Антигельминтные средства в эндемичных по гельминтам районах: влияние на прогрессирование ВИЧ‐инфекции
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Trial design: randomized, double‐blind, placebo‐controlled trial. Participants: adults with and without HIV infection who were found to be co‐infected with soil‐transmitted helminths (STHs) and tuberculosis (TB). Data of the HIV seropositive cohort only are included in this analysis | |
| Participants | Number of participants: a total of 140 helminth‐positive TB participants were enrolled and randomized. 72 participants were randomized to the treatment arm and 68 to the placebo arm. We included only data from the 18 HIV‐positive participants in the treatment arm and 14 HIV‐positive participants in the placebo arm in this Cochrane Review. Exclusion criteria: participants that required hospital admission, were pregnant, infected with Schistosoma spp., displayed symptoms of active helminth infection, or displayed signs of any concomitant chronic or infectious disease other than TB/HIV. | |
| Interventions | Intervention: albendazole treatment (400 mg/day) for 3 consecutive days. All helminth‐positive TB participants, including the placebo group, received deworming treatment at week 12. Randomization occurred 2 weeks following TB treatment. Control: identical placebo tablets. | |
| Outcomes | Outcomes included in this review: change in CD4+ T cells after three months, adverse events, and mortality events. Other trial outcomes: the primary outcome of the trial was change in TB score after 2 months. Other secondary outcomes were sputum smear conversion after 2 months, changes in the chest x‐ray pattern at week 12, IgE and eosinophil responses, as well as changes in the frequency of Tregs and IFN‐c, IL‐5, and IL‐10 producing peripheral blood mononuclear cells (PBMCs) after 3 months. | |
| Notes | Location: Gondar, Ethiopia. Participant helminth status: ascertained. Participant antiretroviral (ART) status: 94% and 100% of participants were on ART in the intervention and placebo groups, respectively. Note: this is the only trial in which all participants had a major co‐infection beyond the helminth and HIV relationship of interest, which might limit comparability to other findings in this review. Author contact: we requested additional data for the HIV cohort alone from the trial authors, who provided the data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers were generated in a block size of eight by the Addis Continental Institute of Public Health, Ethiopia." |
| Allocation concealment (selection bias) | Low risk | "All tablets looked identical and were assigned a treatment code by the manufacturer.The treatment allocated for each patient was concealed in an individual envelope." |
| Blinding of participants and personnel (performance bias) | Low risk | "Both the investigators and clinic staff were blinded to the treatment given." |
| Blinding of outcome assessment (detection bias) | Low risk | "The treatment code was kept in a sealed envelope at the manufacturer and opened after the last patient had been to a follow‐up visit and the data had been analysed." |
| Incomplete outcome data (attrition bias) | Low risk | No risk of attrition; 15% lost to follow‐up with 72 participants in the intervention group and 68 participants in the control group. |
| Methods | Trial design: randomized, unblinded, controlled trial. Participants: adults with and without HIV‐1 infection who were found to be infected with schistosomes. We only included data from the HIV seropositive cohort in this analysis. Length of follow‐up: HIV‐1 RNA and CD4+ T cell counts were measured at baseline and after 3 months. Monitoring and diagnostics: from Kallestrup 2005; Status for HIV was determined by a rapid HIV‐1/2 test in the field on a dry blood spot and two enzyme‐linked immunosorbent assays on serum. Infection with Schistosoma haematobium was diagnosed by microscopic identification and quantification of fixed‐volume urine samples filtered on Nytrel filters. Diagnosis of infection with Schistosoma mansoni and other helminth eggs or parasites was assessed by the modified formol‐ether concentration technique on 1 g of stool from each participant. | |
| Participants | Number of participants: 287 individuals were enrolled of whom we included 130 with HIV‐1 and schistosome co‐infection in this analysis. 64 participants received early praziquantel treatment and 66 received delayed treatment. Inclusion criteria: HIV‐1 and schistosomiasis co‐infection, HIV‐negative schistosomiasis infected, HIV‐1 negative but schistosomiasis infected, or neither infection. Exclusion criteria: pregnant women and participants presenting with clinical signs/symptoms of TB, terminal stages of schistosomiasis, or severe anaemia. | |
| Interventions | Intervention: participants received a single oral dose of praziquantel (40 mg/kg) at enrolment or after a delay of 3 months. Control: participants received a single oral dose of praziquantel (40 mg/kg) after a delay of 3 months following enrolment. | |
| Outcomes | Outcomes included in this review: changes in plasma HIV‐1 RNA levels, CD4+ T cell count, and haemoglobin levels between individuals randomized to early versus delayed treatment. Other trial outcomes: none | |
| Notes | Location: Shamva District, Zimbabwe. Participant helminth status: ascertained. Participant ART status: not stated. Author contact: we requested additional haemoglobin data from the trial authors for this update, who provided the data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Exact random sequence generation used unclear. From Kallestrup 2005: "Allocation of participants to these 3 control groups was done randomly: 1 participant with schistosomiasis was selected for every 2 coinfected participants, and 1 HIV‐1–positive participant or 1 healthy participant was selected for every 4 coinfected participants." |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not done. "On inclusion, all participants infected with schistosomes within each HIV‐1 group were openly randomised". |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded; "On inclusion, all participants infected with schistosomes within each HIV‐1 group were openly randomised into 2 equally sized groups". |
| Blinding of outcome assessment (detection bias) | Low risk | From Kallestrup 2005: "At all stages, when performing parasitological examinations, the technician was blinded to clinical and serological information". |
| Incomplete outcome data (attrition bias) | High risk | There is a risk of attrition as there was < 80% follow‐up. |
| Methods | Trial design: randomized double blind placebo controlled trial. Participants: HIV‐1 or HIV‐2 seropositive adults with persistent diarrhoea. Length of follow‐up: adverse and mortality events were recorded over 6 months of follow‐up. Monitoring and diagnostics: ELISA tests were used to determine HIV seropositivity. Home care staff noted any potential adverse effects. | |
| Participants | Number of participants: 174 participants were initially randomized but only 138 participants were followed‐up after 1 month and considered correctly randomized, with 69 in the intervention group and 69 in the placebo‐controlled group. Inclusion criteria: HIV‐positive adults with persistent diarrhoea (defined as loose but not bloody stools 3 or more times a day for 3 weeks or longer). Exclusion criteria: participants were excluded if they had received antibiotics in the preceding week, or were deteriorating clinically (Karnofsky score ≤ 20). | |
| Interventions | Intervention: 800 mg albendazole twice daily for 14 days for treatment of persistent diarrhoea in HIV‐positive participants. Control: identical placebos twice daily for 14 days. | |
| Outcomes | Outcomes included in this review: incidence of adverse events and mortality. Incidence of adverse events defined as exacerbated diarrhoea, cutaneous reaction, dizziness, headache, cough, and difficulty swallowing. Other trial outcomes: proportion of time periods during which diarrhoea was experienced after completion of treatment and proportion of participants with full remission after completion of treatment. | |
