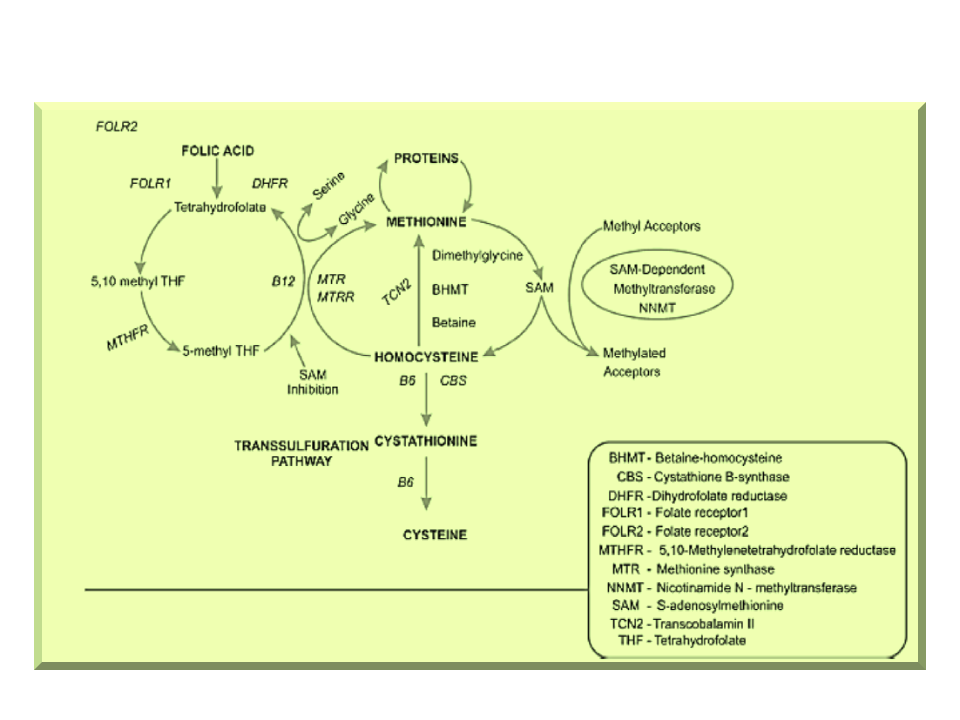
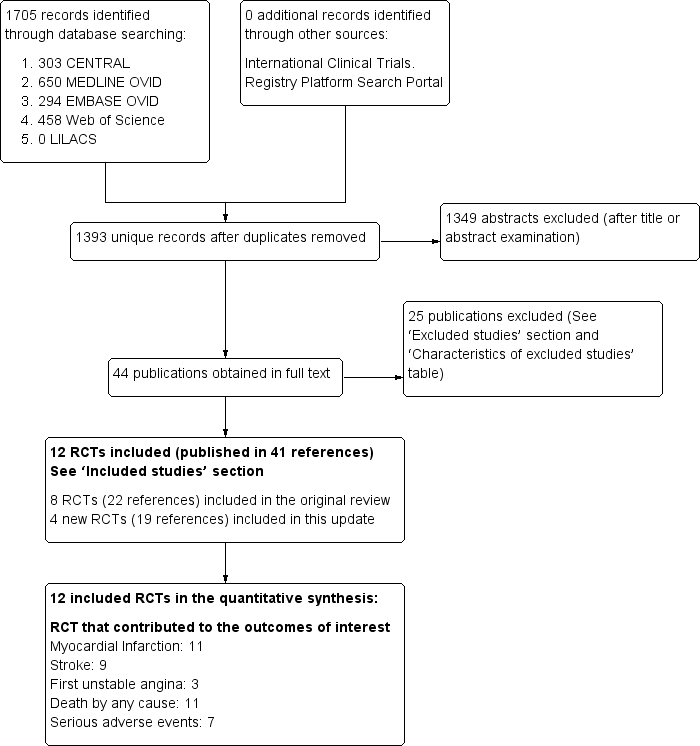
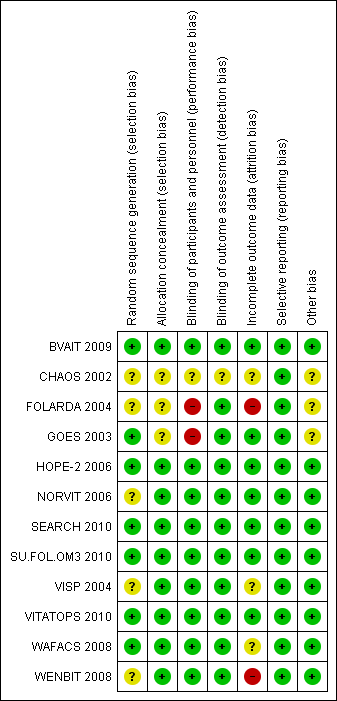
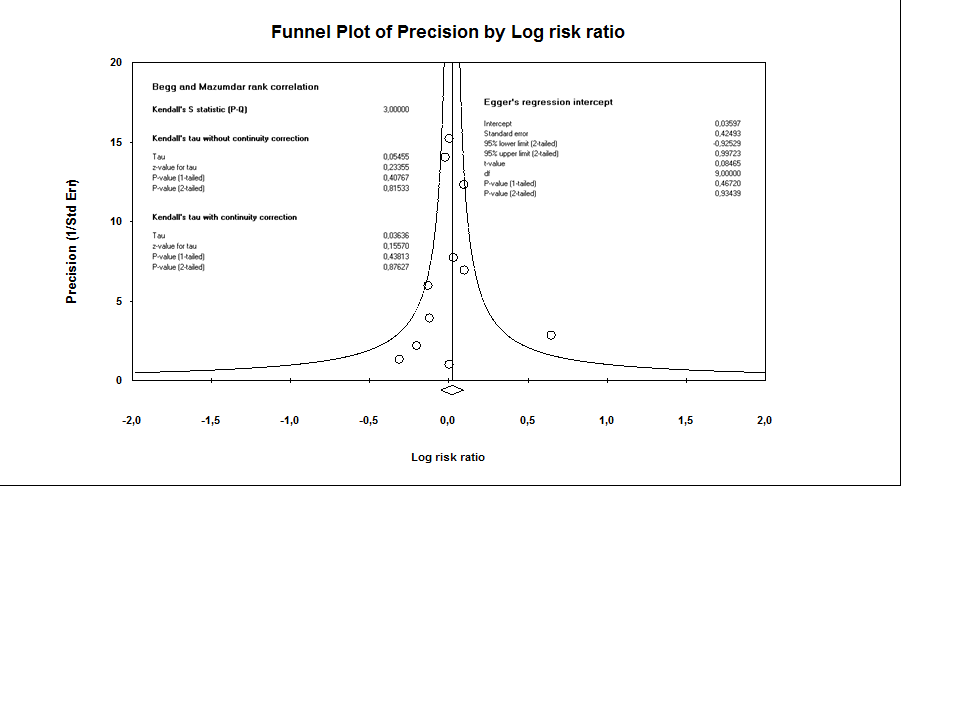
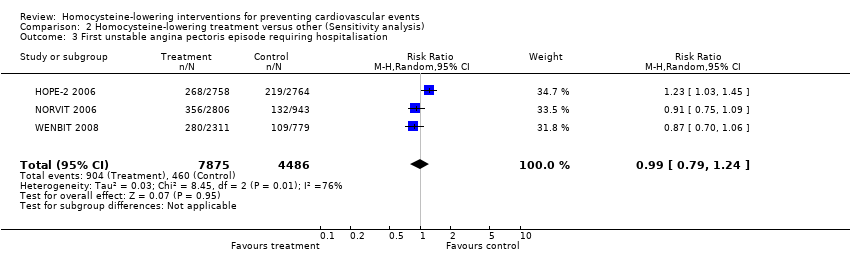
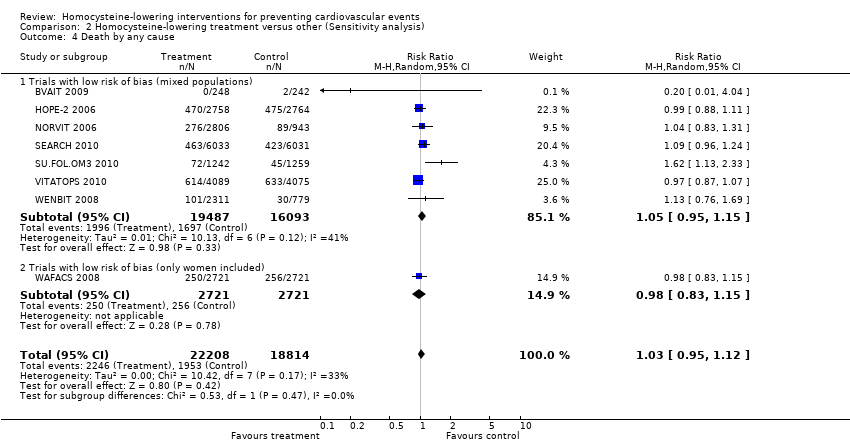
Contenido relacionado
Revisiones y protocolos relacionados
Arturo J Martí-Carvajal, Ivan Solà, Dimitrios Lathyris, Mark Dayer | 17 agosto 2017
Lars G Hemkens, Hannah Ewald, Viktoria L Gloy, Armon Arpagaus, Kelechi K Olu, Mark Nidorf, Dominik Glinz, Alain J Nordmann, Matthias Briel | 27 enero 2016
Deren Wang, Bian Liu, Wendan Tao, Zilong Hao, Ming Liu | 25 octubre 2015
Shipeng Zhan, Min Tang, Fang Liu, Peiyuan Xia, Maoqin Shu, Xiaojiao Wu | 19 noviembre 2018
Amand F Schmidt, John-Paul L Carter, Lucy S Pearce, John T Wilkins, John P Overington, Aroon D Hingorani, JP Casas | 20 octubre 2020
