Intervenciones de reducción de los niveles de homocisteína para la prevención de eventos cardiovasculares
Appendices
Appendix 1. Search strategies 2008
CENTRAL
#1 MeSH descriptor Vitamin B Complex explode all trees
#2 ”vitamin b*“
#3 folic next acid in Title, Abstract or Keywords
#4 folate* in Title, Abstract or Keywords
#5 (homocyst* near/6 lower*)
#6 (homocyst* near/6 reduc*)
#7 pyridoxin*
#8 cobalamin*
#9 cyanocobalamin*
#10 pyridoxol*
#11 MeSH descriptor Vitamins this term only
#12 (vitamin* and homocyst*)
#13 multivitamin*
#14 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13)
#15 MeSH descriptor Cardiovascular Diseases this term only
#16 MeSH descriptor Myocardial Ischemia explode all trees
#17 MeSH descriptor Brain Ischemia explode all trees
#18 MeSH descriptor Cerebrovascular Disorders this term only
#19 (coronary near/6 disease)
#20 angina
#21 myocardial next infarct*
#22 heart next infarct*
#23 (stroke or strokes)
#24 (cerebr* near/6 accident*)
#25 (cerebr* near/6 infarct*)
#26 (brain near/6 infarct*)
#27 apoplexy
#28 cardiovascular next disease*
#29 (cardiovascular near/6 event*)
#30 MeSH descriptor Hyperhomocysteinemia explode all trees
#31 hyperhomocyst*
#32 cva
#33 (#15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25)
#34 (#26 or #27 or #28 or #29 or #30 or #31 or #32)
#35 (#33 or #34)
#36 (#14 and #35)
LILACS (accessed through Biblioteca Virtual em Saúde)
((Pt ENSAYO CONTROLADO ALEATORIO OR Pt ENSAYO CLINICO CONTROLADO OR Mh ENSAYOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUCIÓN ALEATORIA OR Mh METODO DOBLE CIEGO OR Mh METODO SIMPLECIEGO OR Pt ESTUDIO MULTICÉNTRICO) or ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((Ct ANIMALES OR Mh ANIMALES OR Ct CONEJOS OR Ct RATÓN OR MH Ratas OR MH Primates OR MH Perros OR MH Conejos OR MH Porcinos) AND NOT (Ct HUMANO AND Ct ANIMALES)) [Palavras] and MH Vitamina B 12 OR Cobamidas OR Hidroxocobalamina OR Complejo Vitamínico B OR Ácido Fólico OR Ácidos Pteroilpoliglutámicos OR Tetrahidrofolatos OR Formiltetrahidrofolatos OR Vitamina B 6 OR Piridoxal OR Fosfato de Piridoxal OR Piridoxamina OR Piridoxina OR Homocisteína OR Vitaminas or TW vitamin$ or tw cobalamin$ or tw cianocobalamin$ or tw cyanocobalam$ or tw cobamid$ or tw hidroxocobalam$ or tw Hydroxocobalam$ or ((tw complejo or tw complex$) and tw vitamin$ and tw b) or (tw acid$ and (tw folic$ or tw ptero$)) or tw Tetrahidrofolatos or tw Formiltetrahidrofolatos or (tw vitamin$ or (tw b or tw b6 or tw b12)) or tw Piridoxal or tw Pyridoxal or ((tw Fosfat$ or tw phosphate$) and (tw Piridoxal or tw pyridoxal)) or tw Piridox$ or tw Pyridox$ or tw Homocisteína or tw Homocysteine) AND (MH Enfermedades Cardiovasculares or Isquemia Miocárdica or Ex C14.280.647$ or Isquemia Encefálica or Ex C10.228.140.300.150$ or Trastornos Cerebrovasculares or hiperhomocisteinemia or Accidente Cerebrovascular or ((tw apoplexia or tw derrame or tw trastorno$ or tw accident$ or tw acidente or tw stroke$ or tw disease$ or tw enfermedad$ or tw doenca$ or tw event$ or tw infart$ or tw isquemia or tw disorder$) and (tw miocardio or tw myocard$ or tw cerebr$ or tw cardiovascul$ or tw heart or tw cardiovascul$ or tw encefal$)) or tw hyperhomocyst$ or tw hiperhomocisteinemia) [Palavras]
MEDLINE
1 exp Vitamin B Complex/
2 vitamin b.tw.
3 folic acid.tw.
4 folate$.tw.
5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw.
6 pyridoxin$.tw.
7 cobalamin$.tw.
8 cyanocobalamin$.tw.
9 pyridoxol$.tw.
10 Vitamins/
11 or/1‐10
12 Cardiovascular Diseases/
13 exp Myocardial Ischemia/
14 exp Brain Ischemia/
15 Cerebrovascular Disorders/
16 (coronary adj3 disease$).tw.
17 angina.tw.
18 myocardial infarct$.tw.
19 heart infarct$.tw.
20 heart attack$.tw.
21 (stroke or strokes).tw.
22 (cerebr$ adj3 (accident$ or infarct$)).tw.
23 (brain adj3 infarct$).tw.
24 apoplexy.tw.
25 (cardiovascular adj2 (disease$ or event$)).tw.
26 Hyperhomocysteinemia/
27 hyperhomocyst?in?emi$.tw.
28 or/12‐27
29 11 and 28
30 randomized controlled trial.pt.
31 controlled clinical trial.pt.
32 Randomized controlled trials/
33 random allocation/
34 double blind method/
35 single‐blind method/
36 or/30‐35
37 exp animal/ not humans/
38 36 not 37
39 clinical trial.pt.
40 exp Clinical Trials as Topic/
41 (clin$ adj25 trial$).ti,ab.
42 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab.
43 placebos/
44 placebo$.ti,ab.
45 random$.ti,ab.
46 research design/
47 or/39‐46
48 47 not 37
49 38 or 48
50 49 and 29
Embase
1 exp Vitamin B Group/
2 vitamin b.tw.
3 folic acid.tw.
4 folate$.tw.
5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw.
6 pyridoxin$.tw.
7 cobalamin$.tw.
8 cyanocobalamin$.tw.
9 pyridoxol$.tw.
10 Vitamins/
11 or/1‐10
12 Cardiovascular Diseases/
13 exp ischaemic heart disease/
14 exp Coronary Artery Disease/
15 exp Brain Ischemia/
16 cerebrovascular disease/
17 stroke/
18 cerebrovascular accident/
19 (coronary adj3 disease$).tw.
20 angina.tw.
21 myocardial infarct$.tw.
22 heart infarct$.tw.
23 heart attack$.tw.
24 (stroke or strokes).tw.
25 (cerebr$ adj3 (accident$ or infarct$)).tw.
26 (brain adj3 infarct$).tw.
27 apoplexy.tw.
28 (cardiovascular adj2 (disease$ or event$)).tw.
29 Hyperhomocysteinemia/
30 hyperhomocyst?in?emi$.tw.
31 or/12‐30
32 11 and 31
33 controlled clinical trial/
34 random$.tw.
35 randomized controlled trial/
36 follow‐up.tw.
37 double blind procedure/
38 placebo$.tw.
39 placebo/
40 factorial$.ti,ab.
41 (crossover$ or cross‐over$).ti,ab.
42 (double$ adj blind$).ti,ab.
43 (singl$ adj blind$).ti,ab.
44 assign$.ti,ab.
45 allocat$.ti,ab.
46 volunteer$.ti,ab.
47 Crossover Procedure/
48 Single Blind Procedure/
49 or/33‐48
50 32 and 49
Web of Science
# 11 TS=(#10 and (random* or blind* or placebo* or comparative or comparison or prospective or controlled or trial or evaluation or rct))
# 10 #7 or #8 or #9
# 9 TS=(#6 and (”cerebrovascular accident*“ or hyperhomocyst*))
# 8 TS=(#6 and (angina or stroke or strokes or cva or infarction*))
# 7 TS=(#6 and (cardiovascular or myocardial or coronary or cardiac or ”heart disease*“))
# 6 #1 or #2 or #3 or #4 or #5
# 5 TS=(homocyst* same (lower* or reduc*))
# 4 TS=(vitamin* and homocyst*)
# 3 TS=folate*
# 2 TS=”vitamin B“
# 1 TS=(pyridoxin* or cobalamin* or cyanocobalamin* or pyridoxol* or ”folic acid“)
Appendix 2. Search strategies 2012
CENTRAL
#1 MeSH descriptor Vitamin B Complex explode all trees
#2 (vitamin b)
#3 folic acid
#4 folate*
#5 ((homocystein* or homocystin*) near/3 (low* or reduc*))
#6 (pyridoxin*)
#7 (cobalamin*)
#8 (cyanocobalamin*)
#9 (pyridoxol*)
#10 MeSH descriptor Vitamins, this term only
#11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
#12 MeSH descriptor Cardiovascular Diseases, this term only
#13 MeSH descriptor Myocardial Ischemia explode all trees
#14 MeSH descriptor Brain Ischemia explode all trees
#15 MeSH descriptor Cerebrovascular Disorders, this term only
#16 (coronary near/3 disease*)
#17 (angina)
#18 (myocardial infarct*)
#19 (heart infarct*)
#20 (heart attack*)
#21 (stroke or strokes)
#22 (cerebr* near/3 (accident* or infarct*))
#23 (brain near/3 infarct*)
#24 (apoplexy)
#25 (cardiovascular near/2 (disease* or event*))
#26 MeSH descriptor Hyperhomocysteinemia, this term only
#27 hyperhomocyst?in?emi*
#28 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27)
#29 (#11 AND #28)
MEDLINE
1 exp Vitamin B Complex/
2 vitamin b.tw.
3 folic acid.tw.
4 folate$.tw.
5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw.
6 pyridoxin$.tw.
7 cobalamin$.tw.
8 cyanocobalamin$.tw.