| Notes | Location: 3 urban hospitals/health centres in Zambia. Participant helminth status: not ascertained. Participant ART status: not specified, but the study took place before treatment was widely available in Zambia. Author contact: we did not contact the trial authors to provide additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the patients were randomised by allocation of a study pack containing either albendazole or placebo, which had been prepared in London according to a randomisation code." |
| Allocation concealment (selection bias) | Low risk | "The code (constructed so that the numbers of patients randomised to albendazole and placebo balanced every 20 patients) was kept in London during the study". |
| Blinding of participants and personnel (performance bias) | Low risk | "Each pack contained 112 tablets of albendazole or placebo, which were indistinguishable." |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) | High risk | There is a risk of attrition as there was < 80% follow‐up. |
| Methods | Trial design: randomized double‐blind placebo‐controlled cross‐over trial. Participants: HIV‐positive individuals with or without Wuchereria bancrofti co‐infection. Length of follow‐up: all subjects were followed‐up at 1, 12, 13, and 24 weeks after the 1st round of treatment. The data presented in the published manuscript considered the 12‐week visit as the initial visit and the 24‐week visit as the final visit. Individuals treated at the initial visit with diethylcarbamazine (DEC) were then considered as the 'placebo' arm and those who were not treated until the 12‐week visit were the 'treatment' arm. For the purposes of this analysis, we considered only individuals with confirmed HIV‐1 infection who were also filariasis‐infected at the baseline visit. We considered individuals treated with DEC at the initial visit to be in the 'treatment' arm and those who did not receive treatment to be in the 'placebo' arm. We reported outcomes as indicated for the 12‐week visit. As all participants were treated with DEC by the 12 week visit, we did not include data from the 24‐week follow‐up period. Monitoring and diagnostics: diagnosis of infection with lymphatic filariasis (LF) infection with W. bancrofti was performed using immunochromatographic tests (ICT) followed by ELISA testing for circulating filarial antigens (CFA) in serum samples. Participants were also screened for malaria and intestinal helminth eggs. | |
| Participants | Number of participants: the trial authors screened 858 adults and 34 HIV‐1 infected individuals were enrolled and randomized in the trial, of which 27 were followed‐up. Eighteen were co‐infected with W. bancrofti, and 16 were not co‐infected. In the co‐infected group, 10 participants were randomized to early treatment and 8 participants were randomized to delayed treatment. Twelve of these participants were followed‐up, 6 in each treatment group. The HIV RNA from one of the participants in the delayed treatment arm could not be amplified. Inclusion criteria: HIV‐positive individuals without clinical manifestations of HIV, with and without LF infection. Exclusion criteria: none specified. | |
| Interventions | Intervention: DEC (6 mg/kg) at randomization. Control: equivalent placebo and treatment after a delay of 3 months. | |
| Outcomes | Outcomes included in this review: plasma HIV‐1 RNA levels, CD4+ cell count, CD4 percent, serum concentrations of ferritin, adverse events. Other trial outcomes: CD4/CD8 ratio between individuals randomized to early versus delayed treatment (3 months later) (Nielsen 2007 TZA). Baseline and change in serum retinol, β‐carotene, α‐tocopherol, and the acute phase reactant a‐1 antichymotrypsin after 3 months (Nielsen 2009). | |
| Notes | Location: Northeastern Tanzania Participant helminth status: ascertained Participation ART status: the trial authors did not specify if any participants were receiving ART treatment at the start or during the trial. Author contact: we requested additional CD4 and HIV‐1 RNA data from the trial authors, who provided the data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Individuals were randomised (1:1). "The identical DEC and placebo tablets were packed in containers with different color codes (“red” or “blue”). Individuals were randomised (1:1) to receive treatment in the order of “red” followed by “blue” or “blue” followed by “red” by using a list of random numbers.The selected study participants were listed and numbered from 1 to 34, and the first 17 numbers (between 1 and 34) encountered in the table (when starting on a randomly chosen figure) were assigned to receive treatment in the order red–blue, and the remaining 17 received treatment in the order blue–red." |
| Allocation concealment (selection bias) | Unclear risk | This was not specified. |
| Blinding of participants and personnel (performance bias) | Low risk | "All study personnel and participants were blinded to treatment assignment throughout the study." |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) | High risk | There is a risk of attrition as there was < 80% follow‐up. However, this reflects 7 participants who were lost to follow‐up and therefore excluded from the analyses. The excluded participants did not significantly differ at baseline from the included participants. |
| Methods | Trial design: randomized, non‐blinded study. Participants: adult participants with chronic strongyloidiasis. Length of follow‐up: participants were followed up 2 weeks after treatment initiation, then 1 month, 3 months, 6 months, 9 months, and 1 year post‐treatment. Monitoring and diagnostics: infection with Strongyloides stercoralis was ascertained using the direct smear, formol‐ether concentration method, and modified Koga agar plate culture method. | |
| Participants | Number of participants: 90 adult participants with chronic Strongyloides infection were recruited. There were 10 participants with HIV co‐infections, with 3 HIV‐positive participants randomized to albendazole, 2 HIV‐positive participants randomized to single dose ivermectin, and 5 HIV‐positive participants randomized to double dose ivermectin. Inclusion criteria: adult participants with characteristic rhabditiform larvae of S. stercoralis present on faecal microscopy. Exclusion criteria: history of allergic reaction to either study medication, treatment within the month prior to the trial with any drug known to have anti‐Strongyloides activity, pregnancy, or lactation, and any suggestion of disseminated strongyloidiasis. | |
| Interventions | Intervention: Group 1: ivermectin delivered as a single dose of 200 µg/kg; Intervention. Group 2: 2 doses of ivermectin (200 µg/kg) delivered 2 weeks apart. For the purpose of this analysis we considered both groups that received ivermectin together. Control: participants received 7 days of albendazole (800 mg per day). | |
| Outcomes | Outcomes included in this review: incidence of adverse events defined as "symptoms or signs that developed after the study drug administration and had not been reported prior to the administration of the first dose of the antihelmintic." Other trial outcomes: treatment cure (defined as clinical improvement (if symptomatic before treatment) and the absence of rhabditiform larvae in the stool at day 14 of treatment and throughout the follow‐up period) and treatment failure (defined as the presence of larvae two weeks after initiation of treatment or the reappearance of larvae during follow‐up). | |
| Notes | Location: Siriraj Hospital, Thailand Participant helminth status: ascertained Participant ART status: it was unspecified if any individuals were receiving ART treatment at the start or during the trial. Author contact: we requested additional data regarding the incidence of adverse events in the HIV‐positive participants specifically from the trial authors, who provided this information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated, simple, random allocation sequences were prepared for 3 study groups by the investigator team." |
