Surgery for tubal infertility
Appendices
Appendix 1. Gynaecology and Fertility Group Specialised Register
(searched from inception to 19 October 2016) (PROCITE platform)
Keywords CONTAINS "Fallopian tube obstruction" or "tubal factor" or "tubal flushing" or "tubal infertility" or "tubal inflation" or "tubal occlusion" or "tubal occlusion ‐ proximal" or "tubal patency" or "tubal reconstruction" or "tubal subfertility" or "tube drainage" or "tuboplasty" or "Fallopian Tube Fixation" or "fallopian tubes" or "tubal anastomosis" or "tubal disorders" or "tubo‐ovarian abscess" or "salpingectomy" or "Salpingitis‐Physiopathology" or "salpingo‐oopherectomy" or "Salpingolysis" or "*Salpingostomy‐"or "salpingotomy" or "Hydrosalpinx" or "hydrosalpingies" or "hydrosalpinges" or "falloscopy" or "laparoscopic salpingectomy" or "laparoscopic salpingoovolysis" or "laparoscopic salpingotomy" or "laparoscopic tubal fulguration" or "microsurgery" or "microscopic" or "microdiathermy" or "microlaparoscopy" or "hydrotubation" (446 hits)
Appendix 2. CENTRAL CRSO search strategy
(searched from inception to 19 October 2016) (CRSO web platform)
#1 MESH DESCRIPTOR Fallopian Tube Diseases EXPLODE ALL TREES 137
#2 MESH DESCRIPTOR Pelvic Inflammatory Disease EXPLODE ALL TREES 422
#3 MESH DESCRIPTOR Salpingitis EXPLODE ALL TREES 42
#4 (tubal infertility):TI,AB,KY 52
#5 ( tubal factor):TI,AB,KY 55
#6 (disten* adj3 tub*):TI,AB,KY 5
#7 (tubal subfertility):TI,AB,KY 2
#8 (tub* adj3 occlusion*):TI,AB,KY 163
#9 (tube* adj3 damage*):TI,AB,KY 7
#10 (tubal adj3 damage*):TI,AB,KY 12
#11 (adhesion* adj3 tub*):TI,AB,KY 22
#12 fallopian:TI,AB,KY 534
#13 (peritubal adj3 adhesion*):TI,AB,KY 3
#14 (tub* adj3 block*):TI,AB,KY 78
#15 hydrosalpin*:TI,AB,KY 53
#16 (Tub* adj3 lesion*):TI,AB,KY 46
#17 (disease* adj3 tub*):TI,AB,KY 222
#18 (occlu* adj3 oviduct*):TI,AB,KY 2
#19 (adhesion* adj3 oviduct*):TI,AB,KY 2
#20 (Tub* adj3 obstruction*):TI,AB,KY 61
#21 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 1531
#22 MESH DESCRIPTOR Gynecologic Surgical Procedures EXPLODE ALL TREES 3630
#23 MESH DESCRIPTOR Salpingectomy EXPLODE ALL TREES 22
#24 MESH DESCRIPTOR salpingostomy EXPLODE ALL TREES 38
#25 MESH DESCRIPTOR Hand‐Assisted Laparoscopy EXPLODE ALL TREES 7
#26 MESH DESCRIPTOR Laparoscopy EXPLODE ALL TREES 4243
#27 Laparoscop*:TI,AB,KY 9365
#28 MESH DESCRIPTOR Laparotomy EXPLODE ALL TREES 622
#29 Laparotomy:TI,AB,KY 1812
#30 electrosurg*:TI,AB,KY 352
#31 MESH DESCRIPTOR Electrosurgery EXPLODE ALL TREES 204
#32 MESH DESCRIPTOR Microsurgery EXPLODE ALL TREES 518
#33 microsurg*:TI,AB,KY 732
#34 minilaparotom*:TI,AB,KY 106
#35 (tubo‐cornual anastomosis):TI,AB,KY 0
#36 fimbrioplasty:TI,AB,KY 6
#37 adhesiolysis:TI,AB,KY 85
#38 reconstruction:TI,AB,KY 3866
#39 (recanalizing or recanalising):TI,AB,KY 7
#40 (recanalisation or recanalization):TI,AB,KY 872
#41 (salpingostomy or salpingectomy):TI,AB,KY 142
#42 aspiration:TI,AB,KY 3801
#43 electrocoagulation:TI,AB,KY 716
#44 MESH DESCRIPTOR Sclerotherapy EXPLODE ALL TREES 432
#45 Sclerotherap*:TI,AB,KY 1153
#46 emboli?ation:TI,AB,KY 1111
#47 excision*:TI,AB,KY 3333
#48 #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 27783
#49 #21 AND #48 358
Appendix 3. MEDLINE search strategy
(searched form 1946 to 19 October 2016) (Ovid platform)
1 exp fallopian tube diseases/ or pelvic inflammatory disease/ or salpingitis/ (11952)
2 tubal infertility.tw. (707)
3 tubal subfertility.tw. (14)
4 tubal factor.tw. (719)
5 tubal fibrosis.tw. (6)
6 (disten$ adj3 tube).tw. (70)
7 (disten$ adj3 tubal).tw. (10)
8 tubal occlusion.tw. (912)
9 (occlusion adj3 tubes).tw. (70)
10 (occlusion adj3 tube).tw. (306)
11 ((tube adj3 damage) or (tubal adj3 damage)).tw. (426)
12 (tube adj3 damage).tw. (124)
13 (adhesion$ adj3 tubal).tw. (199)
14 (adhesion$ adj3 tube).tw. (216)
15 (adhesion$ adj3 tubes).tw. (66)
16 fallopian.tw. (9086)
17 (peritubal adj3 adhesion$).tw. (117)
18 (tube adj3 block$).tw. (530)
19 (tubal adj3 block$).tw. (160)
20 (tubes adj3 block$).tw. (206)
21 hydrosalpin$.tw. (842)
22 ((Tubal adj3 lesion$) or (Tube adj3 lesion$)).tw. (240)
23 ((disease$ adj3 tubal) or (disease$ adj3 tubes)).tw. (576)
24 (oviduct$ adj3 damage$).tw. (33)
25 (oviduct$ adj3 fibrosis).tw. (4)
26 (disten$ adj3 oviduct$).tw. (7)
27 (occlu$ adj3 oviduct$).tw. (48)
28 (adhesion$ adj3 oviduct$).tw. (24)
29 ((Tubal adj3 obstruction$) or (Tube adj3 obstruction$)).tw. (1058)
30 1 and 29 (266)
31 or/1‐28,30 (21711)
32 gynecologic surgical procedures/ or salpingectomy/ or salpingostomy/ (9590)
33 (surgery or surgical).tw. (1441006)
34 32 and 33 (6300)
35 laparoscopy/ or hand‐assisted laparoscopy/ (70173)
36 Laparoscop$.tw. (101252)
37 Laparotomy/ (17222)
38 Laparotomy.tw. (40722)
39 electrosurgery/ or microsurgery/ (28971)
40 microsurg$.tw. (22043)
41 minilaparotom$.tw. (994)
42 tubo‐cornual anastomosis.tw. (1)
43 fimbrioplasty.tw. (71)
44 adhesiolysis.tw. (1226)
45 reconstruction.tw. (160461)
46 (recanalizing or recanalising).tw. (173)
47 (recanalisation or recanalization).tw. (9700)
48 (salpingostomy or salpingectomy).tw. (1786)
49 aspiration.tw. (68032)
50 electrocoagulation.tw. (2763)
51 Sclerotherapy/ (4766)
52 Sclerotherap$.tw. (6126)
53 emboli?ation.tw. (39239)
54 or/32,34‐53 (469023)
55 31 and 54 (5028)
56 randomized controlled trial.pt. (432907)
57 controlled clinical trial.pt. (91818)
58 randomized.ab. (373391)
59 randomised.ab. (76600)
60 placebo.tw. (185046)
