Растворы для пероральной регидратации на основе полимеров в лечении острой водянистой диареи
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomized controlled trial (RCT) Generation of allocation sequence: block randomization Allocation concealment: code broken at the end of the study Blinding: participants, providers, outcome assessors Inclusion of participants in analysis: 81% (maltodextrin group 33/43, 77%; glucose group 36/43, 84%) Duration: 20 months, from January 1987 to August 1988 | |
| Participants | Number of participants: 86 enrolled Inclusion criteria: male; 4 to 36 months; diarrhoea < 3 days; mild to moderate dehydration Exclusion criteria: bloody diarrhoea; antibiotic treatment in the last 3 days; severe malnutrition; presence of systemic illness | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Dhaka, Bangladesh | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization |
| Allocation concealment (selection bias) | Low risk | Code broken at the end of the study |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, providers, and outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Participants, providers, and outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Inadequate inclusion of randomized participants in analysis |
| Selective reporting (reporting bias) | High risk | 81% (maltodextrin group 33/43, 77%; glucose group 36/43, 84%) |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: permuted block design Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: 13 months, from April 1983 to April 1984 | |
| Participants | Number of participants: 72 enrolled Inclusion criteria: age 1 to 8 years; watery diarrhoea < 3 days; presence of moderate to severe dehydration Exclusion criteria: antibiotic treatment before admission; severe malnutrition; presence of systemic illness | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Dhaka, Bangladesh | |
| Notes | Participants given rice ORS were less dehydrated compared to those given glucose ORS, but the difference was not statistically significant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block design |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Rice arm of Alam 1987 | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block design |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Wheat arm of Alam 1987 | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block design |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: random numbers Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: 30 months, from July 1986 to December 1988 | |
| Participants | Number of participants: 182 enrolled Inclusion criteria: age 15 to 60 years; acute watery diarrhoea; presence of dehydration; positive for Vibrio cholerae Exclusion criteria: history of antidiarrhoeal or antimicrobial intake before admission | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Dhaka, Bangladesh | |
| Notes | Analysed separately with or without food intake | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT by random number |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial included over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: permuted blocks of variable length Allocation concealment: sealed, opaque envelopes Blinding: unclear Inclusion of participants in analysis: > 90% Duration: 17 months, from March 2001 to July 2002 | |
| Participants | Number of participants: 101 enrolled Inclusion criteria: age 1 to 48 months; acute watery diarrhoea < 7 days; presence of dehydration but without hypovolaemic shock Exclusion criteria: malnourished, kwashiorkor type; presence of paralytic ileus | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Medellin, Colombia | |
| Notes | Data on total stool output in first 24 hours are skewed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted blocks of variable length |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: randomly assigned using sealed envelopes Allocation concealment: sealed envelopes Blinding: none Inclusion of participants in analysis: > 90% Duration: not specified; only stated that trial was done for 10 consecutive months | |
| Participants | Number of participants: 93 enrolled Inclusion criteria: males; age 3 months to 5 years; watery diarrhoea < 5 days; presence of dehydration; weight for height > 70% of 50th centile of reference standard Exclusion criteria: female; persistent vomiting; bloody diarrhoea; temperature > 39°C; other associated medical illness; intake of antibiotics during illness | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: New Delhi, India | |
| Notes | Participants who were given glucose ORS were more malnourished as compared to the treatment groups, but the difference was not statistically significant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT: randomly assigned using sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Mung bean ORS arm of Bhan 1987 | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT: randomly assigned using sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Pop rice ORS arm of Bhan 1987 | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT: the trial used sealed envelopes to randomly assign participants. |
| Allocation concealment (selection bias) | Unclear risk | The trial used sealed envelopes to randomly assign participants. |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The trial authors included all randomized participants in the analysis. |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% participants in the final analysis. |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: permuted block of random numbers Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: 32 months, from August 1993 to March 1996 | |
| Participants | Number of participants: 123 enrolled Inclusion criteria: adult males; acute watery diarrhoea; presence of severe dehydration; no antibiotic or intravenous fluid intake; no systemic illness Exclusion criteria: presence of systemic illness; use of intravenous fluid before admission | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L and ≤ 270 mOsm/L | |
| Setting | Hospital‐based trial Location: Calcutta, India | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: random‐numbers table Allocation concealment: not reported Blinding: unclear Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 105 enrolled Inclusion criteria: age 4 months to 4 years; males; acute watery diarrhoea; presence of severe dehydration Exclusion criteria: presence of systemic illness; antibiotic intake before admission | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Calcutta, India | |
| Notes | Results of rice ORS and pop rice ORS were combined both for the continuous and dichotomous outcomes, and compared with glucose ORS. These were all reported as rice‐based ORS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: random‐numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: permuted block of random numbers Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: 14 months, from May 1995 to June 1996 | |
| Participants | Number of participants: 50 adults and 20 children enrolled Inclusion criteria: age 3 to 12 years for children, and 18 to 55 years for adults; acute watery diarrhoea; severe dehydration Exclusion criteria: presence of systemic illness; with intake of drug or intravenous fluid before admission | |
| Interventions | Adults
Children
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Calcutta, India | |
| Notes | Children and adults were randomized separately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Adult arm of Dutta 1998 | |
| Participants |
| |
| Interventions | — | |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Children arm of Dutta 1998 | |
| Participants |
| |
| Interventions | — | |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: permuted blocks of random numbers Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: 34 months, from August 1995 to May 1998 | |
| Participants | Number of participants: 58 enrolled Inclusion criteria: age 2 to 10 years; acute watery diarrhoea; presence of severe dehydration; positive for V. cholerae Exclusion criteria: presence of systemic illness; with intake of drug or intravenous fluid before admission | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L and ≤ 270 mOsm/L | |
| Setting | Hospital‐based trial Location: Calcutta, India | |