| Allocation concealment (selection bias) | Low risk | "These were sealed in an opaque envelope and numbered. The investigator assigned study participants to their respective treatment group after opening the sealed envelope." |
| Blinding of participants and personnel (performance bias) | High risk | "prospective open‐label, randomised, controlled study". |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | There was 10% loss to follow‐up; "Ten patients were excluded from analysis because they did not receive or complete the study treatment (3 in albendazole group, 2 in ivermectin‐II group), or they were lost to follow‐up immediately after treatment (3 in albendazole group, 1 each in ivermectin‐I and ivermectin‐II respectively)." |
| Methods | Trial design: randomized double‐blind placebo‐controlled trial. Partcipants: HIV‐1 positive adults with evidence of co‐infection with albendazole‐treatable STHs. Length of follow‐up: all subjects were followed‐up at 12 weeks post‐randomization. Monitoring and diagnostics: helminth diagnosis was performed using stool samples processed and evaluated with wet preparation, Kato‐Katz, and formol‐ether concentration techniques. HIV‐1 was diagnosed using Determine™ rapid test qualitative immunoassay. The CD4 lymphocyte count was determined using Multiset™ software on a FACSCalibur machine. Plasma HIV‐1 RNA was quantified using the Gen‐Probe HIV‐1 viral load assay. | |
| Participants | Number of participants: the trial screened 1551 adults attending HIV care clinics and 299 were infected with at least 1 helminth species. Regarding enrolment, 234 ART‐naive individuals were enrolled, of whom 208 HIV and STH co‐infected (hookworm, Ascaris, Trichuris, or Strongyloides) individuals were included in the final analysis, 108 were randomized to receive early treatment and 100 to receive placebo. Inclusion criteria: HIV‐1 seropositive adults, not pregnant, and ineligible for initiation of ART based on WHO guidelines (CD4 < 200 cells/mm³, any stage 4 and some stage 3 disease). Exclusion criteria: ever used ART drugs, took medicine for helminth infection in the preceding 6 months, evidence of active TB or TB treatment in the past 3 months, and clinical signs of severe anaemia. | |
| Interventions | Intervention: albendazole (400 mg per day) for 3 days versus placebo at enrolment. Control: placebo at enrolment. After a delay of 3 months, all participants showing evidence of helminth infection were treated with albendazole, regardless of randomization arm. | |
| Outcomes | Outcomes included in this review: changes in plasma HIV‐1 RNA levels and CD4+ count between individuals randomized to early versus delayed treatment (3 months later) and adverse events. Other trial outcomes: none. | |
| Notes | Location: 10 sites throughout Kenya. Participant helminth status: ascertained. Participant ART status: treatment naive. Author contact: the trial author provided additional CD4 and HIV‐1 RNA data for inclusion in this Cochrane Review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned to two groups using a 1:1 allocation scheme with block randomisation of 30 patients and following a random‐allocation list generated independently." |
| Allocation concealment (selection bias) | Low risk | "Pre‐labeled, sequentially numbered treatment packs were used. Both the active drug (albendazole) and an identical appearing placebo were provided by the drug manufacturer." |
| Blinding of participants and personnel (performance bias) | Low risk | "Investigators, clinic staff and patients were blinded to study‐group assignment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No risk of attrition; 0.5% lost to follow‐up, with 4 in the intervention arm and 5 in the control arm. |
| Methods | Trial design: non‐blinded randomized study. Partcipants: HIV‐positive ART naive adults in Kenya. Length of follow‐up: CD4+ cell counts measured every 6 months and plasma RNA measured every 12 months. Participants were followed for 24 months. Monitoring and diagnostics: HIV serological testing was conducted with Determine™ rapid tests, CD4 cell counts using a FACSCalibur™, plasma HIV RNA assays with the COBAS® Amplicor assay. | |
| Participants | Number of participants: 979 individuals were screened for enrolment in the trial and 917 individuals were enrolled and eligible, with 449 participants randomized to the treatment group and 468 randomized to the control group. All participants were administered cotrimoxazole prophylaxis. Inclusion criteria: adults ≥ 18 years, HIV seropositive, and do not meet criteria for ART initiation (on the basis of documented WHO disease stage and CD4+ cell count within the previous 3 months and a clinical assessment at enrolment). Exclusion criteria: pregnancy, reports of taking antihelminthics in the previous 6 months, reports of having previously received ART ((except for the prevention of mother‐to‐child transmission). | |
| Interventions | Intervention: empiric deworming with repeat single‐dose albendazole (400 mg) given every 3 months plus single dose praziquantel (25 mg/kg) given annually. Participants were excluded if they missed two or more consecutive doses of study drug. Control: standard of care. | |
| Outcomes | Outcomes included in this review: changes in plasma HIV‐1 RNA levels, CD4+ cell count, non‐traumatic death, and adverse events between individuals randomized to treatment versus no intervention. Other trial outcomes: time to CD4+ count of < 350 cells/μL and first occurrence of any of the following: CD4+ count < 350 cells/μL, first reported use of ART (excluding that used for the prevention of mother‐to‐child transmission), or non‐traumatic death. Secondary analyses included time to death and ART initiation separately. | |
| Notes | Location: 3 sites in Kenya Participant helminth status: helminth status of participants was not evaluated at baseline. Participant ART status: treatment naive Author contact: the trial author provided additional CD4+ and HIV‐1 RNA data for inclusion in this Cochrane Review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a computer‐generated randomisation sequence, we assigned participants (1:1) to either the treatment group or control group." |
| Allocation concealment (selection bias) | Low risk | "We used a computerised database to ensure that treatment allocation was not disclosed to study staff and participants until randomisation was complete." |
| Blinding of participants and personnel (performance bias) | High risk | The trial participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | There is no risk of attrition; 4.2% of participants were lost to follow‐up with 16 in the intervention arm and 24 in the control arm. |
| Methods | Trial design: 2 x 2 randomized double‐blind placebo controlled trial. Partcipants: HIV‐positive ART‐naive pregnant women. Length of follow‐up: viral load was measured 6 weeks post‐treatment and at delivery. However, this analysis included data at 6 weeks post‐treatment only in order to minimize the effects of prenatal HIV care provided to study participants. Monitoring and diagnostics: stool samples were processed and examined for helminth ova using a duplicate Kato‐Katz method and by charcoal culture for Strongyloides. | |
| Participants | Number of participants: 2507 women were enrolled in the parent study (EMaBS), of whom 299 tested positive for HIV. Of these, 222 participants were randomized and followed‐up to 6 weeks post‐enrolment. Sixty‐four participants were randomized to single‐dose albendazole, 54 participants to praziquantel, 67 participants to both albendazole and praziquantel, and 70 participants to placebo only. Inclusion criteria: pregnant women presenting at the government‐funded antenatal clinic at Entebbe General Hospital, who were resident in the study area, planning to deliver in the hospital, willing to know their HIV status, and in the 2nd or 3rd trimester of pregnancy. Exclusion criteria: evidence of possible helminth‐induced pathology (haemoglobin < 8 g/dL, clinically apparent severe liver disease, diarrhoea with blood in stool), history of adverse reaction to antihelminthics, prior enrolment in an earlier pregnancy, or abnormal pregnancy as assessed by the midwife. | |
