Tratamientos de desintoxicación para adolescentes dependientes de opiáceos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial. Recruitment modality: self‐referred participants. | |
| Participants | 36 adolescents (13‐18 years) who met the DSM‐IV criteria for opioid dependence. Pregnant women and patients with significant psychiatric disorders (e.g. psychosis) or medical illnesses (e.g. cardiovascular disease) were excluded. Mean age. 17.35 years; 39% male; 97% white. Injection route of opiate use: 36%; other drug dependence alcohol: 17.5%, cannabis: 17%, cocaine: 10%, amphetamine: 6%. | |
| Interventions | (1) Buprenorphine detoxification: sublingual buprenorphine tablets daily with flexible dosing procedure based on weight and self‐reported opiate use at intake (starting dose range: 6 mg‐ 8 mg). Buprenorhine dose that decreased by 2 mg every 7 days. Behavioural therapy 3 one‐hour individual sessions per week. Contingency management approach: participants could earn a voucher on the provision of opioid negative urine samples. At the end of the study, participants were offered naltrexone. (2) Transdermal clonidine patches 0.1 mg on intake day and day 1; a second patch was added on day 2 and worn until day 6. An optional third patch (depending on the severity of withdrawal symptoms) may have been added on day 4 and worn until day 6. All patches were removed on day 7 and replaced with a 0.2 mg doses. On day 14 the patches were removed again and replaced with a 0.1 mg dose patch. On day 21 the patches were removed again and replaced with a 0 mg dose. Behavioural therapy 3 one‐hour individual session per week. Contingency management approach: participants could earn a voucher on the provision of opioid negative urine samples. At the end of the study, participants were offered naltrexone. Durattion of the trials: 28 days. | |
| Outcomes | Drop out from treatment measured as the percentage of patients who did not complete the entire detoxification treatment. Time retained in treatment. Opiate abstinence as the percentage of scheduled urine samples opiate negative. Other drug use as percentage of urine samples positives. Acceptability of the treatment: withdrawal effect measured by the Adjective rating scale. Initiation of naltrexone treatment as percentage of patients who initiated. | |
| Notes | Country: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants were randomly assigned to either detoxification with clonidine or with buprenorphine. In this process participants were stratified for sex and past month route of opiate use (injection vs intranasal)" |
| Allocation concealment (selection bias) | Unclear risk | "participants were randomly assigned to either detoxification with clonidine or with buprenorphine. In this process participants were stratified for sex and past month route of opiate use (injection vs intranasal)" |
| Blinding (performance bias and detection bias) | Low risk | "The study used a parallel group, double blind, double dummy design". Participants in the clonidine group received placebo buprenorphine tablets and patients in the buprenorphine group received placebo clonidine patches" |
| Blinding (performance bias and detection bias) | Low risk | "The study used a parallel group, double blind, double dummy design". Participants in the clonidine group received placebo buprenorphine tablets and patients in the buprenorphine group received placebo clonidine patches" |
| Incomplete outcome data (attrition bias) | Low risk | "the primary analysis (drop out, time retained, use of substance) included all participants randomised independently to drop out/non compliance, consistent with an intention to treat approach. All secondary outcomes (withdrawals symptoms and signs) were confined to the data from treatment intake to the end of the first week when retention was still high in both condition" |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Multicentre randomised controlled trial. Recruitment modality described. | |
