Anticoagulación para pacientes con cáncer y catéteres venosos centrales
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomized controlled trial | |
| Participants | 121 participants with solid or hematologic (or both) cancers Mean age: 56 years for warfarin; 60.6 years for no warfarin Minimum life expectancy: 3 months | |
| Interventions | Intervention: warfarin 1 mg/day; 3 days prior to CVC placement and continued for 90 days | |
| Outcomes | Follow‐up: 90 days
Screening test for CRT: venography | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Assignment to receive or not receive the drug was made according to a previously established yes or no code." |
| Allocation concealment (selection bias) | Low risk | Communication with author: "yes or no code held in sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | No placebo used Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The radiologists who interpreted the venograms were blinded to the patients' randomization status." Comment: definitely blinded |
| Incomplete outcome data addressed? | Low risk | Comment: judgment based on comparison between MPD rate (VKA: 0%; no VKA: 5/61 = 8.2%) and event rate (mortality: VKA: 12/60 = 20%; no VKA: 14/56 = 25%) |
| Free of selective reporting? | Unclear risk | Study not registered. No published protocol. No complete list of outcomes provided in the methods section. Deaths mentioned to be equal between the 2 groups but not reported numerically. |
| Free of other bias? | Low risk | Study not stopped early for benefit |
| Methods | Randomized double‐blind placebo‐controlled study | |
| Participants | 255 participants from 3 centers with biopsy‐confirmed cancer and with an indwelling CVC for ≥ 7 days. 152 men and 103 women, median age 52 years 166 participants had solid tumors and 89 participants had leukemia or the CVC was inserted prior to high‐dose therapy and transplant. There were 138 Hickman type, 67 PICC‐type, 46 Portacath‐type and 4 Passport‐type CVCs. No differences in the participant or CVC characteristics between the 2 treatment groups. | |
| Interventions | Intervention: warfarin 1 mg/day orally started within 72 hours of CVC insertion Control: identical placebo | |
| Outcomes | Follow‐up: 90 days
Diagnosis of symptomatic CVC‐associated thrombosis: compression ultrasonography, venogram | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients at each center were randomized centrally in permuted blocks of up to six patients." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients at each center were randomized centrally in permuted blocks of up to six patients." |
| Blinding of participants and personnel (performance bias) | Low risk | Study design noted as double‐blind placebo controlled. Comment: definitely blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All suspected primary outcome events, bleeding episodes, and deaths were adjudicated centrally by two individuals blinded to the treatment assignments." Comment: definitely blinded |
| Incomplete outcome data addressed? | Low risk | Study reported complete follow‐up |
| Free of selective reporting? | Low risk | Study not registered. No published protocol. Outcomes listed in the methods section were reported on in the results sections. |
| Free of other bias? | Low risk | Study not stopped early for benefit |
| Methods | Randomized controlled trial | |
| Participants | 450 participants aged ≥ 18 years with solid or hematologic (or both) cancers Mean age: 55.5 years for acenocumarine group; 55.3 years for dalteparin group; 55.1 years for no anticoagulant group Expected life expectancy ≥ 3 months Discontinued treatment: "The primary efficacy population was 77.3% (348 of 450 patients): 76% in group A [dalteparin], 80% in group D [acenocumarine] and 76% in group NT [no treatment]." | |
| Interventions | Intervention 1: dalteparin prophylactic dose; started 2 hours before and daily for 8 days after CVC insertion Intervention 2: acenocumarine 1 mg/day for 3 days before and 8 days after CVC insertion Intervention 3: no anticoagulant treatment | |
| Outcomes | Follow‐up: 2 months for 3 visits after initial 30 days, therefore, 7 months total
Screening test for CRT: venography on days 8 and 30 after insertion and then every 2 months for three times or earlier if there was a clinical suspicion Screening test for PE: high‐probability V/Q lung scanning or by multislice CT | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was carried out ‡4 days before CVC insertion. Permuted blocks of four were used for treatment allocation." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | No placebo used Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Venograms were evaluated independently by two radiologists who were unaware of the patients' clinical status and the assigned treatment, immediately after venography had been carried out." Comment: definitely blinded |
| Incomplete outcome data addressed? | High risk | Comment: judgment based on comparison between MPD rate (acenocumarine: 24%; dalteparin: 20%; no anticoagulant: 24%) and event rate (CVC‐related DVT: acenocumarine: 21.9%, dalteparin: 40%; no anticoagulant: 52.6%) |
| Free of selective reporting? | Low risk | Study not registered. No published protocol |
| Free of other bias? | Low risk | Study not stopped early for benefit |
| Methods | Randomized controlled trial | |
| Participants | 88 participants with hematologic cancers Mean age: 43 years | |
| Interventions | Intervention: warfarin 1 mg/day with CVC placement | |
| Outcomes | Follow‐up: 90 days
Diagnostic test for CRT: venography | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communication with the author: "Randomisation was from computer generated sequentially numbered sealed opaque envelope." |
| Allocation concealment (selection bias) | Low risk | Communication with the author: "Randomisation was from computer generated sequentially numbered sealed opaque envelope." |
| Blinding of participants and personnel (performance bias) | High risk | No placebo used Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo used Comment: probably not blinded; knowledge of the assigned intervention may not have impacted the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data addressed? | Low risk | Complete follow‐up |