| Interventions | Intervention 1: albendazole (400 mg). Intervention 2: praziquantel (40 mg/kg). Intervention 3: both albendazole (400 mg) and praziquantel (40 mg/kg). Control: equivalent placebos. | |
| Outcomes | Outcomes included in this review: changes in plasma HIV‐1 RNA levels 6 weeks post‐treatment, adverse events (defined as post‐treatment hospitalizations), and mortality. Other trial outcomes: primary outcomes for EMaBS were response to immunisation and incidence of infectious diseases in the offspring, and vertical transmission of HIV (Webb 2011). Secondary outcomes in this analysis included viral load at delivery. | |
| Notes | Location: Entebbe, Uganda. Participant helminth status: ascertained. Participant ART status: ART naive. However, in accordance with guidelines at the time, HIV‐positive women were counselled and given intrapartum and neonatal single dose nevirapine for prevention of mother‐to‐child HIV transmission. After enrolment, women received standard antenatal care, including haematinics, tetanus immunization, and intermittent presumptive treatment for malaria. Author contact: we requested additional HIV‐1 RNA and adverse events data from the trial authors, who provided this information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were then randomised in a 1:1:1:1 ratio to single‐dose albendazole 400 mg or matching placebo and praziquantel 40 mg/kg or matching placebo in a 2×2 factorial design. The randomisation code was generated by the trial statistician in blocks of 100." |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes containing the study intervention were prepared by colleagues at the Medical Research Council Unit in Entebbe with no other involvement in the trial. Treatments were allocated in numerical order by trained interviewer‐counsellors and taken under observation." |
| Blinding of participants and personnel (performance bias) | Low risk | "All participants and staff were blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | There is no risk of attrition; 10% of participants were lost to follow‐up. |
Abbreviations: antiretroviral treatment (ART), circulating filarial antigens (CFA), diethylcarbamazine (DEC), human immunodeficiency virus (HIV), immunochromatographic tests (ICT), soil‐transmitted helminths (STHs), tuberculosis (TB)
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This manuscript presents further analyses using data already presented in Walson 2008 KEN | |
| Observational, prospective cohort study. | |
| Observational, prospective cohort study. | |
| Observational, prospective cohort study. | |
| Study protocol for included study Webb 2012 UGA. | |
| This manuscript presents further analyses using data already presented in Kallestrup 2005 ZWE. | |
| A RCT with non‐relevant intervention (that is, iron supplementation and multivitamins) to assess effects of anaemia in children 6 to 59 months of age. | |
| Observational, prospective cohort study. | |
| Observational, prospective cohort study. | |
| Observational, retrospective cohort study. | |
| Observational, prospective cohort study. | |
| Observational, prospective case‐control study, with irrelevant outcomes to this Cochrane Review. | |
| Case study of a single participant. | |
| Observational, cross‐sectional study. | |
| Observational, cross‐sectional study. | |
| Observational, prospective cohort study. | |
| This manuscript presents further analyses using data already presented in Kallestrup 2005 ZWE. | |
| Observational, prospective cohort study. | |
| Observational, prospective cohort study. | |
| Observational, prospective cohort study. | |
| Observational, case‐control study. | |
| Observational retrospective cohort study. | |
| Observational, prospective cohort study with no comparison group. | |
| Observational, cross‐sectional study. | |
| Observational, prospective cohort study. | |
| Observational, prospective cohort study. | |
| Observational, prospective cohort study | |
| Observational, prospective cohort study. | |
| A RCT with irrelevant outcomes to this Cochrane Review. The study authors hypothesize that antihelminthic treatment in pregnancy and early childhood would improve responses to immunization and modulate disease incidence in early childhood. | |
| Observational, prospective cohort study. | |
| Observational, prospective cohort study. | |
| Observational, prospective cohort study. | |
| Observational, prospective cohort study. |
Abbreviations: RCT: randomized controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Viral load: change in log10 HIV‐1 RNA Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Deworming drugs versus placebo in people with unknown helminth infection status, Outcome 1 Viral load: change in log10 HIV‐1 RNA. | ||||
| 1.1 At 6 weeks after one dose | 1 | 166 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.35, 0.07] |
| 1.2 At 2 years after multiple doses | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2 Change in CD4 count Show forest plot | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐10.15, 15.35] |
| Analysis 1.2  Comparison 1 Deworming drugs versus placebo in people with unknown helminth infection status, Outcome 2 Change in CD4 count. | ||||
| 2.1 At 2 years after multiple doses | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐10.15, 15.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Viral load: change in log10 HIV‐1 RNA Show forest plot | 4 | 445 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.26, ‐0.00] |
| Analysis 2.1  Comparison 2 Deworming drugs versus placebo in people with confirmed helminth infection, Outcome 1 Viral load: change in log10 HIV‐1 RNA. | ||||
| 1.1 Soil‐transmitted helminths | 2 | 273 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.31, 0.12] |
| 1.2 Schistosomiasis | 2 | 161 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.36, 0.10] |
| 1.3 Lymphatic filariasis | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.93, 0.77] |
| 2 Change in CD4 count Show forest plot | 3 | 358 | Mean Difference (IV, Random, 95% CI) | 37.86 [7.36, 68.35] |
| Analysis 2.2  Comparison 2 Deworming drugs versus placebo in people with confirmed helminth infection, Outcome 2 Change in CD4 count. | ||||
| 2.1 Soil‐transmitted helminths | 2 | 228 | Mean Difference (IV, Random, 95% CI) | 40.69 [1.51, 79.87] |
| 2.2 Schistosomiasis | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 33.5 [‐15.06, 82.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in log10 HIV RNA, by antihelminthic Show forest plot | 5 | 1534 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.19, 0.01] |
| Analysis 3.1  Comparison 3 Deworming drugs versus placebo; all trials, Outcome 1 Change in log10 HIV RNA, by antihelminthic. | ||||
| 1.1 Albendazole | 2 | 302 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.37, 0.02] |
| 1.2 Praziquantel | 2 | 213 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.32, ‐0.03] |
| 1.3 Diethylcarbamazine | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.93, 0.77] |
| 1.4 Albendazole and praziquantel | 2 | 1008 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.05] |
| 2 Difference in adverse events between treatment and no treatment groups Show forest plot | 7 | 1649 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.53, 2.83] |
| Analysis 3.2 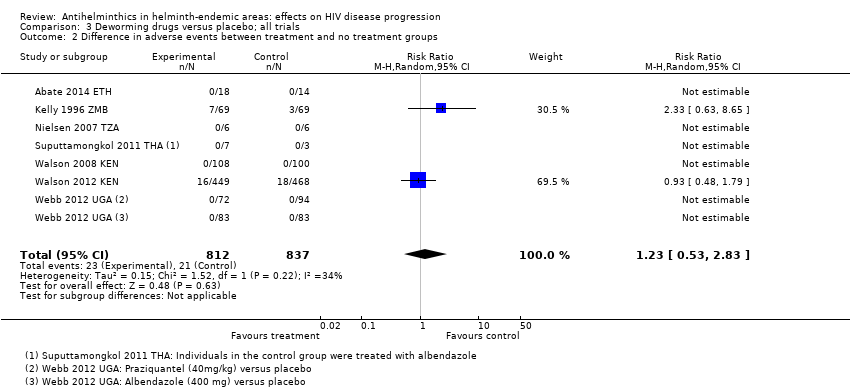 Comparison 3 Deworming drugs versus placebo; all trials, Outcome 2 Difference in adverse events between treatment and no treatment groups. | ||||
| 3 Difference in mortality events between treatment and no treatment groups Show forest plot | 5 | 1627 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.52, 1.14] |
| Analysis 3.3  Comparison 3 Deworming drugs versus placebo; all trials, Outcome 3 Difference in mortality events between treatment and no treatment groups. | ||||

Study flow diagram.
