نقش مکملهای خوراکی آهن برای کودکان در مناطق اندمیک مالاریا
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Cluster randomized controlled trial (RCT) Trial years: May 1993 to October 1995 Unit of randomization: household Number of units randomized: not stated Average cluster size: not stated Adjustment for clustering: none Methods of adjustment: not stated | |
| Participants | Number of children: 841 randomized, 738 evaluated Age: mean 45.2 months (range 6 to 84 months) Setting: school, rural Mean haemoglobin (Hb) (standard deviation (SD)) at baseline: iron arm: 8.27 (1.2) g/dL; placebo: 8.27 (1.3) g/dL Subgroup classification: anaemia % parasitaemia at baseline: 12.35% | |
| Interventions | Ferrous sulfate elixir, about 3 mg/kg/day elemental iron versus placebo elixir Duration of treatment: 12 weeks Duration of follow‐up: 12 months | |
| Outcomes | Main objective/outcome: effect of iron supplementation on malaria Review outcomes reported in the trial.
| |
| Notes | Trial location: north‐western Ethiopia, Shehdi town, and Aftit village Malaria endemicity: mesoendemic (trial included the rainy season) Language of publication: English Exclusion criteria: Hb < 6 g/dL and Hb > 11 g/dL, debilitating chronic disease or acute infection, new residents or about to leave the region PhD dissertation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. Done in random permuted blocks of four households. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding (performance bias and detection bias) | Low risk | Same bottles as intervention used for placebo elixir. Participants and those who supplied the medications were blinded to the intervention. |
| Methods | Individually RCT Trial years: not stated | |
| Participants | 112 randomized, 97 evaluated Age: range 1 to 14 years Setting: community, rural Mean Hb: 10 g/dL Subgroup classification: no anaemia % parasitaemia at baseline: not stated | |
| Interventions | Trial arms.
Duration of treatment: 6 weeks Duration of follow‐up: 6 weeks | |
| Outcomes | Main objective/outcome: to determine more accurately the extent to which folate deficiency contributes to the anaemia of childhood in the community; to find out how the prevalence of anaemia in children can be reduced by 50 % or more; to decide on a cheap and effective supplementation programme as a public health measure applicable in the community Review outcomes reported in the trial.
| |
| Notes | Trial location: Nigeria Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: haemoglobinopathies; refusal of consent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prepared set of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Open. |
| Methods | Individually RCT Trial years: not stated | |
| Participants | 218 randomized (to 2 arms included in review), 202 evaluated Age: 7 to 12 years. Mean age per study arm: iron: 8.40 (SD 1.55), no iron: 8.82 (SD 1.51) Setting: school, urban Mean Hb: (SD) at baseline: iron arm: 10.4 (1.2) g/dL; no iron arm: 10.4 (1.0) g/dL Subgroup classification: no anaemia % parasitaemia at baseline: not stated | |
| Interventions | Trial arms.
Two additional trial arms that we excluded from this Cochrane review compared praziquantel plus multiple micronutrients (including iron); and praziquantel plus multiple micronutrients plus iron Duration of treatment: 12 weeks Duration of follow‐up: 12 weeks | |
| Outcomes | Main objective/outcome: effect of iron supplementation on haematological status Review outcomes reported in the trial.
| |
| Notes | Trial location: Bamako, Mali Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: Hb < 7 g/dL or > 12 g/dL, hookworm infection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized individual randomization within strata of Hb and parasite load in blocks of 4. |
| Allocation concealment (selection bias) | Low risk | The investigator that recruited participants was unaware of allocation assignment. |
| Blinding (performance bias and detection bias) | High risk | Only laboratory personnel who performed hematological and biochemical determinations were blinded. |
| Methods | Individually RCT Trial years: not stated | |
| Participants | 197 randomized, 163 evaluated Age: 6 to 36 months. Mean age per study arm: intervention: 22.8, SD 8.42 months, placebo: 24.9, SD 8.3 months Setting: community Mean Hb: iron arm: 9.89 SD 1.16 g/dL, placebo arm: 10.04 SD 1.06 g/dL Subgroup classification: anaemia % parasitaemia at baseline: iron arm: 59.3, placebo arm: 63.6 | |
| Interventions | Iron betainate tablet 2 to 3 mg/kg/day elemental iron versus placebo Duration of treatment: 3 months Duration of follow‐up: 9 months | |
| Outcomes | Main objective/outcome: impact of iron supplementation on haematological status, cell‐mediated immunity and susceptibility to infections Review outcomes reported in the trial:
| |
| Notes | Trial location: sea region, Togo Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: Hb < 8 g/dL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized assignment of children into an intervention and placebo groups. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double blind. |
| Methods | Individually RCT Trial duration: March 1998 to November 1998 | |
| Participants | 988 randomized. 760 to 780 (depending on outcome assessed) evaluated Age: mean 5.9 months (range: 4 to 7 months) Setting: community, rural Mean Hb: 10.9 g/dL Subgroup classification: no anaemia % parasitaemia at baseline: not stated | |
| Interventions | Ferrous sulphate syrup 10 mg/day (about 1.5 mg/kg/day elemental iron) versus zinc versus ferrous sulphate plus zinc versus placebo. 100,000 IU of vitamin A was given to all infants at the start of the study. Duration of treatment: 6 months Duration of follow‐up: 6 months | |
| Outcomes | Main objective/outcome: to evaluate the effect of combined iron–zinc supplementation on micronutrient status, growth and morbidity Review outcomes reported in the trial.
| |
| Notes | Location: district of Que Vo, 50 km northwest of Hanoi in the Red River Delta in Vietnam Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: chronic or acute illness, severe malnutrition or congenital abnormality, Hb < 7 g/dL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double blind. |
| Methods | Individually RCT Trial duration: April to November 1999 | |
| Participants | 546 randomized, 491 evaluated Age range: 2 to 36 months, mean for all groups = 11.6 months Setting: community Mean Hb: 9.5 g/dL. Subgroup classification: anaemia 20% to 28% malaria prevalence at baseline | |
| Interventions | Ferrous sulfate suspension (40 mg/mL) 3 to 6 mg/kg/day elemental iron plus sulfadoxine‐pyrimethamine 25/2.25 mg as a single dose at baseline, week 4 and 8 (intermittent preventive therapy (IPT)) versus IPT versus ferrous sulfate plus sulfadoxine‐pyrimethamine 25/2.25 mg as a single dose at baseline versus placebo plus sulfadoxine‐pyrimethamine 25/2.25 mg as a single dose at baseline Duration of treatment: 8 weeks Duration of follow‐up: 24 weeks | |
| Outcomes | Main objective/outcome: the efficacy of single and combined therapy with iron supplementation and IPT with SP in improving Hb concentrations among anaemic preschool children Review outcomes reported in the trial.
| |
| Notes | Trial location: 15 villages in Asembo, Bondo district, Western Kenya Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: parasite count > 20,000/ µL, sickle cell disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number listing generated independently before the study |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind |
| Methods | Individually RCT Trial duration: not stated | |
| Participants | 177 participants randomized Age range: 3 to 5 years. Mean 46 months Setting: community, rural Mean Hb: 10.5 g/dL % parasitaemia at baseline: not stated | |
| Interventions | Ferrous sulphate 60 mg/day elemental iron (about 4.6 mg/kg/day) plus albendazole 200 mg/day for 3 days; 1 month later same dose versus ferrous sulphate plus placebo plus albendazole plus placebo versus placebo plus placebo Duration of treatment: 3 months Duration of follow‐up: 10 months | |
| Outcomes | Main objective/outcome: the effects of iron and deworming treatments on appetite and physical growth performance in preschool children Review outcomes reported in the trial.
| |
| Notes | Trial location: Agblangandan, south Benin 10 km from Cotonou, Benin Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were selected and randomly assigned to 4 treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding (performance bias and detection bias) | Low risk | Double blind. |
| Methods | Individually RCT Trial duration: not stated | |
| Participants | 154 participants randomized, but only 76 in the relevant intervention groups, 74 were evaluated Age range: 3 to 30 months, mean 22 months Setting: community Mean Hb: 9.5 g/dL % parasitaemia at baseline: not stated | |
| Interventions | Ferrous fumarate 66 mg/day elemental iron (about 7.3 mg/kg/day) versus placebo (Seresta forte). Both arms received mebendazole 200 mg/day for 3 days Duration of treatment duration: 6 weeks Duration of follow‐up: 5.5 months | |
| Outcomes | Main objective/outcome: the effects of iron and deworming treatments on physical growth performance, Hb level, and intestinal helminth egg loads in preschool children Review outcomes reported in the trial.
| |
| Notes | Trial location: Ze, south Benin 50 km from Cotonou, Benin Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Low risk | A researcher not involved in the trial allocated children by the randomization code. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, placebo used. |
| Methods | Individually RCT Trial duration: January 2009 to August 2010 | |
| Participants | 209 participants randomized, 209 evaluated Age range: 6 to 59 months, mean 25.8 months Setting: community (through HIV clinics) Mean Hb: 9.4 g/dL % parasitaemia at baseline: 6% in iron arm, 3.9% in control | |
| Interventions | 3 mg/kg/day elemental iron + multivitamins+ malaria chemoprophylaxis versus multivitamins + malaria chemoprophylaxis. Duration of treatment duration: 3 months Duration of follow‐up: 6 months | |
| Outcomes | Main objective/outcome: determine the effect of iron supplementation on Hb level, HIV disease progression, and morbidity among HIV‐infected children with anaemia Review outcomes reported in the trial.
| |
| Notes | Trial location: Thyolo District, Malawi Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: Hb < 7 Hb > 9.9, non‐human immunodeficiency virus (HIV), severe malnutrition, already receiving micronutrient supplements/fortified diets, gross congenital, cognitive, or neurodevelopmental anomaly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind. |
| Methods | Individually RCT Trial duration: July 1998 until March 1999 | |
| Participants | 800 participants randomized, but only 392 in the relevant intervention groups. All were evaluated Age range: 3 to 6 months, mean 5.1 ± 1.1 months Setting: community Mean Hb: 9.6 g/dL % parasitaemia at baseline: not stated | |
| Interventions | Iron sulfate syrup 10 mg/day (about 2 mg/kg/day elemental iron) plus zinc sulfate versus zinc sulfate versus iron plus zinc plus vitamin (not used in review) versus placebo (not used in review) Duration of treatment duration: 6 months Duration of follow‐up: 12 months | |
| Outcomes | Main objective/outcome: to investigate the effect of supplementation on improving infants' micronutrient status and linear growth Review outcomes reported in the trial.
| |
| Notes | Trial location: East Lombok, West Nusa Tenggara, Indonesia Malaria endemicity: mesoendemic Language of publication: English Exclusion criteria: congenital abnormalities, Hb < 6 g/dL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to supplementation groups was conducted using systematic random sampling in each sex group. The randomization of the subjects in the study was done, firstly, by assigning to each intervention group codes A to D ( randomly assigned to placebo; zinc; zinc plus iron; and zinc plus iron plus vitamin A groups, respectively), then each child was randomly assigned to each A to D category using systematic random sampling. |
| Allocation concealment (selection bias) | Low risk | Central. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind. |
| Methods | Individually RCT Trial duration: February 1994 to July 1994 | |
