some studies based the dose on mg/kg body weight and this review used the study author definitions to classify low dose and standard dose regimens. This may have some limitation in external validity of these recommendations. However we have tried to minimise this by subclassification into four groups and analyse them further into early and late interventions. Most studies were short‐term and did not capture long‐term hard outcomes such as graft survival, patient survival or adverse effects (such as cardiovascular outcomes) and malignancy. The duration of the majority of studies was between six months and three years with only three studies of up to five years duration. This raises the concern of how outcomes might be different after that time, particularly with regards to antibody‐mediated rejection which can be a complication of reduced immune suppression. The only studies that included more than 10 years of follow‐up tended to be much older studies, and compared immunosuppression such as azathioprine which is now largely obsolete or in very little use. The data from these studies is therefore limited by era effect. Studies with longer follow‐up are required to confirm the potential benefits of CNI reduction or risks of long‐term antibody‐mediated rejection, most studies also do not differentiate between patients with high versus low immunological risk.
Agreements and disagreements with other studies or reviews
This is the first review which sub classified studies into four different intervention groups and analysed them as low dose calcineurin inhibitor or CNI withdrawal with or without mTOR‐I substitution. The classification analysed the possible advantages noted in various studies with additional immunosuppressive agent such as mTOR‐I or continuation of CNI at a low dose.
Sharif 2011 (56 studies, 11,337 participants) showed a similar increase in acute rejection without affecting graft survival, infection, and patient survival, it also concluded an increase in graft failure when mTOR‐I was used. The review however did not classify studies into low dose or withdrawal as in our review but performed a pooled analysis which resulted in significant heterogeneity. In contrast to the conclusions of this review, Sharif 2011 reported lower NODAT in the CNI‐sparing group. Moore 2009 included only CNI‐sparing with MMF. The results were not stratified for mTOR‐I; however the studies were classified into those who had de novo CNI minimisation and elective minimisation or elimination of CNI. The results in the withdrawal group were similar to our review but the lower dose of CNI was not beneficial in reduction of acute rejection as we report. A systematic review by Lim 2014 (29 studies, 2350 participants) analysed conversion to an mTOR‐I based immunosuppression from CNI based therapy. They review reported short‐term improvements in GFR with mTOR‐I but increased acute rejections; there were no differences in graft loss or death. The conclusions of Lim 2014 are similar to our analysis of CNI withdrawal with mTOR‐I, however our review also analysed low dose CNI and mTOR‐I substitution.

Flow chart showing number of studies identified

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 1 Death.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 2 Acute rejection.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 3 GFR.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 4 Graft loss.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 5 Serum creatinine.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 6 Adverse events.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 7 Subgroup analysis: acute rejection.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 8 Subgroup analysis: GFR.

Comparison 1 CNI withdrawal versus standard dose CNI, Outcome 9 Subgroup analysis: graft loss.

Comparison 2 Subgroup analysis (antimetabolite): CNI withdrawal versus standard dose CNI, Outcome 1 Acute rejection.

Comparison 3 Subgroup analysis (CNI type): CNI withdrawal versus standard dose CNI, Outcome 1 Acute rejection.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 1 Death.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 2 Acute rejection.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 3 GFR.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 4 Graft loss.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 5 Serum creatinine.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 6 Change in GFR at 12 months.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 7 Adverse events.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 8 Subgroup analysis: acute rejection.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 9 Subgroup analysis: GFR.

Comparison 4 Low dose CNI versus standard dose CNI, Outcome 10 Subgroup analysis: graft loss.

Comparison 5 Subgroup analysis (CNI type): low dose CNI versus standard dose CNI, Outcome 1 Acute rejection.

Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 1 Death.

Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 2 Acute rejection.

Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 3 GFR.

Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 4 Graft loss.

Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 5 Serum creatinine at 1 year.

Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 6 Change in GFR.

Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 7 Adverse events.

Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 8 Subgroup analysis: acute rejection.

Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 9 Subgroup analysis: GFR.

Comparison 6 CNI withdrawal or avoidance + mTOR‐I versus standard dose CNI, Outcome 10 Subgroup analysis: graft loss.

