نقش هپارین با وزن مولکولی پائین در پیشگیری از ترومبوآمبولی وریدی در بیماران با بیحرکتی اندام تحتانی
چکیده
پیشینه
بیحرکتی اندام تحتانی یک عامل خطر برای ترومبوآمبولی وریدی (venous thromboembolism; VTE) است. هپارینهای با وزن مولکولی پائین (low molecular weight heparin; LMWHs) آنتیکوآگولانتهایی هستند، که ممکن است در بیماران بزرگسال با بیحرکت شدن اندام تحتانی برای پیشگیری از ترومبوز ورید عمقی (deep venous thrombosis; DVT) و عوارض آن مورد استفاده قرار گیرند. این یک نسخه بهروز از مروری است که ابتدا در سال 2008 منتشر شد.
اهداف
ارزیابی اثربخشی هپارین با وزن مولکولی پائین برای پیشگیری از ترومبوآمبولی وریدی در بیماران با بیحرکتی اندام تحتانی در وضعیت سرپایی.
روشهای جستوجو
برای بهروز کردن این نسخه، متخصص اطلاعات عروق در کاکرین (Cochrane Vascular Information Specialist)؛ پایگاه ثبت تخصصی؛ CENTRAL و سه پایگاه ثبت کارآزماییها را جستوجو کرد (اپریل 2017).
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) و کارآزماییهای بالینی کنترل شده (controlled clinical trials; CCTs) که ترومبوپروفیلاکسی را به وسیله LMWHها با عدم پروفیلاکسی یا دارونما (placebo) در بیماران بزرگسالی مقایسه کرده بودند که اندام تحتانی آنها بیحرکت شده بود. بیحرکتی با وسایلی مانند یک گچ یا بریس ایجاد شده بود.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم کارآزماییها را انتخاب کردند، خطر سوگیری (bias) را بررسی و دادهها را استخراج کردند. نویسندگان مرور، در صورت لزوم، با نویسندگان کارآزمایی برای دریافت اطلاعات بیشتر تماس گرفتند. تجزیهوتحلیل آماری با استفاده از Review Manager 5 انجام شد.
نتایج اصلی
ما هشت کارآزمایی را وارد کردیم که با 3680 شرکتکننده واجد شرایط ورود به این مرور بودند. کیفیت شواهد، بر اساس سیستم درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE)، به وسیله پیامدها متغیر بوده و از پائین تا متوسط متغیر بودند. بروز DVT را از 4.3% تا 40% در بیمارانی که به دلیل صدمه پا، اندام تحتانیشان در گچ یا بریس برای حداقل یک هفته بیحرکت شده بود، و کسانی که پروفیلاکسی یا دارونما دریافت نکردند، به دست آوردیم. این میزان بهطور قابل توجهی در بیمارانی که روزانه تزریقهای زیر‐جلدی LMWH در طول بیحرکتی دریافت میکردند، پائینتر بود، نرخ حوادث از 0% تا 37% متغیر بود (نسبت شانس (OR): 0.45؛ 95% فاصله اطمینان (CI): 0.33 تا 0.61، با حداقل شواهد از ناهمگونی: I² = %26؛ P = 0.23؛ هفت مطالعه؛ 1676 شرکتکننده؛ شواهد با کیفیت متوسط). نتایج قابل مقایسه در گروههای زیر از شرکتکنندگان دیده شد: بیماران با کستهای زیر زانو، بیماران درمان شده به صورت محافظهکارانه (بیماران غیر‐قابل جراحی)، بیماران جراحی شده، بیماران دچار شکستگی، بیماران با صدمات بافت نرم، و بیماران با ترومبوز دیستال یا پروگزیمال. تفاوت آشکاری بین LMWH و گروههای کنترل برای آمبولی ریوی یافت نشد (OR: 0.50؛ 95% CI؛ 0.17 تا 1.47؛ بدون شواهدی از ناهمگونی: I² = %0؛ P = 0.56؛ پنج مطالعه؛ 2517 شرکتکننده؛ شواهد با کیفیت پائین). مطالعات همچنین VTE علامتدار کمتری را در گروههای LMWH در مقایسه با گروههای کنترل نشان دادند (OR: 0.40؛ 95% CI؛ 0.21 تا 0.76، با حداقل شواهد از ناهمگونی: I² = %16؛ P = 0.31؛ شش مطالعه؛ 2924 شرکتکننده؛ شواهد با کیفیت پائین). یک مورد مرگومیر در مطالعات وارد شده گزارش شد اما هیچ موردی از مرگومیر در اثر آمبولی ریوی رخ نداده بود. عوارض حوادث جانبی ماژور نادر بودند، خونریزی مینور، حوادث جانبی اصلی گزارش شد.
نتیجهگیریهای نویسندگان
شواهد با کیفیت متوسط نشان داد که استفاده از LMWH در بیماران سرپایی که نیاز به بیحرکتی اندام تحتانی داشتند، در مقایسه با عدم پروفیلاکسی یا دارونما، سبب کاهش DVT شد. سطح کیفیت شواهد به دلیل خطر سوگیری انتخاب و سوگیری ریزش نمونه (attrition bias) در مطالعات وارد شده به متوسط کاهش یافت. شواهد با کیفیت پائین نشان میدهد تفاوتهای واضح در PE بین LMWH و گروههای کنترل وجود ندارد اما VTE علامتدار کمتر در گروه LMWH دیده شد. سطح کیفیت شواهد به دلیل خطر سوگیری و عدم دقت کاهش یافت.
PICO
خلاصه به زبان ساده
هپارین با وزن مولکولی پائین برای پیشگیری از ترومبوآمبولی وریدی در بزرگسالان با بیحرکتی اندام تحتانی در وضعیت سرپایی
پیشینه
ترومبوآمبولی وریدی (venous thromboembolism) وضعیتی است که لخته خونی در وریدهای عمقی (deep venous thrombosis; DVT) تشکیل میشود، در پاها شایعتر است. نگرانی این است که این لخته میتواند حرکت کرده و شریانها را در ریهها (آمبولی ریه) ببندد. در بیماران بزرگسال، بیحرکت شدن اندام تحتانی با یک گچ یا برس، یک عامل خطر برای DVT و آمبولی ریوی است. برای پیشگیری از این عوارض، درمان پیشگیرانه با آنتیکوآگولانتها (داروهایی که خون را رقیق میکنند) اغلب استفاده میشود، اغلب، هپارین با وزن مولکولی پائین (low molecular weight heparin; LMWHs). با این حال، در دستورالعملهای ملی موجود توافقی وجود ندارد. بنابراین، به منظور بررسی شواهد، منابع علمی را برای یافتن کارآزماییهای انجام شده درباره این موضوع جستوجو کردیم.
ویژگیهای مطالعه و نتایج کلیدی
در این مرور هشت مطالعه را وارد کردیم (تا اپریل 2017). این مطالعات شامل مجموع 3680 شرکتکننده بودند. شرکتکنندگان یا LMWH زیر‐جلدی را یک بار در روز یا بدون درمان پیشگیرانه یا دارونما (placebo) دریافت کردند. موارد جدید DVT در گروه کنترل از 4.3% تا 40% و در گروه LMWH از 0% تا 37% متغیر بود. خطر ابتلا به DVT در شرکتکنندگان دریافت کننده LMWH پائینتر بود. تجزیهوتحلیلهای بیشتر نیز کاهش بروز DVT را زمانی که استفاده از LMWH با عدم درمان یا دارونما در گروههای زیر از شرکتکنندگان مقایسه شد، نشان داد: بیماران با کستهای زیر زانو، بیماران درمان شده به صورت محافظهکارانه (بیماران جراحی نشده)، بیماران جراحی شده، بیماران با شکستگی، بیماران با آسیبهای بافتنرم، بیماران مبتلا به ترومبوز بالای زانو و بیماران مبتلا به ترومبوز زیر زانو. هیچ تفاوت آشکاری میان گروه LMWH و گروه کنترل برای آمبولی ریه یافت نشد. مطالعات نشان داد ترومبوآمبولی وریدی علامتدار در گروه LMWH کمتر از گروه کنترل است. هیچ موردی از مرگومیر ناشی از آمبولی ریوی گزارش نشده است. یک مطالعه، یک مورد مرگومیر را در گروه کنترل گزارش کرد.