| Notes | Only the data on glucose ORS ≤ 270 were used as this is the one with same electrolyte composition as the rice ORS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted blocks of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: random permuted blocks Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% of randomized participants included in the final analysis Duration: not stated | |
| Participants | Number of participants: 60 enrolled Inclusion criteria: age 4 months to 4 years; males; acute watery diarrhoea; presence of moderate to severe dehydration; on milk formula intake Exclusion criteria: presence of bloody diarrhoea; severe dehydration; febrile (temperature > 38.5°C); marasmic‐kwashiorkor malnutrition | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Cairo, Egypt | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: random permuted blocks |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: random blocks of fixed length Allocation concealment: serially numbered identical ORS packets Blinding: participants, providers, outcome assessors Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 89 enrolled Inclusion criteria: age 3 to 24 months; acute watery diarrhoea; presence of mild to moderate dehydration; non‐cholera diarrhoea Exclusion criteria: presence of bloody diarrhoea; severe malnutrition; with no or severe dehydration | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Cairo, Egypt | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: random blocks of fixed length |
| Allocation concealment (selection bias) | Low risk | Serially numbered identical ORS packets |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, providers, and outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Participants, providers, and outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: randomized Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: 17 months, from August 1990 to December 1991 | |
| Participants | Number of participants: 471 enrolled Inclusion criteria: age 3 to 35 months; acute watery diarrhoea; presence of mild and moderate dehydration Exclusion criteria: presence of severe dehydration; severe malnutrition; intercurrent illness or chronic disease | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Dhaka, Bangladesh | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: random permuted blocks of variable length Allocation concealment: sealed, serially numbered envelopes Blinding: unclear Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 441 enrolled Inclusion criteria: age 3 to 18 months, acute watery diarrhoea < 7 days; presence of dehydration Exclusion criteria: bloody diarrhoea; severe malnutrition; presence of systemic illness; exclusively or mostly breastfed | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Cairo, Egypt | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: random permuted blocks of variable length |
| Allocation concealment (selection bias) | Low risk | Sealed, serially numbered envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: permuted block randomization Allocation concealment: code was kept Blinding: none Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 100 enrolled Inclusion criteria: age 3 to 18 months; acute watery diarrhoea; presence of moderate dehydration; non‐breastfed Exclusion criteria: presence of systemic illness; presence of moderate to severe malnutrition | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Santiago, Chile | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block randomization |
| Allocation concealment (selection bias) | Low risk | Code was kept |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: block randomization Allocation concealment: code was kept until end of trial Blinding: none Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 48 enrolled Inclusion criteria: age 3 to 24 months; acute watery diarrhoea; presence of moderate dehydration; non‐breastfed Exclusion criteria: presence of systemic illness; moderate to severe malnutrition | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Santiago, Chile | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block randomization |
| Allocation concealment (selection bias) | Low risk | Code was kept |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: computer‐generated randomization Allocation concealment: sealed envelopes Blinding: none Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 113 enrolled Inclusion criteria: adult males 18 to 60 years old; acute watery diarrhoea; presence of severe dehydration; positive for V. cholerae Exclusion criteria: presence of concomitant illness; received antibiotic and ORS before admission | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Dhaka, Bangladesh | |
| Notes | Data for primary outcomes reported as median (range) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: computer generated randomization |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 70 enrolled Inclusion criteria: 9 months to 5 years old; acute watery diarrhoea Exclusion criteria: presence of invasive diarrhoea (white blood cell count > 5 cell/high power field (hpf) or red blood cells > 5 cell/hpf from stool examination), profound shock, alteration of consciousness or convulsion, severe electrolyte imbalance, severe malnutrition or malabsorption syndrome, renal failure, severe systemic infection, rice allergy, acute abdominal conditions | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≤ 270 mOsm/L | |
| Setting | Hospital‐based trial Location: Patumthanee, Thailand | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT but generation of allocation sequence is unclear |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: permuted block randomization Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: 14 months, from March 1989 to April 1990 | |
| Participants | Number of participants: 52 enrolled Inclusion criteria: age < 6 months; acute watery diarrhoea; presence of mild to moderate dehydration; weight for height > 75% of 50th centile Exclusion criteria: presence of bloody diarrhoea; systemic illness; unable to take ORS; intake of antibiotic | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial (diarrhoea training unit) Location: Karachi, Pakistan | |
| Notes | Participants who were given rice ORS were younger compared to those given glucose ORS, but the difference is not statistically significant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: Permuted block randomization |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: randomized Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 63 enrolled Inclusion criteria: age < 6 months; loose stools < 7 days' duration Exclusion criteria: presence of systemic illness; intake of antibiotic/anti‐diarrhoeal before admission; severe dehydration | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≤ 270 mOsm/L | |
| Setting | Hospital‐based trial Location: Kuala Lumpur, Malaysia | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: randomly assigned permuted blocks Allocation concealment: serially numbered sealed envelopes Blinding: none Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 97 enrolled Inclusion criteria: age 1 to 6 months; acute watery diarrhoea < 5 days; presence of mild to moderate dehydration Exclusion criteria: presence of bloody diarrhoea; systemic illness; severe malnutrition; history of diarrhoea in the last 2 weeks | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial (emergency department) Location: Mexico City, Mexico | |
| Notes | Results for primary outcome skewed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: randomly assigned permuted blocks |
| Allocation concealment (selection bias) | Low risk | Serially numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: block randomization Allocation concealment: serially numbered sealed envelopes Blinding: none Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 189 enrolled Inclusion criteria: age 3 to 24 months; males; acute watery diarrhoea; presence dehydration Exclusion criteria: presence of bloody diarrhoea; systemic illness; severe malnutrition | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≤ 270 mOsm/L | |
| Setting | Hospital‐based trial Location: Mexico City, Mexico | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: block randomization |
