La función de los alfabloqueantes antes de la retirada de la sonda urinaria para la retención urinaria aguda en los hombres
Resumen
Antecedentes
La retención urinaria aguda es una urgencia urológica en hombres que requiere la colocación inmediata de una sonda. Toda intervención que tenga por objeto mejorar los síntomas urinarios después de un episodio de retención urinaria aguda podría ser potencialmente beneficiosa. Los alfabloqueantes relajan las células del músculo liso prostático y de ese modo reducen la resistencia al flujo urinario, lo que mejora los síntomas urinarios.
Objetivos
Evaluar la efectividad de los alfabloqueantes en la reanudación satisfactoria de la micción tras la retirada de un catéter urinario uretral después de un episodio de retención urinaria aguda en los hombres. A falta de medidas de resultado acordadas internacionalmente para el éxito de un ensayo sin sonda, el éxito se definió como el retorno al vaciado satisfactorio sin necesidad de volver a cateterizar en un plazo de 24 horas.
Métodos de búsqueda
Se realizaron búsquedas en el registro de ensayos especializados del Grupo Cochrane de Incontinencia (Cochrane Incontinence Group), que contiene ensayos identificados en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL), MEDLINE, MEDLINE in process y búsquedas manuales en revistas y actas de congresos (búsqueda del 9 de octubre de 2013), CENTRAL (2013, Número 5) (búsqueda del 5 de junio de 2013), MEDLINE 1946 hasta la cuarta semana de mayo de 2013, MEDLINE in Process (que cubre hasta el 3 de junio de 2013), EMBASE Classic y EMBASE 1947 hasta la semana 22 de 2013 (todas las búsquedas se realizaron el 4 de junio de 2013) y las listas de referencias de los artículos pertinentes. No se impusieron restricciones de idioma o de otra índole en las búsquedas.
Criterios de selección
Sólo se incluyeron ensayos clínicos aleatorizados y cuasialeatorizados de alfabloqueantes para el ensayo sin una sonda urinaria después de un episodio de retención urinaria aguda en hombres.
Obtención y análisis de los datos
Dos autor de la revisión examinaron de forma independiente todas las citas y resúmenes derivados de la estrategia de búsqueda. Cualquier desacuerdo acerca de la inclusión y exclusión de los ensayos se resolvió mediante discusión. Cuando persistían los desacuerdos, se buscaba una tercera opinión independiente. Dos autor de la revisión extrajeron de forma independiente, verificaron de forma cruzada y procesaron los datos según se describe en el Manual Cochrane para las revisiones sistemáticas de la intervención (Cochrane Handbook for Systematic Reviews of Intervention). La calidad de la evidencia de los resultados críticos se evaluó adoptando el enfoque GRADE.
Resultados principales
En esta revisión se incluyeron nueve ensayos clínicos aleatorizados. Ocho ensayos compararon los alfabloqueantes con el placebo (cinco ensayos probaron la alfuzosina y dos ensayos probaron la tamsulosina, un ensayo probó tanto la alfuzosina como la tamsulosina, un ensayo probó la silodosina) y un ensayo comparó un alfabloqueante (doxazosina) con ningún tratamiento. El ensayo sin catéter se realizó después del tratamiento con el fármaco durante uno a tres días en siete ensayos y durante ocho y 32 días en otros dos ensayos, respectivamente. Hubo evidencia de calidad moderada que indicaron que la tasa de ensayos satisfactorios sin catéteres favorecía a los alfabloqueantes en comparación con el placebo ( 366/608, 60,2%, de los hombres que utilizaron un alfabloqueante pudieron evacuar espontáneamente después de la retirada del catéter en comparación con 185/486, 38,1%, que utilizaron placebo, riesgo relativo (RR) 1,55, intervalo de confianza (IC) del 95% 1,36 a 1,76). La incidencia de retención urinaria aguda recurrente fue menor en los grupos tratados con un bloqueador alfa (RR 0,69; IC del 95%: 0,60 a 0,79). Esta evidencia era de calidad moderada y era estadísticamente significativa para la alfuzosina, la tamsulosina y la silodosina, aunque no para la doxazosina. De los ensayos que mencionaban efectos adversos (por ejemplo, hipotensión postural, mareos), no había suficiente información para detectar diferencias estadísticamente significativas entre los grupos (RR 1,19; IC del 95%: 0,75 a 1,89) y la evidencia era de baja calidad. En general, las tasas de efectos adversos eran bajas tanto para el placebo como para los alfabloqueantes y, por ejemplo, los efectos adversos relacionados con la vasodilatación no solían dar lugar a la interrupción. Sin embargo, los datos de esta revisión son limitados debido a la gran cantidad de datos no publicados que no estaban disponibles.
Conclusiones de los autores
Había alguna evidencia que sugería que los alfabloqueantes aumentaban las tasas de éxito de los ensayos sin catéter, y la incidencia de los efectos adversos era baja. Hubo alguna evidencia de una menor incidencia de la retención urinaria aguda. Se desconoce la necesidad de una nueva cirugía, la efectividad en función de los costes y la duración recomendada del tratamiento con alfabloqueantes después de un ensayo satisfactorio sin catéter, ya que ningún ensayo informó al respecto. Faltan medidas de resultado acordadas internacionalmente para lo que constituye un ensayo exitoso sin catéter. Este hecho dificulta el metanálisis. Se requieren ensayos controlados más grandes y bien diseñados, que utilicen las recomendaciones y principios establecidos en la declaración CONSORT, e incluyan medidas de resultados clínicamente importantes.