تعداد کمی از عوارض جانبی گزارش شده در بیماران درمان شده وجود داشت. حوادث جانبی اصلی گزارش شده، موارد خونریزی جزئی مانند خونریزی بینی، خون در ادرار و مدفوع تیره بود.
کیفیت شواهد و نتیجهگیریها
استفاده از LMWH در بیماران بزرگسال، در مقایسه با عدم پیشگیری یا دارونما (placebo)، زمانی که بیحرکتی اندام تحتانی مورد نیاز است، DVT را کاهش میدهد. کیفیت شواهد به دلیل خطر سوگیری (bias) در بعضی از کارآزماییها، از قبیل عدم کورسازی شرکتکنندگان یا دلایل نامشخص برای کنارگذاشتن شرکتکنندگان از تجزیهوتحلیل، به سطح متوسط کاهش یافت. شواهد با کیفیت پائین نشان داد تفاوت قاطعی در آمبولی ریوی بین LMWH و گروه کنترل وجود ندارد، اما ترومبوآمبولی وریدی علامتدار در گروه LMWH کمتر است. کیفیت شواهد به دلیل مسائل روششناسی و عدم دقت نتایج به دست آمده کاهش یافت.
Authors' conclusions
Summary of findings
| Low molecular weight heparin compared to no prophylaxis or placebo in prevention of venous thromboembolism in patients with lower‐limb immobilization | ||||||
| Patient or population: prevention of venous thromboembolism in patients with lower‐limb immobilization | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no prophylaxis or placebo | Risk with low molecular weight heparin | |||||
| Deep venous thrombosis | Study population | OR 0.45 | 1676 | ⊕⊕⊕⊝ | ||
| 174 per 1000 | 87 per 1000 | |||||
| Pulmonary embolism | Study population | OR 0.50 | 2517 | ⊕⊕⊝⊝ | ||
| 7 per 1000 | 4 per 1000 | |||||
| Symptomatic venous thromboembolism | Study population | OR 0.40 | 2924 | ⊕⊕⊝⊝ | ||
| 21 per 1000 | 9 per 1000 | |||||
| Mortality due to pulmonary embolism | Study population | ‐ | 3111 | ‐ | No mortality due to pulmonary embolism was reported | |
| see comment | see comment | |||||
| Mortality due to other causes | Study population | OR 0.33 (0.01 to 8.15) | 3111 | ⊕⊕⊝⊝ | One death (in no prophylaxis/placebo group) was reported in the included studies | |
| 1 per 1000 | 0 per 1000 (0 to 5) | |||||
| Adverse outcomes | Study population | OR 2.01 | 3178 | ⊕⊕⊝⊝ | ||
| 40 per 1000 | 78 per 1000 | |||||
| *We calculated the assumed risk of the no prophylaxis or placebo group from the average risk in the no prophylaxis or placebo groups (i.e. the number of participants with events divided by total number of participants of the no prophylaxis or placebo group included in the meta‐analysis). The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level as 3 out of 7 studies showed considerable risk of bias | ||||||
Background
Description of the condition
Lower‐limb immobilization is associated with deep venous thrombosis (DVT) and pulmonary embolism (PE). Predisposing risk factors for venous thromboembolism (VTE) can be divided into individual patient factors, trauma, or surgery‐related factors. Patient‐related factors include: obesity, thrombophilia (a hereditary or acquired predisposition to thrombosis), a previous thrombosis, age over 40 years, or cardiac or respiratory failure (Anderson 2003; Clagett 1995; Zagrodnick 1990). Immobilization is considered a significant risk factor for the development of DVT and PE (Knudson 1996; Kudsk 1989; Kujath 1991). Other factors associated with an increased risk of VTE include: blood transfusion, surgery, fracture of the pelvis, femur, or tibia, spinal cord injury, head injury, shock on hospital admission, venous injury, more than three days on ventilation, the time from injury to operation, and operation time (Abelseth 1996; Knudson 2004).
In a group of 102 patients with lower‐limb fractures, Abelseth found a rate of DVT of 28% (Abelseth 1996). All patients underwent surgery and were mobilized without the use of a plaster cast. Proximal fractures were associated with a higher risk of DVT compared with more distal fractures (Abelseth 1996). Other reported incidences of venographically‐proven DVT in patients with lower‐limb fractures range from 27% to 78% (Breyer 1984; Geerts 1994; Hjelmstedt 1968; Kudsk 1989; Spieler 1972). The percentages in hospitalized patients are generally higher than in outpatients. In outpatients immobilized in plaster casts without LMWH, the incidence of DVT on ultrasonography ranges from 4.5% to 16.5% (Kock 1995; Kujath 1993; Reilmann 1993; Zagrodnick 1990). The incidence of PE in trauma patients with DVT without prophylaxis is 4.3%, with a high mortality rate (20% to 23.3%). In patients with DVT receiving thromboprophylaxis, this incidence can be lowered to 0.3% to 2.0% (Hill 2002).
Description of the intervention
The primary goal of administering thromboprophylaxis is to prevent PE and DVT and their sequelae. Oral anticoagulants, unfractionated heparin (UFH) and LMWH, have been studied as treatment options for this indication. In clinical guidelines, the recommendations for preventing venous thrombosis in patients with isolated lower‐limb injuries distal to the knee are sparse. The Italian Intersociety Consensus Statement states that most patients in orthopedic and traumatological fields, other than knee and hip replacements, should be considered for thromboprophylaxis after individual assessment of haemorrhagic risk (Della Rocca 2013). Guidelines in Emergency Medicine Network in the United Kingdom (GEMNet) only advises the use of thromboprophylaxis in patients with rigid cast immobilization and a permanent risk factor for VTE (Roberts 2013).
There remains substantial practice variation amongst surgeons regarding the use of anticoagulation measures (Batra 2006). In daily clinical practice, there remains a huge variation in the way thromboprophylaxis is used. A recent survey among Dutch orthopedic and trauma surgeons showed that 60% to 80% always prescribed thromboprophylaxis in patients with lower‐limb immobilization. Up to 8% of the participants never treated their patients with prophylaxis (van Adrichem 2015).
How the intervention might work
Low molecular weight heparin is proven to be effective in the prevention of venous thromboembolism (Weitz 1997). By treating patients with lower‐limb immobilization with LMWH, we expect to see less venous thromboembolism compared to patients who do not receive any protection.
Why it is important to do this review
This is the second update of the Cochrane review first published in 2008 (first update 2014). Since 2014, two additional studies on this subject have been published (Bruntink 2017; Van Adrichem 2017). Therefore, in order to include the most recent information, we updated this review.
Objectives
To assess the effectiveness of low molecular weight heparin for the prevention of venous thromboembolism in patients with lower‐limb immobilization in an ambulant setting.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomized controlled trials (RCTs) and controlled clinical trials (CCTs) that describe thromboprophylaxis, by means of low molecular weight heparin (LMWH), in adults with lower‐limb immobilization in an ambulatory setting. Treatment with LMWH could have started during hospital admission.
