نقش جراحی در درمان ناباروری لولهای
چکیده
پیشینه
علیرغم افزایش استفاده از لقاح آزمایشگاهی (in vitro fertilisation; IVF)، جراحی به عنوان یک شیوه درمانی قابل قبول برای ناباروری لولهای (tubal infertility) باقی مانده است. تخمین نرخ زندهزایی (livebirth) پس از جراحی از 9% برای زنان مبتلا به اختلال لولهای شدید تا 69% برای افراد دچار اختلال خفیف متفاوت است، با این حال، اثربخشی جراحی به طور دقیق در مقایسه با سایر درمانها از جمله IVF و مدیریت انتظاری (expectant management) (عدم درمان) ارزیابی نشده است. نرخ زندهزایی در ارتباط با شدت آسیب لوله، به اندازه کافی مورد ارزیابی قرار نگرفته است. مهم است که به دلیل نگرانیها درباره پیامدهای جانبی، عوارض حین جراحی و هزینههای مرتبط با جراحی لوله و همچنین درمانهای جایگزین (عمدتا IVF)، اثربخشی جراحی را در برابر سایر گزینههای درمان در زنان مبتلا به ناباروری لولهای مشخص کنیم.
اهداف
هدف این مرور، تعیین اثربخشی و ایمنی جراحی در مقایسه با مدیریت انتظاری یا IVF از لحاظ بهبود احتمال زندهزایی در شرایط ناباروری لولهای بود (صرف نظر از درجه شدت).
روشهای جستوجو
ما این بانکهای اطلاعاتی را در اکتبر 2016 جستوجو کردیم: پایگاه ثبت کارآزماییهای گروه زنان و باروری در کاکرین (CGF)، پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL)؛ MEDLINE؛ Embase؛ Cumulative Index to Nursing and Allied Health Literature (CINAHL) و PsycINFO؛ همچنین پایگاههای ثبت کارآزماییهای بالینی، مآخذ منابع علمی منتشر نشده و فهرست منابع کارآزماییهای وارد شده و مرورهای سیستماتیک مرتبط.
معیارهای انتخاب
فقط کارآزماییهای تصادفیسازی و کنترل شده واجد شرایط را برای ورود در نظر گرفتیم که دارای نرخ زندهزایی به ازای هر شرکتکننده به عنوان پیامد مطلوب اولیه بودند.
گردآوری و تجزیهوتحلیل دادهها
ما برنامهریزی کردیم که دو نویسنده مرور بهطور مستقل از هم واجد شرایط بودن کارآزمایی و خطر سوگیری (bias) را ارزیابی کرده و دادههای مطالعه را استخراج کنند. پیامد اولیه این مرور، نرخ زندهزایی تجمعی (cumulative livebirth rate) بود. پیامدهای ثانویه شامل نرخ بارداری و پیامدهای جانبی مانند نرخ سقط جنین، نرخ بارداری خارج رحمی و نرخ عوارض مرتبط با پروسیجر بود. در نظر داشتیم که دادهها را برای محاسبه نسبتهای شانس (ORs) تجمعی و 95% فواصل اطمینان (CIs) ترکیب کنیم. ناهمگونی آماری را با استفاده از آماره I2 ارزیابی کردیم و برای بررسی کیفیت کلی شواهد مربوط به مقایسههای اصلی، از سیستم درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) بهره بردیم.
نتایج اصلی
کارآزمایی تصادفیسازی و کنترل شده مناسبی را شناسایی نکردیم.
نتیجهگیریهای نویسندگان
اثربخشی جراحی لولهای نسبت به مدیریت انتظاری و IVF از نظر نرخ زندهزایی برای زنان مبتلا به ناباروری لولهای همچنان نامشخص باقی مانده است. کارآزماییهای بزرگ با قدرت کافی برای تعیین اثربخشی جراحی در این زنان مورد نیاز هستند. کارآزماییهای آینده نباید فقط نرخ زندهزایی را به ازای هر بیمار گزارش کنند، بلکه باید عوارض جانبی و هزینههای درمان را در یک دوره طولانیتر مقایسه کنند. عواملی مانند درمان باروری، سن همسر زن، مدت ناباروری و سابقه بارداری قبلی که تاثیر عمدهای بر این پیامدها دارند، باید در نظر گرفته شوند. محققان باید نرخ زندهزایی را در ارتباط با شدت آسیب لوله و روشهای متفاوت به کار رفته برای ترمیم لوله شامل جراحی میکروسکوپی و شیوههای لاپاراسکوپیک، گزارش کنند.