| Participants | 500 participants randomized, 480 evaluated Age range: 5 to 14 years, mean 10.3 years Setting: school Mean Hb: 9.5 g/dL Subgroup classification: anaemia % parasitaemia at baseline: 98% with ≥1 episodes) of malaria attack in the past 14 days; negative malaria smears on initial screening for all | |
| Interventions | Ferrous sulphate 60 mg/day elemental iron (about 2.5 mg/kg/day) versus placebo Duration of treatment duration: 3 months Duration of follow‐up: 6 months | |
| Outcomes | Main objective/outcome: To assess the effect of oral iron on host susceptibility to malaria infection in children with mild to moderate iron deficiency anaemia Review outcomes reported in the trial.
| |
| Notes | Trial location: Northwest Ethiopia, Beles Valley (Pawe), Ethiopia Malaria endemicity: mesoendemic Language of publication: English Exclusion criteria: Hb > 12 or < 5, serum ferritin > 12, positive malaria smears on initial screening, concurrent major illnesses; no iron supplementation past 6 m, < 12 m residence in the area | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Central procedure. |
| Blinding (performance bias and detection bias) | Low risk | Field workers, technicians, parents and children blinded. Placebo used in coded bottles. |
| Methods | Cluster RCT Trial duration: 6 months (dates not stated) | |
| Participants | 204 participants randomized, 204 evaluated Age range: 6 months (± 7 days) Setting: community Mean Hb: 10.1 g/dL. Subgroup classification: no anaemia % parasitaemia at baseline: NS | |
| Interventions | Iron fumarate as sprinkles 12.5 mg/day versus placebo (zinc arm not included) Duration of treatment duration: 12 months Duration of follow‐up: 12 months | |
| Outcomes | Main objective/outcome: compare efficacy of two micronutrient sprinkle supplementation on growth, anaemia, and iron deficiency Review outcomes reported in the trial.
| |
| Notes | Trial location: Cambodia, Chhnang Province Malaria endemicity: holoendemic Language of publication: English Exclusion criteria: Hb > 7 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | Field workers, parents and children. |
| Methods | Cluster RCT Trial duration: May to June 1981 Unit of randomization: 12 school classes Average cluster size: 38.7 Adjustment for clustering: none Methods of adjustment: none | |
| Participants | 12 school classes were divided in 2 equal groups according to their listing on the class registers yielding 24 groups, overall 464 children Age range: 5 to 15 years Setting: school, rural Mean Hb: 12.4 g/dL Subgroup classification: no anaemia % parasitaemia at baseline: not stated | |
| Interventions | Iron‐fumarate 66 mg/day on school days (about 2 mg/kd/day elemental iron) plus placebo versus iron‐fumarate plus chloroquine 300 mg at baseline and 28 days plus tetrachlorethylene liquid 2.5 mL at baseline versus iron‐fumarate plus chloroquine versus iron‐fumarate plus tetrachloroethylene Duration of treatment: 6 weeks Duration of follow‐up: 6 weeks | |
| Outcomes | Main objective/outcome: to evaluate association between anaemia and running distance Review outcomes reported in the trial.
| |
| Notes | Trial location: Namwala township in the great plains of the Kafue river, Zambia Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: acute illness, increased reticulocyte count | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers (12 school classes were divided in 2 equal groups according to their listing on the class registers, yielding 24 groups). |
| Allocation concealment (selection bias) | Low risk | Central procedure (at the pharmacy). |
| Blinding (performance bias and detection bias) | Low risk | Open. |
| Methods | Cluster RCT Trial duration: started January 2000 Unit of randomization: school Number of units randomized: 60 schools Average cluster size: authors' statement: "We did not look at size of school or sub‐district. But since they were all community schools, they were all small rural schools". Adjustment for clustering: not mentioned Methods of adjustment: no adjustment method was used | |
| Participants | Number of children: 1201 randomized, 1113 evaluated Age range: mean 11.4 years range (6 to 19 years) Setting: school; rural Mean Hb: 10.5 g/dL. Subgroup classification: no anaemia % parasitaemia at baseline: not stated | |
| Interventions | Trial arms.
All children received vitamin A before intervention Duration of treatment: 10 weeks Duration of follow‐up: 2 weeks after end of treatment, 14 to 16 weeks from baseline survey weeks | |
| Outcomes | Main objective/outcome: to assess the effect of weekly iron on Hb status Review outcomes reported in the trial.
| |
| Notes | Trial location: Kolondieba district in Sikasso region of south eastern Mali Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: severe anaemia (Hb < 8 g/dL) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Nor reported. |
| Blinding (performance bias and detection bias) | High risk | Open. |
| Methods | Individually RCT Trial duration: started June 1985 | |
| Participants | 318 randomized, up to 298 evaluated for malaria outcomes, 318 evaluated for Hb Age: mean 9.7 years (range 8 to 12 years) Setting: school, rural Mean Hb: 10.7 g/dL % parasitaemia at baseline: 70.5% | |
| Interventions | Trial arms.
Duration of treatment: 16 weeks Duration of follow‐up: 24 weeks | |
| Outcomes | Main objective/outcome: to investigate the effects of iron therapy and changes in iron status on malarial infection in children with mild to moderate iron deficiency and some immunity to malaria Review outcomes reported in the trial.
| |
| Notes | Trial location: north coast,, Madang, Papua New Guinea Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: Hb < 8 g/dL or > 12 g/dL, signs of puberty | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Of 318 participants authors formed 156 matched pairs based on Hb, age and oval‐shaped RBC. Members of each pair were randomized to either iron or placebo. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Low risk | Double blind. |
| Methods | Individually RCT Trial duration: 1999 to 2000 | |
| Participants | 169 randomized, 166 evaluated Age: mean 8.5 years (range 5 to 14 years) Setting: school, rural % anaemic at baseline: 85% Mean Hb: 10.9 g/dL Subgroup classification: no anaemia % parasitaemia at baseline: not stated | |
| Interventions | Trial arms.
Half received a single dose of iodinized poppy seed oil containing 200 mg Duration of treatment: 16 weeks Duration of follow‐up: 20 weeks | |
| Outcomes | Main objective/outcome: to investigate change in response to iodine after iron supplementation Review outcomes reported in the trial.
| |
| Notes | Trial location: Danané health district, an area of endemic goitre in the mountains of western Côte d'Ivoire Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: Hb < 8 g/dL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) | Low risk | Double blind |
| Methods | Individually RCT Trial duration: June 2000 to January 2001 | |
| Participants | 169 randomized, 166 evaluated Age: 6 to 12 months Setting: rural, community % anaemic at baseline: Mean Hb: 9.9 g/dL % parasitaemia at baseline: not stated | |
| Interventions | Trial arms.
Duration of treatment: 6 months Duration of follow‐up: 6 months | |
| Outcomes | Main objective/outcome: effect of iron on anaemia and growth. Review outcomes reported in the trial.
| |
| Notes | Trial location: Soscon District, Vietnam Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: severe wasting, fever (> 39°C), premature birth (< 37 weeks) or low birth weight (< 2500 g), and severe anaemia (Hb < 8 g/dL) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number selection. |
| Allocation concealment (selection bias) | Low risk | Central code not on study site. |
| Blinding (performance bias and detection bias) | Low risk | Double blind. |
| Methods | Individually RCT Trial duration: April to November 1986 | |
| Participants | 55 randomized, 54 evaluated Age: mean 8 years Setting: school Mean Hb (SE): iron arm: 11.6 (0.18) g/dL; placebo arm: 11.5 (0.18) g/dL % parasitaemia at baseline: iron arm: 76%, placebo arm: 46% | |
| Interventions | Trial arms.
All groups received albendazole tablets 400 mg single dose once after 32 weeks Duration of treatment: 15 weeks Duration of follow‐up: 32 weeks | |
| Outcomes | Main objective/outcome: to determine whether iron given to school children in Kenya improves growth Review outcomes reported in the trial.
| |
| Notes | Trial location: Kwale district, Coast Province, south of Mombasa, Kenya Malaria endemicity: holoendemic, undertaken during rainy season Language of publication: English Exclusion criteria: haematuria and proteinuria (indicative of Schistosoma haematobium), absence on the day of first examination, serious disease or malnutrition, Hb < 8 g/dL, heavy infections with hookworms (> 10,000 eggs/g stool), and refusal to participate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were paired by gender within the Hb rankings, from each pair one was randomly assigned to placebo and the other to iron. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Saccharin used as placebo. |
| Methods | Individually randomized Trial duration: March to July 1990 | |
| Participants | 87 randomized, 86 evaluated Age: mean 8.7 years (range 6 to 11 years) Setting: school, rural Mean Hb: 11.1 g/dL Subgroup classification: no anaemia % parasitaemia at baseline: not stated | |
| Interventions | Trial arms.
Duration of treatment: 14 weeks Duration of follow‐up: 14 weeks | |
| Outcomes | Main objective/outcome: to determine effects of iron given to school children in Kenya on appetite and growth Review outcomes reported in the trial.
| |
| Notes | Trial location: Coast Province, Shamu village, Kenya Malaria endemicity: holoendemic Language of publication: English Exclusion criteria: Hb < 8 g/dL, heavy hookworm infection (> 10,000 eggs/g faeces), hematuria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind. |
| Methods | Individually randomized Trial duration: April to November 1998 | |
| Participants | 279 randomized, 279 evaluated Age: mean 13.8 years (range 12 to 18 years) Setting: school, urban Mean Hb: 12.8 g/dL % parasitaemia at baseline: 25.4% | |
| Interventions | Trial arms.
Duration of treatment: 5 months Duration of follow‐up: 5 months | |
| Outcomes | Main objective/outcome: to determine effects of iron and vitamin A on Hb, iron status, malaria, and other morbidities in schoolgirls Review outcomes reported in the trial.
| |
| Notes | Trial location: Kisumu City, on shores of lake Victoria, Nyanza province, western Kenya Malaria endemicity: mesoendemic, undertaken during rainy season Language of publication: English Exclusion criteria: Hb < 7 g/dL, severe vitamin A deficiency (xerophthalmia), pregnancy, concomitant disease requiring hospitalization | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind. |
| Methods | Individually RCT Trial duration: June 1999 to May 2000 | |
| Participants | 291 randomized, 291 evaluated Age: mean 14.3 weeks Setting: community, rural Mean Hb: 9.9 g/dL. Subgroup classification: anaemia % parasitaemia at baseline: mean 31.5% | |
| Interventions | Trial arms.
Duration of treatment: 6 months Duration of follow‐up: 10 months | |
| Outcomes | Main objective/outcome: infections Review outcomes reported in the trial.
| |
| Notes | Trial location: Muheza district, north‐eastern Tanzania Malaria endemicity: holoendemic Language of publication: English Exclusion criteria: infants with congenital malformation, conditions that needed hospital treatment, fever within preceding 2 weeks, packed cell volume < 24%, participants on chemoprophylaxis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Central. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind. |
| Methods | Cluster RCT Trial duration: 1996 to 1997 Unit of randomization: household Number of units randomized: 451 households Average cluster size: 1.5 children per household Adjustment for clustering: yes Methods of adjustment: generalized estimating equation approach was used to account for repeated measurements in children | |
| Participants | 684 children randomized, 684 evaluated for mortality, 614 evaluated for malaria, 459 evaluated for anaemia Age: mean 33.4 months (range 4 to 71 months) Setting: community, rural Mean Hb: 8.7 g/dL Subgroup classification: anaemia % parasitaemia at baseline: not stated | |
| Interventions | Trial arms.