| Participants | 154 participants who met the DSM IV diagnostic criteria for opioid dependence and who sought outpatient treatment.152 randomised. Mean age: 19 years. Only one participant was 15 years old and no participants were 14 years old. Male: 59%. White: 56%. | |
| Interventions | (1) Maintenance group:12 weeks buprenorphine. Naloxone: up to 24 mg/day buprenorphine and 0.5 mg naloxone for 9 weeks and then tapered to week 12. :74 patients. (2) Detoxification group: 2 weeks buprenorphine. Naloxone: up to 14 mg/day buprenorphine and then tapered to day 14: 78 patients. Both groups were offered 1 weekly individual and 1 group counselling. | |
| Outcomes | Primary outcome: opioid positive urine test results at weeks 4, 8 and 12. Secondary outcomes: drop out, self‐reported use, enrolment in addiction treatment outside the assigned condition, other drug use, adverse events. Results at 6,9,12 months follow‐up: self‐reported opioid use, self‐reported other drug use, other addiction treatment received. | |
| Notes | Country: USA Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred through an automated 24‐hour service at the Veterans Affairs Cooperative Studies Program in Perry Point, Maryland, that was programmed Age was dichotomised as 14 to 18 years or 18 to 21 years, ethnicity as the majority ethnic group vs all others within the site, and route of administration as injecting or non injecting. |
| Allocation concealment (selection bias) | High risk | Balance was assessed by comparing the group sum of the binary indicators as each new patient was randomised. If both groups were balanced when a new patient was being randomised, then each group had an allocation probability of 1/2; if there was an imbalance, then the group with the higher score on the sum of indicators received an allocation probability of 1/3 and the other group a probability of 2/3. |
| Blinding (performance bias and detection bias) | Low risk | Patients and providers impossible to be blinded for the nature of the intervention (14 days detox vs 12 weeks maintenance). COMMENT: objective outcomes unlikely to be biased by lack of blinding. |
| Blinding (performance bias and detection bias) | High risk | Patients and providers impossible to be blinded for the nature of the intervention (14 days detox vs 12 weeks maintenance) Outcome assessor not blinded: "Research assistant likely knew groups assignment because the study was not blinded" |
| Incomplete outcome data (attrition bias) | Low risk | Number of participants withdrawn from the study reported for each group. Reason for withdrawal given. Analysis on the basis of the Intention‐to‐treat principle: "patients were contacted at all assessment point regardless of whether they remained in treatment". |
| Selective reporting (reporting bias) | Low risk | |
DSM IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edition
vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Type of intervention not in the inclusion criteria: only psychosocial intervention without pharmacological detoxification | |
| Outcome not in the inclusion criteria: baseline patient characteristics of Woody 2008 trial | |
| Study design not in the inclusion criteria: not RCT or CCT | |
| Study design not in the inclusion criteria: not RCT or CCT | |
| Type of intervention not in the inclusion criteria: psychosocial intervention; the same pharmacological intervention given to both groups | |
| Study design not in the inclusion criteria: not RCT or CCT | |
| Outcome not in the inclusion criteria: association between cannabis use during opioid dependence treatment and positive urine drug screens for opioids; no raw data about cannabis use in the two groups provided | |
| Type of intervention not in the inclusion criteria: only psychosocial intervention without pharmacological detoxification | |