| Free of selective reporting? | Low risk | Study not registered. No published protocol. All outcomes listed in the methods section reported on Comment: probably yes |
| Free of other bias? | Low risk | Study not stopped early for benefit |
| Methods | Multinational, double‐blind randomized controlled trial (phase III) | |
| Participants | 425 participants with solid or hematologic (or both) cancers Mean age 56.3 years; minimum age 18 years Minimum life expectancy: 16 weeks | |
| Interventions | Intervention: dalteparin prophylactic dose; started 5‐7 days prior to CVC placement; once daily for 16 weeks Control: placebo Cointervention: chemotherapy for ≥ 12 weeks, administered to both groups | |
| Outcomes | Follow‐up: 16 weeks
Diagnostic test for CRT: screening by venography or Doppler or CT scan (16 weeks); diagnosis by venography or Doppler or CT scan Diagnostic test for PE: V/Q scan or spiral CT | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients with documented cancer were randomly assigned to receive dalteparin or placebo." Comment: probably generated sequence randomly |
| Allocation concealment (selection bias) | Low risk | Quote: "...centralized interactive voice processing system" |
| Blinding of participants and personnel (performance bias) | Low risk | Trial described as double‐blind, placebo‐controlled study Comment: definitely blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "a central adjudication committee reviewed all tests (venograms, CTs, US [ultrasound], etc.) obtained for a suspected CRC and made final judgement, blinded to patient treatment assignment." Comment: definitely blinded |
| Incomplete outcome data addressed? | High risk | Comment: judgment based on comparison between MPD rate (dalteparin: 26/285 = 9.1%; placebo: 12/140 = 8.5%) and event rate (CRC: dalteparin: 7%; placebo: 3.4%) |
| Free of selective reporting? | Low risk | Study not registered. No published protocol. All outcomes listed in the methods section were reported on |
| Free of other bias? | Low risk | Study not stopped early for benefit |
| Methods | Open‐label, randomized controlled trial (phase III) | |
| Participants | 420 participants with histologic evidence of solid invasive cancer, locally advanced or metastatic status Subclavian CVC inserted for < 7 days Receiving first‐line chemotherapy Median age: 61 years, 75% males, 46% had a metastatic extension Life expectancy: > 3 months Setting: Centre Hospitalier Universitaire de Limoges | |
| Interventions | Duration of treatment: first 6 days after central venous access device implantation and prescribed for 90 days Intervention 1: LMWH 2500 U anti‐XA/day (subcutaneous: dalteparin, nadroparin, or enoxaparin, once daily) Intervention 2: warfarin oral 1 mg/day Intervention 3: no anticoagulant Cointervention: chemotherapy, administered to all groups | |
| Outcomes | Duration of follow‐up: 3 months
Diagnostic test for CRT: Doppler ultrasound and venographies | |
| Notes | Funding: not reported in the manuscript; however, in ClinicalTrial.gov "sponsors and collaborators: University Hospital, Limoges" (clinicaltrials.gov/show/NCT00199602) Ethical approval: quote: "The study protocol was approved by the local ethical committee." Conflict of interest: none ITT: quote: "The intention to treat population was evaluated and was defined as all randomized patients." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization list was generated by an independent statistician who used a standard method of permuted block of variable size without stratification." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported. Comment: probably not blinded; knowledge of the assigned intervention may not impact the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data addressed? | Low risk | Comment: judgment based on comparison between MPD rate (13/420 = 3%) and event rate (catheter‐related DVT: no anticoagulant: 20/135 = 14.8%; LMWH: 14/138 = 10.1%) |
| Free of selective reporting? | Low risk | Study registered in ClinicalTrials.gov (clinicaltrials.gov/show/NCT00199602) Some outcomes of interest were poorly reported (e.g. survival) |
| Free of other bias? | Low risk | Study not stopped early for benefit |
| Methods | Open‐label, multicenter, international randomized controlled trial | |
| Participants | 186 participants aged 0 to 18 years 51% had cancer | |
| Interventions | Intervention: reviparin prophylactic dose, started within 12 hours of randomization (randomization could occur up to 5 days after insertion of the CVC) Control: no intervention | |
| Outcomes | Follow‐up: 30 days but varied depending on the time of CVC removal
Diagnostic test for CRT: venography on day 30 or at time of CVC removal | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned by a computer derived protocol" Comment: definitely yes |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded central outcome adjudication Comment: definitely yes |
| Incomplete outcome data addressed? | Low risk | Study reported complete follow‐up |
| Free of selective reporting? | Low risk | Study not registered. No published protocol. All outcomes listed in the methods section were reported on Comment: probably yes |
| Free of other bias? | Low risk | Study stopped early for insufficient accrual but not for benefit |
| Methods | Multicenter, open, parallel‐group randomized controlled study | |
| Participants | 60 participants with solid (non‐hematologic) cancers Minimum age: 18 years; mean age: 58.7 years Minimum life expectancy: 3 months | |
| Interventions | Intervention: prophylactic dose; nadroparin 2850 IU subcutaneously, once daily started 2 hours prior to CVC placement; for 90 days Control: warfarin 1 mg/day; starting 3 days prior to CVC placement; 90 days | |
| Outcomes | Follow‐up: 6 months
Diagnostic test for DVT: venography of the extremities, Doppler or venography of lower limbs (or both) Diagnostic test for PE: V/Q scan, pulmonary angiogram, helical CT | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer derived treatment schedules were used to assign treatment regimens. To obtain a continuing balance of treatments, the randomized list was divided into consecutive blocks." |