Types of participants
Adults treated with a device for lower‐limb immobilization, such as a leg cast or brace, in an ambulatory setting. Weight bearing and duration of leg cast use were not considered criteria for inclusion or exclusion.
Types of interventions
Studies comparing LMWH with no prophylaxis or placebo. Studies including oral anticoagulants, UFH, or aspirin were excluded.
Types of outcome measures
Primary outcomes
Morbidity
-
DVT ‐ confirmed by venography or ultrasonography
-
PE ‐ confirmed by a ventilation‐perfusion scan, a CT scan, or angiography
-
Symptomatic VTE ‐ symptomatic DVT, PE, or combination
Secondary outcomes
-
Mortality ‐ PE‐related
-
Mortality ‐ other causes
-
Adverse outcomes of treatment: bleeding, heparin induced thrombocytopenia (HIT), allergic reaction, others (definitions of adverse outcomes as reported by study authors).
Search methods for identification of studies
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
-
The Cochrane Vascular Specialised Register (19 April 2017).
-
The Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 3) via the Cochrane Register of Studies Online (searched 19 April 2017).
See Appendix 1 for details of the search strategy used for CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, and AMED, as well as through handsearching relevant journals. The full list of the databases, journals, and conference proceedings searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library.
In addition, the CIS searched the following trials registers for details of ongoing and unpublished studies (19 April 2017). See Appendix 2
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch).
-
ClinicalTrials.gov (clinicaltrials.gov)
-
International Standard Randomised Controlled Trial Number (ISRCTN) registry (www.isrctn.com).
Searching other resources
The review authors searched the reference lists of relevant studies.
Data collection and analysis
Selection of studies
Two review authors (KL and MH) independently assessed all studies identified by the literature searches, according to the inclusion criteria. Disagreements were resolved by discussion.
Data extraction and management
Two review authors (MH and AZ) independently extracted data to ensure objectivity and validity of findings. A third review author (HJ) cross checked the information, and disagreements were resolved by discussion. The review authors contacted trial authors for additional information if required.
Assessment of risk of bias in included studies
Two review authors (KL and AZ) independently assessed the risk of bias of the included studies by using Cochrane's 'Risk of bias' tool (Higgins 2011). Random sequence generation, allocation concealment, blinding of participants, personnel, and outcome assessors, incomplete outcome data, selective outcome reporting, and any other relevant biases were classified as 'low risk', 'high risk' or 'unclear risk'. They resolved any disagreement through discussion with review authors HJ and LJ.
Measures of treatment effect
We measured treatment effect by calculating odds ratios (OR) with 95% confidence interval (CI) for dichotomous data.
Unit of analysis issues
The individual participant was considered the unit of analysis.
Dealing with missing data
Where appropriate, we used all randomized participants for the analysis. However, many of the included studies had participants excluded after randomization, creating a disparity between the number of participants randomized and the number available for assessment of VTE outcomes. Therefore, we used the data from the populations as reported by the studies. These generally consisted of all participants who received treatment and had evaluable testing of VTE at the end of the study. If these values were not available, we used the reported per‐protocol data.
Assessment of heterogeneity
Statistical analysis was carried out using Review Manager 5 (RevMan 2014). Review author LJ coordinated the statistical analysis. We searched for clinical and statistical heterogeneity by visually inspecting the forest plots. We quantified statistical heterogeneity by means of an I² test (Deeks 2011; Higgins 2011). We interpreted an I² value higher than 50% as an indicator for substantial heterogeneity.
Assessment of reporting biases
We had planned to perform funnel plot analyses to assess reporting bias, when ten or more studies were included.
Data synthesis
We synthesized available data using Review Manager 5 (RevMan 2014). We investigated pooled estimates of the effects of treatment using a fixed‐effect model to calculate ORs with 95% CIs for dichotomous outcomes. When substantial heterogeneity was detected, we performed a random‐effects model analysis instead. If it was not possible to pool data, we planned to describe the results reported by the studies in the text.
Subgroup analysis and investigation of heterogeneity
We presented data by different groups of participants. These groups were selected, as these particular patient categories may influence the outcome.
-
DVT: regardless of type of plaster, whether operated or not
-
DVT: in below‐knee cast, whether operated or not
-
DVT: only non‐operated patients
-
DVT: only operated patients
-
DVT: fractures
-
DVT: soft‐tissue injuries
-
DVT: distal segment
-
DVT: proximal segment
Sensitivity analysis
If any trials were judged to be of high risk of bias, we planned to perform a sensitivity analysis to assess outcomes with and without trials with high risk of bias.
Summary of findings
We constructed a 'Summary of findings' table for the comparison LMWH compared to no prophylaxis or placebo in prevention of venous thromboembolism with lower‐limb immobilization' using the GRADEpro GDT software to present the main findings of the review (GRADEpro GDT 2015). We judged the outcomes deep venous thrombosis, pulmonary embolism, symptomatic venous thromboembolism, mortality due to pulmonary embolism, mortality due to other causes, and adverse outcomes, to be the most clinically relevant to healthcare professionals and patients. We calculated assumed control intervention risks from the mean number of events in the control groups of the selected studies for each outcome. We used the system developed by the GRADE Working Group to grade the quality of the evidence as high, moderate, low, or very low, based on within‐study risk of bias, directness of evidence, heterogeneity, precision of effects estimates, and risk of publication bias (Atkins 2004).
Results
Description of studies
Results of the search
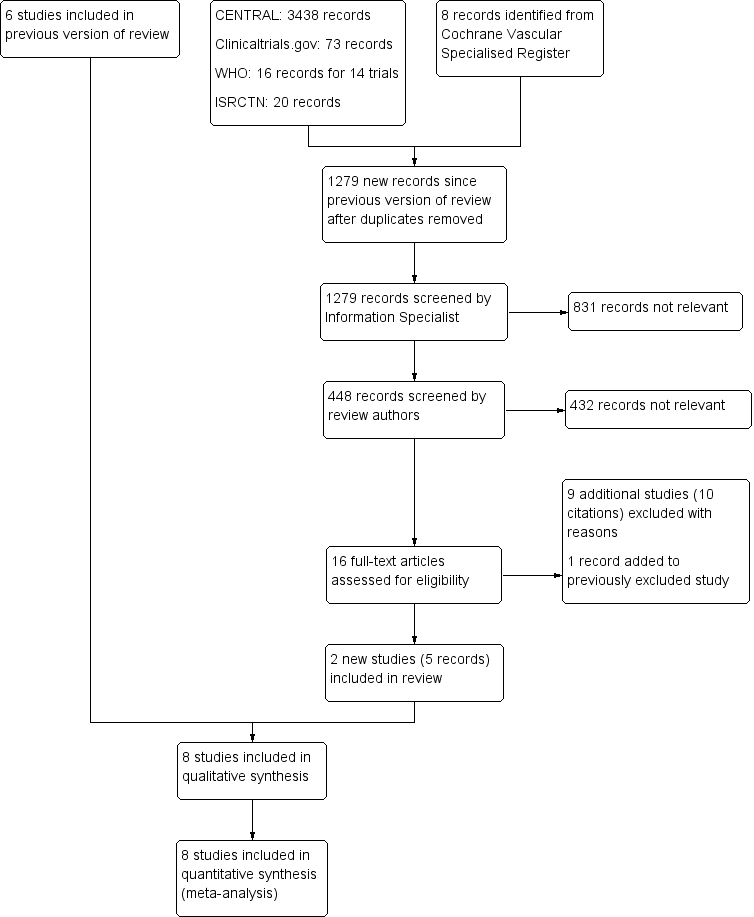
See Figure 1.