Comparison 7 Subgroup analysis (CNI type): CNI withdrawal + mTOR‐I versus standard dose CNI, Outcome 1 Acute rejection.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 1 Death.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 2 Acute rejection.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 3 GFR.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 4 Graft loss.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 5 Serum creatinine at 1 year.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 6 Change in GFR at 2 years.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 7 Adverse events.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 8 Subgroup analysis: graft loss.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 9 Subgroup analysis: GFR.

Comparison 8 Low dose CNI + mTOR‐I versus CNI, Outcome 10 Subgroup analysis: acute rejection.

Comparison 9 Subgroup analysis (CNI type): low dose CNI + mTOR‐I versus standard dose CNI, Outcome 1 Acute rejection.
| CNI withdrawal versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard dose CNI | Risk with CNI withdrawal | ||||
| Death | Study population | RR 1.09 | 2010 (14 ) | ⊕⊕⊕⊝ | |
| 225 per 1,000 | 245 per 1,000 | ||||
| Acute rejection | Study population | RR 2.54 | 1666 (15) | ⊕⊕⊕⊝ | |
| 137 per 1,000 | 348 per 1,000 | ||||
| GFR | The mean GFR in the intervention group was 3.56 mL/min more (1.13 less to 8.25 more) than the control group | ‐ | 910 (8) | ⊕⊕⊝⊝ | |
| Graft loss | Study population | RR 0.85 | 2090 (16) | ⊕⊕⊝⊝ | |
| 236 per 1,000 | 201 per 1,000 | ||||
| Adverse events: hypertension | Study population | RR 0.82 | 950 (5 ) | ⊕⊕⊝⊝ | |
| 555 per 1,000 | 455 per 1,000 | ||||
| Adverse events: CMV infection | Study population | RR 0.87 | 608 (7) | ⊕⊕⊝⊝ | |
| 98 per 1,000 | 86 per 1,000 | ||||
| Adverse events: malignancy | Study population | RR 1.10 | 1079 (6) | ⊕⊕⊝⊝ | |
| 257 per 1,000 | 282 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 despite different follow up times, heterogeneity not noted on analysis 2 Most studies were ITT analysis, some small studies did not specify randomisation and allocation concealment 3 Larger studies closer to pooled estimate on funnel plot 4 Some studies were small with large confidence intervals, CI fails to exclude benefit or harm 5 Heterogeneity low when biopsy‐proven rejections were analysed in subgroup 6 Smaller studies not distributed around point estimate 7 Significant heterogeneity noted despite separating time periods of reporting GFR 8 Only few studies reported GFR with possible attrition bias 9 2 large studies had more than 2 comparison groups 10 Very few studies reported the outcome 11 Symmetric distribution studies around estimate of effect 12 2 studies with high event rates skew the effect | |||||
| Low dose CNI versus standard dose CNI for kidney transplant recipients | ||||||
| Patient or population: kidney transplant recipients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with standard dose CNI | Risk with low dose CNI | |||||
| Death | Study population | RR 0.79 | 3462 (15) | ⊕⊕⊕⊝ | ||
| 23 per 1,000 | 19 per 1,000 | |||||
| Acute rejection | Study population | RR 0.87 | 3757 (19) | ⊕⊕⊕⊝ | ||
| 183 per 1,000 | 159 per 1,000 | |||||
| GFR | The mean GFR in the intervention group was 4.1 mL/min more (2.07 more to 6.12 more) than the control group | ‐ | 2623 (13) | ⊕⊕⊕⊝ | ||
| Graft loss | Study population | RR 0.75 | 3286 (15) | ⊕⊕⊕⊝ | Sensitivity analysis after excluding 1 study which also involved steroid withdrawal; significant reduction in graft loss in the low dose regimen | |
| 58 per 1,000 | 44 per 1,000 | |||||
| Adverse events: hypertension | Study population | RR 0.84 | 1877 (5) | ⊕⊕⊝⊝ | ||
| 218 per 1,000 | 184 per 1,000 | |||||
| Adverse events: CMV infection | Study population | RR 1.23 | 1948 (6) | ⊕⊕⊕⊝ | ||
| 101 per 1,000 | 124 per 1,000 | |||||
| Adverse events: malignancy | Study population | RR 0.90 | 1637 (5) | ⊕⊕⊝⊝ | ||
