Heparina de bajo peso molecular para la prevención del tromboembolismo venoso en pacientes con extremidades inferiores inmovilizadas
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: prospective, randomized, controlled, single‐blind, multicenter study Method of randomization: sealed, numbered envelopes at a ratio of 1:1:1 in blocks of 15, stratified according to centre, to one of the three study groups, by the treating physician at the ED Concealment of allocation: sealed, numbered envelopes by treating physician at the ED, who was not involved in the remainder of the trial Losses to follow‐up: 124 (62 treatment group, 62 control group). Reasons for withdrawal: no fracture, no plaster cast, immobilization < 4 weeks, indication for surgery, no duplex sonography, withdrawal of consent | |
| Participants | Country: The Netherlands Number randomized: 310 (treatment group 154; control group 156) Number completed study and used in analysis, reported in study publication: 186 (treatment group 92; control group 94) Age mean (SD): treatment group 47.7 (16.4); control group 44.5 (17.2) Sex (male/female): treatment group 39/53; control group 38/56 Inclusion criteria: a fracture of the ankle or foot, non‐surgical treatment with immobilization in a below‐knee plaster cast for a minimum of four weeks Exclusion criteria: a delay between injury and the emergency department visit of more than 72 h, a known hypersensitivity to nadroparin or fondaparinux, a history of venous thromboembolism, continuous anticoagulant therapy, hypercoagulability, a bleeding tendency or disorder, pregnancy or lactation, ‘active’ malignancy, a severe hepatic or renal impairment (deficiency of clotting factors or creatinine clearance < 30 mL/min), retinopathy, previous or active bleeding from the digestive tract, a hemorrhagic stroke within the previous two months, major surgery within the previous two months, intraocular, spinal, or brain surgery within the previous year, and severe hypertension (systolic blood pressure above 180 mmHg or diastolic blood pressure above 110 mmHg) | |
| Interventions | Treatment group: Nadroparin 2850 IE anti‐Xa = 0.3 mL, given once daily Control group: no prophylaxis | |
| Outcomes | A venous duplex sonography of the affected leg after removal of the cast on the final day of medication administration, or earlier if thrombosis was suspected | |
| Notes | A second treatment group receiving Fondaparinux was not included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..patients were enrolled and randomly assigned (by use of sealed, numbered envelopes, at a ratio of 1:1:1 in blocks of 15, stratified according to centre) to one of the three study groups, by the treating physician at the ED." |
| Allocation concealment (selection bias) | Low risk | Sealed, numbered envelopes from treating physician at the ED, who was not involved in the remainder of the trial |
| Blinding of participants and personnel (performance bias) | Unclear risk | Reported as a single‐blind study. Blinding of participants was not reported, blinding of personnel other than the ultrasound technician was not reported |
| Blinding of outcome assessment (detection bias) | Low risk | The ultrasound technician who assessed the primary outcome was blinded to the treatment |
| Incomplete outcome data (attrition bias) | High risk | 124/310 participants were excluded from the analysis after randomization, 62 in both treatment and control group. Reasons for withdrawal: no fracture, no plaster cast, immobilization < 4 weeks, indication for surgery, no duplex sonography, withdrawal of consent |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported |
| Other bias | Low risk | No other bias was detected |
| Methods | Study design: randomized, controlled, assessor‐blinded, open, multicenter trial Method of randomization: random numbers Concealment of allocation: sealed envelopes Losses to follow‐up: 95; treatment group 49; control group 46. (discomfort with self‐injection 18, methrorrhagia 1, refused phlebography 12, not possible to perform venography 26, miscellaneous 38 | |
| Participants | Country: Denmark Number randomized: 300 (treatment group 148; control group 152) Number reported, included in analysis, presented in study publication: 205 (treatment group 99; control group 106) Age: adult patients > 18 years (range 18 to 93) Sex (male/female): treatment group 79/69; control group 93/59 Inclusion criteria: planned plaster immobilization of the lower leg for at least 3 weeks Exclusion criteria: pregnancy, allergy to heparin or contrast media, known liver or renal impairment, uncontrolled hypertension, bleeding disorders, recent GI bleeding, or inability to perform self injection | |
