La función de los alfabloqueantes antes de la retirada de la sonda urinaria para la retención urinaria aguda en los hombres
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Prospective, randomised, placebo‐controlled, single centre study | |
| Participants | 150 patients ( 50 alfuzosin, 50 tamsulosin, 50 placebo), mean age 69, 72 and 71 years respectively (aged 48 to 90 years) | |
| Interventions | A (50): Alfuzosin 10 mg OD B (50): Tamsulosin 0.4 mg C (50): Placebo, Then TWOC after 3 days | |
| Outcomes | Number who had successful TWOC: A 33/50, B 35/50, C 18/50 Initial catheterisation volume American Urological Association score Post void residual urinary volume (mL) Peak urinary flow rate (mL/sec) Adverse effects | |
| Notes | Second trial phase: successful TWOC patients continued in respective treatment groups and followed up at 1 week, 2 weeks, 1 month and 3 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified: '...randomized into three groups.' |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | More detail in tables of analysis needed. |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes as detailed in the method section of the trial were reported in the result section. Some outcomes which are considered important from patients’ perspective were not reported. Without the protocol it was difficult to judge whether or not these outcomes were included in the protocol and not reported in the report. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Other bias | Unclear risk | Other aspects of trial design such as baseline comparability, sample size calculation, use of intention‐to‐treat analysis and financial support were inadequately or not at all specified. |
| Methods | Prospective, randomised single centre study | |
| Participants | 72 patients (36 tamsulosin, 36 no treatment) | |
| Interventions | A (36): Tamsulosin 0.4 mg once daily for three days B (36): No treatment, TWOC after 3 days (both groups given prophylactic antibiotics) | |
| Outcomes | TWOC success rates:A 22/36 , B 10/36 | |
| Notes | Full text article in Chinese with English abstract. TWOC success rates stated in English abstract but no other usable data available for any of the specified outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '...were randomly divided into treatment group and control group of 36 patients each.' |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes as detailed in the method section of the trial were reported in the result section. Some outcomes which are considered important from patients’ perspective were not reported. Without the protocol it was difficult to judge whether or not these outcomes were included in the protocol and not reported in the report. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Other bias | Unclear risk | Other aspects of trial design such as baseline comparability, sample size calculation and financial support not specified. |
| Methods | Prospective, randomised placebo‐controlled single centre study | |
| Participants | 60 patients (30 silodosin, 30 placebo). Mean age (SD):
| |
| Interventions | A (30): Silodosin 8 mg once daily for 3 days B (30): placebo | |
| Outcomes | Number who had successful TWOC: A 23/30, B 11/30 Factors influencing TWOC failure Effects of silodosin on uroflowmetry and IPSS in patients who had successful TWOC | |
| Notes | Study conducted in tertiary care regional referral centre in Northern India Successful TWOC:
Failed TWOC:
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘...centrally established computer‐assigned randomization list...’ |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes as detailed in the method section of the trial were reported in the result section. Some outcomes which are considered important from patients’ perspective were not reported. Without the protocol it was difficult to judge whether or not these outcomes were included in the protocol and not reported in the report. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Placebo administered was ‘similar to silodosin capsule in colour, weight, and shape’ Unclear if personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Other bias | Low risk | Study groups were comparable at baseline Power calculation was performed Ethical approval obtained Written informed consent obtained No withdrawals/loss to follow‐up |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multicentre study | |
| Participants | 149 patients (75 tamsulosin, 74 placebo), mean age 69.4 years | |
| Interventions | A (75): Tamsulosin 0.4mg once daily B (74): Placebo TWOC 'after up to 8 doses' | |