61 clinical trials as topic.sh. (180215)
62 randomly.ab. (265326)
63 trial.ti. (163366)
64 (crossover or cross‐over or cross over).tw. (71526)
65 or/56‐64 (1126803)
66 exp animals/ not humans.sh. (4325953)
67 65 not 66 (1039022)
68 55 and 67 (282)
Utilising the Cochrane Highly Sensitive Search Strategies for identifying randomised trials in MEDLINE (Higgins 2011)
Appendix 4. Embase search strategy
(searched from 1974 to 19 October 2016) (Ovid platform)
1 exp uterine tube disease/ or pelvic inflammatory disease/ or salpingitis/ (14863)
2 tubal infertility.tw. (828)
3 tubal subfertility.tw. (15)
4 tubal factor.tw. (875)
5 tubal fibrosis.tw. (6)
6 (disten$ adj3 tube).tw. (84)
7 (disten$ adj3 tubal).tw. (17)
8 tubal occlusion.tw. (949)
9 (occlusion adj3 tubes).tw. (64)
10 (occlusion adj3 tube).tw. (332)
11 ((tube adj3 damage) or (tubal adj3 damage)).tw. (476)
12 (tube adj3 damage).tw. (133)
13 (adhesion$ adj3 tubal).tw. (251)
14 (adhesion$ adj3 tube).tw. (222)
15 (adhesion$ adj3 tubes).tw. (62)
16 fallopian.tw. (9749)
17 (peritubal adj3 adhesion$).tw. (140)
18 (tube adj3 block$).tw. (617)
19 (tubal adj3 block$).tw. (221)
20 (tubes adj3 block$).tw. (242)
21 hydrosalpin$.tw. (1071)
22 ((Tubal adj3 lesion$) or (Tube adj3 lesion$)).tw. (309)
23 ((disease$ adj3 tubal) or (disease$ adj3 tubes)).tw. (649)
24 (oviduct$ adj3 damage$).tw. (26)
25 (oviduct$ adj3 fibrosis).tw. (3)
26 (disten$ adj3 oviduct$).tw. (5)
27 (occlu$ adj3 oviduct$).tw. (41)
28 (adhesion$ adj3 oviduct$).tw. (25)
29 ((Tubal adj3 obstruction$) or (Tube adj3 obstruction$)).tw. (1213)
30 1 and 29 (278)
31 or/1‐28,30 (25812)
32 gynecologic surgical procedures/ or salpingectomy/ or salpingostomy/ (14627)
33 (surgery or surgical).tw. (1658696)
34 32 and 33 (9079)
35 laparoscopy/ or hand‐assisted laparoscopy/ (56876)
36 Laparoscop$.tw. (134431)
37 Laparotomy/ (57049)
38 Laparotomy.tw. (46702)
39 electrosurgery/ or microsurgery/ (28040)
40 microsurg$.tw. (23216)
41 minilaparotom$.tw. (1149)
42 tubo‐cornual anastomosis.tw. (3)
43 fimbrioplasty.tw. (78)
44 adhesiolysis.tw. (1860)
45 reconstruction.tw. (163756)
46 (recanalizing or recanalising).tw. (235)
47 (recanalisation or recanalization).tw. (12911)
48 (salpingostomy or salpingectomy).tw. (2173)
49 aspiration.tw. (79956)
50 electrocoagulatioaintern.tw. (2749)
51 Sclerotherapy/ (8628)
52 Sclerotherap$.tw. (7813)
53 emboli?ation.tw. (47816)
54 or/32,34‐53 (552917)
55 31 and 54 (6793)
56 Clinical Trial/ (848860)
57 Randomized Controlled Trial/ (380154)
58 exp randomization/ (67586)
59 Single Blind Procedure/ (20772)
60 Double Blind Procedure/ (122634)
61 Crossover Procedure/ (43961)
62 Placebo/ (261129)
63 Randomi?ed controlled trial$.tw. (121529)
64 Rct.tw. (17856)
65 random allocation.tw. (1441)
66 randomly.tw. (296704)
67 randomly allocated.tw. (22974)
68 allocated randomly.tw. (2045)
69 (allocated adj2 random).tw. (734)
70 Single blind$.tw. (16181)
71 Double blind$.tw. (153400)
72 ((treble or triple) adj blind$).tw. (471)
73 placebo$.tw. (218534)
74 prospective study/ (302585)
75 or/56‐74 (1662347)
76 case study/ (33245)
77 case report.tw. (287952)
78 abstract report/ or letter/ (933800)
79 or/76‐78 (1248567)
80 75 not 79 (1622351)
81 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5341103)
82 80 not 81 (1508716)
83 55 and 82 (661)
Appendix 5. PsycINFO search strategy
(searched from 1806 to 19 October 2016) (Ovid platform)
1 exp Gynecological Disorders/ (1613)
2 tubal infertility.tw. (2)
3 tubal factor.tw. (4)
4 (disten$ adj3 tube).tw. (1)
5 tubal occlusion.tw. (5)
6 fallopian.tw. (46)
7 ((Tubal adj3 obstruction$) or (Tube adj3 obstruction$)).tw. (6)
8 2 or 3 or 4 or 5 or 6 or 7 (63)
9 1 and 8 (4)
10 8 or 9 (63)
11 exp Gynecology/ or exp Surgery/ (50237)
12 microsurg$.tw. (214)
13 Laparoscop$.tw. (393)
14 Laparotomy.tw. (121)
15 adhesiolysis.tw. (14)
16 reconstruction.tw. (8168)
17 (salpingostomy or salpingectomy).tw. (15)
18 aspiration.tw. (4129)
19 electrocoagulation.tw. (67)
20 emboli?ation.tw. (238)
21 (surgery or surgical).tw. (35051)
22 or/11‐21 (82130)
23 10 and 22 (20)
24 random*.ti,ab,hw,id. (159256)
25 trial*.ti,ab,hw,id. (148083)
26 controlled stud*.ti,ab,hw,id. (10491)
27 placebo*.ti,ab,hw,id. (35395)
28 ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id. (25155)
29 (cross over or crossover or factorial* or latin square).ti,ab,hw,id. (25065)
30 (assign* or allocat* or volunteer*).ti,ab,hw,id. (137432)
31 treatment effectiveness evaluation/ (20480)
32 mental health program evaluation/ (1970)
33 exp experimental design/ (52046)
34 "2000".md. (0)
35 or/24‐34 (434710)
36 23 and 35 (1)
Appendix 6. CINAHL search strategy
(searched from 1982 to 19 October 2016) (EBSCO platform)
| # | Query | Results |
| S57 | S44 AND S56 | 200 |
| S56 | S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 | 1,081,306 |
| S55 | TX allocat* random* | 5,281 |
| S54 | (MH "Quantitative Studies") | 14,919 |
| S53 | (MH "Placebos") | 9,827 |
| S52 | TX placebo* | 39,650 |
| S51 | TX random* allocat* | 5,281 |
| S50 | (MH "Random Assignment") | 41,699 |
| S49 | TX randomi* control* trial* | 110,746 |
| S48 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 857,082 |
| S47 | TX clinic* n1 trial* | 190,012 |
| S46 | PT Clinical trial | 79,719 |
| S45 | (MH "Clinical Trials+") | 203,397 |
| S44 | S22 AND S43 | 1,006 |
| S43 | S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 | 539,009 |
