Antihelmínticos en zonas endémicas de helmintos: efectos sobre la evolución de la enfermedad por VIH
Resumen
Antecedentes
Las infecciones por helmintos, como los helmintos transmitidos por contacto con el suelo, la esquistosomiasis, la oncocercosis y la filariasis linfática, son prevalentes en muchos países donde la infección por el virus de la inmunodeficiencia humana (VIH) también es frecuente. Existe alguna evidencia de estudios observacionales de que el VIH y la coinfección por helmintos se pueden asociar con una carga viral mayor y con recuentos de células CD4+ menores. El tratamiento de las infecciones por helmintos con antihelmínticos (fármacos que eliminan los parásitos) puede tener efectos beneficiosos en los pacientes con VIH más allá de la simple eliminación de dichas infecciones.
Esta es una actualización de una revisión Cochrane publicada en 2009 que se ha ampliado para incluir resultados de anemia y eventos adversos.
Objetivos
Evaluar los efectos de los fármacos que eliminan los parásitos (tratamiento antihelmíntico) sobre marcadores de la evolución de la enfermedad por VIH, la anemia y los eventos adversos en niños y adultos.
Métodos de búsqueda
En esta actualización de la revisión, se hicieron búsquedas de estudios publicados y no publicados en línea en la Cochrane Library, MEDLINE, EMBASE, CENTRAL, la World Health Organization (WHO) International Clinical Trials Registry Platform (ICRTP), ClinicalTrials.gov, y en la WHO Global Health Library hasta el 29 septiembre 2015. También se realizaron búsquedas en las bases de datos de listas de resúmenes de congresos, se examinaron las listas de referencias de artículos y se estableció contacto con los autores de los estudios incluidos.
Criterios de selección
Se buscaron los ensayos controlados aleatorizados (ECA) que compararon fármacos antihelmínticos con placebo o ninguna intervención en pacientes con VIH positivo.
Obtención y análisis de los datos
Dos autores de la revisión extrajeron de forma independiente los datos y evaluaron la elegibilidad y el riesgo de sesgo de los ensayos. Los resultados primarios fueron: cambios en la carga viral del VIH y recuento de células CD4+, y los resultados secundarios fueron: anemia, deficiencia de hierro, eventos adversos y eventos de mortalidad. Los efectos de los antihelmínticos se compararon mediante las diferencias de medias, los riesgos relativos (RR) y los intervalos de confianza (IC) del 95%. La calidad de la evidencia se evaluó mediante el enfoque Grading of Recommendations Assessment, Development and Evaluation (GRADE).
Resultados principales
Ocho ensayos cumplieron los criterios de inclusión de esta revisión, con un total de 1612 participantes. Tres ensayos evaluaron el efecto de administrar antihelmínticos a todos los pacientes adultos con VIH sin conocimiento de su estado de infección por helmintos, y cinco ensayos evaluaron los efectos de administrar fármacos antihelmínticos a pacientes con VIH positivo con infecciones por helmintos confirmadas. Siete ensayos se realizaron en el África subsahariana y uno en Tailandia.
Antihelmínticos para pacientes con un estado de infección por helmintos desconocido
Administrar antihelmínticos (albendazol y praziquantel juntos o por separado) a los adultos con VIH positivo con un estado de infección por helmintos desconocido puede tener un efecto supresivo pequeño sobre la carga viral media a las seis semanas, pero el IC del 95% incluye la posibilidad de ningún efecto (diferencia en el cambio medio: ‐0,14 log10 ARN viral/ml; IC del 95%: ‐0,35 a 0,07; p = 0,19; un ensayo, 166 participantes, evidencia de calidad baja).
Las dosis repetidas de fármacos antihelmínticos durante dos años (albendazol cada tres meses más praziquantel anual), probablemente tienen poco o ningún efecto sobre la carga viral media (diferencia en el cambio medio: 0,01 log10 ARN viral; IC del 95%: −0,03 a −0,05; un ensayo, 917 participantes, evidencia de calidad moderada) y poco o ningún efecto sobre el recuento medio de células CD4+ (diferencia en el cambio medio: 2,60 células CD4+/µl; IC del 95%: ‐10,15 a 15,35; P = 0,7; un ensayo, 917 participantes, evidencia de baja calidad).
Antihelmínticos para pacientes con infecciones confirmadas por helmintos
El tratamiento de las infecciones confirmadas por helmintos en adultos con VIH positivo puede tener un efecto supresivo pequeño sobre la carga viral media a las seis a 12 semanas después de la eliminación de los parásitos (diferencia en el cambio medio: ‐0,13 log10 ARN viral; IC del 95%: ‐0,26 a ‐0,00; P = 0,04; cuatro ensayos, 445 participantes, evidencia de baja calidad). Sin embargo, este resultado está firmemente influenciado por un único estudio de tratamiento con praziquantel para la esquistosomiasis. También puede haber un efecto favorable pequeño sobre el recuento medio de células CD4+ a las 12 semanas después de la eliminación de los parásitos en las poblaciones con VIH positivo con infecciones confirmadas por helmintos (diferencia en el cambio medio: 37,86 células CD4+/µl; IC del 95%: 7,36 a 68,35; P = 0,01;tres ensayos, 358 participantes, evidencia de calidad baja).
Eventos adversos y mortalidad
No hay indicación de que los fármacos antihelmínticos provoquen riesgos adicionales en las poblaciones con VIH positivo. Sin embargo, los eventos adversos no se informaron bien (evidencia de muy baja calidad) y los ensayos tuvieron bajo poder estadístico para evaluar los efectos sobre mortalidad (evidencia de baja calidad).
Conclusiones de los autores
Hay evidencia de muy baja calidad de que el tratamiento de las infecciones confirmadas por helmintos en adultos con VIH positivo puede tener efectos favorables pequeños a corto plazo sobre los marcadores de la evolución de la enfermedad por VIH. Se requieren estudios adicionales para confirmar este hallazgo. La evidencia actual indica que la eliminación de los parásitos con antihelmínticos no tiene efectos perjudiciales, lo que es alentador para el tratamiento estándar de las infecciones confirmadas o presuntas por helmintos en los pacientes con VIH que viven en áreas coendémicas.
Se requieren estudios adicionales a largo plazo para establecer conclusiones fiables sobre la repercusión de la eliminación de los parásitos de forma presuntiva en todos los individuos con VIH positivo independientemente del estado de infección por helmintos, debido a que el único ensayo a largo plazo hasta la fecha no demostró un efecto.
PICO
Resumen en términos sencillos
Antihelmínticos en zonas endémicas de helmintos: efectos sobre la infección por VIH
Esta revisión Cochrane resume los ensayos que evaluaron los efectos beneficiosos y los riesgos potenciales de proporcionarles fármacos que eliminan los parásitos (antihelmínticos) a pacientes infectados por el virus de la inmunodeficiencia humana (VIH). Después de realizar búsquedas de ensayos relevantes hasta el 29 de septiembre 2015, se incluyeron ocho ensayos con 1612 participantes.
Qué son los fármacos antihelmínticos y por qué pueden retardan la evolución del VIH
Los fármacos antihelmínticos se utilizan para tratar diversas infecciones humanas por helmintos, como los helmintos transmitidos por contacto con el suelo, la esquistosomiasis, la oncocercosis y la filariasis linfática. En los lugares donde estas infecciones son frecuentes, la Organización Mundial de la Salud actualmente recomienda que las poblaciones de interés reciban sistemáticamente tratamiento cada seis a 12 meses sin confirmación previa del estado de infección del individuo. Se prefiere la administración de tratamiento empírico, o tratar a todas las poblaciones en riesgo de forma presuntiva, a las estrategias de prueba y tratamiento porque los fármacos antihelmínticos son de bajo costo y se toleran bien. Además, una estrategia de analizar antes del tratamiento se considera menos costo‐efectiva debido a que las pruebas diagnósticas disponibles son relativamente costosas y pueden presentar sensibilidad deficiente.
Se sabe que las infecciones por helmintos afectan al sistema inmunológico humano. En los pacientes con VIH, algunos estudios han indicado que las infecciones por helmintos pueden reducir el número de células CD4+ (que son una parte crítica de la respuesta inmunitaria al VIH) y comprometer la capacidad del paciente de controlar la replicación viral del VIH. Por lo tanto, el tratamiento de las infecciones por helmintos podría tener efectos beneficiosos importantes en los pacientes con VIH más allá de los observados en la población general como resultado de la eliminación de los parásitos.
Lo que indica la evidencia en esta revisión
El tratamiento con fármacos antihelmínticos de todos los adultos con VIH positivo sin conocimiento de su estado de infección por helmintos puede tener un efecto supresivo pequeño sobre la carga viral a las seis semanas (evidencia de baja calidad), pero repetir la dosis durante dos años parece tener poco o ningún efecto sobre la carga viral (evidencia de calidad moderada) o sobre el recuento de células de CD4+ (evidencia de baja calidad). Estas conclusiones se basan en dos estudios incluidos.
La administración de fármacos antihelmínticos a adultos con VIH positivo con un diagnóstico de infección por helmintos puede dar lugar a un efecto supresivo pequeño sobre la carga viral media a las seis a 12 semanas (evidencia de baja calidad) y un efecto favorable pequeño sobre el recuento medio de células CD4+ a las 12 semanas (evidencia de calidad baja). Sin embargo, estos resultados se basan en estudios pequeños y están firmemente influenciados por un único estudio sobre praziquantel para la esquistosomiasis. Se necesitan estudios adicionales en diferentes contextos y poblaciones para confirmación.
Los eventos adversos no se informaron de forma adecuada (evidencia de muy baja calidad), y los ensayos fueron demasiado pequeños para evaluar los efectos sobre la mortalidad (evidencia de calidad baja). Sin embargo, no hay indicios de que los fármacos antihelmínticos sean nocivos en los individuos positivos al VIH.