PICO
خلاصه به زبان ساده
جراحی در برابر IVF یا مدیریت انتظاری برای زنان مبتلا به ناباروری لولهای
سوال مطالعه مروری
نویسنگان مرور کاکرین، اثربخشی جراحی لوله فالوپ را در مقایسه با لقاح آزمایشگاهی (in vitro fertilisation; IVF) یا مدیریت انتظاری در غلبه بر ناباروری ناشی از بیماری لولهای بررسی کردند.
پیشینه
جراحی لولهای برای غلبه بر ناباروری ناشی از بیماری لولهای، تا حدی به دلیل خطرات و هزینههای مرتبط با IVF که گزینه دیگری را برای فائق آمدن بر ناباروری لولهای پیشنهاد میکند، رایج شده است. مزایای حاصل از جراحی لولهای به طور بالقوه طی چرخههای متعدد و سالهای طولانی پایدار میماند، حتی منجر به میزان زندهزاییهای متعدد میشود. با این حال، جراحی لولهای گران است، چراکه نیاز به مهارت و تجربه اضافی متخصص زنان و زایمانی دارد که این پروسیجر را انجام میدهد و میتواند همراه با عوارض جانبی (از جمله بارداریهای خارج رحمی) و خطرات مربوط به جراحی باشد. اثربخشی جراحی لولهای در مقایسه با عدم درمان (مدیریت انتظاری) یا IVF در زنان مبتلا به ناباروری لولهای، نامشخص است.
ویژگیهای مطالعه
این مرور، هیچ کارآزمایی مناسبی را شناسایی نکرد. جستوجوی های ما در منابع علمی تا اکتبر 2016 بهروز هستند.
نتایج کلیدی
در حال حاضر، هیچ شواهد تصادفیسازی شدهای در دسترس نیست. برای دستیابی به اطلاعات در مورد پیامدهای جانبی و هزینهها پژوهش بیشتری لازم است.
Authors' conclusions
Background
Description of the condition
Tubal disease of the fallopian tubes is responsible for 25% to 35% of cases of female infertility (Serafini 1989). Tubal disease can involve the proximal, distal or entire tube and varies in severity. Pelvic inflammatory disease is the most common cause of tubal disease, representing more than 50% of cases, and may affect the fallopian tube at multiple sites (Honore 1999). The Hull & Rutherford classification (2002) is a simple classification system that separates infertile women into three categories according to severity of tubal damage, namely, mild/grade I, moderate/grade II and severe/grade III (Akande 2004). This system is defined in the section on inclusion criteria. Diagnosis is confirmed by hysterosalpingography (HSG) or laparoscopy.
Description of the intervention
Treatment options include surgical tubal repair, expectant management (i.e. waiting/no specific intervention) and in vitro fertilisation (IVF). The effectiveness of these treatments has not been tested rigorously in the context of randomised controlled trials (RCTs).
Surgery
Despite operative risks (general anaesthetic, intraoperative and postoperative) and a high postoperative incidence of ectopic pregnancy, surgery for tubal infertility is considered an effective treatment option. Procedures such as salpingostomy (formation of an opening into a uterine tube for the purpose of drainage) or fimbrioplasty (breaking of scar tissue around the distal end of the tube) are widely performed for distal tubal obstruction. Surgery is considered a viable treatment option for women with mild tubal disease, and for severe disease, laparoscopic salpingectomy before IVF has a role in improving livebirth rates among women with hydrosalpinges (ASRM 2015; Johnson 2010; NICE 2004).