| Interventions | Treatment group: LMWH 3500 IU anti‐Xa of tinzaparin (Innohep) once daily Control group: no prophylaxis | |
| Outcomes | At cast removal, unilateral venography was performed | |
| Notes | Dose of tinzaparin relatively low, contained both operated and non‐operated patients, previous DVT was not excluded, 205/300 were included in final assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Patients were either treated with Tinzaparin, or received no treatment. A placebo was not used. |
| Blinding of outcome assessment (detection bias) | Low risk | ' assessor‐blinded'; two radiologists, unaware of treatment, independently assessed the venograms |
| Incomplete outcome data (attrition bias) | High risk | 95 out of 300 patients were lost to follow‐up. They were evenly divided between groups (treatment group = 49, no treatment group = 46). Reasons for losses to follow‐up were discomfort with self‐injection (18), metrorrhagia (1), refusal of phlebography (12), not possible to perform venography (26), and miscellaneous (38). Reasons varied between the two groups. |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes were not described in methods. Therefore, it was unclear whether all assessed outcomes were reported. |
| Other bias | Low risk | No other bias was detected. |
| Methods | Study design: randomized, controlled, open trial Method of randomization: randomization list stratified for varicose veins and obesity Concealment of allocation: not reported Losses to follow‐up: 5 refused to take part, 32 excluded due to exclusion criteria, data not evaluated from 52: treatment group 21; control group 31 | |
| Participants | Country: Germany Number randomized: 428; 5 refused to take part, 32 excluded due to exclusion criteria, data not evaluated from 52: treatment group 21; control group 31 (no final examination (12 treatment; 16 control), surgery performed before final examination (6 treatment, 12 control), changed groups (3 treatment, 3 control)) Number reported, included in analysis, presented in study publication: 339 (treatment group 176; control group 163) Age mean (range): treatment group 34.1 years (18 to 63); control group 33.5 years (18 to 64) Sex (male/female): treatment group 104/72; control group 104/59 Inclusion criteria: age 18 to 65, conservative treatment of injury with below‐knee cast or cylinder cast Exclusion criteria: previous DVT, pregnancy, clotting disorders or anticoagulation medication, bleeding, chronic venous insufficiency, contraindications for heparin prophylaxis, plaster cast after surgery | |
| Interventions | Treatment group: LMWH 32 mg (certoparin; Mono‐Embolex NM) once daily Control group: no prophylaxis | |
| Outcomes | At randomization and at plaster removal, compression ultrasound and duplex scanning were performed; suspected positive findings were confirmed by phlebography. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization with lists stratified for varicose veins and obesity |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Open study format, in which no placebo was used. The treatment group received injections of LMWH, the control group received none. Blinding of personnel was not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Data of 52 out of 428 randomized participants could not be evaluated, reasons provided |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes were not described. Therefore, it was unclear whether all assessed outcomes were reported. |
| Other bias | Low risk | No other bias was detected. |
| Methods | Study design: randomized, controlled, open trial Method of randomization: randomization plan "after Sachs" Concealment of allocation: not reported Losses to follow‐up: 53 excluded post randomization (12 in treatment group interrupted prophylaxis without permission, 14 control group patients received prophylaxis, 18 lost to follow‐up, 6 participants operated on before 7th day, and 3 participants had cast removed before 7th day | |
| Participants | Country: Germany Number randomized: 306, 53 excluded (12 in treatment group interrupted prophylaxis without permission, 14 control group patients received prophylaxis, 18 lost to follow‐up, 6 participants operated on before 7th day, and 3 participants had cast removed before 7th day Number included in analysis: 253; treatment group 126; control group 127 Age mean (range): treatment group 32.9 years (16 to 70); control group 35.6 years (16 to 76) Sex (male/female): treatment group 69/57; control group 77/50 Inclusion criteria: age over 16 years, injury of the lower limb being treated conservatively, immobilization by a plaster cast applied for at least 7 days Exclusion criteria: known thrombopathy, oral anticoagulation, recent brain or GI bleeding, acute pancreatitis, inflammatory heart disease | |