| S42 | TX salpingo neostom* | 0 |
| S41 | TX recanalisation or TX recanalization | 1,140 |
| S40 | TX salpingostomy or salpingectomy | 231 |
| S39 | TX recanalizing or TX recanalising | 17 |
| S38 | TX lysis N2 adhesion* | 67 |
| S37 | TX reconstruction | 19,742 |
| S36 | TX adhesiolysis | 129 |
| S35 | TX fimbrioplasty | 5 |
| S34 | TX tubo‐cornual anastomosis | 0 |
| S33 | TX excision | 9,388 |
| S32 | TX minilaparotom* | 65 |
| S31 | (MM "Microsurgery+") | 1,269 |
| S30 | TX Laparotomy | 4,259 |
| S29 | (MM "Laparotomy") | 839 |
| S28 | TX microsurg* | 2,853 |
| S27 | TX Laparoscop* | 20,801 |
| S26 | (MH "Surgery, Laparoscopic+") | 4,692 |
| S25 | TX surgical | 160,422 |
| S24 | TX surgery | 467,855 |
| S23 | (MM "Surgery, Gynecologic+") | 6,174 |
| S22 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 | 2,773 |
| S21 | TX disease* N3 tubal | 27 |
| S20 | TX disease* N3 tubes | 322 |
| S19 | TX Tube N3 lesion* | 8 |
| S18 | TX tubes N3 lesion* | 8 |
| S17 | TX Tubal N3 lesion* | 6 |
| S16 | TX hydrosalpin* | 61 |
| S15 | TX tubal N3 block* | 13 |
| S14 | TX tube* N3 block* | 115 |
| S13 | TX pelvic inflammatory | 1,041 |
| S12 | TX peritubal N3 adhesion* | 1 |
| S11 | TX fallopian | 1,155 |
| S10 | TX adhesion* N3 tub* | 30 |
| S9 | TX tubal occlusion | 71 |
| S8 | TX disten* N3 tube* | 10 |
| S7 | TX tubal fibrosis | 2 |
| S6 | TX tub* N3 damage | 271 |
| S5 | (MM "Pelvic Inflammatory Disease") | 416 |
| S4 | TX tubal factor | 113 |
| S3 | TX tubal subfertility | 10 |
| S2 | TX tubal infertility | 112 |
| S1 | (MM "Fallopian Tube Diseases+") | 268 |
Appendix 7. DARE search strategy
Cochrane Library (searched 24 November 2016)
All fields: "Fallopian tube obstruction" or "tubal factor" or "tubal flushing" or "tubal infertility" or "tubal inflation" or "tubal occlusion" or "tubal occlusion ‐ proximal" or "tubal patency" or "tubal reconstruction" or "tubal subfertility" or "tube drainage" or "tuboplasty" or "Fallopian Tube Fixation" or "fallopian tubes" or "tubal anastomosis" or "tubal disorders" or "tubo‐ovarian abcess" or "salpingectomy" or "Salpingitis‐Physiopathology" or "salpingo‐oopherectomy" or "Salpingolysis" or "*Salpingostomy" or "salpingotomy" or "Hydrosalpinx" or "hydrosalpingies" or "hydrosalpinges" or "falloscopy" or "laparoscopic salpingectomy" or "laparoscopic salpingoovolysis" or "laparoscopic salpingotomy" or "laparoscopic tubal fulguration" or "microsurgery" or "microscopic" or "microdiathermy" or "microlaparoscopy" or "hydrotubation"
(142 hits)
Appendix 8. http://www.clinicaltrials.gov search strategy
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| (Infertile OR infertility OR subfertile OR subfertility) AND (Tubal OR tube OR tubes OR oviduct OR oviducts) | Surgery OR surgical OR surgically |
(Infertile OR infertility OR subfertile OR subfertility) AND (Tubal OR tube OR tubes OR oviduct OR oviducts) AND (Surgery OR surgical OR surgically)
(searched 24 November 2016)
45 hits
Appendix 9. World Health Organization International Trials Registry Portal search strategy
(searched 24 November 2016)
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| (Subfertility OR subfertile OR infertility OR infertile) AND (“Fallopian tube” OR “Fallopian tubes” OR oviduct OR oviducts OR tubal) NOT male |
Subfertility AND tubal NOT male (0 trials)
Infertility AND tubal NOT male (11 trials)
Infertile AND tubal NOT male (11 duplicates)
Subfertile AND tubal NOT male (0 trials)
Subfertility AND oviduct* NOT male (0 trials)
Infertility AND oviduct* NOT male (1 trials)
Infertile AND oviduct* NOT male (1 duplicate)
Subfertile AND oviduct* NOT male (0 trials)
Subfertility AND Fallopian tube* NOT male (0 trials)
Infertility AND Fallopian tube* NOT male (2 trials)
Infertile AND Fallopian tube* NOT male (2 duplicate)
Subfertile AND Fallopian tube* NOT male (0)
28 hits (13 hits minus duplicates)
Appendix 10. Web of Science search strategy
Version 5.18, limited to Web of Science Core Collection database
Basic Search option
(searched 24 November 2016)
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| search tag:TOPIC “fallopian tube disease*” or “pelvic inflammatory disease” or “salpingitis” OR “tubal infertil*” OR “tubal subfertil*” OR tubal factor OR “tubal fibrosis” OR (disten* NEAR/3 tube) OR (disten* NEAR/3 tubal) OR (adhesion* NEAR/3 tube) OR (adhesion* NEAR/3 tubes) OR fallopian OR (peritubal NEAR/3 adhesion*) OR (tube NEAR/3 block*) OR (tubal NEAR/3 block*) OR (tubes NEAR/3 block*) OR hydrosalpin* OR ((Tubal NEAR/3 lesion*) or (Tube NEAR/3 lesion*)) OR ((disease* NEAR/3 tubal) or (disease* NEAR/3 tubes)) OR (oviduct* NEAR/3 damage*) OR (oviduct* NEAR/3 fibrosis) OR (disten* NEAR/3 oviduct*) OR (occlu* NEAR/3 oviduct*) OR (adhesion* NEAR/3 oviduct*) OR (((Tubal NEAR/3 obstruction*) or (Tube NEAR/3 obstruction*)) AND (“fallopian tube disease*” or “pelvic inflammatory disease” or salpingitis)) | search tag: TOPIC ((“gynecologic surgical procedures” or salpingectomy or salpingostomy) AND (surgery or surgical)) OR (“gynecologic surgical procedures” or salpingectomy or salpingostomy) or “hand‐assisted laparoscopy“ OR Laparoscop* OR Laparotomy OR electrosurgery or microsurg* OR minilaparotom* OR tubo‐cornual anastomosis OR fimbrioplasty OR adhesiolysis OR reconstruction OR (recanalizing or recanalising) OR (recanalisation or recanalization) OR (salpingostomy or salpingectomy) OR aspiration OR electrocoagulation OR Sclerotherapy OR Sclerotherap* OR emboli?ation | search tag: TOPIC ((randomi* NEAR/1 “controlled trial*”) OR “controlled clinical trial” OR “random allocation*” OR “double‐blind” OR “single‐blind” OR (clin* NEAR/25 trial*) OR ((singl* or doubl* or tripl* or trebl*) NEAR/25 (blind* or mask*)) OR placebo* OR “Research design”) NOT (animal* not human*) |