| Allocation concealment (selection bias) | Low risk | Quote: "...concealment of randomization was achieved through centralized distant randomization." |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial Comment: probably not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All venograms were reviewed by an independent reading committee, the members of which were unaware of the patients' treatment allocation." Comment: probably not blinded |
| Incomplete outcome data addressed? | Low risk | Comment: judgment based on comparison between MPD rate (1/60 = 1.7%) and event rate (upper extremity thrombosis: nadroparin: 6/21 = 28.6%; warfarin: 4/24 = 16.6%) |
| Free of selective reporting? | Low risk | Study not registered. No published protocol. All outcomes listed in the methods section were reported on Comment: probably yes |
| Free of other bias? | Low risk | Study not stopped early for benefit |
| Methods | Open randomized controlled trial | |
| Participants | 32 participants with solid tumors Mean age: 54 years Minimum life expectancy: 3 months 29 participants completed the study (study was stopped prematurely) | |
| Interventions | Intervention: dalteparin (Fragmin; LMWH) 2500 IU subcutaneously; 2 hours prior to CVC placement; 90 days | |
| Outcomes | Follow‐up: 90 days
Diagnostic test for CRT: venography | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...prescribed randomized arrangement to receive or not 2500 IU (subcutaneous) of a low molecular weight heparin." |
| Allocation concealment (selection bias) | High risk | According to the following communication with the author: "We used an open list of random numbers to randomize patients." Comment: there was no allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Interpretation of venograms was done independently by two expert radiologists who were unaware of patients' status and therapy." Comment: definitely blinded |
| Incomplete outcome data addressed? | Low risk | Complete follow‐up |
| Free of selective reporting? | Low risk | Study not registered. No published protocol. All outcomes listed in the methods section were reported on |
| Free of other bias? | High risk | Quote: "Patient recruitment was terminated earlier than planned, after inclusion of 32 patients, because of an excess of thrombotic events in patients without prophylaxis." Study stopped early for benefit |
| Methods | Single‐center, randomized, placebo‐controlled, double‐blind study | |
| Participants | 113 participants with hematologic malignancies Aged: > 18 years; mean age: 56.5 years (nadroparin: 58 years; placebo: 55 years) | |
| Interventions | Intervention: once‐daily nadroparin (LMWH) 2850 IU subcutaneously prophylactic dose; started 2 hours before CVC insertion; for 3 weeks or until the day of CVC removal Control: placebo injections subcutaneously | |
| Outcomes | Follow‐up: 3 weeks
Diagnostic test for DVT: venography | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...prospective, randomized, placebo controlled double‐blinded study" Comment: probably generated sequence randomly |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Placebo controlled double‐blinded study" Comment: definitely blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All venograms were independently adjudicated by an expert radiologist using prior criteria without knowledge of treatment allocation." Comment: definitely blinded |
| Incomplete outcome data addressed? | High risk | Comment: judgment based on comparison between MPD rate (nadroparin: 15/56 = 26.7%; placebo: 15.8%) and event rate (thrombosis rate: nadroparin: 17%; placebo: 9%) |
| Free of selective reporting? | Low risk | Study not registered. No published protocol. All outcomes listed in the methods section were reported on Comment: probably yes |
| Free of other bias? | Low risk | Study not stopped early for benefit |
| Methods | Randomized controlled study | |
| Participants | 73 children with solid or hematologic (or both) cancers Mean age: 6.8 years Setting: 2 university hospitals in Oslo, Norway | |
| Interventions | Intervention: warfarin 0.1 mg/kg starting the day of insertion of CVC (target INR 1.3‐1.9), duration not reported Control: no intervention | |
| Outcomes | Follow‐up: 6 months
CVC characteristics: external tunneled catheters and ports; jugular | |
| Notes | Funding: Norwegian Cancer Society ITT: quote: "Only children who completed the study satisfactorily (n=62) from the basis of our descriptive statistics and analyses." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children were randomized to receive low‐dose warfarin or to a control group." Comment: probably generated sequence randomly |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was practically organized by the primary investigator by drawing closed envelopes from boxes." "We did not use block randomization, but (...) we used stratified randomization (according to whether patient is also receiving asparaginase, a hypothesized significant pro thrombotic factor)." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The children in the standard arm did not receive placebo." Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The radiologists who performed the ultrasonography were blinded to the treatment assignment." Comment: definitely yes |
| Incomplete outcome data addressed? | High risk | Comment: judgment based on comparison between MPD rate (11/73 = 16%) and event rate (CRT: warfarin: 3/30 = 10%; no intervention: 3/33 = 9%) |
| Free of selective reporting? | Low risk | Study not registered. No published protocol. All outcomes listed in the methods section were reported on Comment: probably yes |
| Free of other bias? | Low risk | Study stopped early for harm but not for benefit |
| Methods | Multicenter (11 Italian centers), double‐blind randomized controlled trial | |
| Participants | 385 participants with solid or hematologic (or both) cancers Minimum age: 18 years; mean age: 59.3 years Minimum life expectancy: 3 months | |
| Interventions | Intervention: enoxaparin 40 mg/day subcutaneously prophylactic dose; started 2 hours prior to CVC placement; for 6 weeks Control: placebo (preloaded syringes) | |
| Outcomes | Follow‐up: 3 months