| Interventions | Treatment group: LMWH 36 mg heparin fraction calcium (nadroparin; Fraxiparin) once daily Control group: no prophylaxis | |
| Outcomes | After plaster removal, or at occurrence of symptoms, compression ultrasound to diagnose DVT; in case of doubtful or positive findings, a phlebography was carried out. In case of suspected PE, scintigraphic analysis was performed. | |
| Notes | None of the patients were operated on. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were allocated to two groups according to a random plan after Sachs". Unclear method of randomization |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of personnel was not reported. "Patients of group II did not receive heparin". A placebo was not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | 53 out of 306 patients were excluded for various reasons. It is unclear how those were divided over the two groups. Reasons for exclusion were patient interruption of prophylaxis, patients receiving prophylaxis from co‐treating practitioner, change of treating physician, surgery before 7th day. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes were reported. However, nothing was reported on possible adverse events. |
| Other bias | Low risk | No other bias was detected |
| Methods | Study design: randomized, controlled, double‐blind trial Method of randomization: by computer Concealment of allocation: not specifically reported Losses to follow‐up: 4 (withdrawal of consent treatment group 2, control group 2) and excluded from efficacy analysis | |
| Participants | Country: Sweden Number randomized: 105; treatment group 52; control group 53 Number reported, included in analysis 1 (all participants with negative color duplex sonography, and all participants with DVT verified by phlebography): 91; treatment group 47; control group 44 Number reported, included in analysis 2 (all participants with color duplex sonography for patients with multiple distal DVT or proximal DVT, and all participants with DVT verified by phlebography): 96; treatment group 49; control group 47 Age mean (SD): treatment group 37 years (8); control group 42 years (9) Sex (male/female): treatment group 41/11; control group 42/11 Inclusion criteria: age 18 to 75 years, admitted for an acute (0 to 72 hours) Achilles tendon rupture, accepted for surgery Exclusion criteria: inability or refusal to give informed consent, ongoing treatment with anticoagulant therapy, known allergy for contrast media, kidney disorder, recent thromboembolic event, recent surgery, known malignancy, current bleeding disorder, pregnancy, treatment with platelet inhibitors | |
| Interventions | Treatment group: LMWH dalteparin 5000 units sc once daily until removal of the plaster cast Control group: placebo | |
| Outcomes | Diagnosis of DVT by means of ultrasound and confirmation by venography | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization by computer, no further information provided. However, patients were not included when study personnel were off duty |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study described as double‐blind. Each patient received a box containing 45 prefilled syringes with either Dalteparin or placebo. Syringes of both groups were identical. Blinding of personnel was not explicitly described. |
| Blinding of outcome assessment (detection bias) | Low risk | "evaluation was carried out by an experienced independent radiologist blinded to the randomization and previous phlebographic findings" |
| Incomplete outcome data (attrition bias) | Low risk | 4 patients were lost to follow‐up, evenly divided between the two groups. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were reported, as well as any adverse event. |
| Other bias | Low risk | No other bias was detected. |
| Methods | Study design: randomized, controlled, double‐blind trial Method of randomization: not reported Concealment of allocation: not reported Losses to follow‐up: 75 considered non‐evaluable for primary analysis (35 treatment group and 40 in control group), due to: withdrawal of consent (38), technical failure of phlebography (27), refracture or resurgery (4), failure of protocol compliance (3), minor bleeding (1), inconclusive phlebography (1), never received allocated treatment due to DVT before start of treatment (1). Exclusions were evenly divided over the two groups | |