| Type of intervention not in the inclusion criteria: maintenance treatment | |
| Study design not in the inclusion criteria: not RCT or CCT | |
| Participants not in the inclusion criteria: adults | |
| Secondary analysis of the all sample of the Marsch 2005 study without distinction between experimental and control condition | |
| Study design not in the inclusion criteria: qualitative study | |
| Study design and participants not in the inclusion criteria: observation cohort study on adult population | |
| Outcome not in the inclusion criteria: cost effectiveness analysis of the Woody 2008 trial | |
| Outcome not in the inclusion criteria: Predictors of Abstinence: secondary analysis of the Woody 2008 trial | |
| Outcome not in the inclusion criteria:Predictors of attrition: secondary analysis of the Woody 2008 trial | |
| Outcome not in the inclusion criteria: Concordance between self‐report and urine drug screen data: secondary analysis of the Woody 2008 trial |
CCT: controlledclinical trial
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Double blind randomised controlled trial |
| Participants | 53 opioid dependents adolescents and young adults (age 13‐24 eligible) |
| Interventions | Experimental: buprenorphine taper of 28 days Control: buprenorphine taper of 63 days |
| Outcomes | Retention in treatment; use of primary substance of abuse measured by urine analysis |
| Notes | Author contacted; study ended but definite results not yet published |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 drop out Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.20, 1.04] |
| Analysis 1.1  Comparison 1 Buprenorphine versus clonidine, Outcome 1 drop out. | ||||
| 2 withdrawal score Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 3.97 [‐1.38, 9.32] |
| Analysis 1.2  Comparison 1 Buprenorphine versus clonidine, Outcome 2 withdrawal score. | ||||
| 3 initiation of naltrexone treatment Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [1.58, 76.55] |
| Analysis 1.3  Comparison 1 Buprenorphine versus clonidine, Outcome 3 initiation of naltrexone treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 drop out Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.85, 3.86] |
| Analysis 2.1  Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 1 drop out. | ||||
| 2 patients with positive urine at the end of treatment Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.28] |
| Analysis 2.2  Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 2 patients with positive urine at the end of treatment. | ||||
| 3 self‐reported use at 12 months follow‐ up Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.05, 1.76] |
| Analysis 2.3  Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 3 self‐reported use at 12 months follow‐ up. | ||||
| 4 enrolment in addiction treatment at 12‐month follow‐up Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.53, 1.07] |
| Analysis 2.4  Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 4 enrolment in addiction treatment at 12‐month follow‐up. | ||||
| 5 self‐reported alcohol use Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.63, 2.02] |
| Analysis 2.5  Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 5 self‐reported alcohol use. | ||||
| 6 self‐reported marijuana use Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.83, 3.00] |
| Analysis 2.6  Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 6 self‐reported marijuana use. | ||||
| 7 self‐reported cocaine use Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.54 [1.11, 65.75] |
| Analysis 2.7  Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 7 self‐reported cocaine use. | ||||
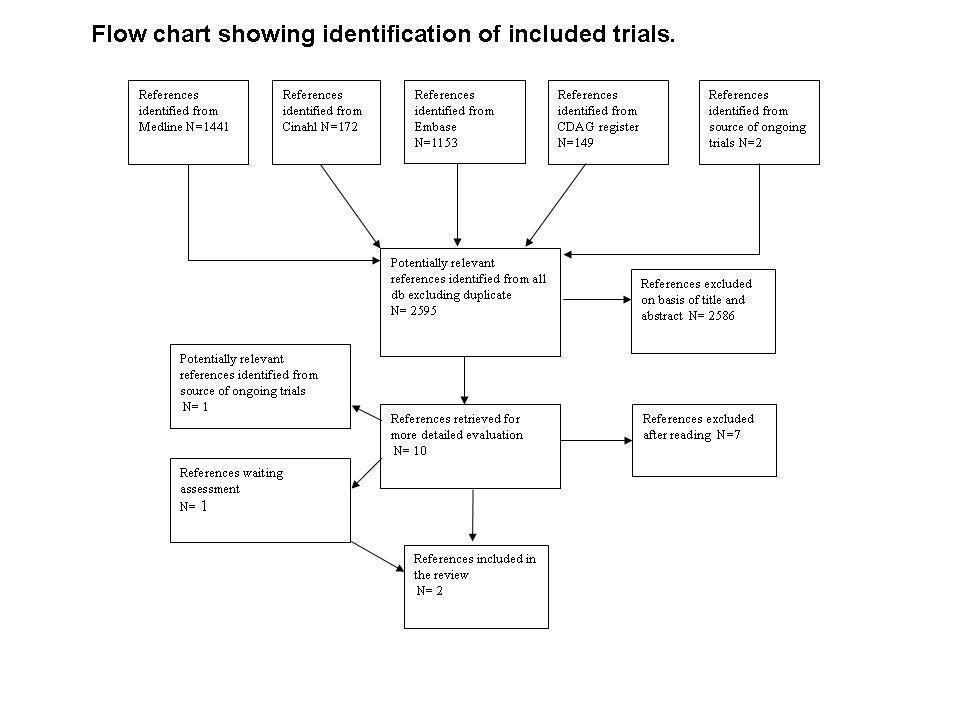
Flow chart of studies of the review published in 2009
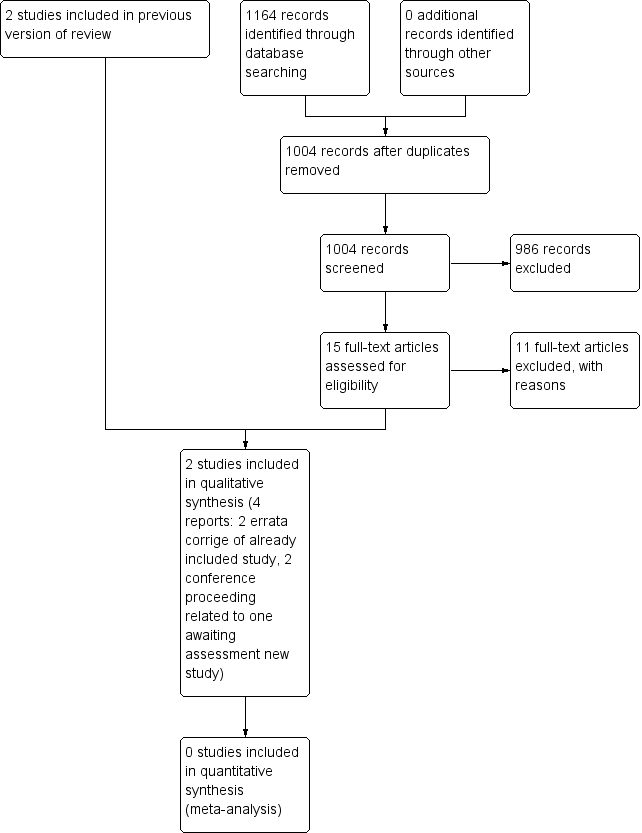
Study flow diagram. 2014 update