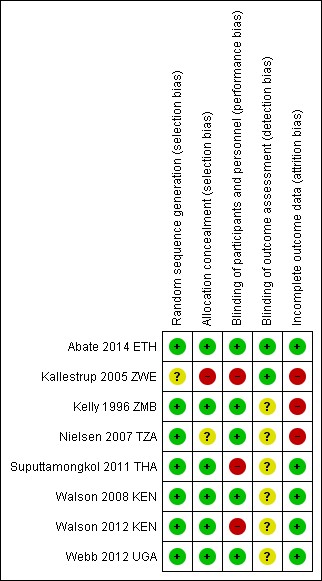
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.

'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included trials.
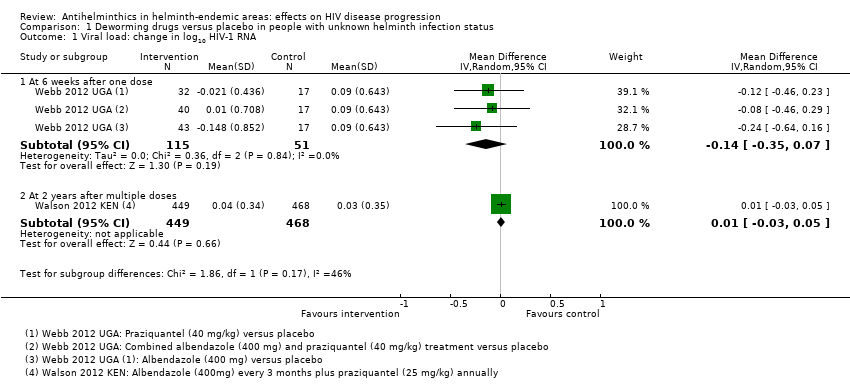
Comparison 1 Deworming drugs versus placebo in people with unknown helminth infection status, Outcome 1 Viral load: change in log10 HIV‐1 RNA.

Comparison 1 Deworming drugs versus placebo in people with unknown helminth infection status, Outcome 2 Change in CD4 count.
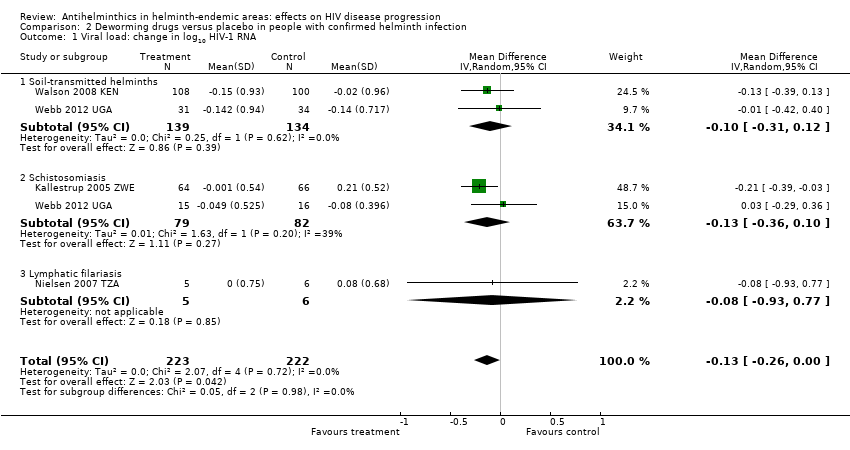
Comparison 2 Deworming drugs versus placebo in people with confirmed helminth infection, Outcome 1 Viral load: change in log10 HIV‐1 RNA.

Comparison 2 Deworming drugs versus placebo in people with confirmed helminth infection, Outcome 2 Change in CD4 count.

Comparison 3 Deworming drugs versus placebo; all trials, Outcome 1 Change in log10 HIV RNA, by antihelminthic.

Comparison 3 Deworming drugs versus placebo; all trials, Outcome 2 Difference in adverse events between treatment and no treatment groups.
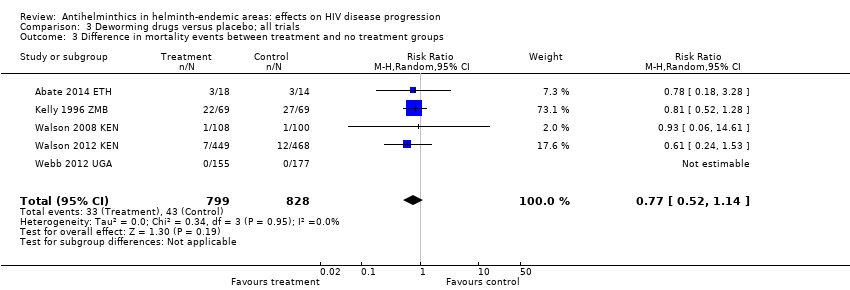
Comparison 3 Deworming drugs versus placebo; all trials, Outcome 3 Difference in mortality events between treatment and no treatment groups.