PRISMA study flow diagram
Included studies
We included eight RCTs (Bruntink 2017; Jorgensen 2002; Kock 1995; Kujath 1993; Lapidus 2007a; Lapidus 2007b; Lassen 2002; Van Adrichem 2017). The characteristics of these eight studies are summarized in the Characteristics of included studies table. All eight studies were reported as full papers and included a total of 3680 participants (range 105 to 1519). The participants included in the trials required lower‐limb immobilization for the treatment of leg injuries such as foot and ankle fractures and achilles ruptures. All studies included participants prospectively, with quite similar exclusion criteria. The most common exclusion criteria were: pregnancy, allergy to heparin or contrast media, uncontrolled hypertension, pre‐existing bleeding disorders, presence of malignancies, recent brain or gastrointestinal bleeding, previous DVT, and chronic venous insufficiency. Different LMWHs were used; they were administered once daily until removal of the plaster cast: nadroparin (2850 anti‐XA IU) (Bruntink 2017; Van Adrichem 2017), nadroparin (36 mg) (Kujath 1993), certoparin (Mono‐Embolex NM; 32 mg) (Kock 1995), tinzaparin (3500 anti‐Xa IU) (Jorgensen 2002), dalteparin (5000 IU) (Lapidus 2007a; Lapidus 2007b), dalteparin (2500 IU or 5000 IU depending on body weight) Van Adrichem 2017), and reviparin (1750 anti‐XA IU) (Lassen 2002). There were no relevant differences between treatment and control groups regarding demographics or risk factors.
In two studies, plaster cast fitted following surgery was used as an exclusion criterion (Kock 1995; Kujath 1993). In one study, patients who underwent surgery before randomization might have had heparin treatment for up to four days before randomization (Lassen 2002). Another study treated all patients for one week with LMWH before randomization (Lapidus 2007b). The included studies differed in the types of plaster cast (upper‐leg, lower‐leg, cylinder, or brace). There was also a variation in the duration of immobilization, ranging from 15 days (Kujath 1993), to 43 days (Lapidus 2007a).
In the included studies, the primary outcome parameter was DVT. Deep venous thrombosis, both symptomatic and asymptomatic, was diagnosed by means of ascending venography (Jorgensen 2002; Lapidus 2007b; Lassen 2002), or ultrasound (Bruntink 2017; Kock 1995; Kujath 1993; Lapidus 2007a). In these seven studies, every participant underwent a diagnostic exam. One study only performed ultrasound in participants who reported symptoms (Van Adrichem 2017).
Clinically‐suspected PE had to be confirmed by ventilation‐perfusion scintigraphy, angiography, or spiral CT‐scanning. Information concerning the number of patients with symptomatic and asymptomatic DVT and the extent of the DVT was collected. Secondary outcome parameters were mortality and side effects in both treatment and control groups.
Excluded studies
For this update, we excluded an additional nine studies, leading to a combined total of 47 excluded articles (Ayhan 2013; Cook 2011; Cvirn 2015; Garcia 2011; Horner 2014; Lim 2015; Samama 2013; Saragas 2014; Warot 2014). We stated the reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies

Risk of bias graph: review authors' judgements about each risk of bias domain, presented as percentages across all included studies
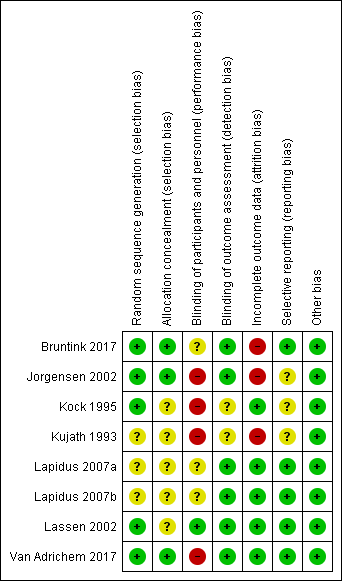
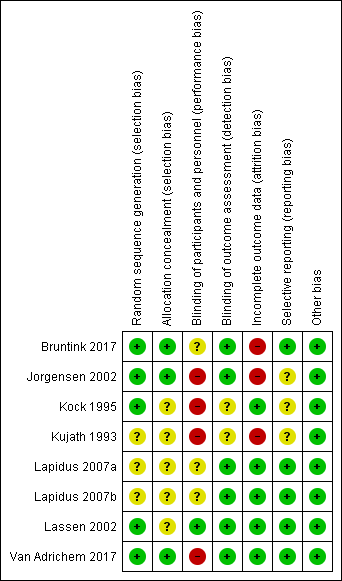
Risk of bias summary: review authors' judgements about each risk of bias domain for each included study
Allocation
We judged five studies at low risk of random sequence generation (Bruntink 2017; Jorgensen 2002; Kock 1995; Lassen 2002; Van Adrichem 2017), and three trials to be of unclear risk of bias, because insufficient information was provided (Kujath 1993; Lapidus 2007a; Lapidus 2007b). In two studies, participants were not recruited when study personnel were off duty (Lapidus 2007a; Lapidus 2007b).
We judged three studies at low risk of random sequence generation (Bruntink 2017; Jorgensen 2002; Van Adrichem 2017), and five trials to be of unclear risk of bias, because information on allocation concealment was not reported (Kock 1995; Kujath 1993; Lapidus 2007a; Lapidus 2007b; Lassen 2002).
Blinding
We judged four studies at high risk of performance bias, as they were open‐label studies, with the control group receiving no prophylaxis (Jorgensen 2002; Kock 1995; Kujath 1993; Van Adrichem 2017). We judged one study at low risk of performance bias, as participants and personnel were blinded to treatment assignment (Lassen 2002). We judged three studies to be of unclear risk of bias, as the blinding of study personnel was not explicitly reported (Bruntink 2017; Lapidus 2007a; Lapidus 2007b).
We judged two studies to be of unclear risk of detection bias, because no information on blinding of outcome assessors was provided (Kock 1995; Kujath 1993; Lassen 2002). We judged the remainder of the included studies to be at low risk of detection bias (Bruntink 2017; Jorgensen 2002; Lapidus 2007a; Lapidus 2007b; Van Adrichem 2017).
Incomplete outcome data
We judged three studies at high risk of attrition bias, because the number of participants excluded from analysis was either high (higher than 30%) or reasons were not clearly described per treatment assignment (Bruntink 2017; Jorgensen 2002; Kujath 1993). We judged the remainder of the included studies to be at low risk of attrition bias, as the number of participants excluded from analyses was either low or clearly described (Kock 1995; Lapidus 2007a; Lapidus 2007b; Lassen 2002; Van Adrichem 2017).
Selective reporting
We judged five studies to be at low risk of reporting bias, as all planned outcome measures, including adverse events, were reported (Bruntink 2017; Lapidus 2007a; Lapidus 2007b; Lassen 2002; Van Adrichem 2017). We judged three studies to be of unclear risk of bias, since the methods sections of the study reports did not describe planned primary and secondary outcomes, therefore, we were unable to judge if all outcomes were reported (Jorgensen 2002; Kock 1995). Kujath 1993 reported on primary outcomes, but not on possible adverse events, therefore, we also judged it to be of unclear risk of reporting bias.
Other potential sources of bias
We identified no other bias in the included studies and therefore, judged all included studies at low risk of other bias.
