Izolacija kao način kontroliranja prijenosa infekcije hepatis C virusom (HCV) u hemodijaliznim jedinicama
Abstract
Background
The hepatitis C virus (HCV) infection affects about 2% of the world's population and can cause chronic liver infection and persistent long‐term sequelae such as cirrhosis and liver cancer.
The prevalence of HCV infection among people on haemodialysis is often higher than the general population. The virus is easily transmitted parenterally, and blood transfusions have previously played a significant role in transmission; however, erythropoietin therapy has reduced the need for transfusions, and coupled with improved screening of donated blood, has significantly decreased transmission by transfusion. Although control of hospital‐acquired infection has improved with the advent of biosafety measures, stopping HCV transmission in haemodialysis units remains challenging.
Isolating people infected with HCV involves physical separation from others to limit direct or indirect transmission and includes a number of strategies during dialysis. The evidence for isolating people infected with HCV during haemodialysis is sparse with some inconsistencies.
Objectives
To evaluate the benefits and harms of isolation of HCV‐infected patients during haemodialysis on the transmission of HCV to other patients.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register to 26 November 2015 through contact with the Information Specialist using search terms relevant to this review. We also searched the Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to 2015), Web of Science Conference Proceedings Citation Index‐Science (CPCI‐S, 1990 to 2015), ProQuest Dissertations & Theses Database (1990 to 2015), and Open Grey (1990 to 2015).
Selection criteria
We included randomised controlled trials (RCTs), quasi‐RCTs and cluster RCTs evaluating the clinical benefits and harms of isolating HCV‐infected patients during haemodialysis on the transmission of HCV to other patients. We considered incidence of dialysis‐acquired HCV infection, all‐cause mortality, and adverse effects associated with isolation as the primary outcomes.
Data collection and analysis
Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) or standardised mean difference (SMD) and 95% CI for continuous outcomes.
Main results
Only one study, which included 12 centres was identified: four centres used dedicated haemodialysis machines for HCV‐infected patients and eight centres used non‐dedicated machines. The total number of patients enrolled was 593. One centre was excluded after randomisation. Random sequence generation was not described and allocation concealment was not performed. Participants and personnel were not blinded and blinding of outcome assessors was not reported. Only 74.5% of the patients were followed for 9 months; and 47.3% were followed for an additional 9 months. The authors only reported one outcome, measuring the difference in the incidence of HCV in both groups. The authors did not consider the exposure time, to determine the adjusted rate of seroconversion risk/patient‐year.
The study reported that the incidence of HCV infection during the first follow‐up period (9 months) was 1.6% in the dedicated group, and 4.7% in the non‐dedicated one (446 patients analysed out of 593 randomised; RR 0.34, 95% CI 0.11 to 1.07). During the second follow‐up period (18 months) the incidence was 1.3% in the dedicated group and 5.8% in the control (281 patients analysed out of 593 randomised; RR 0.22, 95% CI 0.05 to 1.02). Therefore, we found no differences in terms of the number of participants developing HCV infection when comparing the dedicated group with the usual care. Moreover, the evidence was of very low quality, which means that we have very little confidence in the effect estimate.
Authors' conclusions
The benefits and harms of isolation of HCV‐infected patients during haemodialysis on the transmission of HCV to other patients are uncertain. Evidence from one short‐duration cluster‐randomised study with a high risk of bias did not find differences in terms of the number of participants developing HCV infection when comparing the use of dedicated haemodialysis machines for HCV infected patients with the use of non‐dedicated machines.