| Participants | Country: Sweden Number randomized: 272; treatment group 136; control group 136 Number reported, included in analysis 1 (assessment using phlebography): 197; treatment group 101; control group 96 Number reported, included in analysis 2 (assessment using phlebography plus color duplex sonography): 226; treatment group 117; control group 109 Age mean (SD): treatment group 49 years (14); control group 48 years (14) Sex (male/female): treatment group 62/74; control group 62/74 Inclusion criteria: age 18 to 75 years, admitted for an acute (0 to 72 hours) ankle fracture and accepted for surgery Exclusion criteria: inability or refusal to give informed consent, ongoing treatment with anticoagulant therapy, known allergy for contrast media, kidney disorder, recent thromboembolic event, recent surgery, known malignancy, current bleeding disorder, pregnancy, treatment with platelet inhibitors, multi‐trauma | |
| Interventions | Treatment group: LMWH dalteparin 5000 units sc once daily until removal of the plaster cast Control group: placebo | |
| Outcomes | Diagnosis of DVT by means of ascending venography | |
| Notes | All patients treated with LMWH one week before randomization | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not described. Patients were not included when study personnel were off duty. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study described as double‐blind. Patients were blinded by using identical prefilled syringes. Blinding of personnel was not explicitly described. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome measures were assessed by a radiologist blinded to randomization and previous imaging findings. |
| Incomplete outcome data (attrition bias) | Low risk | 75 out of 272 patients were considered non‐evaluable for primary analysis due to withdrawal of consent (38), technical failure of phlebography (27), refracture or resurgery (4), failure of protocol compliance (3), minor bleeding (1), inconclusive phlebography (1), never received allocated treatment due to DVT before start of treatment (1). Exclusions were evenly divided over the two groups. |
| Selective reporting (reporting bias) | Low risk | Primary efficacy, secondary efficacy and any adverse events were assessed and reported |
| Other bias | Low risk | No other bias was detected |
| Methods | Study design: randomized, controlled, double‐blind trial Method of randomization: computer (blocks of four) Concealment of allocation: not specifically reported Losses to follow‐up: 69 excluded from efficacy analysis: 2 received no injections (control group), 2 withdrew consent (treatment group), 4 withdrew because of adverse events (1 treatment, 3 control group), 61 did not have venograms that could be evaluated (31 treatment, 30 control group) | |
| Participants | Country: Denmark Number randomized: 440; treatment group 217; control group 223. Number reported, included in analysis, reported in study publication: 371; treatment group 183; control group 188) Age median (interquartile range): treatment group 47 years (37 to 55); control group 47 years (37 to 56). Sex (male/female): treatment group 112/105; control group 114/108. Inclusion criteria: age 18 years or older, fracture of the leg or rupture of the Achilles tendon requiring at least 5 weeks of immobilization in a plaster cast or brace within 4 days of the injury Exclusion criteria: body weight < 35 kg, pre‐existing VTE, systolic BP > 200 mmHg or diastolic BP > 110 mm Hg, cerebral vascular aneurysm, cerebral vascular accident within the preceding 3 weeks, active gastroduodenal ulcer, bacterial endocarditis, platelet count 1000,000/cu mm, previous treatment with UFH or LMWH, fibrinolytic agents, or oral anticoagulants, known hypersensitivity to contrast media, kidney disorder, MI within the preceding 3 months, multiple myeloma, pregnancy, history of drug or alcohol abuse | |
| Interventions | Treatment group: LMWH reviparin (1750 anti‐Xa units) once daily Control group: placebo | |
| Outcomes | To diagnose DVT, venography was performed within one week after removal of the plaster. In cases of suspected PE, ventilation‐perfusion lung scanning or pulmonary angiography was performed. | |
| Notes | Study contained both operated and non‐operated patients; up to four days of LMWH prophylaxis was allowed before randomization. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | " randomization was performed by computer in blocks of four" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind study. Patients received identical prefilled syringes in both groups. Personnel were blinded until database was locked and results were revealed |
| Blinding of outcome assessment (detection bias) | Low risk | Venograms were evaluated by experienced radiologists, blinded to the treatment assignments. |
| Incomplete outcome data (attrition bias) | Low risk | 69/440 participants were excluded, divided evenly over both groups. Exclusion was mainly due to the technical impossibility to perform a venogram in the patient (61 participants). |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | No other bias was detected. |