Randomization was also done by child to oral mebendazole 500 mg every 3 months; versus placebo Duration of treatment: 12 months Duration of follow‐up: 12 months | |
| Outcomes | Main objective/outcome: to assess the effect of low‐dose, long‐term iron supplementation on malaria infection Review outcomes reported in the trial.
| |
| Notes | Trial location: Pemba Island, Tanzania Malaria endemicity: holoendemic Language of publication: English Exclusion criteria: severe anaemia (Hb < 7 g/dL) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Low risk | Pharmacy, sealed envelopes. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind. |
| Methods | Individually RCT Trial duration: 1995 | |
| Participants | 832 randomized, 832 evaluated Age: range 8 to 48 weeks Setting: community, rural Subgroup classification: anaemia (based on population incidence of anaemia in region and age group) % parasitaemia at baseline: not stated | |
| Interventions | Trial arms.
Duration of treatment: iron; 16 weeks, antimalarial; 40 weeks Duration of follow‐up: 1 year | |
| Outcomes | Main objective/outcome: Hb, anaemia and iron‐related outcomes Review outcomes reported in the trial:
| |
| Notes | Trial location: Ifakara, Kilombero District, Morogoro Region, south‐eastern Tanzania Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: packed cell volume < 25% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequential numbers of a randomization code. |
| Allocation concealment (selection bias) | Low risk | Randomization code kept by an independent monitor ‐ central. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double blind. |
| Methods | Individually RCT Trial duration: not stated | |
| Participants | 136 randomized, 135 evaluated Age: mean 10.8 (range 9 to 12 years) Setting: school, rural Mean Hb: 10.5 g/dL Subgroup classification: no anaemia % parasitaemia at baseline: not stated | |
| Interventions | Trial arms.
All subjects were dewormed for helminthiasis 2 weeks before baseline survey Duration of treatment: 3 months Duration of follow‐up: 3 months | |
| Outcomes | Main objective/outcome: effects of dietary supplements on anaemia and growth Review outcomes reported in the trial.
| |
| Notes | Trial location: Bagamoyo District, coastal area of Tanzania Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: chronic illnesses, physical impairments, severe anaemia (Hb < 8 g/dL) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The RAND function of Excel was used to implement randomization. |
| Allocation concealment (selection bias) | Low risk | Pharmacy. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind. |
| Methods | Individually RCT Trial duration: November 1994 to January 1996 | |
| Participants | 231 children randomized, 231 evaluated for mortality, 200 for Hb end and change Age: mean 8.7 years Setting: community Mean Hb: 11.5 g/dL Subgroup classification: no anaemia % parasitaemia at baseline: 60.6% | |
| Interventions | Trial arms.
Duration of treatment: 12 months Duration of follow‐up: 12 months | |
| Outcomes | Main objective/outcome: effect of 12 months of twice weekly iron supplementation on Hb and ferritin Review outcomes reported in the trial.
| |
| Notes | Trial location: Kisumu district of Nyanza province, Kenya Malaria endemicity: mesoendemic Language of publication: English Exclusion criteria: Hb < 8 g/dL, pregnancy and refusal to participate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes kept in a central location. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind. |
| Methods | Individually randomized Trial duration: not stated | |
| Participants | 80 randomized, 40 evaluated Age: range 4 to 12 years Setting: community, rural Mean Hb: 11.1 g/dL Subgroup classification: no anaemia % parasitaemia at baseline: not stated | |
| Interventions | Trial arms.
Duration of treatment: 6 weeks Duration of follow‐up: 6 weeks | |
| Outcomes | Main objective/outcome: haematological status Review outcomes reported in the trial.
| |
| Notes | Trial location: Keneba village, Gambia Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind. |
| Methods | Individually RCT Trial duration: February to September 1998 | |
| Participants | 855 randomized, 836 evaluated for malaria, 748 evaluated for mortality and Hb Age: range 0.5 to 15 years Setting: school, rural Mean Hb: 11.4 g/dL % parasitaemia at baseline: 5% | |
| Interventions | Trial arms.
Duration of treatment: 7 months Duration of follow‐up: 7 months | |
| Outcomes | Main objective/outcome: effect of daily iron or zinc or both on morbidity ‐ malaria, diarrhoea, and respiratory infections Review outcomes reported in the trial.
| |
| Notes | Trial location: Santa Clara Village, Peru Malaria endemicity: mesoendemic Language of publication: English Exclusion criteria: chronic illness (congenital diseases or major illness requiring medical care or medication, or both, determined by the physician at baseline evaluation) or severe malnutrition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Low risk | Triple blinded: participants, study personnel, and data analyst were all blinded. |
| Methods | Cluster RCT Trial duration: February to September 2002 Unit of randomization: schools Number of units randomized: 40 schools Average cluster size: 29 Adjustment for clustering: none Methods of adjustment: not stated | |
| Participants | Number of children: 40 schools, 1160 were tested for Hb at baseline. Number randomized not stated Age: 7 to 8 years and 10 to 12 years Setting: school, rural Mean Hb: 11.8 g/dL Subgroup classification: no anaemia % parasitaemia at baseline: no or little malaria, not reported further | |
| Interventions | Ferrous sulfate tablets 65 mg/week elemental iron (about 0.3 mg/kd/day) + folic acid 0.25 mg / week versus no treatment. In addition all children received praziquantel 600 mg once, 1 week before the beginning of the trial Duration of treatment: 3.5 months Duration of follow‐up: 4.5 months | |
| Outcomes | Main objective/outcome: to evaluate the effectiveness of weekly school‐based iron supplementation: its impact on mean Hb concentration and anaemia prevalence, on school attendance, performance, drop‐out, and repetition rates Review outcomes reported in the trial:
| |
| Notes | Trial location: Mangochi District in Malawi, upland and coastal areas Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table (inside each class 33% of children were selected for the trial ‐ started from a random number and taking every third trial from this number on). |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Open. |
| Methods | Cluster RCT Unit of randomization: households Number of units randomized: 22,959 Average cluster size: 1.4 Adjustment for clustering: was performed for adverse events (episodes of infection) and admissions. For mortality and cause‐specific mortality adjustment for clustering is not reported Methods of adjustment: for analysis of adverse events and admissions, Anderson Gill time‐to‐event survival methods in Cox regression with robust estimation of standard error to account for multiple events per child or within household were used (SAS version 9.0, STATA version 8.2). For total mortality and cause‐specific mortality, Cox regression with exact handling for ties was used Trial duration: January 2002 to August 2003 | |
| Participants | 22,959 units and 32,155 individuals; 15,956 in the 2 arms relevant for this review Age: 1 to 35 months, mean about 18 months Setting: community Hb levels: not reported % parasitaemia at baseline: not stated | |
| Interventions | Iron tablets (preparation not stated) dissolved in water or breast milk 12.5 mg/day plus folic acid 50 μg/day plus vitamin A; versus placebo plus vitamin A; versus iron plus folic acid plus zinc 10 mg/day plus vitamin A (not used in this review); versus zinc plus vitamin A. Children aged 1 to 11 months received a half dose of iron Duration of treatment: not fixed; from < 3 months to maximum of 18 months of age (until the age of 48 months or the discontinuation of the study ). Most participants received the intervention for about 12 months Duration of follow‐up: not fixed. Maximum of 18 months (until age 48 months or study discontinuation) | |
| Outcomes | Main objective/outcome: composite of death or hospital admission (looking very specifically at malaria) Review outcomes reported in the trial.
| |
| Notes | Trial location: Tanzania Malaria endemicity: holoendemic Language of publication: English Exclusion criteria: none Comparison relevant to this review (iron + folic) stopped at interim analysis based on recommendation from the data and safety monitoring board. The board received data from the main trial every month and established at the beginning of the trial that it would do further analysis of the data when the difference in mortality between any 2 groups reached a P value of 0.2 or less. Stopping rules not defined in publication. No statement on sample size and analysis adjustment for interim monthly monitoring and truncation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence generated at the World Health Organization (WHO) controlled by computer (page 136). Permuted in blocks of 16. |
| Allocation concealment (selection bias) | Low risk | Labelled the strips of supplements with 16 letter codes‐ 4 for each of the groups. This letter code was hidden in the batch number on each strip of tablets. |
| Blinding (performance bias and detection bias) | Low risk | Double blind. Strips of supplements coded with 16 letter codes. |
| Methods | Cluster RCT (independent substudy of Sazawal 2006 (C)a Unit of randomization: households Number of units randomized: 2818 before exclusion of anaemic children Average cluster size: 1.2 Adjustment for clustering was performed for adverse events (episodes of infection) and admissions. For mortality and cause‐specific mortality, adjustment for clustering is not reported. Methods of adjustment for the analysis of adverse events and admissions, Anderson Gill time‐to‐event survival methods in Cox regression with robust estimation of SE to account for multiple events per child or within household were used (SAS version 9.0, STATA version 8.2). For total mortality and cause‐specific mortality, Cox regression with exact handling for ties was used. Trial duration: March to November 2002 | |
| Participants | 3171 individuals; 1619 in the 2 arms relevant for this review Age: 1 to 35 months, mean about 22.5 months Setting: community Mean Hb: 9.7 g/dL Subgroup classification: anaemia % parasitaemia at baseline: not stated | |
| Interventions | Iron tablets (preparation not stated) dissolved in water or breast milk 12.5 mg/day plus folic acid 50 μg/day plus vitamin A; versus placebo plus vitamin A; versus iron plus folic acid plus zinc 10 mg/day plus vitamin A (not used in review); versus zinc plus vitamin A. Children aged 1 to 11 months received a half dose of iron. Duration of treatment: not fixed from < 3 months to a maximum of 18 months (until the participants were aged 48 months or the discontinuation of the study). Most received the intervention for about 12 months. Duration of follow‐up: not fixed. Maximum 18 months (until the participants were aged 48 months or the discontinuation of the study). | |
| Outcomes | Main objective/outcome: to make a composite of death or hospital admission (looking very specifically at malaria) Review outcomes reported in the trial.
| |
| Notes | Trial location: Tanzania Malaria endemicity: holoendemic Language of publication: English Exclusion criteria: Hb < 7 g/dL This was a separate, independent, substudy of the bigger Sazawal 2006 (C)a trial. Separate households were randomized to the substudy, where children had baseline blood samples, anaemic children excluded (Hb < 7 g/dL), half‐yearly surveillance for malaria and clinical infections performed, and treatment for malaria offered throughout the trial. Comparison relevant to this review (iron plus folic) stopped at interim analysis based on recommendation from the data and safety monitoring board. The board received data from the main trial every month and established at the beginning of the trial that it would do further analysis of the data when the difference in mortality between any 2 groups reached a P value of 0.2 or less. Stopping rules not defined in publication. No statement on sample size and analysis adjustment for interim monthly monitoring and truncation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence generated at the WHO controlled by computer (page 136). Permuted in blocks of 16. |