Diagnostic test for DVT: screening by venography (6 weeks); diagnosis by venography Diagnostic test for PE: V/Q scan, CT, pulmonary angiogram, autopsy | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to receive drug or placebo permuted blocks of 4 were used for treatment allocation." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind study" Comment: definitely blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Venographies were evaluated by a central adjudication committee consisting of three radiologists who were unaware of the patients' clinical status." Comment: definitely blinded |
| Incomplete outcome data addressed? | High risk | Comment: judgment based on comparison between MPD rate (enoxaparin: 36/191 = 18.9%; placebo: 39/194 = 20%) and event rate (thrombosis rate: enoxaparin: 14.1%; placebo: 18%) |
| Free of selective reporting? | Low risk | Study not registered. No published protocol. All outcomes listed in the methods section were reported on |
| Free of other bias? | Low risk | Study not stopped early for benefit |
| Methods | Open‐label, multicenter, randomized controlled trial Setting: 68 clinical centers in the UK | |
| Participants | 590 participants with solid or hematologic (or both) cancers Minimum age: 16 years; median age: 60.5 years (warfarin: 60 years; no intervention: 61 years) | |
| Interventions | Intervention: warfarin: fixed dose 1 mg/day or dose adjusted to maintain INR 1.5‐2 | |
| Outcomes | Follow‐up: median 45 months (range 26 to 88 months)
Diagnostic test for CRT: venography | |
| Notes | All types of CVC allowed Funding: Medical Research Council and Cancer Research UK Ethical approval: quote: "The clinical centres received ethical approval from the West Midlands multicentre research ethics committee." Conflict of interest: quote: "We declare that we have no conflict of interest." ITT: quote: "Analysis was by intention‐to‐treat basis" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly assigned patients using computerised block algorithm" |
| Allocation concealment (selection bias) | Low risk | Personal communication with the author: "Randomisation was executed via a computerised block algorithm and performed by randomisation officers at the central randomisation office in the departmental trials unit, accessed by telephone and fax." |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | "All thromboses were radiologically confirmed by two investigators, unaware of treatment allocation" Comment: definitely blinded; knowledge of the assigned intervention may not impact the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.) |
| Incomplete outcome data addressed? | Low risk | Comment: judgment based on comparison between MPD rate (warfarin: 13/408 = 3%; no intervention: 1/404 = 0.2%) and event rate (thrombotic event rate: warfarin: 3%; no intervention: 6%; major bleeding rate: warfarin: < 1%; no intervention: 3%) |
| Free of selective reporting? | Low risk | Study registered as ISRCTN50312145. All outcomes listed in the registry were reported on |
| Free of other bias? | Low risk | Study not stopped early for benefit |
ASCO: American Society of Clinical Oncology; CT: computed tomography; CRC: catheter‐related complication; CRT: catheter‐related thrombosis; CVC: central venous catheter; HIT: heparin‐induced thrombocytopenia; INR: international normalized ratio; ITT: intention to treat; IU: international unit; LMWH: low‐molecular‐weight heparin; MPD: missing participant data; PE: pulmonary embolism; TEE: thromboembolic event; U: unit; V/Q: ventilation/perfusion.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Concerns about the accuracy and validity of the data reported, and the practical aspects of the published protocol. When asked by the review authors, the authors were unable to provide evidence that the study had been conducted. | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants with cancer with VTE); includes 2 reports | |
| Not the population of interest (participants without CVC); includes 2 reports | |
| Not an RCT; no control group | |
| Not an RCT; observational study | |
| Not population of interest (participants did not previously have CVC, it was rather inserted for intervention) | |
| Not the population of interest (participants without CVC) | |
| Not an RCT; observational study | |
| Not the population of interest (participants without CVC) | |
| Not an RCT; historic control | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants without CVC); included 3 reports | |
| No obtainable needed data from study authors | |
| Not an RCT; retrospective study | |
| Not the population of interest (participants without CVC) | |
| Inadequate control groups; intervention compared with urokinase | |
| Review | |
| Not the population of interest (participants without CVC) | |
| No obtainable needed data from study authors | |
| Not the population of interest (participants without CVC); included 3 reports | |
| Not intervention of interest (locking solution) | |
| Not the population of interest (participants without CVC); included 2 reports | |
| Not the population of interest (participants without CVC) | |
| Different drug/agent studied | |
| Different drug/agent studied | |
| Not intervention of interest (flushing solution) | |
| Review | |
| Not an RCT; observational study | |
| Review | |
| Not the population of interest (participants without CVC); included 2 reports | |
| Not the population of interest (participants without CVC); included 2 reports | |
| Not the population of interest (participants without CVC); included 2 reports | |
| Review | |
| Letter to the editor | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants with cancer with VTE); included 9 reports | |
| Not an RCT; observational study | |
| Editorial | |
| Not the population of interest (participants without CVC); included 4 reports | |
| Not an RCT; retrospective study | |
| Not an RCT; no control group | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants with cancer with VTE) | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants without CVC) | |
| No obtainable needed data from the authors | |
| Not the population of interest (participants without CVC); included 10 reports | |
| Not the population of interest (participants with cancer with VTE); included 2 reports | |
| Not the population of interest (participants with cancer with VTE); included 3 reports | |
| Not an RCT | |
| Not an RCT | |
| Not the population of interest (participants without CVC) | |
| Not the population of interest (participants with cancer with VTE) | |
| Not the population of interest (participants with cancer with VTE) | |
