نقش متوتروکسات در القای بهبودی در کولیت اولسراتیو
چکیده
پیشینه
کولیت اولسراتیو (ulcerative colitis; UC) یک بیماری التهابی مزمن روده است. کورتیکواستروئیدها و 5‐آمینوسالیسیلاتها (aminosalicylates) شایعترین درمانهای مورد استفاده برای مداوای این بیماری هستند. با این حال، بسیاری از بیماران برای بیماری مقاوم به استروئید و بیماری وابسته به استروئید نیاز به درمان سرکوبکننده سیستم ایمنی دارند. متوتروکسات (methotrexate) دارویی است که برای درمان انواع بیماریهای التهابی، از جمله بیماری کرون (Crohn's disease)، موثر است. این مرور برای تعیین اثربخشی درمان با متوتروکسات در بیماران مبتلا به UC انجام شد. این مرور، نسخه بهروز شده از یک مرور کاکرین است که قبلا منتشر شد.
اهداف
ارزیابی کارآمدی و بیخطری (safety) مصرف متوتروکسات برای القای بهبودی در بیماران مبتلا به UC.
روشهای جستوجو
MEDLINE؛ EMBASE؛ CENTRAL و پایگاه ثبت تخصصی کارآزماییهای گروه IBD/FBD در کاکرین از ابتدا تا 26 جون 2014 جستوجو شدند. منابع مطالعه و مقالات مروری نیز برای یافتن کارآزماییهای بیشتر جستوجو شدند. چکیدههای مقالات به دست آمده از نشستهای علمی اصلی متخصصین گوارش نیز برای شناسایی پژوهشهای منتشرشده فقط به صورت چکیده، مورد جستوجو قرار گرفتند.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترلشدهای که در آنها متوتروکسات با دارونما (placebo) یا یک مقایسهکننده فعال در بیماران مبتلا به کولیت اولسراتیو فعال مقایسه شد، برای گنجاندن در این مرور در نظر گرفته شدند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده بهطور مستقل از هم مطالعات را از نظر واجد شرایط بودن مرور کرده، دادهها را استخراج کرده، و کیفیت مطالعه را با استفاده از ابزار خطر سوگیری (bias) کاکرین بررسی کردند. معیار پیامد اولیه، نسبتی از بیماران بود که به بهبودی بالینی و قطع مصرف استروئیدها، طبق تعریف ارائهشده در مطالعات، دست یافتند و به عنوان درصدی از تعداد کل بیماران تصادفیسازی شده بیان شدند (آنالیز قصد درمان (intention‐to‐treat)). خطر نسبی (RR) و 95% فواصل اطمینان (95% CI) متناظر را برای پیامدهای دو حالتی (dichotomous outcome) محاسبه کردیم. کیفیت کلی شواهد حمایتکننده از پیامد اولیه با استفاده از معیارهای درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (Grading of Recommendations Assessment, Development and Evaluation; GRADE) بررسی شد.
نتایج اصلی
دو مطالعه (101 بیمار) در این مرور گنجانده شدند. یک مطالعه (67 = n)، متوتروکسات خوراکی 12.5 میلیگرم/هفته) را با دارونما مقایسه کرد. مطالعه دیگر (n = 34) متوتروکسات خوراکی (15 میلیگرم/هفته) را با 6‐مرکاپتوپورین (mercaptopurine) (1.5 میلیگرم/کیلوگرم/روز) و 5‐آمینوسالیسیلیک اسید (3 گرم/روز) مقایسه کرد. مطالعه کنترلشده با دارونما، دارای خطر پائین سوگیری ارزیابی شد. مطالعه دیگر، به دلیل طراحی برچسب‐باز (open‐label)، در معرض خطر بالای سوگیری بود. تفاوت در نرخ بهبودی بالینی میان بیماران دریافتکننده متوتروکسات و دارونما از اهمیت آماری برخوردار نبود. چهل و هفت درصد (14/30) از بیماران گروه متوتروکسات در مقایسه با 49% (18/37) از بیماران گروه دارونما، در طول دوره مطالعه به بهبودی بالینی و قطع کامل مصرف استروئیدها دست یافتند (RR: 0.96؛ 95% CI؛ 0.58 تا 1.59). آنالیز GRADE نشان داد که کیفیت کلی شواهد حمایتکننده از این پیامد، با توجه به دادههای بسیار پراکنده (32 رویداد)، در سطح پائین قرار داشت. در مطالعهای که به مقایسه متوتروکسات خوراکی با 6‐مرکاپتوپورین و 5‐آمینوسالیسیلیک اسید پرداخت، تفاوت در نسبتی از بیماران که به بهبودی بالینی و قطع مصرف استروئیدها دست یافتند، دارای اهمیت آماری نبود. در طول 30 هفته، 58% (7/12) از بیماران گروه متوتروکسات در مقایسه با 79% (11/14) از بیماران گروه 6‐مرکاپتوپورین (RR: 0.74؛ 95% CI؛ 0.43 تا 1.29) و 25% از بیماران گروه 5‐آمینوسالیسیلیک اسید (RR: 2.33؛ 95% CI؛ 0.64 تا 8.49) به بهبودی بالینی و قطع مصرف استروئیدها دست یافتند. آنالیزهای GRADE نشان داد که کیفیت کلی شواهد، به دلیل دادههای بسیار پراکنده (به ترتیب 18 و 9 رویداد) و خطر بالای سوگیری، در سطح بسیار پائین قرار داشت. در کارآزمایی کنترلشده با دارونما، دو بیمار (7%) به دلیل عوارض جانبی (لکوپنی (leucopenia)، میگرن (migraine)) از گروه متوتروکسات خارج شدند، و یک بیمار (3%) نیز به دلیل بروز بثورات پوستی (rash) از گروه دارونما خارج شد (RR: 2.47؛ 95% CI؛ 0.23 تا 25.91). عوارض جانبی در بیماران دریافتکننده متوتروکسات در مطالعه انجام شده با مقایسهکننده فعال شامل تهوع و سوءهاضمه (dyspepsia)، آلوپسی خفیف، افزایش خفیف سطح آسپارتات آمینوترانسفراز (aspartate aminotransferase)، آبسه صفاقی، هیپوآلبومینمی (hypoalbuminemia)، بثورات پوستی شدید و پنومونی (pneumonia) آتیپکال بودند.
نتیجهگیریهای نویسندگان
اگرچه متوتروکسات به خوبی تحمل شد، مطالعات هیچ مزیتی را برای متوتروکسات نسبت به دارونما یا گروههای مقایسه فعال نشان ندادند. نتایج برای پیامدهای کارآمدی درمان میان متوتروکسات و دارونما، متوتروکسات و 6‐مرکاپتوپورین، و متوتروکسات و 5‐آمینوسالیسیلیک اسید نامطمئن بودند. مشخص نیست دوز بالاتر یا تجویز وریدی دارو برای درمان القایی موثر است یا خیر. در حال حاضر هیچ شواهدی برای حمایت از استفاده از متوتروکسات در القای بهبودی در کولیت اولسراتیو فعال وجود ندارد. انجام یک کارآزمایی که در آن تعداد بیشتری از بیماران، دوز بالاتر متوتروکسات خوراکی را دریافت کنند، مورد نیاز است. در حال حاضر دو کارآزمایی کنترلشده با دارونما در ابعاد بزرگ در حال انجام هستند (METEOR و MERIT‐UC) که کارآمدی و بیخطری متوتروکسات عضلانی یا زیر جلدی را در بیماران مبتلا به UC فعال ارزیابی کرده و ممکن است به ارائه شواهد حمایتکننده از استفاده از متوتروکسات به عنوان درمان کولیت اولسراتیو فعال کمک کنند.
خلاصه به زبان ساده
تاثیر متوتروکسات در درمان کولیت اولسراتیو فعال مزمن
کولیت اولسراتیو (ulcerative colitis) چیست؟
کولیت اولسراتیو یک بیماری التهابی طولانیمدت (مزمن) روده است که با درد (کرامپهای شکمی)، نیاز به عجله در رفتن به سرویس بهداشتی برای دفع مدفوع (فوریت مدفوع) و اسهال خونی مشخص میشود.
متوتروکسات (methotrexate) چیست؟
متوتروکسات دارویی است که پاسخهای طبیعی سیستم ایمنی بدن را کاهش داده و میتواند باعث کاهش التهاب مرتبط با کولیت اولسراتیو شود. زمانی که افراد مبتلا به کولیت اولسراتیو نشانههایی را از بیماری نشان دهند، به آن بیماری «فعال» گفته میشود؛ دورههای زمانی که نشانهها متوقف میشوند، «بهبودی» نامیده میشوند.
