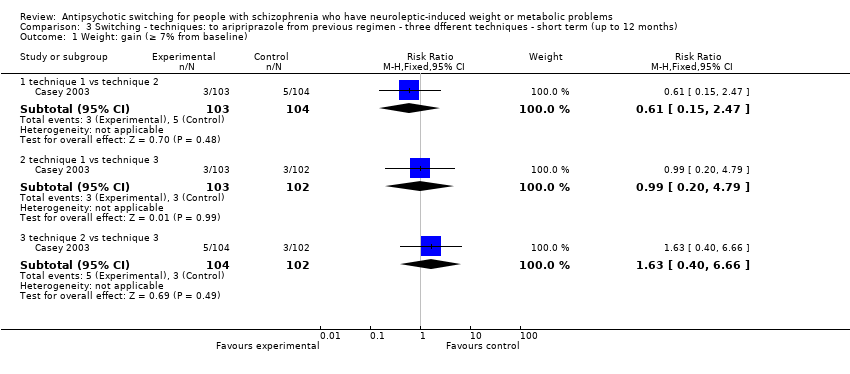
| 1 Weight: gain (≥ 7% from baseline) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.1 technique 1 vs technique 2 | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.47] |
| 1.2 technique 1 vs technique 3 | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.20, 4.79] |
| 1.3 technique 2 vs technique 3 | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.40, 6.66] |
| 2 Global state: 1a. Average change (CGI‐I, high = good) Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 2.1 technique 1 vs technique 2 | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.49, 0.21] |
| 2.2 technique 1 vs technique 3 | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.34, 0.36] |
| 2.3 technique 2 vs technique 3 | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.20, 0.50] |
| 3 Global state: 1b. Average change (CGI‐S, decline = good) Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 3.1 technique 1 vs technique 2 | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.27, 0.15] |
| 3.2 technique 1 vs technique 3 | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.24, 0.16] |
| 3.3 technique 2 vs technique 3 | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.20, 0.24] |
| 4 Mental state: Average change (PANSS, high decline = good) Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 4.1 technique 1 vs technique 2 | 1 | 197 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [‐4.51, 5.69] |
| 4.2 technique 1 vs technique 3 | 1 | 198 | Mean Difference (IV, Fixed, 95% CI) | 2.52 [‐2.39, 7.43] |
| 4.3 technique 2 vs technique 3 | 1 | 201 | Mean Difference (IV, Fixed, 95% CI) | 1.93 [‐2.63, 6.49] |
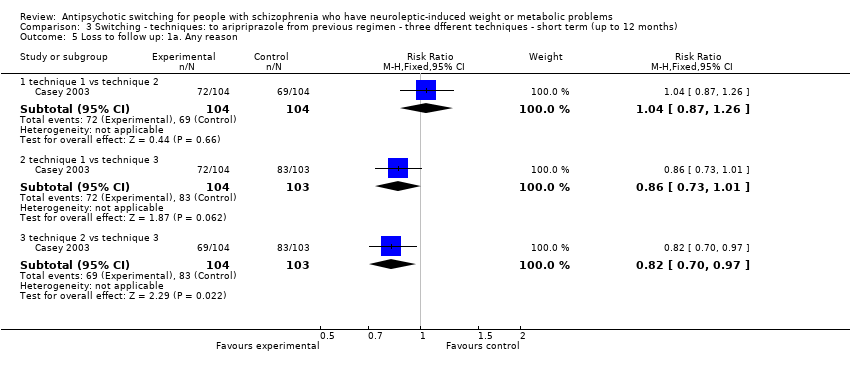
| 5 Loss to follow up: 1a. Any reason Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 5.1 technique 1 vs technique 2 | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.26] |
| 5.2 technique 1 vs technique 3 | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 5.3 technique 2 vs technique 3 | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.70, 0.97] |
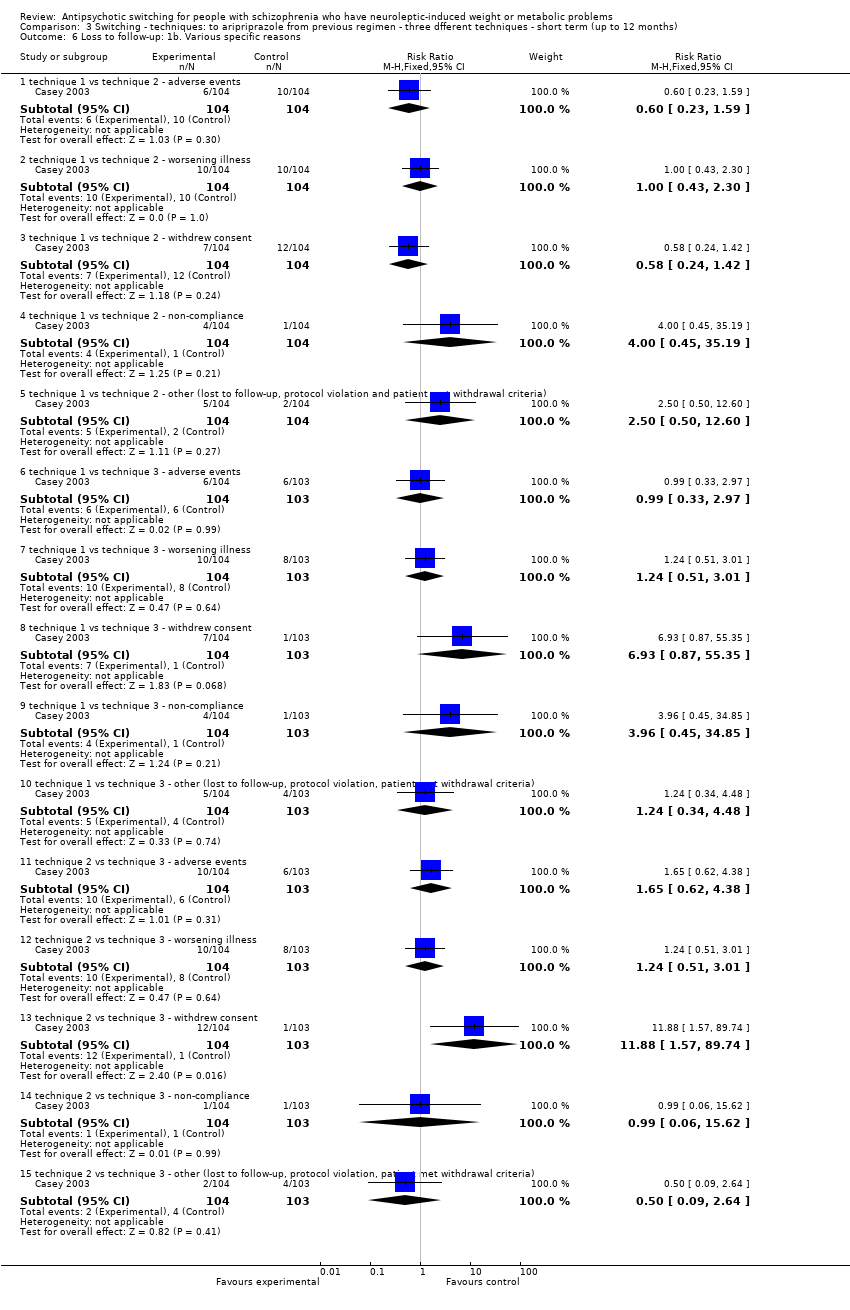
| 6 Loss to follow‐up: 1b. Various specific reasons Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 6.1 technique 1 vs technique 2 ‐ adverse events | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.23, 1.59] |