9 pyridoxol$.tw.
10 Vitamins/
11 or/1‐10
12 Cardiovascular Diseases/
13 exp Myocardial Ischemia/
14 exp Brain Ischemia/
15 Cerebrovascular Disorders/
16 (coronary adj3 disease$).tw.
17 angina.tw.
18 myocardial infarct$.tw.
19 heart infarct$.tw.
20 heart attack$.tw.
21 (stroke or strokes).tw.
22 (cerebr$ adj3 (accident$ or infarct$)).tw.
23 (brain adj3 infarct$).tw.
24 apoplexy.tw.
25 (cardiovascular adj2 (disease$ or event$)).tw.
26 Hyperhomocysteinemia/
27 hyperhomocyst?in?emi$.tw.
28 or/12‐27
29 11 and 28
30 randomized controlled trial.pt.
31 controlled clinical trial.pt.
32 randomized.ab.
33 placebo.ab.
34 drug therapy.fs.
35 randomly.ab.
36 trial.ab.
37 groups.ab.
38 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
39 exp animals/ not humans.sh. (3663238)
40 38 not 39
41 29 and 40
42 (200808* or 200809* or 20081* or 2009* or 2010* or 2011* or 2012*).ed.
43 41 and 42
Embase
1 exp Vitamin B Complex/
2 vitamin b.tw.
3 folic acid.tw.
4 folate$.tw.
5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw.
6 pyridoxin$.tw.
7 cobalamin$.tw.
8 cyanocobalamin$.tw.
9 pyridoxol$.tw.
10 Vitamins/
11 or/1‐10
12 Cardiovascular Diseases/
13 exp Myocardial Ischemia/
14 exp Brain Ischemia/
15 Cerebrovascular Disorders/
16 (coronary adj3 disease$).tw.
17 angina.tw.
18 myocardial infarct$.tw.
19 heart infarct$.tw.
20 heart attack$.tw.
21 (stroke or strokes).tw.
22 (cerebr$ adj3 (accident$ or infarct$)).tw.
23 (brain adj3 infarct$).tw.
24 apoplexy.tw.
25 (cardiovascular adj2 (disease$ or event$)).tw.
26 Hyperhomocysteinemia/
27 hyperhomocyst?in?emi$.tw.
28 or/12‐27
29 11 and 28
30 random$.tw.
31 factorial$.tw.
32 crossover$.tw.
33 cross over$.tw.
34 cross‐over$.tw.
35 placebo$.tw.
36 (doubl$ adj blind$).tw.
37 (singl$ adj blind$).tw.
38 assign$.tw.
39 allocat$.tw.
40 volunteer$.tw.
41 crossover procedure/
42 double blind procedure/
43 randomized controlled trial/
44 single blind procedure/
45 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44
46 (animal/ or nonhuman/) not human/
47 45 not 46
48 29 and 47
49 (200808* or 200809* or 20081* or 2009* or 2010* or 2011* or 2012*).dd.
50 48 and 49
Web of Science
#24 #23 AND #22
#23 Topic=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))
#22 #21 AND #9
#21 #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11
#20 Topic=(hyperhomocyst$in$emi*)
#19 Topic=((cardiovascular near/2 (disease* or event*)))
#18 Topic=(apoplexy)
#17 Topic=((brain near/3 infarct*))
#16 Topic=((cerebr* near/3 (accident* or infarct*)))
#15 Topic=((stroke or strokes))
#14 Topic=(heart attack*)
#13 Topic=(heart infarct*)
#12 Topic=(myocardial infarct*)
#11 Topic=(angina)
#10 Topic=((coronary near/3 disease*))
#9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#8 Topic=(pyridoxol*)
#7 Topic=(cyanocobalamin*)
#6 Topic=(cobalamin*)
#5 Topic=(pyridoxin*)
#4 Topic=(((homocystein*) near/3 (low$ or reduc*))) OR Topic=(((homocystin*) near/3 (low or reduc*)))
#3 Topic=(folate*)
#2 Topic=("folic acid")
#1 Topic=("vitamin b")
Appendix 3. Search strategies 2014
CENTRAL
#1 MeSH descriptor Vitamin B Complex explode all trees
#2 (vitamin b)
#3 folic acid
#4 folate*
#5 ((homocystein* or homocystin*) near/3 (low* or reduc*))
#6 (pyridoxin*)
#7 (cobalamin*)
#8 (cyanocobalamin*)
#9 (pyridoxol*)
#10 MeSH descriptor Vitamins, this term only
#11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
#12 MeSH descriptor Cardiovascular Diseases, this term only
#13 MeSH descriptor Myocardial Ischemia explode all trees
#14 MeSH descriptor Brain Ischemia explode all trees
#15 MeSH descriptor Cerebrovascular Disorders, this term only
#16 (coronary near/3 disease*)
#17 (angina)
#18 (myocardial infarct*)
#19 (heart infarct*)
#20 (heart attack*)
#21 (stroke or strokes)
#22 (cerebr* near/3 (accident* or infarct*))
#23 (brain near/3 infarct*)
#24 (apoplexy)
#25 (cardiovascular near/2 (disease* or event*))
#26 MeSH descriptor Hyperhomocysteinemia, this term only
#27 hyperhomocyst?in?emi*
#28 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27)
#29 (#11 AND #28)
MEDLINE
1 exp Vitamin B Complex/
2 vitamin b.tw.
3 folic acid.tw.
4 folate$.tw.
5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw.
6 pyridoxin$.tw.
7 cobalamin$.tw.
8 cyanocobalamin$.tw.
9 pyridoxol$.tw.
10 Vitamins/
11 or/1‐10
12 Cardiovascular Diseases/
13 exp Myocardial Ischemia/
14 exp Brain Ischemia/
15 Cerebrovascular Disorders/
16 (coronary adj3 disease$).tw.
17 angina.tw.
18 myocardial infarct$.tw.
19 heart infarct$.tw.
20 heart attack$.tw.
21 (stroke or strokes).tw.
22 (cerebr$ adj3 (accident$ or infarct$)).tw.
23 (brain adj3 infarct$).tw.
24 apoplexy.tw.
25 (cardiovascular adj2 (disease$ or event$)).tw.
26 Hyperhomocysteinemia/
27 hyperhomocyst?in?emi$.tw.
28 or/12‐27
29 11 and 28
30 randomized controlled trial.pt.
31 controlled clinical trial.pt.
32 randomized.ab.
33 placebo.ab.
34 drug therapy.fs.
35 randomly.ab.
36 trial.ab.
37 groups.ab.
38 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
39 exp animals/ not humans.sh. (3663238)
40 38 not 39
41 29 and 40
42 (2012* or 2013* or 2014*).ed.
43 41 and 42
Embase
1 exp Vitamin B Complex/
2 vitamin b.tw.
3 folic acid.tw.
4 folate$.tw.
5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw.
6 pyridoxin$.tw.
7 cobalamin$.tw.
8 cyanocobalamin$.tw.
9 pyridoxol$.tw.
10 Vitamins/
11 or/1‐10
12 Cardiovascular Diseases/
13 exp Myocardial Ischemia/
14 exp Brain Ischemia/
15 Cerebrovascular Disorders/
16 (coronary adj3 disease$).tw.
17 angina.tw.
18 myocardial infarct$.tw.
19 heart infarct$.tw.
20 heart attack$.tw.
21 (stroke or strokes).tw.
22 (cerebr$ adj3 (accident$ or infarct$)).tw.
23 (brain adj3 infarct$).tw.
24 apoplexy.tw.
25 (cardiovascular adj2 (disease$ or event$)).tw.
26 Hyperhomocysteinemia/
27 hyperhomocyst?in?emi$.tw.
28 or/12‐27
29 11 and 28
30 random$.tw.
31 factorial$.tw.
32 crossover$.tw.
33 cross over$.tw.
34 cross‐over$.tw.
35 placebo$.tw.
36 (doubl$ adj blind$).tw.
37 (singl$ adj blind$).tw.
38 assign$.tw.
39 allocat$.tw.
40 volunteer$.tw.
41 crossover procedure/
42 double blind procedure/
43 randomized controlled trial/
44 single blind procedure/
45 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44
46 (animal/ or nonhuman/) not human/
47 45 not 46
48 29 and 47
49 (2012* or 2013* or 2014*).dd.
50 48 and 49
Web of Science
#24 #23 AND #22
#23 Topic=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))
#22 #21 AND #9
#21 #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11
#20 Topic=(hyperhomocyst$in$emi*)
#19 Topic=((cardiovascular near/2 (disease* or event*)))
#18 Topic=(apoplexy)
#17 Topic=((brain near/3 infarct*))
#16 Topic=((cerebr* near/3 (accident* or infarct*)))
#15 Topic=((stroke or strokes))
#14 Topic=(heart attack*)
#13 Topic=(heart infarct*)
#12 Topic=(myocardial infarct*)
#11 Topic=(angina)
#10 Topic=((coronary near/3 disease*))
#9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#8 Topic=(pyridoxol*)
#7 Topic=(cyanocobalamin*)
#6 Topic=(cobalamin*)
#5 Topic=(pyridoxin*)
#4 Topic=(((homocystein*) near/3 (low$ or reduc*))) OR Topic=(((homocystin*) near/3 (low or reduc*)))
#3 Topic=(folate*)
#2 Topic=("folic acid")
#1 Topic=("vitamin b")
Appendix 4. Search strategies 2017
CENTRAL
#1 MeSH descriptor Vitamin B Complex explode all trees
#2 (vitamin b)
#3 folic acid
#4 folate*
#5 ((homocystein* or homocystin*) near/3 (low* or reduc*))
#6 (pyridoxin*)
#7 (cobalamin*)
#8 (cyanocobalamin*)
#9 (pyridoxol*)
#10 MeSH descriptor Vitamins, this term only