پژوهشگران چه موضوعی را بررسی کردند؟
پژوهشگران به بررسی این موضوع پرداختند که متوتروکسات منجر به بهبودی افراد مبتلا به کولیت اولسراتیو فعال میشود یا خیر، و اینکه منجر به هر گونه آسیبی (عوارض جانبی) میشود یا خیر. پژوهشگران تا 26 جون 2014، متون علمی پزشکی را بهطور گستردهای جستوجو کردند.
پژوهشگران به چه نتایجی دست یافتند؟
آنها دو مطالعه را با مجموع 101 شرکتکننده شناسایی کردند. یکی از آنها مطالعهای با کیفیت بالا (67 شرکتکننده) بود که متوتروکسات خوراکی (12.5 میلیگرم/هفته) را با دارونما (placebo) (قرص قند یا داروی تقلبی) مقایسه کرد. مطالعه دیگر (34 شرکتکننده) متوتروکسات خوراکی (15 میلیگرم/هفته) را با 6‐مرکاپتوپورین (mercaptopurine) (یک داروی سرکوبکننده سیستم ایمنی با دوز 1.5 میلیگرم/کیلوگرم/روز) و با 5‐آمینوسالیسیلیک اسید (یک داروی ضدالتهابی در دوز 3 گرم/روز) مقایسه کرد.
در مطالعه با کیفیت بالا، هیچ تفاوتی میان گروههای درمان متوتروکسات و دارونما برای تعداد افرادی که بهبود یافتند و توانستند مصرف استروئیدها را متوقف کنند، وجود نداشت. این نشان میدهد که استفاده از دوز پائین (12.5 میلیگرم/هفته) متوتروکسات باعث بهبودی در کولیت اولسراتیو نمیشود. با این حال، به دلیل کم بودن تعداد افرادی که مورد ارزیابی قرار گرفتند، این نتیجهگیری نامطمئن است.
مطالعه کوچکتر دیگر، تفاوتی را میان متوتروکسات و دیگر درمانها از لحاظ نسبتی از شرکتکنندگان که به بهبودی دست یافتند و توانستند مصرف استروئیدها را متوقف کنند، نشان نداد. به دلیل طراحی ضعیف مطالعه و کم بودن تعداد شرکتکنندگان، این نتیجهگیری نیز نامطمئن است.
عوارض جانبی گزارششده در این دو مطالعه عبارت بودند از لوکوپنی (leucopenia) (کاهش تعداد گلبولهای سفید خون)، میگرن، بثورات پوستی (rash)، تهوع و سوءهاضمه (اختلال در هضم)، آلوپسی (alopecia) خفیف (ریزش مو)، افزایش خفیف در سطح آنزیمهای کبدی (آسپارتات آمینوترانسفراز (aspartate aminotransferase))، تجمع چرک در بافت شکم (آبسه صفاقی)، سطوح پائین غیرطبیعی پروتئین آلبومین در خون (هیپوآلبومینمی (hypoalbuminemia))، و پنومونی (pneumonia).
در حال حاضر، نتایج کارآزماییهای پزشکی استفاده از دوز پائین متوتروکسات خوراکی (12.5 تا 15 میلیگرم/هفته) را برای القای بهبودی در کولیت اولسراتیو فعال تایید نمیکنند. مشخص نیست که دوز بالاتر متوتروکسات خوراکی، یا مصرف متوتروکسات از راههای مختلف (مثلا از طریق تزریق)، احتمال بهبودی را افزایش میدهد یا خیر.
در آینده، پژوهشگران باید مطالعهای را با تعداد بیشتری از شرکتکنندگانی انجام دهند که دوز بالاتر متوتروکسات خوراکی را دریافت میکنند. در حال حاضر، دو مطالعه بزرگ در حال انجام است که دوز بالاتر متوتروکسات ‐ تزریقی ‐ را با دارونما در افراد مبتلا به کولیت اولسراتیو فعال (مطالعات METEOR و MERIT‐UC) مقایسه میکنند. نتایج این مطالعات ممکن است عدم قطعیت را در مورد استفاده از متوتروکسات برای درمان کولیت اولسراتیو فعال برطرف کنند.
Authors' conclusions
Summary of findings
| Methotrexate compared to Placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Methotrexate | |||||
| Remission and complete withdrawal from steroids | 486 per 10001 | 467 per 1000 | RR 0.96 | 67 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| Methotrexate compared to 6‐Mercaptopurine for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| 6‐Mercaptopurine | Methotrexate | |||||
| Proportion of patients achieved clinical remission | 786 per 10001 | 581 per 1000 | RR 0.74 | 26 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| Methotrexate compared to 5‐ASA for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| 5‐ASA | Methotrexate | |||||
| Proportion of patients achieved clinical remission | 250 per 1000 | 582 per 1000 | RR 2.33 | 20 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Details of randomization, allocation concealment, and blinding were not described in the study | ||||||
Background
Ulcerative colitis is a chronic inflammatory bowel disease characterized by abdominal cramping, fecal urgency and bloody diarrhea. The most commonly used therapies for patients with ulcerative colitis are 5‐aminosalicylates and corticosteroids. However, many patients require immunosuppressive agents when their disease becomes steroid‐refractory or dependent. Azathioprine, while modestly effective at maintaining remission in patients with quiescent ulcerative colitis (Hawthorne 1992; Jewell 1974; Timmer 2012), has shown mixed results when studied for remission induction in active disease (Ardizzone 2006; Jewell 1974). Cyclosporine may be effective in treating patients with severe disease, but with potentially significant toxicity (Shibolet 2005). More recently, infliximab has been proven to be beneficial for inducing and maintaining remission in patients who have failed other therapies (Lawson 2006; Rutgeerts 2005). However, despite these treatment advances, a proportion of ulcerative colitis patients still require colectomy for refractory disease (Bach 2006), and the identification of other effective therapies is an important area of research.
Methotrexate, a dihydrofolate reductase inhibitor, has been shown to be effective for both induction and maintenance of remission in patients with Crohn's disease (Feagan 1995; Feagan 2000; McDonald 2012; Patel 2014). Although ulcerative colitis shares some clinical and pathological features with Crohn's disease, and some treatments are similar, therapies effective for one type of inflammatory bowel disease are not necessarily effective for the other, and data regarding efficacy of interventions cannot be extrapolated from studies of one disease to the other. This systematic review is an update of a previously published Cochrane review (Chande 2007).
Objectives
To assess the efficacy and safety of methotrexate for induction of remission in patients with ulcerative colitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials comparing methotrexate with placebo or an active comparator were considered for inclusion. For future updates, studies published as abstracts only will be included if the authors can be contacted for further information.
Types of participants
Adult patients with active ulcerative colitis defined by a combination of clinical, radiographic, endoscopic and histological criteria were included.
Types of interventions
Methotrexate given by any route.
Types of outcome measures
The primary outcome measure was the number of patients achieving clinical remission and complete withdrawal from steroids as defined by the studies and expressed as a percentage of the number of patients randomized (intention to treat analysis). Secondary outcomes measures included:
a) Endoscopic remission as defined by the authors;
b) Clinical, histological or endoscopic improvement as defined by the authors;
c) The occurrence of adverse events; and
d) Improvements in quality of life as measured by a validated instrument.
Search methods for identification of studies
See: Inflammatory Bowel Disease and Functional Bowel Disorders Group search strategy.
MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and the Cochrane IBD/FBD group specialized trials register were searched from inception to June 26, 2014 to identify relevant publications. The search strategies are reported in Appendix 1. Review papers on ulcerative colitis, and references from identified papers were also searched in an effort to identify additional studies. Abstracts from major gastroenterological meetings were searched to identify research published in abstract form only.
Data collection and analysis
Study selection
Two authors (YW and JKM) independently reviewed the studies identified by the literature search to determine eligibility for inclusion based on the criteria identified above. Studies published in abstract form only were to be included only if the authors could be contacted for further information.
Data collection
A data extraction form was developed and used to extract data from included studies. Two authors (YW, JKM) independently extracted data. Any disagreements were resolved by consensus.
Statistical analysis
Data were analyzed using Review Manager (RevMan 5.3.3). Data were analyzed on an intention‐to‐treat basis, and treated dichotomously. In the future if any cross‐over studies are identified, only data from the first arm will be included. The primary endpoint was induction of remission, as defined by the studies. Data were to be combined for analysis if they assessed the same treatments (methotrexate versus placebo or other therapy). If a comparison was only assessed in a single trial, the risk ratio (RR) and corresponding 95% confidence interval (95% CI) were calculated and P‐values were derived using the Chi2 test. If the comparison is assessed in more than one trial, summary test statistics were to be derived using the pooled RR and corresponding 95% CI. The presence of heterogeneity among studies was to be assessed using the Chi2 test (a P value of 0.10 was to be regarded as statistically significant). If statistically significant heterogeneity was identified the RR and 95% CI were to be calculated using a random‐effects model.