PICO
Laički sažetak
Izolacija kao način kontroliranja prijenosa infekcije hepatis C virusom (HCV) u hemodijaliznim jedinicama
Istraživačko pitanje
Hepatitis C virus (HCV) lako se prenosi intravenskim putem, primjerice transfuzijama krvi ili uporabom opreme za hemodijalizu. HCV može uzrokovati dugotrajnu infekciju i kroničnu bolest jetre. Učestalost HCV infekcije češća je među osobama koje su na hemodijalizi nego u općoj populaciji i povezana je s povećanim rizikom od smrti uzrokovane bolestima srca i jetre. Cilj ovog Cochrane sustavnog pregleda bio je istražiti može li izolacija osoba zaraženim HCV‐om tijekom hemodijalize (korištenjem druge prostorije, uređaja, osoblja ili posebne smjene) može biti djelotvorno za ograničavanje izravnog ili neizravnog prijenosa virusa na nezaražene pacijente.
Kako je provedeno istraživanje?
U okviru ovog sustavnog pregleda literature provedeno je opsežno pretraživanje medicinske znanstvene literature koja je objavljena do 26. studenoga 2015. Međutim, nađeno je samo 1 kliničko istraživanje koje je ispitalo izolaciju kao način kontroliranja prijenosa infekcije HCV‐om.
Rezultati istraživanja
To jedno istraživanje provedeno je u 12 cenatara i uključilo je 593 ispitanika. U 4 centra su HCV‐om zaraženi pacijenti liječeni na posebnom uređaju za hemodijalizu namijenjenom samo za njih, a u 8 centara nisu. Istraživanje opisuje da se pojavnost HCV infekcija u hemodijaliziranih bolesnika smanjila nakon što su se počeli koristiti uređaji namijenjeni samo HCV‐pozitivnim pacijentima. Međutim, nije bilo moguće procijeniti korisne i štetne učinke povezane s izolacijom, cijenu ili smrtnost od bolesti.
Zaključci
Trenutno nije pronađeno dovoljno dokaza za pouzdani odgovor na postavljeno pitanje. Stoga bi dodatna istraživanja pomogla u razjašnjavanju pitanja može li izolacija smanjti prijenos HCV‐a u hemodijaliziranih bolesnika.
Authors' conclusions
Summary of findings
| Should patients with HCV be isolated in haemodialysis units for controlling the transmission of HCV? | ||||||
| Patient or population: patients in haemodialysis Intervention: isolation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with isolation | |||||
| Incidence of HCV infection (9 months) | Study population | RR 0.34 | 446 (1) | ⊕⊝⊝⊝ | Very low quality of evidence due to high risk of bias and imprecision | |
| 47 per 1.000 | 16 per 1.000 | |||||
| Incidence of HCV infection (18 months) | Study population | RR 0.22 | 281 (1) | ⊕⊝⊝⊝ | Very low quality of evidence due to high risk of bias and imprecision | |
| 58 per 1.000 | 13 per 1.000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
Hepatitis C virus (HCV) infection affects approximately 3% of the global population, or about 170 million people (Lee 2014). HCV causes persistent infection and chronic liver disease; long‐term sequelae include cirrhosis (30%) and liver cancer (1% to 5%) (Hsu 2015; Webster 2015).
Extrahepatic manifestations of chronic HCV infection are considered to be of immunologic origin and include cryoglobulinaemia, membranoproliferative glomerulonephritis, (Morales 2012) and porphyria cutanea tarda.
Prevalence of HCV infection in haemodialysis patients is usually higher than in the general population (Fabrizi 2012). The overall incidence rate of HCV infection is 1.47/100 patient‐years; 4.44/100 patient‐years in low‐ to middle‐income countries, and 0.99/100 patient‐years in high‐income countries (Su 2013). Prevalence ranges from less than 5% in most Northern European countries (Fissell 2004; Schneeberger 2000) to over 70% in many parts of the world, including countries in Asia, Latin America and North Africa (Sun 2009; Vladutiu 2000). However, the prevalence of HCV in European dialysis centres declined sharply in the early 2000s. This was attributed to reduced risk of hospital‐acquired infection and occupational HCV infection (Jadoul 2004), increased mortality rates, and stabilisation of the incidence of acute HCV infection (Espinosa 2004). Falling prevalence rates emphasise the importance of adhering to recommended infection control precautions and virological follow‐up to detect anti‐HCV antibodies using sensitive, specific new‐generation serological tests (Saune 2011).