#11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
#12 MeSH descriptor Cardiovascular Diseases, this term only
#13 MeSH descriptor Myocardial Ischemia explode all trees
#14 MeSH descriptor Brain Ischemia explode all trees
#15 MeSH descriptor Cerebrovascular Disorders, this term only
#16 (coronary near/3 disease*)
#17 (angina)
#18 (myocardial infarct*)
#19 (heart infarct*)
#20 (heart attack*)
#21 (stroke or strokes)
#22 (cerebr* near/3 (accident* or infarct*))
#23 (brain near/3 infarct*)
#24 (apoplexy)
#25 (cardiovascular near/2 (disease* or event*))
#26 MeSH descriptor Hyperhomocysteinemia, this term only
#27 hyperhomocyst?in?emi*
#28 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27)
#29 (#11 AND #28)
MEDLINE OVID
1 exp Vitamin B Complex/
2 vitamin b.tw.
3 folic acid.tw.
4 folate$.tw.
5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw.
6 pyridoxin$.tw.
7 cobalamin$.tw.
8 cyanocobalamin$.tw.
9 pyridoxol$.tw.
10 Vitamins/
11 or/1‐10
12 Cardiovascular Diseases/
13 exp Myocardial Ischemia/
14 exp Brain Ischemia/
15 Cerebrovascular Disorders/
16 (coronary adj3 disease$).tw.
17 angina.tw.
18 myocardial infarct$.tw.
19 heart infarct$.tw.
20 heart attack$.tw.
21 (stroke or strokes).tw.
22 (cerebr$ adj3 (accident$ or infarct$)).tw.
23 (brain adj3 infarct$).tw.
24 apoplexy.tw.
25 (cardiovascular adj2 (disease$ or event$)).tw.
26 Hyperhomocysteinemia/
27 hyperhomocyst?in?emi$.tw.
28 or/12‐27
29 11 and 28
30 randomized controlled trial.pt.
31 controlled clinical trial.pt.
32 randomized.ab.
33 placebo.ab.
34 drug therapy.fs.
35 randomly.ab.
36 trial.ab.
37 groups.ab.
38 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
39 exp animals/ not humans.sh. (3663238)
40 38 not 39
41 29 and 40
42 (2014* or 2015* or 2016*).ed.
43 41 and 42
Embase OVID
1 exp Vitamin B Complex/
2 vitamin b.tw.
3 folic acid.tw.
4 folate$.tw.
5 ((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw.
6 pyridoxin$.tw.
7 cobalamin$.tw.
8 cyanocobalamin$.tw.
9 pyridoxol$.tw.
10 Vitamins/
11 or/1‐10
12 Cardiovascular Diseases/
13 exp Myocardial Ischemia/
14 exp Brain Ischemia/
15 Cerebrovascular Disorders/
16 (coronary adj3 disease$).tw.
17 angina.tw.
18 myocardial infarct$.tw.
19 heart infarct$.tw.
20 heart attack$.tw.
21 (stroke or strokes).tw.
22 (cerebr$ adj3 (accident$ or infarct$)).tw.
23 (brain adj3 infarct$).tw.
24 apoplexy.tw.
25 (cardiovascular adj2 (disease$ or event$)).tw.
26 Hyperhomocysteinemia/
27 hyperhomocyst?in?emi$.tw.
28 or/12‐27
29 11 and 28
30 random$.tw.
31 factorial$.tw.
32 crossover$.tw.
33 cross over$.tw.
34 cross‐over$.tw.
35 placebo$.tw.
36 (doubl$ adj blind$).tw.
37 (singl$ adj blind$).tw.
38 assign$.tw.
39 allocat$.tw.
40 volunteer$.tw.
41 crossover procedure/
42 double blind procedure/
43 randomized controlled trial/
44 single blind procedure/
45 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44
46 (animal/ or nonhuman/) not human/
47 45 not 46
48 29 and 47
49 (2014* or 2015* or 2016*).dd.
50 48 and 49
Web of Science
#26 #25 AND #24
#25 TS=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))
#24 #23 AND #10
#23 #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11
#22 TS=(hyperhomocystein$emi*)
#21 TS=(hyperhomocystin$emi*)
#20 TS=(cardiovascular near/2 (disease* or event*))
#19 TS=(apoplexy)
#18 TS=((brain near/3 infarct*))
#17 TS=((cerebr* near/3 (accident* or infarct*)))
#16 TS=((stroke or strokes))
#15 TS=(heart attack*)
#14 TS=(heart infarct*)
#13 TS=(myocardial infarct*)
#12 TS=(angina)
#11 TS=((coronary near/3 disease*))
#10 #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#9 TS=(pyridoxol*)
#8 TS=(cyanocobalamin*)
#7 TS=(cobalamin*)
#6 TS=(pyridoxin*)
#5 TS=((homocystin*) near/3 (low or reduc*))
#4 TS=((homocystein*) near/3 (low$ or reduc*))
#3 TS=(folate*)
#2 TS=("folic acid")
#1 TS=("vitamin b")
LILACS (accessed through Biblioteca Virtual em Saúde)
((Pt ENSAYO CONTROLADO ALEATORIO OR Pt ENSAYO CLINICO CONTROLADO OR Mh ENSAYOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUCIÓN ALEATORIA OR Mh METODO DOBLE CIEGO OR Mh METODO SIMPLECIEGO OR Pt ESTUDIO MULTICÉNTRICO) or ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((Ct ANIMALES OR Mh ANIMALES OR Ct CONEJOS OR Ct RATÓN OR MH Ratas OR MH Primates OR MH Perros OR MH Conejos OR MH Porcinos) AND NOT (Ct HUMANO AND Ct ANIMALES)) [Palavras] and MH Vitamina B 12 OR Cobamidas OR Hidroxocobalamina OR Complejo Vitamínico B OR Ácido Fólico OR Ácidos Pteroilpoliglutámicos OR Tetrahidrofolatos OR Formiltetrahidrofolatos OR Vitamina B 6 OR Piridoxal OR Fosfato de Piridoxal OR Piridoxamina OR Piridoxina OR Homocisteína OR Vitaminas or TW vitamin$ or tw cobalamin$ or tw cianocobalamin$ or tw cyanocobalam$ or tw cobamid$ or tw hidroxocobalam$ or tw Hydroxocobalam$ or ((tw complejo or tw complex$) and tw vitamin$ and tw b) or (tw acid$ and (tw folic$ or tw ptero$)) or tw Tetrahidrofolatos or tw Formiltetrahidrofolatos or (tw vitamin$ or (tw b or tw b6 or tw b12)) or tw Piridoxal or tw Pyridoxal or ((tw Fosfat$ or tw phosphate$) and (tw Piridoxal or tw pyridoxal)) or tw Piridox$ or tw Pyridox$ or tw Homocisteína or tw Homocysteine) AND (MH Enfermedades Cardiovasculares or Isquemia Miocárdica or Ex C14.280.647$ or Isquemia Encefálica or Ex C10.228.140.300.150$ or Trastornos Cerebrovasculares or hiperhomocisteinemia or Accidente Cerebrovascular or ((tw apoplexia or tw derrame or tw trastorno$ or tw accident$ or tw acidente or tw stroke$ or tw disease$ or tw enfermedad$ or tw doenca$ or tw event$ or tw infart$ or tw isquemia or tw disorder$) and (tw miocardio or tw myocard$ or tw cerebr$ or tw cardiovascul$ or tw heart or tw cardiovascul$ or tw encefal$)) or tw hyperhomocyst$ or tw hiperhomocisteinemia) [Palavras]
Appendix 5. Domains for assessing of risk of bias in included studies
Generation of the allocation sequence
-
Low risk of bias, if the allocation sequence was generated by a computer or random number table, drawing of lots, tossing of a coin, shuffling of cards or throwing dice.
-
Unclear, if the trial was described as randomised but the method used for the allocation sequence generation was not described.
-
High risk of bias, if a system involving dates, names or admittance numbers was used for the allocation of patients. These studies are known as quasi‐randomised and we excluded them from the review when assessing beneficial effects.
Allocation concealment
-
Low risk of bias, if the allocation of patients involved a central independent unit, on‐site locked computer, identical‐appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes.
-
Unclear, if the trial was described as randomised but the method used to conceal the allocation was not described.
-
High risk of bias, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised. We excluded the latter from the review when assessing beneficial effects.
Blinding (or masking)
We assessed each trial (as low, unclear or high risk) with regard to the following levels of blinding.
-
Blinding of clinician (person delivering treatment) to treatment allocation.
-
Blinding of participant to treatment allocation.
-
Blinding of outcome assessor to treatment allocation.
Incomplete outcome data
-
Low risk of bias, if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or it was specified that there were no dropouts or withdrawals.
-
Unclear, if the report gave the impression that there had been no dropouts or withdrawals but this was not specifically stated.
-
High risk of bias, if the number or reasons for dropouts and withdrawals were not described.