Quality assessment
The methodological quality of the included studies was evaluated using the Cochrane risk of bias tool (Higgins 2011). This tool involves rating trials as high, low or unclear risk for each of the following criteria:
-
Randomization sequence generation;
-
Allocation concealment;
-
Blinding;
-
Missing data and attrition;
-
Outcome reporting; and
-
Other sources of bias.
The overall quality of the evidence was evaluated using the GRADE approach (Guyatt 2008; Schünemann 2011). Outcome data are rated as being of high, moderate, low or very low quality evidence. Data from randomized controlled trials begin as high quality but can be downgraded based on the following criteria:
-
Risk of bias in the included trials;
-
Indirect evidence;
-
Inconsistent findings (including unexplained heterogeneity);
-
Imprecision (i.e. sparse data or wide confidence interval or both); and
-
Reporting bias.
The different quality ratings are interpreted as the likelihood that future research would affect the estimate of effect. An estimate of effect based on high quality evidence is unlikely to change with further research. If the overall evidence is of moderate quality further research may have an impact on our confidence in the estimate and may change the estimate. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate when the evidence is rated as low quality. Very low quality research means that we are very uncertain about the finding (Guyatt 2008; Schünemann 2011).
Results
Description of studies
A literature search conducted on June 26, 2014 identified 2776 records. After duplicates were removed, a total of 2481 records remained for review of titles and abstracts. Two authors (YW and JKM) independently reviewed the titles and abstracts of these trials and 30 records were selected for full text review (see Figure 1). Twenty‐eight reports of 24 studies were excluded (See: Characteristics of excluded studies). Egan 1999a was a randomized study but was excluded because there was no placebo or active comparator group. The study compared two doses of subcutaneous methotrexate (15 mg/week versus 25 mg/week). Twenty‐three studies were excluded because they were not randomized controlled trials (Baron 1993; Cummings 2005; Dejica 1998; Egan 1999b; Egan 2000; Fraser 2002; Fraser 2003; Gibson 2006; González‐Lama 2012; Hayes 2014; Herrlinger 2005; Houben 1994; Khan 2013; Kozarek 1989; Kozarek 1992; Mañosa 2011; Paoluzi 2002; Richter 2012; Saibeni 2012; Siveke 2003; Soon 2004; Te 2000; Wahed 2009; see characteristics of excluded studies table and Additional Table 1 ‐ Results from excluded studies). Two studies (total of 101 patients) met the pre‐defined inclusion criteria and were included in the review (Maté‐Jiménez 2000; Oren 1996). The two included studies were sufficiently heterogeneic in terms of comparators, treatment duration, and study design that it was not valid to pool the data. The GRADE analyses were performed on individual studies for each outcome.
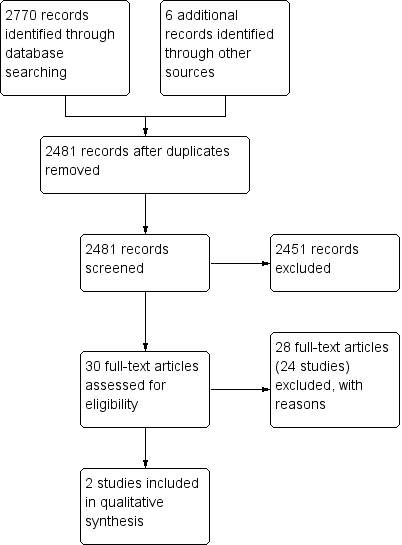
Study flow diagram.
| Study ID | Description | Results |
| Baron 1993 | Open label clinical trial enrolling patients with steroid dependent or steroid refractory IBD (Crohn's n = 10, UC n = 8) Patients received oral methotrexate 15 mg/wk and prednisone The primary outcomes were complete or partial withdrawal from steroids and mean steroid use | UC patients: mean prednisone dose dropped from 26.3 +/‐ 3.2 mg/day to 12.7 +/‐ 2.0 mg/day (P < 0.001) Three patients had a partial response Adverse events were mild |
| Cummings 2005 | Retrospective chart review at two hospitals Steroid dependent or steroid refractory UC patients (n=50) were treated with oral methotrexate (mean dose 19.9 mg/wk for a median of 30 weeks) The primary outcome was remission defined as lack of treatment with steroids for 3 months or more Secondary outcomes: response defined as good, partial or nil, and proportionate reduction of steroids | Remission occurred in 42% of patients The response was good in 54% and partial in 18% Adverse events occurred in 23%; 10% stopped treatment due to adverse events |
| Dejica 1998 | Unrandomized, open label, preliminary trial enrolled twenty‐two patients with chronic active ulcerative colitis, refractory to steroids or sulfasalazine or both for at least 3 months The patients were treated with 25 mg weekly intramuscular injection for 20 weeks The primary outcome was clinical remission with Mayo Clinic Socre ≤ 3, including endoscopy | Clinical remission were obtained in 50% of patients (n=11) Fifteen of 22 patients (68%) had significant clinical improvement in Mayo Clinic score Five patients developed side effects, but the drug‐related adverse effects were not severe enough to warrant discontinuation of therapy |
| Egan 1999a | Randomized, single‐blind trial comparing two doses of subcutaneous methotrexate (15 mg/wk, n=18, versus 25 mg/wk, n=14) in patients with steroid dependent or refractory IBD The primary outcome was remission at 16 weeks defined as the presence of quiescent disease (IBDQ score > or = 170) and discontinuation of prednisone The secondary outcome was partial response defined as ability to discontinue prednisone without a decrease in IBDQ or a clinically significant improvement in disease activity. | After 16 weeks 17% (3/18) of patients in the 15 mg group achieved remission compared to 17% (2/12) of patients in the 25 mg group (P = N.S.) Improvement occurred in 39% (7/18) of the 15 mg group compared to 33% (4/12) of the 25 mg group (P = N.S.) Adverse events occurred in 11% (2/18) of patients in the 15 mg group compared to 17% (2/12) of patients in the 25 mg group (P = N.S.) |
| Egan 1999b | Adenosine was thought to play a major role in anti‐inflammatory mechanism of action of methotrexate in animal models The non‐randomized, open‐label pharmacokinetic study investigating the effects of methotrexate on adenosine concentrations in plasma and at the site of the disease in patients with inflammatory bowel disease In 10 patients with Crohn's disease or ulcerative colitis, rectal adenosine and plasma adenosine concentrations were measured before and immediately after a subcutaneous injection of methotrexate at 15 or 25 mg | There were no significant differences between pre‐injection and post‐injection values in both plasma and rectal adenosine concentrations The mean pre‐dose and post‐dose mean rectal adenosine concentrations were 2.4 µmol/L and 2.1 µmol/L, respectively (P = 0.17) The mean pre‐dose and post ‐dose plasma adenosine concentrations were 3.4 µmol/L and 3.4 µmol/L, respectively (P = 0.95) Therefore, the evidence does not support adenosine as the anti‐inflammatory mediator of methotrexate |
| Egan 2000 | Case series Three patients with steroid refractory UC and 2 patients with steroid refractory Crohn's disease who failed monotherapy with subcutaneous methotrexate 25 mg/week for 16 weeks were treated with the combination of methotrexate and low‐dose oral cyclosporine (3 mg/kg/day) for an additional 16 weeks The primary outcome was remission at 16 weeks defined as the presence of quiescent disease (IBDQ score > or = 170) and discontinuation of prednisone The secondary outcome was partial response defined as ability to discontinue prednisone without a decrease in IBDQ or a clinically significant improvement in disease activity | The three patients with UC experienced clinical improvement with a mean increase in IBDQ score from 164 to 190 points (P = 0.01) One patient developed hypertension |
| Fraser 2002 | Retrospective chart review at two hospitals Seventy patients were reviewed (Crohn's n = 48, UC n = 22) Patients were treated with oral methotrexate (n = 62) or intramuscular methotrexate (n=8) at a mean dose of 20 mg/week for a mean duration of 17.1 months Remission was defined as the lack of a need for oral steroids (either prednisolone or budesonide) for at least 3 months Patients who were well on low doses of prednisolone or budesonide steroids were recorded as ‘remission not achieved’ The continued use of oral 5‐aminosalicylic acid compounds and steroids or 5‐aminosalicylic acid enemas was allowed within the definition of remission Relapse was defined as the need for re‐introduction of steroids, the need for a surgical procedure or the use of infliximab | Remission was achieved in 34 of 55 (62%) of patients who completed more than 3 months of treatment Life‐table analysis showed that the chances of remaining in remission at 12, 24 and 36 months, if treatment was continued, were 90%, 73% and 51% respectively The chances of remaining in remission after stopping treatment at 6, 12 and 18 months were 42%, 21% and 16% respectively |
| Fraser 2003 | Open label clinical trial Eight patients with chronically active moderate to severe UC refractory to corticosteroids and azathioprine or 6‐mercaptopurine were treated with intramuscular methotrexate 25 mg/week (and folic acid) for 16 weeks Efficacy was assessed with the Mayo clinic score | Six of eight patients completed 16 weeks of treatment One patient withdrew due to severe exacerbation and one withdrew due to failure to improve Two patients developed anemia and one patient developed hypertransaminasemia The median Mayo clinic score at 16 weeks was 8 (range 6 to 11) Two patients were referred for colectomy at the end of the study |