Authors' conclusions
Summary of findings
| Deworming drugs compared with placebo for people with HIV and an unknown helminth infection status | ||||
| Participant or population: HIV‐positive people Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole or praziquantel or a combination) Comparison: placebo | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | |||
| Placebo | Deworming drugs | |||
| Viral load | At 6 weeks after a single dose | 166 (1 trial) | ⊕⊕⊝⊝1,2,3,4 | |
| in the control group, the mean change in viral load was an increase of 0.09 log10 viral RNA | On average, with deworming, there was a suppressive effect on mean viral load of 0.14 log10 viral RNA (0.35 benefit to 0.07 harm) | |||
| At 2 years after multiple doses | 917 (1 trial) | ⊕⊕⊕⊝1,2,5,6 | ||
| In the control group, the mean viral load increased by 0.03 log10 viral RNA | On average, with deworming, there was a suppressive effect on mean viral load of 0.01 log10 viral RNA (0.03 benefit to 0.05 harm) | |||
| CD4+ cell count | At 2 years after multiple doses | 917 (1 trial) | ⊕⊕⊝⊝1,2,4,5 | |
| In the control group, the mean CD4+ cell count reduced by 37.3 CD4+ cells/µL | On average, with deworming, there was a favourable effect on mean CD4+ cell count of 2.60 CD4+ cells/µL (15.35 benefit 10.15 harm) | |||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1No serious risk of bias: this single trial was at low risk of selection bias. | ||||
| Deworming drugs compared with placebo for people with HIV and confirmed helminth infections | ||||
| Participant or population: HIV‐positive people with proven helminth infection Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole, praziquantel, albendazole and praziquantel, or diethylcarbamazine) Comparison: placebo | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | |||
| Placebo | Deworming drugs | |||
| Viral load | At 6 to 12 weeks | 445 (4 trials) | ⊕⊕⊝⊝ | |
| In the control groups, the mean change in viral load ranged from a 0.13 decrease to an increase of 0.21 log10 viral RNA | On average, with deworming, there was a small suppressive effect on mean viral load of 0.13 log10 viral RNA (0.26 benefit to 0.00 benefit) | |||
| CD4+ cell count | At 6 to 12 weeks | 358 (3 trials) | ⊕⊕⊝⊝ | |
| In the control groups, the mean change in CD4+ cell count ranged from a decrease of 68 to an increase of 45 CD4+ cells/µL | On average, with deworming, there was favourable effect on mean CD4+ cell count of 37.86 CD4+ cells/µL (7.36 benefit to 68.35 benefit) | |||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1We downgraded by 1 for serious risk of bias: of the five studies, only one had CIs that excluded the possibility of no effect and this study was at high risk of selection bias. | ||||
| Deworming drugs compared with placebo for people with HIV and both known and unknown helminth infection status | |||||
| Participant or population: HIV‐positive people Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole, praziquantel, albendazole and praziquantel, or diethylcarbamazine) Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Albendazole | ||||
| Iron deficiency (serum ferritin) | log10 mean increase of 0.04 µg/L | log10 mean increase of 0.07 µg/L | Deworming drugs associated with a 0.03 higher µg/L log10 mean ferritin measure (0.30 lower to 0.35 higher) | 16 (1 trial) | ⊕⊝⊝⊝1,2,3,4 |
| Anaemia (serum haemoglobin) | Increase in 0.15 g/dL | Decrease in 0.10 g/dL | Deworming drugs associated with a 0.25 lower g/dL haemoglobin (0.58 lower to 0.08 higher) | 130 (1 trial) | ⊕⊝⊝⊝2,4,5,6 |
| Mortality | 41 per 1000 | 52 per 1000 | RR 0.77 (0.52 to 1.14) | 1627 (5 trials) | ⊕⊕⊝⊝7,8,9,10 |
| Adverse events | 32 per 1000 | 35 per 1000 | RR 1.23 (0.53 to 2.83) | 1649 (7 trials) | ⊕⊝⊝⊝4,9,11,12 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1No serious risk of bias: this single trial was at risk of selection bias. However, the outcome is an objective laboratory measure. We did not downgrade the quality of the evidence. | |||||
Background
Description of the condition
Helminths, which include the soil‐transmitted helminths (STH), schistosomiasis, onchocerciasis, and lymphatic filariasis (LF), infect nearly one‐quarter of the world’s population (WHO 2014). Communities with the highest helminth prevalence are often also areas with high prevalence of human immunodeficiency virus (HIV), and over half of HIV‐positive people living in helminth endemic areas are estimated to be co‐infected with at least one helminth infection (UNAIDS 2007).
Helminth infections have a significant effect on the human immune system, and it is hypothesized that helminth infections may modulate the host's ability to control the HIV virus (Lawn 2001; Modjarrad 2010). More specifically, studies have shown that amongst HIV‐positive individuals with helminth co‐infections.
-
There may be a more rapid decline in the CD4+ T‐cells responsible for immunologic function in HIV‐positive people, and increased cellular susceptibility to HIV infection (Eggena 2005; Shapira‐Nahor 1998).
-
CD4+ and CD8 T‐cells and monocytes may exhibit increased expression of HIV chemokine co‐receptors (Chachage 2014; Lawn 2001; Kalinkovich 1999; Kalinkovich 2001; Secor 2003).
-
There may be increased suppression of the antiviral T helper (Th)1 lymphocyte due to helminth‐associated Th2 lymphocyte propagation (Borkow 2006; Brown 2006; Kinter 2007).
-
There may be higher levels of eosinophilia, increased immunoglobulin (Ig) E levels, and stimulation of other immunosuppressive cytokine responses (Bentwich 1996; Blish 2010).
In HIV‐positive individuals, co‐infection with helminths may lead to the accelerated destruction of the host immune system and earlier onset of acquired immunodeficiency syndrome (AIDS)‐defining illnesses and death (Nesheim 2007). Even amongst HIV‐positive patients treated with antiretroviral (ART) drugs for HIV, helminth infections may adversely influence HIV clinical outcomes (Ivan 2015). Given these profound effects on host immunity, helminth infections have been suggested to play an important role in the pathogenesis of HIV in Africa (Bentwich 1995; Fincham 2003).
Description of the intervention
Current World Health Organization (WHO) guidelines recommend that high‐risk populations living in endemic areas participate in regular mass drug administration with antihelminthic medicines (deworming drugs) without the need for prior confirmatory diagnostic testing. This is also known as preventive chemotherapy. In settings where community prevalence of STH infections exceed 20%, the WHO recommends annual treatment with albendazole or mebendazole for all preschool‐age children, school‐age children, and pregnant women in their second and third trimester (WHO 2006).
The rationale for mass treatment rather than a test and treat approach is the safety of the drugs, low cost of the drugs (often donated by pharmaceutical companies), the relatively high cost of diagnostic testing, and the high prevalence of infection in some areas. The cost of these programmes is estimated to be as low as USD 0.25 per treatment, including delivery costs (Bundy 2009; Partnership for Child Development 1998). Mass deworming programs are scaling up rapidly in part due to the WHO NTD Roadmap for Implementation and the 2012 WHO‐endorsed London Declaration, which calls for the control or elimination of 10 neglected tropical diseases including STHs, schistosomiasis, LF, and onchocerciasis by 2020 (Uniting to Combat NTDs 2012).
In research settings deworming treatment may require prior confirmatory testing or treatment may be delivered empirically as customary in preventative chemotherapy campaigns. For example, specific age groups may be targeted and recruited to receive deworming medications during community‐based household recruitment, at HIV clinics, at tuberculosis (TB) clinics, or during antenatal care.
How the intervention might work
If helminth co‐infection plays a significant detrimental role in HIV disease progression it is possible that effective treatment or prevention of helminth infections could slow the progression of HIV (Kallestrup 2005 ZWE; Wolday 2002). Relatively modest increases in viral load (0.3 to 0.5 log10 copies/mL) may increase the annual risk of progression to an AIDS‐defining illness or death by as much as 25% to 44% (Modjarrad 2008). Mathematical modelling suggests that a reduction in HIV‐1 ribonucleic acid (RNA) levels of 0.5 log10 copies/mL could slow the onset of AIDS by 3.5 years and could delay the need for ART medications by almost a full year (Gupta 2007). The potential increases in HIV RNA associated with helminth co‐infection suggest that for every 100 HIV‐positive individuals with an STH infection in sub‐Saharan Africa, there could be 3.1 (95% CI: 0.1 to 14.9) excess HIV‐1 transmission events. The trend is similar for other helminth infections, with 8.5 (95% CI 0.2 to 38.6) excess HIV transmission events attributed to schistosomiasis and 13.3 (95% CI 0.3 to 89.2) to filariasis (Baggaley 2015).
Observational studies of the effects of deworming drugs on markers of HIV disease progression have had conflicting results. Several studies have noted delays in HIV disease progression following deworming (Brown 2005; Ivan 2015; Lankowski 2014; Mulu 2013; Muok 2013; Wolday 2002 ), while others have reported no association (Brown 2004; Elliott 2003; Hosseinipour 2007; Kleppa 2014; Lawn 2000; Modjarrad 2005). In the previous version of this systematic review we found some evidence from randomized controlled trials of short term benefits with deworming but also the need for further larger studies (Walson 2009).
Why it is important to do this review
Given that a substantial number of individuals targeted by helminth control programmes may be exposed to or infected with HIV, it is important to establish the safety of deworming drugs in HIV‐positive populations and the potential for drug interactions, particularly as an increased incidence of drug reactions has been observed in patients with advanced HIV infection (Gordin 1984; Nunn 1991). Available data from a recent systematic review also suggested the potential for an interaction between both mebendazole and albendazole and nucleoside reverse transcriptase inhibitors appears low, although no formal pharmacokinetic studies exist. The review also reported that interactions may exist between praziquantel and protease inhibitors via enzyme inhibition (Seden 2013).
Since the publication of our initial Cochrane Review of this topic in 2009 (Walson 2009), deworming campaigns have been dramatically scaled up and it has become increasingly important to understand not only the effects of deworming on HIV disease progression but also the safety of treating these unique patient populations. In this update, we have expanded the scope of this Cochrane Review to include trials that evaluate the impact of deworming on anaemia and iron deficiency, as well as markers of HIV disease progression and safety.
Objectives
To evaluate the effects of deworming drugs (antihelminthic therapy) on markers of HIV disease progression, anaemia, and adverse events in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) or quasi‐RCTs.
Types of participants
Adults and children (older than one year of age) infected with human immunodeficiency virus (HIV)‐1 or HIV‐2 with and without documented helminth co‐infection.
Relevant helminth‐infections included schistosomiasis (Schistosomiasis mansoni and Schistosomiasis haematobium), STH (Strongyloides stercoralis, Ancylostoma duodenale,Necator americanus, Trichuris trichiura, Ascaris lumbricoides, and Trichostrongylus), lymphatic filariasis (LF) (Wuchereria bancrofti,Brugia malayi, and Brugia timori), onchocerciasis (Onchocerca volvulus), and other filariasis (Mansonella streptocerca).
For trials that included both HIV‐positive and uninfected participants, we contacted the trial authors and requested data relevant to the HIV‐positive participants only.
Types of interventions
Intervention
Any antihelminthic drug therapy recommended by World Health Organization (WHO) guidelines for use in the eradication of helminth infections in humans. This included the benzimidazoles (albendazole or mebendazole), ivermectin, praziquantel, diethylcarbamazine (DEC), bithionol, oxamniquine, pyrantel, and nitazoxanide (WHO 2006).
Control
Placebo or no treatment. For the outcome of adverse events, we also considered alternative antihelminthic drugs.
Types of outcome measures
Primary outcomes
-
Change in plasma viral load (log10copies viral RNA/mL).
-
Change in CD4+ T‐cell count (cells/µL).
Secondary outcomes
-
Change in CD8+ T‐cell count (cells/µL).
-
CD4+/CD8+ T‐cell ratio.
-
Adverse events associated with antihelminthic therapy (if reported in the included trials).
-
Iron deficiency (as defined by the trial authors, based on a biomarker of iron status and tests, at 34 weeks gestation or later in pregnant populations).
-
Anaemia (defined as haemoglobin (Hb) below 110 g/L, adjusted for altitude and smoking as appropriate).
-
Death.