HCV‐infected haemodialysis patients are at increased risk of liver or cardiovascular disease‐related death compared with non‐infected patients (Fabrizi 2012). HCV infection is associated with increased morbidity and mortality in kidney transplant recipients (Batty 2001; Butt 2007; Mahmoud 2004). Anti‐HCV‐positive patients on dialysis are at increased risk compared with anti‐HCV‐negative patients.
Description of the intervention
Isolating HCV‐infected patients (or patients waiting HCV screening results) during haemodialysis is defined as physical segregation from others for the express purpose of limiting direct or indirect transmission of HCV. Isolation policies may include strategies for HCV‐infected patients with different grades of intensity, such as use of a dedicated dialysis machine, personnel, room, or shift, or other barrier precautions (such as aprons, gowns, or gloves), by healthcare professionals attending these patients. These strategies may be implemented in combination.
How the intervention might work
HCV is easily transmitted parenterally and its control has therefore been a challenge in dialysis settings (Natov 2005; Su 2013). More recently erythropoietin therapy, which reduces requirements for transfusions, together with more sensitive tests to detect HCV in donated blood, have significantly reduced transmission via transfusion (Marwaha 2014). The post‐transfusion risk has been calculated at around 0.0001% in the USA (i.e. 1 blood transfusion in 1 million units of blood), with similar dramatic improvements in the viral safety of blood in other western countries (Selvarajah 2012).
The WHO has estimated an overall prevalence of 3% for HCV infection in the global population, but there is a wide geographic variability: fewer than 5% of people in most northern European countries are infected, close to 10% in southern Europe and the USA, and estimates range from 10% to 50% and up to 70% in many low‐ to middle‐income countries. HCV infection incidence has however decreased to less than 1.2% of people in high‐income countries (Espinosa 2004; Finelli 2005; Jadoul 2012).
This reduction was initially attributed to decreased rates of post‐transfusion infection (Djordjevic 2000; Valtuille 2002), but it was later ascribed to other infection control measures used to prevent hospital‐acquired infection rates in dialysis units. Prevalence of HCV infection among people on haemodialysis is generally below 10% in most countries, but may be higher (> 20%) where social crisis, war, or economic downturn exist (Ali 2011; Selm 2010; Voiculescu 2010). In these situations, maintenance of chronic haemodialysis programs is highly challenging, and infection control programs are difficult to maintain.
In spite of reduced rates of infection, HCV transmission in haemodialysis units remains an unsolved problem. Despite advances in screening blood products for HCV people on haemodialysis it remains at a higher risk of infection than in the general population (Ozer Etik 2015)
HCV seroconversion (change from anti‐HCV negative to anti‐HCV positive) has been detected in patients who were never transfused (Agarwal 2011) therefore other mechanisms of transmission occur in dialysis units. Shared haemodialysis machines (Elamin 2011; Sartor 2004) and reprocessing of dialysers from people with HCV have been linked to HCV transmission (Bashiri 2013). Other factors include physical proximity to an infected person and sharing personal items (Al‐Ghamdi 2004; Fabrizi 2008); breakdown in standard infection control practices, including improper handling and preparation of medications (Alter 2008; CDC 2009; Samandari 2005; Thompson 2009; Williams 2004); poor environmental cleaning (CDC 2009; Girou 2008; Kamili 2007; Patel 2010; Thompson 2009) and basic hygiene practices (Alfurayh 2000; Patel 2010); staff numbers and workload (Arenas 2005; CDC 2009; KDIGO 2008; Patel 2010; Shimokura 2011).