The RCT filter was adapted from the Medline RCT filter provided by Cochrane (Higgins 2005)
(193 hits)
Appendix 11. OpenGrey search strategy
(searched 24 November 2016)
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| “fallopian tube disease*” or “pelvic inflammatory disease” or “salpingitis” OR “tubal infertil*” OR “tubal subfertil*” OR tubal factor OR “tubal fibrosis” OR (disten* NEAR/3 tube) OR (disten* NEAR/3 tubal) OR (adhesion* NEAR/3 tube) OR (adhesion* NEAR/3 tubes) OR fallopian OR (peritubal NEAR/3 adhesion*) OR (tube NEAR/3 block*) OR (tubal NEAR/3 block*) OR (tubes NEAR/3 block*) OR hydrosalpin* OR ((Tubal NEAR/3 lesion*) or (Tube NEAR/3 lesion*)) OR ((disease* NEAR/3 tubal) or (disease* NEAR/3 tubes)) OR (oviduct* NEAR/3 damage*) OR (oviduct* NEAR/3 fibrosis) OR (disten* NEAR/3 oviduct*) OR (occlu* NEAR/3 oviduct*) OR (adhesion* NEAR/3 oviduct*) OR (((Tubal NEAR/3 obstruction*) or (Tube NEAR/3 obstruction*)) AND (“fallopian tube disease*” or “pelvic inflammatory disease” or salpingitis)) | ((“gynecologic surgical procedures” or salpingectomy or salpingostomy) AND (surgery or surgical)) OR (“gynecologic surgical procedures” or salpingectomy or salpingostomy) or “hand‐assisted laparoscopy“ OR Laparoscop* OR Laparotomy OR electrosurgery or microsurg* OR minilaparotom* OR tubo‐cornual anastomosis OR fimbrioplasty OR adhesiolysis OR reconstruction OR (recanalizing or recanalising) OR (recanalisation or recanalization) OR (salpingostomy or salpingectomy) OR aspiration OR electrocoagulation OR Sclerotherapy OR Sclerotherap* OR embolization OR embolization | ((randomi* NEAR/1 “controlled trial*”) OR “controlled clinical trial” OR “random allocation*” OR “double‐blind” OR “single‐blind” OR (clin* NEAR/25 trial*) OR ((singl* or doubl* or tripl* or trebl*) NEAR/25 (blind* or mask*)) OR placebo* OR “Research design”) NOT (animal* not human*) |
The RCT filter was adapted from the Medline RCT filter provided by Cochrane (Higgins 2005)
(“fallopian tube disease*” or “pelvic inflammatory disease” or “salpingitis” OR “tubal infertil*” OR “tubal subfertil*” OR tubal factor OR “tubal fibrosis” OR (disten* NEAR/3 tube) OR (disten* NEAR/3 tubal) OR “tubal occlusion” OR (occlusion NEAR/3 tubes) OR (occlusion NEAR/3 tube) OR ((tube NEAR/3 damage) or (tubal NEAR/3 damage)) OR (tube NEAR/3 damage) OR (adhesion* NEAR/3 tubal) OR (adhesion* NEAR/3 tube) OR (adhesion* NEAR/3 tubes) OR fallopian OR (peritubal NEAR/3 adhesion*) OR (tube NEAR/3 block*) OR (tubal NEAR/3 block*) OR (tubes NEAR/3 block*) OR hydrosalpin* OR ((Tubal NEAR/3 lesion*) or (Tube NEAR/3 lesion*)) OR ((disease* NEAR/3 tubal) or (disease* NEAR/3 tubes)) OR (oviduct* NEAR/3 damage*) OR (oviduct* NEAR/3 fibrosis) OR (disten* NEAR/3 oviduct*) OR (occlu* NEAR/3 oviduct*) OR (adhesion* NEAR/3 oviduct*) OR (((Tubal NEAR/3 obstruction*) or (Tube NEAR/3 obstruction*)) AND (“fallopian tube disease*” or “pelvic inflammatory disease” or salpingitis))) AND (((“gynecologic surgical procedures” or salpingectomy or salpingostomy) AND (surgery or surgical)) OR (“gynecologic surgical procedures” or salpingectomy or salpingostomy) or “hand‐assisted laparoscopy“ OR Laparoscop* OR Laparotomy OR electrosurgery or microsurg* OR minilaparotom* OR tubo‐cornual anastomosis OR fimbrioplasty OR adhesiolysis OR reconstruction OR (recanalizing or recanalising) OR (recanalisation or recanalization) OR (salpingostomy or salpingectomy) OR aspiration OR electrocoagulation OR Sclerotherapy OR Sclerotherap* OR embolization OR embolization ) AND (((randomi* NEAR/1 “controlled trial*”) OR “controlled clinical trial” OR “random allocation*” OR “double‐blind” OR “single‐blind” OR (clin* NEAR/25 trial*) OR ((singl* or doubl* or tripl* or trebl*) NEAR/25 (blind* or mask*)) OR placebo* OR “Research design”) NOT (animal* not human*))
0 hits
Appendix 12. LILACS search strategy
(searched 24 November 2016)
Limited to the LILACs database using the "Controlled Clinical Trial" tag as provided by the search portal
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| (TW:Fallopian Tube Disease*) or (TW:tubal infertility) OR (TW:tubal subfertility) OR (TW:tubal factor*) or (TW:Pelvic Inflammatory Disease) OR (TW:tubal factor infertil*) OR (TW:tubal factor subfertil*) OR (TW:tubal damage) OR (TW:tubal fibrosis) OR (TW:tube damage*) OR (TW:tube fibrosis) OR (TW:oviduct* damage*) OR (TW:oviduct* fibrosis) OR (TW:tubal distension*) OR (TW:tube distension*) OR (TW:distended tube) OR (TW:distended tubes) OR (TW:distended oviduct*) OR (TW:oviduct distension*) OR (TW:tubal occlusion) OR (TW:occluded tube) OR (TW:occluded tubes) OR (TW:tube occlu*) OR (TW:occluded oviduct*) OR (TW:oviduct occlu*) OR (TW:tubal adhesion*) OR (TW:tube adhesion*) OR (TW:oviduct adhesion*) | (TW:Laparoscop*) or (TW:Microsurg*) or (TW:tubal surgery) OR (TW:surgery oviduct*) OR (TW:surgical* oviduct*) OR (TW:infertility surgery) OR (TW:surgery infertil*) OR (TW:surgery subfertil*) OR (TW:surgical* infertil*) OR (TW:surgical* subfertil*) |