Search methods for identification of studies
Electronic searches
In coordination with a the Information Specialist of the Cochrane Infectious Diseases Group (CIDG), we searched MEDLINE online (1980 to 2015), EMBASE (1980 to 2015), CENTRAL (1980 to 2015), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (all dates), and clinicaltrials.gov (1980 to 2015) using the strategies documented in Table 1, Table 2, Table 3, Table 4, and Table 5, respectively. As this is a review update, we also searched MEDLINE, EMBASE, CENTRAL, and AIDSEARCH databases using the exact search terms utilized in the previously published in Walson 2009 up to 29 September 2015 (Table 6; Table 7; Table 8; Table 9). We conducted a separate search in the WHO Global Health Library, which accesses AIM (AFRO), LILACS (AMRO/PAHO), IMEMR (EMRO), IMSEAR (SEARO), WPRIM (WPRO), WHOLIS (KMS), and SciELO resources (Table 10).
| Number | Search terms |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) |
| #2 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) |
| #3 | Search (helminths[mh] OR helminth*[tiab] OR nematode*[tiab] OR worm*[tiab] OR parasites[mh] OR parasit*[tiab] OR round worm*[tiab] OR roundworm*[tiab] OR hookworm*[tiab] OR hook worm*[tiab] OR ancylostoma*[tiab] OR cestode*[tiab] OR tapeworm*[tiab] OR tape worm*[tiab] OR trematode*[tiab] OR fluke*[tiab] OR whipworm*[tiab] OR whip worm*[tiab] OR trichuris[tiab] OR ascaris[tiab] OR enterobi*[tiab] OR strongyloide*[tiab] OR mansonell*[tiab] OR taenia[tiab] schistosom*[tiab] OR necator*[tiab] OR paragonim*[tiab] OR hymenolepis[tiab] OR fasciol*[tiab] OR filariasis[tiab] OR trichostrongl*[tiab] OR microfilaria[tiab] OR parasitic diseases[mh:noexp] OR helminthiasis[mh] OR intestinal diseases, parasitic[mh]) |
| #4 | Search (benzimidazoles OR albendazole OR mebendazole OR ivermectin OR praziquantel OR diethylcarbamazine OR bithionol OR oxamniquine OR pyrantel OR nitazoxanide OR anthelmintic OR anthelmintics OR anthelminthic OR anthelminthics OR “anti helminthic” OR “anti helminthics” OR “anti helmintic” OR “anti helmintics” OR antihelminthic OR antihelminthics OR antihelmintic OR antihelmintics) |
| #5 | Search (#1 AND #2 AND #3 AND #4) |
| #6 | Search (((#1 AND #2 AND #3 AND #4))) AND ("1980/01/01"[Date ‐ Publication] : "2015/09/29"[Date ‐ Publication]) |
| Number | Search terms |
| #1 | 'human immunodeficiency virus infection' exp OR 'human immunodeficiency virus infection':ab,ti OR 'hiv infection':ab,ti OR 'hiv infections':ab,ti OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR hiv:ab,ti OR 'hiv 1':ab,ti OR 'hiv 2':ab,ti OR 'human immune deficiency virus':ab,ti OR 'human immuno deficiency virus':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno deficiency syndrome':ab,ti OR 'acquired immune deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti |
| #2 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure'OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl*NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (crossNEXT/1 over*):ab,ti |
| #3 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de |
| #4 | 'human'/de OR 'normal human'/de OR 'human cell'/de |
| #5 | #3 AND #4 |
| #6 | #3 NOT #5 |
| #7 | #2 NOT #6 |
| #8 | 'helminth'/exp OR helminth*:ab,ti OR nematode*:ab,ti OR worm*:ab,ti OR 'parasite'/exp OR parasit*:ab,ti OR roundAND worm*:ab,ti OR roundworm*:ab,ti OR hookworm*:ab,ti OR hookAND worm*:ab,ti OR ancylostoma*:ab,ti OR cestode*:ab,ti OR tapeworm*:ab,ti OR tapeAND worm*:ab,ti OR trematode*:ab,ti OR fluke*:ab,ti OR whipworm*:ab,ti OR whipAND worm*:ab,ti OR trichuris:ab,ti OR ascaris:ab,ti OR enterobi*:ab,ti OR strongyloide*:ab,ti OR mansonell*:ab,ti OR taenia:ab,ti AND schistosom*:ab,ti OR necator*:ab,ti OR paragonim*:ab,ti OR hymenolepis:ab,ti OR fasciol*:ab,ti OR filariasis:ab,ti OR trichostrongl*:ab,ti OR microfilaria:ab,ti OR 'parasitic diseases'/exp OR 'helminthiasis'/de OR 'intestine infection'/de |
| #9 | 'benzimidazoles'/de OR benzimidazolesOR 'albendazole'/de OR albendazoleOR 'mebendazole'/de OR mebendazoleOR 'ivermectin'/de OR ivermectinOR 'praziquantel'/de OR praziquantelOR 'diethylcarbamazine'/de OR diethylcarbamazineOR 'bithionol'/de OR bithionolOR 'oxamniquine'/de OR oxamniquineOR 'pyrantel'/de OR pyrantelOR 'nitazoxanide'/de OR nitazoxanideOR 'anthelmintic'/de OR anthelminticOR 'anthelmintics'/de OR anthelminticsOR 'anthelminthic'/de OR anthelminthicOR anthelminthicsOR 'anti helminthic'OR 'anti helminthics'OR 'anti helmintic'OR 'anti helmintics'OR antihelminthicOR antihelminthicsOR 'antihelmintic'/de OR antihelminticOR 'antihelmintics'/de OR antihelmintics |
| #10 | #1 AND #7 AND #8 AND #9 |
| #11 | #1 AND #7 AND #8 AND #9 AND [1‐1‐1980]/sd NOT [29‐09‐2015]/sd |
| Number | Search terms |
| #1 | MeSH descriptor: [HIV Infections] explode all trees |
| #2 | MeSH descriptor: [HIV] explode all trees |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only |
| #6 | #1 or #2 or #3 or #4 or #5 |
| #7 | [mh helminths] or helminth*:ti,ab,kw or meatode*:ti,ab,kw or worm*:ti,ab,kw or [mh parasites] or parasit*:ti,ab,kw or round worm:ti,ab,kw or roundworm:ti,ab,kw or hook worm*:ti,ab,kw or hookworm*:ti,ab,kw or ancylostoma*:ti,ab,kw or cestode*:ti,ab,kw or tapeworm*:ti,ab,kw or tape worm*:ti,ab,kw or trematode*:ti,ab,kw or fluke*:ti,ab,kw or whipworm*:ti,ab,kw or whip worm*:ti,ab,kw or trichuris:ti,ab,kw or ascaris:ti,ab,kw or enterobi*:ti,ab,kw or strongyloide*:ti,ab,kw or mansonell*:ti,ab,kw or taenia:ti,ab,kw schistosom*:ti,ab,kw or necator*:ti,ab,kw or paragonim*:ti,ab,kw or hymenolepis:ti,ab,kw or fasciol*:ti,ab,kw or filariasis:ti,ab,kw or trichostrongl*:ti,ab,kw or microfilaria:ti,ab,kw or [mh ^"parasitic diseases"] or [mh helminthiasis] or [mh ^"intestinal diseases, parasitic"] (Word variations have been searched) |
| #8 | benzimidazoles or albendazole or mebendazole or ivermectin or praziquantel or diethylcarbamazine or bithionol or oxamniquine or pyrantel or nitazoxanide or anthelmintic or anthelmintics or anthelminthic or anthelminthics or "anti helminthic" or "anti helminthics" or "anti helmintic" or "anti helmintics" or antihelminthic or antihelminthics or antihelmintic or antihelmintics (Word variations have been searched) |
| #9 | #6 and #7 and #8 Publication Year from 1980 to 2015, in Trials |
| Number | Search terms |
| #1 | HIV AND HELMINTH |
| Number | Search terms |
| #1 | HIV AND HELMINTH | Interventional Studies | received from 01/01/1980 to 09/29/2015 |
| Number | Search terms |
| #1 | “HIV Infections”[MeSH] OR “HIV”[MeSH] OR hiv [tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immunodeficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immunodeficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR “Sexually Transmitted Diseases, Viral”[MeSH:NoExp] |
| #2 | randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR (“clinical trial” [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) |
| #3 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CESTODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS OR HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #4 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #5 | #3 AND #4 |
| #6 | #1 AND #2 AND #5 |
| #7 | #1 AND #2 AND #5 Limits: Publication Date from 1980 to 2015/09/29 |
| Number | Search terms |
| #1 | ((’human immunodeficiency virus infection’/exp OR ’human immunodeficiency virus infection’) OR (’human immunodeficiency virus infection’/exp OR ’human immunodeficiency virus infection’)) OR ((’human immunodeficiency virus’/exp OR ’human immunodeficiency virus’) OR (’human immunodeficiency virus’/exp OR ’human immunodeficiency virus’)) OR (hiv:ti OR hiv:ab) OR (’hiv‐1’:ti OR ’hiv‐1’:ab) OR (’hiv‐2’:ti OR ’hiv‐2’:ab) OR (’human immunodeficiency virus’:ti OR ’human immunodeficiency virus’:ab) OR (’human immuno‐deficiency virus’:ti OR ’human immuno‐deficiency virus’:ab) OR (’human immunedeficiency virus’:ti OR ’human immunedeficiency virus’:ab) OR (’human immune‐deficiency virus’:ti OR ’human immune‐deficiency virus’:ab) OR (’acquired immune‐deficiency syndrome’:ti OR ’acquired immune‐deficiency syndrome’:ab) OR (’acquired immunedeficiency syndrome’:ti OR ’acquired immunedeficiency syndrome’:ab) OR (’acquired immunodeficiency syndrome’:ti OR ’acquired immunodeficiency syndrome’:ab) OR (’acquired immuno‐deficiency syndrome’:ti OR ’acquired immuno‐deficiency syndrome’:ab) |
| #2 | random:ti OR random:ab OR factorial:ti OR factorial*:ab OR 'cross over':ti OR 'cross over':ab OR crossover:ti OR crossover:ab OR placebo:ti OR placebo:ab OR (double:ti AND blind:ti) OR (doubl:ab AND blind*:ab) OR (single:ti AND blind:ti) OR (single:ab AND blind:ab) OR assign:ti OR assign:ab OR allocat:ti OR allocate:ab OR volunteer:ti OR volunteer:ab OR 'crossover procedure'/exp OR 'crossover procedure' OR 'double‐blind procedure'/exp OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure' OR 'randomized controlled trial'/exp OR 'randomized controlled trial' |
| #3 | 'helminths' OR 'roundworm' OR 'round worm' OR 'round‐worm' OR roundworms OR 'round worms' OR 'round‐worms' OR nematodes OR 'nematode' OR 'cestode' OR cestodes OR 'tapeworm' OR 'tape worm' OR 'tape‐worm' OR tapeworms OR 'tape worms' OR 'tape‐worms' OR 'trematode' OR trematodes OR 'fluke' OR flukes OR 'worm' OR worms OR 'parasite' OR 'parasites' OR 'ascaris' OR 'trichuris' OR 'enterobius' OR strongyloide OR stronglyloides OR 'ancylostoma' OR ancylostomas OR 'necator' OR necators OR 'hymenolepis' OR 'paragonimus' OR 'fasciola' OR 'taenia' OR 'hookworm' OR 'hook worm' OR 'hook‐worm' OR hookworms OR 'hook worms' OR 'hook‐worms' OR 'whipworm' OR 'whip worm' OR 'whip‐worm' OR whipworms OR 'whip worms' OR 'whip‐worms' OR shistosomiasis OR 'mansonella' OR 'filariasis' OR 'microfilaria' OR 'trichostrongylus' OR trichostronglylosis OR stronglyloidea OR pargonimiasis |
| #4 | ((’benzimidazoles’/exp OR ’benzimidazoles’) OR (’benzimidazoles’/exp OR ’benzimidazoles’)) OR ((’albendazole’/exp OR ’albendazole’) OR (’albendazole’/exp OR ’albendazole’)) OR ((’mebendazole’/exp OR ’mebendazole’) OR (’mebendazole’/exp OR ’mebendazole’)) OR ((’ivermectin’/exp OR ’ivermectin’) OR (’ivermectin’/exp OR ’ivermectin’)) OR ((’praziquantel’/exp OR ’praziquantel’) OR (’praziquantel’/exp OR ’praziquantel’)) OR ((’diethylcarbamazine’/exp OR ’diethylcarbamazine’) OR (’diethylcarbamazine’/exp OR ’diethylcarbamazine’)) OR ((’bithionol’/exp OR ’bithionol’) OR (’bithionol’/exp OR ’bithionol’)) OR ((’oxamniquine’/exp OR ’oxamniquine’) OR (’oxamniquine’/exp OR ’oxamniquine’)) OR ((’pyrantel’/exp OR ’pyrantel’) OR (’pyrantel’/exp OR ’pyrantel’)) OR ((’nitazoxanide’/exp OR ’nitazoxanide’) OR (’nitazoxanide’/exp OR ’nitazoxanide’)) |
| #5 | #3 OR #4 |
| #6 | #1 AND #2 AND #5 [1‐1‐1980]/sd NOT [29‐09‐2015]/sd |
| Number | Search terms |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (VIRAL SEXUALLY TRANSMITTED DISEASES) |
| #2 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CES‐TODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS OR HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #3 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #4 | #2 OR #3 |
| #5 | #1 AND #4 from 1980 to 2015 |
| Number | Search terms |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) |
| #2 | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐ BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (“CLINICAL TRIAL”) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND*)) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN) |
| #3 | #1 AND #2 |
| #4 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CES‐TODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS |
| #5 | HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #6 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #7 | #4 OR #5 OR #6 |
| #8 | #7 AND #3 |
| Number | Search terms |
| #1 | Helminth* AND "HIV Infections" received from 01/01/1980 to 09/29/2015 |
We did not apply any language or date restrictions.