| Not the population of interest (participants with cancer with VTE) | |
| Inadequate control groups; intervention compared with urokinase | |
| Not the population of interest (participants with cancer with VTE) | |
| Not an RCT; retrospective study | |
| Not population of interest (peripheral venous catheter and not CVC) | |
| Not the population of interest (participants with cancer with VTE) | |
| Not an RCT; observational study | |
| Not the population of interest (participants with cancer with VTE) | |
| Not the population of interest (participants with cancer with VTE) | |
| Not the population of interest (participants with cancer with VTE); included 2 reports | |
| Not the population of interest (participants with cancer with VTE) | |
| Not the population of interest (participants with cancer with VTE); included 2 reports |
CVC: central venous catheter; RCT: randomized controlled trial; VTE: venous thromboembolism.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (up to 3 months) Show forest plot | 5 | 1236 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.53, 1.26] |
| Analysis 1.1  Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 1 All‐cause mortality (up to 3 months). | ||||
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 5 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.81] |
| Analysis 1.2  Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months). | ||||
| 3 Asymptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 5 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.62, 1.46] |
| Analysis 1.3  Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 3 Asymptomatic catheter‐related thrombosis (up to 3 months). | ||||
| 4 Major bleeding (up to 3 months) Show forest plot | 4 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.06, 36.28] |
| Analysis 1.4  Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 4 Major bleeding (up to 3 months). | ||||
| 5 Minor bleeding (up to 3 months) Show forest plot | 2 | 544 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.62, 2.92] |
| Analysis 1.5  Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 5 Minor bleeding (up to 3 months). | ||||
| 6 Catheter‐related infection (up to 3 months) Show forest plot | 2 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.79] |
| Analysis 1.6  Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 6 Catheter‐related infection (up to 3 months). | ||||
| 7 Thrombocytopenia (up to 3 months) Show forest plot | 4 | 1002 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.80, 1.33] |
| Analysis 1.7  Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 7 Thrombocytopenia (up to 3 months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (up to 3 months) Show forest plot | 4 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.64, 1.55] |
| Analysis 2.1 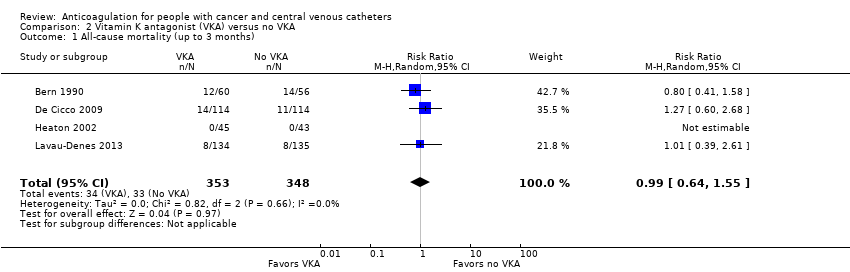 Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 1 All‐cause mortality (up to 3 months). | ||||
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 4 | 1271 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.23, 1.64] |
| Analysis 2.2  Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months). | ||||
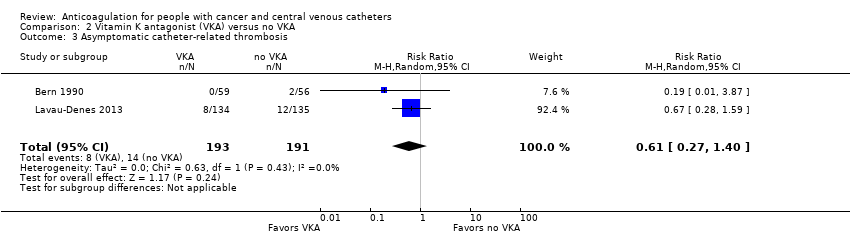
| 3 Asymptomatic catheter‐related thrombosis Show forest plot | 2 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.27, 1.40] |
| Analysis 2.3  Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 3 Asymptomatic catheter‐related thrombosis. | ||||
| 4 Major bleeding (up to 3 months) Show forest plot | 2 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 7.14 [0.88, 57.78] |
| Analysis 2.4  Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 4 Major bleeding (up to 3 months). | ||||
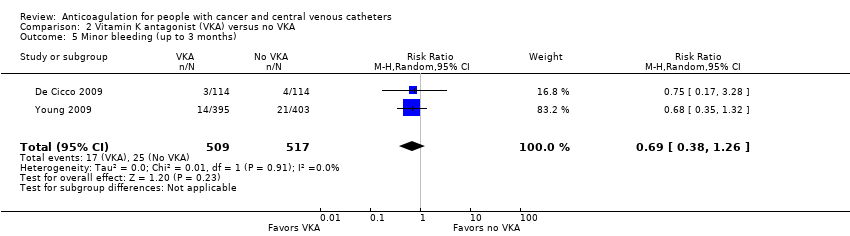
| 5 Minor bleeding (up to 3 months) Show forest plot | 2 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.38, 1.26] |
| Analysis 2.5 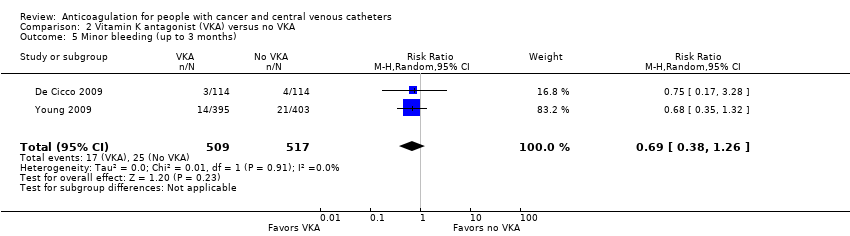 Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 5 Minor bleeding (up to 3 months). | ||||
| 6 Catheter‐related infection Show forest plot | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.74, 1.85] |
| Analysis 2.6 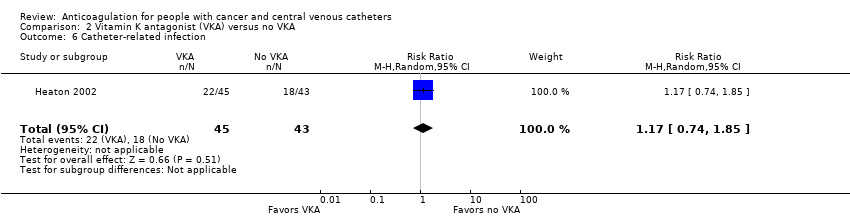 Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 6 Catheter‐related infection. | ||||
| 7 Premature central venous catheter removal Show forest plot | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.30, 2.24] |
| Analysis 2.7  Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 7 Premature central venous catheter removal. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
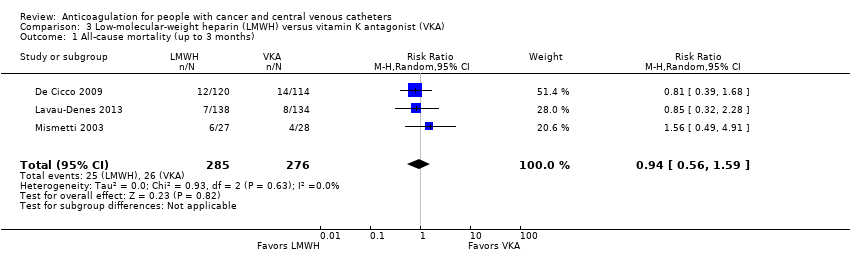
| 1 All‐cause mortality (up to 3 months) Show forest plot | 3 | 561 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.56, 1.59] |
| Analysis 3.1  Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 1 All‐cause mortality (up to 3 months). | ||||
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.44, 7.61] |
| Analysis 3.2 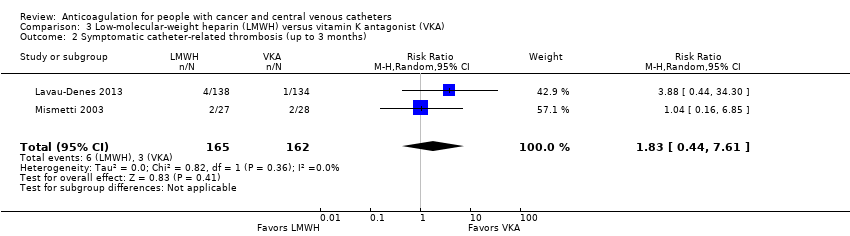 Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months). | ||||
| 3 Asymptomatic catheter‐related thrombosis Show forest plot | 2 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.75, 3.46] |
| Analysis 3.3  Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 3 Asymptomatic catheter‐related thrombosis. | ||||
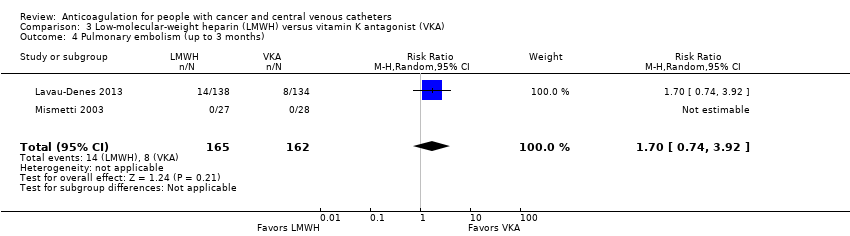
| 4 Pulmonary embolism (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.74, 3.92] |
| Analysis 3.4  Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 4 Pulmonary embolism (up to 3 months). | ||||
| 5 Major bleeding (up to 3 months) Show forest plot | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 3.11 [0.13, 73.11] |
| Analysis 3.5 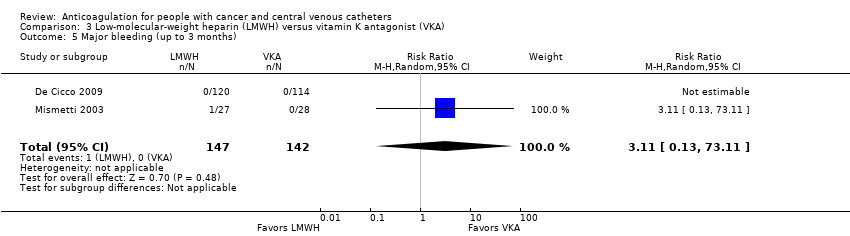 Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 5 Major bleeding (up to 3 months). | ||||
| 6 Minor bleeding (up to 3 months) Show forest plot | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.20, 4.61] |
| Analysis 3.6  Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 6 Minor bleeding (up to 3 months). | ||||
| 7 Thrombocytopenia (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.20, 2.39] |
| Analysis 3.7  Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 7 Thrombocytopenia (up to 3 months). | ||||

Study flow diagram.