((TW:Fallopian Tube Disease*) or (TW:tubal infertility) OR (TW:tubal subfertility) OR (TW:tubal factor*) or (TW:Pelvic Inflammatory Disease) OR (TW:tubal factor infertil*) OR (TW:tubal factor subfertil*) OR (TW:tubal damage) OR (TW:tubal fibrosis) OR (TW:tube damage*) OR (TW:tube fibrosis) OR (TW:oviduct* damage*) OR (TW:oviduct* fibrosis) OR (TW:tubal distension*) OR (TW:tube distension*) OR (TW:distended tube) OR (TW:distended tubes) OR (TW:distended oviduct*) OR (TW:oviduct distension*) OR (TW:tubal occlusion) OR (TW:occluded tube) OR (TW:occluded tubes) OR (TW:tube occlu*) OR (TW:occluded oviduct*) OR (TW:oviduct occlu*) OR (TW:tubal adhesion*) OR (TW:tube adhesion*) OR (TW:oviduct adhesion*)) AND ((TW:Laparoscop*) or (TW:Microsurg*) or (TW:tubal surgery) OR (TW:surgery oviduct*) OR (TW:surgical* oviduct*) OR (TW:infertility surgery) OR (TW:surgery infertil*) OR (TW:surgery subfertil*) OR (TW:surgical* infertil*) OR (TW:surgical* subfertil*))
5 hits
Appendix 13. PubMed search strategy
(From 2012 to 24 November 2016) (limit to last 5 years)
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| Fallopian Tube Disease*[tw] or tubal infertility[all] OR tubal subfertility[all] OR tubal factor*[all] or Pelvic Inflammatory Disease[tw] OR tubal factor infertil*[all] OR tubal factor subfertil*[all] OR tubal damage[all] OR tubal fibrosis[all] OR tube damage*[all] OR tube fibrosis[all] OR oviduct* damage*[all] OR oviduct* fibrosis[all] OR tubal distension*[all] OR tube distension*[all] OR distended tube[all] OR distended tubes[all] OR distended oviduct*[all] OR oviduct distension* [all] OR tubal occlusion[all] OR occluded tube[all] OR occluded tubes[all] OR tube occlu* [all] OR occluded oviduct*[all] OR oviduct occlu* [all] OR tubal adhesion*[all] OR tube adhesion*[all] OR oviduct adhesion*[all] OR hydrosalpin* [all] OR fallopian [all] OR oviducts)Fallopian Tube Disease*[tw] or tubal infertility[all] OR tubal subfertility[all] OR tubal factor*[all] or Pelvic Inflammatory Disease[tw] OR tubal factor infertil*[all] OR tubal factor subfertil*[all] OR tubal damage[all] OR tubal fibrosis[all] OR tube damage*[all] OR tube fibrosis[all] OR oviduct* damage*[all] OR oviduct* fibrosis[all] OR tubal distension*[all] OR tube distension*[all] OR distended tube[all] OR distended tubes[all] OR distended oviduct*[all] OR oviduct distension* [all] OR tubal occlusion[all] OR occluded tube[all] OR occluded tubes[all] OR tube occlu* [all] OR occluded oviduct*[all] OR oviduct occlu* [all] OR tubal adhesion*[all] OR tube adhesion*[all] OR oviduct adhesion*[all] OR hydrosalpin* [all] OR fallopian [all] | Laparoscop*[tw] or Microsurg*[tw] or laparotomy*[tw] or aspiration [tw] or tubal surgery[all] OR surgery oviduct*[all] OR surgical* oviduct*[all] OR infertility surgery[all] OR surgery infertil*[all] OR surgery subfertil*[all] OR surgical* infertil*[all] OR surgical* subfertil*[all] OR adhesiolysis [all] OR salpingostomy [all] OR salpingectomy [all] OR embolisation[all] OR embolization[all] OR reconstruction[all] OR surgical OR surgically | (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tw] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT humans[mh]) |
(Fallopian Tube Disease*[tw] or tubal infertility[all] OR tubal subfertility[all] OR tubal factor*[all] or Pelvic Inflammatory Disease[tw] OR tubal factor infertil*[all] OR tubal factor subfertil*[all] OR tubal damage[all] OR tubal fibrosis[all] OR tube damage*[all] OR tube fibrosis[all] OR oviduct* damage*[all] OR oviduct* fibrosis[all] OR tubal distension*[all] OR tube distension*[all] OR distended tube[all] OR distended tubes[all] OR distended oviduct*[all] OR oviduct distension* [all] OR tubal occlusion[all] OR occluded tube[all] OR occluded tubes[all] OR tube occlu* [all] OR occluded oviduct*[all] OR oviduct occlu* [all] OR tubal adhesion*[all] OR tube adhesion*[all] OR oviduct adhesion*[all] OR hydrosalpin* [all] OR fallopian [all]) AND (Laparoscop*[tw] or Microsurg*[tw] or laparotomy*[tw] or aspiration [tw] or tubal surgery[all] OR surgery oviduct*[all] OR surgical* oviduct*[all] OR infertility surgery[all] OR surgery infertil*[all] OR surgery subfertil*[all] OR surgical* infertil*[all] OR surgical* subfertil*[all] OR adhesiolysis [all] OR salpingostomy [all] OR salpingectomy [all] OR embolisation[all] OR embolization[all] OR reconstruction[all]) AND ((randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tw] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT humans[mh]))
This search utilised the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision) (Higgins 2011)
(76 hits)
Appendix 14. Google Scholar search strategy
The Google Scholar search was run via the Publish or Perish program. (Harzing 2007)
(searched 24 November 2016)
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| (tubal OR fallopian OR oviduct) AND (infertility OR infertile OR subfertile OR subfertility) NOT male NOT men NOT animal | surgery | Random | pregnancy |
Year of publication between: 2016 and 2016
1. Search field (all of the words): tubal, infertility, surgery, random, pregnancy AND Search Field (none of the words): male, men, animal (107 hits)
2. Search field (all of the words): tubal, infertile, surgery, random, pregnancy AND Search Field (none of the words): male, men, animal (87 hits)
3. Search field (all of the words): tubal, subfertile, surgery, random, pregnancy AND Search Field (none of the words): male, men, animal (11 hits)