| Allocation concealment (selection bias) | Low risk | Serially numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants, providers. and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Participants, providers. and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: randomized Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 50 enrolled Inclusion criteria: age 3 to 36 months, acute watery diarrhoea, presence of dehydration Exclusion criteria: none reported | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: New Delhi, India | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT: randomized |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers. and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers. and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: predetermined random numbers Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: 4 months, from December 1982 to March 1983 | |
| Participants | Number of participants: 342 enrolled Inclusion criteria: children aged < 10 years and adults; acute watery diarrhoea; presence of moderate and severe dehydration Exclusion criteria: presence of systemic illness; intake of antibiotics and ORS before admission | |
| Interventions | Adults
Children
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Dhaka, Bangladesh | |
| Notes | Separate analysis for children and adults | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: predetermined random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Adult trial arm of Molla 1985 | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: predetermined random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Children trial arm of Molla 1985 | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: predetermined random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: randomly assigned Allocation concealment: not reported Blinding: unclear Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 93 enrolled Inclusion criteria: children aged < 5 years; acute watery diarrhoea; presence of moderate and severe dehydration; positive for V. cholerae Exclusion criteria: breastfed; those with previous treatment | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Dhaka, Bangladesh | |
| Notes | Data on total stool output in first 24 hours are skewed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT: randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants, providers, and outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Participants, providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: permuted block design Allocation concealment: not reported Blinding: participants and providers not blinded; outcome assessors unclear Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 276 enrolled Inclusion criteria: age 1 to 5 years; acute watery diarrhoea < 48 hours; presence of moderate to severe dehydration; no complications Exclusion criteria: none reported | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Dhaka, Bangladesh | |
| Notes | Study with 6 treatment groups versus 1 control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block design |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and providers not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Rice arm of Molla 1989b | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block design |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and providers not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Sorghum arm of Molla 1989b | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block design |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and providers not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Wheat arm of Molla 1989b | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: permuted block design |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and providers not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: randomly assigned Allocation concealment: not reported Blinding: unclear Inclusion of participants in analysis: > 90% Duration: 9 months, from April to December 1990 | |
| Participants | Number of participants: 96 enrolled Inclusion criteria: males aged < 5 years; acute watery diarrhoea; presence of moderate and severe dehydration Exclusion criteria: presence of bloody diarrhoea; no systemic illness | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Khartoum, Sudan | |
| Notes | Study with 3 treatment arms: 2 polymer‐based ORS versus 1 control group. Data on duration of diarrhoea are skewed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT: randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Rice arm of Mustafa 1995 | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT: randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Sorghum arm of Mustafa 1995 | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT: randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: randomly assigned Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: 88% (rice group, 48/56, 86%; glucose group, 51/57, 89%) Duration: 12 months, from 1 May 1995 to 1 May 1996 | |
| Participants | Number of participants: 113 enrolled Inclusion criteria: age 1 to 12 months; acute watery diarrhoea; presence of mild or moderate dehydration; weight for age > 80% of 50th centile Exclusion criteria: newborn; presence of bloody diarrhoea; systemic illness; intake of antibiotics; severe dehydration; moderate to severe malnutrition | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≤ 270 mOsm/L | |
| Setting | Paediatric clinic Location: Ciuj‐Napoca, Romania | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT: randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers. and outcome assessors not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers. and outcome assessors not blinded |
| Incomplete outcome data (attrition bias) | High risk | The trial included only 88% of the randomized participants in the analysis |
| Selective reporting (reporting bias) | High risk | The trial reported only 88% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: randomly assigned Allocation concealment: sealed envelopes Blinding: none Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 52 participants Inclusion criteria: age 3 months to 5 years; acute watery diarrhoea; presence of moderate to severe dehydration Exclusion criteria: none reported | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Calcutta, India | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT: randomly assigned |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: block randomization Allocation concealment: not reported Blinding: participants and providers partially blinded; outcome assessors unclear Inclusion of participants in analysis: > 90% Duration: 27 months, from May 1994 to July 1996 | |
| Participants | Number of participants: 48 enrolled Inclusion criteria: age 14 to 58 years old; acute watery diarrhoea < 72 hours; positive for V. cholerae Exclusion criteria: presence of systemic illness; intake of antibiotics | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Vellore, India | |
| Notes | Study with 3 treatment arms: 2 polymer‐based ORS versus glucose ORS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: block randomization |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and providers partially blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Amylase arm of Ramakrishna 2000 | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: block randomization |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and providers partially blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | Rice arm of Ramakrishna 2000 | |
| Participants | — | |
| Interventions |
| |
| Outcomes | — | |
| Glucose‐based ORS osmolarity | — | |
| Setting | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: block randomization |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and providers partially blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: table of random numbers Allocation concealment: serially numbered ORS packages Blinding: assessors but not the participants or providers were blinded because of the nature of the study Inclusion of participants in analysis: 100% Duration: not stated | |
| Participants | Number of participants: 50 enrolled Inclusion criteria: males; 18 to 65 years old; acute watery diarrhoea Exclusion criteria: presence of bloody diarrhoea; presence of systemic illness | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≤ 270 mOsm/L | |
| Setting | Hospital‐based trial Location: Vellore, India | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Serially numbered ORS packages |
| Blinding of participants and personnel (performance bias) | High risk | Participants and providers were not blinded because of the nature of the study |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: randomized Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: 27 months, from January 1988 to March 1990 | |