Searching other resources
Reference lists
We examined all references cited by included trials to identify any further studies for inclusion.
Data collection and analysis
Selection of studies
Two review authors (ARM and PB) independently read the titles, abstracts, and descriptor terms of all downloaded material from the electronic searches and discarded any irrelevant reports. When there was uncertainty as to the relevance of the study, we maintained the citation for further review. Two review authors (ARM and PB) then independently evaluated the full‐text articles of all identified citations to establish relevance of the article according to the pre‐specified criteria.
We reviewed the studies for relevance based on study design, types of participants, exposures, and outcome measures. ARM and PB independently applied the inclusion criteria for this Cochrane Review update. There were three differences that required a third review author (JW) to resolve.
Data extraction and management
Two review authors (ARM and PB) independently extracted data from the included trials using standardized data extraction forms for randomized trials, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We extracted the following characteristics from each included trial.
-
Administrative details: identification; author(s); published or unpublished; year of publication; year in which study was conducted; funding source.
-
Details of study: study design; randomization method; duration; completeness of follow‐up; country and location of the study; setting (for example, urban or rural, hospital or clinic); method(s) of recruitment; number of participants by trial arm.
-
Characteristics of participants: age; gender; socioeconomic status; HIV clinical staging (if available); antiretroviral (ART) status.
-
Details of intervention: medication; dose; duration; number of treatments; control group.
-
Details of outcomes: primary outcome; unit of measurement; change in viral load; change in CD4+ count; change in rate of clinical HIV disease progression (changes in the WHO or Centers for Disease Control and Prevention (CDC) staging); nutritional indicators; adverse events; mortality.
Assessment of risk of bias in included studies
Two review authors (ARM and PB) independently evaluated the methodological quality of included clinical trials using the Cochrane 'Risk of bias' assessment tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We followed the guidance to assess whether adequate steps were taken to reduce the risk of bias across five domains: sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessors; and incomplete outcome data.
For sequence generation and allocation concealment, we reported the methods used. For blinding, we described who was blinded and the blinding method. For incomplete outcome data, we reported the percentage and proportion of participants lost to follow‐up. We categorized our 'Risk of bias' judgements as either 'low', 'high', or 'unclear'. Where risk of bias was unclear, we attempted to contact the trial authors for clarification and resolved any differences of opinion through discussion.
Measures of treatment effect
We summarized dichotomous outcomes using risk ratios (RR) with 95% confidence interval (CIs). We summarized continuous outcomes using mean differences with 95% CIs. For some studies we utilized only data from HIV‐positive participants and the trial authors provided the raw data accordingly.
HIV disease progression is associated with a decline in CD4+ cell counts and an increase in viral load. A favourable effect of deworming drugs on CD4+ cell count therefore refers to either a larger increase in CD4+ cell count or a smaller depletion in CD4+ cell count. A suppressive effect on viral load refers to either a reduction in viral load, or a smaller increase in viral load.
Unit of analysis issues
For trials with repeat outcome measurements over time, we utilized and reported the study's final outcome measure reflecting the whole follow‐up time for each individual participant. For studies with 2 x 2 factorial designs in which there were more than one treatment group we chose not to combine experimental intervention groups into a single group and risk loss of information regarding the effects of different antihelminthic treatments. Rather, we chose to present the interventions separately and divide the total number of participants in the control group to account for multiple comparisons in meta‐analyses. The means and standard deviations were left unchanged as recommended in Section 16.5.4 of the Cochrane Handbook (Higgins 2011). This method only partially overcomes the unit‐of‐analysis error (because the resulting comparisons remain correlated). However, an advantage of this approach is that it allows for investigations of heterogeneity across intervention arms.
Dealing with missing data
We contacted trial authors where there were missing or unclear data. We considered missing data resulting from losses to follow‐up to be missing at random unless there were indications otherwise.
Assessment of heterogeneity
We assessed heterogeneity amongst trials by inspecting the forest plots (to detect overlapping CIs) and the I² statistic with a level of 50% to denote moderate levels of heterogeneity, as well as by applying the Chi² test with a P value of 0.10 to indicate statistical significance, as described in Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
We could not perform an assessment of the likelihood of reporting bias due to an insufficient number of included trials.
Data synthesis
We pooled the results from included RCTs in six different meta‐analyses, using a random‐effects method, due to the heterogeneity of the interventions (DerSimonian 1986). We performed pooled meta‐analyses to assess the outcomes of change in mean plasma viral load in populations with unknown helminth infection status, change in mean CD4+ cell count in populations with unknown helminth infection status, change in mean plasma viral load in helminth‐infected populations only, change in mean CD4+ cell count in helminth‐infected populations only, difference in adverse events, and difference in mortality events.
We assessed the quality of evidence by outcome using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. We used GRADEprofiler Guideline Development Tool (GDT) software (GRADEpro GDT 2015) to import data from Review Manager (RevMan) (RevMan 2014) to create three 'Summary of findings' tables. For each of the review outcomes we created a summary of the intervention effect and a measure of quality using the GRADE approach whereby the quality of evidence received a grade of either 'very low', 'low', 'moderate', or 'high', by outcome. We assessed the quality of the evidence based on five criteria: risk of bias, imprecision, indirectness, inconsistency, and publication bias.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses with respect to trials in which participants had confirmed helminth infections, as well as trials in which participants had unknown infection status. We planned to investigate statistical heterogeneity by conducting subgroup analysis with respect to antihelminthic treatment used, participant age, ARV initiation status, and geographic location. However, we could not do so because of the limited number of studies included for meta‐analyses.
Sensitivity analysis
We assessed the robustness of the results by conducting a sensitivity analysis against the 'Risk of bias' criteria.
Results
Description of studies
Results of the search
In this Cochrane Review update, we identified 312 references using the CIDG Information Specialist's search criteria described above and an additional 1936 unique references by applying the search criteria utilized in the 2009 Cochrane Review (Walson 2009). Of the 2248 citations screened, we excluded 2203 citations due to irrelevance to antihelminthics, no inclusion of HIV‐positive participants, or both. We retrieved and formally reviewed 42 full‐text articles for eligibility (see Figure 1 for the study flow diagram).
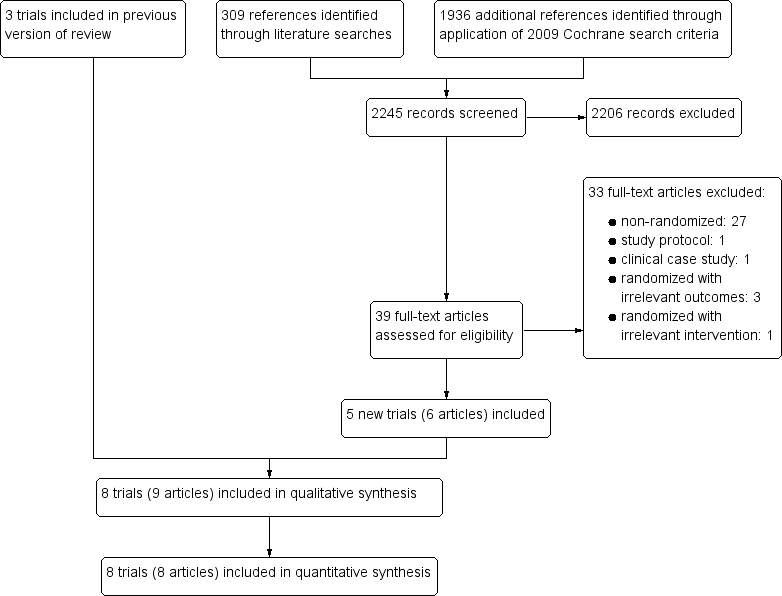
Study flow diagram.
Included studies
Eight randomized controlled trials (RCTs) met the inclusion criteria of this Cochrane Review. Three were included in the 2009 version of this Cochrane Review (Kallestrup 2005 ZWE; Nielsen 2007 TZA; Walson 2008 KEN) and we added five new trials, due to more recent publication dates or the expanded scope of the protocol (Abate 2014 ETH; Kelly 1996 ZMB; Suputtamongkol 2011 THA; Walson 2012 KEN; Webb 2012 UGA).
Seven trials were conducted in sub‐Saharan Africa, and one in Thailand (Suputtamongkol 2011 THA). All eight trials included adults infected with HIV‐1 or HIV‐2, and one trial was limited to pregnant adult women (Webb 2012 UGA). One small trial from Zambia recruited only adults with chronic diarrhoea (Kelly 1996 ZMB). There were no trials in preschool or school‐age children.
The RCTs aimed to evaluate a range of primary outcomes: changes in tuberculosis (TB) morbidity factors (Abate 2014 ETH), diarrhoea duration (Kelly 1996 ZMB), clearance of Strongyloides infection (Suputtamongkol 2011 THA), CD4+/CD8+ cell ratios (Nielsen 2007 TZA), CD4+ cell count (Kallestrup 2005 ZWE; Nielsen 2007 TZA; Walson 2008 KEN; Walson 2012 KEN), and HIV‐1 RNA viral load (Kallestrup 2005 ZWE; Nielsen 2007 TZA; Walson 2008 KEN; Walson 2012 KEN; Webb 2012 UGA).
In two trials, HIV‐positive participants were not taking antiretroviral (ART) drugs (Walson 2008 KEN; Walson 2012 KEN), in one trial ART use was limited to prevention of mother‐to‐child transmission of HIV (Webb 2012 UGA), and in one trial ART use was very high (Abate 2014 ETH). Four trials did not specify if the HIV‐positive individuals were currently taking ART (Kallestrup 2005 ZWE; Kelly 1996 ZMB; Nielsen 2007 TZA; Suputtamongkol 2011 THA).
Three trials evaluated deworming drugs provided presumptively to participants with unknown helminth infection status: Walson 2012 KEN administered albendazole (400 mg) every three months and praziquantel (40 mg/kg) annually for two years; Webb 2012 UGA administered either albendazole (800 mg), praziquantel (40 mg/kg), or both to pregnant women and followed up for six weeks and at delivery; and Kelly 1996 ZMB administered albendazole (800 mg) twice daily for two weeks to adults with chronic diarrhoea (see Table 11 and Table 12).
| Trial | Country | Setting | Year enrolment completed | Inclusion criteria | Age | Total randomized | Number HIV‐positive participants | Taking ART | Number co‐infected with helminths at baseline |
| Zambia | Urban | Not stated | HIV‐infected adults with persistent diarrhoea | > 18 years | 174 | 174 | Not stated (probably low) | Unknown | |
| Uganda | Urban | 2005 | HIV‐infected pregnant women | Not stated | 264 | 264 | < 3% | 67% had at least 1 helminth species | |
| Kenya | Urban/rural | 2011 | HIV‐infected, not on ART | > 18 years | 948 | 948 | None at baseline | Unknown |
Abbreviations: HIV: human immunodeficiency virus; ART: antiretroviral.