Resumen en términos sencillos
Tratamiento con alfabloqueantes para hombres para que aumenten las perspectivas de un retiro exitoso de la sonda urinaria
Antecedentes de la enfermedad
La retención urinaria aguda en hombres es una emergencia médica caracterizada por la incapacidad súbita, y a menudo dolorosa, para orinar. Hay muchas causas conocidas, incluyendo la obstrucción de la próstata (debido al agrandamiento de la próstata o al cáncer), estenosis uretral (un estrechamiento de la uretra debido al tejido cicatrizante), infección de orina, estreñimiento y afecciones neurológicas. Un tubo de drenaje estrecho (sonda urinaria) se inserta temporalmente en la vejiga a través del pene para permitir el drenaje de orina. Una vez retirada la sonda, algunos hombres no pueden orinar y requieren la reinserción de la sonda. En estos hombres, las opciones de tratamiento estándar son el uso continuado de sondas o la cirugía de próstata. Las sondas se asocian con riesgos como la infección y pueden afectar la calidad de vida. Por lo tanto, resultan potencialmente beneficiosas las medidas para aumentar la tasa de extracción exitosa de la sonda, es decir, que permitan a los pacientes volver a orinar espontáneamente. Los alfabloqueantes (por ejemplo, tamsulosina, alfuzosín) son un grupo de fármacos conocidos por tener efectos positivos sobre los síntomas urinarios, como el flujo urinario deficiente. Se cree que su efecto de relajación sobre la próstata también puede aumentar las perspectivas de evacuar nuevamente después de la extracción de la sonda. Esta revisión evaluó la evidencia disponible para apoyar esta práctica.
Principales hallazgos de la revisión
En nueve ensayos clínicos se administró a los hombres una tableta ficticia (placebo, fármaco inactivo), un bloqueador alfa durante uno a tres días (en un estudio hasta un máximo de ocho días y en otro durante 32 días) o ningún tratamiento antes de que se retirara el catéter. En circunstancias ideales, ni los pacientes ni los médicos sabían qué tipo de pastilla se administraba, para evitar el sesgo en el informe de los resultados. Los resultados indicaban que el tratamiento con alfabloqueantes aumentaba las posibilidades de retirar con éxito el catéter y volver a orinar, aunque la evidencia científica general de que se disponía para apoyar esta afirmación era limitada. Se probaron cuatro alfabloqueantes diferentes (alfuzosina, tamsulosina, doxazosina y silodosina). Sus resultados fueron similares, salvo en el caso de la doxazosina, que no pareció marcar una diferencia significativa.
Efectos adversos
Los efectos secundarios causados por los bloqueadores alfa fueron pocos y comparables a los de un placebo o a los de ningún tratamiento, aunque esta evidencia fue limitada. Incluyeron eyaculación retrógrada, mareos, baja presión sanguínea, desmayos, somnolencia, malestar y dolor de cabeza.
Conclusiones
Hubo alguna evidencia que indicaba que los alfabloqueantes también reducen el riesgo de sufrir otro episodio (recurrente) de retención urinaria después de la retirada exitosa del catéter, aunque sigue siendo incierto si reducen la necesidad de una futura cirugía de la próstata. Por lo tanto, no está claro si el tratamiento con alfabloqueantes debe continuarse, o por cuánto tiempo, después de la extracción exitosa de la sonda y si se justifican los costes del tratamiento en tales situaciones. Se necesitan más estudios de investigación para responder a estar preguntas.
Authors' conclusions
Summary of findings
| Alpha blocker versus placebo or control for acute urinary retention in adult men | ||||||
| Patient or population: acute urinary retention in adult men | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Aalpha blocker versus placebo or control | |||||
| Ability to void spontaneously after TWOC without the need for re‐catheterisation | Study population | RR 1.55 | 1044 | ⊕⊕⊕⊝ | ||
| 383 per 1000 | 582 per 1000 | |||||
| Incidence of recurrent urinary retention | Study population | RR 0.69 | 1023 | ⊕⊕⊝⊝ | ||
| 507 per 1000 | 350 per 1000 | |||||
| Need for prostatic surgery ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Condition‐specific QoL ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Cost effectiveness ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Number with adverse effects | Study population | RR 1.2 | 657 | ⊕⊕⊝⊝ | ||
| 74 per 1000 | 89 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation unclear in 4 studies and high risk in 1 study. Allocation concealment unclear in 6 studies and high risk in 1 study. | ||||||
Background
Description of the condition
According to International Continence Society, urinary retention is defined as the inability to pass urine despite persistent effort (Haylen 2010). Acute urinary retention in men is a urological emergency. It is characterised by a sudden and often very painful inability to pass urine. The standard first‐line management is bladder catheterisation (insertion of a drainage tube into the bladder). Reported incidence rates of acute urinary retention vary from 0.4% to 25% per year in men seen in urological practice every year (Schulman 2001). One large US cohort study estimated the risk of a retention episode at 23% for a 60‐year old male if he lived for another 20 years (Jacobsen 1997). The incidence of acute urinary retention in the overall male population is low. It increases with age and various studies have reported incidence rates ranging from 2.2 to 6.8 out of 1000 man‐years in the general male population (Jacobsen 1997; Meigs 1999; Temml 2003; Verhamme 2005). This rises to 17 to 25 in 1000 man‐years in men with lower urinary tract symptoms (LUTS) presumed secondary to benign prostatic enlargement (BPE) (Barry 1997; McConnell 1998; Roerborn 2000). It has been claimed that emergency admissions due to retention have a larger adverse impact on patients' health‐related quality of life than emergency admissions for renal colic or elective admission for surgery for LUTS or BPE. This is mainly due to the higher number of investigations and recurrent emergency attendances in the acute urinary retention group compared to the other two groups (Thomas 2005).
The causes of acute urinary retention can be classified into three categories. The first one is characterised by any condition that causes an increased resistance to urinary flow (for example, BPE, bladder neck stenosis, urethral stricture). The second category is characterised by weakness of the detrusor muscle or interruption of the sensory bladder innervation (for example, spinal cord injuries, neurological disorders such as multiple sclerosis). The third category is caused by any event leading to bladder overdistention (for example, following surgery or as a side effect of drug treatment) (Emberton 1999). Retention is also commonly associated with urinary tract infection.
The definitive treatment depends on the underlying cause. The principal aim of treatment is to restore spontaneous voiding without the need for re‐catheterisation and, by doing so, avoiding the risk of catheter‐related complications (such as causing a false urethral passage, infection, pain, haematuria (blood in the urine), urethral stricture formation) and worsening renal function (acute or chronic renal failure) to improve quality of life. Catheters are either introduced by the transurethral or suprapubic route and can be used short term, long term or intermittently.
Description of the intervention
There are three approaches to treatment once symptoms have been controlled by bladder catheterisation.
-
The first is early surgery, such as transurethral resection of the prostate, within a few days or semi‐elective within a few weeks of the first episode of retention. Surgery carries the risk of bleeding, infection, retrograde ejaculation, erectile dysfunction, urinary incontinence, treatment failure and recurrence of lower urinary tract symptoms (Omar 2014).
