Postępowanie przy wgłobieniu jelita u dzieci
Abstract
Background
Intussusception is a common abdominal emergency in children with significant morbidity. Prompt diagnosis and management reduces associated risks and the need for surgical intervention. Despite widespread agreement on the use of contrast enema as opposed to surgery for initial management in most cases, debate persists on the appropriate contrast medium, imaging modality, pharmacological adjuvant, and protocol for delayed repeat enema, and on the best approach for surgical management for intussusception in children.
Objectives
To assess the safety and effectiveness of non‐surgical and surgical approaches in the management of intussusception in children.
Search methods
We searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library; Ovid MEDLINE (1950 to September 2016); Ovid Embase (1974 to September 2016); Science Citation Index Expanded (via Web of Science) (1900 to September 2016); and BIOSIS Previews (1969 to September 2016).
We examined the reference lists of all eligible trials to identify additional studies. To locate unpublished studies, we contacted content experts, searched the World Health Organization International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov (September 2016), and explored proceedings from meetings of the British Association of Paedatric Surgeons (BAPS), the American Soceity of Pediatric Surgery, and the World Congress of Pediatric Surgery.
Selection criteria
We included all randomised controlled trials comparing contrast media, imaging modalities, pharmacological adjuvants, protocols for delayed repeat enema, and/or surgical approaches for the management of intussusception in children. We applied no language, publication date, or publication status restrictions.
Data collection and analysis
Two review authors independently conducted study selection and data extraction and assessed risk of bias using a standardised form. We resolved disagreements by consensus with a third review author when necessary. We reported dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs). We analysed data on an intention‐to‐treat basis and evaluated the overall quality of evidence supporting the outcomes by using GRADE criteria.
Main results
We included six randomised controlled trials (RCTs) with a total of 822 participants. Two trials compared liquid enema reduction plus glucagon versus liquid enema alone. One trial compared liquid enema plus dexamethasone versus liquid enema alone. Another trial compared air enema plus dexamethasone versus air enema alone, and two trials compared use of liquid enema versus air enema.
We identified three ongoing trials.
We judged all included trials to be at risk of bias owing to omissions in reported methods. We judged five of six trials as having high risk of bias in at least one domain. Therefore, the quality of the evidence (GRADE) for outcomes was low. Interventions and data presentation varied greatly across trials; therefore meta‐analysis was not possible for most review outcomes.
Enema plus glucagon versus enema alone
It is uncertain whether use of glucagon improves the rate of successful reduction of intussusception when compared with enema alone (reported in two trials, 218 participants; RR 1.09, 95% CI 0.94 to 1.26;low quality of evidence). No trials in this comparison reported on the number of children with bowel perforation(s) nor on the number of children with recurrent intussusception.
Enema plus dexamethasone versus enema alone
Use of the adjunct, dexamethasone, may be beneficial in reducing intussusception recurrence with liquid or air enema (two trials, 299 participants; RR 0.14, 95% CI 0.03 to 0.60; low quality of evidence). This equates to a number needed to treat for an additional beneficial outcome of 13 (95% CI 8 to 37). It is uncertain whether use of the adjunct, dexamethasone, improves the rate of successful reduction of intussusception when compared with enema alone (reported in two trials, 356 participants; RR 1.01, 95% CI 0.92 to 1.10;low quality of evidence).
Air enema versus liquid enema
Air enema may be more successful than liquid enema for reducing intussusception (two trials, 199 participants; RR 1.28, 95% CI 1.10 to 1.49; low quality of evidence). This equates to a number needed to treat for an additional beneficial outcome of 6 (95% CI 4 to 19). No trials in this comparison reported on the number of children with bowel perforation(s) or on the number of children with recurrent intussusception nor any intraoperative complications, such as bowel perforation, or other adverse effects. Only one trial reported postoperative complications, but owing to the method of reporting used, a quantitative analysis was not possible. We identified no studies that exclusively evaluated surgical interventions for management of intussusception.
Authors' conclusions
This review identified a small number of trials that assessed a variety of interventions. All included trials provided evidence of low quality and were subject to serious concerns about imprecision, high risk of bias, or both. Air enema may be superior to liquid enema for successfully reducing intussusception in children; however, this finding is based on a few studies including small numbers of participants. Dexamethasone as an adjuvant may be more effective in reducing intussusception recurrence rates following air enema or liquid enema, but these results are also based on a few studies of small numbers of participants. This review highlights several points that need to be addressed in future studies, including reducing the risk of bias and including relevant outcomes. Specifically, surgical trials are lacking, and future research is needed to address this evidence gap.
PICO
Streszczenie prostym językiem
Postępowanie przy wgłobieniu jelita u dzieci
Pytanie badawcze
Jakie jest najlepsze postępowanie przy wgłobieniu jelita u dzieci?
Wprowadzenie
Wgłobienie jelita jest ostrym stanem medycznym występującym u dzieci, w którym część jelita wsuwa się w inny fragment jelita. Powoduje to ból, wymioty i zaparcie utrudniające prawidłowy pasaż pokarmu w jelicie. Nieleczone wgłobienie może prowadzić do przedziurawienia jelita i w konsekwencji do przemieszczenia się jego zawartości do jamy brzusznej powodując dalsze powikłania. W rzadkich przypadkach takie zdarzenia mogą prowadzić do śmierci. Szybkie rozpoznanie i odpowiednie leczenie wgłobienia jelita zmniejsza ryzyko z nim związane oraz konieczność operacji.
Przy rozpoznaniu wgłobienia większość lekarzy proponuje zastosowanie lewatywy jako leczenia początkowego. Procedura ta polega na wprowadzeniu substancji (powietrza lub cieczy) do jelita przez odbyt z określonym ciśnieniem, co prowadzi do powrotu wgłobionego jelita do normalnej pozycji.
Toczą się dyskusje na temat rodzaju substancji, która powinna być użyta przy lewatywie, w jaki sposób jest ona uwidoczniana podczas zabiegu, czy powinno się podawać dodatkowe leki, aby zwiększyć skuteczność leczenia, jak sobie radzić z niepowodzeniem terapii oraz jakie jest najlepsze postępowanie chirurgiczne przy wgłobieniu jelita u dzieci.
Charakterystyka badań
Dane naukowe są aktualne do września 2016 roku. Zidentyfikowaliśmy 6 badań z randomizacją obejmujących 822 osoby. W badaniach oceniano postępowanie lecznicze przy wgłobieniu jelita u dzieci oraz różne typy interwencji. Zidentyfikowaliśmy również 3 badania, które jeszcze trwały.
