La función de los alfabloqueantes antes de la retirada de la sonda urinaria para la retención urinaria aguda en los hombres
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006744.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 10 junio 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Incontinencia
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
For the 2014 update, two review authors (EF, MO) independently assessed the identified trials, extracted data and performed 'Risk of bias' assessment. EF and MO assessed the quality of evidence of the critical outcomes. All review authors contributed to the analysis of data. EF took the lead in modifying the manuscript and all review authors contributed to writing the manuscript.
Hans‐Joerg Zeif conceived, drafted and wrote the original review. Kesavapillai Subramonian critically revised and approved the review. Both review authors independently examined all the citations and abstracts derived from the search strategy and extracted, cross‐checked and processed data independently as described in the Cochrane Collaboration Handbook.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
The National Institute for Health Research, UK.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Incontinence Group.
Declarations of interest
The review authors have no conflict of interest to declare.
Acknowledgements
The review authors would like to acknowledge Hans‐Joerg Zeif for his contribution to the first version of the review (Zeif 2009). Our sincere gratitude goes to Malik Mukhitdinov for his services in translation of a Russian study (Perepanova 2001) and for the kind responses of the trial authors Dr Luis Prieto Chaparro and Dr Marlon Jay Tibung to our questions. We would also like to express our thanks to the members of the Cochrane Incontinence Review Group.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Jun 10 | The role of alpha blockers prior to removal of urethral catheter for acute urinary retention in men | Review | Euan Fisher, Kesavapillai Subramonian, Muhammad Imran Omar | |
| 2009 Oct 07 | Alpha blockers prior to removal of a catheter for acute urinary retention in adult men | Review | Hans‐Joerg Zeif, Kesavapillai Subramonian | |
| 2009 Jul 08 | Alpha blockers for removal of urethral catheter after acute urinary retention in men | Protocol | Hans‐Joerg Zeif, Kesavapillai Subramonian | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Disease;
- Adrenergic alpha-Antagonists [adverse effects, *therapeutic use];
- *Device Removal;
- Doxazosin [therapeutic use];
- Indoles [therapeutic use];
- Quinazolines [adverse effects, therapeutic use];
- Randomized Controlled Trials as Topic;
- Recovery of Function [drug effects];
- Sulfonamides [adverse effects, therapeutic use];
- Tamsulosin;
- Urinary Catheterization [*instrumentation];
- Urinary Retention [*therapy];
- Urination [drug effects];
Medical Subject Headings Check Words
Adult; Humans; Male;

PRISMA study flow diagram
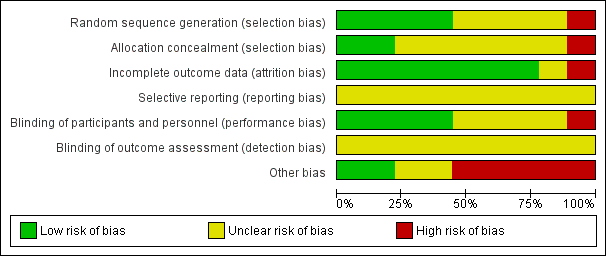
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
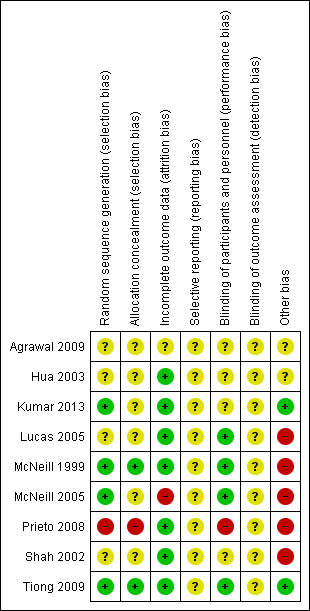
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Alpha blocker versus placebo or control, Outcome 1 Ability to void spontaneously after TWOC without the need for re‐catheterisation.

Comparison 1 Alpha blocker versus placebo or control, Outcome 2 Incidence of recurrent urinary retention.

Comparison 1 Alpha blocker versus placebo or control, Outcome 3 Number with adverse effects due to alpha‐blocker treatment.