| Outcomes | Number who had successful TWOC: A 34/75, B 18/74 | |
| Notes | Qmax, PVR volume and spontaneously voided volume analysed but not separately (in groups of 'any two successful criteria') | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '...randomized, double‐blind, placebo‐controlled, parallel‐group, multicentre study' |
| Allocation concealment (selection bias) | Unclear risk | Not specified ('...randomly assigned to receive...') |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes as detailed in the method section of the trial were reported in the result section. Some outcomes which are considered important from patients’ perspective were not reported. Without the protocol it was difficult to judge whether or not these outcomes were included in the protocol and not reported in the report. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Other bias | High risk | 'This study was sponsored by a grant from Yamanouchi Pharma Ltd.' |
| Methods | Prospective, randomised, placebo‐controlled multicentre trial | |
| Participants | 81 patients (40 alfuzosin, 41 placebo), mean age 68.4 years | |
| Interventions | A(40): Alfuzosin SR 5 mg twice daily B (41): Placebo TWOC after 24 hours | |
| Outcomes | Number who had successful TWOC: A 22/40, B 12/41 Factors affecting successful TWOC Incidence of adverse events Incidence of repeat episode of AUR in patients who had successful TWOC Need for surgery in patients who had successful TWOC | |
| Notes | Allocation concealment well explained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...tablets was allocated a study number generated randomly by computer' |
| Allocation concealment (selection bias) | Low risk | 'A sealed copy of the code was held by each participating pharmacy, ...' |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes as detailed in the method section of the trial were reported in the result section. Some outcomes which are considered important from patients’ perspective were not reported. Without the protocol it was difficult to judge whether or not these outcomes were included in the protocol and not reported in the report. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded. 'A central pharmacy...packaged the SR alfuzosin and placebo to appear identical...' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified if assessors were blinded at time of TWOC. |
| Other bias | High risk | High risk:
Low risk:
|
| Methods | Randomised, double‐blind, placebo‐controlled multicentre trial | |
| Participants | 363 patients randomised (A 238, B 122. See notes) | |
| Interventions | A (238): Alfuzosin 10 mg once daily B (122): Placebo Then TWOC after 3 days | |
| Outcomes | Number who had successful TWOC: A 146/236, B 58/121 Factors influencing TWOC success | |
| Notes | Second phase of trial: successful TWOC patients again randomised into alfuzosin versus placebo for 6 months (AUR relapse/ BPH surgery need). Data missing for 3 patients therefore results for primary outcome were reported as sub‐totals without these patients (A 236, B 121). Adverse events were however reported as sub‐totals including these patients (A 238, B 122) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomized...according to a centrally established randomization list.' |
| Allocation concealment (selection bias) | Unclear risk | Not explained |
| Incomplete outcome data (attrition bias) | High risk | Incomplete data for 3 patients |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes as detailed in the method section of the trial were reported in the result section. Some outcomes which are considered important from patients’ perspective were not reported. Without the protocol it was difficult to judge whether or not these outcomes were included in the protocol and not reported in the report. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Other bias | High risk | High risk:
Low risk:
|
| Methods | Randomised, controlled single centre study | |
| Participants | 46 patients (23 doxazosin, 23 no treatment), mean age 74 years | |
| Interventions | A (23): Doxazosin modified release 4 mg once daily B (23): No treatment TWOC on day 32. | |
| Outcomes | TWOC success rates: A 13/22, B 13/24 Maximum urinary flow rate in patients with spontaneous micturition Post‐void residual volume in patients with spontaneous micturition | |
| Notes | Second phase trial: successful TWOC patients review at 6, 12 and 24 months for recurrence of AUR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 'Two groups were formed.' Patients 'born in even‐numbered years' (doxazosin group) versus 'born in odd‐numbered years' (no treatment group) |