-
The second approach is long‐term catheterisation, using either an in‐dwelling transurethral or suprapubic catheter, or intermittent catheterisation. This approach has disadvantages such as urinary tract infection (Niël‐Weise 2005).
-
The third option is to remove the catheter again to see whether normal micturition resumes, a trial without catheter (TWOC). This can be done in an ambulant or in‐patient hospital setting.
Success rates reported for TWOC in observational studies range from 23% to 28% (Murray 1984; Taube 1989a). Failure requires re‐catheterisation and re‐assessment of future management options, such as prostate surgery or long‐term catheterisation. There are advantages both for the patient and healthcare system from a successful trial period without a catheter. Apart from not having to spend time with a catheter at home, successful resumption of voiding could mean delaying or ideally avoiding the need for surgery. It has also been shown that surgery, where necessary, is safer in the absence of an indwelling catheter (Pickard 1998).
How the intervention might work
Any intervention which aims to increase the rate of successful TWOC following an acute urinary retention episode is considered potentially beneficial. Alpha1‐adrenoreceptors are highly prevalent in the prostate and bladder neck (Caine 1975; Caine 1987). The principle behind the use of alpha1‐adrenoreceptor antagonists (alpha1 blockers) is that they reduce prostatic smooth muscle tonus and thus resistance to urinary flow (Fulton 1995). This should result in an improvement of urinary symptoms. Caine and colleagues suggested that alpha blockers could improve the chances of successful TWOC, assuming there is adequate detrusor function (Caine 1976). However, their wide use at that time was restricted mainly because of cardiovascular side effects. Functionally more uroselective agents have been developed. Alpha1 blockers such as alfuzosin, doxazosin, indoramin, prazosin, silodosin, tamsulosin and terazosin are commonly used as first‐line treatment for lower urinary tract symptoms associated with BPE.
Despite their greater selectivity, alpha1 blockers can still interact with many other drugs. Their main pharmacological adverse effect, apart from retrograde ejaculation, remains an additive or enhancing hypotensive effect especially when used with other antihypertensive drugs. Known adverse effects include drowsiness, (postural) hypotension, syncope, asthenia, depression, headache, dry mouth, nausea, vomiting, diarrhoea, constipation (gastro‐intestinal disturbances), oedema, blurred vision, rhinitis, erectile disorders (including priapism), tachycardia and palpitations. They should, therefore, be avoided in patients with a history of postural hypotension and micturition syncope. Patients on antihypertensive treatment may require reduced dosage. Alpha blockers are also associated with hypersensitivity reactions including rash, pruritus and angioedema. Caution is advised in elderly patients and those with hepatic or severe renal impairment. Caution is also in elderly people undergoing cataract surgery as there is risk of intra‐operative floppy iris syndrome (most strongly associated with tamsulosin) (BNF December 2013).
Why it is important to do this review
A systematic review of the available evidence from randomised controlled trials (RCTs), periodically updated, is needed to find out whether alpha blockers improve the chances of successful TWOC.
Objectives
To assess the effectiveness of alpha blockers on successful resumption of micturition following removal of a urethral urinary catheter after an episode of acute urinary retention in men. In the absence of internationally agreed outcome measures for the success of a trial without catheter (TWOC), success was defined as the return to satisfactory voiding without need for re‐catheterisation within 24 hours.
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi‐randomised controlled trials (Higgins 2011) of alpha blockers for TWOC after acute urinary retention in men.
Types of participants
Adult men of any age who needed to be catheterised transurethrally because of an episode of acute urinary retention.
Types of interventions
At least one trial group managed with alpha blockers versus any other type of management. Other types of management included placebo or no treatment, or other active treatments such as another alpha blocker.
The following comparisons were addressed:
-
an alpha blocker versus placebo or no treatment;
-
one alpha blocker versus another alpha blocker;
-
an alpha blocker versus another active treatment.
Types of outcome measures
Primary outcomes
-
Ability to void spontaneously after TWOC without the need for re‐catheterisation
-
Incidence of recurrent urinary retention
-
Need for prostatic surgery
-
Adverse effects of alpha blockers
-
Condition‐specific quality of life measures (e.g. IPSS (International Prostate Symptom Score)) (Batista‐Miranda 1995)
-
Cost effectiveness
These six outcomes were considered 'critical' for the purpose of analysis using GRADE.
Secondary outcomes
-
Drop‐out/discontinuation rates (due to adverse effects or lack of therapeutic effect)
-
Persistent lower urinary tract symptoms (LUTS)
-
Quality of life (QoL) (psychological)
-
General quality of life health measures (e.g. SF12, SF36 questionnaires)
Quality of evidence
The quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) (Guyatt 2011; Guyatt 2011a; Guyatt 2013a; Guyatt 2013b). The following five domains were assessed for evaluating the quality of evidence.
-
Limitations of study design
-
Inconsistency of results
-
Indirectness of evidence
-
Imprecision of measurements
-
Publication bias
The primary outcomes listed above were categorised as 'critical' for decision‐making from the male patient's perspective: the remaining secondary outcomes were categorised as 'important' or 'not important'. Evidence related to outcomes deemed 'critical' was then rated as high, moderate, low or very low quality. This rating indicates confidence in the accuracy of the measured effect of intervention compared to the true effect. The quality of evidence was downgraded if the randomisation process (random sequence generation and allocation concealment) of the trial was considered to be inadequate and/or if the effect estimate crossed the line of 'no effect' on either side by 25% or 50% (i.e. an effect estimate with a wide confidence interval). Further reasons to downgrade were if findings between trials were inconsistent or if trials measured outcomes not directly corresponding to the pre‐specified review outcomes. Publication bias was only assessed if there were 10 or more trials. The GRADE working group recommended including up to seven critical outcomes in a systematic review. Quality of evidence was assessed for all primary outcomes if reported (Guyatt 2011; Guyatt 2011a; Guyatt 2013a; Guyatt 2013b).
Search methods for identification of studies
We did not impose any language or other restrictions on the searches.