Effects of interventions
Van Adrichem 2017 used a different study protocol, in which only patients with symptoms were examined using ultrasound. This can lead to an underestimation of the number of DVTs. Furthermore, primary asymptomatic DVT can still lead to late post‐thrombotic syndrome. Therefore, we considered asymptomatic DVT to be a relevant clinical outcome. However, due to the large number of included participants, we deemed this study valuable for this review. In order not to obscure the primary outcome, we only used the data from Van Adrichem 2017 in the pulmonary embolism and symptomatic VTE analyses.
Morbidity
Deep venous thrombosis (DVT)
Kujath and colleagues assessed 253 participants in their study, 126 of whom received a subcutaneous injection of Fraxiparin daily; 127 participants received no prophylaxis. Incidences of DVT were 16.5% (n = 21) in the control group and 4.8% (n = 6) in the LMWH group (odds ratio (OR) 0.25, 95% confidence interval (CI) 0.10 to 0.65; Kujath 1993). Kock and colleagues assessed 163 participants in the control group (no treatment), and 176 participants in the LMWH once daily group. The incidence of DVT in the prophylaxis group was 0% versus 4.3% (n = 7) in the control group (OR 0.06, 95% CI 0.00 to 1.04; Kock 1995). In 2002, Jorgensen and colleagues published the results of their venographic‐controlled study, and diagnosed DVT in 10 out of 99 participants in the treatment group and in 18 out of 106 participants in the control group. This difference was not significant (OR 0.55, 95% CI 0.24 to 1.26; Jorgensen 2002). In 2002, Lassen and colleagues found an incidence of 35 of 188 participants randomly assigned to receive placebo (18.6%) and in 17 of 183 participants (9%) in the LMWH group (OR 0.45, 95% CI 0.24 to 0.83; Lassen 2002). Lapidus and colleagues published two studies in 2007. The study on thromboprophylaxis after surgical treatment of Achilles tendon rupture revealed a high incidence of thromboembolic events: 37% (18/49) in the treatment group versus 40% (19/47) in the placebo group (OR 0.86, 95% CI 0.38 to 1.95; Lapidus 2007a). The study on prolonged thromboprophylaxis during immobilization after ankle surgery also showed a high incidences without a significant difference between groups: 21% in the treatment group versus 31% in the placebo group (OR 0.57, 95% CI 0.31 to 1.04; Lapidus 2007b). In the first study published following the most recent update of this review in 2014, 719 participants received either dalteparin or nadroparin, 716 participants did not receive prophylaxis (Van Adrichem 2017). Incidences of clinical relevant DVT were 1.0% (n = 7) in the treatment group and 1.3% (n = 9) in the control group (OR 0.77, 95% CI 0.29 to 2.09). The most recent study assessed 186 participants, 92 of whom received nadroparin, and 94 of whom received no prophylaxis. The treatment group showed a DVT incidence of 2.2% (n = 2), which was significantly lower than the DVT incidence of 11.7% (n = 11) in the control group (OR 0.17, 95% CI 0.04 to 0.78; Bruntink 2017).
Meta‐analysis
We conducted a meta‐analysis to establish whether there was evidence of a thromboprophylactic effect of LMWH, to estimate the size of this effect, and to investigate whether it was consistent across the included studies. We combined all participants, and subsequently assessed the effect for different groups of participants: surgically‐treated patients, patients with conservative treatment, patients with below‐knee casts, patients with cylinder or above‐knee casts, patients with fractures, patients with soft‐tissue injuries, PE, distal or proximal DVT, and finally, the number of patients with symptomatic VTE.
All participants, regardless of type of plaster, whether operated or not
Seven studies, with a total of 1676 participants, had injuries of the lower limb immobilized by a plaster cast or brace (Bruntink 2017; Jorgensen 2002; Kock 1995; Kujath 1993; Lapidus 2007a; Lapidus 2007b; Lassen 2002). The control group (N = 834) received no prophylaxis or placebo; the prophylaxis group received LMWH once daily (N = 842). The incidence of thromboembolic events ranged from 4.3% to 40% in the control group (145/834), and from 0% to 37% (77/842) in the prophylaxis group (OR 0.45, 95% CI 0.33 to 0.61, P < 0.001; Analysis 1.1).
Participants with below‐knee casts, whether operated or not
We were able to obtain data on specific analyses of DVT in below‐knee casts or braces from six studies (Bruntink 2017; Jorgensen 2002; Kock 1995; Lapidus 2007a; Lapidus 2007b; Lassen 2002). Lassen 2002 and Kujath 1993 did not study the relationship between the type of cast and occurrence of thrombosis as part of their study designs. However, we could still add data from the group of participants with ruptured Achilles tendons from Lassen 2002. The incidences of DVT ranged from 0% to 37% in the LMWH groups, and from 3.6% to 40% in the control groups (OR 0.49, 95% CI 0.34 to 0.72; P < 0.001; Analysis 1.2; N = 1080).
Only Kock 1995 provided data on participants with cylinder or above‐knee casts, with a DVT incidence of 0/24 (0%) in the LMWH group and 2/24 (8.3%) in the control group.
Only participants with conservative treatment (i.e. non‐operated participants)
Five studies provided details of DVT in conservatively treated participants (i.e. non‐operated participants; Bruntink 2017; Jorgensen 2002; Kock 1995; Kujath 1993; Lassen 2002). When analyzed without consideration of type of cast or brace, the incidence ranged from 0% to 11.8% in the LMWH groups and from 4.3% to 17.3% in controls (OR 0.31, 95% CI 0.18 to 0.53, P < 0.001; Analysis 1.3; N = 974).
Only surgically‐treated participants
We obtained information about surgically‐treated participants from four studies (Jorgensen 2002; Lapidus 2007a; Lapidus 2007b; Lassen 2002). The incidence of DVT in surgically‐treated participants ranged from 7.2% to 37% in the LMWH group, and from 18.0% to 40% in the control group (OR 0.54, 95% CI 0.37 to 0.80, P = 0.002; Analysis 1.4; N = 699).
Fractures or soft‐tissue injuries
Six studies provided information on participants with fractures (Bruntink 2017; Jorgensen 2002; Kock 1995; Kujath 1993; Lapidus 2007b; Lassen 2002). These groups contained both surgically and conservatively treated participants. We found results in favour of the LMWH groups (OR 0.48, 95% CI 0.33 to 0.70, P < 0.001; Analysis 1.5; N = 1003). In participants with soft‐tissue injury, we again found results in favour of the LMWH groups (OR 0.39, 95% CI 0.22 to 0.68, P < 0.001; Analysis 1.6; N = 658; Jorgensen 2002; Kock 1995; Kujath 1993; Lapidus 2007a; Lassen 2002).
Distal or proximal deep vein thrombosis
Five studies provided information on the segment in which the thrombus was located (Jorgensen 2002; Kock 1995; Lapidus 2007a; Lapidus 2007b; Lassen 2002). The incidence of distal segment DVT, defined as below‐knee DVT, ranged from 0% to 34.7% in participants who received LMWH, and from 2.5% to 34.0% in the control groups (OR 0.61, 95% CI 0.42 to 0.89, P = 0.009; Analysis 1.7; N = 1208). Proximal DVT (above knee) was rare; there were eight events in a total of 614 participants who received LMWH (incidence ranged from 0% to 4.0%) versus 20/603 events in the controls (incidence ranged from 0.9% to 6.4%, OR 0.41, 95% CI 0.19 to 0.91, P = 0.03; Analysis 1.8; N = 1217).