| 15 per 1,000 | 14 per 1,000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies with ITT analysis, randomisation procedure and allocation concealment not clear from most publications 2 Minimal heterogeneity noted on analysis 3 Several small studies with wide confidence intervals 4 Despite studies with or without induction, sensitivity analysis made no difference to outcome 5 Heterogeneity noted only between subgroups 6 Only 2/15 studies had more than 2 comparison groups 7 Industry sponsored 8 1/6 studies did not report some outcomes due to high dropout 9 Only 5 studies reported the outcome and had wide CI 10 Few studies reported the outcome | ||||||
| CNI withdrawal + mTORi versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard dose CNI | Risk with CNI withdrawal + mTOR | ||||
| Death | Study population | RR 0.99 | 5427 (23) | ⊕⊕⊕⊝ | |
| 26 per 1,000 | 26 per 1,000 | ||||
| Acute rejection | Study population | RR 1.43 | 5903 (30) | ⊕⊕⊕⊝ | |
| 134 per 1,000 | 191 per 1,000 | ||||
| Graft loss | Study population | RR 0.94 | 5446 (25) | ⊕⊕⊝⊝ | |
| 53 per 1,000 | 50 per 1,000 | ||||
| Adverse events: hypertension | Study population | RR 0.86 | 2207 (7) | ⊕⊕⊝⊝ | |
| 218 per 1,000 | 187 per 1,000 | ||||
| Adverse events: CMV Infection | Study population | RR 0.60 | 2503 (13) | ⊕⊕⊕⊝ | |
| 150 per 1,000 | 90 per 1,000 | ||||
| Adverse events: malignancy | Study population | RR 0.69 | 3699 (14) | ⊕⊕⊝⊝ | |
| 54 per 1,000 | 38 per 1,000 | ||||
| Adverse events: lymphocele | Study population | RR 1.45 | 1926 (8) | ⊕⊕⊝⊝ | |
| 100 per 1,000 | 144 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Randomisation method and allocation concealment performed in most studies 2 No significant heterogeneity noted in analysis 3 Only 2 studies had more than 2 comparison arms 4 Many studies with small events and wide CI 5 Significant heterogeneity in studies in biopsy‐proven acute rejection 6 Funnel plot skewed 7 Significant heterogeneity noted 8 Few studies reported this outcome 9 Moderate heterogeneity but follow‐up times are variable 10 Not all studies reported the outcome 11 Heterogeneity is not significant when 1 long‐term study was excluded | |||||
| Low dose CNI + mTORi versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard dose CNI | Risk with low dose CNI + mTORi | ||||
| Death | Study population | RR 1.16 | 2750 (11) | ⊕⊕⊕⊝ | |
| 22 per 1,000 | 26 per 1,000 | ||||
| Acute rejection | Study population | RR 1.13 | 3300 (16) | ⊕⊕⊕⊝ | |
| 132 per 1,000 | 149 per 1,000 | ||||
| GFR | The mean GFR in the intervention group was 6.24 mL/min more (3.28 more to 9.19 more) than the control group | ‐ | 1749 (11) | ⊕⊕⊕⊝ | |
| Graft loss | Study population | RR 0.67 | 3304 (16) | ⊕⊕⊕⊝ | |
| 38 per 1,000 | 25 per 1,000 | ||||
| Adverse events: hypertension | Study population | RR 0.98 | 1421 (5) | ⊕⊕⊝⊝ | |
| 203 per 1,000 | 199 per 1,000 | ||||
| Adverse events: CMV infection | Study population | RR 0.41 | 1250 (5) | ⊕⊕⊝⊝ | |
| 105 per 1,000 | 43 per 1,000 | ||||
| Adverse events: malignancy | Study population | RR 1.22 | 1074 (5) | ⊕⊕⊝⊝ | |
| 11 per 1,000 | 14 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Randomisation and allocation process not clear in some studies 2 No significant heterogeneity 3 Only 2 of the studies had more than 2 comparisons 4 Some small studies with wide CI 5 Substantial heterogeneity noted due to recording at different time periods 6 Small number of events and some small studies with wide CI 7 Only few studies reported this outcome 8 95% CI fails to exclude benefit or harm 9 Heterogeneity present but when abstract only studies are removed, heterogeneity is zero | |||||
| Subgroup analysis: CNI avoidance and late withdrawal versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard dose CNI | Risk with CNI avoidance and withdrawal | ||||
| Acute rejection: avoidance | Study population | RR 2.16 | 238 (3) | ⊕⊕⊝⊝ | |
| 344 per 1,000 | 744 per 1,000 | ||||
| Acute rejection: late withdrawal | Study population | RR 3.21 | 1428 (12) | ⊕⊕⊕⊝ | |
| 102 per 1,000 | 328 per 1,000 | ||||