Electronic searches
This review has drawn on the search strategy developed for the Cochrane Incontinence Review Group. Relevant trials were identified from the Group's Specialised Register of controlled trials which is described, along with the group search strategy, under the Incontinence Group's module in The Cochrane Library. The register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, and handsearching of journals and conference proceedings. The Incontinence Group Specialised Register was searched using the Group's own keyword system. The date of the last search was 9 October 2013.
The terms used to search the Incontinence Group Specialised Register are given below.
({DESIGN.CCT*} OR {DESIGN.RCT*})
AND
({INTVENT.MECH.CATH.TrialOffCatheter.prostate.} OR {INTVENT.MECH.CATH.trialWithoutCath.})
(All searches were of the keyword field of Reference Manager 2012).
For the current version of the review the following databases were also searched (all on OVID SP), the date of the last search for all except CENTRAL was 4 June 2013. The search terms are given in Appendix 1:
-
CENTRAL (2013, Issue 5) was last searched on 5 June 2013
-
MEDLINE 1946 to May Week 4 2013
-
MEDLINE in Process (covering to 3 June 2013)
-
EMBASE Classic andEMBASE 1947 to 2013 Week 22
For the original version of this review (Zeif 2009) extra, specific searches were performed. For details of this search please see Appendix 2.
Searching other resources
The reference lists of relevant articles were checked for other potentially relevant trial reports.
Data collection and analysis
Selection of studies
Only randomised or quasi‐randomised trials were included in this systematic review. Two review authors (EF and MO) independently examined all the citations and abstracts derived from the search strategy. Full reports of potentially relevant trials were then retrieved and examined independently for eligibility in the same manner. Review authors were not blinded to the names of the trials' authors, institutions or the journals. We contacted authors of trials under consideration for additional information when required. Trials in languages other than English were translated as appropriate. Any disagreement about trial selection and inclusion was resolved by discussion. An independent judgment was sought where disagreement persisted. Excluded studies considered formally for this review were also listed along with reasons for their exclusion. Studies were excluded from the review if they were not randomised or quasi‐randomised controlled trials of treatment with alpha blockers for urinary retention, or if they made comparisons other than those pre‐specified. Trials were also excluded if the participants were not relevant.
The process of trial selection is documented in a PRISMA flow chart (Figure 1).

PRISMA study flow diagram
Data extraction and management
Two review authors (EF and MO) independently extracted data from included trials using a standardised form. Any differences of opinion relating to study inclusion, methodological flaws or data extraction were resolved by discussion among the review authors and, when necessary, were referred for independent arbitration. Trial authors were contacted when there was insufficient information. All data entry was done using Review Manager software (RevMan 5.2). Quality of evidence was assessed by adopting GRADE approach by two review authors (EF and MO) using GRADEpro software. Data from included trials were processed according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
The Cochrane 'Risk of bias' assessment tool (Higgins 2011) was used to assess the risk of bias in included trials. Domains of bias included the following.
-
Sequence generation
-
Allocation concealment
-
Blinding of participants or staff
-
Blinding of outcome assessors
-
Incomplete outcome data
-
Selective reporting of outcomes
-
Any other potential sources of bias
These domains were independently assessed by two review authors. Any difference of opinion was resolved by discussion. Where disagreement persisted, a third party was consulted.
Measures of treatment effect
The analyses in this systematic review were based upon available data from all included trials relevant to the comparisons and outcomes being studied. When trials were reported in multiple publications, only the most current or complete data were included for each outcome. When appropriate, meta‐analysis was performed using a fixed‐effect model for calculation of pooled estimates and their 95% confidence intervals (CI).
For categorical outcomes, the number of participants reporting an outcome was related to the number at risk in each group to derive the risk ratio (RR). If continuous variables had been identified, we planned to use the mean and standard deviation to derive a mean difference. For similar outcomes reported using different scales, the standard mean difference (SMD) would have been calculated. When data used to calculate RRs were unavailable, the most detailed available numerical data were used to calculate actual numbers or mean and standard deviations (e.g. P values). Differences between trials were further investigated when significant heterogeneity was found or appeared obvious from visual inspection of the results.
Unit of analysis issues
Analysis of the primary outcome was per man randomised.
Dealing with missing data
As far as possible, data were analysed on an "intention‐to‐treat" basis, so that participants were analysed in the groups to which they were originally randomised. Trials which did not do this were considered for exclusion. In the case of missing data, attempts were made to obtain these from the original trialists (see Acknowledgements). Data were reported as shown in the trials if this was possible except in the case of evidence of differential loss to follow‐up, for which imputation of missing data was considered.
Assessment of heterogeneity
Only trials deemed clinically similar were combined. Heterogeneity between trials was evaluated by visual inspection of forest plots and the I2 test. Thresholds for interpretation of the I2 test were defined according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
In light of the difficulties associated with the detection of, and correction for, publication bias and other biases, the authors strived to minimise their impact by ensuring a fully comprehensive search strategy was used and by monitoring for duplication of data.
Data synthesis
Trials were combined for analysis of similar interventions based upon clinical criteria.
Subgroup analysis and investigation of heterogeneity
Data were subgrouped by the type of alpha blocker received by participants.
-
Alfuzosin
-
Tamsulosin
-
Doxazosin
-
Silodosin
Test for subgroup differences was carried out with Chi2 and I2 tests.
If heterogeneity between trials was evident, we planned investigation to identify its causes.
Sensitivity analysis
We planned to perform sensitivity analyses to determine the effects of including or excluding trials at high risk of bias, but this was not possible due to lack of trials.
Results
Description of studies
Full characteristics of the trials are presented in the tables 'Characteristics of included studies'' and 'Characteristics of excluded studies'.
Results of the search
The literature search resulted in 34 records to assess. The flow of literature through the assessment process is shown in the PRISMA flowchart (Figure 1).
Included studies
Nine randomised controlled trials were included in the systematic review. The trials involved a total of 1044 men who received either an alpha blocker, placebo or no treatment for one to three days in six trials, a maximum of eight days in one trial and 32 days in the other trial, before trial without catheter (TWOC) following an acute urinary retention episode. The number of study participants in each trial ranged from 44 to 360. The trials were conducted in China, India, Singapore, Spain, the UK and USA.
-
Five trials compared alfuzosin versus placebo (Agrawal 2009; McNeill 1999; McNeill 2005; Shah 2002; Tiong 2009).
-
Two trials compared tamsulosin versus placebo (Agrawal 2009; Lucas 2005).