| Deworming drugs compared with placebo for people with HIV and an unknown helminth infection status | ||||
| Participant or population: HIV‐positive people Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole or praziquantel or a combination) Comparison: placebo | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | |||
| Placebo | Deworming drugs | |||
| Viral load | At 6 weeks after a single dose | 166 (1 trial) | ⊕⊕⊝⊝1,2,3,4 | |
| in the control group, the mean change in viral load was an increase of 0.09 log10 viral RNA | On average, with deworming, there was a suppressive effect on mean viral load of 0.14 log10 viral RNA (0.35 benefit to 0.07 harm) | |||
| At 2 years after multiple doses | 917 (1 trial) | ⊕⊕⊕⊝1,2,5,6 | ||
| In the control group, the mean viral load increased by 0.03 log10 viral RNA | On average, with deworming, there was a suppressive effect on mean viral load of 0.01 log10 viral RNA (0.03 benefit to 0.05 harm) | |||
| CD4+ cell count | At 2 years after multiple doses | 917 (1 trial) | ⊕⊕⊝⊝1,2,4,5 | |
| In the control group, the mean CD4+ cell count reduced by 37.3 CD4+ cells/µL | On average, with deworming, there was a favourable effect on mean CD4+ cell count of 2.60 CD4+ cells/µL (15.35 benefit 10.15 harm) | |||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1No serious risk of bias: this single trial was at low risk of selection bias. | ||||
| Deworming drugs compared with placebo for people with HIV and confirmed helminth infections | ||||
| Participant or population: HIV‐positive people with proven helminth infection Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole, praziquantel, albendazole and praziquantel, or diethylcarbamazine) Comparison: placebo | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | |||
| Placebo | Deworming drugs | |||
| Viral load | At 6 to 12 weeks | 445 (4 trials) | ⊕⊕⊝⊝ | |
| In the control groups, the mean change in viral load ranged from a 0.13 decrease to an increase of 0.21 log10 viral RNA | On average, with deworming, there was a small suppressive effect on mean viral load of 0.13 log10 viral RNA (0.26 benefit to 0.00 benefit) | |||
| CD4+ cell count | At 6 to 12 weeks | 358 (3 trials) | ⊕⊕⊝⊝ | |
| In the control groups, the mean change in CD4+ cell count ranged from a decrease of 68 to an increase of 45 CD4+ cells/µL | On average, with deworming, there was favourable effect on mean CD4+ cell count of 37.86 CD4+ cells/µL (7.36 benefit to 68.35 benefit) | |||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1We downgraded by 1 for serious risk of bias: of the five studies, only one had CIs that excluded the possibility of no effect and this study was at high risk of selection bias. | ||||
| Deworming drugs compared with placebo for people with HIV and both known and unknown helminth infection status | |||||
| Participant or population: HIV‐positive people Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole, praziquantel, albendazole and praziquantel, or diethylcarbamazine) Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Albendazole | ||||
| Iron deficiency (serum ferritin) | log10 mean increase of 0.04 µg/L | log10 mean increase of 0.07 µg/L | Deworming drugs associated with a 0.03 higher µg/L log10 mean ferritin measure (0.30 lower to 0.35 higher) | 16 (1 trial) | ⊕⊝⊝⊝1,2,3,4 |
| Anaemia (serum haemoglobin) | Increase in 0.15 g/dL | Decrease in 0.10 g/dL | Deworming drugs associated with a 0.25 lower g/dL haemoglobin (0.58 lower to 0.08 higher) | 130 (1 trial) | ⊕⊝⊝⊝2,4,5,6 |
| Mortality | 41 per 1000 | 52 per 1000 | RR 0.77 (0.52 to 1.14) | 1627 (5 trials) | ⊕⊕⊝⊝7,8,9,10 |
| Adverse events | 32 per 1000 | 35 per 1000 | RR 1.23 (0.53 to 2.83) | 1649 (7 trials) | ⊕⊝⊝⊝4,9,11,12 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1No serious risk of bias: this single trial was at risk of selection bias. However, the outcome is an objective laboratory measure. We did not downgrade the quality of the evidence. | |||||
| Number | Search terms |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) |
| #2 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) |
| #3 | Search (helminths[mh] OR helminth*[tiab] OR nematode*[tiab] OR worm*[tiab] OR parasites[mh] OR parasit*[tiab] OR round worm*[tiab] OR roundworm*[tiab] OR hookworm*[tiab] OR hook worm*[tiab] OR ancylostoma*[tiab] OR cestode*[tiab] OR tapeworm*[tiab] OR tape worm*[tiab] OR trematode*[tiab] OR fluke*[tiab] OR whipworm*[tiab] OR whip worm*[tiab] OR trichuris[tiab] OR ascaris[tiab] OR enterobi*[tiab] OR strongyloide*[tiab] OR mansonell*[tiab] OR taenia[tiab] schistosom*[tiab] OR necator*[tiab] OR paragonim*[tiab] OR hymenolepis[tiab] OR fasciol*[tiab] OR filariasis[tiab] OR trichostrongl*[tiab] OR microfilaria[tiab] OR parasitic diseases[mh:noexp] OR helminthiasis[mh] OR intestinal diseases, parasitic[mh]) |
| #4 | Search (benzimidazoles OR albendazole OR mebendazole OR ivermectin OR praziquantel OR diethylcarbamazine OR bithionol OR oxamniquine OR pyrantel OR nitazoxanide OR anthelmintic OR anthelmintics OR anthelminthic OR anthelminthics OR “anti helminthic” OR “anti helminthics” OR “anti helmintic” OR “anti helmintics” OR antihelminthic OR antihelminthics OR antihelmintic OR antihelmintics) |
| #5 | Search (#1 AND #2 AND #3 AND #4) |
| #6 | Search (((#1 AND #2 AND #3 AND #4))) AND ("1980/01/01"[Date ‐ Publication] : "2015/09/29"[Date ‐ Publication]) |
| Number | Search terms |
| #1 | 'human immunodeficiency virus infection' exp OR 'human immunodeficiency virus infection':ab,ti OR 'hiv infection':ab,ti OR 'hiv infections':ab,ti OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR hiv:ab,ti OR 'hiv 1':ab,ti OR 'hiv 2':ab,ti OR 'human immune deficiency virus':ab,ti OR 'human immuno deficiency virus':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno deficiency syndrome':ab,ti OR 'acquired immune deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti |
| #2 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure'OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl*NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (crossNEXT/1 over*):ab,ti |
| #3 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de |
| #4 | 'human'/de OR 'normal human'/de OR 'human cell'/de |
| #5 | #3 AND #4 |
| #6 | #3 NOT #5 |
| #7 | #2 NOT #6 |
| #8 | 'helminth'/exp OR helminth*:ab,ti OR nematode*:ab,ti OR worm*:ab,ti OR 'parasite'/exp OR parasit*:ab,ti OR roundAND worm*:ab,ti OR roundworm*:ab,ti OR hookworm*:ab,ti OR hookAND worm*:ab,ti OR ancylostoma*:ab,ti OR cestode*:ab,ti OR tapeworm*:ab,ti OR tapeAND worm*:ab,ti OR trematode*:ab,ti OR fluke*:ab,ti OR whipworm*:ab,ti OR whipAND worm*:ab,ti OR trichuris:ab,ti OR ascaris:ab,ti OR enterobi*:ab,ti OR strongyloide*:ab,ti OR mansonell*:ab,ti OR taenia:ab,ti AND schistosom*:ab,ti OR necator*:ab,ti OR paragonim*:ab,ti OR hymenolepis:ab,ti OR fasciol*:ab,ti OR filariasis:ab,ti OR trichostrongl*:ab,ti OR microfilaria:ab,ti OR 'parasitic diseases'/exp OR 'helminthiasis'/de OR 'intestine infection'/de |