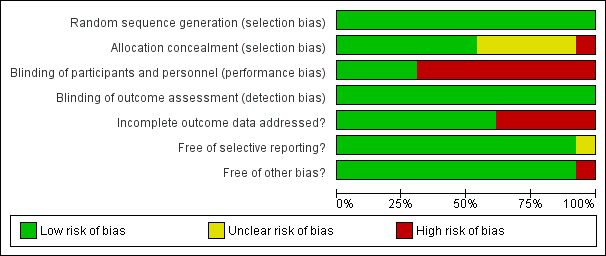
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
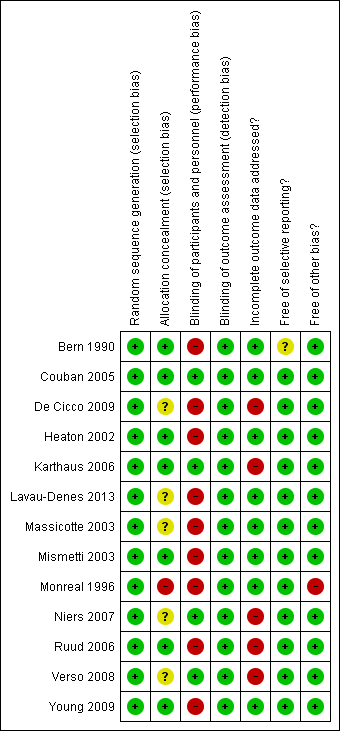
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 1 All‐cause mortality (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 3 Asymptomatic catheter‐related thrombosis (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 4 Major bleeding (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 5 Minor bleeding (up to 3 months).
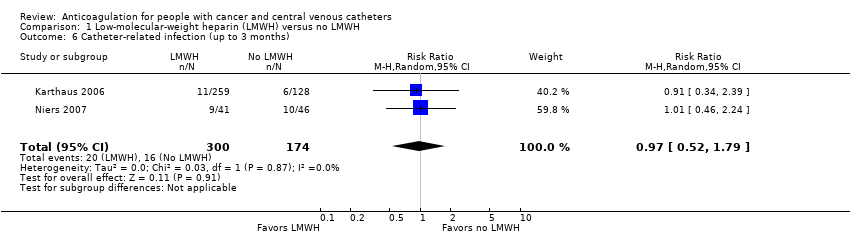
Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 6 Catheter‐related infection (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 7 Thrombocytopenia (up to 3 months).

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 1 All‐cause mortality (up to 3 months).

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months).
Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 3 Asymptomatic catheter‐related thrombosis.

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 4 Major bleeding (up to 3 months).
Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 5 Minor bleeding (up to 3 months).

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 6 Catheter‐related infection.

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 7 Premature central venous catheter removal.
Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 1 All‐cause mortality (up to 3 months).

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months).

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 3 Asymptomatic catheter‐related thrombosis.
Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 4 Pulmonary embolism (up to 3 months).

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 5 Major bleeding (up to 3 months).
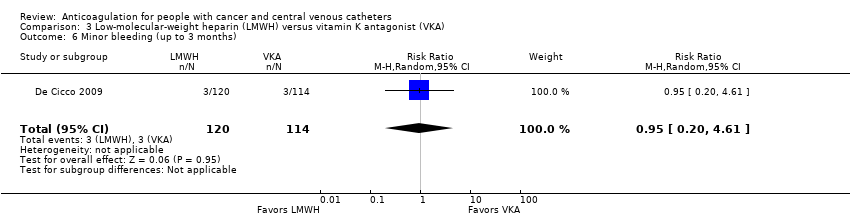
Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 6 Minor bleeding (up to 3 months).

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 7 Thrombocytopenia (up to 3 months).