-
One trial compared tamsulosin versus no treatment (Hua 2003).
-
One trial compared doxazosin versus no treatment (Prieto 2008).
-
One trial compared silodosin versus placebo (Kumar 2013).
The primary endpoint of what constitutes successful TWOC was defined differently by the trialists, reflecting the lack of internationally agreed outcome measures.
-
Agrawal 2009 and Prieto 2008 defined it simply as the return to satisfactory voiding after catheter removal.
-
No definition was given by Hua 2003 and McNeill 1999.
-
McNeill 2005 defined successful TWOC as the return to satisfactory voiding without a need for re‐catheterisation within 24 hours.
-
Shah and colleagues (Shah 2002) defined it as the ability to void with a residual volume of 200 mL or less
-
Lucas 2005 used a flow rate greater than 5 mL/sec with a voided volume of greater than 100 mL and residual volume of 200 mL or less.
-
Tiong 2009 and Kumar 2013 defined it as successful voiding with a post void residual volume of less than 150 mL.
The trials used a variety of outcome measures. Apart from successful TWOC, these included:
-
prevention of recurrent urinary retention after successful TWOC (Lucas 2005; McNeill 2005; Shah 2002);
-
need for prostatic surgery (Agrawal 2009; McNeill 2005; Shah 2002);
-
persistent lower urinary tract symptoms (McNeill 2005);
-
post‐void residual volumes (McNeill 1999);
-
condition‐specific QoL (IPSS scores) (McNeill 2005);
-
alpha blocker adverse effects (Lucas 2005; McNeill 1999; McNeill 2005; Tiong 2009);
-
alpha blocker serious adverse effects (McNeill 2005);
-
maximum urinary flow rate (Qmax) (Agrawal 2009; Prieto 2008);
-
drop‐out rates (Lucas 2005; McNeill 1999; McNeill 2005; Prieto 2008; Tiong 2009).
None of the eligible trials addressed outcome measures such as spontaneously voided volume, cost effectiveness, improvement in QoL (other than condition‐specific QoL as assessed by IPSS score) or general health measures (for example SF12 or SF36 questionnaires).
Excluded studies
Nine studies were excluded (Joo 2008; Kraus 2010; Lim 1999; Liu 2009; Lorente 2004; Martov 2010; McNeill 2004; Taube 1989b; Wang 2009). One study involved elective catheterisation prior to spinal surgery (Joo 2008). Four studies did not involve use of a catheter (Kraus 2010; Liu 2009; Martov 2010; Wang 2009). Two studies addressed TWOC in patients without any alpha blocker or other treatment (Lim 1999; Taube 1989b), and one study used increasing dosages of alpha blocker in failed TWOC patients (Lorente 2004). One study involved long‐term follow‐up of patients who had previously had successful TWOC (McNeill 2004).
A 2006 report detailing preliminary results of a trial had been originally included in this review but was superceded by a full publication (Tiong 2009).
Lack of clarity regarding the process of randomisation in one trial (Perepanova 2001) meant that we needed to contact the trialists for additional information. This study was therefore moved into the 'Characteristics of studies awaiting classification' section pending a response.
Risk of bias in included studies
Allocation
Random sequence generation
Four of the included trials clearly described the method of randomisation. These were done by computer‐based randomisation in three trials (Kumar 2013; McNeill 1999; Tiong 2009) and in one trial by a 'centrally established randomization list' (McNeill 2005). Four trials did not adequately specify their methods of randomisation (Agrawal 2009; Hua 2003; Lucas 2005; Shah 2002), and one study (Prieto 2008) was deemed high risk (quasi‐randomised) due to the allocation of patients by year of birth (odd years versus even).
Concealment of allocation
Methods of allocation concealment were inadequately described in six of the included trials (Agrawal 2009; Hua 2003; Kumar 2013; Lucas 2005; McNeill 2005; Shah 2002). One low risk study (McNeill 1999) used a 'sealed copy of the code' to conceal randomisation and another stated that allocation was blinded (Tiong 2009). One study (Prieto 2008) was deemed high risk as allocation concealment was judged to be highly unlikely given the method of randomisation (described above, quasi‐randomised).
Blinding
Blinding of participants and personnel (performance bias)
Blinding of participants and personnel was adequately described in four trials (Lucas 2005; McNeill 1999; McNeill 2005; Tiong 2009), one of which (McNeill 1999) stating that a central pharmacy 'packaged the SR alfuzosin and placebo to appear identical'. This aspect of study design was not adequately described in a further four trials (Agrawal 2009; Hua 2003; Kumar 2013; Shah 2002), and in one trial (Prieto 2008) was deemed to be high risk due to the method of randomisation (described earlier, quasi‐randomised).
Blinding of outcome assessors (detection bias)
None of the included trials (Agrawal 2009; Hua 2003; Kumar 2013; Lucas 2005; McNeill 1999; McNeill 2005; Prieto 2008; Shah 2002; Tiong 2009) gave details of blinding of outcome assessment. Therefore all these trials were judged as "unclear" risk of bias for this domain.
Incomplete outcome data
Seven of the included trials were classified as low risk for incomplete outcome data (Hua 2003; Kumar 2013; Lucas 2005; McNeill 1999; Prieto 2008; Shah 2002; Tiong 2009). It was unclear in one trial (Agrawal 2009) if data were complete due to lack of detail in tables of analysis. One trial was classified high risk due to incomplete data for three patients (McNeill 2005).
Selective reporting
Due to lack of availability of the study protocols, it was not clear if selective reporting was present in any of the included trials (Agrawal 2009; Hua 2003; Kumar 2013; Lucas 2005; McNeill 1999; McNeill 2005; Prieto 2008; Shah 2002; Tiong 2009).
Other potential sources of bias
Two trials (Kumar 2013; Tiong 2009) were classified as low risk of other bias, two trials (Agrawal 2009; Hua 2003) as unclear risk, and five trials (Lucas 2005; McNeill 1999; McNeill 2005; Prieto 2008; Shah 2002) as high risk. Both Kumar 2013 and Tiong 2009 had comparable groups at baseline, had obtained ethical approval and informed consent and had a low withdrawal rate/no withdrawals at all and were judged at low risk of bias. The trials deemed unclear risk did not adequately explain such aspects of study design. All trials deemed high risk received financial support from pharmaceutical companies. Further reasons for this classification included lack of baseline comparability in trials groups (McNeill 1999), lack of justification for reporting both intention‐to‐treat and per‐protocol analyses (McNeill 2005), and inconsistencies in description of trial interventions (Prieto 2008).