4. Search field (all of the words): tubal, subfertility, surgery, random, pregnancy AND Search Field (none of the words):male, men, animal (106 hits)
5. Search field (all of the words): fallopian, infertility, surgery, random, pregnancy AND, Search field (none of the words): male, men, animal (77 hits)
6. Search field (all of the words): fallopian, infertile, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (61 hits)
7. Search field (all of the words): fallopian, subfertile, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (9 hits)
8. Search field (all of the words): fallopian, subfertility, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (79 hits)
9. Search field (all of the words): oviduct, infertility, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (5 hits)
10. Search field (all of the words): oviduct, infertile, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (3 hits)
11. Search field (all of the words): oviduct, subfertile, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (0 hits)
12. Search field (all of the words):oviduct, subfertility, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (5 hits)
Total: 550 hits (146 hits excluding duplicates)
Appendix 15. ProQuest Dissertations & Theses Global search strategy
Searched 24th November 2016
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| “fallopian tube disease*” or “pelvic inflammatory disease” or salpingitis OR “tubal infertil*” OR” tubal subfertil*” OR “tubal factor” OR “tubal fibrosis” OR (disten* NEAR/3 tube) OR (disten* NEAR/3 tubal) OR “tubal occlusion” OR (occlusion NEAR/3 tubes) OR (occlusion NEAR/3 tube) OR ((tube NEAR/3 damage) or (tubal NEAR/3 damage)) OR (tube NEAR/3 damage) OR (adhesion* NEAR/3 tubal) OR (adhesion* NEAR/3 tube) OR (adhesion* NEAR/3 tubes) OR fallopian OR (peritubal NEAR/3 adhesion*) OR (tube NEAR/3 block*) OR (tubal NEAR/3 block*) OR (tubes NEAR/3 block*) OR hydrosalpin* OR ((Tubal NEAR/3 lesion*) or (Tube NEAR/3 lesion*)) OR ((disease* NEAR/3 tubal) or (disease* NEAR/3 tubes)) OR (oviduct* NEAR/3 damage*) OR (oviduct* NEAR/3 fibrosis) OR (disten* NEAR/3 oviduct*) OR (occlu* NEAR/3 oviduct*) OR (adhesion* NEAR/3 oviduct*) OR (((Tubal NEAR/3 obstruction*) or (Tube NEAR/3 obstruction*)) AND (exp fallopian tube diseases or pelvic inflammatory disease or salpingitis)) NOT male NOT male | ((“gynecologic surgical procedure*” or salpingectomy or salpingostomy) AND (surgery or surgical)) OR (“gynecologic surgical procedure*” or salpingectomy or salpingostomy) OR “hand‐assisted laparoscopy” OR Laparoscop* OR Laparotomy OR electrosurgery or microsurg* OR minilaparotom* OR tubo‐cornual anastomosis OR fimbrioplasty OR adhesiolysis OR reconstruction OR (recanalizing or recanalising) OR (recanalisation or recanalization) OR (salpingostomy or salpingectomy) OR aspiration OR electrocoagulation OR Sclerotherap* OR emboli?ation | ((randomi* NEAR/1 “controlled trial*”) OR “controlled clinical trial” OR “random allocation*” OR “double‐blind” OR “single‐blind” OR (clin* NEAR25 trial*) OR ((singl* or doubl* or tripl* or trebl*) NEAR/25 (blind* or mask*)) OR placebo* OR “Research design”) NOT (animal* not human*) | Pregnan* OR birth* |
(“fallopian tube disease*” or “pelvic inflammatory disease” or salpingitis OR “tubal infertil*” OR” tubal subfertil*” OR “tubal factor” OR “tubal fibrosis” OR (disten* NEAR/3 tube) OR (disten* NEAR/3 tubal) OR “tubal occlusion” OR (occlusion NEAR/3 tubes) OR (occlusion NEAR/3 tube) OR ((tube NEAR/3 damage) or (tubal NEAR/3 damage)) OR (tube NEAR/3 damage) OR (adhesion* NEAR/3 tubal) OR (adhesion* NEAR/3 tube) OR (adhesion* NEAR/3 tubes) OR fallopian OR (peritubal NEAR/3 adhesion*) OR (tube NEAR/3 block*) OR (tubal NEAR/3 block*) OR (tubes NEAR/3 block*) OR hydrosalpin* OR ((Tubal NEAR/3 lesion*) or (Tube NEAR/3 lesion*)) OR ((disease* NEAR/3 tubal) or (disease* NEAR/3 tubes)) OR (oviduct* NEAR/3 damage*) OR (oviduct* NEAR/3 fibrosis) OR (disten* NEAR/3 oviduct*) OR (occlu* NEAR/3 oviduct*) OR (adhesion* NEAR/3 oviduct*) OR (((Tubal NEAR/3 obstruction*) or (Tube NEAR/3 obstruction*)) AND (exp fallopian tube diseases or pelvic inflammatory disease or salpingitis)) NOT male) AND (((“gynecologic surgical procedure*” or salpingectomy or salpingostomy) AND (surgery or surgical)) OR (“gynecologic surgical procedure*” or salpingectomy or salpingostomy) OR “hand‐assisted laparoscopy” OR Laparoscop* OR Laparotomy OR electrosurgery or microsurg* OR minilaparotom* OR tubo‐cornual anastomosis OR fimbrioplasty OR adhesiolysis OR reconstruction OR (recanalizing or recanalising) OR (recanalisation or recanalization) OR (salpingostomy or salpingectomy) OR aspiration OR electrocoagulation OR Sclerotherap* OR emboli?ation ) AND (Pregnan* OR birth*) AND (((controli* NEAR/1 “controlled trial*”) OR “controlled clinical trial” OR “control allocation*” OR “double‐blind” OR “single‐blind” OR (clin* NEAR25 trial*) OR ((singl* or doubl* or tripl* or trebl*) NEAR/25 (blind* or mask*)) OR placebo* OR “Research design”) NOT (animal* not human*))
The RCT filter was adapted from the Medline RCT filter provided by Cochrane (Higgins 2005)
132 hits
Appendix 16. ESHRE and ASRM search strategy
Handsearching of the ESHRE 2007, ESHRE 2015 and ASRM 2008 conference abstracts as these are not covered by the search of the Gynaecology and Fertility Group specialised register.