Tubal ectopic pregnancy ‐ pregnancy in the fallopian tubes ‐ is a potential adverse effect of tubal surgery. A large retrospective regional study from Denmark of 236 women who underwent tubal surgery or adhesiolysis (a procedure performed to remove scar tissue around the tube) reported an ectopic pregnancy rate of 16% (Mosgaard 1996). Higher ectopic pregnancy rates have been associated with increasing severity of tubal damage (Akande 2004). Compared with the 2% incidence of ectopic pregnancy reported in the general population, rates of ectopic pregnancy after surgical correction of tubal abnormalities are reported to be 1% to 10% in mild tubal disease, up to 40% in severe pathology and 2.1% to 11% when IVF is performed in patients with tubal infertility (Schippert 2012).
Expectant management
Pregnancies do occur without treatment in women with a diagnosis of tubal blockage (Collins 1983; Evers 1998; NICE 2004; Wiedemann 1996). It has been suggested that this could be the result of beneficial effects of diagnostic tests required to establish infertility and the therapeutic value of counselling provided during outpatient visits (Collins 1983). In addition, chance inclusion of normal couples (i.e. those at the boundaries of normal reference ranges of fertility) with infertile couples during clinical studies may be contributory. It is likely that "post hoc ergo propter hoc" (i.e. the temporal association between event A and event B immediately implies causation of event B by event A) does not apply for some types of infertility, as the widely held assumption that infertile women serve as their own controls and hence any pregnancy after treatment can be attributed to said treatment may not hold true.
IVF
Tubal infertility remains a major indication for IVF, which completely bypasses tubal blockage and offers an 18% to 29% livebirth rate per cycle (AIHW 2012; SART 2014). As IVF involves manual fertilisation outside the normal reproductive system, it is expensive, invasive and not available to all infertile patients. IVF is associated with several potential complications, including multiple births and foetal anomalies (ASRM 2015; El‐Chaar 2009). Ovarian hyperstimulation syndrome (OHSS) is a potentially life‐threatening adverse effect of ovulation induction. The intravascular depletion associated with OHSS can lead to dehydration, hypovolaemia (low volume of fluid in veins), electrolyte disturbances and thrombosis due to haemoconcentration. In IVF cycles, the rate of severe OHSS requiring hospitalisation is less than 0.01% (AIHW 2012; HFEA 2015). This figure increases with the number of oocytes retrieved at each cycle, reaching 4.0% when more than 20 oocytes have been retrieved (AIHW 2012; HFEA 2015). Older women have been shown to have poor success rates, and increased recognition of factors such as parity (number of children to whom a patient has given birth), duration of infertility, coexisting infertility factors and local IVF success rates can influence outcomes (AIHW 2012; SART 2014).
How the intervention might work
Surgery
The largest case series to date reported an impressive intrauterine pregnancy rate of 72.8% (2369/3254) for terminal salpingo‐neostomy and salpingo‐ovariolysis performed in patients with tubo‐peritoneal infertility (Ponomarev 2009). However, the time period over which the pregnancy rate was measured was not mentioned in the study publication, which was provided in the form of a conference abstract. A recent meta‐analysis combining 22 observational studies of women (N = 2810) undergoing salpingostomy for hydrosalpinges revealed a cumulative pregnancy rate of 20.0% (95% confidence interval (CI) 17.5% to 22.8%) at one year and 25.5% (95% CI 22.2% to 29.4%) at two years (Chu 2015). Although surgical techniques, participant characteristics and duration of follow‐up were heterogeneous, study authors cited these as reasons for generalisability.
The second largest case series to date (N = 1669), which stratified participants according to severity of tubal disease, reported favourable pregnancy outcomes of 55% to 80% for those with mild tubal disease, including prior tubal ligation (n = 1517), and poor pregnancy outcomes of 10% for participants with severe disease (e.g. concurrent proximal and distal lesions, extended dense adhesions, sclerohypertrophic tube, intra‐ampullary adhesions) (n = 152) at a minimum of two years of follow‐up (Tran 2010). However, the significance of these positive results is limited by the risk of bias inherent to retrospective case series.