| Gibson 2006 | Retrospective chart review at a single IBD clinic including 65 patients (Crohn's n=45, UC n=20) The initial weekly dose was 25 mg in 29 patients, 20 mg in 16 patients, 15 mg in 7 patients or 10 mg in 3 Eighty‐four percent received methotrexate by subcutaneous injection All patients received folate supplementation Response was defined as improvement in bowel symptoms or ability to reduce the dose of steroids Remission was defined as improvement in symptoms with no requirement for steroids for 3 months, or ability to wean off steroids | Remission was achieved by 12 of 19 (63%) patients with UC ‐ an additional patient with UC had a response to treatment The median duration of treatment was 11 months (range 3 to 36) in responders and 6 months (range 1.5 to 10) in non‐responders Fifteen per cent of patients experienced adverse events |
| González‐Lama 2012 | Retrospective chart review of IBD patients treated with methotrexate in eight hospitals in Madrid, Spain Seventy‐seven patients were included (Crohn's disease n = 62, ulcerative colitis n = 15) Methotrexate was initiated at a mean dose of 21 mg/week (range: 15‐30), using parenteral administration in 67% of cases and oral route in 33% of patients Partial response was defined as a decrease in the Harvey‐Bradshaw index of more than three points Remission was defined as a Harvey‐Bradshaw index without steroid treatment below or equal to four | Fourteen out of 15 UC patients received parenteral methotrexate Two patients achieved clinical remission with induction therapy, and 12 (71%) patients gained some response and started maintenance treatment Among the twelve patients, five required dose modification during the follow‐up, three showed loss of response after a mean of 28 weeks, and three more patients achieved clinical remission Adverse events led to methotrexate withdrawal in 5% (4/77) of patients |
| Hayes 2014 | Retrospective chart review of UC patients treated with infliximab (IFX) at a regional referral center Eighty‐five patients with UC were included in the analysis Duration of efficacious IFX therapy, and serum IFX and antibody‐to‐IFX (ATI) levels were compared between patients, who received IFX as monotherapy (n = 39) and in combination with an immunosuppressant (n = 46) Immunosuppressants included azathioprine (65.2% of combination group), mercaptopurine (28.3%), and methotrexate (6.5%) | Concomitant immunosuppressant use was associated with increased duration of IFX therapy (90% in combination group vs. 61% of patients in monotherapy group at 1 year, P = 0.016); greater IFX levels (20.4 mg/L vs. 10.5 mg/L, P = 0.025); and less frequent ATI formation (4.5% vs. 33.3%, P = 0.031) |
| Herrlinger 2005 | Case control study of pharmacogenetics of Mtx therapy in IBD Allele frequencies were assessed in 102 IBD patients treated with methotrexate, 202 patients with Crohn's disease, 205 patients with UC and 189 healthy volunteers All subjects were genotyped for four polymorphisms | No significant difference in allele frequencies were detected between Crohn's disease, UC and healthy volunteers Twenty‐one per cent of methotrexate treated patients experienced adverse events |
| Houben 1994 | Case series of 15 IBD patients (Crohn's disease n=13, UC n=2) treated with intramuscular methotrexate 25 mg/week for 12 weeks, followed by a tapering oral dose One patient was treated twice Disease activity was determined after 1, 2 and 3 months of treatment | The mean defecation frequency went down from 7 to 2 times daily after 12 weeks and prednisone dose could be lowered from 22 mg to 15 mg after 3 months Subjective and objective improvement was noted in 12/15 patients No serious adverse events were reported |
| Khan 2013 | Retrospective cohort study using the nationwide Veterans Affairs database to describe the efficacy of methotrexate in achieving steroid‐free remission Ninety‐one patients with UC were included and they were followed for 15 months after methotrexate initiation by tracking prednisone, methotrexate, thiopurines, and infliximab dispensing records Endpoints were: 1) successful remission (cessation of prednisone filling activity while continuing methotrexate); 2) failure with continuance, failure to be weaned off steroids while continuing methotrexate; 3) failure with discontinuance, cessation of methotrexate while continuing steroids | The average weekly prescription dose for oral and parenteral methotrexate was 14 mg/week (range: 2.3‐31.25) and 25 mg/week (range: 5.8‐70), respectively The mean daily prescription dose for oral prednisone within the oral methotrexate group was 12 mg/day (range 0.7‐68 mg/day) and 25 mg/day (range: 5‐113 mg/day) in the parenteral methotrexate group At the twelfth month, 37% of patients on oral methotrexate and 30% of patients on parenteral methotrexate were able to discontinue steroids |
| Kozarek 1989 | Open label clinical trial including 21 patients with refractory IBD (Crohn's n=14, UC n=7) Patients received intramuscular methotrexate 25 mg/week for 12 weeks After 12 weeks, patients were switched to a tapering oral dose if clinical and objective improvement was noted | Five of 7 UC patients had an objective response as measured by the Ulcerative Colitis Activity Index (13.3 to 6.3, P=0.007) Prednisone dosage decreased from 38.6 mg +/‐ 6.35 (SEM) to 12.9 mg +/‐ 3.4, P=0.01 Five of 7 had histological improvement. None of the UC patients had normal flexible sigmoidoscopy results. Adverse events included mild rises in transaminase levels in 2 patients, transient leukopenia in 1, self‐limited diarrhea and nausea in 2 patients, brittle nails (1 case) and atypical pneumonitis (1 case). |
| Kozarek 1992 | Retrospective chart review, over a 4 year period (1987 to 1991) 86 patients with refractory IBD (Crohn's n=37, UC n=30) were started on 25 mg/week parenteral methotrexate Those patients who responded clinically at 12 weeks were offered weekly oral methotrexate therapy (7.5 to 15 mg) Outcomes included the DAI (scored 0 to 15), prednisone dose, and Mtx toxicity | Seventy per cent of UC patients had a symptomatic and objective response At a mean follow‐up of 59 weeks, only 40% of UC patients continued to respond to Mtx (DAI 5.0 +/‐ 0.9; prednisone 12 +/‐ 3.9 mg), 15 of 30 UC patients required colectomy and one patient stopped methotrexate due to hypersensitivity pneumonitis |
| Mañosa 2011 | Retrospective chart review to evaluate the efficacy and safety of methotrexate in UC patients Patients were included in the study if they received methotrexate for steroid dependency or steroid refractoriness and for maintenance of remission Forty patients were identified from databases of 8 Spanish IBD referral hospitals and followed for at least 6 months Therapeutic success was defined as the absence of UC‐related symptoms, complete steroid withdrawal and no‐requirement of rescue therapies within the first 6 months after starting methotrexate | At 6 months, 45% (18/40) achieved therapeutic success Treatment failure were mainly due to inefficacy (11/22, 50%) or intolerance (8/22, 36%) After a median follow‐up of 28 months. 38% (7) of patients with initial therapeutic success required new steroid courses, 22% (4) started biological therapy and 1 of them required colectomy The cumulative probability of maintaining steroid‐free clinical remission was 60%, 48%, and 35% at 6, 12, 24 months after starting methotrexate, respectively In all, 11 out of 40 patients (27.5%) experienced adverse effects, leading to methotrexate discontinuation in 8 patients |
| Paoluzi 2002 | Open label clinical trial including 42 patients with steroid dependent or steroid resistant active UC Patients were treated with a daily dose of azathioprine (2 mg/kg) and, if intolerant or not responding, with intramuscular methotrexate (12.5 mg/week) Efficacy was assessed by clinical, endoscopic and histological examinations at 6 months Patients achieving clinical remission continued with treatment and were followed up Ten patients received methotrexate The achievement of complete remission with the ability to discontinue oral steroids was defined as the primary outcome Response to treatment was defined as follows: complete remission equals achievement of clinical, endoscopic and histological remission; improvement equals disappearance of symptoms (clinical remission) with endoscopic and histological improvement of inflammatory changes; failure equals worsening, no benefit or clinical improvement with the persistence of unmodified inflammatory changes of the mucosa | Methotrexate induced complete remission in six patients (60%) and improvement in four (40%) During follow‐up, a larger number of patients on azathioprine relapsed in comparison with patients on methotrexate [16/28 (57%) vs. 2/10 (20%), respectively; P < 0.05] |