| Allocation concealment (selection bias) | Low risk | Labelled the strips of supplements with 16 letter codes‐ 4 for each of the groups. This letter code was hidden in the batch number on each strip of tablets. |
| Blinding (performance bias and detection bias) | Low risk | Double blind. Strips of supplements coded with 16 letter codes. |
| Methods | Cluster RCT Trial duration: July to August 1983 Unit of randomization: household Number of units randomized: not stated Average cluster size: not stated Adjustment for clustering: none Methods of adjustment: not stated | |
| Participants | Number of participants: 213 children Age: 6 months to 5 years, mean about 2.7 years Setting: community Mean Hb: 9.3 g/dL Subgroup classification: anaemia % parasitaemia at baseline: not stated | |
| Interventions | Ferrous sulphate elixir of crushed tablets in orange juice 3 to 6 mg/kg/day elemental iron versus orange juice (placebo) Duration of treatment: 12 weeks Duration of follow‐up: 13 weeks | |
| Outcomes | Main objective/outcome: Hb/iron + malaria status Review outcomes reported in the trial.
| |
| Notes | Trial location: Gambia Malaria endemicity: hyperendemic Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The first compound on the compound list for each village was randomly assigned and compounds were assigned alternately thereafter. |
| Allocation concealment (selection bias) | High risk | Alternation. |
| Blinding (performance bias and detection bias) | Low risk | Parents, field workers, and study investigator blinded. |
| Methods | Individually RCT Trial duration: November 2004 to May 2005. | |
| Participants | Number of participants: 168 children Age: mean 87 months Setting: community Mean Hb: 10.8 g/dL. Subgroup classification: no anaemia % parasitaemia at baseline: not stated | |
| Interventions | Iron fumarate tablets 200 mg and mebendazole versus placebo and mebendazole Duration of treatment: 6 months Duration of follow‐up: 6 months | |
| Outcomes | Main objective/outcome: Hb Review outcomes reported in the trial.
| |
| Notes | Trial location: Phu Tho Province, Vietnam. Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: Hb < 7 g/dL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "carried out by a researcher" ‐ method not described. |
| Allocation concealment (selection bias) | Unclear risk | Methods not described. |
| Blinding (performance bias and detection bias) | High risk | Not described. |
| Methods | Individually RCT Trial duration: 1998 to 2000 | |
| Participants | In total 328 randomized Age: 2 to 36 months, mean about 18 months Setting: community Mean Hb: 9.6 g/dL Subgroup classification: anaemia % parasitaemia at baseline: as indicated by a dipstick test result, 31% in this age group from an earlier survey | |
| Interventions | Ferrous fumarate suspension 6 mg/kg/week elemental iron (about 0.86 mg/kg/day) given in two doses (twice a week) plus sulfadoxine/pyrimethamine 25/1.25 mg/kg once every 4 weeks versus ferrous fumarate plus placebo; versus sulfadoxine‐pyrimethamine plus placebo versus placebo Duration of treatment: 3 months Duration of follow‐up: 3 months | |
| Outcomes | Main objective/outcome: effect of intermittent iron and sulfadoxine‐pyrimethamine on Hb in symptom‐free children Review outcomes reported in the trial.
| |
| Notes | Trial location: Kenya Malaria endemicity: mesoendemic Language of publication: English Exclusion criteria: Hb < 6 or >11 g/dL, axillary temp > 37.5 °C, symptoms suggestive of malaria or anaemia, or any systemic illness occurring in combination with a blood dipstick test result indicating current or recent malaria infection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Tables with randomized permutations. |
| Allocation concealment (selection bias) | Low risk | The order of children listed was concealed from the person generating the allocation schedule. |
| Blinding (performance bias and detection bias) | Low risk | Double blind: field investigators, participants. |
| Methods | Individually RCT Trial duration: October 1999 to March 2000 | |
| Participants | 437 randomized, 165 evaluated Age: mean 16.5 ± 3.9 months for iron drops versus 15.4 ± 4.4 for iron sprinkles versus 15.2 ± 4.1 for placebo Setting: community Mean Hb: 12.7 g/dL. Subgroup classification: no anaemia % parasitaemia at baseline: 62.3% (202/324 children who completed the intervention) | |
| Interventions | Ferrous sulphate drops 12.5 mg/day elemental iron (about 1.25 mg/kd/day) versus iron fumarate sprinkles 40 mg/day versus placebo versus iron fumarate sprinkles (not used in this review as could not compare two iron treatment group to one placebo group) plus vitamin A (not used in this review) Duration of treatment: 6 months Duration of follow‐up: 18 months (only children who were not anaemic at the end of supplementation were followed‐up for the additional period of time) | |
| Outcomes | Main objective/outcome: to compare the efficacy of microencapsulated iron fumarate sprinkles ± Vit A with iron sulphate drops with placebo in preventing recurrent anaemia and to determine the long‐term haematological outcome Review outcomes reported in the trial.
| |
| Notes | Trial location: Ghana Malaria endemicity: hyperendemic Language of publication: English Exclusion criteria: Hb < 10 g/dL, age 8 to 20 months, only breast feeding children | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding (performance bias and detection bias) | High risk | Open trial, intervention and control arms different. |
| Methods | Cluster RCT Trial duration: March 2010 to September 2010 | |
| Participants | 1958 randomized, 1958 evaluated Age: mean 19.5 ± 8.6 months and 19.4 ± 8.6 months for iron versus placebo Setting: community Mean Hb: 10.3 g/dL % parasitaemia at baseline: 31% | |
| Interventions | Microencapsulated ferrous fumarate 12.5 mg and micronutrients versus micronutrients alone Duration of treatment: 5 months Duration of follow‐up: 6 months | |
| Outcomes | Main objective/outcome: malaria Review outcomes reported in the trial.
| |
| Notes | Trial location: Ghana Malaria endemicity: hyperendemic. Bed nets and antimalarials available. Language of publication: English Exclusion criteria: Hb < 7, severe malnutrition, receipt of iron supplements within the past 6 months, or chronic illness | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Central. |
| Blinding (performance bias and detection bias) | Unclear risk | Trial team, caregivers, and data analysts blinded but packages marked with A/B for fortification/no fortification. |
Abbreviations: RCT: randomized controlled trial; Hb: haemoglobin; SD: standard deviation; HIV: human immunodeficiency virus; TIBC: total iron binding capacity; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomized controlled trial (RCT). | |
| I ncompatible intervention. | |
| Study not in children (participants' age 14 to 19 years and results for children not separated). | |
| Incompatible intervention (iron + other micronutrients). | |
| Editorial (not a RCT). | |
| incompatible intervention, insufficient dose. | |
| Not a RCT. | |
| Incompatible intervention (iron + other micronutrients) iron + vitamin C + riboflavin versus placebo. | |
| Incompatible intervention (i ron + other micronutrients) iron + multivitamin tablet. | |
| Incompatible intervention (iron + other micronutrients: iron versus B12). | |
| RCT conducted in non‐endemic area: Germany. | |
| Not a RCT. | |
| None of the reported outcomes relevant/usable for the review. | |
| RCT, blood transfusion versus iron (parenteral administration of iron). | |
| Not a RCT. | |
| Non‐endemic area (Chile); iron administered as fortification of milk. | |
| RCT, all groups received iron. | |
| RCT, all groups received iron. | |
| Incompatible intervention (i ron + other micronutrients). Comparison between basal and rich fortification including multiple vitamins + iron. | |
| RCT, all groups received iron. | |
| Dose comparison, all groups given iron. | |
| Stated specifically in study that the area wa s malaria‐free. | |
| Not a RCT (correspondence). | |
| Incompatible intervention (iron + other micronutrients: multivitamins versus promethazine hydrochloride). | |
| Not a malaria‐ endemic area: California. | |
| RCT that assessed iron as part of a treatment for malaria. | |
| All children were given iron supplementation. | |
| Not a RCT. | |
| Stated specifically in study that the area wa s malaria‐free. | |
| RCT, parenteral iron. | |
| RCT with intramuscular iron. | |
| Not a RCT (review article). | |
| No relevant outcome. In correspondence with study author, who stated that the study was not adequately completed, therefore results will not be analysed. | |
| Randomization to antimalaria treatment. All children received iron. | |
| Not a RCT. | |
| Cluster‐RCT with less than 2 units per arm. | |
| Not a RCT. | |
| Not a RCT. | |
| Not a RCT. | |
| Not a RCT. | |
| Comparison of different iron administration schedules. No placebo group. | |
| Dose comparison, all groups given iron. | |
| Incompatible intervention (iron + other micronutrients). | |
| None of the reported outcomes relevant/usable for the review. | |
| Not a RCT (cross‐sectional survey). | |
| Stated specifically in study that the area wa s malaria‐free. | |
| Stated specifically in study that the area wa s malaria‐free. | |
| No placebo group. | |
| Incompatible intervention (dose of iron administered was 0.08 mg/kg/day, too low for consideration as supplementation. | |
| RCT that included adults. | |
| Incompatible intervention (iron versus vitamin C). | |
| No relevant outcome (study assessed geophagy as outcome), | |
| No relevant outcome. The pharmaceutical company that supplied the drugs, placebo, and drug blinding codes did not provide the investigators with the codes (author correspondence). The authors stated that "Should the drug company come forth with the codes we will certainly share the results with you". | |
| Incompatible interventions: group 1 placebo, group 2 iron, group 3 daily iron, group 4 weekly iron. Only groups 3 and 4 were assigned randomly. | |
| RCT that assessed iron as part of treatment for malaria. | |
| RCT, parenteral iron. | |
| RCT, parenteral iron. | |
| Not a RCT. | |
| Incompatible intervention (i ron + other micronutrients). Intervention included iron, zinc, vitamin A, vitamin C and iodinedes MM . (TO AUTHORS: please write in full at first mention) | |
| Not a RCT. | |
| Not a RCT. | |
| Stated specifically in study that the area wa s malaria‐free. | |
| None of the reported outcomes were relevant/usable for the review. | |
| Insufficient iron dose. | |
| Stated by the author: specific area wa s hypoendemic. | |
| RCT, all groups received iron. | |
| Dose comparison, all groups given iron. | |
| All groups given iron (dose, schedule, or other comparisons). | |
| I nsufficient iron dose. | |
| Insuficient iron dose. Non‐endemic areas. Author stated in correspondence that area not endemic for malaria. | |
| None of the reported outcomes relevant/usable for the review. | |
| All groups given iron (dose, schedule, or other comparisons). | |
| Incompatible intervention (iron + other micronutrients: iron + FA + B12 versus placebo). | |
| Stated specifically in study that the area wa s malaria‐free. | |
| Stated specifically in study that the area wa s malaria‐free. | |
| Incompatible intervention (iron + other micronutrients: iron + zinc + retinol + vitamin C versus placebo). | |
| Non‐endemic area, according to correspondence with the author. | |
| RCT, all groups received iron. | |
| Non‐endemic areas. Stated specifically that the area is malaria‐free. Intervention consisted of multiple micronutrients. | |
| RCT that assessed iron as part of treatment for malaria. | |
| RCT that assessed iron as part of treatment for malaria. | |
| None of the reported outcomes were relevant/usable for this review. | |
| Not a RCT. | |
| I nsufficient iron dose. | |
| I nsufficient iron dose. |
Abbreviations : RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Cluster RCT, double‐blind, placebo controlled |
| Participants | Children aged 1 to 35 months living in Pemba, Zanzibar |
| Interventions | In the current version of the review we included two arms of this trial: iron‐ folic acid‐vitamin A versus placebo‐vitamin A, up until the time the iron arms were stopped based on the safety committee decision. Depending on data availability, we plan to add results from the iron‐folic acid plus vitamin A and zinc; versus zinc‐vitamin A arms at the time the iron arms were stopped (and the children receiving iron were transferred to the respective study arms without iron supplementation). |