| Methods | Study design: multicenter, controlled, randomized, open‐label with blinded outcome Method of randomization: computer‐generated block randomization with variable block sizes Concealment of allocation: data management unit, physicians, and researchers were unaware of the randomization scheme and block sizes Losses to follow‐up: after randomization, 33 excluded as either failed inclusion or met exclusion criteria, 23 withdrew consent, and 28 lost to follow‐up | |
| Participants | Country: The Netherlands Number randomized: 1519; treatment 761; control group 758 Number reported ,included in analysis, reported in study publication: 1435; treatment group 719; control group 716 Age mean (SD): treatment group 46.5 (16.5); control group 45.6 (16.4) Sex (male/female): treatment group 347/372; control group 369/347 Inclusion criteria: patients 18 years of age or older who presented to the emergency department, and were treated for at least 1 week with casting of the lower leg (with or without surgery, before or after casting, but without multiple traumatic injuries) Exclusion criteria: history of venous thromboembolism, contraindications to low molecular weight heparin therapy, pregnancy, current use of anticoagulant therapy for other indications (use of antiplatelet drugs was allowed) | |
| Interventions | Treatment group: LMWH once daily SC injection of 2850 IU nadroparin or 2500 IU dalteparin for participants ≤ 100 kg, or a double dose for participants weighing > 100 kg (LMWH (nadroparin or dalteparin) chosen according to preference at the hospital) Control group: no prophylaxis | |
| Outcomes | Cumulative incidence of venous thromboembolism within 3 months of the procedure Safety outcome: cumulative incidence of major bleeding | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization with variable block sizes |
| Allocation concealment (selection bias) | Low risk | "To ensure concealment of treatment assignment the data management unit, physicians, and researchers were unaware of the randomization scheme and block sizes." |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial, participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | All outcomes were assessed by an independent committee whose members were unaware of assignment. |
| Incomplete outcome data (attrition bias) | Low risk | 84/1519 participants lost to follow‐up, withdrew consent, or failed inclusion criteria and were not included in the analysis. 93/719 patients did not adhere to the LMWH trial regimen. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary effective. as well as safety outcomes were reported. |
| Other bias | Low risk | No other bias was detected. |
BP: blood pressure
DVT: deep vein thrombosis
ED: emergency department
GI: gastrointestinal
Hg: mercury
IU: international units
LMWH: low molecular weight heparin
MI: myocardial infarction
PE: pulmonary embolism
sc: subcutaneous
UFH: unfractionated heparin
VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Prospective incidence study, not a randomized or controlled clinical trial, operated patients without plaster immobilization and LMWH, clinical and not outpatients | |
| Survey on thrombosis prophylaxis by Italian orthopedic surgeons | |
| Not a randomized or controlled clinical trial | |
| Not a randomized or controlled clinical trial | |
| Patients operated on for tendon rupture, one group treated with plaster and LMWH, the other group with early functional mobilization without prophylaxis. No clinical DVTs seen. | |
| No LMWH used; trial was on compression stockings. | |
| Not a randomized or controlled clinical trial | |
| Not a randomized or controlled clinical trial | |
| LEAP‐study (LMWH expedited Anticoagulation Program) to decrease number of inpatient days on warfarin, and total hospital days for trauma patients requiring DVT; case‐control study | |
| Trial protocol | |
| Patients included did not meet inclusion criteria; no lower extremity trauma or immobilization, only healthy patients | |
| Randomized controlled trial; comparison of fondaparinux and enoxaparin after hip‐fracture surgery | |
| Not a randomized or controlled clinical trial | |
| Patients included did not meet inclusion criteria: no lower extremity trauma or /immobilization, only ICU patients | |
| Prospective study of VTE after major trauma; no prophylaxis against VTE | |
| Randomized controlled clinical trial of LMWH and low‐dose heparin; focus on major trauma, not outpatients | |
| Prospective clinical study to determine incidence of DVT, no antithrombotic treatment | |
| Randomized controlled clinical trial; comparison of acetylsalicylic acid with LMWH in plaster immobilized trauma patients | |