| Allocation concealment (selection bias) | High risk | Patients 'born in even‐numbered years' (doxazosin group) versus 'born in odd‐numbered years' (no treatment group) |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes as detailed in the method section of the trial were reported in the result section. Some outcomes which are considered important from patients’ perspective were not reported. Without the protocol it was difficult to judge whether or not these outcomes were included in the protocol and not reported in the report. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding is unlikely given the study design. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Other bias | High risk | High risk:
Low risk:
|
| Methods | Randomised, double‐blind, placebo‐controlled single centre study | |
| Participants | 62 patients (34 alfuzosin, 28 placebo), mean age 68.6 years | |
| Interventions | A (34): Alfuzosin SR 5 mg twice daily versus B (28): Placebo, TWOC after minimum of three doses or 36 hours after admission | |
| Outcomes | TWOC success rates: A 17/34, B 16/28 Need for further TURP (phase 2) | |
| Notes | Phase 2 of study; all patients with successful TWOC were given Alfuzosin SR 5 mg twice daily and followed up at 1 year and 2 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '...double blind, placebo controlled study...' 'Patients were randomised to receive ...' |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes as detailed in the method section of the trial were reported in the result section. Some outcomes which are considered important from patients’ perspective were not reported. Without the protocol it was difficult to judge whether or not these outcomes were included in the protocol and not reported in the report. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Other bias | High risk | Funding obtained from Lorex Synthelabo Pharma. |
| Methods | Prospectively, randomised trial single centre study | |
| Participants | 67 patients (35 alfuzosin, 32 placebo) | |
| Interventions | A (35): Alfuzosin XL 10 mg once daily B (32): Placebo '...TWOC at least 2h after taking the second dose of trial medication.' | |
| Outcomes | TWOC success rates: A (21/35), B (11/32) | |
| Notes | Both patient groups offered alpha blocker treatment after successful TWOC | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised '...using a centrally established computer‐assigned randomization list'. |
| Allocation concealment (selection bias) | Low risk | 'The allocation and administration of treatment were double‐blinded'. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes as detailed in the method section of the trial were reported in the result section. Some outcomes which are considered important from patients’ perspective were not reported. Without the protocol it was difficult to judge whether or not these outcomes were included in the protocol and not reported in the report. |
| Blinding of participants and personnel (performance bias) | Low risk | 'The allocation and administration of treatment were double‐blinded'. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Other bias | Low risk |
|
AUR = acute urinary retention
BPH = benign prostatic hyperplasia
IPSS = International Prostate Symptom Score
PVR = post‐void residual
TURP = transurethral resection of the prostate
TWOC = trial without catheter
SD = standard deviation
SR = sustained release
XL = extended release
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Tamsulosin versus no treatment for TWOC in spinal surgery patients electively catheterised perioperatively (not in acute urinary retention prior to surgery). | |
| No catheters involved. Urodynamic assessment of daily tadalafil versus placebo on lower urinary tract symptoms. | |
| No alpha blockers used in study. Study describes outcome of TWOC in 79 untreated patients. | |
| No catheters involved as patients not in acute urinary retention. Randomised trial of amlodipine with or without terazosin for LUTS. | |
| Use of increasing dosage of doxazosin in failed TWOC patients. 40 patients randomly assigned to doxazosin 4 mg once daily (n = 20) or no treatment (n = 20) for seven days before TWOC. Failed TWOC patients either received doxazosin 8mg (treatment group) or 4 mg (control group) once daily before a second TWOC. | |
| No catheters involved as study assessing tamsulosin for stent‐related irritative symptoms. | |