| Participants | Number of participants: 150 enrolled Inclusion criteria: age 6 to 36 months; males; acute watery diarrhoea; mild to moderate dehydration; severe malnutrition < 70% of reference standard Exclusion criteria: presence of bloody diarrhoea; presence of systemic illness; patients in shock | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Antanarivo, Madagascar | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, and outcome assessors not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, and outcome assessors not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: table of random numbers Allocation concealment: code was kept until the end of trial Blinding: participants, providers, outcome assessors Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 120 enrolled Inclusion criteria: age 3 to 36 months; males; acute diarrhoea < 5 days; mild to moderate dehydration Exclusion criteria: presence of bloody diarrhoea; systemic illness; intake of antibiotics; severe dehydration; severe malnutrition; history of diarrhoea in the last 2 weeks | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsml/L | |
| Setting | Hospital‐based trial Location: Manila, Philippines | |
| Notes | Results of total stool output in first 24 hours, total stool output from randomization to discharge, and duration of diarrhoea are skewed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: table of random numbers |
| Allocation concealment (selection bias) | Low risk | Code was kept until the end of trial |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, providers, outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Participants, providers, outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: randomized Allocation concealment: not reported Blinding: none Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 100 enrolled Inclusion criteria: age 7 to 36 months; acute diarrhoea; some dehydration; non‐cholerae; weight > 80% of reference standard Exclusion criteria: presence of bloody diarrhoea; presence of systemic illness; severe dehydration; malnutrition; abdominal distension | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Rohtak, India | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: table of random numbers Allocation concealment: not reported Blinding: participants and providers not blinded; outcome assessors unclear Inclusion of participants in analysis: > 90% Duration: not stated | |
| Participants | Number of participants: 100 enrolled Inclusion criteria: age 4 weeks to 5 years old; acute diarrhoea; mild to moderate dehydration Exclusion criteria: presence of systemic illness; intake of antibiotics/antidiarrhoeals; severe dehydration; previous surgery | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Hospital‐based trial Location: Brisbane, Australia | |
| Notes | Data on duration of diarrhoea are skewed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and providers not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
| Methods | RCT Generation of allocation sequence: table of random numbers Allocation concealment: not specified whether envelope is opaque and sealed Blinding: none Inclusion of participants in analysis: > 90% of randomized participants included in the final analysis Duration: September 1996 to May 1997 | |
| Participants | Number of participants: 167 enrolled Inclusion criteria: age 5 to 15 years; acute diarrhoea; moderate to severe dehydration; purging rate > 2 mL/kg/hour Exclusion criteria: presence of bloody diarrhoea; systemic illness; intake of antibiotics; malnutrition < 65% weight for age | |
| Interventions |
| |
| Outcomes |
| |
| Glucose‐based ORS osmolarity | ≥ 310 mOsm/L | |
| Setting | Rural treatment centre Location: Matlab, Bangladesh | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT: table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not specified whether envelope is opaque and sealed |
| Blinding of participants and personnel (performance bias) | High risk | Participants, providers, outcome assessors were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Participants, providers, outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial included all randomized participants in the analysis |
| Selective reporting (reporting bias) | Low risk | The trial reported over 90% of the included participants in the final analysis |
| Other bias | Unclear risk | We did not detect any other sources of bias |
Abbreviations: RCT: randomized controlled trial; ORS: oral rehydration solution; hpf: high power field.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This was not a clinical trial on oral rehydration solution (ORS). | |
| Guar gum, a soluble fibre and not a polymer, was added to the ORS. | |
| The ORS contained other electrolytes such as zinc, copper, and magnesium, which are not present in the World Health Organization (WHO)‐recommended ORS. | |
| Different electrolyte composition of the 2 groups. | |
| Different electrolyte composition of the 2 groups. | |
| The trial gave the control group oral saline solution, not ORS. | |
| This was not a clinical trial on ORS. | |
| Different electrolyte composition of the 2 groups. | |
| L‐glutamine, an amino acid and not a polymer, was added in the ORS. | |
| Investigated the use of non‐digestible carbohydrates, which are not polymers. | |
| Different electrolyte composition of the 2 groups. | |
| The composition of the home‐made ORS is not known. | |
| The 2 groups had different sources of bicarbonate: polymer‐based ORS used sodium bicarbonate and glucose ORS, trisodium citrate dihydrate. | |
| Polymer‐based ORS has an additional amino acid. | |
| Glucose‐based ORS contained 50 mmol/L sodium. The inclusion criteria of this review specified 90 or 60 to 75 mmol/L of sodium. | |
| This study used a sucrose and not a glucose‐based ORS as a control group. | |
| This was not an efficacy study. The study compared the biochemical analysis of home‐made rice ORS versus glucose‐based ORS. | |
| Different electrolyte composition of the 2 groups. | |
| Unknown electrolyte composition of the wheat‐based ORS. | |
| Treatment group used an amino acid‐based ORS, not a polymer‐based ORS. | |
| This was not a RCT, as the study performed alternate allocation of participants in the 2 interventions. | |
| Different electrolyte composition of the 2 groups. | |
| The primary outcome of interest relevant to the study was not evaluated. | |
| The study had no control group that used glucose‐based ORS. The control group contained L‐histidine, an amino acid. | |
| Polymer was not used in place of glucose. Instead, the amylase‐resistant starch was added to the glucose‐based ORS. | |
| This was not a clinical trial on ORS. | |
| Different electrolyte content of rice ORS and glucose‐based ORS. | |
| Participants with persistent diarrhoea (more than 14 days). | |
| Different electrolyte composition of the 2 groups. | |
| This was not an efficacy but an effectiveness study. | |
| This was a clinical trial on the use of reduced osmolarity ORS in acute diarrhoea. Not a clinical trial on the use of polymer‐based ORS. | |
| Different electrolyte composition of the 2 groups. | |
| The study only observed participants during the rehydration phase. The primary outcome of interest relevant to the study was not evaluated. | |
| This was not a clinical trial on ORS. | |
| Different electrolyte composition of the 2 groups. |
Abbreviations: ORS: oral rehydration solution; RCT: randomized controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total stool output during first 24 hours Show forest plot | 1 | 99 | Mean Difference (IV, Random, 95% CI) | ‐24.60 [‐40.69, ‐8.51] |
| Analysis 1.1  Comparison 1 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≤ 270, Outcome 1 Total stool output during first 24 hours. | ||||
| 1.1 Rice‐based | 1 | 99 | Mean Difference (IV, Random, 95% CI) | ‐24.60 [‐40.69, ‐8.51] |
| 2 Duration of diarrhoea Show forest plot | 5 | 364 | Mean Difference (IV, Random, 95% CI) | ‐8.24 [‐13.17, ‐3.30] |
| Analysis 1.2  Comparison 1 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≤ 270, Outcome 2 Duration of diarrhoea. | ||||
| 2.1 Rice‐based | 5 | 364 | Mean Difference (IV, Random, 95% CI) | ‐8.24 [‐13.17, ‐3.30] |
| 3 Unscheduled use of intravenous fluid Show forest plot | 3 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.43, 1.02] |