| Prospective clinical study to determine incidence of DVT in selected patients, no antithrombotic treatment | |
| A prospective randomized double‐blind controlled trial using LMWH with saline injection as placebo in adults who had sustained an isolated fracture below the knee that required operative fixation. Study authors did not focus on immobilization of the lower leg in plaster‐cast, so this study did not meet our inclusion criteria. The study authors included 238 patients, and all underwent bilateral venography for diagnosis of DVT. There was no statistically significant difference in the incidence of DVT between the patients treated with LMWH or placebo (P = 0.22). However, owing to a cessation of funding, recruitment had to be ended before the necessary sample size was established (another reason for exclusion). The study results could not categorically exclude a potentially beneficial role of LMWH treatment, and the authors recommended a further randomized controlled trial be undertaken. | |
| Randomized controlled clinical trial. Clinical trauma patients were randomized to low‐dose UFH, LMWH, pneumatic compression devices, or foot pumps with or without vena caval filters. | |
| Observational study on the use and tolerance of LMWH in ambulatory patients; not focused on trauma | |
| Prospective cohort study to determine the clinical incidence of VTE and the tolerance to LMWH in operated and not operated surgical and orthopedic patients | |
| Not a randomized or controlled clinical trial | |
| Patients included did not meet inclusion criteria; no lower extremity trauma or immobilization, only patients with DVT | |
| Review on treatment for acute tears of the lateral ligaments of the ankle; not focused on subject of thrombosis prophylaxis | |
| Randomized controlled clinical trial; LMWH in high‐risk trauma patients, compared with mechanical methods of prophylaxis | |
| Retrospective study to identify VTE incidence and risk factors in trauma patients using the American College of Surgeons National Trauma Data Bank | |
| Not a randomized or controlled clinical trial | |
| Not a randomized or controlled clinical trial | |
| Patients included did not meet inclusion criteria; no lower extremity trauma or immobilization, only ICU patients | |
| Not a randomized or controlled clinical trial | |
| This study focused on prolonged thrombosis prophylaxis after arthroscopic surgery rather than immobilization of the lower leg after trauma, and was excluded for that reason. | |
| Not a randomized or controlled clinical trial | |
| Case report, expert opinion | |
| The purpose of this ongoing study sponsored by GlaxoSmithKline is to evaluate the efficacy and safety of fondaparinux in comparison with a LMWH (nadroparin) in preventing VTE in patients with leg injuries below the knee that require a cast or other type of immobilization, but not surgery. This study does not meet our inclusion criteria (LMWH versus placebo or no prophylaxis) | |
| Case report and literature review | |
| Not a randomized or controlled clinical trial | |
| Not a randomized or controlled clinical trial | |
| Treatment protocol did not meet inclusion criteria; comparing fondaparinux with LMWH | |
| Not a randomized or controlled clinical trial | |
| Focus on multiple trauma patients. | |
| In this study (known as the D‐KAF trial), consecutive patients with isolated fractures of the distal leg requiring surgery were randomized to dalteparin 5000 IU or placebo once daily SC. Patients were screened using proximal ultrasound (only of the upper leg, not the calf) at day 14. The researchers were interested in clinically important venous thromboembolism (CIVTE). The study authors found that the overall incidence of CIVTE was so low (1.9%; 95% CI 0.7 to 4.7%), with no observed differences between dalteparin and placebo, that recruitment was stopped early. For this reason, we did not include this study in our meta‐analysis. However, the study demonstrated the large discrepancy between trials that use venographic outcomes (all DVTs) and CIVTE. | |
| Not a randomized or controlled clinical trial | |
| Not a randomized or controlled clinical trial | |
| Cohort of 515 patients with plaster immobilization of the lower leg treated with LMWH; no comparison | |
| Retrospective data and prospective study to evaluate self‐injection of UFH and LMWH. |
CIVTE: clinically important venous thromboembolism
DVT: deep vein thrombosis
HIT: heparin‐induced thrombocytopenia
ICU: intensive care unit
LMWH: low molecular weight heparin
SC: subcutaneous
UFH: unfractionated heparin
VTE: venous thromboembolism