Why it is important to do this review
The evidence for or against the use of isolation of HCV‐infected patients during haemodialysis is weak and certain inconsistencies exist regarding the recommendations for its use among different guidelines. The centres for Disease Control and Prevention (CDC 2001; Mbaeyi 2013) published guidelines to prevent the transmission of HCV and other infections among haemodialysis patients, but did not recommend the isolation of HCV‐infected patients. KDIGO 2008 stated that haemodialysis units should ensure implementation of and adherence to strict infection‐control procedures designed to prevent transmission of blood‐borne pathogens, including HCV, but isolation of HCV‐infected patients was not recommended as an alternative to strict infection‐control procedures (unless in cases of continued hospital‐acquired transmission, where a local isolation policy may be deemed necessary). The UK Renal association stated that patients with HCV patients do not need to be dialysed in a segregated area, however more experienced staff should be assigned. If nosocomial transmission continues to occur, despite reinforcement and audit of the precautions, a local segregation policy may be deemed necessary (Geddes 2011)
The European Best Practice (ERBP) Work Group considers that implementation of universal hygienic measures should be the standard of care. Isolation of positive patients could be considered, but only if this practice does not have a negative impact on the implementation and reinforcement of basic hygienic measures in the unit as a whole (Covic 2009). Some investigators support isolating patients with HCV infection in a specific haemodialysis room (Fabrizi 2008), or suggest that the no isolation policy should not be generalised. Whether or not HCV‐positive patients should be isolated is still debated, particularly since isolation policies to control HCV infection transmission in haemodialysis units involves significant logistic problems that should be considered.
Objectives
This review aimed to evaluate the benefits and harms of isolation of HCV‐infected patients during haemodialysis on the transmission of HCV to other patients.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods), and cluster RCTs and cluster quasi‐RCTs (where centres rather than individual patients were randomised), looking at isolation of HCV‐infected patients during haemodialysis were eligible for inclusion.
Types of participants
All patients (adults and children) undergoing maintenance haemodialysis and dialysed in a haemodialysis centre (e.g. hospital unit, outpatient clinic) were eligible for inclusion.
Types of interventions
Intervention group
Any strategy targeting the isolation during haemodialysis of HCV‐infected patients or patients waiting HCV screening results was eligible. Isolation was defined as the physical segregation of these patients from others with the express purpose of limiting direct or indirect transmission of HCV to other patients. Isolation policies could include a number of strategies with different grades of intensity, such as the use of a dedicated dialysis machine, personnel, room or dialysis shift.
Control group
We considered any control group that enabled comparison to determine the relative effect of the isolation strategy as eligible for inclusion. Studies comparing two types of isolation strategies were also eligible.
Types of outcome measures
Primary outcomes
-
Incidence of dialysis‐acquired HCV infection
-
All‐cause mortality
-
Adverse effects associated to the isolation strategy (such as negative effects on patient mental well‐being, or adverse effects related to supportive care failures).
Secondary outcomes
-
Incidence of dialysis‐acquired non‐HCV infections
-
Patient satisfaction with treatment, measured with a validated tool
-
Isolation costs.
Search methods for identification of studies
Electronic searches
We searched Cochrane Kidney and Transplant's Specialised Register to 26 November 2015 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from the following sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials CENTRAL
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected kidney journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on Cochrane Kidney and Transplant scope. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
We searched the following electronic databases.
-
Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to 2015)
-
Web of Science Conference Proceedings Citation Index‐Science (CPCI‐S, 1990 to 2015) (accessed via ISI Web of Science)
-
ProQuest Dissertations & Theses Database (1990 to 2015)
-
Open Grey (1990 to 2015).
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
-
Reference lists of review articles and relevant studies and clinical practice guidelines.
-
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews thought to include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts, and if necessary the full text, of these studies to determine which satisfied the inclusion criteria.
Data extraction and management
Data extraction was to be carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were to be translated before assessment. Where more than one publication of one study existed, reports were to be grouped together and the publication with the most complete data used in analyses. Where relevant outcomes were only published in earlier versions these data were to be used. Any discrepancy between published versions was to be highlighted.