Główne wyniki
Głównym punktem końcowym była liczba dzieci, u których pomyślnie odprowadzono wgłobione jelito. Ponadto punktami końcowymi była liczba dzieci, które miały nawrót wgłobienia oraz obecność działań szkodliwych (niepożądanych efektów) wynikających z interwencji.
Dane z 2 badań sugerują, że zastosowanie przy lewatywie powietrza jest lepsze niż stosowanie płynu w odprowadzeniu wgłobienia. Dane z 2 badań postulują również, że podanie dziecku leków zawierających steroidy, takie jak deksametazon, może obniżyć ryzyko nawrotu wgłobienia, niezależnie od tego czy podczas lewatywy wykorzystano powietrze, czy płyn.
Zidentyfikowaliśmy nieliczne informacje dotyczące śród‐ i pooperacyjnych powikłań i innych działań niepożądanych.
Jakość danych naukowych
We wszystkich 6 badaniach rozważamy ryzyko związane ze stronniczością ze względu na brak szczegółów dotyczących sposobu przeprowadzenia poszczególnych badań. Stwierdziliśmy brak spójności w sposobie definiowania i mierzenia wyników. Wszystkie włączone badania były przedmiotem poważnych obaw dotyczących braku precyzji opartego ze względu na małą liczbę zdarzeń, szerokie przedziały ufności lub duże ryzyko błędu systematycznego (błędu stronniczości wypaczającego wyniki badania w jednym kierunku ‐ przyp. tłum.). Ostatecznie doszliśmy do wniosku, że jakość dostarczonych dowodów była niska, a realne efekty mogą się różnić znacząco od tych stwierdzonych w przedstawionych badaniach.
Konieczne są dalsze badania aby pomóc lekarzom lepiej zrozumieć najbardziej skuteczny sposób postępowania z wgłobieniem jelita u dzieci.
Authors' conclusions
Summary of findings
| Enema plus glucagon versus enema alone summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with liquid enema alone | Risk with liquid enema plus glucagon | ||||
| Successfully reduced intussusception | Study population | RR 1.09 | 218 | Lowa | |
| 739 per 1000 | 805 per 1000 | ||||
| Moderate | |||||
| 649 per 1000 | 707 per 1000 | ||||
| Bowel perforation(s) | Outcome not reported in any studies | ||||
| Recurrent intussusception (follow‐up: 6 months) | Outcome not reported in any studies | ||||
| Bowel resection | Outcome not reported in any studies | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of selection, attrition, and performance bias | |||||
| Enema plus dexamethasone versus enema alone summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with enema alone | Risk with enema plus dexamethasone | ||||
| Successfully reduced intussusception | Study population | RR 1.01 | 356 | Lowa | |
| 157 per 1000 | 159 per 1000 | ||||
| Moderate | |||||
| 771 per 1000 | 779 per 1000 | ||||
| Bowel perforation(s) | Study population | RR 2.63 | 75 | Lowb,c | |
| 125 per 1000 | 329 per 1000 | ||||
| Moderate | |||||
| 125 per 1000 | 48 per 1000 | ||||
| Recurrent intussusception (follow‐up: 6 months) | Study population | RR 0.14 | 299 | Lowa | |
| 69 per 1000 | 10 per 1000 | ||||
| Moderate | |||||
| 370 per 1000 | 52 per 1000 | ||||
| Bowel resection | Study population | RR 0.88 | 75 | Lowb,c | |
| 86 per 1000 | 75 per 1000 | ||||
| Moderate | |||||
| 375 per 1000 | 330 per 1000 | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of attrition and performance bias bDowngraded one level for serious imprecision (95% CI is wide and includes null effect) cDowngraded one level for concerns for high risk of performance bias | |||||
| Air enema versus liquid enema summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with liquid contrast enema | Risk with air enema | ||||
| Successfully reduced intussusception | Study population | RR 1.28 | 199 | Lowa | |
| 677 per 1000 | 867 per 1000 | ||||
| Moderate | |||||
| 712 per 1000 | 911 per 1000 | ||||
| Bowel perforation(s) | Outcome not reported in any studies | ||||
| Recurrence of intussusception (follow‐up: 6 months) | Outcome not reported in any studies | ||||
| Bowel resection | Outcome not reported in any studies | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of selection, performance, and detection bias | |||||
Background
Description of the condition
Intussusception in children is a medical emergency that requires prompt diagnosis and management. It occurs when a segment of bowel (the intussusceptum) invaginates or telescopes into the lumen of another segment of bowel (the intussuscipiens). Both small and large bowel can be involved, but the most common kind of intussusception arises at the junction between the ileum and the caecum and is called ileocaecal intussusception (Loukas 2011). When untreated, intussusception may cause bowel perforation, peritonitis, and shock (Ko 2007). Mortality is rare, with the USA reporting a stable mortality rate of 2.1 per 1 million live births between 1997 and 2007 (Buttery 2011; Davis 2003; Desai 2012; Parashar 2000). Case fatality rates are higher in developing countries, particularly in Africa (9.4%), than in other regions (< 1%). This may be due to delays in treatment, a higher incidence of non‐viable bowel, and lack of adequate medical care (Iwase 2010; Jiang 2013; Meier 1996).
Intussusception is one of the most common abdominal emergencies for children younger than age three (Applegate 2009). Its incidence varies from 0.24 to 2.4 per 1000 live births (Bines 2002; Eng 2012; Fischer 2004; Huppertz 2006; Samad 2014), although evidence suggests that this rate is higher in developing countries (Ugwu 2000). Boys are affected two to eight times more often than girls (Bines 2002), and peak incidence occurs between five and nine months of age (Daneman 2003; Samad 2012). Vaccination against rotavirus has been shown to increase the risk of intussusception. Currently, the monovalent rotavirus vaccine (Rotarix, GlaxoSmithKline, Abbott Park, North Carolina, USA) accounts for an increase of 5.3 cases of intussusception per 100,000 infants receiving the two doses of vaccine (Weintraub 2014). However, each year, rotavirus infection causes gastroenteritis, resulting in 592,000 deaths among children younger than five years of age, with 82% of deaths reported in developing countries (Parashar 2000). Hence, rotavirus vaccination is considered beneficial. A much stronger link between intussusception and an older rotavirus vaccine (RotaShield, Wyeth Laboratories, Marietta, Pennsylvania, USA) (Kramarz 2001; Murphy 2001; Peter 2002; Soares‐Weiser 2004) led to its worldwide withdrawal in 1999.