Detailed results of the 'Risk of bias' assessment are provided in Figure 2 and Figure 3.
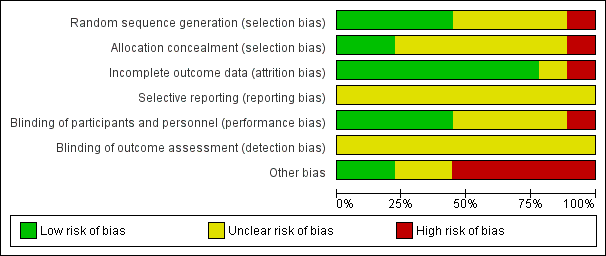
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Nine randomised controlled trials met the inclusion criteria for this review (Agrawal 2009; Hua 2003; Kumar 2013; Lucas 2005; McNeill 1999; McNeill 2005; Prieto 2008; Shah 2002; Tiong 2009). All compared alpha blockers versus placebo. Five trials tested alfuzosin (Agrawal 2009; McNeill 1999; McNeill 2005; Shah 2002; Tiong 2009), three trials tested tamsulosin (Agrawal 2009; Hua 2003; Lucas 2005), one trial tested doxazosin (Prieto 2008) and one trial tested silodosin (Kumar 2013).
Primary outcomes
We applied GRADE to all these primary outcomes and the results are summarised in the summary of findings Table for the main comparison.
Ability to void spontaneously after trial without catheter (TWOC)
All nine trials reported this outcome (Agrawal 2009; Hua 2003; Kumar 2013; Lucas 2005; McNeill 1999; McNeill 2005; Prieto 2008; Tiong 2009; Shah 2002). There was moderate quality evidence that the usage of alpha blockers was better compared to placebo (366/608, 60.2% of men using an alpha blocker were able to void spontaneously after catheter removal compared with 185/486, 38.1% using placebo, risk ratio (RR) 1.55, 95% confidence interval (CI) 1.36 to 1.76) (Analysis 1.1).
On subgroup analysis based upon individual drugs, three types of alpha blockers were statistically significantly better than placebo: alfuzosin RR 1.40 (95% CI 1.19 to 1.64); tamsulosin RR 1.97 (95% CI 1.49 to 2.59); and silodosin RR 2.09 (1.26 to 3.48) (Analysis 1.1). The only trial to investigate doxazosin versus no medication (Prieto 2008) did not show a statistically significant difference (RR 1.09, 0.66 to 1.81, Analysis 1.1.3), but the confidence interval was wide and the trial was small.
Incidence of recurrent acute urinary retention
Eight trials reported this outcome (Agrawal 2009; Kumar 2013; Lucas 2005; McNeill 1999; McNeill 2005; Prieto 2008; Shah 2002; Tiong 2009). There was moderate quality evidence that alpha blockers were effective in reducing the chance of recurrent urinary retention (204/573, 35.6% of men had a further episode of retention after alpha blocker use compared with 228/450, 50.7% after control, RR 0.69, 95% CI 0.60 to 0.79) (Analysis 1.2).
On subgroup analysis based upon individual drugs, statistically significant differences were found to favour three of the drugs trialled (alfuzosin: RR 0.72, 95% CI 0.60 to 0.85; tamsulosin: RR 0.66, 95% CI 0.50 to 0.87; silodosin: RR 0.37, 95% CI 0.18 to 0.74). Only one trial (Prieto 2008) investigated doxazosin and found no difference compared with patients who received no treatment, though the confidence intervals were wide (RR 0.98, 95% CI 0.65 to 1.48)
Three other trials (McNeill 2005; Shah 2002;Tiong 2009) reported recurrent retention (relapse) after successful TWOC but this was carried out during the second phases of the trials after both successful alpha blocker and placebo participants were either re‐randomised or continued on alpha blocker treatment. Therefore, these data could not be used for analysis in this review.
Need for prostatic surgery
Although reported in three trials (McNeill 1999; McNeill 2005; Shah 2002), this outcome measure could not be assessed due to the fact that participants of both the alpha blocker and placebo groups were pooled together in one group following successful TWOC and either randomised again (McNeill 2005), openly followed up (McNeill 1999) or continued (or commenced) on alpha blocker treatment (Shah 2002).
Adverse effects of alpha blockers
Adverse effects described included dizziness, somnolence, fainting, headache, postural hypotension or hypotension, malaise, retrograde ejaculation and syncope. Five trials (Lucas 2005; McNeill 1999; McNeill 2005; Prieto 2008; Tiong 2009) reported this outcome (Analysis 1.3). No statistically significant difference was found in the incidence of adverse effects between alpha blockers and control overall (RR 1.19, 95% CI 0.75 to 1.89), with non‐significant findings also on subgroup analysis (alfuzosin: RR 0.91, 95% CI 0.53 to 1.54; tamsulosin: RR 2.71, 95% CI 0.90 to 8.14); doxazosin: RR 3.13, 95% CI 0.13 to 73.01, though this evidence was of low quality.
Serious adverse effects of alpha blockers
Only one trial (McNeill 2005) mentioned serious alpha blocker adverse effects but did not specify what constituted a 'serious' effect. The number of events was small (3/238 with alfuzosin versus 2/122 with placebo) and the difference was not statistically significant (RR 0.77, 95% CI 0.13 to 4.54) (Analysis 1.4).
Improvement in condition‐specific QoL (IPSS and bother score)
Two trials (Kumar 2013; McNeill 2005) reported IPSS scores.
-
An improvement was described in mean IPSS score with alfuzosin treatment compared with placebo (McNeill 2005) (IPSS: alpha blocker 8.75 versus placebo 11.45, P = 0.012; bother score: alpha blocker 1.66 versus placebo 2.27, P = 0.004), although these patients had been re‐randomised after a successful TWOC.
-
Patients treated with silodosin (Kumar 2013) showed an improvement in mean IPSS scores at TWOC (TWOC: silodosin 25.7 +/‐ 2.5 versus placebo 24.9 +/‐ 1.8, P = 0.02).