Assessment of risk of bias in included studies
The following items were to be independently assessed by two authors using the risk of bias assessment tool (Higgins 2011a) (see Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was recruitment bias adequately prevented?
-
Were baseline imbalances (in terms of either the clusters or the individuals) adequately addressed?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
-
Was the study analysed by correct statistical methods (i.e. taking the clustering into account)?
Measures of treatment effect
For dichotomous data and for counts of rare events and rates (dialysis‐acquired HCV infections, dialysis‐acquired non‐HCV infections, mortality due to dialysis‐acquired infections, all‐cause mortality, adverse effects) we planned to report risk ratios (RR); for continuous data (patient satisfaction with treatment) we planned to use the mean difference (MD) with 95% CI. Where different scales were used to measure continuous outcomes, we planned to calculate a standardised mean difference (SMD).
Unit of analysis issues
We planned to include cluster RCTs, and when possible, extract effect measures and standard error rates from an analysis taking clustering into account. If that was not possible, we planned to extract the number of clusters and estimate the intra‐cluster correlation coefficient to inform a reliable analysis. If this was not possible, we planned to disregard the clustering and investigate the effect of this in a sensitivity analysis (Deeks 2011).
Dealing with missing data
We planned to extract data for intention‐to‐treat analyses (ITT) and contact authors if required information was missing. Where ITT analysis was not possible, we planned to extract data from an available case analysis and assess the risk of bias from attrition.
Assessment of heterogeneity
We planned to analyse heterogeneity using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance, and the I² statistic.
Assessment of reporting biases
We did not expect that a sufficient number of studies would be identified to create a useful funnel plot. Assessing reporting bias is difficult, but we planned to note whether outcomes that we considered important were reported. We planned to contact authors about possible unpublished outcomes.
Data synthesis
We planned to use a random‐effects model and to express the results as both relative risks and number‐needed‐to‐screen to achieve the relevant outcomes, both beneficial and harmful.
Subgroup analysis and investigation of heterogeneity
We planned to perform the subgroup analyses
-
Mean duration of the haemodialysis treatment
-
The degree of missing primary outcome data
-
High prevalence of HCV in the haemodialysis unit: studies implemented in a context of high HCV prevalence (< 5%) versus non‐high HCV prevalence (≥ 5%)
-
-
Outbreak situation: studies implemented when healthcare associated infection (any pathogen) were noted to be increasing or to exceed a recognised benchmark versus non outbreak situation
-
Types of isolation.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors on effect size.
-
Repeating the meta‐analysis to assess the effect of excluding studies with high risk of bias
-
Exploring the impact of the assumptions taken in the available case analysis by performing a sensitivity analysis with imputation of missing data
-
Assessing the effect of the statistical model chosen for meta‐analysis (fixed‐effect model versus random‐effects model)
-
Repeating the meta‐analysis to assess the effect of including only studies with allocation to interventions at the group level (cluster designs) (Ukoumunne 1999).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
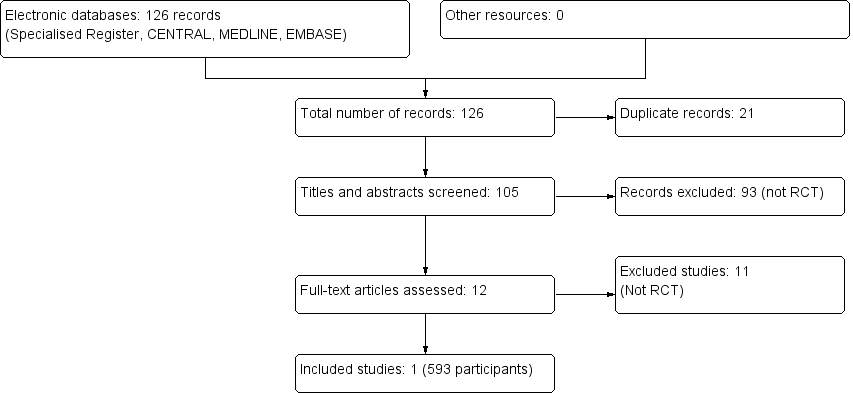
The combined search of MEDLINE, EMBASE and CENTRAL identified a total of 126 articles, of which 125 were excluded.