Asmaa S Abdelhamid, Tracey J Brown, Julii S Brainard, Priti Biswas, Gabrielle C Thorpe, Helen J Moore, Katherine HO Deane, Carolyn D Summerbell, Helen V Worthington, Fujian Song, Lee Hooper | 29 febrero 2020
Carlos A Salazar, Juan E Basilio Flores, Liz E Veramendi Espinoza, Jhon W Mejia Dolores, Diego E Rey Rodriguez, César Loza Munárriz | 8 febrero 2017
Chen Mao, Xiao‐Hong Fu, Jin‐Qiu Yuan, Zu‐Yao Yang, Vincent CH Chung, Ying Qin, Yafang Huang, Wilson Wai San Tam, Joey SW Kwong, Wei Xie, Jin‐Ling Tang | 21 mayo 2015
Noah Vale, Alain J Nordmann, Gregory G Schwartz, James de Lemos, Furio Colivicchi, Frank den Hartog, Petr Ostadal, Stella M Macin, Anho H Liem, Edward J Mills, Neera Bhatnagar, Heiner C Bucher, Matthias Briel | 1 septiembre 2014
Lee Hooper, Lena Al‐Khudairy, Asmaa S Abdelhamid, Karen Rees, Julii S Brainard, Tracey J Brown, Sarah M Ajabnoor, Alex T O'Brien, Lauren E Winstanley, Daisy H Donaldson, Fujian Song, Katherine HO Deane | 29 noviembre 2018