| Richter 2012 | Retrospective study using a large U.S. health insurance database to document treatment of new‐onset ulcerative colitis (UC) and ulcerative proctitis (UP) in routine clinical practice One thousand five hundred and sixteen UC patients and 636 UP patients were included in the analysis New‐onset UC or UP were identified based on: 1) initial receipt of an oral 5‐ASA, mesalazine suppository, 5‐ASA enema, steroid, antimetabolite, budesonide or TNF inhibitor; 2) sigmoidoscopy/colonoscopy in prior 30 days resulting in a new diagnosis of UC or UP and 3) no prior encounters for Crohn's disease | In UC, initial therapies most frequently used were oral 5‐ASAs (53%), oral 5‐ASAs and systemic steroids (12%), systemic steroids (8%) and mesalazine suppositories (6%); in UP, mesalazine suppositories (42%) and oral 5‐ASAs (19%), combination therapy (14%), mesalazine enema (11%) and rectal steroids (10%) were the mostly frequently used therapies Few patients received maintenance therapy, and there was a limited use of antimetabolites (0.3% in UC, and lower in UP ‐ no specific figures were provided) and biological agents (0.1% in UC) |
| Saibeni 2012 | Retrospective, observational study using 5420 case histories from 8 referral centres in Italy, to evaluate frequency, indications, efficacy and safety of methotrexate in IBD patients One hundred and twelve patients received methotrexate (2.1%, 89 Crohn's disease, 23 ulcerative colitis) | Indications: first‐line immunosuppressant in 32 (28.6%), alternative (second‐line) to thiopurines in 80 (71.4%) Efficacy: optimal in 39/112 (34.8%), partial in 29/112 (25.9%), absent in 22/112 (19.6%), not assessable in 22/122 (19.6%) Side effects happened in 49/112 patients (43.7%, 39 Crohn's disease, 10 ulcerative colitis), leading to drug discontinuation in 38 patients (33.9%) Folic acid use was related to the lower side effects (35/93, 37.6% in those who received folic acid vs. 14/19, 73.7% in those who did not) |
| Siveke 2003 | Case series of 3 patients with steroid dependent or steroid resistant UC Patients were treated with intramuscular methotrexate 25 mg/week These patients received 10 mg of folate orally on the day after injection An additional patient received 15 mg of methotrexate, with the dose being adjusted to 25 mg following increased activity of colitis | Three of 4 patients achieved remission One patient had to discontinue methotrexate due to an increase in aspartate aminotransferase and alanine aminotransferase levels despite dose reduction and prophylactic supplementation of folate |
| Soon 2004 | Retrospective chart review including 72 patients (Crohn's n=66, UC n=6) Patients were treated with mean dose of 18.2 mg/week of methotrexate for six months Methotrexate was given orally in 64 patients and intramuscularly in eight patients Clinical response was defined as sustained withdrawal of oral steroids within 3 months of starting treatment and sustained for a further 3 months or fistula improvement New episodes of steroid therapy, infliximab or surgery during the first 6 months were considered as failure to achieve clinical response | Fifty‐four patients completed six months of treatment Clinical response was achieved in 22 (40.7%) patients [19 of 48 (39.6%) with CD and three of six (50%) with UC] |
| Te 2000 | Retrospective chart review looking at hepatotoxicity among IBD patients who had received a minimum cumulative dose of 1500 mg of methotrexate | In 20 patients who had liver biopsies, the mean cumulative methotrexate dose was 2633 mg (range, 1500–5410 mg), given for a mean of 131.7 wk (range, 66–281 weeks) Nineteen of 20 patients (95%) had mild histological abnormalities (Roenigk’s grade I and II), and one patient had hepatic fibrosis (Roenigk’s grade IIIB) |
| Wahed 2009 | Retrospective chart review to examine the efficacy and safety profile of methotrexate in patients with CD or UC who were either intolerant or non‐responsive to azathioprine/mercaptopurine (AZA/MP) One hundred and thirty‐one patients with IBD treated with MTX were included (99 CD, 32 UC) Clinical response was assessed at 6 months and it was defined as steroid withdrawal, normalization of previously raised CRP or physician's clinical assessment of improvements | In CD, clinical response occurred in 18/29 patients (62%) refractory to AZA/MP and 42/70 patients (60%) intolerant to AZA/MP (P = 1.0) In UC, clinical response occurred in 7/9 patients (78%) refractory to AZA/MP and 15/23 (65%) intolerant to AZA/MP Side effects were seen in 23 (17.4%) patients and led to discontinuation in 11 (8.3%) patients |
Methotrexate versus placebo
Oren 1996
This trial included 67 patients (35 male, 32 female) with chronic active steroid‐dependent ulcerative colitis (defined by typical clinical, radiographic, endoscopic, and pathological criteria). Disease chronicity was defined by the requirement of steroid therapy (minimum 7.5 mg/day) for 4 months of the preceding 12 months. Current use of mesalamine or steroids was permitted. Steroid therapy was to be tapered and discontinued within 2 to 3 months of study entry, but could be restarted or the dose increased as clinically indicated. No immunosuppressive agents could be used in the three months prior to entry . Active disease was defined by a Mayo clinic score of > 7 at study entry. The patients were randomized to oral methotrexate 12.5 mg/week (n = 30) or identical placebo (n = 37) for 9 months. The patients were seen at regular intervals during the nine months. At each visit, the Mayo clinic score was calculated, and a sigmoidoscopy was also performed every three months. The primary outcomes were the proportion of patients who achieved their first remission as well as the maintenance of remission in those patients. The definition of remission was a Mayo clinic score of < 3 (or < 2 without sigmoidoscopy results), and complete withdrawal from steroid therapy.
Methotrexate versus active comparators
Maté‐Jiménez 2000
This study enrolled 34 patients with ulcerative colitis (and 38 patients with Crohn's disease). All patients had steroid‐dependent disease (Mayo clinic score of > 7 despite prednisone > 20 mg/day), but all other therapies were stopped at least 6 months before study entry. The patients were randomized in a 2:2:1 ratio to 6‐mercaptopurine 1.5 mg/kg/day (n = 14), methotrexate 15 mg weekly (n = 12), or 5‐aminosalicylic acid 3 g/day (n = 8) for 30 weeks. There was no placebo comparator. All medications were given orally, and prednisone was tapered by 8 mg/week if clinically appropriate. If remission was achieved the methotrexate dose was reduced to 10 mg/week and the 6‐mercaptopurine dose to 1 mg/kg/day. Patients in the 5‐aminosalicylic acid group who achieved remission continued to receive the same dose (3 g/day) as maintenance therapy. Follow‐up occurred regularly over the study period, and the Mayo clinic score was calculated at weeks 12 and 30. Patients in remission and off steroids at the end of 30 weeks then entered a 76 week maintenance phase. The primary outcome measure was the proportion of patients in remission at 30 weeks, defined by a Mayo clinic score of < 3 and withdrawal from steroid therapy.
Risk of bias in included studies
The risk of bias results are summarized in Figure 2. Oren 1996 used adequate methods of randomization, blinding, and allocation concealment and was rated as low risk of bias for these items. Maté‐Jiménez 2000 was an open‐label study and was rated as high risk of bias for blinding. Moreover, Maté‐Jiménez 2000 did not report the methods used for randomization and allocation concealment and these items were rated as unclear risk of bias. Both of the included trials were rated as low risk of bias for incomplete outcome data (Maté‐Jiménez 2000; Oren 1996). No other issues were found with the trials and they were rated as low risk of bias for the other bias item (Maté‐Jiménez 2000; Oren 1996).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Summary of findings for the main comparison Methotrexate compared to placebo for induction of remission in ulcerative colitis; Summary of findings 2 Methotrexate compared to 6‐mercaptopurine for induction of remission in ulcerative colitis; Summary of findings 3 Methotrexate compared to 5‐aminosalicylic acid (5‐ASA) for induction of remission in ulcerative colitis
Methotrexate versus placebo
One study (N = 67) compared methotrexate to placebo (Oren 1996). There was no statistically significant difference in clinical remission rates between methotrexate and placebo patients. Forty‐seven per cent (14/30) of methotrexate patients achieved clinical remission and complete withdrawal from steroids during the study period compared to 49% (18/37) of placebo patients (RR 0.96, 95% CI 0.58 to 1.59; See Figure 3). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (32 events; See summary of findings Table for the main comparison). The mean time to remission was 4.1 months in the methotrexate group compared to 3.4 months in the placebo group. There was no statistically significant difference in withdrawals due to adverse events (RR 2.47, 95% CI 0.23 to 25.91). Two patients (7%) were withdrawn from the methotrexate group due to adverse events (leucopenia, migraine) compared to one patient (3%) from the placebo group (rash). The Oren 1996 study did not report on any of the other secondary outcomes including endoscopic remission, clinical, histological or endoscopic improvement or improvements in quality of life.