| GFR: avoidance | The mean GFR for avoidance studies in the intervention group was 2.22 mL/min lower (14.84 less to 10.4 more) than the control group | ‐ | 242 (3) | ⊕⊝⊝⊝ | |
| GFR: late withdrawal | The mean GFR for late withdrawal studies in the intervention group was 5.54 mL/min more (1.66 more to 9.43 more) than the control group | ‐ | 668 (5) | ⊕⊕⊝⊝ | |
| Graft loss: avoidance | Study population | RR 0.96 | 566 (4) | ⊕⊕⊝⊝ | |
| 355 per 1,000 | 341 per 1,000 | ||||
| Graft loss: late withdrawal | Study population | RR 0.84 | 1831 (13) | ⊕⊕⊕⊝ | |
| 260 per 1,000 | 219 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 3 small studies with one study including a non‐randomised arm 2 Significant heterogeneity 3 Several small studies with wide confidence intervals 4 Small numbers to make a judgement of difference 5 Skewed funnel plot 6 Substantial heterogeneity 7 2/4 are small studies with wide CI 8 4 studies included with one study with high event rate 9 No heterogeneity identified on analysis 10 Larger studies are not industry sponsored | |||||
| Subgroup analysis: CNI avoidance and late withdrawal + mTORi versus standard dose CNI for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard dose CNI | Risk with CNI avoidance and withdrawal + mTORi | ||||
| Acute rejection: avoidance | Study population | RR 1.27 | 1844 (11) | ⊕⊕⊕⊝ | |
| 234 per 1,000 | 297 per 1,000 | ||||
| Acute rejection: late withdrawal | Study population | RR 1.90 | 3636 (17) | ⊕⊕⊕⊝ | |
| 65 per 1,000 | 124 per 1,000 | ||||
| GFR: avoidance | The mean GFR for avoidance studies in the intervention group was 6.45 mL/min higher (1.33 higher to 11.58 higher) than the control group | ‐ | 1748 (9) | ⊕⊕⊝⊝ | |
| GFR: late withdrawal | The mean GFR for late withdrawal studies in the intervention group was MD 4.55 higher | ‐ | 2679 (14) | ⊕⊕⊝⊝ | |
| Graft loss: avoidance | Study population | RR 1.03 | 1420 (8) | ⊕⊕⊕⊝ | |
| 74 per 1,000 | 76 per 1,000 | ||||
| Graft loss: late withdrawal | Study population | RR 0.92 | 4026 (17) | ⊕⊕⊕⊝ | |
| 46 per 1,000 | 42 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Several smaller studies with wide CI 2 Significant heterogeneity | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 14 | 2010 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.93, 1.54] |
| 1.1 Avoidance | 4 | 566 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.85, 2.01] |
| 1.2 Late withdrawal | 10 | 1444 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.83, 1.56] |
| 2 Acute rejection Show forest plot | 15 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.56, 4.12] |
| 2.1 Unspecified | 7 | 1066 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.08, 2.75] |
| 2.2 Biopsy‐proven | 8 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 4.48 [2.10, 9.55] |
| 3 GFR Show forest plot | 8 | 910 | Mean Difference (IV, Random, 95% CI) | 3.56 [‐1.13, 8.25] |
| 3.1 One year | 5 | 653 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐5.38, 4.94] |
| 3.2 Two years | 1 | 108 | Mean Difference (IV, Random, 95% CI) | 7.90 [1.43, 14.37] |
| 3.3 Over 5 years | 2 | 149 | Mean Difference (IV, Random, 95% CI) | 11.09 [4.81, 17.37] |
| 4 Graft loss Show forest plot | 16 | 2090 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.74, 0.98] |
| 5 Serum creatinine Show forest plot | 4 | 189 | Mean Difference (IV, Random, 95% CI) | 19.17 [5.89, 32.44] |
| 5.1 Six months | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 31.78 [10.58, 52.98] |
| 5.2 One year | 3 | 165 | Mean Difference (IV, Random, 95% CI) | 11.04 [‐5.99, 28.06] |
| 6 Adverse events Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Hypertension | 5 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.95] |
| 6.2 Hyperlipidaemia | 3 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.21] |
| 6.3 CMV infection | 7 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.52, 1.45] |
| 6.4 Diabetes | 6 | 810 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.62, 1.42] |