| Long‐term follow‐up study of patient cohort who had successful TWOC following an episode of acute urinary retention (alfuzosin versus placebo). | |
| No alpha‐blockers used in study. Study describes outcome of TWOC immediately, after 24 and 48 hours in 60 untreated patients (subdivided into three groups). | |
| No catheters involved as study assessing effect of tamsulosin in patients with indwelling double‐J ureteral stents. |
LUTS = lower urinary tract symptoms
TWOC = trial without catheter
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Prospective, randomised, controlled trial. |
| Participants | Patients admitted on an emergency basis with AUR due to benign prostatic hyperplasia. |
| Interventions | Group A: Doxazosin 4 mg Group B: Placebo Catheters removed after 12 hours. |
| Outcomes | Number with successful TWOC: A 19/30, B 1/6 Need for further surgery: A10/30, B 5/6 |
| Notes | Paper translated from Russian. Method of randomisation and allocation concealment unclear: 30 patients in Group A, 6 patients in Group B. Which patient received which surgery unclear. No statistical analysis was performed on outcomes. |
AUR = acute urinary retention
TWOC = trial without catheter
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ability to void spontaneously after TWOC without the need for re‐catheterisation Show forest plot | 9 | 1094 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.36, 1.76] |
| Analysis 1.1  Comparison 1 Alpha blocker versus placebo or control, Outcome 1 Ability to void spontaneously after TWOC without the need for re‐catheterisation. | ||||
| 1.1 Alfuzosin versus control | 5 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.19, 1.64] |
| 1.2 Tamsulosin versus control | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.49, 2.59] |
| 1.3 Doxazosin versus control | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.66, 1.81] |
| 1.4 Silodosin versus control | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [1.26, 3.48] |
| 2 Incidence of recurrent urinary retention Show forest plot | 8 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.60, 0.79] |
| Analysis 1.2  Comparison 1 Alpha blocker versus placebo or control, Outcome 2 Incidence of recurrent urinary retention. | ||||
| 2.1 Alfuzosin versus control | 5 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.85] |
| 2.2 Tamsulosin versus control | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.50, 0.87] |
| 2.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.48] |
| 2.4 Silodosin versus control | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.74] |
| 3 Number with adverse effects due to alpha‐blocker treatment Show forest plot | 5 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.75, 1.89] |
| Analysis 1.3  Comparison 1 Alpha blocker versus placebo or control, Outcome 3 Number with adverse effects due to alpha‐blocker treatment. | ||||
| 3.1 Alfuzosin versus control | 3 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.54] |
| 3.2 Tamsulosin versus control | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.90, 8.14] |
| 3.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 73.01] |
| 4 Number with serious adverse effects due to alpha‐blocker treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Alpha blocker versus placebo or control, Outcome 4 Number with serious adverse effects due to alpha‐blocker treatment. | ||||
| 4.1 Alfuzosin versus control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of drop‐outs due to adverse effects of alpha blockers treatment Show forest plot | 5 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.94 [1.28, 12.12] |
| Analysis 1.5  Comparison 1 Alpha blocker versus placebo or control, Outcome 5 Number of drop‐outs due to adverse effects of alpha blockers treatment. | ||||
| 5.1 Alfuzosin versus control | 3 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.62, 12.93] |
| 5.2 Tamsulosin versus control | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.91 [0.87, 54.76] |
| 5.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 73.01] |

PRISMA study flow diagram
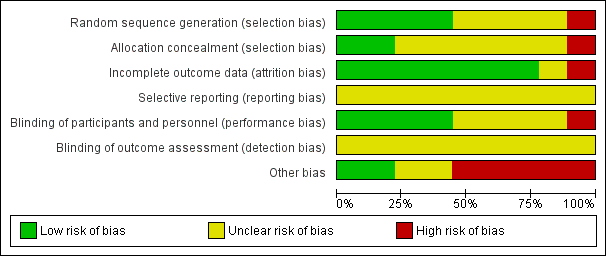
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
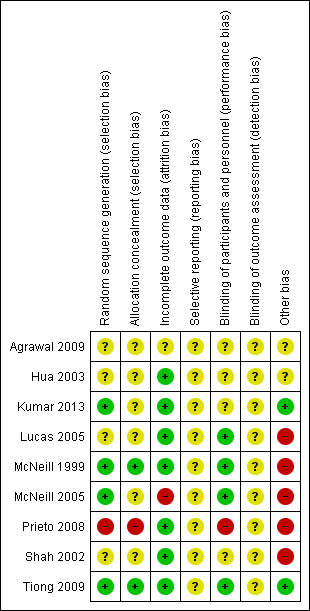
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Alpha blocker versus placebo or control, Outcome 1 Ability to void spontaneously after TWOC without the need for re‐catheterisation.

Comparison 1 Alpha blocker versus placebo or control, Outcome 2 Incidence of recurrent urinary retention.

Comparison 1 Alpha blocker versus placebo or control, Outcome 3 Number with adverse effects due to alpha‐blocker treatment.