-
Twenty one records were duplicates
-
After reviewing the abstracts, 93 records were excluded because they did not meet our inclusion criteria
-
After full text review an additional 11 records, which were not RCTs, were excluded.
We therefore included one study enrolling 593 patients (Shamshirsaz 2004). A flow chart of the study selection process is shown in Figure 1
Included studies
Shamshirsaz 2004 was a prospective RCT evaluating the effect of dialysis machine separation in reducing HCV transmission to haemodialysis patients. Selected centres were randomly divided into dedicated and non‐dedicated haemodialysis machine groups. Positive cases were confirmed by RT‐PCR. Information regarding age, sex, occupation (health care personnel, surgeons and dentists), HCV‐infected relatives, previous peritoneal dialysis, surgery during last 2 months, duration of haemodialysis, number of blood product transfusions, history of organ transplantation, and the causes of ESRD was collected. Outcomes included incidence of HCV infection in both groups.
Patients were dialyzed for 4 to 4.5 hours, 2 or 3 times/week, using standard haemodialysis techniques. All included haemodialysis patients were HIV and hepatitis B surface antigen (HBsAg) negative. Dialysis membranes were low pressure and used only once and haemodialysis machines were bleached and rinsed between dialysis sessions according to the manufacturers' instruction. All machines were located in dialysis wards and not in separate rooms for both groups. Patient to staff ratio in the dedicated and non‐dedicated groups was not statistically different (3.1 and 3.4 respectively) and all staff members were negative for anti‐HCV. Education courses hygiene guidelines the CDC were conducted for all personnel involved in patient care, a checklist of the practice was used. In all centres the patients had specific dialysis place. It was specified how often the tests were performed HCV patients during the study or whether this was routinely carried out.
The patients were followed for 9 months (first follow‐up population) and 281 patients who remained within the study were followed for an additional 9 months (second follow‐up population). See Characteristics of included studies.
Excluded studies
Eleven studies were not RCTs (Agarwal 2009; Barril 2003; Gallego 2006; Garcia‐Valdecasas 1994; Huang 1995; Mohamed 2010; Ross 2009; Saxena 2003; Shebeb 2006; Valtuille 1998; Yang 2003)
Risk of bias in included studies
See risk of bias in Figure 2 and Figure 3.
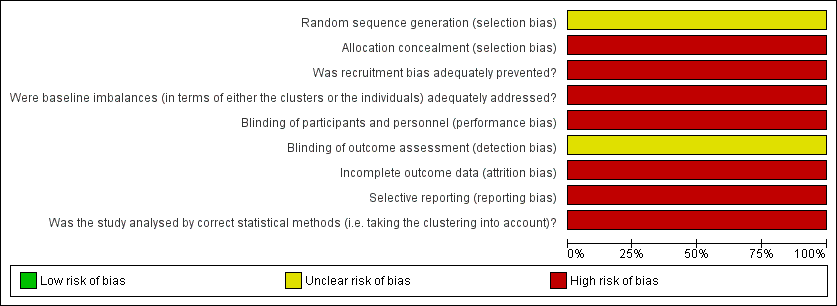
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across one included study.

Risk of bias summary: review authors' judgements about each risk of bias item for one included study.
Allocation
This study did not describe the use of a random number table to generate the allocation sequence. Allocation concealment was not performed
Randomisation was performed by dialysis centre. It was a cluster RCT, which included four centres (dedicated) compared with eight centres (non‐dedicated).
Blinding
Participants (patients and investigators) were not blinded. Blinding of outcome assessors was not reported.