| 6.5 Malignancy | 6 | 1079 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.93, 1.30] |
| 6.6 Infection | 6 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.61, 1.51] |
| 7 Subgroup analysis: acute rejection Show forest plot | 15 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.56, 4.12] |
| 7.1 Avoidance | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [0.85, 5.49] |
| 7.2 Late withdrawal | 12 | 1428 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [1.59, 6.48] |
| 8 Subgroup analysis: GFR Show forest plot | 8 | 910 | Mean Difference (IV, Random, 95% CI) | 3.56 [‐1.13, 8.25] |
| 8.1 Avoidance | 3 | 242 | Mean Difference (IV, Random, 95% CI) | ‐2.22 [‐14.84, 10.40] |
| 8.2 Late withdrawal | 5 | 668 | Mean Difference (IV, Random, 95% CI) | 5.54 [1.66, 9.43] |
| 9 Subgroup analysis: graft loss Show forest plot | 16 | 2414 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 0.99] |
| 9.1 Avoidance | 4 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.16] |
| 9.2 Late withdrawal | 13 | 1848 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Acute rejection Show forest plot | 15 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.56, 4.12] |
| 1.1 MMF/MPA | 10 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 3.51 [1.79, 6.88] |
| 1.2 AZA | 5 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.78, 4.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Acute rejection Show forest plot | 15 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.56, 4.12] |
| 1.1 CSA | 11 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.31, 3.48] |
| 1.2 TAC | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 5.65 [1.96, 16.27] |
| 1.3 Either CSA or TAC | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.52, 156.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 15 | 3462 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.50, 1.27] |
| 1.1 Early intervention | 13 | 3272 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.48, 1.27] |
| 1.2 Late intervention | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.15, 6.94] |
| 2 Acute rejection Show forest plot | 19 | 3757 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 2.1 Unspecified | 8 | 2028 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.74, 1.03] |
| 2.2 Biopsy‐proven | 11 | 1729 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.64, 1.16] |
| 3 GFR Show forest plot | 13 | 2623 | Mean Difference (IV, Random, 95% CI) | 4.10 [2.07, 6.12] |
| 3.1 Six months | 5 | 812 | Mean Difference (IV, Random, 95% CI) | 1.96 [‐1.35, 5.28] |
| 3.2 One year | 7 | 1710 | Mean Difference (IV, Random, 95% CI) | 4.30 [1.78, 6.82] |
| 3.3 Two years | 1 | 101 | Mean Difference (IV, Random, 95% CI) | 11.10 [4.14, 18.06] |
| 4 Graft loss Show forest plot | 15 | 3286 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.02] |
| 5 Serum creatinine Show forest plot | 6 | 742 | Mean Difference (IV, Random, 95% CI) | ‐4.28 [‐14.65, 6.10] |
| 5.1 Six months | 4 | 530 | Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐11.25, 8.33] |
| 5.2 One year | 2 | 212 | Mean Difference (IV, Random, 95% CI) | ‐23.18 [‐46.12, ‐0.23] |
| 6 Change in GFR at 12 months Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Adverse events Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Hypertension | 5 | 1877 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.70, 1.00] |
| 7.2 Hyperlipidaemia | 3 | 1443 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.90, 1.19] |
| 7.3 CMV infection | 6 | 1948 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.94, 1.62] |
| 7.4 Diabetes | 5 | 1292 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.50, 1.34] |
| 7.5 Malignancy | 5 | 1637 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.41, 1.97] |
| 7.6 Infection | 9 | 1437 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 8 Subgroup analysis: acute rejection Show forest plot | 18 | 2968 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.70, 1.02] |