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Deep venous thrombosis: regardless of type of plaster, whether operated or not Show forest plot | 7 | 1676 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.33, 0.61] |
| Analysis 1.1 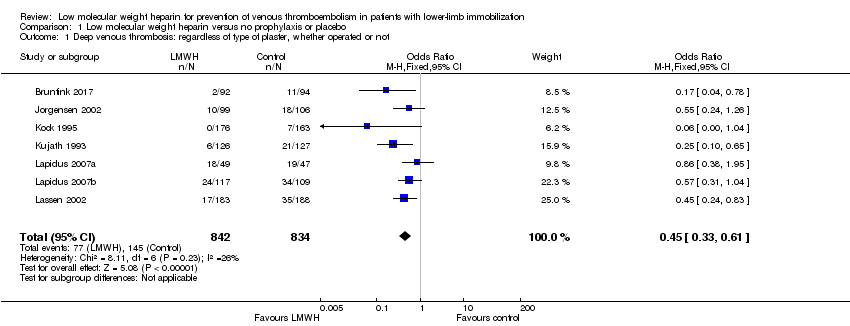 Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 1 Deep venous thrombosis: regardless of type of plaster, whether operated or not. | ||||
| 2 Deep venous thrombosis: in below‐knee cast, whether operated or not Show forest plot | 6 | 1080 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.34, 0.72] |
| Analysis 1.2  Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 2 Deep venous thrombosis: in below‐knee cast, whether operated or not. | ||||
| 3 Deep venous thrombosis: conservative treatment (i.e. non‐operated patients) Show forest plot | 5 | 974 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.18, 0.53] |
| Analysis 1.3  Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 3 Deep venous thrombosis: conservative treatment (i.e. non‐operated patients). | ||||
| 4 Deep venous thrombosis: operated patients Show forest plot | 4 | 699 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.37, 0.80] |
| Analysis 1.4 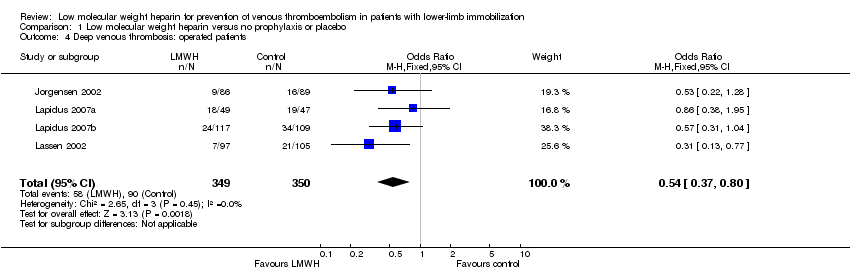 Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 4 Deep venous thrombosis: operated patients. | ||||
| 5 Deep venous thrombosis: fractures Show forest plot | 6 | 1003 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.33, 0.70] |
| Analysis 1.5  Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 5 Deep venous thrombosis: fractures. | ||||
| 6 Deep venous thrombosis: soft‐tissue injuries Show forest plot | 5 | 658 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.22, 0.68] |
| Analysis 1.6 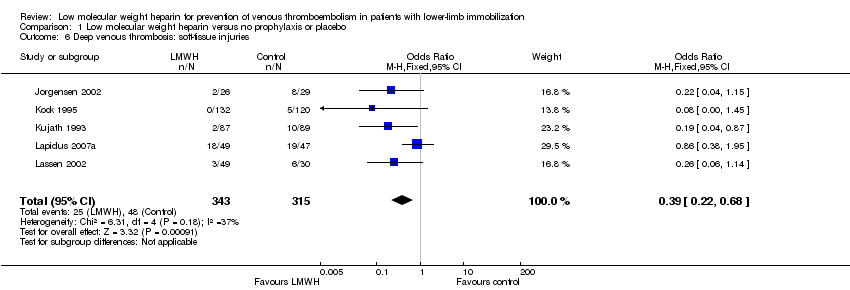 Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 6 Deep venous thrombosis: soft‐tissue injuries. | ||||
| 7 Deep venous thrombosis: distal segment Show forest plot | 5 | 1208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.42, 0.89] |
| Analysis 1.7  Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 7 Deep venous thrombosis: distal segment. | ||||
| 8 Deep venous thrombosis: proximal segment Show forest plot | 5 | 1217 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.91] |
| Analysis 1.8 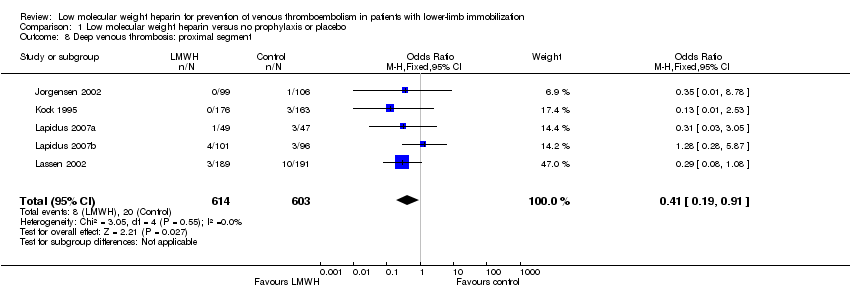 Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 8 Deep venous thrombosis: proximal segment. | ||||
| 9 Pulmonary embolism Show forest plot | 5 | 2517 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.47] |
| Analysis 1.9  Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 9 Pulmonary embolism. | ||||
| 10 Symptomatic venous thromboembolism Show forest plot | 6 | 2924 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.21, 0.76] |
| Analysis 1.10 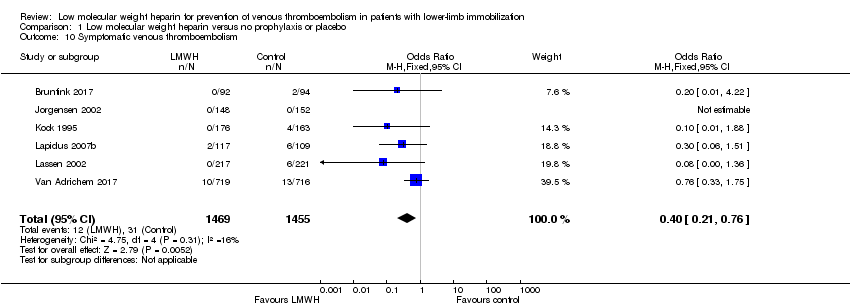 Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 10 Symptomatic venous thromboembolism. | ||||
| 11 Mortality due to pulmonary embolism Show forest plot | 8 | 3111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.11  Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 11 Mortality due to pulmonary embolism. | ||||
| 12 Mortality due to other causes Show forest plot | 8 | 3111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| Analysis 1.12  Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 12 Mortality due to other causes. | ||||
| 13 Adverse outcomes Show forest plot | 8 | 3178 | Odds Ratio (M‐H, Random, 95% CI) | 2.01 [0.83, 4.86] |
| Analysis 1.13 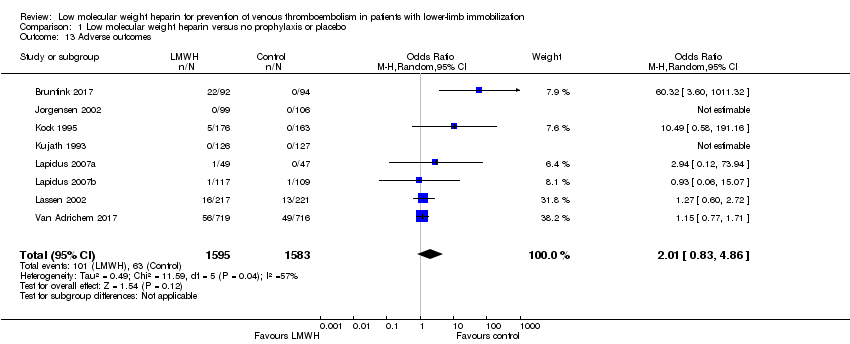 Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 13 Adverse outcomes. | ||||
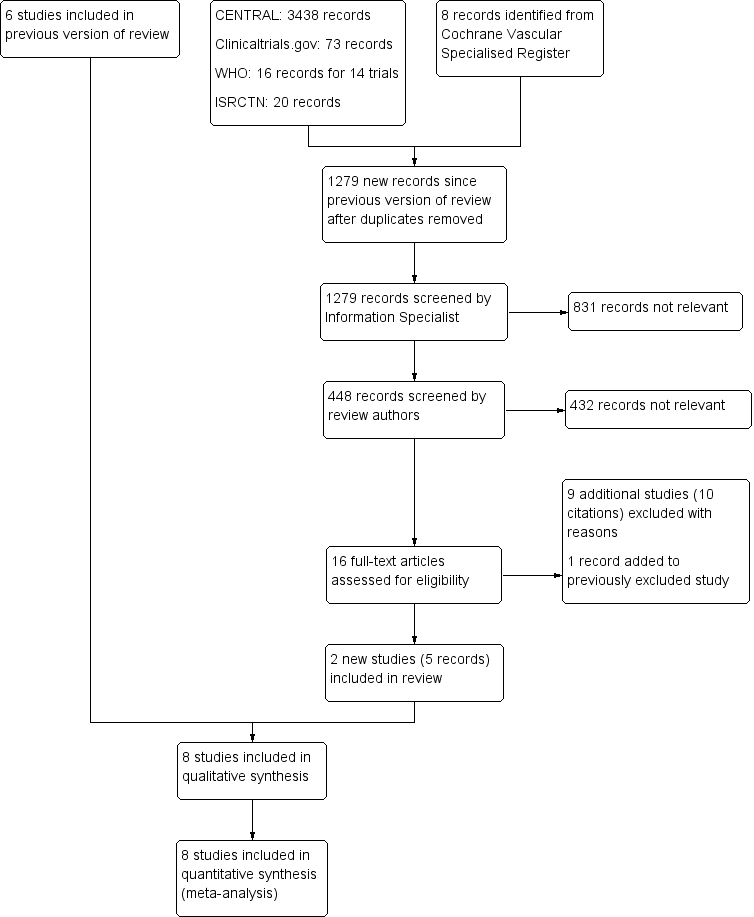
PRISMA study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias domain, presented as percentages across all included studies
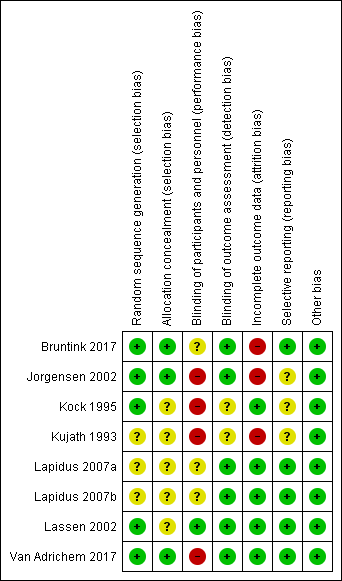
Risk of bias summary: review authors' judgements about each risk of bias domain for each included study

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 1 Deep venous thrombosis: regardless of type of plaster, whether operated or not.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 2 Deep venous thrombosis: in below‐knee cast, whether operated or not.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 3 Deep venous thrombosis: conservative treatment (i.e. non‐operated patients).
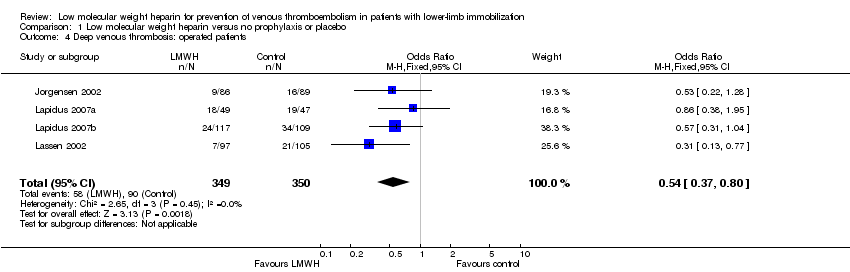
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 4 Deep venous thrombosis: operated patients.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 5 Deep venous thrombosis: fractures.
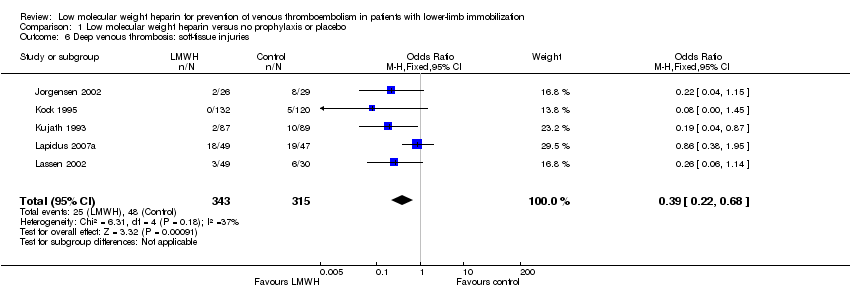
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 6 Deep venous thrombosis: soft‐tissue injuries.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 7 Deep venous thrombosis: distal segment.