| Trial | Intervention | Control | Outcomes | Timing | ||
| Drug | Dose | Frequency | ||||
| Albendazole | 400 mg | Once daily for 3 days | Placebo | Viral load CD4 Adverse events Mortality events | 12 weeks | |
| Albendazole | 400 mg | Once daily for 3 days | Placebo | CD4 Adverse events Mortality events | 12 weeks | |
| Praziquantel | 40 mg/kg | Once only | No intervention | Viral load CD4 | 12 weeks | |
| Diethylcarbamazine | 6 mg/kg | Once only | Placebo | Viral load CD4 Adverse events | 12 weeks | |
| Albendazole | 800 mg | Twice daily for 14 days | Placebo | Adverse events Mortality events | 6 months | |
| Ivermectin | 200 mg/kg | Single or double dose 2 weeks apart | Albendazole | Adverse events | 1 year | |
| Albendazole Praziquantel | 400 mg 40 mg/kg | Once only Once only | Placebo | Viral load Adverse events Mortality events | 6 weeks | |
| Albendazole Praziquantel | 400 mg 25 mg/kg | Every 3 months Annually | No intervention | Viral load CD4 Adverse events Mortality events | 2 years | |
Five RCTs treated HIV‐positive adults with confirmed helminth infections; two trials treated soil‐transmitted helminth (STH) infections with albendazole (400 mg) once daily for three days (Abate 2014 ETH; Walson 2008 KEN), one study treated schistosomiasis with praziquantel (40 mg/kg) once only (Kallestrup 2005 ZWE), one study treated STH or schistosomiasis with albendazole, praziquantel, or both (Webb 2012 UGA), and one trial treated lymphatic filariasis (LF) with diethylcarbamazine (DEC) (Nielsen 2007 TZA). These trials followed participants for six to 12 weeks (see Table 13 and Table 12).
| Trial | Country | Setting | Year enrolment completed | Inclusion criteria | Age | Total randomized | Number of HIV‐positive participants | Taking ART | Number co‐infected with helminths at baseline |
| Kenya | Urban/rural | 2007 | HIV‐infected with at least one helminth co‐infection | > 18 years | 234 | 234 | None at baseline | All | |
| Ethiopia | Urban | 2012 | Newly diagnosed TB participants with helminth co‐infection | 15 to 60 years | 140 | 32 | 94% of intervention group, 100% of controls | All | |
| Zimbabwe | Rural | 2003 | Adults infected with schistosomiasis | > 18 years | 287 | 130 | Not stated | All | |
| Tanzania | Rural | 2002 | HIV‐infected adults | 22 to 70 years | 34 | 34 | Not stated | 18 | |
| Thailand | Urban | 2009 | Adults with characteristic strongyloides infection | > 18 years | 100 | 10 | Not stated | All |
Abbreviations: HIV: human immunodeficiency virus; ART: antiretroviral; TB: tuberculosis.
Five trials measured and reported HIV‐1 RNA viral load (Kallestrup 2005 ZWE; Nielsen 2007 TZA; Walson 2008 KEN; Walson 2012 KEN; Webb 2012 UGA), and four trials reported CD4+ cell count (Kallestrup 2005 ZWE; Nielsen 2007 TZA; Walson 2008 KEN; Walson 2012 KEN). One study reported CD4+/CD8+ cell ratio, seven trials reported adverse events, one trial reported measures of iron deficiency and anaemia respectively, and six trials reported mortality.
We requested unpublished data from all but one trial author (Kelly 1996 ZMB), and we included these data in the analyses.
Excluded studies
We excluded 33 studies due to their observational designs or irrelevance to the intervention or outcomes of interest. See the 'Characteristics of excluded studies' table. We identified two ongoing or planned RCTs, and have provided descriptions of these trials in Table 14.
| Trial name | Relevant intervention | Relevant outcome | Target population | Location(s) | Estimated completion date |
| Reduction of EArly mortaLITY in HIV‐infected Adults and Children Starting Antiretroviral Therapy (REALITY) | Immediate enhanced opportunistic infections (OI) prophylaxis with isoniazid/pyridoxine and cotrimoxazole, plus 12 weeks fluconazole, 5 days azithromycin, and a single dose of albendazole versus cotrimoxazole prophylaxis alone for the first 12 weeks followed by isoniazid and any prophylaxis and/or treatment prescribed at screening | • Change in CD4 count • Adverse events | HIV‐infected individuals ages ≥ 5 years | Kenya, Malawi, Uganda, Zimbabwe | February 2016 |
| Can Anthelminthic Treatment Delay the Progression of HIV? Randomised Open‐label Trial Testing Presumptive Anthelminthic Treatment on Progression of HIV in ART‐naïve HIV‐positive Patients in a Rural African Setting With Presumed High Prevalence of Helminth Infections | Standard HIV care with provision of praziquantel, albendazole, and ivermectin at baseline, after 6 months, and after 12 months versus standard HIV care with no anthelminthic treatment | • Change in viral load • Change in CD4 count • Adverse event | HIV‐infected individuals aged ≥ 18 years | Tanzania | Terminated prematurely due to recruitment difficulties |
Abbreviations: RCT: randomized controlled trial; HIV: human immunodeficiency virus; ART: antiretroviral.
Risk of bias in included studies
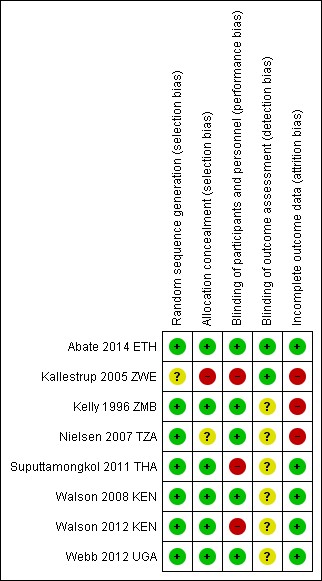
We have presented a summary of our 'Risk of bias' assessments in Figure 2 and Figure 3.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.

'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included trials.
Allocation
Five trials adequately described both random sequence generation and allocation concealment and we judged them at low risk of selection bias (Abate 2014 ETH; Suputtamongkol 2011 THA; Walson 2008 KEN; Walson 2012 KEN; Webb 2012 UGA). One trial did not describe allocation concealment and was at unclear risk of selection bias (Nielsen 2007 TZA), and one stated that allocation was 'open' and was at high risk of selection bias (Kallestrup 2005 ZWE). All included trials appeared to have similar reported baseline characteristics between randomization groups.
Blinding
Five trials used placebos and we judged them to be at low risk of performance bias (systematic differences between groups in the care that is provided, or in exposure to factors other than the interventions of interest) (Abate 2014 ETH; Kelly 1996 ZMB; Nielsen 2007 TZA; Walson 2008 KEN; Webb 2012 UGA). We judged the remaining trials to be at high risk of performance bias (Kallestrup 2005 ZWE; Suputtamongkol 2011 THA; Walson 2012 KEN). In addition, individual participants who received more frequent treatment doses had more opportunities to interface with health facilities as an inherent aspect of the intended trial design and may have non‐differentially had access to improved care.
Only two trials adequately described blinding of outcomes assessment to prevent detection bias (Abate 2014 ETH; Kallestrup 2005 ZWE). The remaining trials were at unclear risk of bias. Two trials utilized diagnostic methods with low sensitivity, such as duplicate Kato‐Katz from a single stool sample (Walson 2008 KEN; Webb 2012 UGA), which might affect the total sample size of helminth‐infected participants, but not the fidelity of the intervention or outcome measures.
Incomplete outcome data
Attrition bias may have been an issue in one included trial, Nielsen 2007 TZA, where there appeared to be differential loss to follow‐up between the groups. In addition, two trials reported that less than 80% of the established participant cohort were followed‐up (Kallestrup 2005 ZWE; Kelly 1996 ZMB), although Kallestrup 2005 ZWE reported that reasons for study drop‐out were evenly distributed, with the exception of a single group which had a higher number of losses to follow‐up due to migration.
Selective reporting
We could not perform an assessment of the likelihood of reporting bias due to an insufficient number of included trials.
Five trials performed and reported an intention‐to‐treat analysis (Abate 2014 ETH; Kallestrup 2005 ZWE; Walson 2008 KEN; Walson 2012 KEN; Webb 2012 UGA). The three remaining trials performed outcome comparisons only for participants for whom follow‐up data were available or relevant (Kelly 1996 ZMB; Nielsen 2007 TZA; Suputtamongkol 2011 THA).
Effects of interventions
See: Summary of findings for the main comparison Summary of findings for participants with unknown helminth infection status; Summary of findings 2 Summary of findings for participants with confirmed helminth infections; Summary of findings 3 Summary of findings for secondary outcomes
Comparison: antihelminthic drugs versus placebo or no intervention
Primary outcomes: markers of HIV disease progression
Participants with unknown helminth infection status
Two trials evaluated the effects of antihelminthic drugs versus placebo on HIV disease progression amongst HIV‐positive adults in sub‐Saharan Africa with unknown helminth infection status. One trial administered either albendazole (800 mg), praziquantel (40 mg/kg), or both, to pregnant women and followed up for six weeks (Webb 2012 UGA), and one administered albendazole (400 mg) every three months and praziquantel (40 mg/kg) annually for two years (Walson 2012 KEN).
Viral load
In the single included trial of pregnant women, the mean viral load at six weeks was lower with each of the interventions, but the confidence intervals (CIs) included the possibility of no difference (difference in mean change −0.14 log10 viral RNA, 95% CI −0.35 to 0.07; P = 0.19, one trial, 166 participants, Analysis 1.1).
In the larger trial from Kenya, there was no substantial difference in mean change in viral load between treatment and control groups after two years of repeated drug administration (difference in mean change 0.01 log10 viral RNA, 95% CI −0.03 to 0.05; P = 0.66, one trial, 917 participants, Analysis 1.1).
CD4+ cell count
Only the long‐term trial from Kenya reported mean change in CD4+ cell counts. There was no substantial difference in mean change in CD4+ count between the treatment and control groups over two years of repeated drug administration (difference in mean change 2.60 cells/µL/year, 95% CI −10.15 to 15.35; P = 0.7, one trial, 917 participants, Analysis 1.2).
Participants with confirmed helminth infections
Five trials treated HIV‐positive adults known to be infected with helminths; two trials treated STH infection with albendazole (400 mg) once daily for three days (Abate 2014 ETH; Walson 2008 KEN), one trial treated schistosomiasis with praziquantel (40 mg/kg) once only (Kallestrup 2005 ZWE), and one trial treated LF with DEC (Nielsen 2007 TZA). These trials reported these outcomes at 12 weeks. One trial also provided outcome data at six weeks for participants infected with schistosomiasis and STHs (Webb 2012 UGA).
Viral load
In a pooled analysis across four trials with different helminth infections and different drug regimens, there was an overall suppressive effect on viral load after six to 12 weeks in the intervention groups compared to controls (difference in mean change −0.13 log10 viral RNA, 95% CI −0.26 to −0.00; P = 0.04, four trials, 445 participants, Analysis 2.1). However the CIs of the individual trials are wide and the CIs of all but one trial, Kallestrup 2005 ZWE, included the possibility of no effect. In this trial of HIV and schistosomiasis co‐infected participants, those treated with praziquantel had little change in mean plasma viral load at 12 weeks (−0.001 log10 viral RNA), while the mean viral load substantially increased in the untreated group (0.21 log10 viral RNA, P = 0.03). This trial was at high risk of selection bias, and exclusion of this trial broadens the CI of the pooled estimate to allow the possibility of no difference.
CD4+ cell count
In three trials mean CD4+ cell count declined in both treated and control groups over 12 weeks, with a smaller decrease in the treated groups (Analysis 2.2). However in one trial of TB‐infected individuals who initiated both TB treatment and ART at baseline, CD4+ cell counts increased in both treated and control groups with a larger increase in those treated with deworming drugs (difference in mean change 43.00 cells/µL, 95% CI 1.14 to 84.86; one trial, 208 participants). In the pooled analysis, the average change in CD4+ cell count was more favourable in the group treated with deworming drugs, and the CI excludes the possibility of no effect (difference in mean change 37.86 cells/µL, 95% CI 7.36 to 68.35; P = 0.01; three trials, 358 participants, Analysis 2.2).
Nielsen 2007 TZA presented results as declines in CD4+ per cent, and thus we did not include the results in the pooled meta‐analysis (Analysis 2.2).