We further examined the percentage of dropouts overall in each trial and per randomisation arm and we evaluated whether intention‐to‐treat analysis was performed or could be performed from the published information.
Selective outcome reporting
-
Low risk of bias, if pre‐defined or clinically relevant and reasonably expected outcomes were reported on.
-
Unclear, if not all pre‐defined or clinically relevant and reasonably expected outcomes were reported on or were not reported on fully, or it was unclear whether data on these outcomes were recorded or not.
-
High risk of bias, if one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded.
Other bias
-
Low risk of bias, the trial appeared to be free of other components that could put it at risk of bias.
-
Unclear, the trial may or may not be free of other components that could put it at risk of bias.
-
High risk of bias, there were other factors in the trial that could put it at risk of bias.
Overall risk of bias
We considered studies to have an overall low risk of bias if they did not have high risk of bias in any of six individual domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data or selective reporting), and if a definitive risk of bias assessment could be made for the majority (at least five of six) of domains. We did not include ‘Other bias’ in our overall assessment.
Appendix 6. Definitions of myocardial infarction (MI), stroke, unstable angina and death
| Trial | Myocardial infarction | Stroke | Angina pectoris | Death | |
|---|---|---|---|---|---|
| Not available | Not available | Not available | Not available | ||
| Not available | Not available | Not available | |||
| Criteria for ischaemic symptoms or corresponding electrocardiographic changes plus evidence of myocardial damage. | Medical records and imaging data | Not measured | Evidence for death included death certificates from hospitals or reports of home visit by investigators | ||
| 2 of the following 3 criteria were met: typical symptoms, increased cardiac‐enzyme levels and diagnostic electrocardiographic changes. | Focal neurologic deficit lasting more than 24 hours. Computed tomography or magnetic resonance imaging was recommended to identify the type of stroke (ischaemic or haemorrhagic). When these tools were not available, the stroke was classified as of uncertain type | Not available | Cardiovascular causes were unexpected deaths presumed to be due to ischaemic cardiovascular disease and occurring within 24 hours after the onset of symptoms without clinical or postmortem evidence of another cause, deaths from myocardial infarction or stroke within 7 days after the event, deaths associated with cardiovascular interventions within 30 days after cardiovascular surgery or within 7 days after percutaneous interventions, and deaths from congestive heart failure, arrhythmia, pulmonary embolism or ruptured aortic aneurysm. Deaths from uncertain causes were presumed to be due to cardiovascular causes | Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined ‐ a consensus document of the joint European Society of Cardiology/American College of Cardiology Committee for the | |
| Not measured | Not available | Not measured | Not measured | ‐ | |
| See supplementary appendix: www.nejm.org | See supplementary appendix: www.nejm.org | See supplementary appendix: www.nejm.org | See supplementary appendix: www.nejm.org | Definitions are too long to summarise in this table | |
| https://www.ctsu.ox.ac.uk/research/research-archive/searchs/search-study-protocol/view Accessed: 7 January 2015 | https://www.ctsu.ox.ac.uk/research/research-archive/searchs/search-study-protocol/view Accessed: 7 January 2015 | https://www.ctsu.ox.ac.uk/research/research-archive/searchs/search-study-protocol/view Accessed: 7 January 2015 | https://www.ctsu.ox.ac.uk/research/research-archive/searchs/search-study-protocol/view Accessed: 7 January 2015 | Definitions are too long to summarise in this table | |
| Myocardial infarction (ICD‐10 (International Classification of Diseases, 10th revision) codes I21.0–I21.9) was defined on the basis of 2 or more of the criteria: typical chest pain, electrocardiographic changes consistent with myocardial infarction and cardiac enzyme increase | An acute cerebral ischaemic event was defined as an ischaemic cerebrovascular accident based on clinical criteria confirmed by computed tomography or magnetic resonance imaging and a Rankin score 3 at inclusion (ICD‐10 codes I63.0–I63.9) | Acute coronary syndrome without myocardial infarction (ICD‐10 codes I20.0–I20.1) was initially defined by the presence of 3 criteria: typical chest pain, electrocardiographic changes consistent with coronary artery disease without myocardial infarction and evidence of coronary artery disease (myocardial infarction, angina with angiographic evidence of stenosis > 50% in one or more coronary arteries, or angina pectoris corroborated by coronary angiography or exercise testing, or coronary angioplasty or coronary artery bypass graft procedure). Suspected acute coronary syndrome without characteristic electrocardiographic evidence of myocardial infarction provided there was angiographic evidence of coronary artery disease | |||
| New ECG changes including Q waves or marked ST‐T changes plus abnormal cardiac enzymes, cardiac symptoms plus abnormal enzymes or symptoms plus hyperacute ECG changes resolving with thrombolysis | Evidence of sudden onset of focal neurologic deficit lasting at least 24 hours accompanied by an increased NIHSS Score in an area that was previously normal. When the sudden onset of symptoms lasting at least 24 hours was not accompanied by an increased NIHSS Score in an area that was previously normal, then recurrent stroke was diagnosed using cranial CT or MRI evidence of new infarction consistent with the clinical presentation | Not available | Not available | ||
| According to World Health Organization criteria | A new neurologic deficit of sudden onset that persisted for more than 24 hours or until death within 24 hours | Not available | Death due to cardiovascular disease was confirmed by examinations of autopsy reports, death certificates, medical records and information obtained from the next kin or other family members. Death from any cause was confirmed by the endpoint committee on the basis of a death certificate | ||
| According to the Joint European Society of Cardiology/American College of Cardiology Committee. Eur Heart J. 2000;21:1502‐13 | According to Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, et al. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol. 2001;38:2114‐30 | According to Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR et al. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol. 2001; 38:2114‐30 | If death occurred within 28 days after the onset of an event, the event was classified as fatal |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Trial Sequential Analysis for homocysteine‐lowering interventions versus placebo on myocardial infarction. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 10% from proportion event in control (Pc) group of 5.95% with an alpha of 5% and beta of 20%. Cumulative Z‐curve (blue line) reached futility area which means that no more trials are needed.
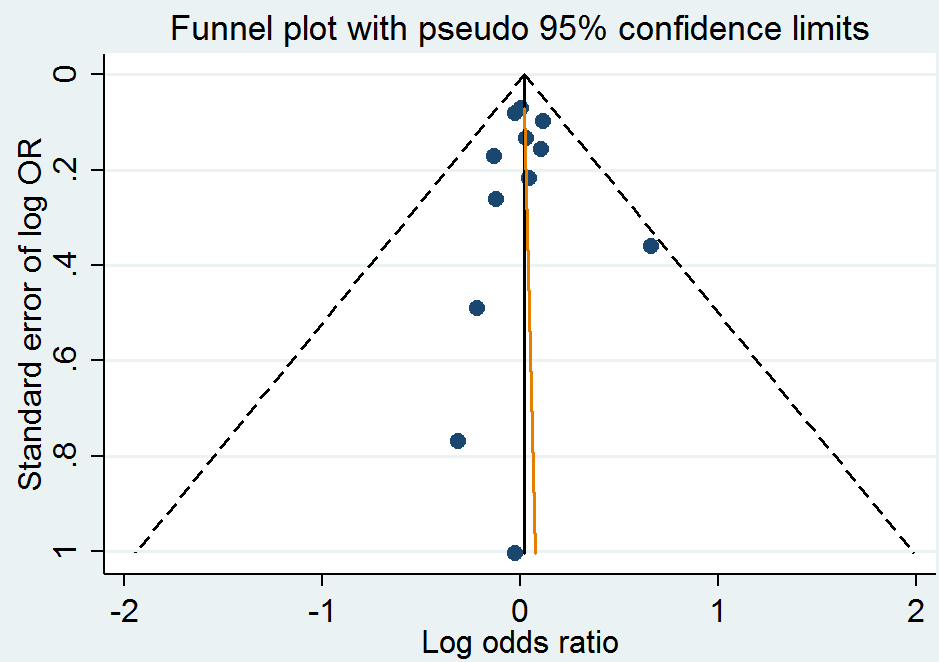
Funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.1 Myocardial infarction.