| Analysis 1.3  Comparison 1 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≤ 270, Outcome 3 Unscheduled use of intravenous fluid. | ||||
| 3.1 Rice‐based | 3 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.08] |
| 3.2 Amylase‐resistant starch | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.39, 1.46] |
| 4 Vomiting (number of participants) Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.34] |
| Analysis 1.4  Comparison 1 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≤ 270, Outcome 4 Vomiting (number of participants). | ||||
| 4.1 Rice‐based | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.34] |
| 5 Hyponatraemia (number of participants) Show forest plot | 3 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.82] |
| Analysis 1.5  Comparison 1 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≤ 270, Outcome 5 Hyponatraemia (number of participants). | ||||
| 5.1 Rice‐based | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.34, 1.72] |
| 5.2 Amylase‐resistant starch | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total stool output during first 24 hours Show forest plot | 16 | 1483 | Mean Difference (IV, Random, 95% CI) | ‐65.47 [‐83.92, ‐47.03] |
| Analysis 2.1  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 1 Total stool output during first 24 hours. | ||||
| 1.1 Rice‐based | 12 | 1160 | Mean Difference (IV, Random, 95% CI) | ‐61.36 [‐80.61, ‐42.11] |
| 1.2 Wheat‐based | 2 | 129 | Mean Difference (IV, Random, 95% CI) | ‐119.85 [‐124.97, ‐114.73] |
| 1.3 Sorghum‐based | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐128.0 [‐207.66, ‐48.34] |
| 1.4 Maltodextrin‐based | 1 | 117 | Mean Difference (IV, Random, 95% CI) | 27.40 [‐17.58, 72.38] |
| 2 Total stool output during the first 24 hours; rice‐based ORS subgrouped by age group Show forest plot | 12 | 1160 | Mean Difference (IV, Random, 95% CI) | ‐61.36 [‐80.61, ‐42.11] |
| Analysis 2.2  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 2 Total stool output during the first 24 hours; rice‐based ORS subgrouped by age group. | ||||
| 2.1 Paediatric | 10 | 914 | Mean Difference (IV, Random, 95% CI) | ‐59.19 [‐80.87, ‐37.51] |
| 2.2 Adults | 2 | 246 | Mean Difference (IV, Random, 95% CI) | ‐87.98 [‐184.72, 8.76] |
| 3 Total stool output during the first 24 hours; rice‐based ORS subgrouped by pathogen Show forest plot | 11 | 1092 | Mean Difference (IV, Random, 95% CI) | ‐46.03 [‐68.36, ‐23.70] |
| Analysis 2.3  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 3 Total stool output during the first 24 hours; rice‐based ORS subgrouped by pathogen. | ||||
| 3.1 Cholera | 3 | 304 | Mean Difference (IV, Random, 95% CI) | ‐110.49 [‐214.58, ‐6.40] |
| 3.2 Mixed pathogens | 8 | 728 | Mean Difference (IV, Random, 95% CI) | ‐21.88 [‐53.80, 10.04] |
| 3.3 Pathogen not reported | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐81.80 [‐93.75, ‐69.85] |
| 4 Duration of diarrhoea Show forest plot | 16 | 1187 | Mean Difference (IV, Random, 95% CI) | ‐8.47 [‐12.86, ‐4.08] |
| Analysis 2.4  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 4 Duration of diarrhoea. | ||||
| 4.1 Rice‐based | 13 | 957 | Mean Difference (IV, Random, 95% CI) | ‐8.25 [‐13.19, ‐3.30] |
| 4.2 Wheat‐based | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐10.86, ‐9.14] |
| 4.3 Sorghum‐based | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐16.4 [‐33.57, 0.77] |
| 4.4 Maltodextrin‐based | 1 | 116 | Mean Difference (IV, Random, 95% CI) | ‐4.60 [‐17.28, 8.08] |
| 5 Duration of diarrhoea; rice‐based ORS subgrouped by age group Show forest plot | 13 | 904 | Mean Difference (IV, Random, 95% CI) | ‐7.38 [‐12.34, ‐2.43] |
| Analysis 2.5  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 5 Duration of diarrhoea; rice‐based ORS subgrouped by age group. | ||||
| 5.1 Paediatrics | 10 | 733 | Mean Difference (IV, Random, 95% CI) | ‐7.31 [‐12.84, ‐1.77] |
| 5.2 Adults | 3 | 171 | Mean Difference (IV, Random, 95% CI) | ‐5.77 [‐11.47, ‐0.07] |
| 6 Duration of diarrhoea; rice‐based ORS subgrouped by type of pathogen Show forest plot | 13 | 888 | Mean Difference (IV, Random, 95% CI) | ‐7.31 [‐12.47, ‐2.15] |
| Analysis 2.6  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 6 Duration of diarrhoea; rice‐based ORS subgrouped by type of pathogen. | ||||
| 6.1 Cholera | 7 | 453 | Mean Difference (IV, Random, 95% CI) | ‐4.79 [‐7.99, ‐1.59] |
| 6.2 Mixed pathogens | 6 | 435 | Mean Difference (IV, Random, 95% CI) | ‐8.88 [‐14.97, ‐2.78] |
| 7 Unscheduled use of intravenous fluid Show forest plot | 19 | 1877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 0.98] |
| Analysis 2.7  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 7 Unscheduled use of intravenous fluid. | ||||
| 7.1 Rice‐based | 16 | 1698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.60, 1.03] |
| 7.2 Wheat‐based | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.53] |
| 7.3 Sorghum‐based | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Maltodextrin‐based | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.32] |
| 7.5 Mung beans | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.01] |
| 8 Unscheduled use of intravenous fluid, subgrouped by type of pathogen Show forest plot | 19 | 1877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 0.98] |
| Analysis 2.8  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 8 Unscheduled use of intravenous fluid, subgrouped by type of pathogen. | ||||
| 8.1 Cholera | 7 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.34] |
| 8.2 Mixed pathogens | 11 | 928 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.41, 0.96] |
| 8.3 Pathogen not reported | 1 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.59] |
| 9 Vomiting (number of participants) Show forest plot | 10 | 584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.14] |
| Analysis 2.9  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 9 Vomiting (number of participants). | ||||
| 9.1 Rice‐based | 9 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.12] |
| 9.2 Sorghum‐based | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.59, 2.22] |
| 10 Hyponatraemia (number of participants) Show forest plot | 4 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.52, 6.44] |
| Analysis 2.10  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 10 Hyponatraemia (number of participants). | ||||
| 10.1 Rice‐based | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.34, 14.92] |
| 10.2 Maize‐based | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.22] |
| 11 Hypokalaemia (number of participants) Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.74, 2.25] |
| Analysis 2.11  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 11 Hypokalaemia (number of participants). | ||||
| 11.1 Rice‐based | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.74, 2.25] |
| 12 Developed persistent diarrhoea (number of participants) Show forest plot | 2 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.68, 2.41] |
| Analysis 2.12  Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 12 Developed persistent diarrhoea (number of participants). | ||||
| 12.1 Rice‐based | 2 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.68, 2.41] |

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Funnel plot of comparison: 2 Polymer‐based ORS versus glucose‐based ORS, osmolarity ≥ 310, outcome: 2.7 Unscheduled use of intravenous fluid.

Comparison 1 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≤ 270, Outcome 1 Total stool output during first 24 hours.

Comparison 1 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≤ 270, Outcome 2 Duration of diarrhoea.

Comparison 1 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≤ 270, Outcome 3 Unscheduled use of intravenous fluid.
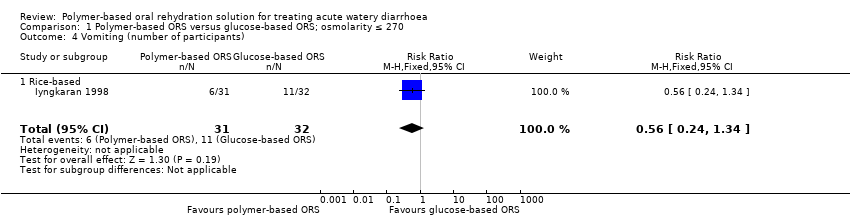
Comparison 1 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≤ 270, Outcome 4 Vomiting (number of participants).

Comparison 1 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≤ 270, Outcome 5 Hyponatraemia (number of participants).

Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 1 Total stool output during first 24 hours.

Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 2 Total stool output during the first 24 hours; rice‐based ORS subgrouped by age group.

Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 3 Total stool output during the first 24 hours; rice‐based ORS subgrouped by pathogen.
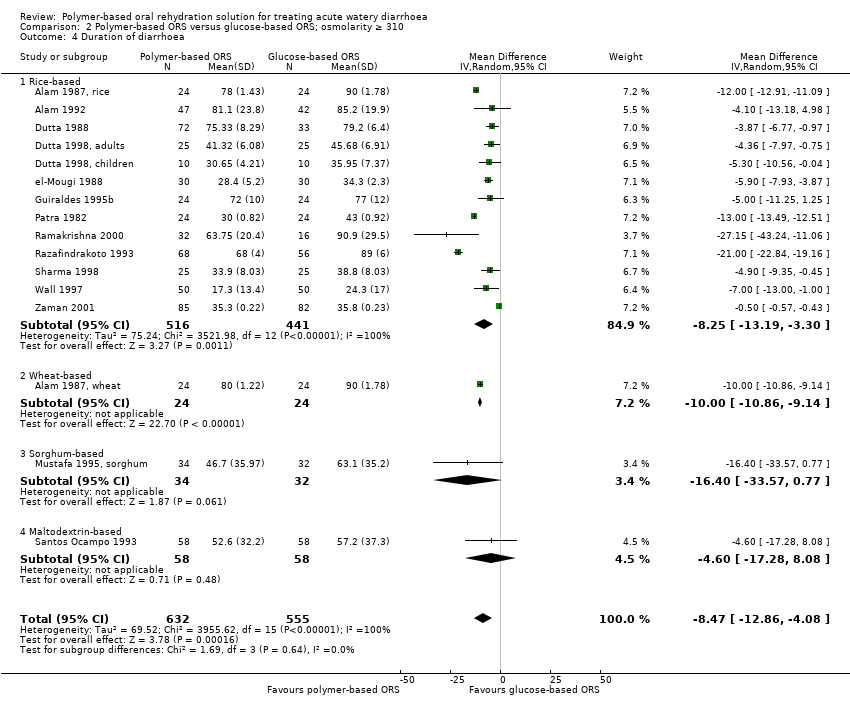
Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 4 Duration of diarrhoea.

Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 5 Duration of diarrhoea; rice‐based ORS subgrouped by age group.

Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 6 Duration of diarrhoea; rice‐based ORS subgrouped by type of pathogen.

Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 7 Unscheduled use of intravenous fluid.

Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 8 Unscheduled use of intravenous fluid, subgrouped by type of pathogen.
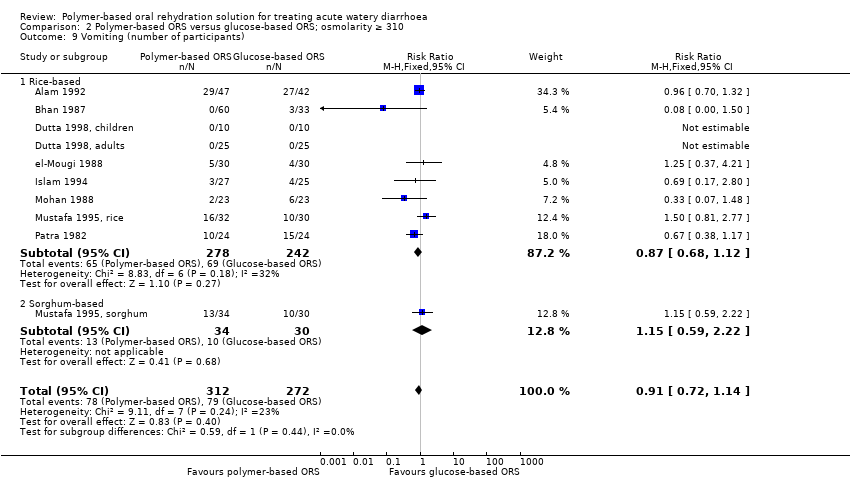
Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 9 Vomiting (number of participants).

Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 10 Hyponatraemia (number of participants).
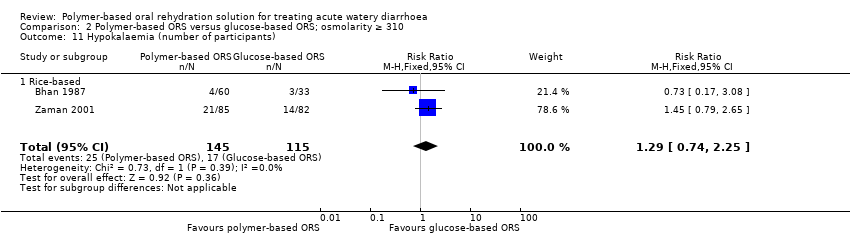
Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 11 Hypokalaemia (number of participants).

Comparison 2 Polymer‐based ORS versus glucose‐based ORS; osmolarity ≥ 310, Outcome 12 Developed persistent diarrhoea (number of participants).
| Polymer‐based ORS compared to glucose‐based ORS ≤ 270 mOsm/L for treating acute watery diarrhoea | |||||
| Patient or population: adults and children with acute watery diarrhoea | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Glucose‐based ORS | Polymer‐based ORS | ||||
| Total stool output during first 24 hours | The mean stool output in the control group was 102 mL/kg | The mean stool output in the intervention group was | — | 99 | ⊕⊕⊝⊝ |
| Duration of diarrhoea | The mean duration in the control groups ranged from | The mean duration of diarrhoea in the intervention groups was | — | 364 | ⊕⊕⊝⊝ |
| Unscheduled use of intravenous fluid | 9 per 100 | 6 per 100 | RR 0.66 | 376 | ⊕⊝⊝⊝ |
| Vomiting | 35 per 100 | 20 per 100 (8 to 47) | RR 0.56 (0.24 to 1.34) | 63 (1 trial) | ⊕⊝⊝⊝8,9,10 very low |
| Hyponatraemia | 23 per 100 | 18 per 100 (8 to 40) | RR 0.88 (0.43 to 1.82) | 145 (3 trials) | ⊕⊝⊝⊝1,7,11 very low |
| The assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 for serious risk of bias: the method of allocation concealment was unclear, and there was no blinding. | |||||
| Polymer‐based ORS compared to glucose‐based ORS ≥ 310 mOsm/L for treating acute watery diarrhoea | |||||
| Patient or population: adults and children with acute watery diarrhoea | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Glucose‐based ORS | Polymer‐based ORS | ||||
| Total stool output during first 24 hours | The mean stool output in the control groups ranged from 81 to 366 mL/kg | The mean stool output in the intervention groups was | — | 1483 | ⊕⊕⊝⊝ |
| Duration of diarrhoea | The mean duration in the control groups ranged from | The mean duration of diarrhoea in the intervention groups was | — | 1187 | ⊕⊕⊝⊝ |
| Unscheduled use of intravenous fluid | 102 per 1000 | 79 per 1000 | RR 0.75 | 1877 | ⊕⊕⊝⊝ |
| Unscheduled use of intravenous fluid in those with mixed pathogen | 101 per 1000 | 63 per 1000 (31 to 60) | RR 0.63 (0.49 to 0.96) | 928 (11 trials) | ⊕⊕⊝⊝ low1,3,4,5 |
| Unscheduled use of intravenous fluid in those with Cholera | 159 per 1000 | 150 per 1000 (105 to 213) | RR 0.94 (0.66 to 1.34) | 535 (7 trials) | ⊕⊕⊝⊝ low1,3,4,5 |
| Vomiting | 313 per 1000 | 250 per 1000 (197 to 313) | RR 0.91 (0.72 to 1.14) | 584 (10 trials) | ⊕⊝⊝⊝ |
| Hyponatraemia | 16 per 1000 | 30 per 1000 (9 to 104) | RR 1.82 (0.52 to 6.44) | 385 (4 trials) | ⊕⊝⊝⊝ |
| Hypokalaemia | 148 per 1000 | 191 per 1000 (110 to 333) | RR 1.29 (0.74 to 2.25) | 260 (2 trials) | ⊕⊕⊝⊝ low1,6,8,9 |
| Development of persistent diarrhoea | 17 per 1000 | 21 per 1000 (6 to 78) | RR 1.28 (0.68 to 2.41) | 885 (2 trials) | ⊕⊝⊝⊝6,10,11 |
| The assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 for serious risk of bias: most trials were at high risk of selection bias due to a lack of allocation concealment, and most were at high risk of detection or reporting bias due to a lack of blinding. | |||||
| Trial | Unit of reporting total stool output during the first 24 hours |
| ORS ≤ 270 | |
| Mean (SD) L | |
| Median (range) | |
| Mean (SD) L | |
| Geometric mean (95% CI) but during maintenance phase | |
| ORS ≥ 310 | |
| Median (range) | |
| Geometric mean (95% CI) | |
| Geometric mean (95% CI) but during maintenance phase | |
| Median (range) | |
| Mean (SD) but reported only in g | |
| Mean (SD) in g | |
| Abbreviations: SD: standard deviation; CI: confidence interval; ORS: oral rehydration solution. | |
| Trial | Unit of reporting duration of diarrhoea |
| ORS ≤270 | |
| Reported only as shorter for rice ORS | |
| Median (range) | |
| ORS ≥310 | |
| Median (hours) | |
| Geometric mean (95% CI) | |
| Mean (SD) but during maintenance phase | |
| Median (range) | |
| Not reported as patients were observed only for 24 hours | |
| Abbreviations: CI: confidence interval; SD: standard deviation; ORS: oral rehydration solution. | |
| Trial | Type of polymer ORS | Age of participants | Cause of diarrhoea | Level of dehydration | Malnutrition | Country | Year of study |
| Rice ORS | 1 month to 5 years | Rotavirus | Mild and moderate | Not stated | Australia | Not stated | |
| Rice ORS | Adult males | Vibrio cholerae | Severe | Not stated | India | 1993 to 1996 | |
| Rice ORS | < 6 months | Mixed pathogen | Mild and moderate | Not stated | Malaysia | Not stated | |
| Rice ORS | 1 to 12 months | Mixed pathogens | Mild and moderate | Included those with weight for age > 80% of 50th percentile | Northern Romania | 1995 to 1996 | |
| Cooked rice | 2 to 10 years | V. cholerae | Severe | Not stated | India | 1995 to 1998 | |
| Premixed rice | 3 to 24 months | Rotavirus (43%) | Mild, moderate, and severe | Excluded those with severe malnutrition | Mexico | 1994 to 1995 | |
| Amylase‐resistant starch | 12 to 65 years | Mixed pathogens | Moderate and severe | Not stated | India | 2003 to 2005 | |
| Rice ORS | 9 months to 5 years old | Rotavirus (60%) | Mild and Moderate | Not stated | Thailand | 2007 to 2008 | |
| Abbreviation: ORS: oral rehydration solution. | |||||||
| Trial | Type of polymer ORS | Age of participants | Cause of diarrhoea | Level of dehydration | Malnutrition | Country | Year of study |
| Maltodextrin | 4 to 36 months | Rotavirus and ETEC | Mild and moderate | Excluded severe | Bangladesh | 1987 to 1988 | |
| Wheat and rice | 1 to 8 years | Not stated | Moderate and severe | Excluded severe malnutrition (< 60% weight for age of 50th centile National Center for Health Statistics (NCHS) | Bangladesh | 1984 | |
| Rice | 15 to 60 years | Cholera (positive for Vibrio cholerae) | Not stated | Not stated | Bangladesh | 1988 | |
| Plantain flour | 1 to 48 months | Mixed pathogens | "Presence", but without shock | Not stated | Colombia | Not stated | |
| Pop Rice and mung bean | 3 months to 5 years | Mixed pathogens | "Presence", level not stated | Included those with weight for height (wt/ht) > 70% of 50th centile of reference standard | India | Not stated | |
| Rice and pop rice | 4 months to 4 years | Not stated | Severe | Not stated | India | Not stated | |
| Rice | 3 to 12 years; 18 to 55 years | Cholera (positive for V. cholerae) | Severe | Not stated | India | 1995 to 1996 | |
| Rice | 4 months to 4 years | Not stated | Moderate and severe | Not stated. Excluded marasmic‐kwashiorkor | Egypt | Not stated | |
| Maltodextrin | 3 to 24 months | Not stated | Moderate | Not stated. Excluded severe malnutrition | Egypt | Not stated | |
| Rice | 3 to 35 months | Mixed pathogens | Mild and Moderate | Excluded severe malnutrition | Bangladesh | 1990 to 1991 | |
| Rice | 3 to 18 months | Not stated | "Presence", level not stated | Excluded severe malnutrition | Egypt | 1990 to 1992 | |
| Rice | 3 to 18 months | Mixed pathogens | Moderate | Excluded moderate to severe malnutrition | Chile | Not stated | |
| Rice | 3 to 24 months | Mixed pathogens | Moderate | Excluded moderate to severe malnutrition | Chile | Not stated | |
| Rice | 18 to 60 years | V. cholerae | Severe | Not stated | Bangladesh | 1995 | |
| Rice | < 6 months | Not stated | Mild and moderate | Included those with wt/age > 75% of 50th centile | Pakistan | 1990 | |
| Precooked rice | 1 to 6 months | Mixed pathogens | Mild and moderate | Excluded severe malnutrition | Mexico | Not stated | |
| Rice | 3 to 36 months | Not stated | "Presence", level not stated | Not stated | India | Not stated | |
| Rice | "adults" | Not stated | Moderate and severe | Not stated | Bangladesh | 1983 | |
| Rice | < 10 years | Not stated | Moderate and severe | Not stated | Bangladesh | 1983 | |
| Rice | < 5 years | V. cholerae | Moderate and severe | Not stated | Bangladesh | Not stated | |
| Rice, maize, sorghum, millet, wheat, potatoes | 1 to 5 years | Not stated | Moderate and severe | Not stated | Bangladesh | Not stated | |
| Rice, sorghum | 6 to 40 months | Not stated | Moderate and severe | Included normal and underweight children | Sudan | 1990 | |
| Rice | 3 months to 5 years | Not stated | Moderate and severe | Not stated | India | Not stated | |
| Rice flour, amylase resistant starch | 14 to 58 years | V. cholerae | Not stated | Not stated | India | 1994 to 1996 | |
| Rice | 6 to 36 months | Not stated | Mild and moderate | Excluded severe malnutrition, < 70% of reference standard | Madagascar | 1990 | |
| Maltodextrin | 3 to 36 months | Mixed pathogens | Mild and moderate | Excluded severe malnutrition | Philippines | Not stated | |
| Rice | 7 to 36 months | Non‐cholerae | Some (mild and moderate) | Included children > 80% as per Indian Academy of Pediatrics (IAP) classification | India | Not stated | |
| Rice | 5 to 15 years | Not stated | Moderate to severe | Not stated. Exclusion criteria: malnutrition < 65% weight for age | Bangladesh | 1997 | |
| Abbreviations: ORS: oral rehydration solution.NCHS: National Center for Health Statistics; IAP: Indian Academy of Pediatrics; wt/ht: weight for height. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total stool output during first 24 hours Show forest plot | 1 | 99 | Mean Difference (IV, Random, 95% CI) | ‐24.60 [‐40.69, ‐8.51] |
| 1.1 Rice‐based | 1 | 99 | Mean Difference (IV, Random, 95% CI) | ‐24.60 [‐40.69, ‐8.51] |
| 2 Duration of diarrhoea Show forest plot | 5 | 364 | Mean Difference (IV, Random, 95% CI) | ‐8.24 [‐13.17, ‐3.30] |
| 2.1 Rice‐based | 5 | 364 | Mean Difference (IV, Random, 95% CI) | ‐8.24 [‐13.17, ‐3.30] |
| 3 Unscheduled use of intravenous fluid Show forest plot | 3 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.43, 1.02] |
| 3.1 Rice‐based | 3 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.08] |
| 3.2 Amylase‐resistant starch | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.39, 1.46] |
| 4 Vomiting (number of participants) Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.34] |
| 4.1 Rice‐based | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.34] |
| 5 Hyponatraemia (number of participants) Show forest plot | 3 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.82] |
| 5.1 Rice‐based | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.34, 1.72] |
| 5.2 Amylase‐resistant starch | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total stool output during first 24 hours Show forest plot | 16 | 1483 | Mean Difference (IV, Random, 95% CI) | ‐65.47 [‐83.92, ‐47.03] |
| 1.1 Rice‐based | 12 | 1160 | Mean Difference (IV, Random, 95% CI) | ‐61.36 [‐80.61, ‐42.11] |
| 1.2 Wheat‐based | 2 | 129 | Mean Difference (IV, Random, 95% CI) | ‐119.85 [‐124.97, ‐114.73] |
| 1.3 Sorghum‐based | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐128.0 [‐207.66, ‐48.34] |
| 1.4 Maltodextrin‐based | 1 | 117 | Mean Difference (IV, Random, 95% CI) | 27.40 [‐17.58, 72.38] |
| 2 Total stool output during the first 24 hours; rice‐based ORS subgrouped by age group Show forest plot | 12 | 1160 | Mean Difference (IV, Random, 95% CI) | ‐61.36 [‐80.61, ‐42.11] |
| 2.1 Paediatric | 10 | 914 | Mean Difference (IV, Random, 95% CI) | ‐59.19 [‐80.87, ‐37.51] |
| 2.2 Adults | 2 | 246 | Mean Difference (IV, Random, 95% CI) | ‐87.98 [‐184.72, 8.76] |
| 3 Total stool output during the first 24 hours; rice‐based ORS subgrouped by pathogen Show forest plot | 11 | 1092 | Mean Difference (IV, Random, 95% CI) | ‐46.03 [‐68.36, ‐23.70] |
| 3.1 Cholera | 3 | 304 | Mean Difference (IV, Random, 95% CI) | ‐110.49 [‐214.58, ‐6.40] |
| 3.2 Mixed pathogens | 8 | 728 | Mean Difference (IV, Random, 95% CI) | ‐21.88 [‐53.80, 10.04] |
| 3.3 Pathogen not reported | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐81.80 [‐93.75, ‐69.85] |
| 4 Duration of diarrhoea Show forest plot | 16 | 1187 | Mean Difference (IV, Random, 95% CI) | ‐8.47 [‐12.86, ‐4.08] |
| 4.1 Rice‐based | 13 | 957 | Mean Difference (IV, Random, 95% CI) | ‐8.25 [‐13.19, ‐3.30] |
| 4.2 Wheat‐based | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐10.86, ‐9.14] |
| 4.3 Sorghum‐based | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐16.4 [‐33.57, 0.77] |
| 4.4 Maltodextrin‐based | 1 | 116 | Mean Difference (IV, Random, 95% CI) | ‐4.60 [‐17.28, 8.08] |
| 5 Duration of diarrhoea; rice‐based ORS subgrouped by age group Show forest plot | 13 | 904 | Mean Difference (IV, Random, 95% CI) | ‐7.38 [‐12.34, ‐2.43] |
| 5.1 Paediatrics | 10 | 733 | Mean Difference (IV, Random, 95% CI) | ‐7.31 [‐12.84, ‐1.77] |
| 5.2 Adults | 3 | 171 | Mean Difference (IV, Random, 95% CI) | ‐5.77 [‐11.47, ‐0.07] |
| 6 Duration of diarrhoea; rice‐based ORS subgrouped by type of pathogen Show forest plot | 13 | 888 | Mean Difference (IV, Random, 95% CI) | ‐7.31 [‐12.47, ‐2.15] |
| 6.1 Cholera | 7 | 453 | Mean Difference (IV, Random, 95% CI) | ‐4.79 [‐7.99, ‐1.59] |
| 6.2 Mixed pathogens | 6 | 435 | Mean Difference (IV, Random, 95% CI) | ‐8.88 [‐14.97, ‐2.78] |
| 7 Unscheduled use of intravenous fluid Show forest plot | 19 | 1877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 0.98] |
| 7.1 Rice‐based | 16 | 1698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.60, 1.03] |
| 7.2 Wheat‐based | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.53] |
| 7.3 Sorghum‐based | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Maltodextrin‐based | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.32] |
| 7.5 Mung beans | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.01] |
| 8 Unscheduled use of intravenous fluid, subgrouped by type of pathogen Show forest plot | 19 | 1877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 0.98] |
| 8.1 Cholera | 7 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.34] |
| 8.2 Mixed pathogens | 11 | 928 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.41, 0.96] |
| 8.3 Pathogen not reported | 1 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.59] |
| 9 Vomiting (number of participants) Show forest plot | 10 | 584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.14] |
| 9.1 Rice‐based | 9 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.12] |
| 9.2 Sorghum‐based | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.59, 2.22] |
| 10 Hyponatraemia (number of participants) Show forest plot | 4 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.52, 6.44] |
| 10.1 Rice‐based | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.34, 14.92] |
| 10.2 Maize‐based | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.22] |
| 11 Hypokalaemia (number of participants) Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.74, 2.25] |
| 11.1 Rice‐based | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.74, 2.25] |
| 12 Developed persistent diarrhoea (number of participants) Show forest plot | 2 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.68, 2.41] |
| 12.1 Rice‐based | 2 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.68, 2.41] |