| #9 | 'benzimidazoles'/de OR benzimidazolesOR 'albendazole'/de OR albendazoleOR 'mebendazole'/de OR mebendazoleOR 'ivermectin'/de OR ivermectinOR 'praziquantel'/de OR praziquantelOR 'diethylcarbamazine'/de OR diethylcarbamazineOR 'bithionol'/de OR bithionolOR 'oxamniquine'/de OR oxamniquineOR 'pyrantel'/de OR pyrantelOR 'nitazoxanide'/de OR nitazoxanideOR 'anthelmintic'/de OR anthelminticOR 'anthelmintics'/de OR anthelminticsOR 'anthelminthic'/de OR anthelminthicOR anthelminthicsOR 'anti helminthic'OR 'anti helminthics'OR 'anti helmintic'OR 'anti helmintics'OR antihelminthicOR antihelminthicsOR 'antihelmintic'/de OR antihelminticOR 'antihelmintics'/de OR antihelmintics |
| #10 | #1 AND #7 AND #8 AND #9 |
| #11 | #1 AND #7 AND #8 AND #9 AND [1‐1‐1980]/sd NOT [29‐09‐2015]/sd |
| Number | Search terms |
| #1 | MeSH descriptor: [HIV Infections] explode all trees |
| #2 | MeSH descriptor: [HIV] explode all trees |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only |
| #6 | #1 or #2 or #3 or #4 or #5 |
| #7 | [mh helminths] or helminth*:ti,ab,kw or meatode*:ti,ab,kw or worm*:ti,ab,kw or [mh parasites] or parasit*:ti,ab,kw or round worm:ti,ab,kw or roundworm:ti,ab,kw or hook worm*:ti,ab,kw or hookworm*:ti,ab,kw or ancylostoma*:ti,ab,kw or cestode*:ti,ab,kw or tapeworm*:ti,ab,kw or tape worm*:ti,ab,kw or trematode*:ti,ab,kw or fluke*:ti,ab,kw or whipworm*:ti,ab,kw or whip worm*:ti,ab,kw or trichuris:ti,ab,kw or ascaris:ti,ab,kw or enterobi*:ti,ab,kw or strongyloide*:ti,ab,kw or mansonell*:ti,ab,kw or taenia:ti,ab,kw schistosom*:ti,ab,kw or necator*:ti,ab,kw or paragonim*:ti,ab,kw or hymenolepis:ti,ab,kw or fasciol*:ti,ab,kw or filariasis:ti,ab,kw or trichostrongl*:ti,ab,kw or microfilaria:ti,ab,kw or [mh ^"parasitic diseases"] or [mh helminthiasis] or [mh ^"intestinal diseases, parasitic"] (Word variations have been searched) |
| #8 | benzimidazoles or albendazole or mebendazole or ivermectin or praziquantel or diethylcarbamazine or bithionol or oxamniquine or pyrantel or nitazoxanide or anthelmintic or anthelmintics or anthelminthic or anthelminthics or "anti helminthic" or "anti helminthics" or "anti helmintic" or "anti helmintics" or antihelminthic or antihelminthics or antihelmintic or antihelmintics (Word variations have been searched) |
| #9 | #6 and #7 and #8 Publication Year from 1980 to 2015, in Trials |
| Number | Search terms |
| #1 | HIV AND HELMINTH |
| Number | Search terms |
| #1 | HIV AND HELMINTH | Interventional Studies | received from 01/01/1980 to 09/29/2015 |
| Number | Search terms |
| #1 | “HIV Infections”[MeSH] OR “HIV”[MeSH] OR hiv [tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immunodeficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immunodeficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR “Sexually Transmitted Diseases, Viral”[MeSH:NoExp] |
| #2 | randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR (“clinical trial” [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) |
| #3 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CESTODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS OR HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #4 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #5 | #3 AND #4 |
| #6 | #1 AND #2 AND #5 |
| #7 | #1 AND #2 AND #5 Limits: Publication Date from 1980 to 2015/09/29 |
| Number | Search terms |
| #1 | ((’human immunodeficiency virus infection’/exp OR ’human immunodeficiency virus infection’) OR (’human immunodeficiency virus infection’/exp OR ’human immunodeficiency virus infection’)) OR ((’human immunodeficiency virus’/exp OR ’human immunodeficiency virus’) OR (’human immunodeficiency virus’/exp OR ’human immunodeficiency virus’)) OR (hiv:ti OR hiv:ab) OR (’hiv‐1’:ti OR ’hiv‐1’:ab) OR (’hiv‐2’:ti OR ’hiv‐2’:ab) OR (’human immunodeficiency virus’:ti OR ’human immunodeficiency virus’:ab) OR (’human immuno‐deficiency virus’:ti OR ’human immuno‐deficiency virus’:ab) OR (’human immunedeficiency virus’:ti OR ’human immunedeficiency virus’:ab) OR (’human immune‐deficiency virus’:ti OR ’human immune‐deficiency virus’:ab) OR (’acquired immune‐deficiency syndrome’:ti OR ’acquired immune‐deficiency syndrome’:ab) OR (’acquired immunedeficiency syndrome’:ti OR ’acquired immunedeficiency syndrome’:ab) OR (’acquired immunodeficiency syndrome’:ti OR ’acquired immunodeficiency syndrome’:ab) OR (’acquired immuno‐deficiency syndrome’:ti OR ’acquired immuno‐deficiency syndrome’:ab) |
| #2 | random:ti OR random:ab OR factorial:ti OR factorial*:ab OR 'cross over':ti OR 'cross over':ab OR crossover:ti OR crossover:ab OR placebo:ti OR placebo:ab OR (double:ti AND blind:ti) OR (doubl:ab AND blind*:ab) OR (single:ti AND blind:ti) OR (single:ab AND blind:ab) OR assign:ti OR assign:ab OR allocat:ti OR allocate:ab OR volunteer:ti OR volunteer:ab OR 'crossover procedure'/exp OR 'crossover procedure' OR 'double‐blind procedure'/exp OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure' OR 'randomized controlled trial'/exp OR 'randomized controlled trial' |
| #3 | 'helminths' OR 'roundworm' OR 'round worm' OR 'round‐worm' OR roundworms OR 'round worms' OR 'round‐worms' OR nematodes OR 'nematode' OR 'cestode' OR cestodes OR 'tapeworm' OR 'tape worm' OR 'tape‐worm' OR tapeworms OR 'tape worms' OR 'tape‐worms' OR 'trematode' OR trematodes OR 'fluke' OR flukes OR 'worm' OR worms OR 'parasite' OR 'parasites' OR 'ascaris' OR 'trichuris' OR 'enterobius' OR strongyloide OR stronglyloides OR 'ancylostoma' OR ancylostomas OR 'necator' OR necators OR 'hymenolepis' OR 'paragonimus' OR 'fasciola' OR 'taenia' OR 'hookworm' OR 'hook worm' OR 'hook‐worm' OR hookworms OR 'hook worms' OR 'hook‐worms' OR 'whipworm' OR 'whip worm' OR 'whip‐worm' OR whipworms OR 'whip worms' OR 'whip‐worms' OR shistosomiasis OR 'mansonella' OR 'filariasis' OR 'microfilaria' OR 'trichostrongylus' OR trichostronglylosis OR stronglyloidea OR pargonimiasis |
| #4 | ((’benzimidazoles’/exp OR ’benzimidazoles’) OR (’benzimidazoles’/exp OR ’benzimidazoles’)) OR ((’albendazole’/exp OR ’albendazole’) OR (’albendazole’/exp OR ’albendazole’)) OR ((’mebendazole’/exp OR ’mebendazole’) OR (’mebendazole’/exp OR ’mebendazole’)) OR ((’ivermectin’/exp OR ’ivermectin’) OR (’ivermectin’/exp OR ’ivermectin’)) OR ((’praziquantel’/exp OR ’praziquantel’) OR (’praziquantel’/exp OR ’praziquantel’)) OR ((’diethylcarbamazine’/exp OR ’diethylcarbamazine’) OR (’diethylcarbamazine’/exp OR ’diethylcarbamazine’)) OR ((’bithionol’/exp OR ’bithionol’) OR (’bithionol’/exp OR ’bithionol’)) OR ((’oxamniquine’/exp OR ’oxamniquine’) OR (’oxamniquine’/exp OR ’oxamniquine’)) OR ((’pyrantel’/exp OR ’pyrantel’) OR (’pyrantel’/exp OR ’pyrantel’)) OR ((’nitazoxanide’/exp OR ’nitazoxanide’) OR (’nitazoxanide’/exp OR ’nitazoxanide’)) |
| #5 | #3 OR #4 |
| #6 | #1 AND #2 AND #5 [1‐1‐1980]/sd NOT [29‐09‐2015]/sd |
| Number | Search terms |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (VIRAL SEXUALLY TRANSMITTED DISEASES) |
| #2 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CES‐TODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS OR HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #3 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #4 | #2 OR #3 |