Pulmonary embolism (PE)
In the studies under review, PE was a rare complication in immobilization of the lower extremity. Lassen 2002 reported that one participant in the treatment group and four participants in the control group showed clinical signs of PE; in two of them, both in the control group, PE was confirmed by a ventilation‐perfusion scan. Kujath 1993 reported that one participant in the group without prophylaxis showed clinical signs of a PE, but this diagnosis could not be proven by scintigraphic imaging. Van Adrichem 2017 reported that four participants in the treatment group and five in the control group developed a PE, diagnosed with a spiral CT scan. In the PROTECT study, two participants in the control group developed a pulmonary embolism (Bruntink 2017). Jorgensen 2002 reported no cases of pulmonary embolism. Overall, no clear differences were found between the treatment and control groups (OR 0.50, 95% CI 0.17 to 1.47, P = 0.21; Analysis 1.9; N = 2517).
Symptomatic venous thromboembolism (VTE) ‐ symptomatic deep venous thrombosis (DVT), pulmonary embolism (PE), or combination
All studies but one reported on participants with symptomatic VTE. Lapidus 2007a did not report on participants with a symptomatic DVT, because it was not clinically possible to differentiate symptoms of a possible DVT from those of normal postoperative findings. Lapidus 2007b reported on two events in the LMWH group and six events in the placebo group. Lassen 2002 mentioned two participants with PE and four with symptomatic DVT, all of them in the placebo group. Kujath 1993 reported on nine symptomatic participants, however, it was not clear whether they were in the treatment group or not. For that reason, this study was not included in the pooled analysis. Kock 1995 did not report on symptomatic participants in their Lancet publication, but additional information from participants with thrombosis was found in an earlier publication. Four participants in the control group showed symptoms of DVT. Jorgensen 2002 did not find any symptomatic VTEs. Van Adrichem 2017 reported six participants with symptomatic DVT, three with PE and one with both DVT and PE in the LMWH group, and eight with DVT, four with PE, and one participant with both, in the control group. Bruntink 2017 reported two participants with a PE in the control group.
Symptomatic VTE was observed in 12 of 1469 (0.8%) participants receiving LMWH compared with 31 out of 1455 (2.1%) participants in the control group (OR 0.40, 95% CI 0.21 to 0.76; Analysis 1.10; six RCTs; N = 2924).
Mortality (Pulmonary embolism‐ (PE)‐related and other causes)
Van Adrichem 2017 reported one death in the no treatment group during their three‐month follow‐up after treatment; they reported that the death was assessed as possibly due to pulmonary embolism. However, a conclusive diagnosis could not be made because no autopsy was performed; the participant was over 90 years old and suffered from heart failure. The remaining seven studies reported no deaths due to PE (Analysis 1.11; N = 3111), or other causes (Analysis 1.12; N = 3111).
Adverse outcomes of treatment
Major side effects, such as hematoma, acute major bleeding, allergic reaction, and thrombocytopenia were rare.
Lassen 2002 reported 14 participants in the LMWH group and 12 in the placebo group had a bleeding event. Lassen 2002 also reported major bleeding occurred in two participants in the LMWH group (retroperitoneal bleeding in one and permanent discontinuation of LMWH due to minor bleeding in another) and one in the placebo group (permanent discontinuation of study medication due to minor bleeding). Lassen 2002 reported no cases of heparin induced thrombocytopenia (HIT). Kock 1995 reported on five participants with minor complications (four small local hematomas, one facial eczema). Jorgensen 2002 reported no cases of HIT, hematomas, or severe bleeding. Kujath 1993 did not observe any side effects. Lapidus and colleagues did not report any cases of major bleeding (Lapidus 2007a; Lapidus 2007b). Lapidus 2007a reported one participant had a nosebleed after two days of dalteparin treatment. In Lapidus 2007b, two participants (one in each group) discontinued treatment due to minor bleeding. Van Adrichem 2017 reported no major bleeds, one clinically relevant non‐major bleed in the LMWH group, 55 minor bleeds in the treatment group, and 49 minor bleeds in the control group. Bruntink 2017 reported no major complications. Twenty‐two participants in the treatment group reported minor bleeding, hematuria, or dark stool (Bruntink 2017).
Combining all reported adverse events into a random‐effects model meta‐analysis showed an OR of 2.01, 95% CI 0.83 to 4.86, I² = 57%, 3178 participants; 8 studies; Analysis 1.13).
Discussion
Summary of main results
We included eight studies in this updated review, with a total of 3680 participants. From these studies, we found that the incidence of deep venous thrombosis (DVT), diagnosed by compression ultrasound, venography, or both, in participants with a leg injury who were immobilized in a plaster cast or brace for at least one week and received no thromboprophylaxis (or placebo) was 4.3% to 40%. This was significantly higher than for participants who received daily subcutaneous injections of low molecular weight heparin (LMWH) during the entire period of immobilization (0% to 37%).
Comparable results were seen in the following groups of participants: patients with a below‐knee cast, surgically treated patients, conservatively treated patients (not surgically treated), patients with fractures, patients with soft‐tissue injuries, and patients with proximal or distal DVT. The odds ratios (OR) between the individual participant groups were similar, with an overlap of the confidence intervals (CI). Therefore, it was not possible to indicate a participant or patient group where prophylaxis was not indicated. No clear differences were found for PE between LMWH and the control groups. The studies found less symptomatic venous thromboembolism (VTE) in the LMWH groups compared with the control groups. One death (in the control group) was reported in the included studies. Major adverse events were rare; the main adverse events reported were cases of minor bleeding.
Overall completeness and applicability of evidence
The studies included in this review all assessed participants with lower‐limb immobilization. Some studies only included conservatively treated participants, whereas, others also included surgically treated participants. Studies included participants with both fractures and soft‐tissue injuries. These differences in inclusion criteria may have led to a lower external validity.
In addition to using Cochrane's 'Risk of bias' tool to assess methodological quality, we also assessed the validity and quality of the included randomized controlled trials using the scoring scheme provided on the website of Cochrane Netherlands (www.cochrane.nl). The overall quality of the eight included studies according to this scoring system, was rated 'good', although some remarks should be added.
The true thromboembolic rate in unprotected patients remains unknown, as high‐risk patients were excluded from participation in all eight studies, underestimating the incidence of thromboembolism in unprotected patients and the potentially beneficial effect of LMWHs. Although there is consensus among surgeons that thromboprophylaxis should be initiated in patients with a moderate to high risk for thromboembolism, there is no uniform definition of this patient group, leading to practice variation. Several attempts have been made to stratify the risk for VTE of immobilized patients (Gearhart 2000; Zagrodnick 1990). Knudson 2004 developed a scheme, based on analysis of 1602 episodes of VTE using the National Trauma Data Bank, to identify trauma patients with a high risk of thrombosis. Lower‐limb fracture and age over 40 years were among the factors used for selection. However, this study also stated that with this scheme, 90% of patients with VTE would be identified, and 10% would be missed. By contrast, in another study, patients under 40 years of age, with soft‐tissue injuries also developed DVT (Kock 1995). Up to now, no stratification method has proven its superiority or has gained general acceptance. In most studies in this review, investigators did not include patients with a high risk of DVT. So even in patients with a low to average risk of DVT, LMWH still provided significant protection.
In five studies, the included participants had a wide variety of trauma, ranging from fractures to soft‐tissue injuries, and tendon ruptures (Jorgensen 2002; Kock 1995; Kujath 1993; Lassen 2002; Van Adrichem 2017). Since the risk of DVT is found to be related to the presence and type of fracture, the extent of soft‐tissue injury, and type and duration of surgery, heterogeneity might have been introduced. However, randomization should have minimized this effect. Three studies focused on participants with a specific trauma (Bruntink 2017; Lapidus 2007a; Lapidus 2007b). We also observed a high drop‐out rate.