Secondary outcomes
Adverse events
Seven trials commented on adverse events, of which five reported that no adverse events occurred (Abate 2014 ETH; Nielsen 2007 TZA; Suputtamongkol 2011 THA; Walson 2008 KEN; Webb 2012 UGA).
Kelly 1996 ZMB administered albendazole twice daily for two weeks to adults with chronic diarrhoea, and reported seven adverse events in the treatment group and three in the control group. Of the seven adverse events with albendazole, one participant had a cutaneous reaction that resolved within one week of discontinuing the drug, and four complained of dizziness, headache, cough, and difficulty swallowing. The trial authors noted that the symptoms were indistinguishable from symptoms of the underlying illness. Walson 2012 KEN administered praziquantel annually and albendazole every three months for two years, and reported 16 severe adverse events in the treatment group and 18 in the control group. The trial authors determined that none of the adverse events were directly related to the study drug.
The pooled analysis of these two trials does not suggest a significant increase in relative risk for adverse events in HIV‐positive individuals who receive antihelminthics relative to those who do not (RR 1.23, 95% CI 0.53 to 2.83; P = 0.63, seven trials, 1649 participants, Analysis 3.2).
Mortality
Six included trials recorded mortality events, of which five trials observed at least one death (Abate 2014 ETH; Kelly 1996 ZMB; Suputtamongkol 2011 THA; Walson 2008 KEN; Walson 2012 KEN). In the pooled analysis, treatment was associated with a point estimate of a 33% reduction in mortality, but the CIs included no effect (RR 0.77, 95% CI 0.52 to 1.14; P = 0.19, five trials, 1627 participants, Analysis 3.3). Most deaths occurred in the oldest study where participants were chronically unwell (had chronic diarrhoea) and were probably not on ART (Kelly 1996 ZMB). None of the deaths appeared to be directly associated with the antihelminthic interventions, as reported by the trial authors.
Anaemia and iron deficiency
Only one trial captured data regarding changes in serum haemoglobin levels (Kallestrup 2005 ZWE). The trial authors reported a mean difference in haemoglobin of −0.25 g/dL (95% CI −0.58 to −0.08) in the HIV‐positive participants that received praziquantel relative to the untreated participants over three months follow‐up (unpublished data).
A secondary analysis of the primary Nielsen 2007 TZA trial examined the effect of antihelminthic treatment on micronutrient indicators in LF and HIV co‐infected individuals (Nielsen 2009). The study found that serum ferritin levels increased in co‐infected individuals 12 weeks after treatment. The study authors observed a log mean increase of 0.07 µg/L in the treatment group, while they noted a log mean increase of 0.04 µg/L in the non‐treated group. The mean difference in log ferritin levels between baseline and 12 weeks follow‐up in the treated group relative to the control group was thus 0.03 µg/L (95% CI −0.30 to 0.35) (unpublished data provided by the study authors). As the study authors did not observe the same increase in ferritin in the HIV‐positive LF‐uninfected groups, the study authors hypothesize that the serum ferritin changes may be related to the immune mechanisms involved in killing of filaria.
Discussion
Summary of main results
In human immunodeficiency virus (HIV)‐infected antiretroviral (ART)‐naive adults with unknown helminth infection status, provision of deworming drugs (albendazole and praziquantel together or separately) may have a small suppressive effect on viral load at six weeks. However, these data come from a small single trial and the 95% confidence interval (CI) includes the possibility of no effect (low quality evidence). Repeated dosing over two years appears to have little or no effect in HIV‐positive ART‐naive adults (moderate quality evidence).
Treating helminth infections in HIV‐positive individuals with confirmed helminth infections may have a small suppressive effect on mean viral load at six to 12 weeks (low quality evidence). However, this finding is strongly influenced by a single study of praziquantel for schistosomiasis and further studies from different settings and populations are needed for validation. There may also be a small favourable effect on mean CD4+ cell count at 12 weeks.
Most of the included trials did not consistently or rigorously report adverse events (very low quality evidence) and trials were underpowered to evaluate effects on mortality (low quality evidence). However, there is no indication of excessive risk associated with deworming HIV‐positive populations.
Overall completeness and applicability of evidence
Despite the publication of five new RCTs since the previous edition of this Cochrane Review, Walson 2009, there is still insufficient evidence to make definitive conclusions about the effects of deworming drugs on markers of HIV disease progression. The eight trials included evaluate different drugs used to treat different helminth infections, which limits our ability to make broad generalizations.
For preventive chemotherapy programmes, in which deworming drugs are provided presumptively to all individuals, this Cochrane Review includes only two relevant trials and only one that reported long‐term effects. In the short‐term trial there is a suggestion of a short‐term benefit to deworming, however the trial is too small to prove an effect. Similarly, while the single long‐term trial found no evidence of benefit, further trials from other settings with different helminth endemicities are necessary before the possibility of long‐term beneficial effects can be fully excluded.
Only short‐term evidence is available when we limit the analysis to HIV‐positive adults with confirmed helminth infections. The finding of a suppressive effect on viral load in this group is highly reliant on a single trial of praziquantel for schistosomiasis. While the effect estimate of the pooled analysis is small (−0.13 log10), mathematical modelling exercises estimate that a decrease in viral load of 0.3 log10 in heterosexual couples could decrease HIV transmission by an average of 20% and AIDS‐related illnesses or death by 25% (Modjarrad 2008).
It is also important to note that none of the trials included children, and only one trial included a significant number of people taking ART (Abate 2014 ETH). These are therefore important groups for further research.
While overall few adverse events were reported in the included trials (23 in total in treated participants and 21 in total in non‐treated participants), it is important to note that only three trials defined adverse events, one with a detailed definition of qualifying adverse events (Kelly 1996 ZMB), and two with more generalized definitions (Suputtamongkol 2011 THA; Webb 2012 UGA). Only one trial reported how adverse events were monitored, using patient record‐keeping cards and house visits (Kelly 1996 ZMB). Despite these biases, most randomized controlled trials (RCTs) reported no adverse events following antihelminthic therapy and there is no evidence of excessive harm associated with deworming in HIV‐positive populations. To validate review findings, larger RCTs should be conducted with well‐documented and active adverse event identification, as well as frequent follow‐up points to assess how deworming may affect HIV‐positive populations of all ages over time.
Quality of the evidence
We have presented the GRADE assessment of the quality of evidence in the included trials in the 'Summary of findings' tables (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3). The overall assessment of quality ranges from moderate (representing a moderate level of certainty in the result) to very low (where we are very uncertain of the result).
The primary reason for downgrading the quality of evidence was 'indirectness', where we had too few trials to make broad generalizations about the potential effects. This is a particular problem for trials that evaluate deworming in populations with unknown helminth infection status as helminth endemicity varies substantially across settings, undoubtedly influencing observed findings. Although we had more data from trials that evaluated deworming drugs in participants with documented helminth infections, the substantial heterogeneity in populations and deworming drugs used again decreased our confidence in making broad generalizations. The second most common reason for downgrading the quality of evidence was 'imprecision' in effect estimates. Many of the included trials were small, and had wide 95% CIs, which included the possibility of both clinically important effects and no effect.
Potential biases in the review process
Judd Walson is a co‐author of this Cochrane Review and was Primary Investigator on two of the included RCTs. However, he was not involved in conducting any of the 'Risk of bias' assessments or in data extraction for this Cochrane Review.
There were not enough trials available in the published literature to be able to assess for publication bias.
Agreements and disagreements with other studies or reviews
The first review on this topic, Walson 2007a, identified only one RCT for inclusion that evaluated the potential benefit of treating helminth co‐infection in HIV‐1‐infected individuals (Kallestrup 2005 ZWE). Subsequently two additional RCTs were published (Nielsen 2007 TZA; Walson 2008 KEN), and a Cochrane Review of all three RCTs concluded that treatment of helminth co‐infection appeared to result in favourable effects on markers of HIV‐1 disease progression (Walson 2009). Specifically, the pooled analysis of the data suggested that treatment of helminth co‐infection may attenuate increases in HIV‐1 RNA and result in slower decline in CD4+ counts.
The findings of this Cochrane Review update are consistent with the previous edition (Walson 2009). However, we used the GRADE approach to assess the quality of evidence, which caused us to downgrade the quality of the evidence. As we stratified by helminth infection status, we also separately provide evidence for individuals who were helminth‐infected or of unknown infection status.

Study flow diagram.
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.

'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included trials.
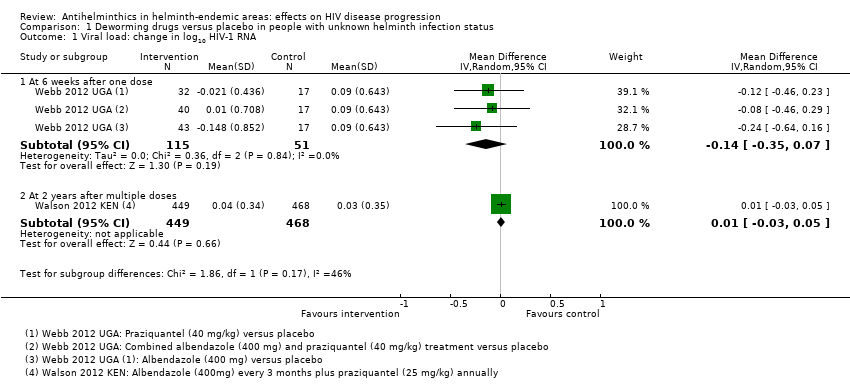
Comparison 1 Deworming drugs versus placebo in people with unknown helminth infection status, Outcome 1 Viral load: change in log10 HIV‐1 RNA.

Comparison 1 Deworming drugs versus placebo in people with unknown helminth infection status, Outcome 2 Change in CD4 count.
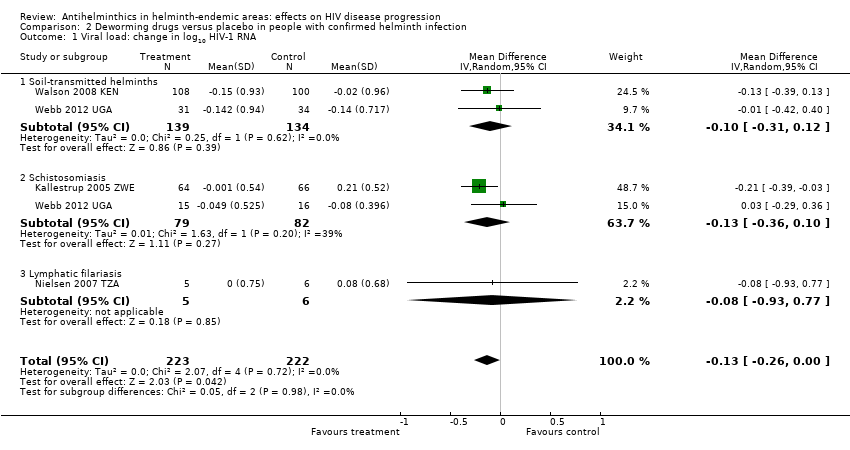
Comparison 2 Deworming drugs versus placebo in people with confirmed helminth infection, Outcome 1 Viral load: change in log10 HIV‐1 RNA.

Comparison 2 Deworming drugs versus placebo in people with confirmed helminth infection, Outcome 2 Change in CD4 count.

Comparison 3 Deworming drugs versus placebo; all trials, Outcome 1 Change in log10 HIV RNA, by antihelminthic.

Comparison 3 Deworming drugs versus placebo; all trials, Outcome 2 Difference in adverse events between treatment and no treatment groups.