| Outcomes | Admissions for malaria Cerebral malaria Hospital admissions Mortality |
| Notes | Data correspondence with Professor Sazawal. FInal study published as Sazawal 2007 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical malaria (grouped by presence of anaemia) Show forest plot | 14 | Risk Ratio (Fixed, 95% CI) | 0.93 [0.87, 1.00] | |
| Analysis 1.1 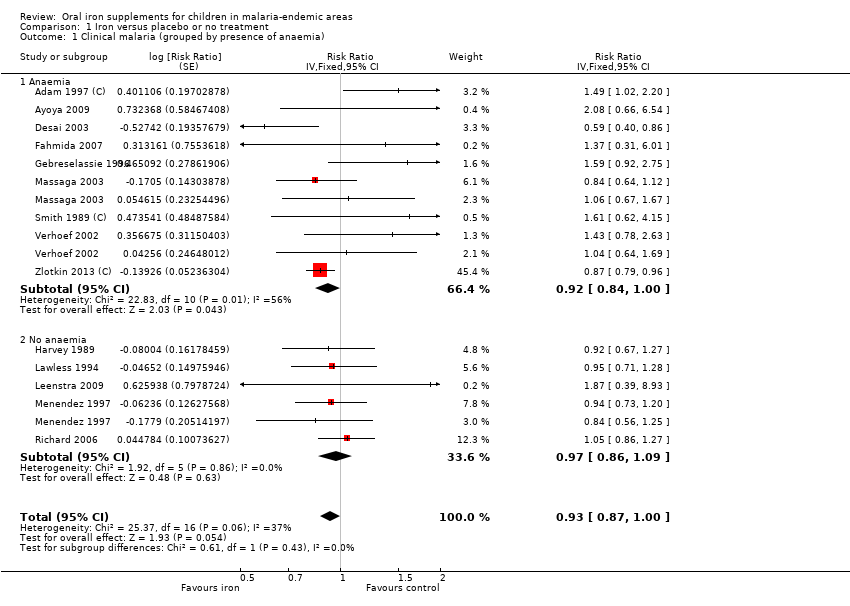 Comparison 1 Iron versus placebo or no treatment, Outcome 1 Clinical malaria (grouped by presence of anaemia). | ||||
| 1.1 Anaemia | 9 | Risk Ratio (Fixed, 95% CI) | 0.92 [0.84, 1.00] | |
| 1.2 No anaemia | 5 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.86, 1.09] | |
| 2 Clinical malaria (grouped by age) Show forest plot | 14 | Risk Ratio (Fixed, 95% CI) | 0.93 [0.87, 1.00] | |
| Analysis 1.2 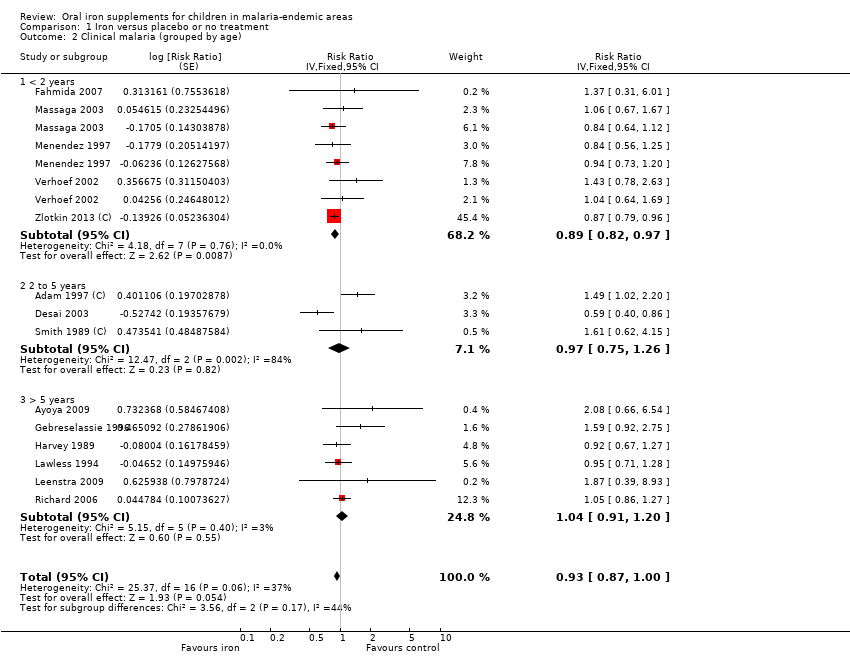 Comparison 1 Iron versus placebo or no treatment, Outcome 2 Clinical malaria (grouped by age). | ||||
| 2.1 < 2 years | 5 | Risk Ratio (Fixed, 95% CI) | 0.89 [0.82, 0.97] | |
| 2.2 2 to 5 years | 3 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.75, 1.26] | |
| 2.3 > 5 years | 6 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.91, 1.20] | |
| 3 Clinical malaria (P. falciparum only) Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 0.91 [0.84, 0.99] | |
| Analysis 1.3  Comparison 1 Iron versus placebo or no treatment, Outcome 3 Clinical malaria (P. falciparum only). | ||||
| 4 Any parasitaemia, end of treatment (by anaemia at baseline) Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 1.11 [1.00, 1.23] | |
| Analysis 1.4  Comparison 1 Iron versus placebo or no treatment, Outcome 4 Any parasitaemia, end of treatment (by anaemia at baseline). | ||||
| 4.1 Anaemia | 6 | Risk Ratio (Fixed, 95% CI) | 1.07 [0.94, 1.23] | |
| 4.2 No anaemia | 3 | Risk Ratio (Fixed, 95% CI) | 1.17 [0.99, 1.40] | |
| 5 All‐cause mortality Show forest plot | 18 | 7576 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.00, 0.01] |
| Analysis 1.5 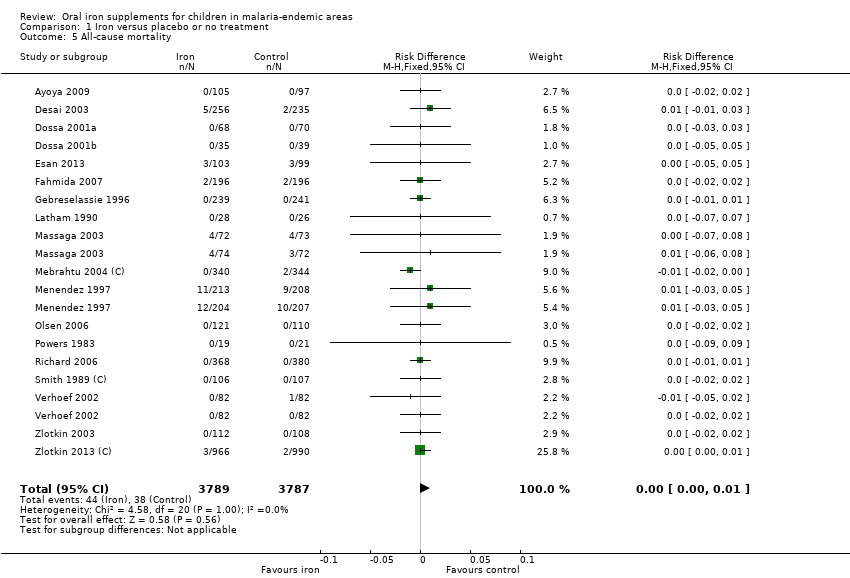 Comparison 1 Iron versus placebo or no treatment, Outcome 5 All‐cause mortality. | ||||
| 6 Clinical malaria with high‐grade parasitaemia or requiring admission Show forest plot | 5 | Risk Ratio (Fixed, 95% CI) | 0.90 [0.81, 0.98] | |
| Analysis 1.6  Comparison 1 Iron versus placebo or no treatment, Outcome 6 Clinical malaria with high‐grade parasitaemia or requiring admission. | ||||
| 7 Any parasitaemia, end of treatment ( by age) Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 1.11 [1.00, 1.23] | |
| Analysis 1.7 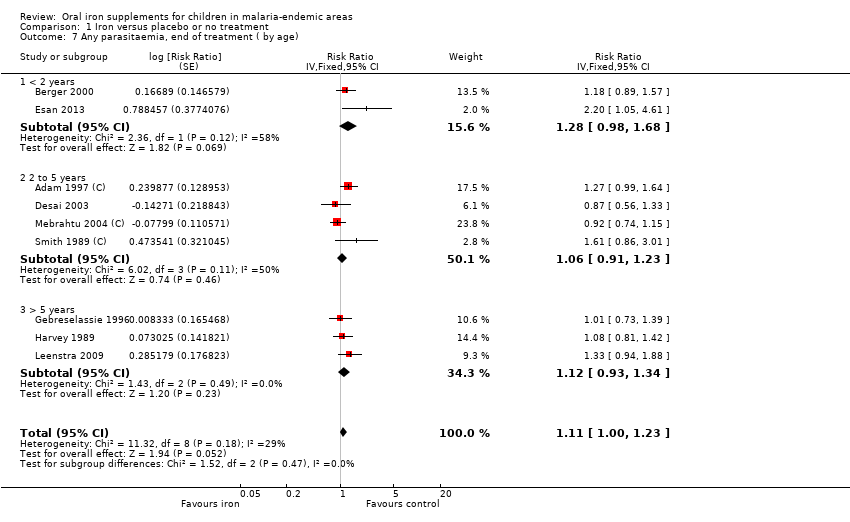 Comparison 1 Iron versus placebo or no treatment, Outcome 7 Any parasitaemia, end of treatment ( by age). | ||||
| 7.1 < 2 years | 2 | Risk Ratio (Fixed, 95% CI) | 1.28 [0.98, 1.68] | |
| 7.2 2 to 5 years | 4 | Risk Ratio (Fixed, 95% CI) | 1.06 [0.91, 1.23] | |
| 7.3 > 5 years | 3 | Risk Ratio (Fixed, 95% CI) | 1.12 [0.93, 1.34] | |
| 8 Any parasitaemia, end of treatment (P. falciparum only) Show forest plot | 7 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.97, 1.23] | |
| Analysis 1.8  Comparison 1 Iron versus placebo or no treatment, Outcome 8 Any parasitaemia, end of treatment (P. falciparum only). | ||||
| 8.1 Iron versus placebo/no treatment | 5 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.96, 1.24] | |
| 8.2 Iron + antimalarial versus antimalarial | 2 | Risk Ratio (Fixed, 95% CI) | 1.10 [0.76, 1.59] | |
| 9 Any parasitaemia, end of treatment (by allocation concealment) Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 1.11 [1.00, 1.23] | |
| Analysis 1.9  Comparison 1 Iron versus placebo or no treatment, Outcome 9 Any parasitaemia, end of treatment (by allocation concealment). | ||||
| 9.1 Adequate | 4 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.83, 1.15] | |
| 9.2 Unclear | 5 | Risk Ratio (Fixed, 95% CI) | 1.22 [1.06, 1.40] | |
| 10 High‐grade parasitaemia Show forest plot | 5 | Risk Ratio (Fixed, 95% CI) | 1.13 [0.93, 1.37] | |
| Analysis 1.10  Comparison 1 Iron versus placebo or no treatment, Outcome 10 High‐grade parasitaemia. | ||||
| 11 Any parasitaemia, end of follow‐up Show forest plot | 5 | 1150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.09, 1.40] |
| Analysis 1.11 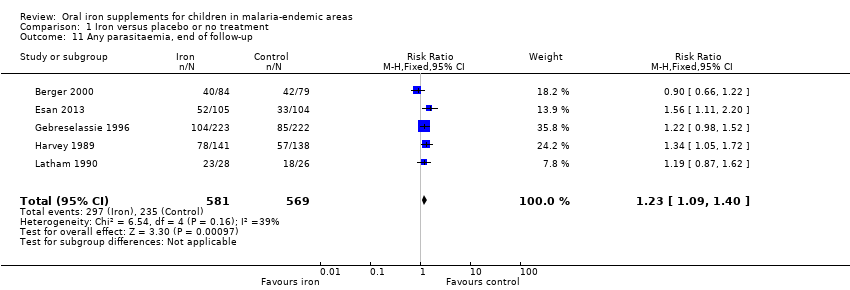 Comparison 1 Iron versus placebo or no treatment, Outcome 11 Any parasitaemia, end of follow‐up. | ||||
| 12 Hospitalizations and clinic visits Show forest plot | 7 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.95, 1.04] | |
| Analysis 1.12 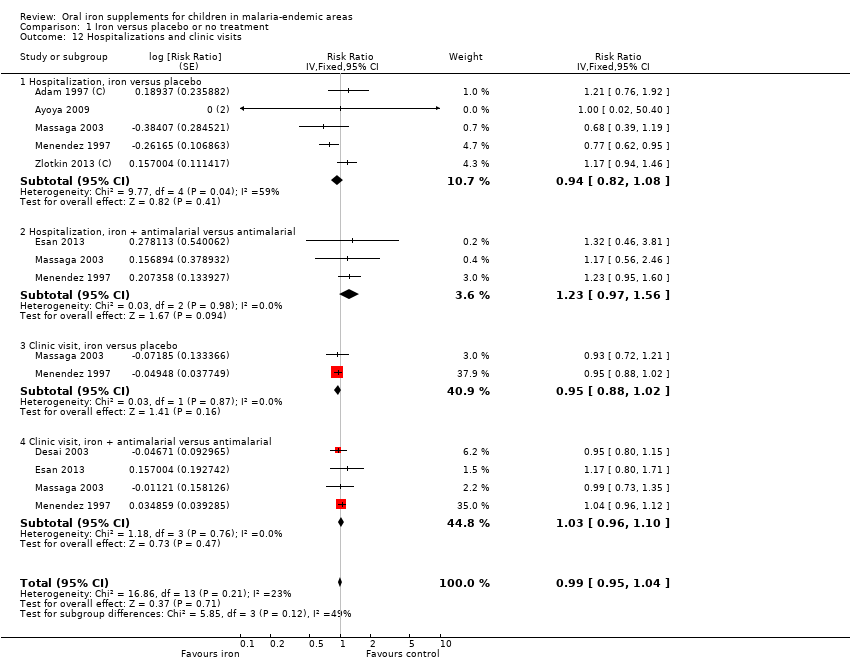 Comparison 1 Iron versus placebo or no treatment, Outcome 12 Hospitalizations and clinic visits. | ||||
| 12.1 Hospitalization, iron versus placebo | 5 | Risk Ratio (Fixed, 95% CI) | 0.94 [0.82, 1.08] | |
| 12.2 Hospitalization, iron + antimalarial versus antimalarial | 3 | Risk Ratio (Fixed, 95% CI) | 1.23 [0.97, 1.56] | |
| 12.3 Clinic visit, iron versus placebo | 2 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.88, 1.02] | |
| 12.4 Clinic visit, iron + antimalarial versus antimalarial | 4 | Risk Ratio (Fixed, 95% CI) | 1.03 [0.96, 1.10] | |
| 13 Haemoglobin, end of treatment (by anaemia at baseline) Show forest plot | 16 | 5261 | Mean Difference (IV, Random, 95% CI) | 0.75 [0.48, 1.01] |
| Analysis 1.13  Comparison 1 Iron versus placebo or no treatment, Outcome 13 Haemoglobin, end of treatment (by anaemia at baseline). | ||||
| 13.1 Anaemia | 7 | 2481 | Mean Difference (IV, Random, 95% CI) | 0.95 [0.38, 1.51] |
| 13.2 No anaemia | 9 | 2780 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.38, 0.85] |
| 14 Weight, end value Show forest plot | 5 | 1830 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.12, 0.06] |
| Analysis 1.14  Comparison 1 Iron versus placebo or no treatment, Outcome 14 Weight, end value. | ||||
| 15 Anaemia, end of treatment Show forest plot | 15 | 3784 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.82] |
| Analysis 1.15 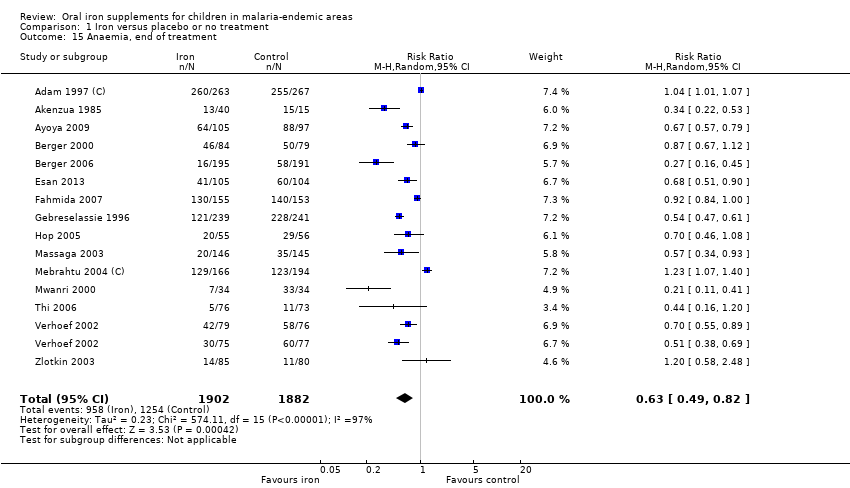 Comparison 1 Iron versus placebo or no treatment, Outcome 15 Anaemia, end of treatment. | ||||
| 16 Weight, change from baseline Show forest plot | 4 | 486 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.13, 0.49] |
| Analysis 1.16  Comparison 1 Iron versus placebo or no treatment, Outcome 16 Weight, change from baseline. | ||||
| 17 Diarrhoeal episodes per patient‐month (by zinc administration) Show forest plot | 8 | 23912 | Risk Ratio (Fixed, 95% CI) | 1.15 [1.06, 1.26] |
| Analysis 1.17  Comparison 1 Iron versus placebo or no treatment, Outcome 17 Diarrhoeal episodes per patient‐month (by zinc administration). | ||||
| 17.1 Without zinc | 7 | 17566 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.87, 1.13] |
| 17.2 With zinc | 3 | 6346 | Risk Ratio (Fixed, 95% CI) | 1.29 [1.15, 1.44] |
| 18 Infections per patient‐month Show forest plot | 8 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.18  Comparison 1 Iron versus placebo or no treatment, Outcome 18 Infections per patient‐month. | ||||
| 18.1 Febrile episodes | 6 | 15531 | Risk Ratio (Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 18.2 Days with fever | 1 | 110 | Risk Ratio (Fixed, 95% CI) | 8.37 [1.91, 36.58] |
| 18.3 All disease episodes | 1 | 1395 | Risk Ratio (Fixed, 95% CI) | 1.15 [0.91, 1.46] |
| 19 Haemoglobin, change from baseline, end of treatment Show forest plot | 12 | 2462 | Mean Difference (IV, Random, 95% CI) | 0.67 [0.42, 0.92] |
| Analysis 1.19  Comparison 1 Iron versus placebo or no treatment, Outcome 19 Haemoglobin, change from baseline, end of treatment. | ||||
| 20 URTI/pneumonia episodes per patient‐month Show forest plot | 6 | 21767 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.85, 1.15] |
| Analysis 1.20  Comparison 1 Iron versus placebo or no treatment, Outcome 20 URTI/pneumonia episodes per patient‐month. | ||||
| 21 Height, end value Show forest plot | 5 | 2102 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.08, 0.10] |
| Analysis 1.21  Comparison 1 Iron versus placebo or no treatment, Outcome 21 Height, end value. | ||||
| 22 Height, change from baseline Show forest plot | 4 | 486 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.09, 0.27] |
| Analysis 1.22  Comparison 1 Iron versus placebo or no treatment, Outcome 22 Height, change from baseline. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severe malaria (malaria requiring admission) Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 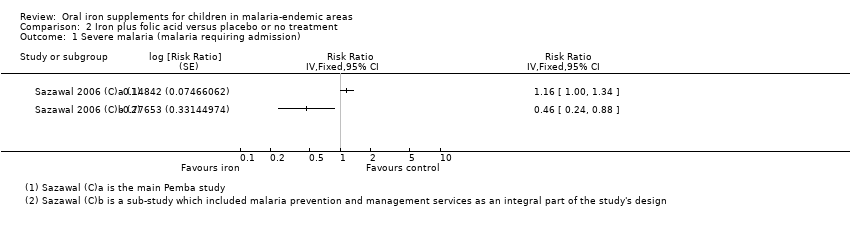 Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 1 Severe malaria (malaria requiring admission). | ||||
| 2 Severe malaria (cerebral malaria) Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 2 Severe malaria (cerebral malaria). | ||||
| 3 All‐cause mortality Show forest plot | 5 | 18034 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.00, 0.01] |
| Analysis 2.3 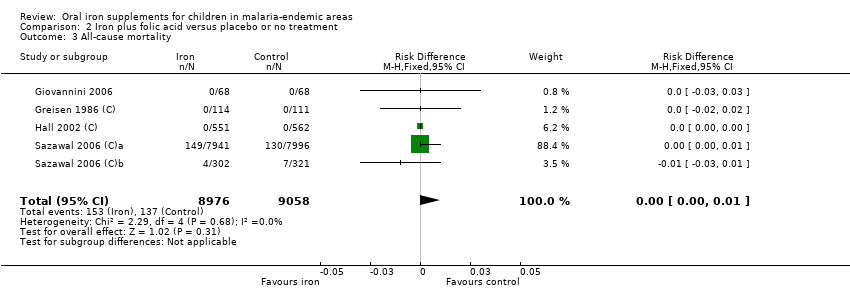 Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 3 All‐cause mortality. | ||||
| 4 Any hospitalization Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4 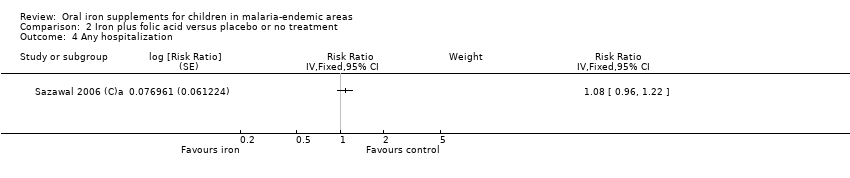 Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 4 Any hospitalization. | ||||
| 5 Haemoglobin, end of treatment Show forest plot | 1 | 124 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.51, 1.29] |
| Analysis 2.5  Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 5 Haemoglobin, end of treatment. | ||||
| 6 Anaemia, end of treatment Show forest plot | 3 | 633 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.99] |
| Analysis 2.6  Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 6 Anaemia, end of treatment. | ||||
| 7 Weight, end value Show forest plot | 2 | 1080 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.18, 0.06] |
| Analysis 2.7  Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 7 Weight, end value. | ||||
| 8 Height, end value Show forest plot | 2 | 1082 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.12, 0.12] |
| Analysis 2.8  Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 8 Height, end value. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical malaria (grouped by presence of malaria prevention or management) Show forest plot | 16 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.91, 1.03] | |
| Analysis 3.1 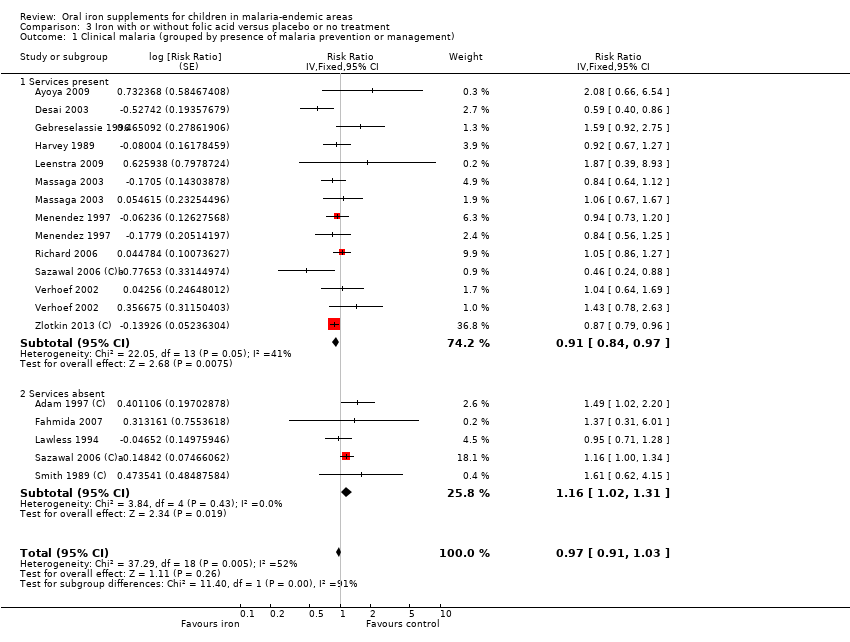 Comparison 3 Iron with or without folic acid versus placebo or no treatment, Outcome 1 Clinical malaria (grouped by presence of malaria prevention or management). | ||||
| 1.1 Services present | 11 | Risk Ratio (Fixed, 95% CI) | 0.91 [0.84, 0.97] | |
| 1.2 Services absent | 5 | Risk Ratio (Fixed, 95% CI) | 1.16 [1.02, 1.31] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical malaria Show forest plot | 3 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.43, 0.67] |
| Analysis 4.1  Comparison 4 Iron plus antimalarial versus placebo, Outcome 1 Clinical malaria. | ||||
| 2 All‐cause mortality Show forest plot | 3 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.52, 2.11] |
| Analysis 4.2  Comparison 4 Iron plus antimalarial versus placebo, Outcome 2 All‐cause mortality. | ||||
| 3 Hospitalizations and clinic visits Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Iron plus antimalarial versus placebo, Outcome 3 Hospitalizations and clinic visits. | ||||
| 3.1 Hospitalization, iron + antimalarial versus placebo | 2 | 5904 | Risk Ratio (Fixed, 95% CI) | 0.59 [0.48, 0.73] |
| 3.2 Clinic visit, iron + antimalarial versus placebo | 2 | 5904 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.82, 0.95] |
| 4 Haemoglobin at end of treatment Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.4 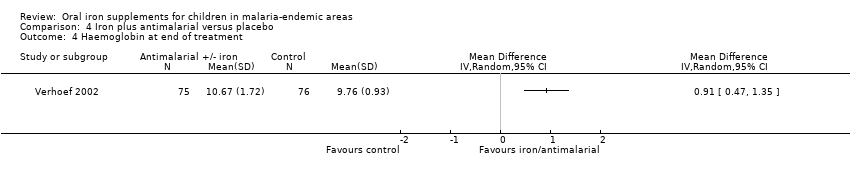 Comparison 4 Iron plus antimalarial versus placebo, Outcome 4 Haemoglobin at end of treatment. | ||||
| 5 Anaemia Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.5 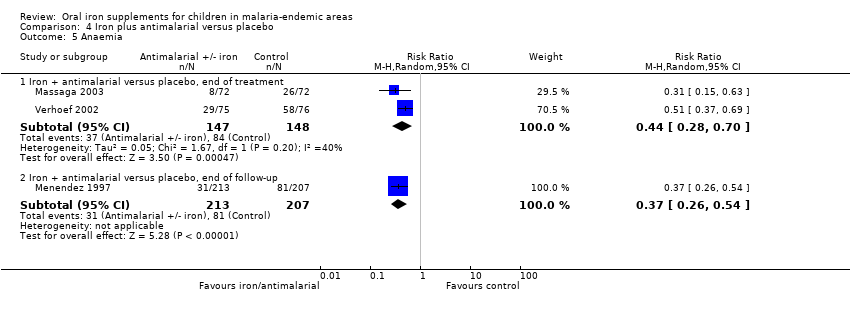 Comparison 4 Iron plus antimalarial versus placebo, Outcome 5 Anaemia. | ||||
| 5.1 Iron + antimalarial versus placebo, end of treatment | 2 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.28, 0.70] |
| 5.2 Iron + antimalarial versus placebo, end of follow‐up | 1 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.26, 0.54] |