Incomplete outcome data
Only 74.5% (446/593) of the patients were followed for 9 months, and 47.3% (281/593) were followed for an additional 9 months.
One centre was excluded after randomisation causing a deviation from protocol.
Selective reporting
The authors only reported one outcome, measuring the difference in the incidence of HCV in both groups. No secondary outcomes were reported.
The authors did not consider the exposure time, to determine the adjusted rate of seroconversion risk/patient‐year.
Other potential sources of bias
The assessments of the risk of bias domains relative to cluster designs are detailed in the risk of bias table (see Characteristics of included studies).
Effects of interventions
See: Summary of findings for the main comparison Dialysis machine separation versus usual care
Incidence of dialysis‐acquired HCV infection
Shamshirsaz 2004 reported the incidence of HCV infection was 1.6% in the dedicated group and 4.7% in the non‐dedicated one (Analysis 1.1 (1 study 446 participants): RR 0.34, 95% CI 0.11 to 1.07) during the first follow‐up period (9 months). During the second follow‐up period (18 months), the incidence was 1.3% in the dedicated group and 5.8% in the non‐dedicated group (Analysis 1.2 (1 study, 281 participants): RR 0.22, 95% CI 0.05 to 1.02). Therefore, we found no differences in terms of the number of participants developing HCV infection when comparing the dedicated group with the usual care. Moreover, the evidence was of very low quality, which means that we have very little confidence in the effect estimate and that the true effect is likely to be substantially different from the estimate of effect.
All‐cause mortality
Mortality was not reported.
Adverse effects
Adverse effects associated with the isolation strategy were not reported.
Incidence of dialysis‐acquired non‐HCV infections
Incidence of dialysis‐acquired non‐HCV infections were not reported.
Patient satisfaction with treatment
Patient satisfaction with treatment was not reported.
Isolation costs
Isolation costs were not reported.
Discussion
Summary of main results
Only one study meeting our inclusion criteria was identified (Shamshirsaz 2004). This study selected haemodialysis centres, one by one to reach a total number of 593 patients (12 centres). Selected centres were randomly divided in to dedicated and non‐dedicated haemodialysis machine groups, including 297 patients in the dedicated group (4 centres) and 296 patients in the non‐dedicated group (8 centres). This study found that the use of dialysis machines dedicated for HCV infected individuals, as compared with the use of non‐dedicated dialysis machines, made no difference in terms of reducing the incidence incidence of HCV infection during the first (9 months) or second (18 months) follow‐up periods. The quality of the evidence was very low (see summary of findings Table for the main comparison).
This study did not report any of our other primary outcomes of interest (all‐cause mortality, adverse effects associated with the strategy of isolation).
Overall completeness and applicability of evidence
We planned to include patients (adults and children) undergoing maintenance haemodialysis and dialysed in a haemodialysis centre and assess whether strategies isolation machine, room, staff or dialysis shift influence the transmission of hepatitis C. Our search just found one study where the separation of machines is evaluated as a form of isolation.
Consideration should also be given to both the prevalence of hepatitis C and the geographical region as this may influence the possibility of seroconversion. Regions with high prevalence may require isolation combined strategies: room, machine, and personnel. No costs are included for the isolation strategy.
Methods of randomisation were unclear, participants and patients were not blinded, so that the results of this study must be considered with some caution. Confirmatory research is required. Any further studies conducted in this area must be well designed RCTs assessing these primary outcomes.
Quality of the evidence
This review is based on the evidence of one RCT (Shamshirsaz 2004) that included a total of 12 haemodialysis centres (593 patients) divided into a group of dedicated dialysis machines involving four centres: 297 patients, 267 negative and 30 positive for HCV and a group of centres with non‐dedicated machines: 8 centres: 296 patients, 275 negative and 21 positive for HCV.