The cause of intussusception is often idiopathic (Staatz 1998), although any condition that produces pathological lead points (lesions in the bowel) can cause intussusception (Loukas 2011). Of these conditions, lymphoid hypertrophy seems to be the most common (Applegate 2009; Staatz 1998), implicating a viral or bacterial origin for most cases (Nylund 2010; Okimoto 2011; Parashar 2000; Staatz 1998). Other potential causes of pathological lead points include Meckel's diverticulum, duplication cyst, polyp, and lymphoma (Daneman 2003; Daneman 2004). Compared with idiopathic intussusception, intussusception caused by lead points is associated with poorer outcomes and may not be amenable to standard treatment owing to different intussusception locations (Applegate 2009; Loukas 2011).
Diagnosis is challenging because the symptoms of intussusception are wide‐ranging and non‐specific (Beasley 1988); the classic triad of symptoms associated with intussusception comprises vomiting, colicky abdominal pain, and bloody stool, but this triad is noted in less than half of cases (Blanch 2007; Lehnert 2009; Samad 2012). Three studies found that physicians correctly diagnosed intussusception in less than half of initial clinical encounters (Beasley 1988; Blanch 2007; Budwig 1994). Following successful reduction of the intussusception, early recurrence is rare, with rates ranging from 2.7% to 5.4% (Beres 2014; Gray 2014). Diagnostic delay increases the risk of surgical intervention (Lehnert 2009), thus emphasising the importance of prompt and effective management.
Description of the intervention
Non‐surgical management of intussusception in children consists of contrast enema (Applegate 2009; Daneman 2004; Ito 2012; Ko 2007), which involves instilling contrast medium (i.e. air, saline, or barium) into the rectum via a rectal tube to reduce the intussusceptum by increasing intraluminal pressure (Davis 2003). Fluoroscopy or, in the case of liquid contrast media, ultrasonography can guide the procedure and monitor the reduction. Ultrasonography avoids the radiation exposure associated with fluoroscopy and is an effective diagnostic tool (del‐Pozo 1999).
Pharmacological adjuvants can facilitate non‐surgical management, but their efficacy remains controversial. For example, glucagon is an antispasmodic adjuvant used by 10% to 21% of surveyed practitioners (Cachat 2012; Katz 1992; Meyer 1992; Rosenfeld 1999). It provides analgesia (Lappas 1995) and reduces colonic muscle tone (Skucas 1994). However, a recent narrative review suggests that glucagon does not improve the rate of reduction in the non‐surgical management of intussusception (Cachat 2012). Other adjuvants include antibiotics (Ein 2006; Moss 2000; Pepper 2012). One prospective study concluded that the actual risk of bacteraemia following fluoroscopically guided air reduction is low (Somekh 1996), although two other studies reported an elevated risk for intussusception following antibiotic administration (Hviid 2009; Spiro 2003).
Surgical management entails open laparotomy with manual reduction of the intussusception, although case series and retrospective studies show that laparoscopy may be safer and just as effective and may result in shorter hospitalisation (Bailey 2007; Bonnard 2008; Kia 2005; Sklar 2014). Surgical management is generally indicated only if peritonitis, bowel perforation, or shock occurs; when appropriate radiological facilities are unavailable; or when contrast enema fails (American College of Radiology 2007; Daneman 2004). However, because non‐surgical management may be associated with lower morbidity and shorter hospitalisation (Bruce 1987), delayed repeat attempts at contrast enema may be preferred to surgical management (Gonzalez‐Spinola 1999; Navarro 2004; Sandler 1999).
Why it is important to do this review
Intussusception is a common abdominal emergency in children with significant morbidity. Despite widespread agreement on the use of contrast enema for initial management, debate persists on the appropriate contrast medium, imaging modality, pharmacological adjuvant, and protocol to be used for delayed repeat enema (i.e. duration of delay and number of repeated attempts) (Beasley 1998; Daneman 2004; Davis 2003; del‐Pozo 1999; Littlewood 1998; Liu 1986; Schmit 1999). Debate also surrounds the best approach for its surgical management (i.e. open laparotomy vs laparoscopy). Prior reviews of non‐surgical management (Applegate 2009; Cachat 2012; Daneman 2003; Gray 2014; Ko 2007) are narrative in nature. In contrast to narrative reviews, systematic reviews use transparent, objective, and reproducible methods to locate and assess studies (Borenstein 2009).
To the best of our knowledge, this is the first systematic review of non‐surgical and surgical approaches in the management of intussusception in children.
Objectives
To assess the safety and effectiveness of non‐surgical and surgical approaches in the management of intussusception in children.
Methods
Criteria for considering studies for this review
Types of studies
We considered for inclusion all randomised controlled trials (RCTs) comparing contrast media, imaging modalities, pharmacological adjuvants, protocols for delayed repeat enema, surgical approaches, or other curative techniques for the management of intussusception in children. Both quasi‐RCTs and cluster‐RCTs were eligible for inclusion.
Types of participants
Any child, younger than age 18, with a clinical diagnosis of intussusception as determined by study authors. For this review, we considered intussusception at any point in the gastrointestinal tract distal to the pylorus. Although the Brighton Collaboration established a validated and standardised case definition (Bines 2004a; Bines 2004b; Kohl 2008; Tapiainen 2006), this definition has been used only in the context of rotavirus vaccine post licensure monitoring. We have not used the Brighton Collaboration case definition in assessing eligibility of participants for inclusion in this review.
Types of interventions
We included all trials that compared different contrast media, imaging modalities, pharmacological adjuvants, protocols for delayed repeat enemas, and/or surgical approaches.
Types of outcome measures
When possible, we extracted the following primary and secondary outcome measures. We assessed outcomes at the time points reported by study authors unless otherwise noted. As recurring intussusception is associated with various outcomes (Applegate 2009), we conducted our assessment by using the participant as the unit of analysis. If we identified cluster trials, we planned to involve a statistician to ensure that we did not create unit of analysis errors.
Primary outcomes
-
Number of children with successfully reduced intussusception, characterised by radiologically confirmed passage of contrast media into the ileum
-
Number of children with radiologically confirmed or clinically suspected (intraoperative or endoscopic) bowel perforation(s)
-
Number of children with recurrent intussusception (recurrence is defined as occurring after a minimum of 12 hours following a successful reduction)
Secondary outcomes
-
Number of children who underwent a bowel resection (defined by any transection of the lumen, with removal of a segment of bowel)
-
Number of children with a diagnosis of sepsis (defined as life‐threatening organ dysfunction caused by a dysregulated host response to infection (Singer 2016))
-
Radiation exposure (measured in milli‐Sieverts (mSv)) resulting from intervention
-
Length of hospitalisation (measured in days) associated with intervention
-
Intraluminal pressure (measured in mm Hg) used to achieve reduction
-
Number of attempts required to achieve successful reduction
-
Length of operation (measured in minutes) in the case of surgical intervention
-
Number of intraoperative complications (as defined by study authors) in the case of surgical intervention
-
Number of postoperative complications (as defined by study authors) in the case of surgical intervention
-
Number of intraoperative conversions (i.e. open laparotomy required) in the case of laparoscopic intervention
-
Time to resumption of full diet (measured in hours), as defined by study authors
Search methods for identification of studies
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished randomised controlled trials with no language or date of publication restrictions. We searched the following electronic databases for relevant studies.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library (Appendix 1).
-
MEDLINE Ovid (1950 to 16 September 2016) (Appendix 2).
-
Embase Ovid (1974 to 16 September 2016) (Appendix 3).
-
Science Citation Index (via Web of Science) (1900 to 16 September 2016) (Appendix 4).
-
BIOSIS Previews (1969 to 16 September 2016) (Appendix 5).
Our subject search in MEDLINE followed the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE (Lefebvre 2011). Similarly, our subject search in Embase followed sensitivity‐maximising strategy as recommended by Cochrane (Wong 2006).
Searching other resources
Two review authors (SG and RGM) searched the reference lists of all eligible trials and contemporary reviews to identify further trials. To identify unpublished studies, we contacted content experts and searched the World Health Organization International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en) and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (http://www.clinicaltrials.gov) up to 16 September 2016. We also examined proceedings from meetings of the British Association of Paedatric Surgeons (BAPS), the American Society of Pediatric Surgery, and the World Congress of Pediatric Surgery (2009‐2015).
Data collection and analysis
Selection of studies
Two review authors (SG and RGM) screened titles and abstracts for study eligibility using the inclusion criteria of this review. When necessary, we read the full text of the paper or requested additional data from study authors. A third review author (ACW) adjudicated disagreements about study eligibility. We were not blinded to study details during this process.
Data extraction and management
Two review authors (SG and RGM) independently extracted data and assessed risk of bias using a standardised data extraction form. We resolved disagreements by consensus, involving a third review author (ACW) when required. Review authors were not blinded to study details during this process.
We extracted the following data.
-
General information: study author(s), title, source, contact address, country of study, language of publication, year of publication, any author conflicts of interest, study setting (e.g. hospital emergency department, specialised paediatric hospital).
-
Study characteristics and eligibility for review: study design, randomisation method, allocation concealment, recruitment method, duration of trial, study location, length of follow‐up, operator allocation, any obvious concerns of bias.
-
Participants: inclusion and exclusion criteria, age, gender, presence of pathological lead points, anatomical location of intussusception, criteria used to diagnose intussusception, total number of participants, country of origin, number of dropouts or withdrawals and reasons if recorded.
-
Interventions: number of participants for each intervention, a detailed description of interventions and comparison interventions including, when relevant, type, dose, concentration, and duration of application.
-
Outcomes: specific outcomes reported and rates of recurrence, perforation, resection, sepsis, and, when applicable, operative complications and intraoperative conversions.
We entered relevant data into Review Manager software (RevMan version 5.3) (RevMan 2014).
We contacted study authors via email when data were unclear or missing. Study authors provided no new information.
Assessment of risk of bias in included studies
We assessed risk of bias using the 'Risk of bias' tool of the Cochrane Collaboration, as detailed in Section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (see Appendix 6). We assessed the following domains: selection bias (due to inadequate random sequence generation or allocation concealment); performance bias (due to inadequate blinding of participants or personnel); detection bias (due to inadequate blinding of outcome assessment and data analysis); attrition bias (due to incomplete outcome data); reporting bias (due to selective reporting); and other potential biases. We planned to assess publication bias by visually inspecting funnel plots and using Egger's linear regression (minimum 10 studies required). When we assessed studies as having 'unclear risk' in any domain, we attempted to contact study authors for clarification.
We planned to perform sensitivity analyses using risk of bias as one of the sensitivity factors (see Subgroup analysis and investigation of heterogeneity).
Summary of findings
Two review authors (SG and RGM) assessed the overall quality of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Schünemann 2008) and presented these results in 'Summary of findings' tables. We resolved disagreements by consensus, involving a third review author (ACW) when required. In the 'Summary of findings' tables, we included all primary outcomes, as well as secondary outcomes, reported by included studies for the following comparisons: enema plus glucagon versus enema alone; enema plus dexamethasone versus enema alone; and air enema versus liquid enema. We calculated baseline risk using the event rate in the control group.
The GRADE system classifies the quality of evidence as one of four grades.
| Grade | Definition |
| High | Further research is very unlikely to change our confidence in the estimate of effect |
| Moderate | Further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate |
| Low | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| Very low | Any estimate of effect is very uncertain |
We judged the quality of evidence according to the following factors.
| Downgrades the evidence |
| Risk of bias |
| Inconsistency of results |
| Indirectness of evidence |
| Imprecision |
| Publication bias |
We described results while taking into account the quality of evidence and the importance (size) of the effect as follows.
| Important benefit or harm | Less important benefit or harm | No important benefit/harm or null effect | |
| High‐quality evidence | Improves/decreases/prevents/leads to [outcome] | Improves slightly/decreases slightly/leads to slightly fewer (more) [outcome] | Results in little or no difference in [outcome] |
| Moderate‐quality evidence | Probably improves/decreases/prevents/leads to [outcome] | Probably improves slightly/decreases slightly/leads to slightly fewer (more) [outcome] | Probably leads to little or no difference in [outcome] |
| Low‐quality evidence | May improve/decrease/prevent/lead to [outcome] | May slightly improve/slightly decrease/lead to slightly fewer (more) [outcome] | May lead to little or no difference in [outcome] |
| Very low‐quality evidence | It is uncertain whether [intervention] improves, decreases, prevents, leads to [outcome] because the quality of the evidence is very low | ||
| No data or no studies | [Outcome] was not measured or was not reported, or no studies were found that evaluated the impact of [intervention] on [outcome] | ||
Measures of treatment effect
We conducted our analysis according to the guidelines set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We presented results for dichotomous data as summary risk ratios (RRs) with 95% confidence intervals (CIs) and as number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) as appropriate. NNTB and NNTH reflect the numbers of participants who need to be treated for an additional beneficial and harmful outcome, respectively. For continuous data, we planned to present results as mean differences (MDs), if outcomes were measured in the same way between trials. We planned to use standardised mean differences (SMDs) to combine studies that measured the same outcome but used different methods. For rate data, we planned to present results as rate ratios with 95% CIs, and for survival data, we planned to present results as hazard ratios (HRs) with 95% CIs.