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included trials. The unclear category for incomplete outcome data represents trials that did not report this outcome.

Methodological quality summary: review authors' judgements about each methodological quality item for each included trial.

Funnel plot of comparison: 1. Iron versus placebo or no treatment, outcome: 1.1 Clinical malaria (grouped by presence of anaemia).

Comparison 1 Iron versus placebo or no treatment, Outcome 1 Clinical malaria (grouped by presence of anaemia).

Comparison 1 Iron versus placebo or no treatment, Outcome 2 Clinical malaria (grouped by age).

Comparison 1 Iron versus placebo or no treatment, Outcome 3 Clinical malaria (P. falciparum only).

Comparison 1 Iron versus placebo or no treatment, Outcome 4 Any parasitaemia, end of treatment (by anaemia at baseline).

Comparison 1 Iron versus placebo or no treatment, Outcome 5 All‐cause mortality.

Comparison 1 Iron versus placebo or no treatment, Outcome 6 Clinical malaria with high‐grade parasitaemia or requiring admission.

Comparison 1 Iron versus placebo or no treatment, Outcome 7 Any parasitaemia, end of treatment ( by age).

Comparison 1 Iron versus placebo or no treatment, Outcome 8 Any parasitaemia, end of treatment (P. falciparum only).

Comparison 1 Iron versus placebo or no treatment, Outcome 9 Any parasitaemia, end of treatment (by allocation concealment).

Comparison 1 Iron versus placebo or no treatment, Outcome 10 High‐grade parasitaemia.

Comparison 1 Iron versus placebo or no treatment, Outcome 11 Any parasitaemia, end of follow‐up.

Comparison 1 Iron versus placebo or no treatment, Outcome 12 Hospitalizations and clinic visits.
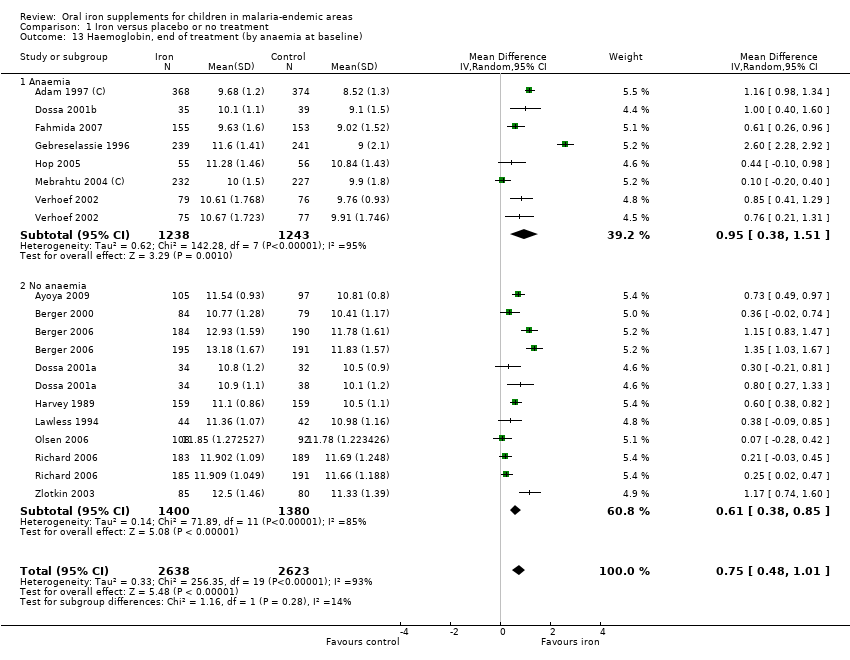
Comparison 1 Iron versus placebo or no treatment, Outcome 13 Haemoglobin, end of treatment (by anaemia at baseline).

Comparison 1 Iron versus placebo or no treatment, Outcome 14 Weight, end value.

Comparison 1 Iron versus placebo or no treatment, Outcome 15 Anaemia, end of treatment.

Comparison 1 Iron versus placebo or no treatment, Outcome 16 Weight, change from baseline.
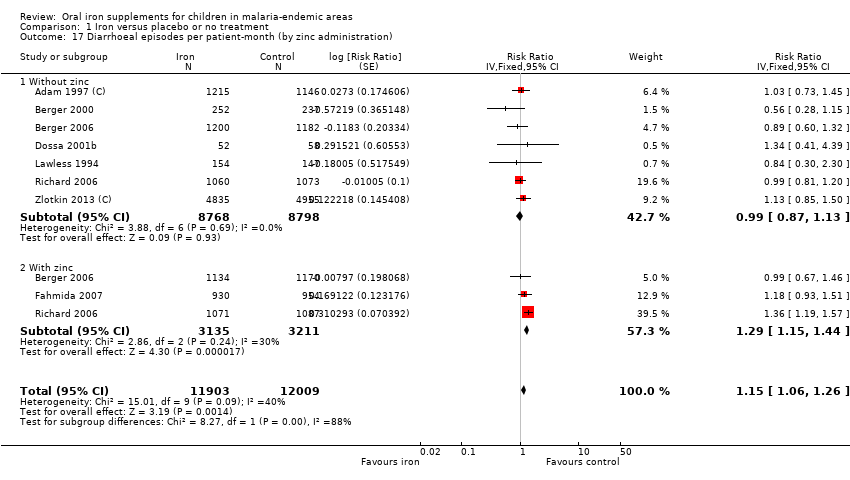
Comparison 1 Iron versus placebo or no treatment, Outcome 17 Diarrhoeal episodes per patient‐month (by zinc administration).

Comparison 1 Iron versus placebo or no treatment, Outcome 18 Infections per patient‐month.

Comparison 1 Iron versus placebo or no treatment, Outcome 19 Haemoglobin, change from baseline, end of treatment.

Comparison 1 Iron versus placebo or no treatment, Outcome 20 URTI/pneumonia episodes per patient‐month.
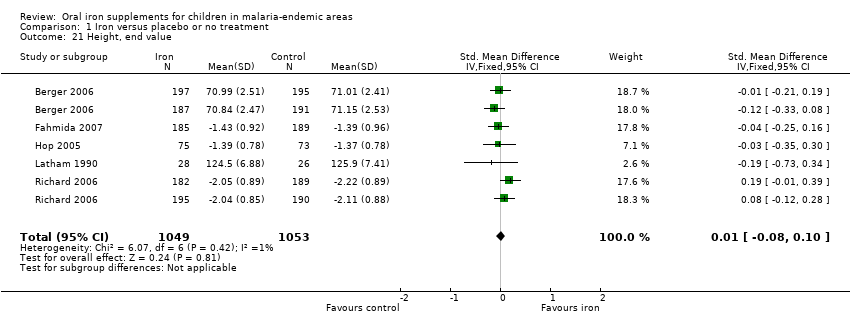
Comparison 1 Iron versus placebo or no treatment, Outcome 21 Height, end value.

Comparison 1 Iron versus placebo or no treatment, Outcome 22 Height, change from baseline.
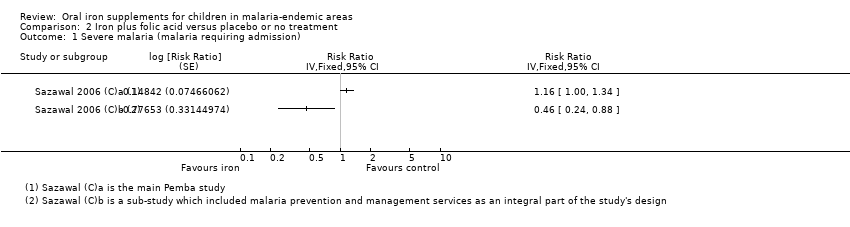
Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 1 Severe malaria (malaria requiring admission).

Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 2 Severe malaria (cerebral malaria).

Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 3 All‐cause mortality.

Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 4 Any hospitalization.

Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 5 Haemoglobin, end of treatment.

Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 6 Anaemia, end of treatment.

Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 7 Weight, end value.

Comparison 2 Iron plus folic acid versus placebo or no treatment, Outcome 8 Height, end value.
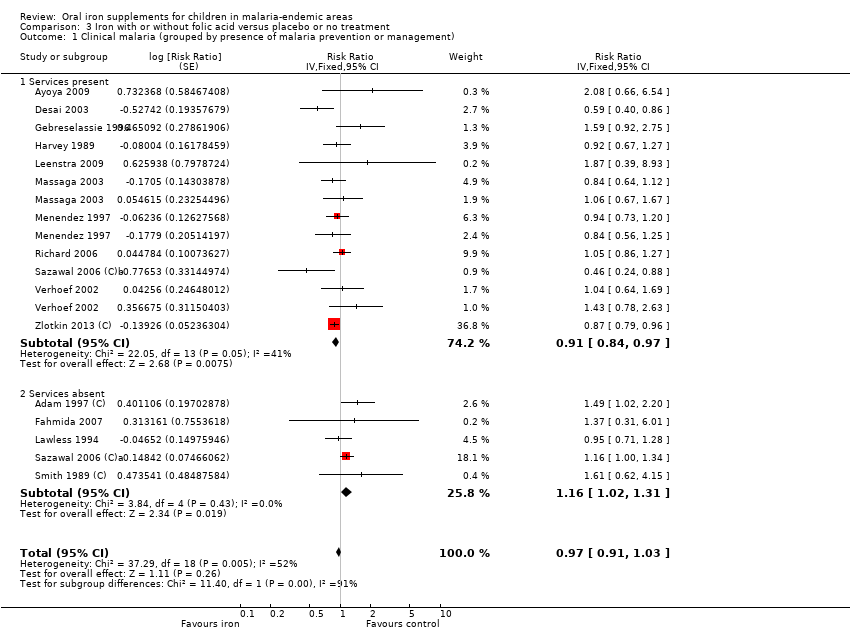
Comparison 3 Iron with or without folic acid versus placebo or no treatment, Outcome 1 Clinical malaria (grouped by presence of malaria prevention or management).
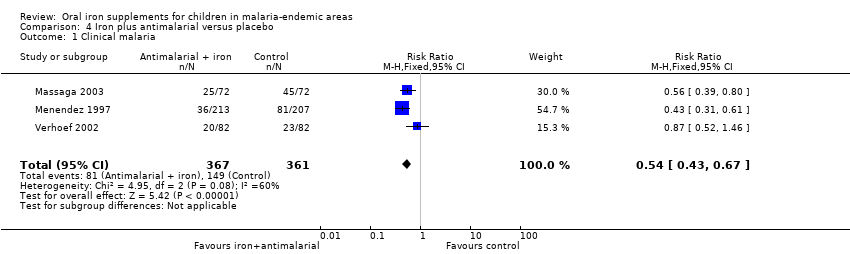
Comparison 4 Iron plus antimalarial versus placebo, Outcome 1 Clinical malaria.
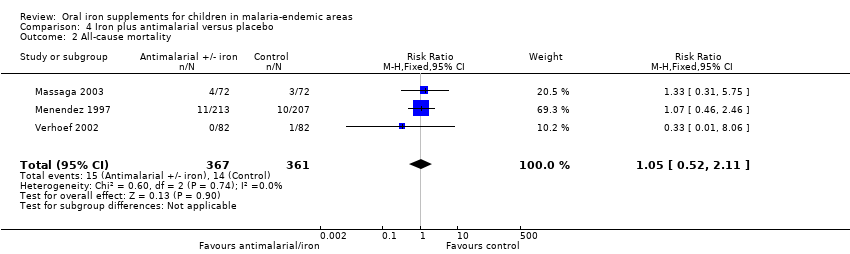
Comparison 4 Iron plus antimalarial versus placebo, Outcome 2 All‐cause mortality.
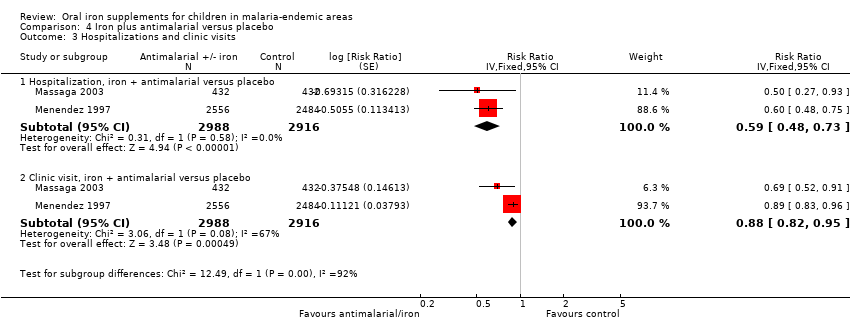
Comparison 4 Iron plus antimalarial versus placebo, Outcome 3 Hospitalizations and clinic visits.