In both trials, IPSS scores were only collected in those men who had a successful TWOC. These data were therefore not relevant to the analyses of this review, and the quality of evidence could not be estimated.
Cost effectiveness
No trials reported any information about cost effectiveness.
Secondary outcomes:
The following secondary outcomes were reported in the included trials.
Drop‐out/discontinuation rates (due to adverse effects or lack of therapeutic effect)
Five trials described drop‐out rates due to alpha blocker side effects involving alfuzosin, tamsulosin and doxazosin (Lucas 2005; McNeill 1999; McNeill 2005; Prieto 2008; Tiong 2009). There were lower drop‐out rates for placebo compared to alpha blocker treatment with a statistically significant outcome in favour of placebo (RR 3.94, 95% CI 1.28 to 12.12) (Analysis 1.5). Subgroup analysis showed no statistically significant difference for individual drugs however (alfuzosin: RR 2.83, 95% CI 0.62 to 12.93; tamsulosin: RR 6.91, 95% CI 0.87 to 54.76; doxazosin: RR 3.13, 95% CI 0.13 to 73.01).
Persistent lower urinary tract symptoms (LUTS)
In the second phase (re‐randomisation of successful TWOC patients) of a trial (McNeill 2005), a significant improvement of LUTS was described with alpha blocker treatment compared with placebo at six months follow‐up (IPSS 8.75 versus 11.45, P = 0.012 ; bother score 1.66 versus 2.27, P = 0.004). Due to re‐randomisation, we could not use this data as part of our pre‐defined secondary outcomes.
Discussion
This systematic review suggests that some alpha blockers may help men by increasing the rate of successful trial without catheter (TWOC) after an acute urinary retention episode. However, the current evidence is limited in terms of the number of trials and participants. A major limitation of this review is the lack of standardisation of the definition of a successful TWOC. When is a TWOC considered successful? Does it depend on maximum flow rate, residual volume, or both? Or is it simply no need for re‐catheterisation within 24 hours? Does re‐catheterisation after two or three days following a successful TWOC constitute a failed trial or is it recurrent urinary retention, that is a new event?
Costs and cost effectiveness of alpha blocker treatment is important for both patients and healthcare providers. It would probably make little sense if alpha blockers only delayed prostate surgery for a few months, with the added cost and distress of going back into retention. Because this outcome was not reported in the trials, this review was unable to answer whether alpha blockers before catheter removal are cost effective in this clinical scenario. A few trials tried to address these issues but we could not assess the information as part of a meta‐analysis because men had been treated both with the active drug and a placebo. A review of the literature showed one retrospective study from Belgium that evaluated costs for the first six months after a failed TWOC following a first episode of acute urinary retention (Lamotte 2005). No alpha blockers were used in the study, which came to the conclusion that delayed transurethral resection of the prostate (TURP) during a subsequent or scheduled hospitalisation was less expensive compared to TURP performed during the initial emergency hospital admission.
A follow‐on study to McNeill 2005 examined whether use of alfuzosin during hospitalisation for acute urinary retention and for six months after successful TWOC was cost effective compared to placebo and immediate prostatectomy (Annemans 2005). Alfuzosin treatment was associated with a lower rate of prostatectomy after hospital discharge following successful TWOC. Alfuzosin treatment generated cost savings of £349 relative to placebo and £892 relative to immediate prostatectomy (both statistically significant, P < 0.05). However, this is limited evidence considering the possible implications of this important issue.
Another unanswered question is how long alpha blockers should be continued after successful TWOC? If future trials reporting on longer‐term outcomes suggest that most men on alpha blockers end up having prostate surgery within one year, then this could lead to a change in management strategy where men could be listed for prostate surgery regardless of a successful TWOC. In order to answer these questions, rigorous well‐designed randomised controlled trials, which use the recommendations set out in the CONSORT statement and reporting all critical outcomes, are required.
Summary of main results
The critical outcomes were assessed using the GRADE approach. There was moderate quality evidence that alpha blockers increase success rates of a trial without a catheter. There was low quality evidence that alpha blockers may reduce the risk of recurrent urinary retention. There was insufficient evidence to determine whether the use of alpha blockers reduces the need for prostatic surgery. The effects on QoL, cost effectiveness and recommended duration of alpha blocker treatment after successful trial without catheter remain unknown, as these outcomes were not reported.
Overall completeness and applicability of evidence
Four of the pre‐specified primary outcomes were reported. All of the included trials reported successful TWOC though only three trials addressed incidence of recurrent acute urinary retention directly (Agrawal 2009; Lucas 2005; Prieto 2008).Only one of these reported any useable data on long‐term outcomes according to original allocation status (Prieto 2008). Other trials reported need for re‐catheterisation as a TWOC failure endpoint (Agrawal 2009; Kumar 2013; Lucas 2005; McNeill 1999; McNeill 2005; Prieto 2008; Shah 2002; Tiong 2009). We therefore combined these data by considering need for re‐catheterisation following TWOC as a surrogate marker of relapse of acute urinary retention.
Four trials also addressed the issue of adverse effects due to alpha blocker treatment ( Lucas 2005; McNeill 1999; McNeill 2005; Tiong 2009), with one trial (McNeill 2005) reporting serious (but undefined) adverse effects. Five trials reported drop out due to adverse effects (Lucas 2005; McNeill 1999; McNeill 2005; Prieto 2008; Tiong 2009) but these were rare (less than 5% of men on active treatment).
A condition‐specific quality of life measure (IPSS score) was also reported by two trials (Kumar 2013; McNeill 2005) but the data were only reported in men who had had a successful TWOC, so were not relevant to a comparison between the groups. Other generic measures of quality of life (e.g. SF36) were not reported by any of the included trials.
None of the included trials reported useable data regarding the need for further surgery according to original allocation status. Similarly, other pre‐specified secondary outcomes including urinary flow rate, cost effectiveness or costs were not reported. The incidence of LUTS was reported in the second phase of one trial (McNeill 2005), though again the data were not usable.