Unit of analysis issues
As recurring intussusception is associated with differing outcomes (Applegate 2009), when possible we conducted our assessment with the participant as the unit of analysis. If we had identified cluster‐randomised trials, we had planned to involve a statistician, to ensure that we did not create unit of analysis errors. However, we did not identify any cluster‐randomised trials for this systematic review.
Dealing with missing data
We analysed data for all participants in the group to which they were allocated, regardless of whether they received the allocated intervention. If in the original reports, participants were not analysed in the group to which they were randomised, and if information in the trial report was sufficient, we attempted to restore these participants to the correct group, that is, we conducted intention‐to‐treat analysis when it was possible to do so. When data were missing, we sought clarification from the authors of the trial. When intention‐to‐treat analysis was not possible, we conducted available‐case analysis or per‐protocol analysis.
Assessment of heterogeneity
To deal with clinical heterogeneity, we analysed studies of each intervention and presented them separately. We conducted subgroup analyses when required to deal with variations in the study population age (Subgroup analysis and investigation of heterogeneity).
To deal with statistical heterogeneity, we used the I² statistic and Chi² statistics to measure the proportion of total variation in estimates of treatment effect that was due to heterogeneity beyond chance (Borenstein 2009; Higgins 2003). We judged statistical heterogeneity to be substantial for I² values greater than 50% or Chi² P values less than 0.10. In the case of substantial statistical heterogeneity, we planned to perform prespecified subgroup and sensitivity analyses (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We planned to investigate publication bias by visually assessing funnel plots for the primary outcome if the number of identified and included trials exceeded 10. However, this review included only six trials.
Data synthesis
We analysed data using Review Manager software (RevMan Version 5.3) (RevMan 2014). For trials judged to have similar interventions, populations, and outcomes, we used fixed‐effect model meta‐analysis, as random‐effects models produce poor estimates with small numbers of studies (Higgins 2011), and we considered a P value of 0.05 or less to be statistically significant.
Subgroup analysis and investigation of heterogeneity
We expected the following areas to contribute to study heterogeneity, and we planned to conduct subgroup analyses of relevant models when necessary.
-
Care setting. Different care settings, such as tertiary care centres, are associated with differing outcomes (Bratton 2001; Calder 2001; Rosenfeld 1999).
-
Participants with confirmed presence of pathological lead point. The presence of lead points is associated with differing outcomes (Loukas 2011).
-
Participants with previous intussusceptions. Recurrence is associated with different patient characteristics and outcomes (Applegate 2009).
-
Bowel structures involved in the intussusception. Intussusception involving different bowel structures (e.g. ileocaecal vs ileoileal) are associated with different outcomes (Loukas 2011).
-
Studies with high risk of bias. We identified these studies as having one or more domains judged 'high risk' by the risk of bias tool, as suggested in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
-
Quasi‐randomised trials. These studies by their design fail to implement optimal sequence generation and so are prone to bias (Higgins 2011).
-
Age. Children younger than one year of age or older than three years of age are more likely to possess pathological lead points (Applegate 2009).
-
Geographical region. Regional differences in epidemiology, equipment availability, and operator experience are known (Beasley 1998; Liu 1986; Schmit 1999; Ugwu 2000).
We planned to assess differences among subgroups using analysis of variance (Altman 1996).
However, we could not perform any of the planned subgroup analyses owing to the limited number of included studies.
Sensitivity analysis
We planned to conduct sensitivity analysis when unforeseen or arbitrary decisions were made, as per the guidance provided in Section 9.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
However, we were unable to perform the planned sensitivity analysis owing to the limited number of included studies.
Results
Description of studies
We included six RCTs with a total of 822 participants (Essa 2011; Franken 1983; Hadidi 1999; Lin 2000; Meyer 1993; Mortensson 1984), and we identified three ongoing trials (El Fiky 2016; Mehraeen 2011; Zhang 2015).
Please see Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies.
Results of the search
We outlined in Figure 1 (study flow diagram) the process of identifying RCTs for inclusion in the review.

Study flow diagram for identification of randomised trials exploring management of intussusception in children.
Electronic searches of the Conchrane Central Register of Controlled Trials (n = 59), MEDLINE (n = 307), Embase (n = 140), BIOSIS (n = 94), and the Science Citation Index (n = 158) yielded a total of 758 publications. We identified three additional ongoing trials through trial registries and found no additional trials by searching conference proceedings and reference lists, or by contacting content experts. After exclusion of duplicates and ongoing trials, 435 unique records remained. Of these, we excluded 423 after reviewing titles and abstracts. We examined the full text of the remaining 12 publications and excluded five additional trials ‐ four because they were not RCTs (Diaz‐Aldagalan 2012; Guo 2010; Hsiao 1988; Morrison 2009) and one because we could not obtain a translation of the trial and classification is pending (Zhang 2014a). Two of the seven remaining publications were duplicates; thus we included them as one trial (Lin 2000). In summary, we included six RCTs (Essa 2011; Franken 1983; Hadidi 1999; Lin 2000; Meyer 1993; Mortensson 1984) in the review. These six completed trials were published in five different journals.
Searches for ongoing trials revealed three (El Fiky 2016; Mehraeen 2011; Zhang 2015), for which no results were available.
No disagreements about trial selection among review authors required adjudication.
Included studies
Included trials assessed a wide range of treatments.
-
Essa 2011 compared use of saline enema plus dexamethasone versus saline enema alone in 75 participants.
-
Franken 1983 and Mortensson 1984 compared use of liquid contrast enema plus glucagon versus liquid contrast enema alone in 30 and 188 participants, respectively.
-
Hadidi 1999 and Meyer 1993 compared use of liquid contrast enemas versus air enemas in 147 and 101 participants, respectively.
-
Lin 2000 compared use of air enema plus dexamethasone versus air enema alone in 281 participants.
All six included trials recruited participants referred for management of intussusception in a hospital setting. Two of these studies were performed in the USA (Franken 1983; Meyer 1993). One trial was performed in Taiwan (Lin 2000), two in Egypt (Essa 2011; Hadidi 1999), and one in Sweden (Mortensson 1984).
Only one trial (Essa 2011) reported adverse outcomes for surgical interventions, including number of participants requiring manual reduction and number requiring bowel resection.
This review used subsets of data from two trials (Meyer 1993; Mortensson 1984). Meyer 1993 examined liquid enema versus air enema; however, not all participants who were initially randomised had intussusception at the time of intervention. Therefore, it was necessary to extrapolate data from those with confirmed intussusception. Mortensson 1984 conducted this study in three stages. We have included data only for the first stage, as this was the only stage that met our inclusion criteria (Characteristics of included studies).