Forest plot of comparison: 1 Methotrexate versus placebo, outcome: 1.1 Remission and complete withdrawal from steroids.
Methotrexate versus active comparators
One study (N = 34) compared methotrexate to 6‐mercaptopurine and 5‐aminosalicylic acid (Maté‐Jiménez 2000). There were no statistically significant differences in the proportion of patients who achieved clinical remission and withdrawal from steroids. At 30 weeks 58% (7/12) of methotrexate patients achieved clinical remission and withdrawal from steroids compared to 79% (11/14) of 6‐mercaptopurine patients (RR 0.74, 95% CI 0.43 to 1.29; See Figure 4). Twenty‐five per cent (2/8) of 5‐aminosalicylic acid patients achieved remission and withdrawal of steroids after completing 30 weeks of induction treatment compared to 58% (7/12) of methotrexate patients (RR 2.33, 95% CI 0.64 to 8.49; See Figure 5). GRADE analyses indicated that the overall quality of the evidence was very low due to very sparse data and high risk of bias (see summary of findings Table 2; summary of findings Table 3). Adverse events experienced by methotrexate patients included nausea and dyspepsia, mild alopecia, mild increase in aspartate aminotransferase levels, peritoneal abscess, hypoalbuminemia, severe rash and atypical pneumonia. Three of 26 patients treated with methotrexate withdrew due to adverse events compared to 4 of 30 patients treated with 6‐mercaptopurine.
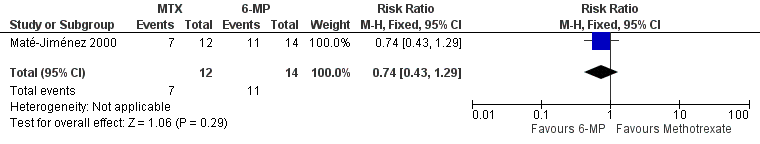
Forest plot of comparison: 2 Methotrexate versus 6‐Mercaptopurine, outcome: 2.1 Proportion of patients achieved clinical remission.
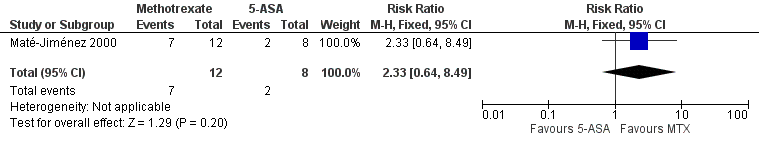
Forest plot of comparison: 3 Methotrexate versus 5‐ASA, outcome: 3.1 Proportion of patients achieved clinical remission.
Discussion
The treatment of patients with ulcerative colitis often involves 5‐aminosalicylic acid and corticosteroids. However, some patients require immunosuppressive therapy when their disease becomes dependent on or refractory to steroid therapy. Unfortunately, there are a limited number of therapeutic options for these patients. Azathioprine has traditionally been the next choice of therapy for these patients, and although it may be effective for maintenance of remission (Timmer 2012), it does not appear to provide any benefit for induction of remission in these patients (Hawthorne 1992; Jewell 1974; Ardizzone 2006). Infliximab has recently been shown to be effective for induction of remission in patients with active ulcerative colitis who have failed steroid therapy (Lawson 2006; Rutgeerts 2005). Cyclosporine may be effective for treating some patients with severe disease (Shibolet 2005). Failing these medications, surgery is usually considered the next therapeutic option for these patients.
Methotrexate has been shown to be effective for both induction of remission (at a dose of 25 mg intramuscular weekly) and maintenance of remission (15 mg intramuscular weekly) in patients with Crohn's disease (Feagan 1995; Feagan 2000; McDonald 2012; Patel 2014). There has only been one well‐designed, placebo‐controlled, randomized trial assessing methotrexate for induction of remission in ulcerative colitis.
Oren 1996 was designed to assess the utility of methotrexate for induction of remission in patients with active steroid‐dependent ulcerative colitis. In this study, no benefit for methotrexate over placebo was found. The dose of methotrexate used (12.5 mg orally weekly) was lower than the dose used in the trial assessing methotrexate for induction of remission in Crohn's disease (Feagan 1995), and was administered orally rather than parenterally. The low dose oral regimen utilized by Oren 1996 is effective in patients with rheumatoid arthritis (Lopez‐Olivo 2014). Since methotrexate is absorbed in the small bowel, oral administration should be appropriate in ulcerative colitis. A parenteral route is preferred in Crohn's disease, where drug absorption may be affected by disease activity. However, whether or not a higher dose or parenteral administration of methotrexate in ulcerative colitis patients would be more effective is unknown.
One other small (N =34), poor quality randomized trial assessing methotrexate, 6‐mercaptopurine and 5‐aminosalicylic acid in ulcerative colitis has been published (Maté‐Jiménez 2000). No statistically significant differences in clinical remission rates were found. The results for efficacy outcomes between methotrexate and 6‐mercaptopurine and methotrexate and 5‐aminosalicylic acid were uncertain as GRADE analyses rated the overall quality of evidence from this study as very low. Thus no firm conclusions can be drawn from this study.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Methotrexate versus placebo, outcome: 1.1 Remission and complete withdrawal from steroids.

Forest plot of comparison: 2 Methotrexate versus 6‐Mercaptopurine, outcome: 2.1 Proportion of patients achieved clinical remission.

Forest plot of comparison: 3 Methotrexate versus 5‐ASA, outcome: 3.1 Proportion of patients achieved clinical remission.

Comparison 1 Methotrexate versus placebo, Outcome 1 Remission and complete withdrawal from steroids.

Comparison 1 Methotrexate versus placebo, Outcome 2 Withdrawal due adverse events.

Comparison 2 Methotrexate versus 6‐Mercaptopurine, Outcome 1 Proportion of patients achieved clinical remission.

Comparison 3 Methotrexate versus 5‐ASA, Outcome 1 Proportion of patients achieved clinical remission.