Five studies used ultrasound (Bruntink 2017; Kock 1995; Kujath 1993, Lapidus 2007a; Van Adrichem 2017), and three used venography (Jorgensen 2002; Lapidus 2007b; Lassen 2002), to diagnose DVT. The latter is considered the 'gold standard', but is rarely used in routine practice as the first line of investigation for DVT. Duplex ultrasonography and compression ultrasonography have a lower sensitivity and specificity compared to venography, especially for diagnosing calf vein thrombosis (CBO 2008; Lensing 1989). This might be the reason for the differences in the results between Kock 1995 and the studies that used venography (Jorgensen 2002; Lapidus 2007b; Lassen 2002), since there were no other major differences reported in participant characteristics or intervention. However, it does not explain the differences between the results of Kock 1995, Kujath 1993 and Lapidus 2007a. By only examining participants reporting symptoms, Van Adrichem 2017 only reported clinically‐relevant DVTs, therefore underestimating the number of DVTs in the study population. Due to this difference in study protocol, we only used the results of this study in the analyses for pulmonary embolism (Analysis 1.9) and symptomatic VTE (Analysis 1.10).
Lassen 2002 included participants who received up to four days of LMWH (32% of participants). Another study treated all participants with LMWH for one week prior to randomization (Lapidus 2007b). This might equalize the effects in the two groups and lead to an underestimation of the treatment effect. Therefore, Lapidus 2007b only focused on the duration of treatment, and not on the indication of treatment itself.
The dose of 3500 anti‐Xa IU of tinzaparin that Jorgensen 2002 used might have been too low, since another study showed equal antithrombotic effect using 4500 anti‐Xa IU of tinzaparin compared with 40 mg of enoxaparin, which is the standard dose in orthopedic surgery (Eriksson 2001). This possibly creates an underestimation of the effect of prophylaxis.
Quality of the evidence
The quality of evidence according GRADE varied by outcome and ranged from low to moderate with reasons for downgrading being risk of bias due to attrition and performance bias (DVT), imprecision and risk of bias due to attrition and performance bias (PE, symptomatic VTE and adverse outcomes) and low number of events and imprecision (mortality due to other causes).
Potential biases in the review process
Two review authors independently carried out study selection, data extraction, and study quality assessment in order to reduce bias and subjectivity. We are confident that all potential studies are included in this review. However, the possibility remains that relevant data exist which have not been published or were not found in the search.
The total number of included studies in this review was eight. Since ten studies are required to perform a funnel plot analysis, reliable funnel plot analysis could not be performed.
By only examining patient reporting symptoms Van Adrichem 2016 only reported clinical relevant DVTs, therefore underestimating the number of DVTs in the study population. Due to this difference in study protocol the results of this study were only used in the analyses for 'pulmonary embolism' (Analysis 1.9) and 'symptomatic VTE' (Analysis 1.10).
Agreements and disagreements with other studies or reviews
As early as 1944, the first study on deep venous thrombosis (DVT) following leg injuries was published, reporting incidence rates of 7% to 18% (Bauer 1944). Nonfatal and fatal pulmonary embolism (PE) complicate DVT of the lower extremities. Fatal PE used to be a common cause of death in hospitals, because of the often clinically occult nature of DVT.
The discussion on the use of LMWH in immobilization of the lower limb focuses on the following issues: the reduction of symptomatic VTE, the reduction of asymptomatic DVT, the relevance of asymptomatic DVT, and the incidence of complications. With meta‐analyses, we were able to show that the use of LMWH in immobilization of the lower limb following leg injury results in a reduction of symptomatic VTE and of (asymptomatic) DVT.
Over 80% of DVTs diagnosed were located distally. Calf vein thrombosis propagates and becomes proximal in between 0% and 25% of patients, accounting for a mean of 10%. Up to 10% of proximal DVTs embolize massively, and are potentially fatal (Anonymous 1986; Schellong 2007). Opinions differ about the risk of post‐thrombotic syndrome (PTS) after distal DVT. It is stated that the risk is considerably lower than in proximal DVT. Results are inconsistent for the relationship between the location of the initial thrombus and the subsequent development of PTS. Some prospective studies reported rates of PTS after distal DVT that were as high as 20% to 80% (McLafferty 1998; Schulman 1986). Hence, distal DVT appears to be associated with a substantial risk of subsequent PTS. Further research is indicated to elicit the exact role of distal DVT in the development of PTS (Kahn 2006).
The incidence of complications in the review seemed to be low compared with data in the literature. Major bleeding was reported in 0.27% (two out of 1469 participants) and minor bleeding in up to 7.8% of participants (Van Adrichem 2017). In contrast, Reilmann 1993 reported up to 14% of participants with hematomas due to injections. Another study even described it up to 28%, although some of the participants were not treated with LMWH, but with unfractionated heparin (Zagrodnick 1990). In our seven included studies, no cases of heparin‐induced thrombocytopenia were described, which is in concordance with the observation that this condition is seldom seen in combination with LMWH (Bloemen 2012).
The eight studies used different types of LMWH. The total number of participants was insufficient to evaluate which type of LMWH to choose. Evidence published so far indicates that any differences between LMWH preparations, if they exist, must be extremely small (Geerts 2004). It is unlikely that a properly sized and designed study comparing the various LMWHs will ever take place. Sample size calculations quickly reach over 10,000 if one considers appropriate definitions of 'non‐inferiority' when comparing the various antithrombotic regimens (Vaitkus 2004).
Venography is considered the most accurate method of diagnosing DVT but is now rarely used in clinical practice (Abelseth 1996; Bergqvist 2002). It is an invasive procedure, and there is a reported incidence of serious adverse reactions to the contrast media in the range of 0.4% to 2% (Lensing 1990). Duplex ultrasound is the most common non‐invasive test used to diagnose venous thrombosis of the extremities. Compared with venography, ultrasound has been shown to be reliable in the diagnosis of proximal symptomatic DVT, with a sensitivity and specificity of over 90%. However, for distal thrombosis, a sensitivity of 73% can be reached when combining compression ultrasound with color‐doppler ultrasound (CBO 2008). Considering the properties of the imaging methods used to diagnose PE, a recent systematic review showed a sensitivity of 86% for the ventilation‐perfusion scintigraphy and a specificity of 46% compared with pulmonary angiography. For the CT scan, the percentages were 85% sensitivity and 94% specificity (Hayashino 2005). Consequently, the number of instances of missed DVT and PE seems to be acceptable when including studies with different diagnostic procedures, and should not influence our conclusions to a major extent. However, an underestimation of the incidence of VTE can occur when using ultrasound, CT‐scan, or ventilation‐perfusion scanning.
Increased incidence of DVT in an untreated population calls for measures, and thromboprophylaxis seems necessary if immobilization in a cast or brace is needed. Post‐traumatic DVT can lead to PE or long‐term damage in the form of PTS (Kakkar 1994). Low molecular weight heparin has been shown to be effective in reducing the incidence of VTE. The possible complications of major bleeding events (2/750 participants or 0.3%), and heparin‐induced thrombopenia (none in this review) have been shown to be extremely rare, and do not outweigh the beneficial effect of the reduction of thromboses.