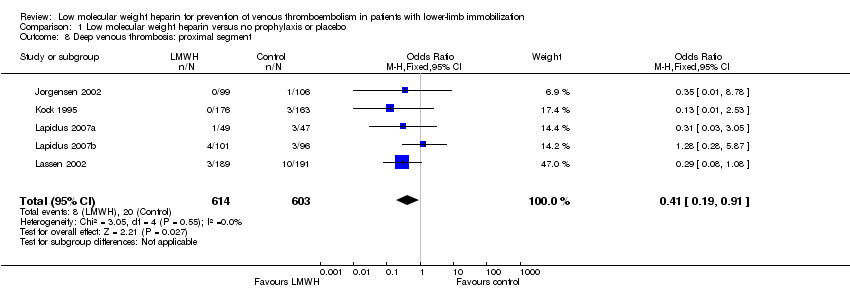
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 8 Deep venous thrombosis: proximal segment.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 9 Pulmonary embolism.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 10 Symptomatic venous thromboembolism.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 11 Mortality due to pulmonary embolism.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 12 Mortality due to other causes.
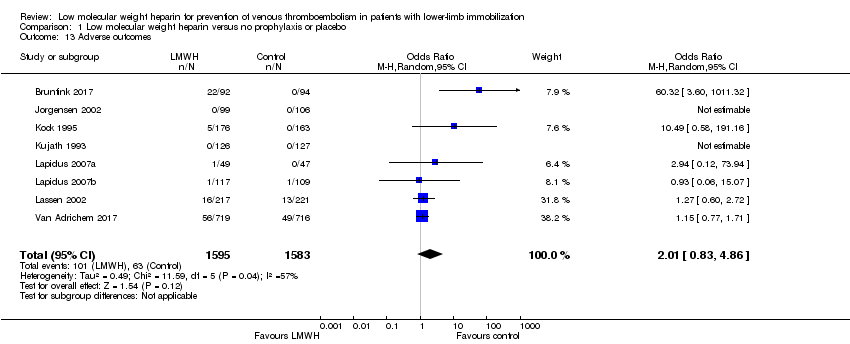
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 13 Adverse outcomes.
| Low molecular weight heparin compared to no prophylaxis or placebo in prevention of venous thromboembolism in patients with lower‐limb immobilization | ||||||
| Patient or population: prevention of venous thromboembolism in patients with lower‐limb immobilization | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no prophylaxis or placebo | Risk with low molecular weight heparin | |||||
| Deep venous thrombosis | Study population | OR 0.45 | 1676 | ⊕⊕⊕⊝ | ||
| 174 per 1000 | 87 per 1000 | |||||
| Pulmonary embolism | Study population | OR 0.50 | 2517 | ⊕⊕⊝⊝ | ||
| 7 per 1000 | 4 per 1000 | |||||
| Symptomatic venous thromboembolism | Study population | OR 0.40 | 2924 | ⊕⊕⊝⊝ | ||
| 21 per 1000 | 9 per 1000 | |||||
| Mortality due to pulmonary embolism | Study population | ‐ | 3111 | ‐ | No mortality due to pulmonary embolism was reported | |
| see comment | see comment | |||||
| Mortality due to other causes | Study population | OR 0.33 (0.01 to 8.15) | 3111 | ⊕⊕⊝⊝ | One death (in no prophylaxis/placebo group) was reported in the included studies | |
| 1 per 1000 | 0 per 1000 (0 to 5) | |||||
| Adverse outcomes | Study population | OR 2.01 | 3178 | ⊕⊕⊝⊝ | ||
| 40 per 1000 | 78 per 1000 | |||||
| *We calculated the assumed risk of the no prophylaxis or placebo group from the average risk in the no prophylaxis or placebo groups (i.e. the number of participants with events divided by total number of participants of the no prophylaxis or placebo group included in the meta‐analysis). The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level as 3 out of 7 studies showed considerable risk of bias | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Deep venous thrombosis: regardless of type of plaster, whether operated or not Show forest plot | 7 | 1676 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.33, 0.61] |
| 2 Deep venous thrombosis: in below‐knee cast, whether operated or not Show forest plot | 6 | 1080 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.34, 0.72] |
| 3 Deep venous thrombosis: conservative treatment (i.e. non‐operated patients) Show forest plot | 5 | 974 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.18, 0.53] |
| 4 Deep venous thrombosis: operated patients Show forest plot | 4 | 699 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.37, 0.80] |
| 5 Deep venous thrombosis: fractures Show forest plot | 6 | 1003 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.33, 0.70] |
| 6 Deep venous thrombosis: soft‐tissue injuries Show forest plot | 5 | 658 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.22, 0.68] |
| 7 Deep venous thrombosis: distal segment Show forest plot | 5 | 1208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.42, 0.89] |
| 8 Deep venous thrombosis: proximal segment Show forest plot | 5 | 1217 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.91] |
| 9 Pulmonary embolism Show forest plot | 5 | 2517 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.47] |
| 10 Symptomatic venous thromboembolism Show forest plot | 6 | 2924 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.21, 0.76] |
| 11 Mortality due to pulmonary embolism Show forest plot | 8 | 3111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Mortality due to other causes Show forest plot | 8 | 3111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 13 Adverse outcomes Show forest plot | 8 | 3178 | Odds Ratio (M‐H, Random, 95% CI) | 2.01 [0.83, 4.86] |