Hadidi 1999 conducted a three‐arm trial to assess the efficacy of air, barium, and saline enemas. Review authors combined barium and saline into a liquid enema group for comparison with air enema.
Excluded studies
We excluded five full‐text articles (see Characteristics of excluded studies).
Risk of bias in included studies
Reporting of methods was incomplete for most trials, as shown in Figure 2 and Figure 3. We judged five trials as having at least one domain at high risk of bias, and we judged Franken 1983 as having unclear risk of bias.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
No study reported adequate sequence generation or adequate allocation concealment. Both Hadidi 1999 and Meyer 1993 used random number tables that may have allowed for prediction of intervention by participants. It is unclear whether this was adequate to ensure random sequence generation in Hadidi 1999; in Meyer 1993, the randomisation process was compromised by the need to extrapolate data for participants with confirmed intussusception; and Mortensson 1984 applied inadequate sequence generation by using birth dates to randomly allocate participants. The remaining studies (three studies for sequence generation and five for allocation concealment) used unclear methods. Both Essa 2011 and Franken 1983 referred to the random allocation used but provided no details.
Blinding
Owing to the nature of some treatments, blinding was not possible, for example, liquid versus air enema in Hadidi 1999 and Meyer 1993. Therefore, we reported four trials as having inadequate blinding of participants and personnel. One trial (Franken 1983) successfully blinded participants and personnel through the use of pre‐made identical appearing vials of drug and placebo. The remaining trial used unclear methods.
We judged two trials as having inadequate blinding of outcome assessors because treating personnel recorded the results and thus were unable to be blinded (Hadidi 1999; Meyer 1993). For the remaining four trials, it was unclear whether outcome assessors were study personnel (i.e. paediatricians, radiologists, or surgeons) or independent assessors.
Incomplete outcome data
Four studies adequately addressed incomplete outcome data (no missing data in the trials). Essa 2011 explicitly referred to reporting on all participants included in this trial, and Franken 1983, Hadidi 1999, and Meyer 1993 avoided attrition bias by randomising participants after completing an exclusion process. Two studies reported incomplete outcome data inadequately (Lin 2000; Mortensson 1984), when data were not available for unexplained reasons.
Selective reporting
We judged only two studies (Essa 2011; Franken 1983) as being free of selective reporting bias (all outcomes were reported). We judged the remaining four trials as having unclear risk. None of the trials included a protocol. Lin 2000 did not report how data were collected after participants were discharged, and Meyer 1993 and Mortensson 1984 did not report all expected outcomes.
Other potential sources of bias
No other biases were evident as judged by review authors (e.g. pharmaceutical funding).
We attempted to contact study authors to clarify all areas of unclear risk, but we received no replies and acquired no new information.
We could not assess publication bias as planned because of the small number of included studies.
Effects of interventions
See: Summary of findings for the main comparison Enema plus glucagon versus enema alone; Summary of findings 2 Enema plus dexamethasone versus enema alone; Summary of findings 3 Air enema versus liquid enema
Interventions and outcomes reported across trials varied greatly; therefore, meta‐analysis was not possible for many outcomes.
Enema plus glucagon versus enema alone
1. Primary outcomes
1.1 Number of children with successfully reduced intussusception
It is uncertain whether use of liquid enema plus glucagon improved the rate of successful reduction of intussusception when compared with enema alone because the quality of the evidence is low (reported in two trials, 218 participants; RR 1.09, 95% CI 0.94 to 1.26; I² = 0%; Analysis 1.1).
1.2 Number of children with bowel perforation or perforations
This outcome was not reported for this comparison.
1.3 Number of children with recurrent intussusception
This outcome was not reported for this comparison.
2. Secondary outcomes
2.1 Number of children who undergo a bowel resection
This outcome was not reported for this comparison.
2.2 Number of children with a diagnosis of sepsis
This outcome was not reported in any trial.
2.3 Radiation exposure from intervention
This outcome was not reported in any trial.
2.4 Length of hospitalisation
This outcome was not reported in any trial.
2.5 Intraluminal pressure
This outcome was not reported in any trial.
2.6 Number of attempts required to achieve successful reduction
This outcome was not reported in any trial.
2.7 Length of operation, in the case of surgical intervention
This outcome was not reported in any trial.
2.8 Number of intraoperative complications
This outcome was not reported in any trial.
2.9 Number of postoperative complications
This outcome was not reported for this comparison.
2.10 Number of intraoperative conversions
This outcome was not reported in any trial.
2.11 Time to resumption of full diet
This outcome was not reported in any trial.
Enema plus dexamethasone versus enema alone
1. Primary outcomes
1.1 Number of children with successfully reduced intussusception
It is uncertain whether use of liquid enema plus dexamethasone improved the rate of successful reduction of intussusception when compared with enema alone because the quality of the evidence is low (reported in two trials, 356 participants; RR 1.01, 95% CI 0.92 to 1.10; I² = 0%; Analysis 2.1).
1.2 Number of children with bowel perforation or perforations
It is uncertain whether use of enema plus dexamethasone reduced the number of participants with bowel perforation or perforations because the quality of the evidence is low (reported in one trial, 75 participants; RR 2.63, 95% CI 0.11 to 62.66; Analysis 2.2).
1.3 Number of children with recurrent intussusception
Treatment with enema plus dexamethasone compared with enema alone may reduce the recurrence rate of intussusception (reported in two trials, 299 participants; RR 0.14, 95% CI 0.03 to 0.60; I² = 0%; Analysis 2.3). This equates to an NNTB of 13 (95% CI 8 to 37).
2. Secondary outcomes
2.1 Number of children who undergo a bowel resection
It is uncertain whether use of liquid enema plus dexamethasone reduced the number of participants who underwent bowel resection (an unwanted complication) (reported in one trial, 75 participants; RR 0.88, 95% CI 0.19 to 4.06; Analysis 2.4).
2.2 Number of children with a diagnosis of sepsis
This outcome was not reported for this comparison.
2.3 Radiation exposure from intervention
This outcome was not reported in any trial.
2.4 Length of hospitalisation
This outcome was not reported in any trial.
2.5 Intraluminal pressure
This outcome was not reported in any trial.
2.6 Number of attempts required to achieve successful reduction
This outcome was not reported in any trial.
2.7 Length of operation, in the case of surgical intervention
This outcome was not reported in any trial.
2.8 Number of intraoperative complications
This outcome was not reported in any trial.
2.9 Number of postoperative complications
Only one trial reported on postoperative complications (Essa 2011) when comparing use of enema plus dexamethasone versus enema alone. We did not perform a quantitative analysis of this outcome owing to poor reporting and high risk of bias. A small sample of 15 children underwent surgical intervention ‐ nine underwent manual reduction and the remaining six had a bowel resection. However, data on postoperative complications for the nine children undergoing manual reduction were not available. We contacted the study authors for clarification but received no response.