Contour‐enhanced funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.1 Myocardial infarction.

Trial Sequential Analysis for homocysteine‐lowering interventions versus placebo on stroke. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 10% from proportion event in control (Pc) group of 5% with an alpha of 5% and beta of 20%.
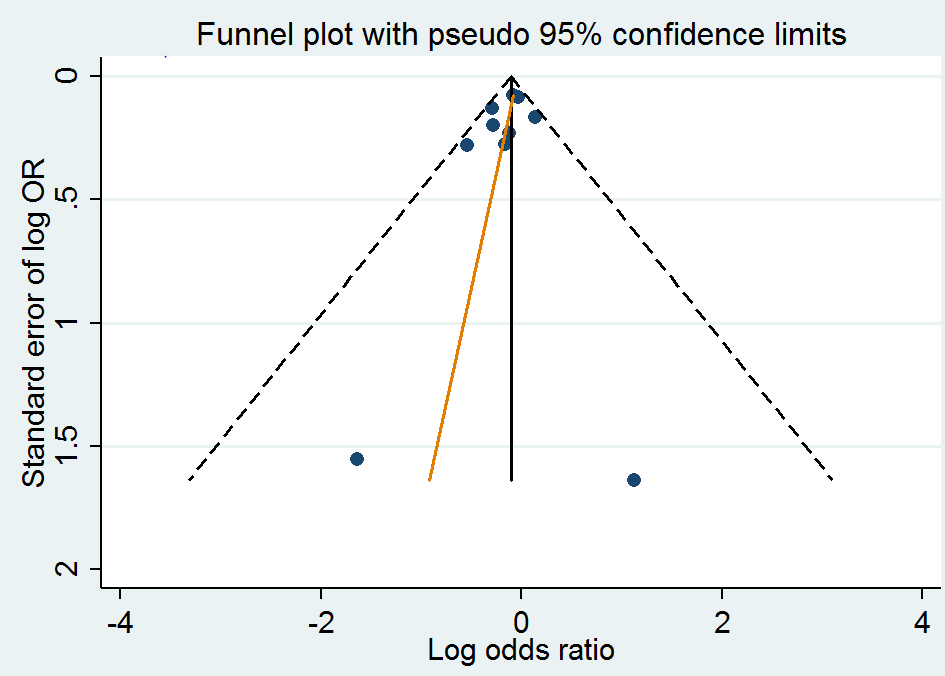
Funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.2 Stroke.