Expectant management
A retrospective analysis of 109 women with proximal tubal occlusion reported a spontaneous pregnancy rate of 10% per patient and 1.6% per month (Wiedemann 1996). This study showed that when assisted reproductive technology – in particular, gamete intrafallopian transfer (GIFT) – was used as a subsequent treatment, the pregnancy rate was increased to 50%. A retrospective cohort study of 562 couples with tubal factor infertility who were on the waiting list for IVF found that the 12‐month cumulative spontaneous pregnancy rate was only 2.4% (95% CI 1.2% to 3.9%). More than 75% of these pregnancies occurred during the first three months on the waiting list (Evers 1998). Another study followed 1145 couples with infertility and noted that 61% (26/43) of conceptions among patients with infertility of tubal origin were treatment independent, defined as pregnancies that occur after no treatment, three months after medical management or 12 months after surgical management, respectively. Of note, a significant percentage of non‐treated infertile couples also conceived ‐ 35% (191/548). However, subgroup analysis revealed that pregnancies unrelated to treatment were less likely to occur in women with bilateral tubal occlusion (0%; 0/5) than in women with other less severe tubal lesions (68%; 26/38), further emphasising the need for comparative studies stratifying participants according to severity of disease (Collins 1983).
IVF
Analysis of cumulative data showed that women with tubal factor, both with and without other coexisting infertility factors, had a pregnancy rate in excess of 70% after four cycles of IVF and embryo transfer (Benadiva 1995). A meta‐analysis of 14 retrospective studies compared pregnancy rates in women with tubal infertility with and without hydrosalpinx (accumulation of watery fluid in the tube as a consequence of distal obstruction) and revealed the pregnancy rate to be 31.2% for the 4588 women without hydrosalpinx who underwent IVF (Camus 1999). Most of the studies included in this meta‐analysis did not specify the number of IVF cycles completed.
Why it is important to do this review
Considerable uncertainty remains about whether surgical treatment is superior to expectant management and IVF in women with tubal factor infertility. Surgery is still commonly performed, especially in areas where reimbursement for IVF is not available. This systematic review evaluated the effectiveness and safety of surgery in comparison with other available treatments for women with tubal infertility.
Objectives
The aim of this review was to determine the effectiveness and safety of surgery compared with expectant management or IVF in improving the probability of livebirth in the context of tubal infertility (regardless of grade of severity).
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs) comparing the clinical effectiveness of tubal surgery versus expectant management or IVF. We included cross‐over trials if pre‐cross‐over data were available.
Types of participants
Inclusion criteria
Participants were required to meet all the criteria listed below.
-
Subfertile couples with infertility of at least one year’s duration.
-
Women younger than 40 years of age.
-
Women with minor/grade I, moderate/grade II or severe/grade III tubal damage confirmed before tubal surgery by hysterosalpingography (HSG) or laparoscopy.
-
Women who had undergone tubal surgery for minor/grade I, moderate/grade II or severe/grade III tubal damage after investigation.
According to the Hull & Rutherford 2002 classification of tubal damage (Akande 2004), minor/grade I tubal damage is defined as:
-
tubal fibrosis absent even if tube occluded (proximally);
-
tubal distension absent even if tube occluded (distally);
-
mucosal appearances favourable; and
-
flimsy adhesions (peritubal‐ovarian).
Moderate/grade II tubal damage is defined as:
-
unilateral severe tubal damage;
-
contralateral minor disease present or absent; and
-
'limited' dense adhesions of tubes and/or ovaries.
Severe/grade III tubal damage is defined as:
-
bilateral tubal damage;
-
extensive tubal fibrosis;
-
tubal distension greater than 1.5 cm;
-
abnormal mucosal appearance;
-
bipolar occlusion; and
-
'extensive' dense adhesions.
Exclusion criteria
-
Women 40 years of age or older.
-
Women with multiple or other causes of infertility such as ovulatory or sperm dysfunction.
-
Women who had undergone tubal sterilisation.
When trials included couples with infertility of various categories, we included only couples with tubal infertility. We excluded participants with other causes of infertility because their inclusion could have confounded outcomes. When we had doubt about the definitions of various grades of tubal infertility, we requested more information from study authors. If extraction of data is not possible for any reason, we will exclude that trial and will state the reason for exclusion. We will include in the review trials that cannot be included in the meta‐analysis owing to insufficient data.