2.10 Number of intraoperative conversions
This outcome was not reported in any trial.
2.11 Time to resumption of full diet
This outcome was not reported in any trial.
Air enema versus liquid enema
1. Primary outcomes
1.1 Number of children with successfully reduced intussusception
Air enema may be superior to liquid enema for successfully reducing intussusception in children (reported in two trials, 199 participants; RR 1.28, 95% CI 1.10 to 1.49; I² = 0%; Analysis 3.1). This equates to an NNTB of 6 (95% CI 4 to 17).
1.2 Number of children with bowel perforation or perforations
This outcome was not reported for this comparison.
1.3 Number of children with recurrent intussusception
This outcome was not reported for this comparison.
2. Secondary outcomes
2.1 Number of children who undergo a bowel resection
This outcome was not reported for this comparison.
2.2 Number of children with a diagnosis of sepsis
This outcome was not reported in any trial.
2.3 Radiation exposure from intervention
This outcome was not reported in any trial.
2.4 Length of hospitalisation
This outcome was not reported in any trial.
2.5 Intraluminal pressure
This outcome was not reported in any trial.
2.6 Number of attempts required to achieve successful reduction
This outcome was not reported in any trial.
2.7 Length of operation, in the case of surgical intervention
This outcome was not reported in any trial.
2.8 Number of intraoperative complications
This outcome was not reported in any trial.
2.9 Number of postoperative complications
This outcome was not reported for this comparison.
2.10 Number of intraoperative conversions
This outcome was not reported in any trial.
2.11 Time to resumption of full diet
This outcome was not reported in any trial.
GRADE analysis indicated that the quality of evidence supporting all reported outcomes was low (see summary of findings Table for the main comparison, summary of findings Table 2, and summary of findings Table 3).
Although none of the included trials reported Outcome 2.3, four trials made use of fluoroscopy (Franken 1983; Hadidi 1999; Meyer 1993; Mortensson 1984), and one trial made use of ultrasound guidance alone (Essa 2011). One trial did not stipulate whether fluoroscopy or ultrasound guidance was used (Lin 2000).
Discussion
Summary of main results
We identified six completed trials of 822 participants in which all children had presented for management of intussusception. Investigators used a wide range of treatments, and this prevented meta‐analysis for most of our outcomes. In particular, many review outcomes related to adverse effects (e.g. number of intraoperative complications) were not reported.
We could make few direct comparisons of interventions. However, air enema may be superior to liquid enema for successfully reducing intussusception in children. Use of dexamethasone as an adjunct may reduce the rate of recurrence of intussusception. No other results were statistically significant. See summary of findings tables (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3). Of note, we downgraded many of the recommendations provided in these tables owing to the small numbers of included trials and the small participant numbers.
It is important to note that surgical intervention was not the primary study question for any of the included studies. Lack of trials on surgical management might reflect the nature of treatment of children with intussusception, and might suggest that cases are managed largely by non‐surgical means, although this suggestion does not seem to be based on trial evidence.
Overall completeness and applicability of evidence
The internal validity of the design, conduct, and analysis of included studies was difficult to assess because important methodological details were omitted from the study reports. No single study adequately reported all domains of the risk of bias assessment (Figure 2). We judged most trials as having high risk of bias in at least one domain, and omissions in methods were evident in all included studies. Selection bias was generally addressed adequately.Although detection and performance biases are difficult to mitigate for researchers in this field, it may be possible to overcome such biases, for example, Franken 1983 used identical appearing vials for injection in both intervention and control groups. Reporting bias was also difficult to address, although with adequate reporting of protocols and reporting of all expected outcomes, as in Essa 2011 and Franken 1983, this may be mitigated. Although postoperative complications were reported (Outcome 2.9), Essa 2011 presented data in such a way that analysis was not possible. Data for one subgroup of children, specifically those undergoing manual reduction, were not available. We attempted to contact study authors but received no response. Thus data were provided by only one study, and for only a subgroup of children receiving surgical intervention, and data were not sufficient to permit an analysis of this outcome. Again, trials infrequently reported data related to adverse events and harms. These situations might reflect missing data, which may have implications for analysis.
Included trials largely assessed participants of varied ethnic and cultural backgrounds from single centres; this fact may influence the comparability of results between studies. However, given the small quantity of evidence and our inability to perform a meta‐analysis, we could not assess the implications of population differences for applicability of the evidence.
Again we wish to highlight the lack of evidence on surgical interventions and on different imaging modalities and protocols used for delayed repeat enemas.
Quality of the evidence
We have summarised the quality of evidence for each outcome in summary of findings tables (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3), which present evidence of low quality for all outcomes examined. We obtained only data for the outcomes 'liquid enema plus glucagon versus liquid enema alone' and 'air enema versus liquid enema' from two trials each, and data for all other outcomes from single trials only, most with small sample sizes. In summary of findings Table for the main comparison, summary of findings Table 2, and summary of findings Table 3, we downgraded quality of trial evidence for serious to very serious concerns of imprecision or wide confidence intervals, or because trials were subject to serious to very serious concerns of high risk of bias. This limits the strength of our conclusions and our ability to investigate both clinical and statistical heterogeneity. The limited number of included studies and the heterogeneity between them precluded performance of sensitivity and subgroup analyses.
As all examined outcomes were subject to a GRADE assessment of low quality, the true effect of outcomes measured may be substantially different from the estimates; therefore, these estimates can be accepted only with limited confidence.