| Methotrexate compared to Placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Methotrexate | |||||
| Remission and complete withdrawal from steroids | 486 per 10001 | 467 per 1000 | RR 0.96 | 67 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| Methotrexate compared to 6‐Mercaptopurine for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| 6‐Mercaptopurine | Methotrexate | |||||
| Proportion of patients achieved clinical remission | 786 per 10001 | 581 per 1000 | RR 0.74 | 26 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of study | ||||||
| Methotrexate compared to 5‐ASA for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| 5‐ASA | Methotrexate | |||||
| Proportion of patients achieved clinical remission | 250 per 1000 | 582 per 1000 | RR 2.33 | 20 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Details of randomization, allocation concealment, and blinding were not described in the study | ||||||
| Study ID | Description | Results |
| Baron 1993 | Open label clinical trial enrolling patients with steroid dependent or steroid refractory IBD (Crohn's n = 10, UC n = 8) Patients received oral methotrexate 15 mg/wk and prednisone The primary outcomes were complete or partial withdrawal from steroids and mean steroid use | UC patients: mean prednisone dose dropped from 26.3 +/‐ 3.2 mg/day to 12.7 +/‐ 2.0 mg/day (P < 0.001) Three patients had a partial response Adverse events were mild |
| Cummings 2005 | Retrospective chart review at two hospitals Steroid dependent or steroid refractory UC patients (n=50) were treated with oral methotrexate (mean dose 19.9 mg/wk for a median of 30 weeks) The primary outcome was remission defined as lack of treatment with steroids for 3 months or more Secondary outcomes: response defined as good, partial or nil, and proportionate reduction of steroids | Remission occurred in 42% of patients The response was good in 54% and partial in 18% Adverse events occurred in 23%; 10% stopped treatment due to adverse events |
| Dejica 1998 | Unrandomized, open label, preliminary trial enrolled twenty‐two patients with chronic active ulcerative colitis, refractory to steroids or sulfasalazine or both for at least 3 months The patients were treated with 25 mg weekly intramuscular injection for 20 weeks The primary outcome was clinical remission with Mayo Clinic Socre ≤ 3, including endoscopy | Clinical remission were obtained in 50% of patients (n=11) Fifteen of 22 patients (68%) had significant clinical improvement in Mayo Clinic score Five patients developed side effects, but the drug‐related adverse effects were not severe enough to warrant discontinuation of therapy |
| Egan 1999a | Randomized, single‐blind trial comparing two doses of subcutaneous methotrexate (15 mg/wk, n=18, versus 25 mg/wk, n=14) in patients with steroid dependent or refractory IBD The primary outcome was remission at 16 weeks defined as the presence of quiescent disease (IBDQ score > or = 170) and discontinuation of prednisone The secondary outcome was partial response defined as ability to discontinue prednisone without a decrease in IBDQ or a clinically significant improvement in disease activity. | After 16 weeks 17% (3/18) of patients in the 15 mg group achieved remission compared to 17% (2/12) of patients in the 25 mg group (P = N.S.) Improvement occurred in 39% (7/18) of the 15 mg group compared to 33% (4/12) of the 25 mg group (P = N.S.) Adverse events occurred in 11% (2/18) of patients in the 15 mg group compared to 17% (2/12) of patients in the 25 mg group (P = N.S.) |
| Egan 1999b | Adenosine was thought to play a major role in anti‐inflammatory mechanism of action of methotrexate in animal models The non‐randomized, open‐label pharmacokinetic study investigating the effects of methotrexate on adenosine concentrations in plasma and at the site of the disease in patients with inflammatory bowel disease In 10 patients with Crohn's disease or ulcerative colitis, rectal adenosine and plasma adenosine concentrations were measured before and immediately after a subcutaneous injection of methotrexate at 15 or 25 mg | There were no significant differences between pre‐injection and post‐injection values in both plasma and rectal adenosine concentrations The mean pre‐dose and post‐dose mean rectal adenosine concentrations were 2.4 µmol/L and 2.1 µmol/L, respectively (P = 0.17) The mean pre‐dose and post ‐dose plasma adenosine concentrations were 3.4 µmol/L and 3.4 µmol/L, respectively (P = 0.95) Therefore, the evidence does not support adenosine as the anti‐inflammatory mediator of methotrexate |
| Egan 2000 | Case series Three patients with steroid refractory UC and 2 patients with steroid refractory Crohn's disease who failed monotherapy with subcutaneous methotrexate 25 mg/week for 16 weeks were treated with the combination of methotrexate and low‐dose oral cyclosporine (3 mg/kg/day) for an additional 16 weeks The primary outcome was remission at 16 weeks defined as the presence of quiescent disease (IBDQ score > or = 170) and discontinuation of prednisone The secondary outcome was partial response defined as ability to discontinue prednisone without a decrease in IBDQ or a clinically significant improvement in disease activity | The three patients with UC experienced clinical improvement with a mean increase in IBDQ score from 164 to 190 points (P = 0.01) One patient developed hypertension |
| Fraser 2002 | Retrospective chart review at two hospitals Seventy patients were reviewed (Crohn's n = 48, UC n = 22) Patients were treated with oral methotrexate (n = 62) or intramuscular methotrexate (n=8) at a mean dose of 20 mg/week for a mean duration of 17.1 months Remission was defined as the lack of a need for oral steroids (either prednisolone or budesonide) for at least 3 months Patients who were well on low doses of prednisolone or budesonide steroids were recorded as ‘remission not achieved’ The continued use of oral 5‐aminosalicylic acid compounds and steroids or 5‐aminosalicylic acid enemas was allowed within the definition of remission Relapse was defined as the need for re‐introduction of steroids, the need for a surgical procedure or the use of infliximab | Remission was achieved in 34 of 55 (62%) of patients who completed more than 3 months of treatment Life‐table analysis showed that the chances of remaining in remission at 12, 24 and 36 months, if treatment was continued, were 90%, 73% and 51% respectively The chances of remaining in remission after stopping treatment at 6, 12 and 18 months were 42%, 21% and 16% respectively |
| Fraser 2003 | Open label clinical trial Eight patients with chronically active moderate to severe UC refractory to corticosteroids and azathioprine or 6‐mercaptopurine were treated with intramuscular methotrexate 25 mg/week (and folic acid) for 16 weeks Efficacy was assessed with the Mayo clinic score | Six of eight patients completed 16 weeks of treatment One patient withdrew due to severe exacerbation and one withdrew due to failure to improve Two patients developed anemia and one patient developed hypertransaminasemia The median Mayo clinic score at 16 weeks was 8 (range 6 to 11) Two patients were referred for colectomy at the end of the study |
| Gibson 2006 | Retrospective chart review at a single IBD clinic including 65 patients (Crohn's n=45, UC n=20) The initial weekly dose was 25 mg in 29 patients, 20 mg in 16 patients, 15 mg in 7 patients or 10 mg in 3 Eighty‐four percent received methotrexate by subcutaneous injection All patients received folate supplementation Response was defined as improvement in bowel symptoms or ability to reduce the dose of steroids Remission was defined as improvement in symptoms with no requirement for steroids for 3 months, or ability to wean off steroids | Remission was achieved by 12 of 19 (63%) patients with UC ‐ an additional patient with UC had a response to treatment The median duration of treatment was 11 months (range 3 to 36) in responders and 6 months (range 1.5 to 10) in non‐responders Fifteen per cent of patients experienced adverse events |
| González‐Lama 2012 | Retrospective chart review of IBD patients treated with methotrexate in eight hospitals in Madrid, Spain Seventy‐seven patients were included (Crohn's disease n = 62, ulcerative colitis n = 15) Methotrexate was initiated at a mean dose of 21 mg/week (range: 15‐30), using parenteral administration in 67% of cases and oral route in 33% of patients Partial response was defined as a decrease in the Harvey‐Bradshaw index of more than three points Remission was defined as a Harvey‐Bradshaw index without steroid treatment below or equal to four | Fourteen out of 15 UC patients received parenteral methotrexate Two patients achieved clinical remission with induction therapy, and 12 (71%) patients gained some response and started maintenance treatment Among the twelve patients, five required dose modification during the follow‐up, three showed loss of response after a mean of 28 weeks, and three more patients achieved clinical remission Adverse events led to methotrexate withdrawal in 5% (4/77) of patients |
| Hayes 2014 | Retrospective chart review of UC patients treated with infliximab (IFX) at a regional referral center Eighty‐five patients with UC were included in the analysis Duration of efficacious IFX therapy, and serum IFX and antibody‐to‐IFX (ATI) levels were compared between patients, who received IFX as monotherapy (n = 39) and in combination with an immunosuppressant (n = 46) Immunosuppressants included azathioprine (65.2% of combination group), mercaptopurine (28.3%), and methotrexate (6.5%) | Concomitant immunosuppressant use was associated with increased duration of IFX therapy (90% in combination group vs. 61% of patients in monotherapy group at 1 year, P = 0.016); greater IFX levels (20.4 mg/L vs. 10.5 mg/L, P = 0.025); and less frequent ATI formation (4.5% vs. 33.3%, P = 0.031) |
| Herrlinger 2005 | Case control study of pharmacogenetics of Mtx therapy in IBD Allele frequencies were assessed in 102 IBD patients treated with methotrexate, 202 patients with Crohn's disease, 205 patients with UC and 189 healthy volunteers All subjects were genotyped for four polymorphisms | No significant difference in allele frequencies were detected between Crohn's disease, UC and healthy volunteers Twenty‐one per cent of methotrexate treated patients experienced adverse events |