Types of interventions
Included studies performed one or more comparisons of effectiveness of tubal surgery versus expectant management, or of tubal surgery versus IVF. We considered a variety of techniques for tubal surgery to be eligible, including microsurgery or macrosurgery, laparoscopy and minilaparotomy or laparotomy. No treatment for infertility was administered to couples undergoing expectant management. For women undergoing IVF, a standard IVF procedure was carried out according to standard protocols for controlled ovarian stimulation, oocyte retrieval under ultrasound guidance, insemination, embryo culture and transcervical replacement of embryos, most often between pro‐nucleate and eight‐cell stages. Embryo transfer up to the blastocyst stage and frozen replacement cycles were eligible for inclusion.
Types of outcome measures
Primary outcomes
-
Cumulative livebirth rate per couple, where cumulative refers to time‐specific or cycle‐specific rates over a given time or number of cycles, and livebirth is defined as the delivery of one or more living infants after 20 completed weeks of gestational age.
Secondary outcomes
-
Cumulative pregnancy rate per participant/couple (evidence of a gestational sac, confirmed on ultrasonography, defines clinical pregnancy).
-
Pregnancy rate per participant/couple (evidence of clinical pregnancy ‐ evidence of a gestational sac, confirmed on ultrasonography), including ectopic pregnancy, although multiple gestational sacs in one individual count as one clinical pregnancy).
-
Livebirth rate per cycle commenced.
-
Ectopic pregnancy rate per participant.
-
Multiple pregnancy rate per participant (demonstration of more than one sac with foetal pole on ultrasonographic scan defines multiple pregnancy).
-
Incidence of OHSS per participant.
Search methods for identification of studies
We searched for all published and unpublished RCTs comparing tubal surgery versus expectant management or IVF, without language restriction, and in consultation with the Gynaecology and Fertility Group (CGF) Information Specialist.
Electronic searches
We searched the following databases.
-
Gynaecology and Fertility Group (CGFG) Specialised Register of Controlled Trials Procite (searched 19 October 2016) (Appendix 1).
-
Central Register of Controlled Trials (CENTRAL) Ovid (searched 19 October 2016) (Appendix 2).
-
MEDLINE Ovid (1946 to 19 October 2016) (Appendix 3).
-
Embase Ovid (1974 to 19 October 2016) (Appendix 4).
-
PsycINFO Ovid (1806 to 19 October 2016) (Appendix 5).
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO (1982 to 19 October 2016) (Appendix 6).
-
Database of Abstracts of Reviews of Effects (DARE) in the Cochrane Library (for reference lists from relevant non‐Cochrane reviews) (searched 17 August 2015) (Appendix 7).
-
Trial registries for ongoing and registered trials.
-
http://www.clinicaltrials.gov (a service of the US National Institutes of Health) (searched 24 November 2016) (Appendix 8).
-
http://www.who.int/trialsearch/Default.aspx (World Health Organization International Trials Registry Platform search portal) (searched 24 November 2016) (Appendix 9).
-
-
Web of Science (searched 24 November 2016) (Appendix 10).
-
OpenGrey (unpublished literature from Europe) (searched 24 November 2016) (Appendix 11).
-
Latin American Caribbean Health Sciences Literature (LILACS, trials from the Portuguese and Spanish speaking world) (searched 24 November 2016) (Appendix 12).
-
PubMed and Google Scholar (for recent trials not yet indexed in MEDLINE) (searched 24 November 2016) (Appendix 13; Appendix 14).
-
ProQuest Dissertations & Theses Global (searched 17 August to 24 November 2016) (Appendix 15).
We combined the MEDLINE search with the Cochrane highly sensitive search strategy, which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.0.2, Chapter 6, 6.4.11) (Higgins 2011), to identify randomised trials. We combined the Embase, PsycINFO and CINAHL searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN).
We designed a new search strategy that differed from the strategy used in the previous version of this review, necessitating database searches covering inception up to October 2015. All searches were current to 19 October 2016.