Contour‐enhanced funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.2 Stroke.

Trial Sequential Analysis for homocysteine‐lowering interventions versus placebo on death from any cause. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 12% from proportion event in control (Pc) group of 11.7% with an alpha of 5% and beta of 20%. Cumulative Z‐curve (blue line) reached futility area which means that no more trials are needed.
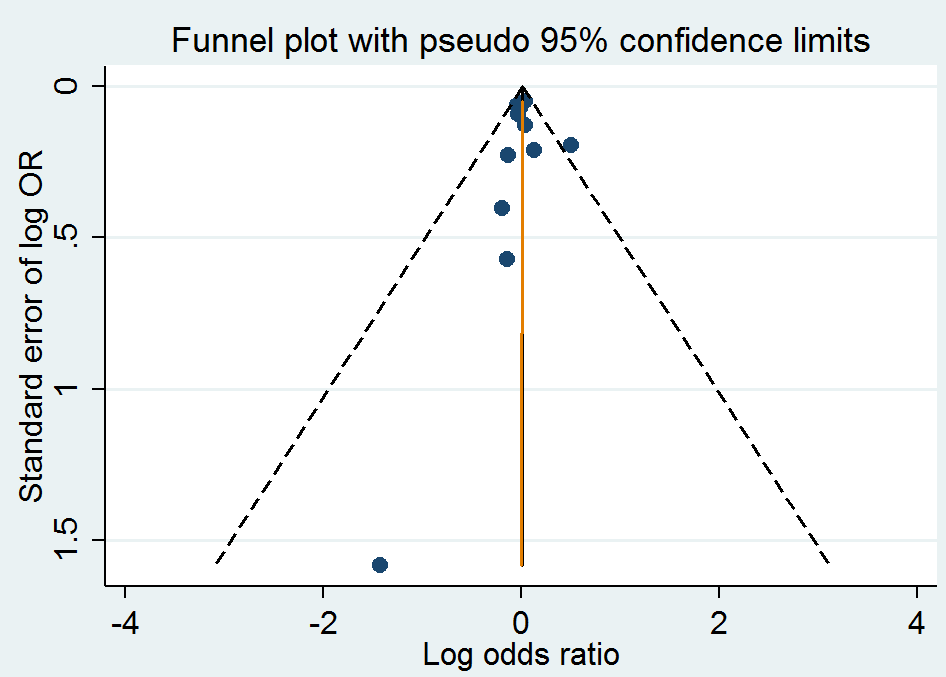
Funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.4 Death from any cause.
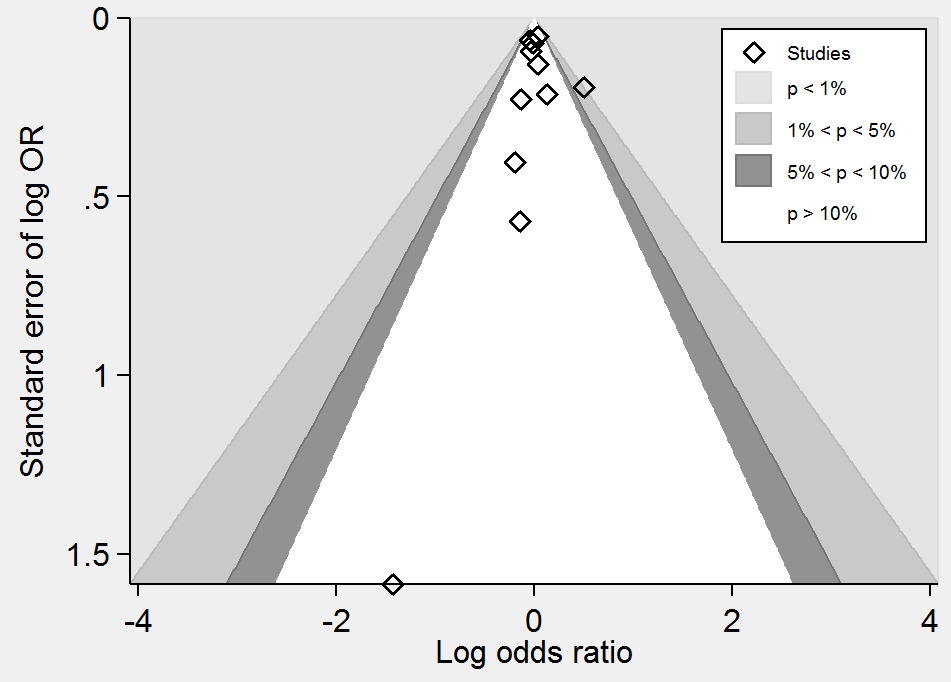
Contour‐enhanced funnel plot of comparison: 1 Homocysteine‐lowering interventions versus placebo, outcome: 1.4 Death from any cause.
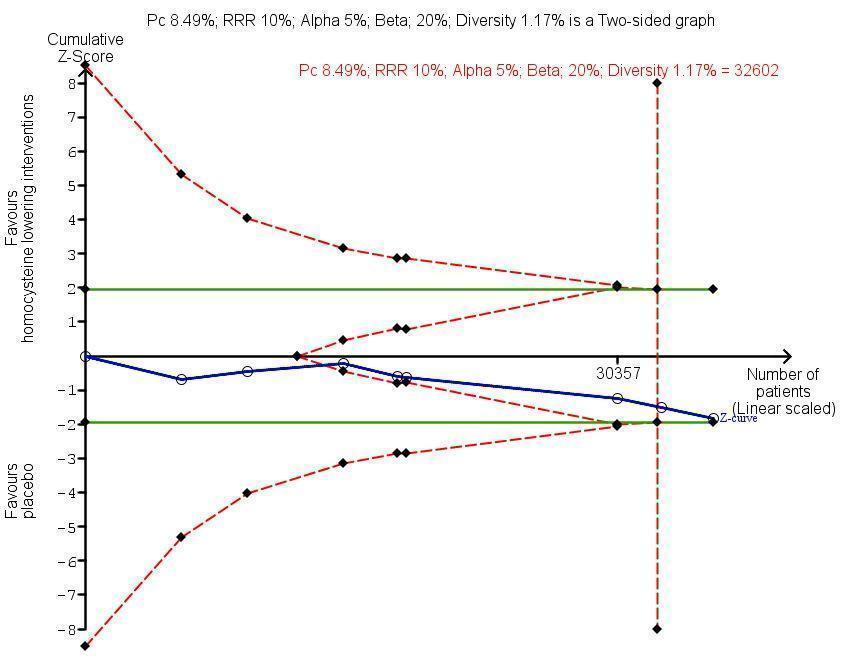
Trial Sequential Analysis for homocysteine‐lowering interventions versus placebo on adverse events (cancer). The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 10% from proportion event in control (Pc) group of 8.49% with an alpha of 5% and beta of 20%. Cumulative Z‐curve (blue line) reached futility area which means that no more trials are needed.