| #5 | #1 AND #4 from 1980 to 2015 |
| Number | Search terms |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) |
| #2 | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐ BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (“CLINICAL TRIAL”) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND*)) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN) |
| #3 | #1 AND #2 |
| #4 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CES‐TODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS |
| #5 | HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #6 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #7 | #4 OR #5 OR #6 |
| #8 | #7 AND #3 |
| Number | Search terms |
| #1 | Helminth* AND "HIV Infections" received from 01/01/1980 to 09/29/2015 |
| Trial | Country | Setting | Year enrolment completed | Inclusion criteria | Age | Total randomized | Number HIV‐positive participants | Taking ART | Number co‐infected with helminths at baseline |
| Zambia | Urban | Not stated | HIV‐infected adults with persistent diarrhoea | > 18 years | 174 | 174 | Not stated (probably low) | Unknown | |
| Uganda | Urban | 2005 | HIV‐infected pregnant women | Not stated | 264 | 264 | < 3% | 67% had at least 1 helminth species | |
| Kenya | Urban/rural | 2011 | HIV‐infected, not on ART | > 18 years | 948 | 948 | None at baseline | Unknown | |
| Abbreviations: HIV: human immunodeficiency virus; ART: antiretroviral. | |||||||||
| Trial | Intervention | Control | Outcomes | Timing | ||
| Drug | Dose | Frequency | ||||
| Albendazole | 400 mg | Once daily for 3 days | Placebo | Viral load CD4 Adverse events Mortality events | 12 weeks | |
| Albendazole | 400 mg | Once daily for 3 days | Placebo | CD4 Adverse events Mortality events | 12 weeks | |
| Praziquantel | 40 mg/kg | Once only | No intervention | Viral load CD4 | 12 weeks | |
| Diethylcarbamazine | 6 mg/kg | Once only | Placebo | Viral load CD4 Adverse events | 12 weeks | |
| Albendazole | 800 mg | Twice daily for 14 days | Placebo | Adverse events Mortality events | 6 months | |
| Ivermectin | 200 mg/kg | Single or double dose 2 weeks apart | Albendazole | Adverse events | 1 year | |
| Albendazole Praziquantel | 400 mg 40 mg/kg | Once only Once only | Placebo | Viral load Adverse events Mortality events | 6 weeks | |
| Albendazole Praziquantel | 400 mg 25 mg/kg | Every 3 months Annually | No intervention | Viral load CD4 Adverse events Mortality events | 2 years | |
| Trial | Country | Setting | Year enrolment completed | Inclusion criteria | Age | Total randomized | Number of HIV‐positive participants | Taking ART | Number co‐infected with helminths at baseline |
| Kenya | Urban/rural | 2007 | HIV‐infected with at least one helminth co‐infection | > 18 years | 234 | 234 | None at baseline | All | |
| Ethiopia | Urban | 2012 | Newly diagnosed TB participants with helminth co‐infection | 15 to 60 years | 140 | 32 | 94% of intervention group, 100% of controls | All | |
| Zimbabwe | Rural | 2003 | Adults infected with schistosomiasis | > 18 years | 287 | 130 | Not stated | All | |
| Tanzania | Rural | 2002 | HIV‐infected adults | 22 to 70 years | 34 | 34 | Not stated | 18 | |
| Thailand | Urban | 2009 | Adults with characteristic strongyloides infection | > 18 years | 100 | 10 | Not stated | All | |
| Abbreviations: HIV: human immunodeficiency virus; ART: antiretroviral; TB: tuberculosis. | |||||||||
| Trial name | Relevant intervention | Relevant outcome | Target population | Location(s) | Estimated completion date |
| Reduction of EArly mortaLITY in HIV‐infected Adults and Children Starting Antiretroviral Therapy (REALITY) | Immediate enhanced opportunistic infections (OI) prophylaxis with isoniazid/pyridoxine and cotrimoxazole, plus 12 weeks fluconazole, 5 days azithromycin, and a single dose of albendazole versus cotrimoxazole prophylaxis alone for the first 12 weeks followed by isoniazid and any prophylaxis and/or treatment prescribed at screening | • Change in CD4 count • Adverse events | HIV‐infected individuals ages ≥ 5 years | Kenya, Malawi, Uganda, Zimbabwe | February 2016 |
| Can Anthelminthic Treatment Delay the Progression of HIV? Randomised Open‐label Trial Testing Presumptive Anthelminthic Treatment on Progression of HIV in ART‐naïve HIV‐positive Patients in a Rural African Setting With Presumed High Prevalence of Helminth Infections | Standard HIV care with provision of praziquantel, albendazole, and ivermectin at baseline, after 6 months, and after 12 months versus standard HIV care with no anthelminthic treatment | • Change in viral load • Change in CD4 count • Adverse event | HIV‐infected individuals aged ≥ 18 years | Tanzania | Terminated prematurely due to recruitment difficulties |
| Abbreviations: RCT: randomized controlled trial; HIV: human immunodeficiency virus; ART: antiretroviral. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Viral load: change in log10 HIV‐1 RNA Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 At 6 weeks after one dose | 1 | 166 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.35, 0.07] |
| 1.2 At 2 years after multiple doses | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2 Change in CD4 count Show forest plot | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐10.15, 15.35] |
| 2.1 At 2 years after multiple doses | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐10.15, 15.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Viral load: change in log10 HIV‐1 RNA Show forest plot | 4 | 445 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.26, ‐0.00] |
| 1.1 Soil‐transmitted helminths | 2 | 273 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.31, 0.12] |
| 1.2 Schistosomiasis | 2 | 161 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.36, 0.10] |
| 1.3 Lymphatic filariasis | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.93, 0.77] |
| 2 Change in CD4 count Show forest plot | 3 | 358 | Mean Difference (IV, Random, 95% CI) | 37.86 [7.36, 68.35] |
| 2.1 Soil‐transmitted helminths | 2 | 228 | Mean Difference (IV, Random, 95% CI) | 40.69 [1.51, 79.87] |
| 2.2 Schistosomiasis | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 33.5 [‐15.06, 82.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in log10 HIV RNA, by antihelminthic Show forest plot | 5 | 1534 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.19, 0.01] |
| 1.1 Albendazole | 2 | 302 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.37, 0.02] |
| 1.2 Praziquantel | 2 | 213 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.32, ‐0.03] |
| 1.3 Diethylcarbamazine | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.93, 0.77] |
| 1.4 Albendazole and praziquantel | 2 | 1008 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.05] |
| 2 Difference in adverse events between treatment and no treatment groups Show forest plot | 7 | 1649 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.53, 2.83] |
| 3 Difference in mortality events between treatment and no treatment groups Show forest plot | 5 | 1627 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.52, 1.14] |