Comparison 4 Iron plus antimalarial versus placebo, Outcome 4 Haemoglobin at end of treatment.
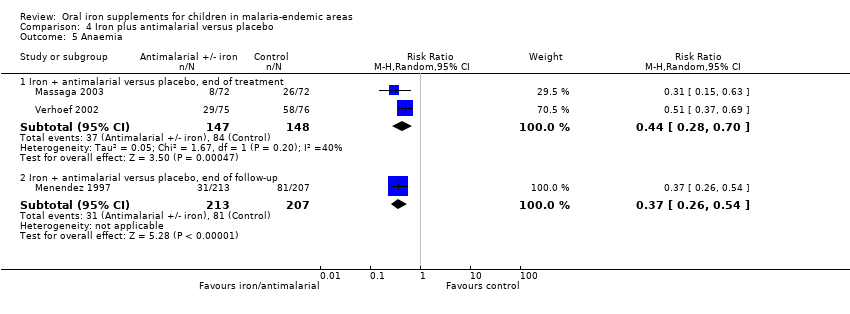
Comparison 4 Iron plus antimalarial versus placebo, Outcome 5 Anaemia.
| Does iron supplementation or fortification increase malaria and related morbidity and mortality among children in malaria‐endemic areas? | ||||||
| Participant or population: children in malaria‐endemic areas | ||||||
| Subgroup | Anticipated absolute effects* (95% CI) | Relative effect results | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo or no treatment | Risk with iron supplementation | |||||
| Clinical malaria | 27/100 | 25/100 (23 to 27) | RR 0.93 (0.87 to 1.00) | 7168 (14 RCTs) | ⊕⊕⊕⊕ High1 | Overall, among anaemic or non‐anaemic children, iron does not cause an excess of clinical malaria |
| Clinical malaria Subgrouped by population anaemia (trial level) | Anaemic at baseline | RR 0.92 | 7168 (14 RCTs) | ⊕⊕⊕⊝ | In populations where anaemia is common, iron probably does not cause an excess of clinical malaria | |
| 256 per 1000 | 236 per 1000 | |||||
| Not anaemic at baseline | RR 0.97 | 2112 | ⊕⊕⊕⊝ | In populations where anaemia is uncommon, iron probably does not cause an excess of clinical malaria | ||
| 326 per 1000 | 316 per 1000 | |||||
| Severe malaria Defined as clinical malaria with high‐grade parasitaemia or requiring admission | 397 per 1000 | 357 per 1000 | RR 0.90 | 3421 | ⊕⊕⊕⊕ | Iron supplementation does not cause an excess of severe malaria |
| Death | 10 per 10000 | 10 per 1000 (10 to 10) | Not estimated | 7576 (18 RCTs) | ⊕⊕⊝⊝ Low4 | Iron may have no effect on mortality |
| Hospitalization plus clinic visits | 295 per 1000 | 295 per 1000 | RR 0.99 | 12,578 | ⊕⊝⊝⊝ | It is uncertain whether iron affects hospitalizations or clinic visits |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Funnel plot asymmetry favouring the control arm, publication bias not suspected. The CIs range from important benefits of iron supplementation in reducing clinical malaria to no excess of clinical malaria. | ||||||
| Does iron with or without folic acid increase malaria among children in malaria‐endemic areas? | ||||||
| Participant or population: children in malaria‐endemic areas | ||||||
| Subgroup | Anticipated absolute effects* (95% CI) | Relative effect results | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo or no treatment | Risk with iron supplementation | |||||
| Clinical malaria Subgrouped by presence of malaria prevention or management services | Malaria prevention or management services present | RR 0.91 (0.84 to 0.97) | 5586 | ⊕⊕⊝⊝ | In areas where there are prevention and management services for malaria, iron supplementation may reduce clinical malaria | |
| 24 per 100 | 22 per 1000 | |||||
| Malaria prevention or management services not present | RR 1.16 | 19,086 | ⊕⊕⊝⊝ | In areas where there are no prevention and management services for malaria, iron may increase the number of children with clinical malaria | ||
| 6 per 100 | 7 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by 1 for inconsistency and 1 for funnel plot asymmetry and suspected publication bias. | ||||||
| Is iron supplementation with antimalarial treatment safe and beneficial for children living in malaria‐endemic areas? | ||||||
| Participant or population: children with or without anaemia at baseline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Iron supplementation plus antimalarial | |||||
| Clinical malaria | 41 per 100 | 22 per 100 | RR 0.54 | 728 | ⊕⊕⊕⊕ | Iron given together with antimalarial antimicrobials reduce malaria |
| All‐cause mortality | 42 per 1000 | 44 per 1000 (23 to 85) | RR 1.05 | 728 (3 (RCTs) | ⊕⊕⊝⊝ | Iron given together with antimalarial antimicrobials may have no effect on mortality |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1All trials were individually randomized, with adequate concealment, double‐blinded, and no loss to follow‐up. | ||||||
| Area definition | Parasite rates | Description | Geographical location |
| Hypoendemicity (also called designated unstable malaria) | 10% or fewer children aged 2 to 9 years, but may be higher for part of the year | Areas where there is little transmission and during the average year the effects upon the general population are unimportant | AFRO: Chad |
| Mesoendemicity (also called unstable and stable malaria) | 11 to 50% of children aged 2 to 9 years | Typically found among rural communities in subtropical zones where wide geographical variations in transmission exist | AFRO: Angola, Botswana, Cape Verde, Chad, Eritrea, Ethiopia, Kenya (considered hyper‐ or holoendemic in review, as indicated in most of the trials), Mauritania, Namibia, Niger, Zambia, Zimbabwe |
| Hyperendemicity (also called stable malaria) | Consistently > 50% among children aged 2 to 9 years | Areas where transmission is intense but seasonal; immunity is insufficient in all age groups | AFRO: Angola, Benin, Burkina Faso, Cameroon, Central African Republic, Chad, Congo, Côte d'Ivoire, Equatorial Guinea, Gabon, Gambia, Ghana, Guinea, Guinea‐Bissau, Liberia, Madagascar, Malawi, Mali, Mozambique, Nigeria, Sao Tome and Principe, Senegal, Sierra Leone, Togo, Uganda,Tanzania, Zambia |
| Holoendemicity (also called stable malaria) | Consistently > 75% among infants aged 0 to 11 months | Intense transmission resulting in a considerable degree of immunity after early childhood | AFRO: Central African Republic, Democratic Republic of Congo, Tanzania, Uganda, Burundi, Madagascar, Malawi, Mozambique |
| Abbreviations: AFRO: WHO African Regional Office; AMRO: WHO Americas Regional Office; EMRO: WHO Eastern Mediterranean Regional Office; EURO: WHO Europe Regional Office; SEARO: WHO South East Asian Regional Office; WPRO: WHO Western Pacific Regional Office. | |||
| Age group | Minimal daily dose |
| < 6 months | 2 mg/kg |
| 6 to 24 months | 12.5 mg or 2 mg/kg |
| 2 to 5 years | 20 mg |
| 6 to 11 years | 30 mg |
| 11 to 18 years | 60 mg |
| Abbreviations: WHO: World Health Organization; RDA: recommended dietary allowance. | |
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | iron | iron | iron | iron | iron |
| 2 | ferrous | ferrous | ferrous | FERROUS‐SULPHATE | ferrous |
| 3 | 1 or 2 | IRON COMPOUNDS | IRON COMPOUNDS | 1 or 2 | 1 or 2 |
| 4 | malaria | 1 or 2 or 3 | 1 or 2 or 3 | supplem$ | malaria |
| 5 | anaemia | supplem* | supplem* | 3 and 4 | anaemia |
| 6 | anaemia | 4 and 5 | 4 and 5 | malaria | anaemia |
| 7 | 4 or 5 or 6 | malaria | malaria | anaemia | 4 or 5 or 6 |
| 8 | 3 and 7 | anaemia | anaemia | 6 or 7 | 3 and 7 |
| 9 | — | anaemia | anaemia | 5 and 8 | — |
| 10 | — | 7 or 8 or 9 | 7 or 8 or 9 | child$ | — |
| 11 | — | 6 and 10 | 6 and 10 | infant$ | — |
| 12 | — | — | child* | 10 or 11 | — |
| 13 | — | — | infant* | 9 and 12 | — |
| 14 | — | — | 12 or 13 | — | — |
| 15 | — | — | 11 and 14 | — | — |
| aCochrane Infectious Diseases Group Specialized Register. | |||||
| Trial ID | Clinical definition | Laboratory definition | Malaria‐related outcomes reported | Time of assessment | Malaria prevention or management strategies | Malaria prevention or management |
| Physician's diagnosis of malaria | Any parasitaemia (all malaria species, assumed most Plasmodium falciparum since trial conducted in same region as Gebreselassie 1996) | Clinical malaria; any parasitaemia; malaria necessitating hospitalization (used as severe malaria); parasite density (all N events/N individuals, unadjusted for clustering) | 3 months to end of treatment | Blood smears for malaria obtained before, during and after treatment. Children with clinical malaria referred to local hospital and treated | No | |
| Fever > 37.5°C (axillary) | Any parasitaemia (P. falciparum) | Clinical malaria; clinical malaria with parasitaemia ≥ 5000/μL (used as severe malaria); parasite density | 3 months to end of treatment | Malaria screening was done at baseline for all children and repeated throughout the study in children who had fever. Children infected with P. falciparum also were treated with sulfadoxine‐pyrimethamine | Yes | |
| Isolated fever | Parasite density > 3000 (P. falciparum, Plasmodium malariae and Plasmodium ovale assessed. Over 97% were P. falciparum) | Parasite index (%, used as parasitaemia); parasitaemia above 3000 /µL (used as severe malaria) and 10,000 / µL (%); parasite density | 3 months to end of treatment 9 months to end of follow‐up (FU) | Blood smears for malaria obtained at baseline, end of treatment (3 months) and end of FU (6 months). Chloroquine treatment given for all isolated fevers | Yes | |
| Fever ≥37.5°C | Any parasitaemia (P. falciparum) with fever or parasitaemia > 5000/mm3 alone | Clinical malaria; any parasitaemia; hazard ratios for these; parasite density | 3 months to end of treatment | Blood smears at baseline and every 4 weeks. Oral quinine given for any fever with parasitaemia and cases of severe malaria referred for further treatment | Yes | |
| No clinical definition | Any parasitaemia | All cause sick visits (including malaria), | 3 months to end of treatment 6 months to end of FU | Routine trimethoprim ‐ sulfamethoxazole prophylaxis | Yes | |
| Not stated | Not stated | Participants with "malaria" (used primarily as clinical malaria) | 6 months to end of treatment | Not stated | No | |
| Fever ≥ 37.5°C with signs and symptoms suggestive of malaria and other diagnoses ruled out | Presence of parasites in blood (all species, P. falciparum 88.9%) | Children with at least one episode of clinical malaria; cumulative incidence of parasitaemia; parasite density > 5000 µL (used as severe malaria); parasite density | 3 months to end of treatment 6 months to end of FU | Blood smears negative at baseline and repeated weekly. Chloroquine with or without primaquine given for any positive smear | Yes | |
| Fever and headache at the same time | Any parasitaemia (P. falciparum 67%, P. vivax 26.4%, P. malariae 6.6%) | First episodes of clinically suspected malaria (used primarily as clinical malaria); any parasitaemia | 4 months to end of treatment 6 months to end of FU | Blood smears for malaria obtained at 0, 6, 16, and 24 weeks. Chloroquine given for any illness reported as fever or headache, or both | Yes | |
| Not assessed | Any positive smear (malaria species not stated) | Any positive smear; parasite density | 8 months to end of FU | Blood smears for malaria obtained at baseline and end of treatment. Treatment not stated | Yes | |
| Child's recall of clinical illness | Any positive blood smear (malaria species not stated) | Malaria is not defined (used as clinical malaria) | 3.5 months to end of treatment | No blood smears at baseline or during the trial (only at end of treatment). Treatment not stated | No | |
| Fever ≥ 37.5°C | Positive blood smear (malaria species not stated) | Episodes of clinical malaria and RRs adjusted for school; episodes of malaria parasitaemia and parasitaemia > 500 parasites/mm3 (used as severe malaria) and RRs adjusted for school, age, and baseline parasitaemia | 5 months to end of treatment | Blood smears for malaria at baseline (1/4 of participants positive) and monthly during the trial. No treatment offered for positive smears; symptomatic cases referred to physician | Yes | |
| History of fever in the previous 24 to 72 hours or measured temperature of ≥ 37.5°C | Any level of parasitaemia (P. falciparum only) | Clinical malaria as first or only episode per participant (used as clinical malaria) and episodes of clinical malaria; episodes of clinical malaria associated with parasitaemia > 5000 parasites/μL (used as severe malaria) | 6 months to end of treatment | Blood smears for malaria at baseline and every 2 weeks. Sulfadoxine‐pyrimethamine treatment given for uncomplicated cases; complicated and severe malaria referred to the hospital | Yes | |
| Not assessed | Any positive smear (P. falciparum only) | Parasitaemia as OR (95% CI) adjusted for repeated measurements in each child | 12 months to end of treatment | Blood smears for malaria at baseline and end of treatment. In addition, monthly smears from a random sample (50% of randomized). Treatment not stated | Yes | |
| Fever ≥ 37.5°C | Parasitaemia of any density (P. falciparum only) | First or only episode of clinical malaria | 1 year (6 months after end of treatment) | Blood smears for malaria at baseline, week 8 and for any fever. Chloroquine treatment given for clinical malaria | Yes | |
| Any fever within the previous 72 hours | P. falciparum (29%) or P. vivax (71%), any density | Episodes of falciparum or vivax malaria, or both (used primarily as clinical malaria) | 7 months to end of treatment | Blood smears for malaria at baseline and whenever febrile. Treatment given for all clinical cases | Yes | |
| Fever > 38°C and | Parasitaemia > 1000 or history of fever and parasitaemia > 3000 or parasitaemia > 10,000 parasites/mm3 regardless of fever (mostly P. falciparum) | Malaria‐related adverse events, defined as hospital admission or death due to malaria (used primarily as clinical malaria). RRs with 95% CI adjusted for multiple events per child and clustering; cerebral malaria (used as severe malaria) | Not fixed. End of treatment about 1 year and end of FU about 18 months | No baseline or routine surveillance for malaria during the trial. Treatment given only if admitted to the hospital and malaria diagnosed | No | |
| Fever > 38°C | Parasitaemia > 1000 or history of fever and parasitaemia > 3000 or parasitaemia > 10,000 parasites/mm3 regardless of fever (mostly P. falciparum) | Malaria‐related adverse events, defined as hospital admission or death due to malaria (used primarily as clinical malaria). RRs with 95% CI adjusted for multiple events per child and clustering; cerebral malaria (used as severe malaria) | Not fixed. End of treatment about 1 year and end of FU about 18 months | Blood smear for malaria at baseline, and at 6 and 12 months. Sulfadoxine‐pyrimethamine treatment delivered to home to all slide‐confirmed malaria participants or clinical disease presenting during the study | Yes | |