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Buprenorphine versus clonidine, Outcome 1 drop out.

Comparison 1 Buprenorphine versus clonidine, Outcome 2 withdrawal score.

Comparison 1 Buprenorphine versus clonidine, Outcome 3 initiation of naltrexone treatment.

Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 1 drop out.

Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 2 patients with positive urine at the end of treatment.

Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 3 self‐reported use at 12 months follow‐ up.

Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 4 enrolment in addiction treatment at 12‐month follow‐up.

Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 5 self‐reported alcohol use.
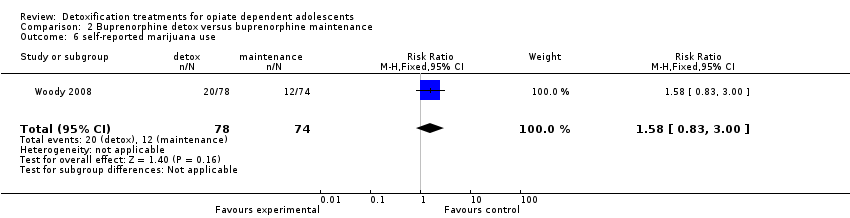
Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 6 self‐reported marijuana use.
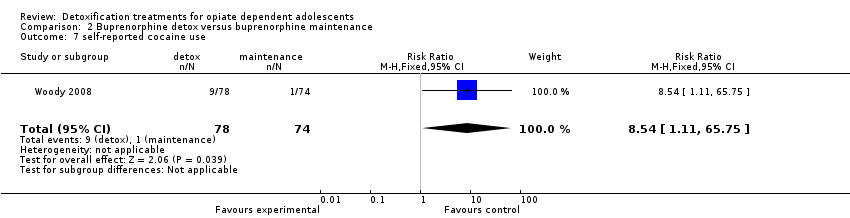
Comparison 2 Buprenorphine detox versus buprenorphine maintenance, Outcome 7 self‐reported cocaine use.
| Buprenorphine versus clonidine for opiate dependent adolescents | ||||||
| Patient or population: patients with opiate dependent adolescents | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Buprenorphine versus clonidine | |||||
| Drop out | Study population | RR 0.45 | 36 | ⊕⊕⊕⊝ | ||
| 611 per 1000 | 275 per 1000 | |||||
| Moderate | ||||||
| 611 per 1000 | 275 per 1000 | |||||
| Duration and severity of signs and symptoms of withdrawal | The mean duration and severity of signs and symptoms of withdrawal in the control groups was | The mean duration and severity of signs and symptoms of withdrawal in the intervention groups was | 32 | ⊕⊕⊕⊝ | ||
| Initiation of naltrexone treatment | Study population | RR 11 | 36 | ⊕⊕⊕⊝ | ||
| 56 per 1000 | 611 per 1000 | |||||
| Moderate | ||||||
| 56 per 1000 | 616 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 only one study included | ||||||
| Buprenorphine detox compared with buprenorphine maintenance for opiate dependent adolescents | ||||||
| Patient or population: patients with opiate dependent adolescents | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Buprenorphine maintenance | Buprenorphine detox | |||||
| Drop out | Study population | RR 2.67 | 152 | ⊕⊕⊝⊝ | ||
| 297 per 1000 | 794 per 1000 | |||||
| Moderate | ||||||
| 297 per 1000 | 793 per 1000 | |||||
| Patients with positive urine at the end of treatment | Study population | RR 1.03 | 152 | ⊕⊕⊝⊝ | ||
| 662 per 1000 | 682 per 1000 | |||||
| Moderate | ||||||
| 662 per 1000 | 682 per 1000 | |||||
| Self‐reported use at 12 months follow‐up | Study population | RR 1.36 | 152 | ⊕⊕⊝⊝ | ||
| 527 per 1000 | 717 per 1000 | |||||
| Moderate | ||||||
| 527 per 1000 | 717 per 1000 | |||||
| Enrolment in addiction treatment at 12 month follow‐up | Study population | RR 0.75 | 152 | ⊕⊕⊝⊝ | ||
| 527 per 1000 | 395 per 1000 | |||||
| Moderate | ||||||
| 527 per 1000 | 395 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 no allocation concealment | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 drop out Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.20, 1.04] |
| 2 withdrawal score Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 3.97 [‐1.38, 9.32] |
| 3 initiation of naltrexone treatment Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [1.58, 76.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 drop out Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.85, 3.86] |
| 2 patients with positive urine at the end of treatment Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.28] |
| 3 self‐reported use at 12 months follow‐ up Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.05, 1.76] |
| 4 enrolment in addiction treatment at 12‐month follow‐up Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.53, 1.07] |
| 5 self‐reported alcohol use Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.63, 2.02] |
| 6 self‐reported marijuana use Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.83, 3.00] |
| 7 self‐reported cocaine use Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.54 [1.11, 65.75] |