| Houben 1994 | Case series of 15 IBD patients (Crohn's disease n=13, UC n=2) treated with intramuscular methotrexate 25 mg/week for 12 weeks, followed by a tapering oral dose One patient was treated twice Disease activity was determined after 1, 2 and 3 months of treatment | The mean defecation frequency went down from 7 to 2 times daily after 12 weeks and prednisone dose could be lowered from 22 mg to 15 mg after 3 months Subjective and objective improvement was noted in 12/15 patients No serious adverse events were reported |
| Khan 2013 | Retrospective cohort study using the nationwide Veterans Affairs database to describe the efficacy of methotrexate in achieving steroid‐free remission Ninety‐one patients with UC were included and they were followed for 15 months after methotrexate initiation by tracking prednisone, methotrexate, thiopurines, and infliximab dispensing records Endpoints were: 1) successful remission (cessation of prednisone filling activity while continuing methotrexate); 2) failure with continuance, failure to be weaned off steroids while continuing methotrexate; 3) failure with discontinuance, cessation of methotrexate while continuing steroids | The average weekly prescription dose for oral and parenteral methotrexate was 14 mg/week (range: 2.3‐31.25) and 25 mg/week (range: 5.8‐70), respectively The mean daily prescription dose for oral prednisone within the oral methotrexate group was 12 mg/day (range 0.7‐68 mg/day) and 25 mg/day (range: 5‐113 mg/day) in the parenteral methotrexate group At the twelfth month, 37% of patients on oral methotrexate and 30% of patients on parenteral methotrexate were able to discontinue steroids |
| Kozarek 1989 | Open label clinical trial including 21 patients with refractory IBD (Crohn's n=14, UC n=7) Patients received intramuscular methotrexate 25 mg/week for 12 weeks After 12 weeks, patients were switched to a tapering oral dose if clinical and objective improvement was noted | Five of 7 UC patients had an objective response as measured by the Ulcerative Colitis Activity Index (13.3 to 6.3, P=0.007) Prednisone dosage decreased from 38.6 mg +/‐ 6.35 (SEM) to 12.9 mg +/‐ 3.4, P=0.01 Five of 7 had histological improvement. None of the UC patients had normal flexible sigmoidoscopy results. Adverse events included mild rises in transaminase levels in 2 patients, transient leukopenia in 1, self‐limited diarrhea and nausea in 2 patients, brittle nails (1 case) and atypical pneumonitis (1 case). |
| Kozarek 1992 | Retrospective chart review, over a 4 year period (1987 to 1991) 86 patients with refractory IBD (Crohn's n=37, UC n=30) were started on 25 mg/week parenteral methotrexate Those patients who responded clinically at 12 weeks were offered weekly oral methotrexate therapy (7.5 to 15 mg) Outcomes included the DAI (scored 0 to 15), prednisone dose, and Mtx toxicity | Seventy per cent of UC patients had a symptomatic and objective response At a mean follow‐up of 59 weeks, only 40% of UC patients continued to respond to Mtx (DAI 5.0 +/‐ 0.9; prednisone 12 +/‐ 3.9 mg), 15 of 30 UC patients required colectomy and one patient stopped methotrexate due to hypersensitivity pneumonitis |
| Mañosa 2011 | Retrospective chart review to evaluate the efficacy and safety of methotrexate in UC patients Patients were included in the study if they received methotrexate for steroid dependency or steroid refractoriness and for maintenance of remission Forty patients were identified from databases of 8 Spanish IBD referral hospitals and followed for at least 6 months Therapeutic success was defined as the absence of UC‐related symptoms, complete steroid withdrawal and no‐requirement of rescue therapies within the first 6 months after starting methotrexate | At 6 months, 45% (18/40) achieved therapeutic success Treatment failure were mainly due to inefficacy (11/22, 50%) or intolerance (8/22, 36%) After a median follow‐up of 28 months. 38% (7) of patients with initial therapeutic success required new steroid courses, 22% (4) started biological therapy and 1 of them required colectomy The cumulative probability of maintaining steroid‐free clinical remission was 60%, 48%, and 35% at 6, 12, 24 months after starting methotrexate, respectively In all, 11 out of 40 patients (27.5%) experienced adverse effects, leading to methotrexate discontinuation in 8 patients |
| Paoluzi 2002 | Open label clinical trial including 42 patients with steroid dependent or steroid resistant active UC Patients were treated with a daily dose of azathioprine (2 mg/kg) and, if intolerant or not responding, with intramuscular methotrexate (12.5 mg/week) Efficacy was assessed by clinical, endoscopic and histological examinations at 6 months Patients achieving clinical remission continued with treatment and were followed up Ten patients received methotrexate The achievement of complete remission with the ability to discontinue oral steroids was defined as the primary outcome Response to treatment was defined as follows: complete remission equals achievement of clinical, endoscopic and histological remission; improvement equals disappearance of symptoms (clinical remission) with endoscopic and histological improvement of inflammatory changes; failure equals worsening, no benefit or clinical improvement with the persistence of unmodified inflammatory changes of the mucosa | Methotrexate induced complete remission in six patients (60%) and improvement in four (40%) During follow‐up, a larger number of patients on azathioprine relapsed in comparison with patients on methotrexate [16/28 (57%) vs. 2/10 (20%), respectively; P < 0.05] |
| Richter 2012 | Retrospective study using a large U.S. health insurance database to document treatment of new‐onset ulcerative colitis (UC) and ulcerative proctitis (UP) in routine clinical practice One thousand five hundred and sixteen UC patients and 636 UP patients were included in the analysis New‐onset UC or UP were identified based on: 1) initial receipt of an oral 5‐ASA, mesalazine suppository, 5‐ASA enema, steroid, antimetabolite, budesonide or TNF inhibitor; 2) sigmoidoscopy/colonoscopy in prior 30 days resulting in a new diagnosis of UC or UP and 3) no prior encounters for Crohn's disease | In UC, initial therapies most frequently used were oral 5‐ASAs (53%), oral 5‐ASAs and systemic steroids (12%), systemic steroids (8%) and mesalazine suppositories (6%); in UP, mesalazine suppositories (42%) and oral 5‐ASAs (19%), combination therapy (14%), mesalazine enema (11%) and rectal steroids (10%) were the mostly frequently used therapies Few patients received maintenance therapy, and there was a limited use of antimetabolites (0.3% in UC, and lower in UP ‐ no specific figures were provided) and biological agents (0.1% in UC) |
| Saibeni 2012 | Retrospective, observational study using 5420 case histories from 8 referral centres in Italy, to evaluate frequency, indications, efficacy and safety of methotrexate in IBD patients One hundred and twelve patients received methotrexate (2.1%, 89 Crohn's disease, 23 ulcerative colitis) | Indications: first‐line immunosuppressant in 32 (28.6%), alternative (second‐line) to thiopurines in 80 (71.4%) Efficacy: optimal in 39/112 (34.8%), partial in 29/112 (25.9%), absent in 22/112 (19.6%), not assessable in 22/122 (19.6%) Side effects happened in 49/112 patients (43.7%, 39 Crohn's disease, 10 ulcerative colitis), leading to drug discontinuation in 38 patients (33.9%) Folic acid use was related to the lower side effects (35/93, 37.6% in those who received folic acid vs. 14/19, 73.7% in those who did not) |
| Siveke 2003 | Case series of 3 patients with steroid dependent or steroid resistant UC Patients were treated with intramuscular methotrexate 25 mg/week These patients received 10 mg of folate orally on the day after injection An additional patient received 15 mg of methotrexate, with the dose being adjusted to 25 mg following increased activity of colitis | Three of 4 patients achieved remission One patient had to discontinue methotrexate due to an increase in aspartate aminotransferase and alanine aminotransferase levels despite dose reduction and prophylactic supplementation of folate |
| Soon 2004 | Retrospective chart review including 72 patients (Crohn's n=66, UC n=6) Patients were treated with mean dose of 18.2 mg/week of methotrexate for six months Methotrexate was given orally in 64 patients and intramuscularly in eight patients Clinical response was defined as sustained withdrawal of oral steroids within 3 months of starting treatment and sustained for a further 3 months or fistula improvement New episodes of steroid therapy, infliximab or surgery during the first 6 months were considered as failure to achieve clinical response | Fifty‐four patients completed six months of treatment Clinical response was achieved in 22 (40.7%) patients [19 of 48 (39.6%) with CD and three of six (50%) with UC] |
| Te 2000 | Retrospective chart review looking at hepatotoxicity among IBD patients who had received a minimum cumulative dose of 1500 mg of methotrexate | In 20 patients who had liver biopsies, the mean cumulative methotrexate dose was 2633 mg (range, 1500–5410 mg), given for a mean of 131.7 wk (range, 66–281 weeks) Nineteen of 20 patients (95%) had mild histological abnormalities (Roenigk’s grade I and II), and one patient had hepatic fibrosis (Roenigk’s grade IIIB) |
| Wahed 2009 | Retrospective chart review to examine the efficacy and safety profile of methotrexate in patients with CD or UC who were either intolerant or non‐responsive to azathioprine/mercaptopurine (AZA/MP) One hundred and thirty‐one patients with IBD treated with MTX were included (99 CD, 32 UC) Clinical response was assessed at 6 months and it was defined as steroid withdrawal, normalization of previously raised CRP or physician's clinical assessment of improvements | In CD, clinical response occurred in 18/29 patients (62%) refractory to AZA/MP and 42/70 patients (60%) intolerant to AZA/MP (P = 1.0) In UC, clinical response occurred in 7/9 patients (78%) refractory to AZA/MP and 15/23 (65%) intolerant to AZA/MP Side effects were seen in 23 (17.4%) patients and led to discontinuation in 11 (8.3%) patients |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Remission and complete withdrawal from steroids Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.58, 1.59] |
| 2 Withdrawal due adverse events Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.23, 25.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients achieved clinical remission Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.43, 1.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients achieved clinical remission Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.64, 8.49] |