The authors did not disclose the details of the method of randomisation, participants and patients were not blinded, and blinding of outcome assessors was not reported. The authors decided to exclude, after randomisation, one of dialysis centres in the non‐dedicated group due to non‐adherence to CDC hygienic guidelines early in the study. There was a high risk of bias due to incomplete outcome data: only 74.5% of patients were followed for 9 months, and 47.3% were followed for an additional 9 months. In addition, the estimation of the effect of the intervention was imprecise. This makes the quality of evidence very low (see summary of findings Table for the main comparison).
Potential biases in the review process
We followed the Cochrane Collaboration guidelines for conducting this systematic review and meta‐analysis. Strengths of our review include the searching of several databases. Study selection, assessment of risk of bias, and data extraction were performed by two authors, which reduced the risk of error and bias. Although efforts were made to collect relevant data, the possibility of missing data cannot be excluded. Publication bias remains a possible source of important bias. Meanwhile, interpretation of the result should be done with extreme caution.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic review on this topic; many authors have reported some reduction (but not full prevention) of HCV transmission in haemodialysis, and after the adoption of an isolation policy, most of the studies have compared their results with their own historical control (before‐and‐after). Thus, it is unclear whether the reported improvement resulted from the adoption of an isolation policy or rather from the simultaneous raising of awareness and reinforcement of the application of hygienic precautions. These isolation policies include: implementing the isolation of a room (Agarwal 2009; Barril 2003); using exclusive machines (Garcia‐Valdecasas 1994); and isolating machines, room and staff (Gallego 2006; Huang 1995; Saxena 2003); and Mohamed 2010, Ross 2009 and Shebeb 2006, compared isolation versus universal precautions. In all cases isolation was found to decrease the rate of seroconversion. In addition, Yang 2003 conducted a study with three sets of patients: one set without isolation, a second set with a dedicated area and a dedicated machine in the same room and a third set of patients isolated in a separate room, and showed that isolation in a different room was better than dedicated machines.
In contrast, a DOPPS multicentre study concluded that isolation does not protect against transmission of HCV in haemodialysis patients (Fissell 2004). A prospective observational study by Jadoul 1998 showed a reduction from 1.4% to 0% of the annual incidence of HCV seroconversion. They have reported a reduction of HCV transmission after the reinforcement of basic hygienic precautions, without any isolation measures.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across one included study.

Risk of bias summary: review authors' judgements about each risk of bias item for one included study.

Comparison 1 Dialysis machine separation vs Usual care, Outcome 1 Incidence of HCV infection: 9 months.
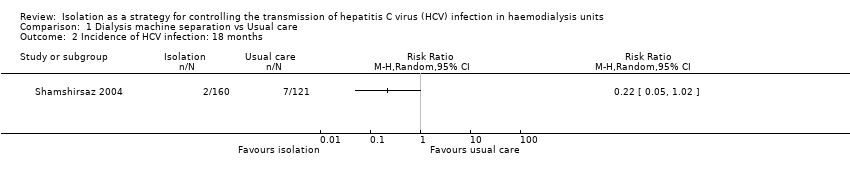
Comparison 1 Dialysis machine separation vs Usual care, Outcome 2 Incidence of HCV infection: 18 months.
| Should patients with HCV be isolated in haemodialysis units for controlling the transmission of HCV? | ||||||
| Patient or population: patients in haemodialysis Intervention: isolation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with isolation | |||||
| Incidence of HCV infection (9 months) | Study population | RR 0.34 | 446 (1) | ⊕⊝⊝⊝ | Very low quality of evidence due to high risk of bias and imprecision | |
| 47 per 1.000 | 16 per 1.000 | |||||
| Incidence of HCV infection (18 months) | Study population | RR 0.22 | 281 (1) | ⊕⊝⊝⊝ | Very low quality of evidence due to high risk of bias and imprecision | |
| 58 per 1.000 | 13 per 1.000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of HCV infection: 9 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Incidence of HCV infection: 18 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |