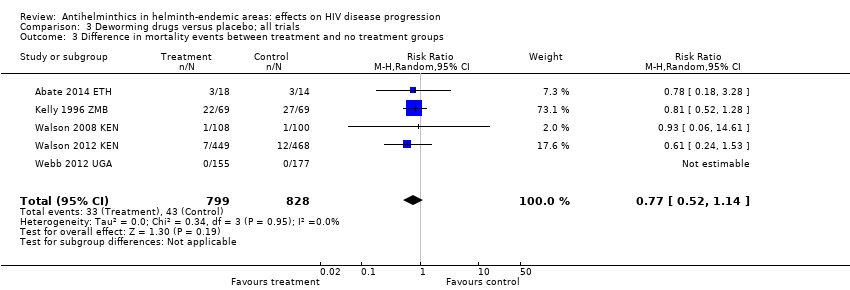
Comparison 3 Deworming drugs versus placebo; all trials, Outcome 3 Difference in mortality events between treatment and no treatment groups.
| Deworming drugs compared with placebo for people with HIV and an unknown helminth infection status | ||||
| Participant or population: HIV‐positive people Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole or praziquantel or a combination) Comparison: placebo | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | |||
| Placebo | Deworming drugs | |||
| Viral load | At 6 weeks after a single dose | 166 (1 trial) | ⊕⊕⊝⊝1,2,3,4 | |
| in the control group, the mean change in viral load was an increase of 0.09 log10 viral RNA | On average, with deworming, there was a suppressive effect on mean viral load of 0.14 log10 viral RNA (0.35 benefit to 0.07 harm) | |||
| At 2 years after multiple doses | 917 (1 trial) | ⊕⊕⊕⊝1,2,5,6 | ||
| In the control group, the mean viral load increased by 0.03 log10 viral RNA | On average, with deworming, there was a suppressive effect on mean viral load of 0.01 log10 viral RNA (0.03 benefit to 0.05 harm) | |||
| CD4+ cell count | At 2 years after multiple doses | 917 (1 trial) | ⊕⊕⊝⊝1,2,4,5 | |
| In the control group, the mean CD4+ cell count reduced by 37.3 CD4+ cells/µL | On average, with deworming, there was a favourable effect on mean CD4+ cell count of 2.60 CD4+ cells/µL (15.35 benefit 10.15 harm) | |||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1No serious risk of bias: this single trial was at low risk of selection bias. | ||||
| Deworming drugs compared with placebo for people with HIV and confirmed helminth infections | ||||
| Participant or population: HIV‐positive people with proven helminth infection Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole, praziquantel, albendazole and praziquantel, or diethylcarbamazine) Comparison: placebo | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | |||
| Placebo | Deworming drugs | |||
| Viral load | At 6 to 12 weeks | 445 (4 trials) | ⊕⊕⊝⊝ | |
| In the control groups, the mean change in viral load ranged from a 0.13 decrease to an increase of 0.21 log10 viral RNA | On average, with deworming, there was a small suppressive effect on mean viral load of 0.13 log10 viral RNA (0.26 benefit to 0.00 benefit) | |||
| CD4+ cell count | At 6 to 12 weeks | 358 (3 trials) | ⊕⊕⊝⊝ | |
| In the control groups, the mean change in CD4+ cell count ranged from a decrease of 68 to an increase of 45 CD4+ cells/µL | On average, with deworming, there was favourable effect on mean CD4+ cell count of 37.86 CD4+ cells/µL (7.36 benefit to 68.35 benefit) | |||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1We downgraded by 1 for serious risk of bias: of the five studies, only one had CIs that excluded the possibility of no effect and this study was at high risk of selection bias. | ||||
| Deworming drugs compared with placebo for people with HIV and both known and unknown helminth infection status | |||||
| Participant or population: HIV‐positive people Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole, praziquantel, albendazole and praziquantel, or diethylcarbamazine) Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Albendazole | ||||
| Iron deficiency (serum ferritin) | log10 mean increase of 0.04 µg/L | log10 mean increase of 0.07 µg/L | Deworming drugs associated with a 0.03 higher µg/L log10 mean ferritin measure (0.30 lower to 0.35 higher) | 16 (1 trial) | ⊕⊝⊝⊝1,2,3,4 |
| Anaemia (serum haemoglobin) | Increase in 0.15 g/dL | Decrease in 0.10 g/dL | Deworming drugs associated with a 0.25 lower g/dL haemoglobin (0.58 lower to 0.08 higher) | 130 (1 trial) | ⊕⊝⊝⊝2,4,5,6 |
| Mortality | 41 per 1000 | 52 per 1000 | RR 0.77 (0.52 to 1.14) | 1627 (5 trials) | ⊕⊕⊝⊝7,8,9,10 |
| Adverse events | 32 per 1000 | 35 per 1000 | RR 1.23 (0.53 to 2.83) | 1649 (7 trials) | ⊕⊝⊝⊝4,9,11,12 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1No serious risk of bias: this single trial was at risk of selection bias. However, the outcome is an objective laboratory measure. We did not downgrade the quality of the evidence. | |||||
| Number | Search terms |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) |
| #2 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) |
| #3 | Search (helminths[mh] OR helminth*[tiab] OR nematode*[tiab] OR worm*[tiab] OR parasites[mh] OR parasit*[tiab] OR round worm*[tiab] OR roundworm*[tiab] OR hookworm*[tiab] OR hook worm*[tiab] OR ancylostoma*[tiab] OR cestode*[tiab] OR tapeworm*[tiab] OR tape worm*[tiab] OR trematode*[tiab] OR fluke*[tiab] OR whipworm*[tiab] OR whip worm*[tiab] OR trichuris[tiab] OR ascaris[tiab] OR enterobi*[tiab] OR strongyloide*[tiab] OR mansonell*[tiab] OR taenia[tiab] schistosom*[tiab] OR necator*[tiab] OR paragonim*[tiab] OR hymenolepis[tiab] OR fasciol*[tiab] OR filariasis[tiab] OR trichostrongl*[tiab] OR microfilaria[tiab] OR parasitic diseases[mh:noexp] OR helminthiasis[mh] OR intestinal diseases, parasitic[mh]) |
| #4 | Search (benzimidazoles OR albendazole OR mebendazole OR ivermectin OR praziquantel OR diethylcarbamazine OR bithionol OR oxamniquine OR pyrantel OR nitazoxanide OR anthelmintic OR anthelmintics OR anthelminthic OR anthelminthics OR “anti helminthic” OR “anti helminthics” OR “anti helmintic” OR “anti helmintics” OR antihelminthic OR antihelminthics OR antihelmintic OR antihelmintics) |
| #5 | Search (#1 AND #2 AND #3 AND #4) |
| #6 | Search (((#1 AND #2 AND #3 AND #4))) AND ("1980/01/01"[Date ‐ Publication] : "2015/09/29"[Date ‐ Publication]) |
| Number | Search terms |
| #1 | 'human immunodeficiency virus infection' exp OR 'human immunodeficiency virus infection':ab,ti OR 'hiv infection':ab,ti OR 'hiv infections':ab,ti OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR hiv:ab,ti OR 'hiv 1':ab,ti OR 'hiv 2':ab,ti OR 'human immune deficiency virus':ab,ti OR 'human immuno deficiency virus':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno deficiency syndrome':ab,ti OR 'acquired immune deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti |
| #2 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure'OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl*NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (crossNEXT/1 over*):ab,ti |
| #3 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de |
| #4 | 'human'/de OR 'normal human'/de OR 'human cell'/de |
| #5 | #3 AND #4 |
| #6 | #3 NOT #5 |
| #7 | #2 NOT #6 |
| #8 | 'helminth'/exp OR helminth*:ab,ti OR nematode*:ab,ti OR worm*:ab,ti OR 'parasite'/exp OR parasit*:ab,ti OR roundAND worm*:ab,ti OR roundworm*:ab,ti OR hookworm*:ab,ti OR hookAND worm*:ab,ti OR ancylostoma*:ab,ti OR cestode*:ab,ti OR tapeworm*:ab,ti OR tapeAND worm*:ab,ti OR trematode*:ab,ti OR fluke*:ab,ti OR whipworm*:ab,ti OR whipAND worm*:ab,ti OR trichuris:ab,ti OR ascaris:ab,ti OR enterobi*:ab,ti OR strongyloide*:ab,ti OR mansonell*:ab,ti OR taenia:ab,ti AND schistosom*:ab,ti OR necator*:ab,ti OR paragonim*:ab,ti OR hymenolepis:ab,ti OR fasciol*:ab,ti OR filariasis:ab,ti OR trichostrongl*:ab,ti OR microfilaria:ab,ti OR 'parasitic diseases'/exp OR 'helminthiasis'/de OR 'intestine infection'/de |
| #9 | 'benzimidazoles'/de OR benzimidazolesOR 'albendazole'/de OR albendazoleOR 'mebendazole'/de OR mebendazoleOR 'ivermectin'/de OR ivermectinOR 'praziquantel'/de OR praziquantelOR 'diethylcarbamazine'/de OR diethylcarbamazineOR 'bithionol'/de OR bithionolOR 'oxamniquine'/de OR oxamniquineOR 'pyrantel'/de OR pyrantelOR 'nitazoxanide'/de OR nitazoxanideOR 'anthelmintic'/de OR anthelminticOR 'anthelmintics'/de OR anthelminticsOR 'anthelminthic'/de OR anthelminthicOR anthelminthicsOR 'anti helminthic'OR 'anti helminthics'OR 'anti helmintic'OR 'anti helmintics'OR antihelminthicOR antihelminthicsOR 'antihelmintic'/de OR antihelminticOR 'antihelmintics'/de OR antihelmintics |
| #10 | #1 AND #7 AND #8 AND #9 |
| #11 | #1 AND #7 AND #8 AND #9 AND [1‐1‐1980]/sd NOT [29‐09‐2015]/sd |
| Number | Search terms |
| #1 | MeSH descriptor: [HIV Infections] explode all trees |
| #2 | MeSH descriptor: [HIV] explode all trees |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only |
| #6 | #1 or #2 or #3 or #4 or #5 |
| #7 | [mh helminths] or helminth*:ti,ab,kw or meatode*:ti,ab,kw or worm*:ti,ab,kw or [mh parasites] or parasit*:ti,ab,kw or round worm:ti,ab,kw or roundworm:ti,ab,kw or hook worm*:ti,ab,kw or hookworm*:ti,ab,kw or ancylostoma*:ti,ab,kw or cestode*:ti,ab,kw or tapeworm*:ti,ab,kw or tape worm*:ti,ab,kw or trematode*:ti,ab,kw or fluke*:ti,ab,kw or whipworm*:ti,ab,kw or whip worm*:ti,ab,kw or trichuris:ti,ab,kw or ascaris:ti,ab,kw or enterobi*:ti,ab,kw or strongyloide*:ti,ab,kw or mansonell*:ti,ab,kw or taenia:ti,ab,kw schistosom*:ti,ab,kw or necator*:ti,ab,kw or paragonim*:ti,ab,kw or hymenolepis:ti,ab,kw or fasciol*:ti,ab,kw or filariasis:ti,ab,kw or trichostrongl*:ti,ab,kw or microfilaria:ti,ab,kw or [mh ^"parasitic diseases"] or [mh helminthiasis] or [mh ^"intestinal diseases, parasitic"] (Word variations have been searched) |
| #8 | benzimidazoles or albendazole or mebendazole or ivermectin or praziquantel or diethylcarbamazine or bithionol or oxamniquine or pyrantel or nitazoxanide or anthelmintic or anthelmintics or anthelminthic or anthelminthics or "anti helminthic" or "anti helminthics" or "anti helmintic" or "anti helmintics" or antihelminthic or antihelminthics or antihelmintic or antihelmintics (Word variations have been searched) |
| #9 | #6 and #7 and #8 Publication Year from 1980 to 2015, in Trials |
| Number | Search terms |
| #1 | HIV AND HELMINTH |
| Number | Search terms |
| #1 | HIV AND HELMINTH | Interventional Studies | received from 01/01/1980 to 09/29/2015 |
| Number | Search terms |
| #1 | “HIV Infections”[MeSH] OR “HIV”[MeSH] OR hiv [tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immunodeficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immunodeficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR “Sexually Transmitted Diseases, Viral”[MeSH:NoExp] |
| #2 | randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR (“clinical trial” [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) |
| #3 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CESTODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS OR HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #4 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #5 | #3 AND #4 |
| #6 | #1 AND #2 AND #5 |
| #7 | #1 AND #2 AND #5 Limits: Publication Date from 1980 to 2015/09/29 |
| Number | Search terms |