| Low‐molecular‐weight heparin (LMWH) compared to no LMWH for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: LMWH Comparison: no LMWH | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with no LMWH | Risk difference with LMWH | ||||
| All‐cause mortality (up to 3 months) | 1236 | ⊕⊕⊝⊝ | RR 0.82 | Study population | |
| 77 per 1000 | 14 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 1089 | ⊕⊕⊕⊝ | RR 0.43 | Study population | |
| 67 per 1000 | 38 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 1089 | ⊕⊝⊝⊝ | RR 0.95 | Study population | |
| 96 per 1000 | 5 fewer per 1000 | ||||
| Major bleeding (up to 3 months) | 1018 | ⊕⊝⊝⊝ | RR 1.49 | Study population | |
| 0 per 1000 | 0 fewer per 1000 | ||||
| Low | |||||
| 1 per 1000 | 0 fewer per 1000 | ||||
| Minor bleeding (up to 3 months) | 544 | ⊕⊕⊝⊝ | RR 1.35 | Study population | |
| 41 per 1000 | 14 more per 1000 | ||||
| Catheter‐related infection (up to 3 months) | 474 | ⊕⊕⊝⊝ | RR 0.97 | Study population | |
| 92 per 1000 | 3 fewer per 1000 | ||||
| Thrombocytopenia (up to 3 months) | 1002 | ⊕⊕⊝⊝ | RR 1.03 | Study population | |
| 176 per 1000 | 5 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low‐molecular‐weight heparin; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to serious risk of bias; lack of blinding in participants and personnel in three studies, incomplete outcome data not addressed in three studies, and unclear or no allocation concealment in four out of five studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (36 per 1000 absolute reduction) and the possibility of important harm (20 per 1000 absolute increase), including 79 events in total. cDowngraded by one level due to serious risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in three studies; and unclear or no allocation concealment in four out of five studies. dDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. eDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (36 per 1000 absolute reduction) and the possibility of important harm (40 per 1000 absolute increase), including 94 events in total. fDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in three studies; and unclear or no allocation concealment in four out of five studies. gDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in one study; incomplete outcome data not addressed in four studies; and unclear or no allocation concealment in three out of four studies. hDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for benefit (1 per 1000 absolute reduction) and the possibility of important harm (35 per 1000 absolute increase), including five events in total. Given the observed baseline risk of 0% we used 0.1% to generate an absolute effect and a confidence interval. iDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in one study; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in two out of two studies. jDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (16 per 1000 absolute reduction) and the possibility of important harm (79 per 1000 absolute increase), including 26 events in total. kDowngraded by one level due to concern about risk of bias; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in one out of two studies. lDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (35 per 1000 absolute reduction) and the possibility of important harm (58 per 1000 absolute increase), including 36 events in total. mDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in three out of four studies. nDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (35 per 1000 absolute reduction) and the possibility of important harm (58 per 1000 absolute increase) including 163 events in total. | |||||
| Vitamin K antagonist (VKA) compared to no VKA for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: VKA Comparison: no VKA | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with no VKA | Risk difference with VKA | ||||
| All‐cause mortality (up to 3 months) | 701 | ⊕⊕⊝⊝ | RR 0.99 | Study population | |
| 95 per 1000 | 1 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 1271 | ⊕⊕⊝⊝ | RR 0.61 | Study population | |
| 80 per 1000 | 31 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 384 | ⊕⊝⊝⊝ | RR 0.61 | Study population | |
| 73 per 1000 | 29 fewer per 1000 | ||||
| Major bleeding (up to 3 months) | 1026 | ⊕⊕⊝⊝ | RR 7.14 | Study population | |
| 2 per 1000 | 12 more per 1000 | ||||
| Minor bleeding (up to 3 months) | 1026 | ⊕⊕⊝⊝ | RR 0.69 | Study population | |
| 48 per 1000 | 15 fewer per 1000 | ||||
| Catheter‐related infection | 88 | ⊕⊕⊝⊝ | RR 1.17 | Study population | |
| 419 per 1000 | 71 more per 1000 | ||||
| Premature CVC removal | 88 | ⊕⊕⊝⊝ | RR 0.82 | Study population | |
| 163 per 1000 | 29 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVC: central venous catheter; RCT: randomized controlled trial; RR: risk ratio; VKA: vitamin K antagonist. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in four studies and unclear or no allocation concealment in two out of four studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (34 per 1000 absolute reduction) and the possibility of important harm (52 per 1000 absolute increase), including 67 events in total. cThe trial WARP showed no overall survival advantage in participants taking warfarin compared with participants in the no‐warfarin group (hazard ratio 0.98, 95% CI 0.77 to 1.25; P = 0.26) (Young 2009). dDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in four studies and no clear information concerning allocation concealment in one out of four studies). eDowngraded by one level due to unexplained inconsistency (I2 = 70%). Imprecision was partially driven by the inconsistency between the studies and was taken into consideration when downgrading by two levels for serious risk of bias and serious inconsistency. fDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (63 per 1000 absolute reduction) and the possibility of important harm (57 per 1000 absolute increase), including 87 events in total. gDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and no clear information about allocation concealment in one out of two studies. hDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. iDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (54 per 1000 absolute reduction) and the possibility of important harm (29 per 1000 absolute increase), including 22 events in total. jDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and no clear information about allocation concealment in one out of two studies. kDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for no effect (0 per 1000 absolute reduction) and the possibility of important harm (120 per 1000 absolute increase), including eight events in total. lDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in three studies; and unclear or no allocation concealment in two out of three studies. mDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (30 per 1000 absolute reduction) and the possibility of important harm (16 per 1000 absolute increase), including 42 events in total. nDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in the included study). oDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (109 per 1000 absolute reduction) and the possibility of important harm (356 per 1000 absolute increase), including 40 events in total. pDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (114 per 1000 absolute reduction) and the possibility of important harm (202 per 1000 absolute increase), including 13 events in total. | |||||
| Low‐molecular‐weight heparin (LMWH) compared to vitamin K antagonist (VKA) for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: LMWH Comparison: VKA | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with VKA | Risk difference with LMWH | ||||
| All‐cause mortality (up to 3 months) | 561 | ⊕⊕⊝⊝ | RR 0.94 | Study population | |
| 94 per 1000 | 6 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 327 | ⊕⊝⊝⊝ | RR 1.83 | Study population | |
| 19 per 1000 | 15 more per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 317 | ⊕⊝⊝⊝ | RR 1.61 | Study population | |
| 63 per 1000 | 39 more per 1000 | ||||
| Pulmonary embolism (up to 3 months) | 327 | ⊕⊕⊝⊝ | RR 1.70 | Study population | |
| 49 per 1000 | 35 more per 1000 | ||||
| Major bleeding (up to 3 months) | 289 | ⊕⊝⊝⊝ | RR 3.11 | Study population | |
| 0 per 1000 | 0 fewer per 1000 | ||||
| Low | |||||
| 1 per 1000 | 2 more per 1000 | ||||
| Minor bleeding (up to 3 months) | 234 | ⊕⊝⊝⊝ | RR 0.95 | Study population | |
| 26 per 1000 | 1 fewer per 1000 | ||||
| Thrombocytopenia (up to 3 months)n | 327 | ⊕⊕⊕⊝ | RR 1.69 | Study population | |