Comparison 1 Alpha blocker versus placebo or control, Outcome 4 Number with serious adverse effects due to alpha‐blocker treatment.
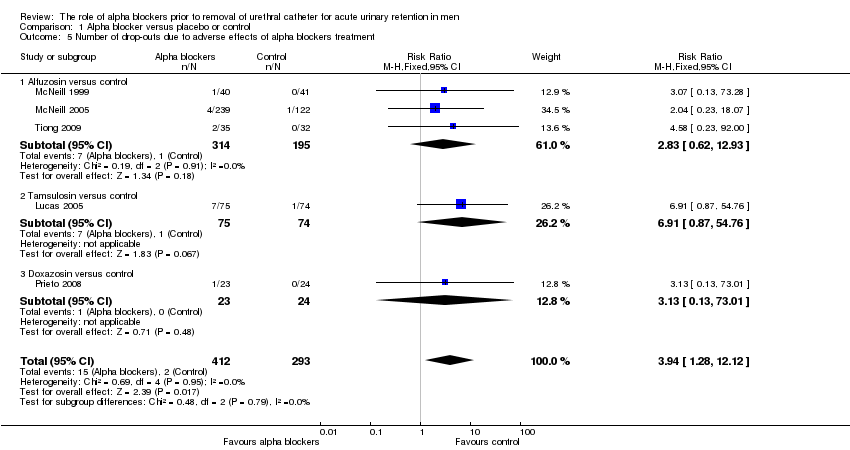
Comparison 1 Alpha blocker versus placebo or control, Outcome 5 Number of drop‐outs due to adverse effects of alpha blockers treatment.
| Alpha blocker versus placebo or control for acute urinary retention in adult men | ||||||
| Patient or population: acute urinary retention in adult men | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Aalpha blocker versus placebo or control | |||||
| Ability to void spontaneously after TWOC without the need for re‐catheterisation | Study population | RR 1.55 | 1044 | ⊕⊕⊕⊝ | ||
| 383 per 1000 | 582 per 1000 | |||||
| Incidence of recurrent urinary retention | Study population | RR 0.69 | 1023 | ⊕⊕⊝⊝ | ||
| 507 per 1000 | 350 per 1000 | |||||
| Need for prostatic surgery ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Condition‐specific QoL ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Cost effectiveness ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Number with adverse effects | Study population | RR 1.2 | 657 | ⊕⊕⊝⊝ | ||
| 74 per 1000 | 89 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation unclear in 4 studies and high risk in 1 study. Allocation concealment unclear in 6 studies and high risk in 1 study. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ability to void spontaneously after TWOC without the need for re‐catheterisation Show forest plot | 9 | 1094 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.36, 1.76] |
| 1.1 Alfuzosin versus control | 5 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.19, 1.64] |
| 1.2 Tamsulosin versus control | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.49, 2.59] |
| 1.3 Doxazosin versus control | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.66, 1.81] |
| 1.4 Silodosin versus control | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [1.26, 3.48] |
| 2 Incidence of recurrent urinary retention Show forest plot | 8 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.60, 0.79] |
| 2.1 Alfuzosin versus control | 5 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.85] |
| 2.2 Tamsulosin versus control | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.50, 0.87] |
| 2.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.48] |
| 2.4 Silodosin versus control | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.74] |
| 3 Number with adverse effects due to alpha‐blocker treatment Show forest plot | 5 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.75, 1.89] |
| 3.1 Alfuzosin versus control | 3 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.54] |
| 3.2 Tamsulosin versus control | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.90, 8.14] |
| 3.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 73.01] |
| 4 Number with serious adverse effects due to alpha‐blocker treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Alfuzosin versus control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of drop‐outs due to adverse effects of alpha blockers treatment Show forest plot | 5 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.94 [1.28, 12.12] |
| 5.1 Alfuzosin versus control | 3 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.62, 12.93] |
| 5.2 Tamsulosin versus control | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.91 [0.87, 54.76] |
| 5.3 Doxazosin versus control | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 73.01] |