| #1 | ((’human immunodeficiency virus infection’/exp OR ’human immunodeficiency virus infection’) OR (’human immunodeficiency virus infection’/exp OR ’human immunodeficiency virus infection’)) OR ((’human immunodeficiency virus’/exp OR ’human immunodeficiency virus’) OR (’human immunodeficiency virus’/exp OR ’human immunodeficiency virus’)) OR (hiv:ti OR hiv:ab) OR (’hiv‐1’:ti OR ’hiv‐1’:ab) OR (’hiv‐2’:ti OR ’hiv‐2’:ab) OR (’human immunodeficiency virus’:ti OR ’human immunodeficiency virus’:ab) OR (’human immuno‐deficiency virus’:ti OR ’human immuno‐deficiency virus’:ab) OR (’human immunedeficiency virus’:ti OR ’human immunedeficiency virus’:ab) OR (’human immune‐deficiency virus’:ti OR ’human immune‐deficiency virus’:ab) OR (’acquired immune‐deficiency syndrome’:ti OR ’acquired immune‐deficiency syndrome’:ab) OR (’acquired immunedeficiency syndrome’:ti OR ’acquired immunedeficiency syndrome’:ab) OR (’acquired immunodeficiency syndrome’:ti OR ’acquired immunodeficiency syndrome’:ab) OR (’acquired immuno‐deficiency syndrome’:ti OR ’acquired immuno‐deficiency syndrome’:ab) |
| #2 | random:ti OR random:ab OR factorial:ti OR factorial*:ab OR 'cross over':ti OR 'cross over':ab OR crossover:ti OR crossover:ab OR placebo:ti OR placebo:ab OR (double:ti AND blind:ti) OR (doubl:ab AND blind*:ab) OR (single:ti AND blind:ti) OR (single:ab AND blind:ab) OR assign:ti OR assign:ab OR allocat:ti OR allocate:ab OR volunteer:ti OR volunteer:ab OR 'crossover procedure'/exp OR 'crossover procedure' OR 'double‐blind procedure'/exp OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure' OR 'randomized controlled trial'/exp OR 'randomized controlled trial' |
| #3 | 'helminths' OR 'roundworm' OR 'round worm' OR 'round‐worm' OR roundworms OR 'round worms' OR 'round‐worms' OR nematodes OR 'nematode' OR 'cestode' OR cestodes OR 'tapeworm' OR 'tape worm' OR 'tape‐worm' OR tapeworms OR 'tape worms' OR 'tape‐worms' OR 'trematode' OR trematodes OR 'fluke' OR flukes OR 'worm' OR worms OR 'parasite' OR 'parasites' OR 'ascaris' OR 'trichuris' OR 'enterobius' OR strongyloide OR stronglyloides OR 'ancylostoma' OR ancylostomas OR 'necator' OR necators OR 'hymenolepis' OR 'paragonimus' OR 'fasciola' OR 'taenia' OR 'hookworm' OR 'hook worm' OR 'hook‐worm' OR hookworms OR 'hook worms' OR 'hook‐worms' OR 'whipworm' OR 'whip worm' OR 'whip‐worm' OR whipworms OR 'whip worms' OR 'whip‐worms' OR shistosomiasis OR 'mansonella' OR 'filariasis' OR 'microfilaria' OR 'trichostrongylus' OR trichostronglylosis OR stronglyloidea OR pargonimiasis |
| #4 | ((’benzimidazoles’/exp OR ’benzimidazoles’) OR (’benzimidazoles’/exp OR ’benzimidazoles’)) OR ((’albendazole’/exp OR ’albendazole’) OR (’albendazole’/exp OR ’albendazole’)) OR ((’mebendazole’/exp OR ’mebendazole’) OR (’mebendazole’/exp OR ’mebendazole’)) OR ((’ivermectin’/exp OR ’ivermectin’) OR (’ivermectin’/exp OR ’ivermectin’)) OR ((’praziquantel’/exp OR ’praziquantel’) OR (’praziquantel’/exp OR ’praziquantel’)) OR ((’diethylcarbamazine’/exp OR ’diethylcarbamazine’) OR (’diethylcarbamazine’/exp OR ’diethylcarbamazine’)) OR ((’bithionol’/exp OR ’bithionol’) OR (’bithionol’/exp OR ’bithionol’)) OR ((’oxamniquine’/exp OR ’oxamniquine’) OR (’oxamniquine’/exp OR ’oxamniquine’)) OR ((’pyrantel’/exp OR ’pyrantel’) OR (’pyrantel’/exp OR ’pyrantel’)) OR ((’nitazoxanide’/exp OR ’nitazoxanide’) OR (’nitazoxanide’/exp OR ’nitazoxanide’)) |
| #5 | #3 OR #4 |
| #6 | #1 AND #2 AND #5 [1‐1‐1980]/sd NOT [29‐09‐2015]/sd |
| Number | Search terms |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (VIRAL SEXUALLY TRANSMITTED DISEASES) |
| #2 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CES‐TODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS OR HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #3 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #4 | #2 OR #3 |
| #5 | #1 AND #4 from 1980 to 2015 |
| Number | Search terms |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) |
| #2 | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐ BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (“CLINICAL TRIAL”) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND*)) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN) |
| #3 | #1 AND #2 |
| #4 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CES‐TODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS |
| #5 | HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #6 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #7 | #4 OR #5 OR #6 |
| #8 | #7 AND #3 |
| Number | Search terms |
| #1 | Helminth* AND "HIV Infections" received from 01/01/1980 to 09/29/2015 |
| Trial | Country | Setting | Year enrolment completed | Inclusion criteria | Age | Total randomized | Number HIV‐positive participants | Taking ART | Number co‐infected with helminths at baseline |
| Zambia | Urban | Not stated | HIV‐infected adults with persistent diarrhoea | > 18 years | 174 | 174 | Not stated (probably low) | Unknown | |
| Uganda | Urban | 2005 | HIV‐infected pregnant women | Not stated | 264 | 264 | < 3% | 67% had at least 1 helminth species | |
| Kenya | Urban/rural | 2011 | HIV‐infected, not on ART | > 18 years | 948 | 948 | None at baseline | Unknown | |
| Abbreviations: HIV: human immunodeficiency virus; ART: antiretroviral. | |||||||||
| Trial | Intervention | Control | Outcomes | Timing | ||
| Drug | Dose | Frequency | ||||
| Albendazole | 400 mg | Once daily for 3 days | Placebo | Viral load CD4 Adverse events Mortality events | 12 weeks | |
| Albendazole | 400 mg | Once daily for 3 days | Placebo | CD4 Adverse events Mortality events | 12 weeks | |
| Praziquantel | 40 mg/kg | Once only | No intervention | Viral load CD4 | 12 weeks | |
| Diethylcarbamazine | 6 mg/kg | Once only | Placebo | Viral load CD4 Adverse events | 12 weeks | |
| Albendazole | 800 mg | Twice daily for 14 days | Placebo | Adverse events Mortality events | 6 months | |
| Ivermectin | 200 mg/kg | Single or double dose 2 weeks apart | Albendazole | Adverse events | 1 year | |
| Albendazole Praziquantel | 400 mg 40 mg/kg | Once only Once only | Placebo | Viral load Adverse events Mortality events | 6 weeks | |
| Albendazole Praziquantel | 400 mg 25 mg/kg | Every 3 months Annually | No intervention | Viral load CD4 Adverse events Mortality events | 2 years | |
| Trial | Country | Setting | Year enrolment completed | Inclusion criteria | Age | Total randomized | Number of HIV‐positive participants | Taking ART | Number co‐infected with helminths at baseline |
| Kenya | Urban/rural | 2007 | HIV‐infected with at least one helminth co‐infection | > 18 years | 234 | 234 | None at baseline | All | |
| Ethiopia | Urban | 2012 | Newly diagnosed TB participants with helminth co‐infection | 15 to 60 years | 140 | 32 | 94% of intervention group, 100% of controls | All | |
| Zimbabwe | Rural | 2003 | Adults infected with schistosomiasis | > 18 years | 287 | 130 | Not stated | All | |
| Tanzania | Rural | 2002 | HIV‐infected adults | 22 to 70 years | 34 | 34 | Not stated | 18 | |
| Thailand | Urban | 2009 | Adults with characteristic strongyloides infection | > 18 years | 100 | 10 | Not stated | All | |
| Abbreviations: HIV: human immunodeficiency virus; ART: antiretroviral; TB: tuberculosis. | |||||||||
| Trial name | Relevant intervention | Relevant outcome | Target population | Location(s) | Estimated completion date |
| Reduction of EArly mortaLITY in HIV‐infected Adults and Children Starting Antiretroviral Therapy (REALITY) | Immediate enhanced opportunistic infections (OI) prophylaxis with isoniazid/pyridoxine and cotrimoxazole, plus 12 weeks fluconazole, 5 days azithromycin, and a single dose of albendazole versus cotrimoxazole prophylaxis alone for the first 12 weeks followed by isoniazid and any prophylaxis and/or treatment prescribed at screening | • Change in CD4 count • Adverse events | HIV‐infected individuals ages ≥ 5 years | Kenya, Malawi, Uganda, Zimbabwe | February 2016 |
| Can Anthelminthic Treatment Delay the Progression of HIV? Randomised Open‐label Trial Testing Presumptive Anthelminthic Treatment on Progression of HIV in ART‐naïve HIV‐positive Patients in a Rural African Setting With Presumed High Prevalence of Helminth Infections | Standard HIV care with provision of praziquantel, albendazole, and ivermectin at baseline, after 6 months, and after 12 months versus standard HIV care with no anthelminthic treatment | • Change in viral load • Change in CD4 count • Adverse event | HIV‐infected individuals aged ≥ 18 years | Tanzania | Terminated prematurely due to recruitment difficulties |
| Abbreviations: RCT: randomized controlled trial; HIV: human immunodeficiency virus; ART: antiretroviral. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Viral load: change in log10 HIV‐1 RNA Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 At 6 weeks after one dose | 1 | 166 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.35, 0.07] |
| 1.2 At 2 years after multiple doses | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2 Change in CD4 count Show forest plot | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐10.15, 15.35] |
| 2.1 At 2 years after multiple doses | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐10.15, 15.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Viral load: change in log10 HIV‐1 RNA Show forest plot | 4 | 445 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.26, ‐0.00] |
| 1.1 Soil‐transmitted helminths | 2 | 273 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.31, 0.12] |
| 1.2 Schistosomiasis | 2 | 161 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.36, 0.10] |
| 1.3 Lymphatic filariasis | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.93, 0.77] |
| 2 Change in CD4 count Show forest plot | 3 | 358 | Mean Difference (IV, Random, 95% CI) | 37.86 [7.36, 68.35] |
| 2.1 Soil‐transmitted helminths | 2 | 228 | Mean Difference (IV, Random, 95% CI) | 40.69 [1.51, 79.87] |
| 2.2 Schistosomiasis | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 33.5 [‐15.06, 82.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in log10 HIV RNA, by antihelminthic Show forest plot | 5 | 1534 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.19, 0.01] |
| 1.1 Albendazole | 2 | 302 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.37, 0.02] |
| 1.2 Praziquantel | 2 | 213 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.32, ‐0.03] |
| 1.3 Diethylcarbamazine | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.93, 0.77] |
| 1.4 Albendazole and praziquantel | 2 | 1008 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.05] |
| 2 Difference in adverse events between treatment and no treatment groups Show forest plot | 7 | 1649 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.53, 2.83] |
| 3 Difference in mortality events between treatment and no treatment groups Show forest plot | 5 | 1627 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.52, 1.14] |