-
ESHRE 2007 (2)
-
Gordts S, Campo R, Puttemans P, Valkenburg M, Brosens I, Gordts S. Microsurgical reversal of tubal sterilisation: to be preferred? Hum Reprod. 2007;22(Suppl 1):i227.
-
Hotineanu AL, Moshin VN, Hotineanu AV, Croitor ME. The effect of the proximal tubal “clamping” prior to the IVF in patients with distal tubal occlusion. Hum Reprod. 2007;22(Suppl 1):i126.
-
-
ASRM 2008 (5)
-
Fukuda A, Hamada A, Sawabe M, Sonoda M, Nakaoka Y, Morimoto Y. Pregnancy rate of bilateral tubal occlusion patients by IVF improves after recovery of tubal patency by falloposcopic tuboplasty. Fertility and sterility. 2008;90(Supplement):S155.
-
Poncelet C, Ducarme G, Yazbeck C, Uzan M, Madelenat P, Carbonnel M. Efficacy and safety of transient ovariopexy in severe endometriotic patients. A ten year experience. . Fertility and sterility. 2008;90(Supplement):S167‐8.
-
Sawabe M, Fukuda A, Hamada A, Sonoda M, Nakaoka Y, Morimoto Y. Experience of 1000 falloposcopic tuboplasty (FT) cases: FT is a novel, patient friendly and effective regimen for tubal factor infertility before ART. Fertility and sterility. 2008;90(Supplement):S40.
-
Jindal UN, Verma YB, Sodhi S, Verma S. Comparative evaluation of laparoscopy and endometrial polymerase chain reaction for the diagnosis of female genital tuberculosis in infertile women in India. Fertility and sterility. 2008;90(Supplement):S152.
-
Hirano Y, Shibahara H, Shimada K, Suzuki T, Takamizawa S, Suzuki M. Clinical role of transvaginal hydrolaparoscopy for the diagnosis of early stage endometriosis. Fertility and sterility. 2008;90(Supplement):S441.
-
-
ESHRE 2015 (3)
-
Chu J, Harb HM, Gallos ID, Dhillon RK, Al‐Rshoud FM, Robinson L, et al. Salpingostomy in the treatment of hydrosalpinx: a systematic review and meta‐analysis. Hum Reprod. 2015;30(Supp 1):i448.
-
Wang XR, Bao HC, Hao CF. Core‐pulling Salpingectomy: A Novel Surgical for Hydrosalpinx before IVF‐ET. Hum Reprod. 2015;30(Supp 1):i33‐4.
-
Lind T, Olofsson JI, Holte J, Hadziosmanovic N, Berglund L, Gudmundsson J, et al. Reduced clinical pregnancy rates by ART in women with a history of unilateral oophorectomy. Results of a large multi‐center cohort study. Hum Reprod. 2015;30(Supp 1):i33.
-
Total = 10 abstracts
Appendix 17. Reference lists of included trials and related reviews
1. Boer‐Meisel, ME, te Velde, ER, Habbema, JD & Kardaun, JW 1986, 'Predicting the pregnancy outcome in patients treated for hydrosalpinx: a prospective study', Fertil Steril, vol. 45, no. 1, Jan, pp. 23‐29.
2. Vasquez, G, Boeckx, W & Brosens, I 1995, 'Prospective study of tubal mucosal lesions and fertility in hydrosalpinges', Hum Reprod, vol. 10, no. 5, May, pp. 1075‐1078.
3. Marcoux, S, Maheux, R & Berube, S 1997, 'Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis', N Engl J Med, vol. 337, no. 4, Jul 24, pp. 217‐222.
4. Hughes, EG, Fedorkow, DM & Collins, JA 1993, 'A quantitative overview of controlled trials in endometriosis‐associated infertility', Fertil Steril, vol. 59, no. 5, May, pp. 963‐970.
5. Parazzini, F 1999, 'Ablation of lesions or no treatment in minimal‐mild endometriosis in infertile women: a randomized trial. Gruppo Italiano per lo Studio dell'Endometriosi', Hum Reprod, vol. 14, no. 5, May, pp. 1332‐1334.
6. Bontis JN, Dinas KD. Management of hydrosalpinx: reconstructive surgery or IVF? Ann NY Acad Sci 2000;900:260 –71.
7. Murray DL, Sagoskin AW, Widra EA, Levy MJ. The adverse effect of hydrosalpinges on in vitro fertilization pregnancy rates and the benefit of surgical correction. Fertil Steril 1998;69:41–5.
8. Sagoskin AW, Lessey BA, Mottla GL, Richter KS, Chetkowski RJ, Chang AS, et al. Salpingectomy or proximal tubal occlusion of unilateral hydrosalpinx increases the potential for spontaneous pregnancy. Hum Reprod 2003;18:2634 –7.