Searching other resources
We searched reference lists of articles retrieved by the search, along with conference abstracts not covered in the CGFG register, in liaison with the Information Specialist (Appendix 16). We communicated with trial authors and experts in the field regarding additional trials.
Data collection and analysis
Selection of studies
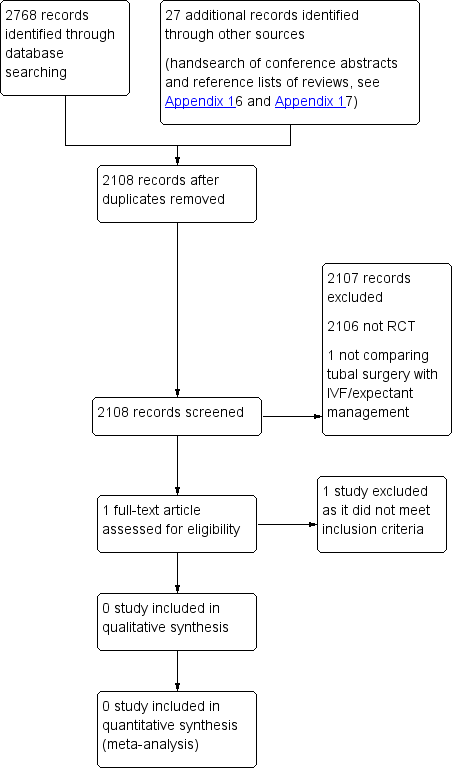
We planned that two review authors (SC and BM) would independently undertake selection of studies after an initial screen of titles and abstracts retrieved (by SC), employing the search strategy outlined above. We planned that study investigators would be contacted, as required, to clarify study eligibility. We resolved discrepancies by discussion. Review authors identified no RCTs via the search strategy. We documented the selection process on a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow chart (see Figure 1).
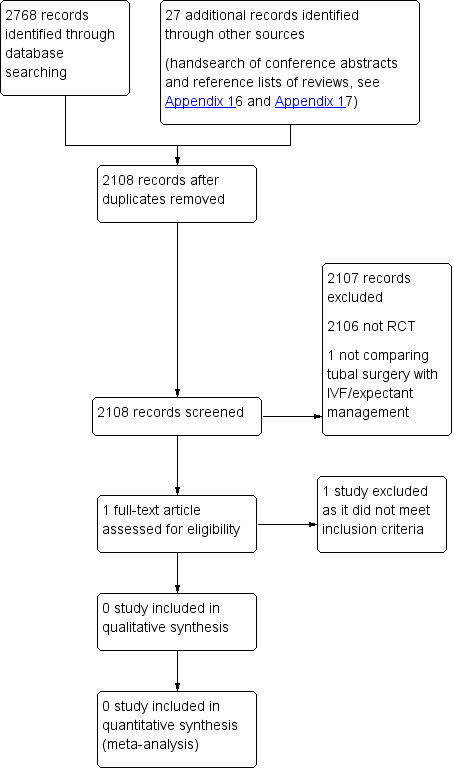
PRISMA study flow diagram.
Data extraction and management
We planned that two review authors (SC and BM) would independently extract data from eligible studies and would resolve disagreements by discussion or by consultation with the third review author. Data extracted would include study characteristics and outcome data (see data extraction form for details; Appendix 18). We would collate studies involving multiple publications in such a way that each study, with its unique study identifier and multiple references, rather than each report, would be considered a single unit of interest in the review.
We planned to extract the following data from studies selected for inclusion in the review.
-
Trial characteristics.
-
Characteristics of study participants.
-
Outcomes.
-
Analysis.
Assessment of risk of bias in included studies
We planned that two review authors (SC and BM) would independently assess included studies for risk of bias using the Cochrane risk of bias assessment tool (www.cochrane‐handbook.org) to assess selection (random sequence generation and allocation concealment), performance (blinding of participants and personnel), detection (blinding of outcome assessors), attrition (incomplete outcome data), reporting (selective reporting) and other bias. We planned to resolve disagreements by discussion with the third review author. We described all judgements fully and presented our conclusions in the risk of bias table.