| 8.1 Immediate intervention | 12 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 1.00] |
| 8.2 Late intervention | 6 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.61, 1.81] |
| 9 Subgroup analysis: GFR Show forest plot | 12 | 2443 | Mean Difference (IV, Random, 95% CI) | 4.21 [1.90, 6.51] |
| 9.1 Immediate intervention | 9 | 2200 | Mean Difference (IV, Random, 95% CI) | 3.09 [0.95, 5.23] |
| 9.2 Late intervention | 3 | 243 | Mean Difference (IV, Random, 95% CI) | 8.81 [3.79, 13.83] |
| 10 Subgroup analysis: graft loss Show forest plot | 14 | 3106 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
| 10.1 Immediate intervention | 11 | 2800 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
| 10.2 Late intervention | 3 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.12, 7.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Acute rejection Show forest plot | 19 | 3757 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 1.1 CsA | 16 | 2906 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.01] |
| 1.2 TAC | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.61, 3.83] |
| 1.3 Either CsA or TAC | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.34, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 23 | 5427 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.68, 1.36] |
| 1.1 Avoidance | 9 | 1689 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.47] |
| 1.2 Withdrawal | 14 | 3738 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.59, 1.76] |
| 2 Acute rejection Show forest plot | 30 | 5903 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.15, 1.78] |
| 2.1 Unspecified | 4 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.12, 1.60] |
| 2.2 Biopsy‐proven | 26 | 4966 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.10, 1.85] |
| 3 GFR Show forest plot | 23 | 4427 | Mean Difference (IV, Random, 95% CI) | 5.29 [2.08, 8.51] |
| 3.1 Six months | 2 | 187 | Mean Difference (IV, Random, 95% CI) | 5.22 [‐0.02, 10.46] |
| 3.2 One year | 16 | 3144 | Mean Difference (IV, Random, 95% CI) | 5.02 [0.59, 9.45] |
| 3.3 Two years | 4 | 796 | Mean Difference (IV, Random, 95% CI) | 6.08 [‐0.85, 13.01] |
| 3.4 Five years | 1 | 300 | Mean Difference (IV, Random, 95% CI) | 6.30 [2.43, 10.17] |
| 4 Graft loss Show forest plot | 25 | 5446 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.19] |
| 5 Serum creatinine at 1 year Show forest plot | 12 | 1702 | Mean Difference (IV, Random, 95% CI) | ‐17.10 [‐26.95, ‐7.25] |
| 6 Change in GFR Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 One year | 1 | 246 | Mean Difference (IV, Random, 95% CI) | 6.10 [0.01, 12.19] |
| 6.2 Two years | 2 | 521 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐15.00, 15.56] |
| 7 Adverse events Show forest plot | 24 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Hypertension | 7 | 2207 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.64, 1.15] |
| 7.2 Hyperlipidaemia | 13 | 3494 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.40, 2.20] |
| 7.3 CMV infection | 13 | 2503 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.82] |
| 7.4 Diabetes | 11 | 2833 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.97, 1.66] |
| 7.5 Malignancy | 14 | 3699 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.47, 1.00] |
| 7.6 Infection | 9 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.92, 1.07] |
| 7.7 Lymphocele | 8 | 1926 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.95, 2.21] |
| 8 Subgroup analysis: acute rejection Show forest plot | 28 | 5480 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.27, 1.91] |
| 8.1 Avoidance | 11 | 1844 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.98, 1.65] |
| 8.2 Late withdrawal | 17 | 3636 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.44, 2.51] |
| 9 Subgroup analysis: GFR Show forest plot | 23 | 4427 | Mean Difference (IV, Random, 95% CI) | 5.29 [2.08, 8.51] |
| 9.1 Avoidance | 9 | 1748 | Mean Difference (IV, Random, 95% CI) | 6.45 [1.33, 11.58] |