Potential biases in the review process
We undertook an extensive literature search to examine different aspects of surgical and non‐surgical management of intussusception in children, and we sought data from each identified study. In particular, we attempted to contact study authors to gain further information, and we identified ongoing trials. Two independent review authors undertook searching and data extraction and analysis, and a third review author provided arbitration. However, we could not contact study authors to obtain the data that we required. Individuals who apply the results of this review need to acknowledge the limitations of available data derived from few trials.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the only systematic review of RCTs related to this topic, including unpublished data and ongoing trials. However, several narrative reviews have included comparative studies and RCTs (Applegate 2009; Cachat 2012; Daneman 2003; Ko 2007; Sadigh 2015). Applegate 2009 included comparative trials as well as RCTs to examine the role of ultrasonography, air versus liquid enema for reduction, and risk of bowel perforation in children with intussusception. This review concurred that air enema was superior to liquid enema for successful reduction of intussusception in children ‐ a fact that review authors attribute to speed, cost of the procedure, and safety. Daneman 2003 similarly included comparative studies and RCTs, highlighting in their review the ongoing debate regarding fluoroscopy versus ultrasound‐guided enema reduction, suggesting that greater accuracy can be afforded with ultrasound‐guided reduction. Ko 2007 also examined the role of fluoroscopy versus ultrasound‐guided enema reduction by examining both comparative studies and RCTs; these review authors concluded that ultrasound‐guided reduction is superior to fluoroscopy owing to its greater accuracy, lack of ionising radiation, lower costs, and no need for sedation. The authors of the current review could not perform the comparison offered in both Daneman 2003 and Ko 2007 but agree with the findings of Ko 2007, which suggest that lack of standardisation among single studies makes objective comparison difficult. Cachat 2012 performed a meta‐analysis of studies examining children with radiologically confirmed intussusception, including RCTs and retrospective comparative studies, to compare rates of recurrence. Although Cachat found that dexamethasone was beneficial in reducing rates of recurrence of intussusception among children, review findings suggest that risk of recurrence of intussusception is low, and that regardless of the technique used for successful reduction, it is safe to discharge a patient after performing successful reduction. Sadigh 2015 compared the efficacy of air versus liquid enema for reduction of intussusception in children and found that air enema was superior to liquid enema. These results are similar to those of the current review. Applegate 2009, Cachat 2012, Daneman 2003, Ko 2007, and Sadigh 2015 included no relevant randomised trial that was not included in our review.

Study flow diagram for identification of randomised trials exploring management of intussusception in children.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
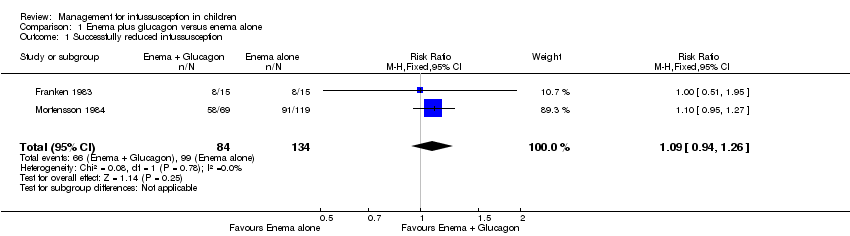
Comparison 1 Enema plus glucagon versus enema alone, Outcome 1 Successfully reduced intussusception.
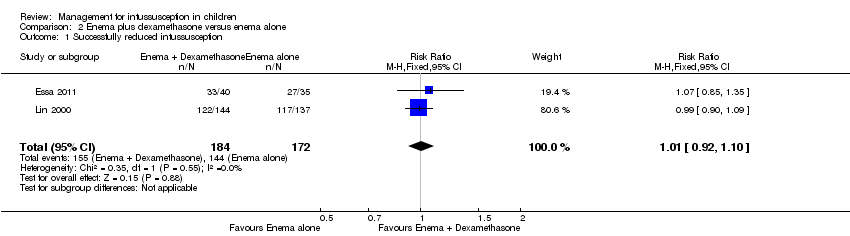
Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 1 Successfully reduced intussusception.

Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 2 Bowel perforation(s).

Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 3 Recurrent intussusception.

Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 4 Bowel resection.
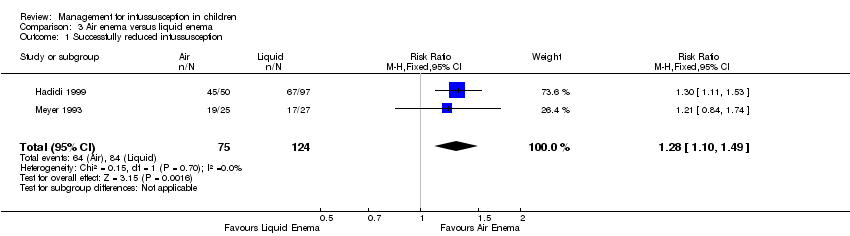
Comparison 3 Air enema versus liquid enema, Outcome 1 Successfully reduced intussusception.
| Enema plus glucagon versus enema alone summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with liquid enema alone | Risk with liquid enema plus glucagon | ||||
| Successfully reduced intussusception | Study population | RR 1.09 | 218 | Lowa | |
| 739 per 1000 | 805 per 1000 | ||||
| Moderate | |||||
| 649 per 1000 | 707 per 1000 | ||||
| Bowel perforation(s) | Outcome not reported in any studies | ||||
| Recurrent intussusception (follow‐up: 6 months) | Outcome not reported in any studies | ||||
| Bowel resection | Outcome not reported in any studies | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of selection, attrition, and performance bias | |||||
| Enema plus dexamethasone versus enema alone summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with enema alone | Risk with enema plus dexamethasone | ||||
| Successfully reduced intussusception | Study population | RR 1.01 | 356 | Lowa | |
| 157 per 1000 | 159 per 1000 | ||||
| Moderate | |||||
| 771 per 1000 | 779 per 1000 | ||||
| Bowel perforation(s) | Study population | RR 2.63 | 75 | Lowb,c | |
| 125 per 1000 | 329 per 1000 | ||||
| Moderate | |||||
| 125 per 1000 | 48 per 1000 | ||||
| Recurrent intussusception (follow‐up: 6 months) | Study population | RR 0.14 | 299 | Lowa | |
| 69 per 1000 | 10 per 1000 | ||||
| Moderate | |||||
| 370 per 1000 | 52 per 1000 | ||||
| Bowel resection | Study population | RR 0.88 | 75 | Lowb,c | |
| 86 per 1000 | 75 per 1000 | ||||
| Moderate | |||||
| 375 per 1000 | 330 per 1000 | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of attrition and performance bias bDowngraded one level for serious imprecision (95% CI is wide and includes null effect) cDowngraded one level for concerns for high risk of performance bias | |||||
| Air enema versus liquid enema summary of findings table | |||||
| Patient or population: children with intussusception | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with liquid contrast enema | Risk with air enema | ||||
| Successfully reduced intussusception | Study population | RR 1.28 | 199 | Lowa | |
| 677 per 1000 | 867 per 1000 | ||||
| Moderate | |||||
| 712 per 1000 | 911 per 1000 | ||||
| Bowel perforation(s) | Outcome not reported in any studies | ||||
| Recurrence of intussusception (follow‐up: 6 months) | Outcome not reported in any studies | ||||
| Bowel resection | Outcome not reported in any studies | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels for serious concerns for high risk of selection, performance, and detection bias | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.10] |
| 2 Bowel perforation(s) Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [0.11, 62.66] |
| 3 Recurrent intussusception Show forest plot | 2 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.60] |
| 4 Bowel resection Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.19, 4.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Successfully reduced intussusception Show forest plot | 2 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.10, 1.49] |