| Fever > 37.5°C | > 500 parasites/mm3 (mostly P. falciparum) | Visits for clinical malaria; parasitaemia > 500/µL; fever with parasitaemia > 5000 parasites/mm3 (used as severe malaria (all N events/N individuals, unadjusted for clustering) | 3 months to end of treatment | Blood smear for malaria at baseline, 2 weeks and end of treatment. No treatment at baseline; clinical malaria referred to local healthcare services | No | |
| Axillary temperature ≥ 37.5°C | Dipstick test for P. falciparum | Number of children with malaria infection (used primarily as clinical malaria) | 3 months to end of treatment | Dipstick for P. falciparum tested at baseline, 4, 8, and 12 weeks. Confirmed with blood smear if febrile and treated with sulfadoxine‐pyrimethamine, amodiaquine or halofantrine | Yes | |
| Axillary temperature ≥ 37.5°C | Parasitaemia of any density (mostly P. falciparum) | Incidence of clinical malaria, malaria with parasite density > 5000/µL, cerebral malaria | 5 months to end of treatment 6 months to end of FU | Insecticide‐treated bed nets supplied with instructions for use. Children with malaria treated with artemisinin combination therapy | Yes | |
| Time of assessment: refers to time from randomization. | ||||||
| Trial ID | Outcome | n Int reported | N Int reported | n Cont reported | N Cont reported | Average cluster size | DE | Unadjusted RR (95% CI) | ln(RR) | Unadjusted SE(lnRR) | Adjusted SE(lnRR)/ sample size |
| Clinical malaria | 72 | 366 | 49 | 372 | Household (used 1.5) | 1.34 | 1.49 (1.07 to 2.08) | 0.40 | 0.17 | 0.20 | |
| Parasitaemia | 127 | 368 | 101 | 372 | Household (used 1.5) | 1.34 | 1.27 (1.02 to 1.58) | 0.24 | 0.11 | 0.13 | |
| Clinical malaria necessitating hospitalization | 41 | 405 | 32 | 382 | Household (used 1.5) | 1.34 | 1.21 (0.78 to 1.88) | 0.19 | 0.22 | 0.26 | |
| Parasitaemia | — | 307 | — | 307 | 1.5 | 1.34 | OR 0.9 (0.72 to 1.19) Converted to RR 0.98 | 0.47 | 0.28 | 0.32 | |
| High‐grade parasitaemia | — | 307 | — | 307 | 1.5 | 1.34 | OR 1.04 (0.82 to 1.34) Converted to RR 1.03 | 0.03 | 0.12 | 0.14 | |
| Clinical malaria | 467 | 7950 | 411 | 8006 | 1.4 | — | 1.16 (1.00 to 1.34) | 0.15 | — | 0.07 | |
| Severe malaria (cerebral) | — | 7950 | — | 8006 | 1.4 | — | 1.32 (1.02 to 1.70) | 0.28 | — | 0.13 | |
| Clinical malaria | 14 | 815 | 30 | 804 | 1.2 | — | 0.46 (0.24 to 0.88) | ‐0.78 | — | 0.33 | |
| Severe malaria (cerebral) | 4 | 815 | 15 | 804 | 1.2 | — | 0.26 (0.09 to 0.81) | ‐1.35 | — | 0.56 | |
| Clinical malaria | 14 | 97 | 8 | 89 | Household (used 1.5) | 1.34 | 1.60 (0.42 to 0.71) | 0.47 | 0.42 | 0.48 | |
| Parasitaemia | 28 | 97 | 16 | 89 | Household (used 1.5) | 1.34 | 1.61 (0.93 to 2.76) | 0.47 | 0.28 | 0.32 | |
| High‐grade parasitaemia | 17 | 97 | 11 | 89 | Household (used 1.5) | 1.34 | 1.42 (0.70 to 2.86) | 0.35 | 0.13 | 0.15 | |
| Clinical malaria | 338 | 966 | 392 | 989 | Compounds | — | 0.87 (0.79 to 0.97) | ‐0.14 | — | 0.05 | |
| Clinical malaria with high grade parasitaemia | 273 | 966 | 308 | 989 | Compounds | — | 0.89 (0.80 to 1.00) | ‐0.12 | — | 0.06 | |
| Text in bold;results provided in publication or from authors adjusted for clustering. | |||||||||||
| Study ID | Outcome | n Int reported | N Int reported | n Cont reported | N Cont reported | Average cluster size | DE | n Int adjusted | N Int adjusted | n Cont adjusted | N Cont adjusted |
| Anaemia | 364 | 368 | 357 | 374 | Household (used 1.5) | 1.4 | 260 | 263 | 255 | 267 | |
| Anaemia | 273 | 551 | 356 | 562 | 20 | 2.77 | 99 | 199 | 129 | 203 | |
| All‐cause mortality | 0 | 340 | 2 | 344 | 1.5 | 1.001 | 0 | 340 | 2 | 344 | |
| Anaemia | 180 | 232 | 172 | 272 | 1.5 | 1.4 | 129 | 166 | 123 | 194 | |
| Anaemia | 133 | 224 | 110 | 203 | 30 | 3.70 | 36 | 61 | 30 | 55 | |
| All‐cause mortality | 149 | 7950 | 130 | 8006 | 1.4 | 1.001 | 149 | 7941 | 130 | 7996 | |
| All‐cause mortality | 8 | 815 | 9 | 804 | 1.2 | 1.0004 | 8 | 815 | 9 | 804 | |
| Anaemia | 4 | 308 | 7 | 327 | 1.2 | 1.4 | 3 | 220 | 5 | 234 | |
| All‐cause mortality | 3 | 967 | 2 | 991 | Compound | 1.001 | 3 | 966 | 2 | 990 | |
| None of the trials provided results adjusted for clustering for the outcomes reported in the table. | |||||||||||
| Trial ID | Intervention | Unit of measurement | Iron | Control | No. iron | No. control | Favours |
| For prevention or treatment of anaemia | |||||||
| Iron versus placebo | Geometric mean, parasites/μL | 15,059 | 8225 | 368 slides | 372 slides | Control | |
| Iron versus placebo | Geometric mean, parasites/μL ± SD | 2733 ± 1459 | 2648 ± 1562 | 105 children | 97 children | Control | |
| Iron versus placebo | Geometric mean, RBC/mm3 | 61.2 | 25.7 | 49 children with malarial index | 39 children with malarial index | Controlor similar | |
| Iron plus antimalaria versus antimalaria Iron versus placebo (with single‐dose antimalarial treatment) | Geometric mean, parasites/mm3 | 1705 2569 | 2485 3778 | 129 children 127 children | 127 children 108 children | Iron | |
| Iron versus placebo | Average parasite density class (parasite density classified in ascending order from 1 to 10) | 5.2 | 5.0 | 239 children | 241 children | Control or similar | |
| Iron versus placebo | Geometric mean, infected RBCs/100 WBC | 4.8 | 1.9 | 28 children | 26 children | Control | |
| Iron versus placebo | Geometric mean, parasites/μL (counting against 200 to 500 WBC, assuming 8000 WBC/μL | Age < 30 months 3402 Age > 30 months 2188 | Age < 30 months 3422 Age > 30 months 2046 | 273 children (225 households) | 265 children (225 households) | Similar | |
| For treatment of malaria | |||||||
| Iron daily plus antimalarial versus iron weekly plus antimalarial versus antimalarial | Mean, parasites/μL (counting against 300 WBC, assuming 6000 WBC/μL | 4927 (daily) 2207 (weekly) | 1812 | 77 (daily) 63 (weekly) children | 75 children | Control | |
| Iron plus antimalarial plus folic acid versus antimalarial plus folic acid | Geometric mean, parasites/μL | 5308 | 9302 | 48 children | 47 children | Iron (at baseline groups unbalanced favouring placebo) | |
| Abbreviations: RBC: red blood cell; WBC: white blood cell. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical malaria (grouped by presence of anaemia) Show forest plot | 14 | Risk Ratio (Fixed, 95% CI) | 0.93 [0.87, 1.00] | |
| 1.1 Anaemia | 9 | Risk Ratio (Fixed, 95% CI) | 0.92 [0.84, 1.00] | |
| 1.2 No anaemia | 5 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.86, 1.09] | |
| 2 Clinical malaria (grouped by age) Show forest plot | 14 | Risk Ratio (Fixed, 95% CI) | 0.93 [0.87, 1.00] | |
| 2.1 < 2 years | 5 | Risk Ratio (Fixed, 95% CI) | 0.89 [0.82, 0.97] | |
| 2.2 2 to 5 years | 3 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.75, 1.26] | |
| 2.3 > 5 years | 6 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.91, 1.20] | |
| 3 Clinical malaria (P. falciparum only) Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 0.91 [0.84, 0.99] | |
| 4 Any parasitaemia, end of treatment (by anaemia at baseline) Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 1.11 [1.00, 1.23] | |
| 4.1 Anaemia | 6 | Risk Ratio (Fixed, 95% CI) | 1.07 [0.94, 1.23] | |
| 4.2 No anaemia | 3 | Risk Ratio (Fixed, 95% CI) | 1.17 [0.99, 1.40] | |
| 5 All‐cause mortality Show forest plot | 18 | 7576 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.00, 0.01] |
| 6 Clinical malaria with high‐grade parasitaemia or requiring admission Show forest plot | 5 | Risk Ratio (Fixed, 95% CI) | 0.90 [0.81, 0.98] | |
| 7 Any parasitaemia, end of treatment ( by age) Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 1.11 [1.00, 1.23] | |
| 7.1 < 2 years | 2 | Risk Ratio (Fixed, 95% CI) | 1.28 [0.98, 1.68] | |
| 7.2 2 to 5 years | 4 | Risk Ratio (Fixed, 95% CI) | 1.06 [0.91, 1.23] | |
| 7.3 > 5 years | 3 | Risk Ratio (Fixed, 95% CI) | 1.12 [0.93, 1.34] | |
| 8 Any parasitaemia, end of treatment (P. falciparum only) Show forest plot | 7 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.97, 1.23] | |
| 8.1 Iron versus placebo/no treatment | 5 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.96, 1.24] | |
| 8.2 Iron + antimalarial versus antimalarial | 2 | Risk Ratio (Fixed, 95% CI) | 1.10 [0.76, 1.59] | |
| 9 Any parasitaemia, end of treatment (by allocation concealment) Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 1.11 [1.00, 1.23] | |
| 9.1 Adequate | 4 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.83, 1.15] | |
| 9.2 Unclear | 5 | Risk Ratio (Fixed, 95% CI) | 1.22 [1.06, 1.40] | |
| 10 High‐grade parasitaemia Show forest plot | 5 | Risk Ratio (Fixed, 95% CI) | 1.13 [0.93, 1.37] | |
| 11 Any parasitaemia, end of follow‐up Show forest plot | 5 | 1150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.09, 1.40] |
| 12 Hospitalizations and clinic visits Show forest plot | 7 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.95, 1.04] | |
| 12.1 Hospitalization, iron versus placebo | 5 | Risk Ratio (Fixed, 95% CI) | 0.94 [0.82, 1.08] | |
| 12.2 Hospitalization, iron + antimalarial versus antimalarial | 3 | Risk Ratio (Fixed, 95% CI) | 1.23 [0.97, 1.56] | |
| 12.3 Clinic visit, iron versus placebo | 2 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.88, 1.02] | |
| 12.4 Clinic visit, iron + antimalarial versus antimalarial | 4 | Risk Ratio (Fixed, 95% CI) | 1.03 [0.96, 1.10] | |
| 13 Haemoglobin, end of treatment (by anaemia at baseline) Show forest plot | 16 | 5261 | Mean Difference (IV, Random, 95% CI) | 0.75 [0.48, 1.01] |
| 13.1 Anaemia | 7 | 2481 | Mean Difference (IV, Random, 95% CI) | 0.95 [0.38, 1.51] |
| 13.2 No anaemia | 9 | 2780 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.38, 0.85] |
| 14 Weight, end value Show forest plot | 5 | 1830 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.12, 0.06] |
| 15 Anaemia, end of treatment Show forest plot | 15 | 3784 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.82] |
| 16 Weight, change from baseline Show forest plot | 4 | 486 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.13, 0.49] |
| 17 Diarrhoeal episodes per patient‐month (by zinc administration) Show forest plot | 8 | 23912 | Risk Ratio (Fixed, 95% CI) | 1.15 [1.06, 1.26] |
| 17.1 Without zinc | 7 | 17566 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.87, 1.13] |
| 17.2 With zinc | 3 | 6346 | Risk Ratio (Fixed, 95% CI) | 1.29 [1.15, 1.44] |
| 18 Infections per patient‐month Show forest plot | 8 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 18.1 Febrile episodes | 6 | 15531 | Risk Ratio (Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 18.2 Days with fever | 1 | 110 | Risk Ratio (Fixed, 95% CI) | 8.37 [1.91, 36.58] |
| 18.3 All disease episodes | 1 | 1395 | Risk Ratio (Fixed, 95% CI) | 1.15 [0.91, 1.46] |
| 19 Haemoglobin, change from baseline, end of treatment Show forest plot | 12 | 2462 | Mean Difference (IV, Random, 95% CI) | 0.67 [0.42, 0.92] |
| 20 URTI/pneumonia episodes per patient‐month Show forest plot | 6 | 21767 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.85, 1.15] |
| 21 Height, end value Show forest plot | 5 | 2102 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.08, 0.10] |
| 22 Height, change from baseline Show forest plot | 4 | 486 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.09, 0.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severe malaria (malaria requiring admission) Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Severe malaria (cerebral malaria) Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 3 All‐cause mortality Show forest plot | 5 | 18034 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.00, 0.01] |
| 4 Any hospitalization Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 5 Haemoglobin, end of treatment Show forest plot | 1 | 124 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.51, 1.29] |
| 6 Anaemia, end of treatment Show forest plot | 3 | 633 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.99] |
| 7 Weight, end value Show forest plot | 2 | 1080 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.18, 0.06] |
| 8 Height, end value Show forest plot | 2 | 1082 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.12, 0.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical malaria (grouped by presence of malaria prevention or management) Show forest plot | 16 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.91, 1.03] | |
| 1.1 Services present | 11 | Risk Ratio (Fixed, 95% CI) | 0.91 [0.84, 0.97] | |
| 1.2 Services absent | 5 | Risk Ratio (Fixed, 95% CI) | 1.16 [1.02, 1.31] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical malaria Show forest plot | 3 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.43, 0.67] |
| 2 All‐cause mortality Show forest plot | 3 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.52, 2.11] |
| 3 Hospitalizations and clinic visits Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Hospitalization, iron + antimalarial versus placebo | 2 | 5904 | Risk Ratio (Fixed, 95% CI) | 0.59 [0.48, 0.73] |
| 3.2 Clinic visit, iron + antimalarial versus placebo | 2 | 5904 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.82, 0.95] |
| 4 Haemoglobin at end of treatment Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Anaemia Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Iron + antimalarial versus placebo, end of treatment | 2 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.28, 0.70] |
| 5.2 Iron + antimalarial versus placebo, end of follow‐up | 1 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.26, 0.54] |