| 6.2 technique 1 vs technique 2 ‐ worsening illness | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.43, 2.30] |
| 6.3 technique 1 vs technique 2 ‐ withdrew consent | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.24, 1.42] |
| 6.4 technique 1 vs technique 2 ‐ non‐compliance | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.45, 35.19] |
| 6.5 technique 1 vs technique 2 ‐ other (lost to follow‐up, protocol violation and patient met withdrawal criteria) | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.50, 12.60] |
| 6.6 technique 1 vs technique 3 ‐ adverse events | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.33, 2.97] |
| 6.7 technique 1 vs technique 3 ‐ worsening illness | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.51, 3.01] |
| 6.8 technique 1 vs technique 3 ‐ withdrew consent | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.93 [0.87, 55.35] |
| 6.9 technique 1 vs technique 3 ‐ non‐compliance | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [0.45, 34.85] |
| 6.10 technique 1 vs technique 3 ‐ other (lost to follow‐up, protocol violation, patient met withdrawal criteria) | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.34, 4.48] |
| 6.11 technique 2 vs technique 3 ‐ adverse events | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.62, 4.38] |
| 6.12 technique 2 vs technique 3 ‐ worsening illness | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.51, 3.01] |
| 6.13 technique 2 vs technique 3 ‐ withdrew consent | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.88 [1.57, 89.74] |
| 6.14 technique 2 vs technique 3 ‐ non‐compliance | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.62] |
| 6.15 technique 2 vs technique 3 ‐ other (lost to follow‐up, protocol violation, patient met withdrawal criteria) | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.64] |
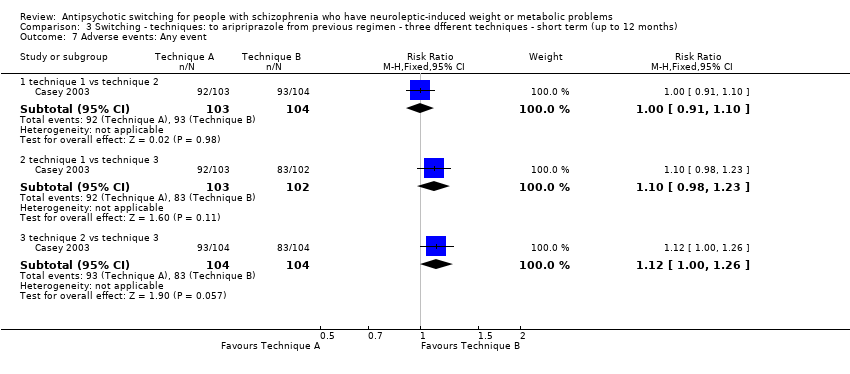
| 7 Adverse events: Any event Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 7.1 technique 1 vs technique 2 | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.10] |
| 7.2 technique 1 vs technique 3 | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.98, 1.23] |
| 7.3 technique 2 vs technique 3 | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.00, 1.26] |