| 9.2 Late withdrawal | 14 | 2679 | Mean Difference (IV, Random, 95% CI) | 4.55 [0.26, 8.85] |
| 10 Subgroup analysis: graft loss Show forest plot | 25 | 5446 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.19] |
| 10.1 Avoidance | 8 | 1420 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.72, 1.48] |
| 10.2 Late withdrawal | 17 | 4026 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.65, 1.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Acute rejection Show forest plot | 30 | 5903 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.15, 1.78] |
| 1.1 CsA | 18 | 3463 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.15, 1.76] |
| 1.2 TAC | 7 | 753 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [1.43, 3.49] |
| 1.3 Either CsA or TAC | 5 | 1687 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.40, 2.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 11 | 2750 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.71, 1.90] |
| 1.1 Low dose CNI + immediate mTOR | 9 | 2182 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.62, 1.87] |
| 1.2 low dose CNI + late mTOR | 2 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.52, 4.59] |
| 2 Acute rejection Show forest plot | 16 | 3300 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.40] |
| 2.1 Unspecified | 3 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.90, 2.09] |
| 2.2 Biopsy‐proven | 13 | 2804 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.83, 1.37] |
| 3 GFR Show forest plot | 11 | 1749 | Mean Difference (IV, Random, 95% CI) | 6.24 [3.28, 9.19] |
| 3.1 Six months | 4 | 244 | Mean Difference (IV, Random, 95% CI) | 5.79 [‐3.57, 15.15] |
| 3.2 One year | 6 | 1293 | Mean Difference (IV, Random, 95% CI) | 6.63 [4.11, 9.14] |
| 3.3 Two years | 1 | 212 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐3.00, 6.16] |
| 4 Graft loss Show forest plot | 16 | 3304 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 1.01] |
| 5 Serum creatinine at 1 year Show forest plot | 6 | 1320 | Mean Difference (IV, Random, 95% CI) | ‐14.14 [‐22.55, ‐5.72] |
| 6 Change in GFR at 2 years Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Adverse events Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Hypertension | 5 | 1421 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.80, 1.20] |
| 7.2 Hyperlipidaemia | 8 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.89, 1.28] |
| 7.3 CMV infection | 5 | 1250 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.16, 1.06] |
| 7.4 Diabetes | 5 | 686 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.81, 2.27] |
| 7.5 Malignancy | 5 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.42, 3.52] |
| 7.6 Infection | 5 | 1271 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.83, 1.08] |
| 8 Subgroup analysis: graft loss Show forest plot | 16 | 3304 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 1.01] |
| 8.1 Immediate mTOR | 14 | 2736 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.18] |
| 8.2 Late mTOR | 2 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.15, 1.04] |
| 9 Subgroup analysis: GFR Show forest plot | 11 | 1749 | Mean Difference (IV, Random, 95% CI) | 6.24 [3.28, 9.19] |
| 9.1 Immediate mTOR | 10 | 1537 | Mean Difference (IV, Random, 95% CI) | 6.91 [3.86, 9.96] |
| 9.2 Late mTOR | 1 | 212 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐3.00, 6.16] |
| 10 Subgroup analysis: acute rejection Show forest plot | 16 | 3300 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.40] |
| 10.1 Immediate mTOR | 14 | 2736 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.86, 1.39] |
| 10.2 Late mTOR | 2 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.82, 2.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Acute rejection Show forest plot | 16 | 3300 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.40] |
| 1.1 CsA | 11 | 2232 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.78, 1.22] |
| 1.2 TAC | 5 | 1068 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.16, 2.13] |