9. Nackley AC, Muasher SJ. The significance of hydrosalpinx in in vitro fertilization. Fertil Steril 1998;69:373–84.
10. Strandell A, Lindhard A. Why does hydrosalpinx reduce fertility? The importance of hydrosalpinx fluid. Hum Reprod 2002;17:1141–5.
11. Eytan O, Azem F, Gull I, Wolman I, Elad D, Jaffa AJ. The mechanism of hydrosalpinx in embryo implantation. Hum Reprod 2001;16:2662–7.
12. Bildirici I, Bukulmez O, Ensari A, Yarali H, Gurgan T. A prospective evaluation of the effect of salpingectomy on endometrial receptivity in cases of women with communicating hydrosalpinges. Hum Reprod 2001;16:2422– 6.
13. Zeyneloglu HB. Hydrosalpinx and assisted reproduction: options andrationale for treatment. Curr Opin Obstet Gynecol 2001;13:281–6.
14. Dechaud H. Hydrosalpinx and ART: hydrosalpinges suitable for salpingectomy before IVF. Hum Reprod 2000;15:2464–5
15. Choe J, Check JH. Salpingectomy for unilateral hydrosalpinx may improve in vivo fecundity. Gynecol Obstet Invest 1999;48:285–7.
16. Barmat LI, Rauch E, Spandorfer S, Kowalik A, Sills ES, Schattman G, et al. The effect of hydrosalpinges on IVF‐ET outcome. J Assist Reprod Genet 1999;16:350–4.
17. Camus E, Poncelet C, Goffinet F, Wainer B, Merlet F, Nisand I, et al. Pregnancy rates after in‐vitro fertilization in cases of tubal infertility with and without hydrosalpinx: a meta‐analysis of published comparative studies. Hum Reprod 1999;14:1243–9.
17 articles identified
Appendix 18. Data extraction
The following information was extracted from the studies selected for the review:
Trial characteristics
1. Method of randomisation
a. Third‐party randomisation: e.g. computer, telephone randomisation.
b. True randomisation by trialist: e.g. sequentially numbered, sealed, opaque envelopes, register, on‐site computer system.
c. Method not stated.
2. Study design
a. Cross‐over or parallel design.
b. Duration of follow up.
c. Type of follow up.
3. Size of the studies
a. Number of women recruited.
b. Number of women randomised.
c. Number of women excluded.
d. Number of women analysed.
e. Number of women lost to follow up.
f. Details of drop‐outs given.
g. Duration of follow up.
4. Study setting
a. Single or multi‐ centred.
b. Location.
c. Timing.
5. Analysis
a. Sample size with power calculation.
b. Whether or not analysed by intention‐to‐treat:
b1. done;
b2. not done, but possible;
b3. not possible;
b4. uncertain.
6. The extent to which the Consolidated Standards of Reporting Trials criteria (CONSORT) are met.
Characteristics of the study participants
a. Subfertile couples with at least one year’s duration of infertility.
b. Females under forty years of age.
c. Minor/grade I, moderate/grade II, or severe/grade III tubal damage confirmed prior to tubal surgery by means of HSG or laparoscopy.
d. Women who have had tubal surgery for minor/grade I, moderate/grade II, or severe/grade III tubal damage carried out following investigation.
1. Baseline characteristics
a. Age of the female partner.
b. Primary or secondary infertility.
c. Duration of subfertility.
d. Previous fertility treatment.
2. Interventions used
a. Tubal surgery.
b. Expectant management.
c. IVF.
Outcomes
1. Primary
Cumulative livebirth rate per couple.
2. Secondary
a. Cumulative pregnancy rate per patient/couple.
b. Pregnancy rate per patient/ couple.
c. Livebirth rate per treatment cycle commenced.
d. Ectopic pregnancy rate per patient.
e. Multiple pregnancy rate per patient.
f. Incidence of OHSS per patient.
All assessments of trial quality and data extraction will be independently performed by three review authors (SJ, VA, BM) using forms designed according to Cochrane guidelines. Any discrepancies will be resolved by a senior review author (BM). Additional information on trial methodology or actual original trial data will be sought from the corresponding authors of trials which appear to meet the eligibility criteria but are unclear in aspects of methodology, or where the data are in a form unsuitable for meta‐analysis.
Analysis
Should suitable trials become available in future, statistical analysis will be performed in accordance with the guidelines developed by the Gynaecology and Fertility group. Heterogeneity between the results of different studies will be examined by inspecting the scatter in the data points and the overlap in their confidence intervals and, more formally, using I2 tests. The possible contribution of differences in trial design to any heterogeneity identified in this manner, will be investigated. Where possible, the outcomes will be pooled statistically.
For cross‐over trials, only the data from the first phase (i.e. before cross‐over) will be used.
For dichotomous data (e.g. pregnancy rate), results for each study will be expressed as an odds ratio with 95% confidence interval and combined for meta‐analysis where appropriate, with RevMan software using the Peto‐modified Mantel‐Haenzel method. If possible, a sub‐group analysis will be performed to assess the clinical effectiveness of tubal surgery in women with grades I, II and III tubal damage separately. Sensitivity analysis will be undertaken to examine the stability of the results in relation to a number of factors including study quality and the source of the data.
Time line
The review is expected to be updated within two years of publication on the Cochrane Library or earlier should a seminal piece of research become available. New searches for RCTs will be performed every two years thereafter, and the review updated accordingly.
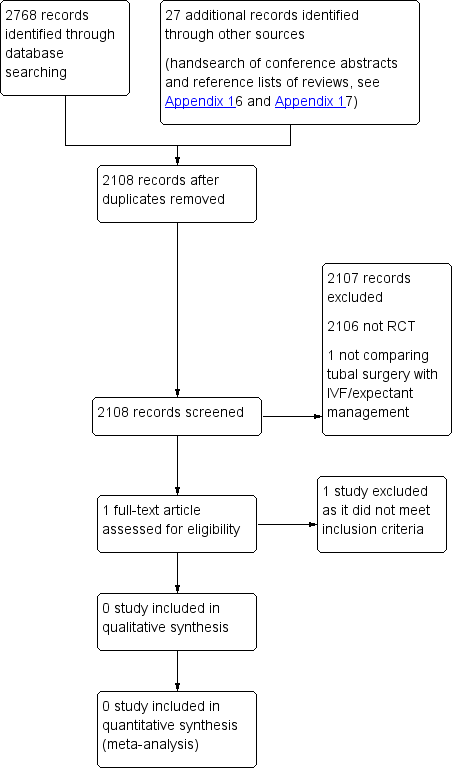
PRISMA study flow diagram.