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 1: Myocardial infarction

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 2: Stroke

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 3: First unstable angina pectoris episode requiring hospitalisation

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 4: Death from any cause

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 5: Serious adverse events (cancer)

Comparison 1: Homocysteine‐lowering treatment versus other (any comparisons), Outcome 6: Adverse events (serious and non‐serious) excluding cancer

Comparison 2: Homocysteine‐lowering treatment versus placebo or standard care (Sensitivity analysis), Outcome 1: Myocardial infarction

Comparison 2: Homocysteine‐lowering treatment versus placebo or standard care (Sensitivity analysis), Outcome 2: Stroke

Comparison 2: Homocysteine‐lowering treatment versus placebo or standard care (Sensitivity analysis), Outcome 3: Death from any cause

Comparison 3: Homocysteine‐lowering treatment versus placebo (Subgoup analysis), Outcome 1: Myocardial Infarction

Comparison 3: Homocysteine‐lowering treatment versus placebo (Subgoup analysis), Outcome 2: Stroke

Comparison 3: Homocysteine‐lowering treatment versus placebo (Subgoup analysis), Outcome 3: Death

Comparison 4: Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) (Sensitivity analysis), Outcome 1: Myocardial infarction

Comparison 4: Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) (Sensitivity analysis), Outcome 2: Stroke

Comparison 4: Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) (Sensitivity analysis), Outcome 3: Death from any cause

Comparison 5: Homocysteine‐lowering treatment at high dose versus low dose (Subgoup analysis), Outcome 1: Stroke

Comparison 6: Homocysteine‐lowering treatment (high dose) versus Homocysteine‐lowering treatment (low dose) (Sensitivity analysis), Outcome 1: Stroke
| Homocysteine‐lowering interventions (vitamins B6 (pyridoxine; pyridoxal); B9 (folic acid) or B12 (cyanocobalamin) compared with placebo or standard care for preventing cardiovascular events | ||||||
| Patient or population: adults at risk of or with established cardiovascular disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Homocysteine‐lowering interventions | |||||
| Myocardial infarction | 60 per 1000 | 61 per 1000 | RR 1.02 | 46,699 | ⊕⊕⊕⊕ | |
| Stroke | 51 per 1000 | 46 per 1000 | RR 0.90 | 44,224 | ⊕⊕⊕⊕ | |
| Death by any cause | 123 per 1000 | 124 per 1000 | RR 1.01 | 44,817 | ⊕⊕⊕⊕ | |
| Adverse events | 85 per 1000 | 91 per 1000 | RR 1.07 | 35,788 | ⊕⊕⊕⊕ | Cancer is the only reported adverse event. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the outcomes of the study control arms. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Homocysteine‐lowering interventions (high dose) compared with homocysteine lowering interventions (low dose) for preventing cardiovascular events | ||||||
| Patient or population: adults at risk of or with established cardiovascular disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Homocysteine‐lowering interventions (low‐dose) | Homocysteine‐lowering interventions (high‐dose) | |||||
| Myocardial infarction | 44 per 1000 | 40 per 1000 | RR 0.90 | 3649 | ⊕⊕⊕⊝ |
|
| Stroke | 112 per 1000 | 101 per 1000 | RR 0.90 | 3929 | ⊕⊝⊝⊝ | 1. Li 2015a was conducted including only Chinese elderly females.Trial used only folic acid as homocysteine‐lowering intervention.
2. VISP 2004:
|
| Death by any cause | 64 per 1000 | 55 per 1000 | RR 0.86 | 3649 | ⊕⊕⊕⊝ |
|
| Cancer | Not estimable | ‐ | Li 2015a and VISP 2004 reported no information on this outcome. | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the outcomes of the study control arms. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for imprecision due to low number of events | ||||||
| Enalapril plus folic acid compared with folic acid for adults with hypertension | ||||||
| Patient or population: adults with hypertension | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Folic acid | Enalapril plus folic acid | |||||
| Myocardial infarction | 2 per 1000 | 2 per 1000 | RR 1.04 | 20,702 | ⊕⊕⊕⊝ | |
| Stroke | 34 per 1000 | 27 per 1000 | RR 0.79 | 20,702 | ⊕⊕⊕⊕ | |
| First unstable angina pectoris episode requiring hospitalisation | Not estimable | ‐ | CSPPT 2015 did not assess this outcome. | |||
| Death from any cause | 31 per 1000 | 29 per 1000 | RR 0.94 | 20,702 | ⊕⊕⊕⊕ | |
| Serious adverse event (cancer) | 8 per 1000 | 8 per 1000 | RR 0.96 | 20,243 | ⊕⊕⊕⊝ | CSPPT 2015 included either neoplasms benign, malignant or unspecified |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the outcomes of the study control arms. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for imprecision due to low number of events. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Myocardial infarction Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Homocysteine‐lowering versus placebo | 12 | 46699 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.10] |
| 1.1.2 Homocysteine‐lowering treatment at high dose versus low dose | 1 | 3649 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.23] |
| 1.1.3 Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.60, 1.82] |
| 1.2 Stroke Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Homocysteine‐lowering treatment versus placebo | 10 | 44224 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.82, 0.99] |
| 1.2.2 Homocysteine‐lowering treatment at high dose versus low dose | 2 | 3929 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.22] |
| 1.2.3 Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.68, 0.93] |
| 1.3 First unstable angina pectoris episode requiring hospitalisation Show forest plot | 4 | 12644 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.80, 1.21] |
| 1.4 Death from any cause Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Homocysteine‐lowering treatment versus placebo | 11 | 44817 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.06] |
| 1.4.2 Homocysteine‐lowering treatments at high dose versus low dose | 1 | 3649 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.11] |
| 1.4.3 Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.81, 1.10] |
| 1.5 Serious adverse events (cancer) Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 Homocysteine‐lowering versus placebo | 8 | 35788 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [1.00, 1.14] |
| 1.5.2 Homocysteine‐lowering treatment (folic acid) plus antihypertensive therapy (enalapril) versus antihypertensive therapy (enalapril) | 1 | 20243 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.71, 1.31] |
| 1.6 Adverse events (serious and non‐serious) excluding cancer Show forest plot | 3 | 13802 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.19] |
| 1.6.1 Homocysteine‐lowering versus placebo | 3 | 13802 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Myocardial infarction Show forest plot | 6 | 37442 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 2.1.1 Trials with low risk of bias | 6 | 37442 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 2.2 Stroke Show forest plot | 6 | 37442 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.02] |
| 2.2.1 Trials with low risk of bias | 6 | 37442 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.02] |
| 2.3 Death from any cause Show forest plot | 7 | 37932 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.95, 1.12] |
| 2.3.1 Trials with low risk of bias | 7 | 37932 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.95, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Myocardial Infarction Show forest plot | 12 | 46699 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.10] |
| 3.1.1 Without history of cardiovascular disease | 1 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.87] |
| 3.1.2 With history of cardiovascular disease | 11 | 46209 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.10] |
| 3.2 Stroke Show forest plot | 10 | 44224 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.82, 0.99] |
| 3.2.1 Without history of cardiovascular disease | 1 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.04] |
| 3.2.2 With history of cardiovascular disease | 9 | 43734 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.82, 0.99] |
| 3.3 Death Show forest plot | 11 | 44817 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.06] |
| 3.3.1 Without history of cardiovascular disease | 1 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.04] |
| 3.3.2 With history of cardiovascular disease | 10 | 44327 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Myocardial infarction Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1.1 Per protocol analysis | 1 | 20635 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.60, 1.82] |
| 4.1.2 Best‐worst scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.27, 0.68] |
| 4.1.3 Worst‐best scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [1.48, 3.83] |
| 4.2 Stroke Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.2.1 Per protocol analysis | 1 | 20635 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.68, 0.93] |
| 4.2.2 Best‐worst scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.62, 0.84] |
| 4.2.3 Worst‐best scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.03] |
| 4.3 Death from any cause Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.3.1 Per protocol analysis | 1 | 20635 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.81, 1.10] |
| 4.3.2 Best‐worst scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.99] |
| 4.3.3 Worst‐best scenario | 1 | 20702 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.90, 1.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Stroke Show forest plot | 2 | 3929 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.22] |
| 5.1.1 Combined (folic acid, vitamin B6 and vitamin B12) | 1 | 3649 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.84, 1.29] |
| 5.1.2 Folic acid alone | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.59, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Stroke Show forest plot | 2 | 3929 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.22] |
| 6.1.1 Trials with low risk of bias | 1 | 3649 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.84, 1.29] |
| 6.1.2 Trials with high risk of bias | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.59, 0.98] |