PRISMA study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias domain, presented as percentages across all included studies
Risk of bias summary: review authors' judgements about each risk of bias domain for each included study

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 1 Deep venous thrombosis: regardless of type of plaster, whether operated or not.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 2 Deep venous thrombosis: in below‐knee cast, whether operated or not.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 3 Deep venous thrombosis: conservative treatment (i.e. non‐operated patients).
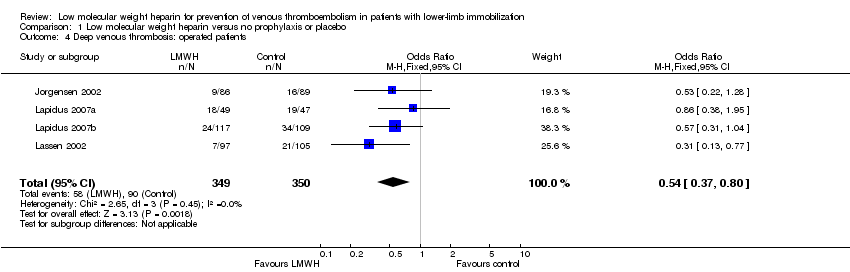
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 4 Deep venous thrombosis: operated patients.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 5 Deep venous thrombosis: fractures.
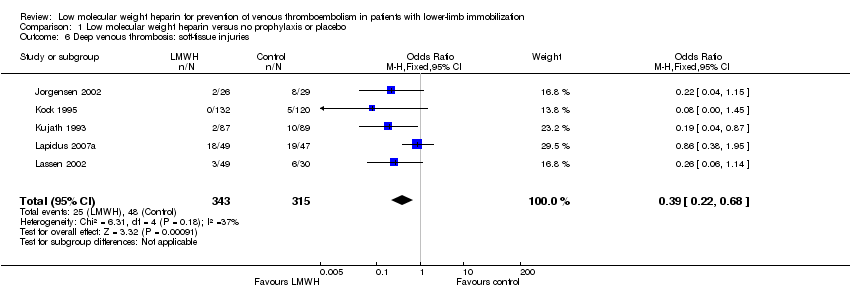
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 6 Deep venous thrombosis: soft‐tissue injuries.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 7 Deep venous thrombosis: distal segment.
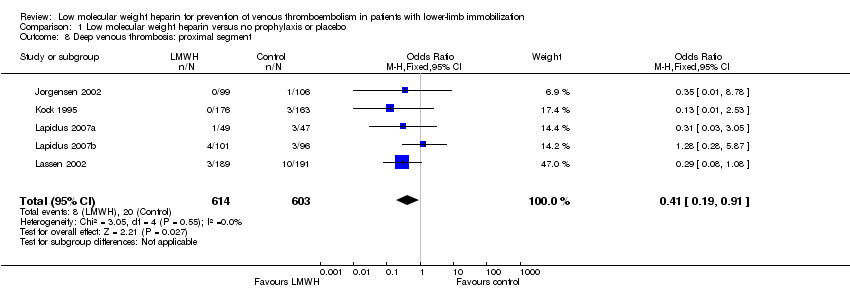
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 8 Deep venous thrombosis: proximal segment.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 9 Pulmonary embolism.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 10 Symptomatic venous thromboembolism.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 11 Mortality due to pulmonary embolism.

Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 12 Mortality due to other causes.
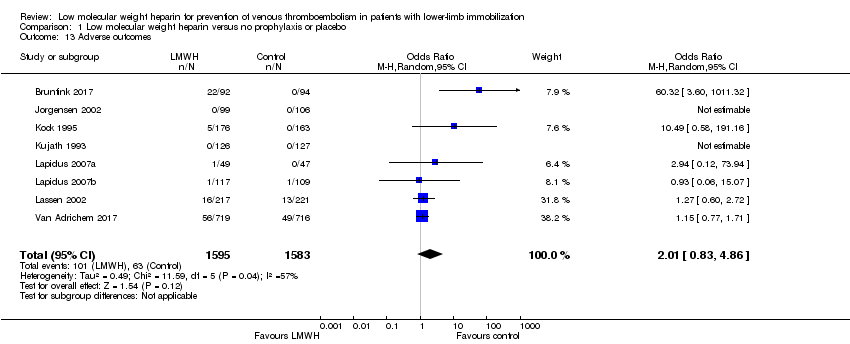
Comparison 1 Low molecular weight heparin versus no prophylaxis or placebo, Outcome 13 Adverse outcomes.
| Low molecular weight heparin compared to no prophylaxis or placebo in prevention of venous thromboembolism in patients with lower‐limb immobilization | ||||||
| Patient or population: prevention of venous thromboembolism in patients with lower‐limb immobilization | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no prophylaxis or placebo | Risk with low molecular weight heparin | |||||
| Deep venous thrombosis | Study population | OR 0.45 | 1676 | ⊕⊕⊕⊝ | ||
| 174 per 1000 | 87 per 1000 | |||||
| Pulmonary embolism | Study population | OR 0.50 | 2517 | ⊕⊕⊝⊝ | ||
| 7 per 1000 | 4 per 1000 | |||||
| Symptomatic venous thromboembolism | Study population | OR 0.40 | 2924 | ⊕⊕⊝⊝ | ||
| 21 per 1000 | 9 per 1000 | |||||
| Mortality due to pulmonary embolism | Study population | ‐ | 3111 | ‐ | No mortality due to pulmonary embolism was reported | |
| see comment | see comment | |||||
| Mortality due to other causes | Study population | OR 0.33 (0.01 to 8.15) | 3111 | ⊕⊕⊝⊝ | One death (in no prophylaxis/placebo group) was reported in the included studies | |
| 1 per 1000 | 0 per 1000 (0 to 5) | |||||
| Adverse outcomes | Study population | OR 2.01 | 3178 | ⊕⊕⊝⊝ | ||
| 40 per 1000 | 78 per 1000 | |||||
| *We calculated the assumed risk of the no prophylaxis or placebo group from the average risk in the no prophylaxis or placebo groups (i.e. the number of participants with events divided by total number of participants of the no prophylaxis or placebo group included in the meta‐analysis). The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level as 3 out of 7 studies showed considerable risk of bias | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Deep venous thrombosis: regardless of type of plaster, whether operated or not Show forest plot | 7 | 1676 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.33, 0.61] |
| 2 Deep venous thrombosis: in below‐knee cast, whether operated or not Show forest plot | 6 | 1080 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.34, 0.72] |
| 3 Deep venous thrombosis: conservative treatment (i.e. non‐operated patients) Show forest plot | 5 | 974 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.18, 0.53] |
| 4 Deep venous thrombosis: operated patients Show forest plot | 4 | 699 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.37, 0.80] |
| 5 Deep venous thrombosis: fractures Show forest plot | 6 | 1003 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.33, 0.70] |
| 6 Deep venous thrombosis: soft‐tissue injuries Show forest plot | 5 | 658 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.22, 0.68] |
| 7 Deep venous thrombosis: distal segment Show forest plot | 5 | 1208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.42, 0.89] |
| 8 Deep venous thrombosis: proximal segment Show forest plot | 5 | 1217 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.91] |
| 9 Pulmonary embolism Show forest plot | 5 | 2517 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.47] |
| 10 Symptomatic venous thromboembolism Show forest plot | 6 | 2924 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.21, 0.76] |
| 11 Mortality due to pulmonary embolism Show forest plot | 8 | 3111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Mortality due to other causes Show forest plot | 8 | 3111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 13 Adverse outcomes Show forest plot | 8 | 3178 | Odds Ratio (M‐H, Random, 95% CI) | 2.01 [0.83, 4.86] |