| 216 per 1000 | 149 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low‐molecular‐weight heparin; RCT: randomized controlled trial; RR: risk ratio; VKA: vitamin K antagonist. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in three studies; and unclear allocation concealment in two out of three studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (41 per 1000 absolute reduction) and the possibility of important harm (56 per 1000 absolute increase), including 51 events in total. cDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and unclear allocation concealment in one out of two studies. dDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (10 per 1000 absolute reduction) and the possibility of important harm (122 per 1000 absolute increase), including nine events in total. eDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and unclear allocation concealment in one out of two studies. fDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. gDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (16 per 1000 absolute reduction) and the possibility of important harm (156 per 1000 absolute increase), including 26 events in total. hDowngraded by one level due to concern both risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. iDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (13 per 1000 absolute reduction) and the possibility of important harm (144 per 1000 absolute increase), including 22 events in total. jDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. kDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for benefit (1 per 1000 absolute reduction) and the possibility of important harm (51 per 1000 absolute increase), including one event in total. Given the observed baseline risk of 0% we used 0.1% to generate an absolute effect and a confidence interval. lDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in the study and unclear allocation concealment). mDowngraded by two levels due to concern about imprecision (95% CI was consistent with the possibility for benefit (21 per 1000 absolute reduction) and the possibility of important harm (95 per 1000 absolute increase), including six events in total. nThe study by Lavau‐Denes and colleagues included all grades of thrombocytopenia (even mild cases) (Lavau‐Denes 2013). oDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. | |||||
| Term | Definition |
| Adjuvant therapy | Assisting in the amelioration, or cure of disease |
| Anticoagulation | Process of hindering the clotting of blood especially by treatment with an anticoagulant. |
| Antithrombotic | Used against or tending to prevent thrombosis (clotting) |
| Bacteremia | Presence of bacteria in the blood |
| Central venous line | Synthetic tube that is inserted into a central (large) vein to provide temporary intravenous access for the administration of fluid, medication, or nutrients. |
| Coagulation | Clotting |
| Deep venous (vein) thrombosis (DVT) | Condition marked by the formation of a thrombus within a deep vein (as of the leg or pelvis) that may be asymptomatic or be accompanied by symptoms (as swelling and pain) and that is potentially life threatening if dislodgment of the thrombus results in pulmonary embolism |
| Fibrin | White insoluble fibrous protein formed from fibrinogen by the action of thrombin especially in the clotting of blood |
| Fondaparinux | Anticoagulant medication |
| Hemostatic system | System that shortens the clotting time of blood and stops bleeding |
| Heparin | Enzyme occurring especially in the liver and lungs that prolongs the clotting time of blood by preventing the formation of fibrin. 2 forms of heparin that are used as anticoagulant medications are: unfractionated heparin (UFH) and low‐molecular‐weight heparin (LMWH) |
| Impedance plethysmography | Technique that measures the change in blood volume (venous blood volume as well as the pulsation of the arteries) for a specific body segment |
| Kappa statistics | Measure of degree of non‐random agreement between observers or measurements of a specific categorical variable or both |
| Metastasis | Spread of cancer cells from the initial or primary site of disease to another part of the body |
| Oncogene | Gene having the potential to cause a normal cell to become cancerous |
| Osteoporosis | Condition that affects especially older women and is characterized by decrease in bone mass with decreased density and enlargement of bone spaces producing porosity and brittleness |
| Parenteral nutrition | Practice of feeding a person intravenously, circumventing the gastrointestinal tract |
| Pulmonary embolism (PE) | Embolism of a pulmonary artery or 1 of its branches that is produced by foreign matter and most often a blood clot originating in a vein of the leg or pelvis and that is marked by labored breathing, chest pain, fainting, rapid heart rate, cyanosis, shock, and sometimes death. |
| Stroma | Supporting framework of an organ typically consisting of connective tissue |
| Thrombin | Proteolytic enzyme formed from prothrombin that facilitates the clotting of blood by catalyzing conversion of fibrinogen to fibrin |
| Thrombocytopenia | Persistent decrease in the number of blood platelets that is often associated with hemorrhagic conditions |
| Thrombosis | Formation or presence of a blood clot within a blood vessel |
| Vitamin K antagonists (VKA) | Anticoagulant medications that are used for anticoagulation. Warfarin is a vitamin K antagonist |
| Warfarin | Anticoagulant medication that is a vitamin K antagonist that is used for anticoagulation |
| Ximelagatran | Anticoagulant medication |
| LMWH | Generic name | Prophylactic dose | Therapeutic dose |
| Lovenox | Enoxaparin | 40 mg once daily | 1 mg/kg twice daily |
| Fragmin | Dalteparin | 2500‐5000 U once daily | 200 U/kg once daily or |
| Innohep | Tinzaparin, logiparin | 4500 U once daily | 90 U/kg twice daily |
| Fraxiparine | Nadroparin | 35‐75 anti‐Xa IU/kg/day | 175 anti‐Xa IU/kg/day |
| Certoparin | Sandoparin | 3000 anti‐Xa IU once daily | – |
| Reviparin | Reviparin | 1750‐4200 anti‐Xa IU | 7000‐12,600 anti‐Xa IU |
| IU: international units; U: units; Xa: factor Xa. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (up to 3 months) Show forest plot | 5 | 1236 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.53, 1.26] |
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 5 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.81] |
| 3 Asymptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 5 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.62, 1.46] |
| 4 Major bleeding (up to 3 months) Show forest plot | 4 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.06, 36.28] |
| 5 Minor bleeding (up to 3 months) Show forest plot | 2 | 544 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.62, 2.92] |
| 6 Catheter‐related infection (up to 3 months) Show forest plot | 2 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.79] |
| 7 Thrombocytopenia (up to 3 months) Show forest plot | 4 | 1002 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.80, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (up to 3 months) Show forest plot | 4 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.64, 1.55] |
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 4 | 1271 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.23, 1.64] |
| 3 Asymptomatic catheter‐related thrombosis Show forest plot | 2 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.27, 1.40] |
| 4 Major bleeding (up to 3 months) Show forest plot | 2 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 7.14 [0.88, 57.78] |
| 5 Minor bleeding (up to 3 months) Show forest plot | 2 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.38, 1.26] |
| 6 Catheter‐related infection Show forest plot | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.74, 1.85] |
| 7 Premature central venous catheter removal Show forest plot | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.30, 2.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (up to 3 months) Show forest plot | 3 | 561 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.56, 1.59] |
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.44, 7.61] |
| 3 Asymptomatic catheter‐related thrombosis Show forest plot | 2 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.75, 3.46] |
| 4 Pulmonary embolism (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.74, 3.92] |
| 5 Major bleeding (up to 3 months) Show forest plot | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 3.11 [0.13, 73.11] |
| 6 Minor bleeding (up to 3 months) Show forest plot | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.20, 4.61] |
| 7 Thrombocytopenia (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.20, 2.39] |