Quality of the evidence
The assessment of trial methodology is crucial in determining the quality of the estimated size of treatment effects of all interventions. In this review, methodological flaws of the included trials were assessed using the reports of the trials. Quality of reporting was therefore fundamental to our judgement of methodological quality and quality of effect estimates. None of the reported outcomes deemed 'critical' were assessed to be of high quality, with one outcome deemed moderate quality and two low quality. The quality of evidence of three of the 'critical' outcomes were not estimable.
Potential biases in the review process
Every effort was made to ensure adherence to the methodological framework set out in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). We were nevertheless mindful of potential biases which may enter into the review process and endeavoured to correct these where possible. We ensured that the entire process (such as abstract and full text screening) was done independently by two review authors. An independent third party was involved in case of disagreement in order to minimise potential bias in the review process.
Agreements and disagreements with other studies or reviews
The review authors are unaware of any similar review relating to the subject investigated.

PRISMA study flow diagram
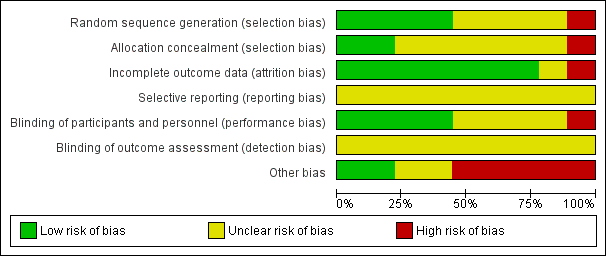
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
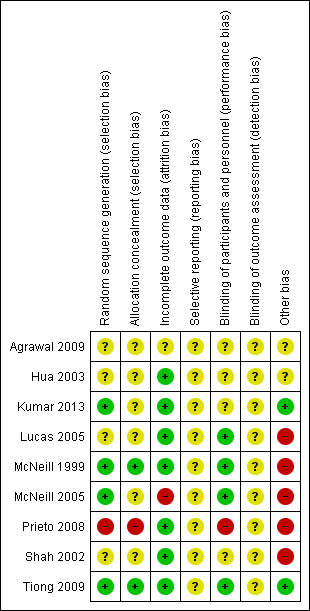
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Alpha blocker versus placebo or control, Outcome 1 Ability to void spontaneously after TWOC without the need for re‐catheterisation.

Comparison 1 Alpha blocker versus placebo or control, Outcome 2 Incidence of recurrent urinary retention.

Comparison 1 Alpha blocker versus placebo or control, Outcome 3 Number with adverse effects due to alpha‐blocker treatment.

Comparison 1 Alpha blocker versus placebo or control, Outcome 4 Number with serious adverse effects due to alpha‐blocker treatment.
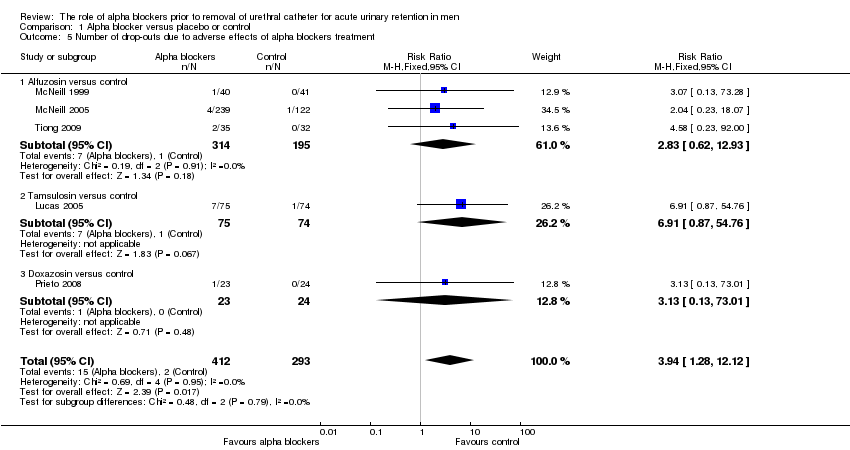
Comparison 1 Alpha blocker versus placebo or control, Outcome 5 Number of drop‐outs due to adverse effects of alpha blockers treatment.
| Alpha blocker versus placebo or control for acute urinary retention in adult men | ||||||
| Patient or population: acute urinary retention in adult men | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Aalpha blocker versus placebo or control | |||||
| Ability to void spontaneously after TWOC without the need for re‐catheterisation | Study population | RR 1.55 | 1044 | ⊕⊕⊕⊝ | ||
| 383 per 1000 | 582 per 1000 | |||||
| Incidence of recurrent urinary retention | Study population | RR 0.69 | 1023 | ⊕⊕⊝⊝ | ||
| 507 per 1000 | 350 per 1000 | |||||
| Need for prostatic surgery ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Condition‐specific QoL ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Cost effectiveness ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Number with adverse effects | Study population | RR 1.2 | 657 | ⊕⊕⊝⊝ | ||
| 74 per 1000 | 89 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation unclear in 4 studies and high risk in 1 study. Allocation concealment unclear in 6 studies and high risk in 1 study. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ability to void spontaneously after TWOC without the need for re‐catheterisation Show forest plot | 9 | 1094 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.36, 1.76] |
| 1.1 Alfuzosin versus control | 5 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.19, 1.64] |
| 1.2 Tamsulosin versus control | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.49, 2.59] |
| 1.3 Doxazosin versus control | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.66, 1.81] |
| 1.4 Silodosin versus control | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [1.26, 3.48] |
| 2 Incidence of recurrent urinary retention Show forest plot | 8 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.60, 0.79] |
| 2.1 Alfuzosin versus control | 5 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.85] |
| 2.2 Tamsulosin versus control | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.50, 0.87] |
| 2.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.48] |
| 2.4 Silodosin versus control | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.74] |
| 3 Number with adverse effects due to alpha‐blocker treatment Show forest plot | 5 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.75, 1.89] |
| 3.1 Alfuzosin versus control | 3 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.54] |
| 3.2 Tamsulosin versus control | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.90, 8.14] |
| 3.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 73.01] |
| 4 Number with serious adverse effects due to alpha‐blocker treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Alfuzosin versus control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of drop‐outs due to adverse effects of alpha blockers treatment Show forest plot | 5 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.94 [1.28, 12.12] |
| 5.1 Alfuzosin versus control | 3 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.62, 12.93] |
| 5.2 Tamsulosin versus control | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.91 [0.87, 54.76] |
| 5.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 73.01] |