Comparison 1 Alpha blocker versus placebo or control, Outcome 4 Number with serious adverse effects due to alpha‐blocker treatment.
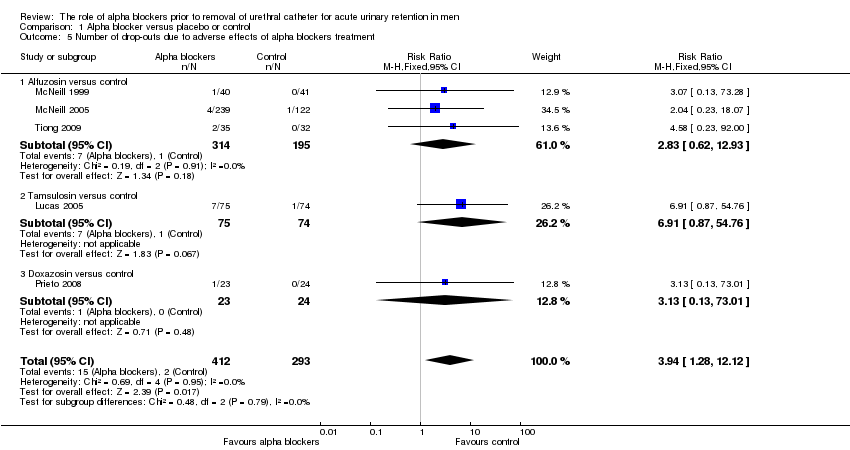
Comparison 1 Alpha blocker versus placebo or control, Outcome 5 Number of drop‐outs due to adverse effects of alpha blockers treatment.
| Alpha blocker versus placebo or control for acute urinary retention in adult men | ||||||
| Patient or population: acute urinary retention in adult men | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Aalpha blocker versus placebo or control | |||||
| Ability to void spontaneously after TWOC without the need for re‐catheterisation | Study population | RR 1.55 | 1044 | ⊕⊕⊕⊝ | ||
| 383 per 1000 | 582 per 1000 | |||||
| Incidence of recurrent urinary retention | Study population | RR 0.69 | 1023 | ⊕⊕⊝⊝ | ||
| 507 per 1000 | 350 per 1000 | |||||
| Need for prostatic surgery ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Condition‐specific QoL ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Cost effectiveness ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Number with adverse effects | Study population | RR 1.2 | 657 | ⊕⊕⊝⊝ | ||
| 74 per 1000 | 89 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation unclear in 4 studies and high risk in 1 study. Allocation concealment unclear in 6 studies and high risk in 1 study. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ability to void spontaneously after TWOC without the need for re‐catheterisation Show forest plot | 9 | 1094 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.36, 1.76] |
| 1.1 Alfuzosin versus control | 5 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.19, 1.64] |
| 1.2 Tamsulosin versus control | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.49, 2.59] |
| 1.3 Doxazosin versus control | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.66, 1.81] |
| 1.4 Silodosin versus control | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [1.26, 3.48] |
| 2 Incidence of recurrent urinary retention Show forest plot | 8 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.60, 0.79] |
| 2.1 Alfuzosin versus control | 5 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.85] |
| 2.2 Tamsulosin versus control | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.50, 0.87] |
| 2.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.48] |
| 2.4 Silodosin versus control | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.74] |
| 3 Number with adverse effects due to alpha‐blocker treatment Show forest plot | 5 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.75, 1.89] |
| 3.1 Alfuzosin versus control | 3 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.54] |
| 3.2 Tamsulosin versus control | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.90, 8.14] |
| 3.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 73.01] |
| 4 Number with serious adverse effects due to alpha‐blocker treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Alfuzosin versus control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of drop‐outs due to adverse effects of alpha blockers treatment Show forest plot | 5 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.94 [1.28, 12.12] |
| 5.1 Alfuzosin versus control | 3 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.62, 12.93] |
| 5.2 Tamsulosin versus control | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.91 [0.87, 54.76] |
| 5.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 73.01] |