We planned to search for within‐trial selective reporting when obvious outcomes were not reported or were not reported in insufficient detail to allow inclusion and to seek published protocols for comparison with the final published study.
Measures of treatment effect
For dichotomous data (e.g. livebirth rates), we planned to use numbers of events in the control and intervention groups of each study to calculate Peto odds ratios (ORs). We planned to present 95% confidence intervals for all outcomes. When data needed to calculate ORs were not available, we planned to utilise the most detailed numerical data available that might facilitate similar analyses of included studies (e.g. test statistics, P values). We planned to assess whether estimates calculated in the review for individual studies were compatible in each case with estimates reported in study publications.
Unit of analysis issues
We planned that the primary analysis would be randomised per woman, and we planned to include per pregnancy data for some outcomes (e.g. miscarriage). We would briefly summarise in an additional table data that did not allow valid analysis (e.g. "per cycle" data) and would not perform meta‐analysis. We would count multiple livebirths (e.g. twins, triplets) as one livebirth event and would include only first‐phase data obtained from cross‐over trials.
Dealing with missing data
We planned to analyse the data on an intention‐to‐treat basis as far as possible, and to attempt to obtain missing data from the original trialists. When these data could not be obtained, we planned to undertake imputation of individual values for the primary outcome only. We planned to assume that livebirths did not occur in participants without a reported outcome. For other outcomes, we planned to analyse only available data. We would perform sensitivity analysis of any imputation undertaken (see below).
Assessment of heterogeneity
We planned to consider whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We would have assessed statistical heterogeneity by using the I2 statistic (I2 greater than 50% would indicate substantial heterogeneity) (Higgins 2003; Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, review authors planned to minimise their potential impact by ensuring a comprehensive search for eligible studies and by staying alert for duplication of data. If we included 10 or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small study effects (the tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
If studies were sufficiently similar, we planned to combine the data using a fixed‐effect model for the following comparisons.
-
Tubal surgery versus expectant management.
-
Tubal surgery versus IVF.
Subgroup analysis and investigation of heterogeneity
We planned no subgroup analyses.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcome to determine whether conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses were to include consideration of whether review conclusions would have differed if:
-
eligibility were restricted to studies without high risk of bias;
-
a random‐effects model had been adopted;
-
alternative imputation strategies had been implemented; or
-
the summary effect measure was relative risk rather than odds ratio.
Overall quality of the body of evidence ‐ 'Summary of findings' table
We planned to prepare a 'Summary of findings' table using GRADEpro software (GRADEpro GDT 2014). This table would have evaluated the overall quality of the body of evidence for the main review outcomes (livebirth rate and pregnancy rate) according to GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We would have justified judgements about evidence quality (high, moderate or low) and would have documented and incorporated these into reporting of results for each outcome. Two review authors (SC and BM) would have made judgements independently as needed.
Results
Description of studies
We identified no eligible trials for inclusion.
Results of the search
We included no RCTs.
Excluded studies
We found one related single‐centre RCT that was performed at a university tertiary care centre in Iran from March 2002 to September 2004 (2.5‐year period). This study included 13 participants with unilateral hydrosalpinx and recurrent abortion, detected on ultrasonography and hysterosalpingography, and thus did not meet our inclusion criteria, which required that couples would be subfertile.
Ongoing studies
We found no currently ongoing studies undertaken to compare the effectiveness of tubal surgery versus expectant management or IVF.
Risk of bias in included studies
We identified no RCTs for inclusion in the review, so we could perform no assessment of methodological quality.
Effects of interventions
We found no RCTs that compared surgery versus expectant management or IVF in women with tubal infertility; therefore, we cannot report study data.
Discussion
Summary of main results
This review shows that evidence on this topic has not been provided by randomised controlled trials (RCTs).
Overall completeness and applicability of evidence
No evidence was available. Despite potential risks of surgery, such as possibly increased risk of ectopic pregnancy, and despite widespread availability of in vitro fertilisation (IVF), surgical treatment remains a popular option. This is reflected by recent epidemiological data on fertility services indicating that although the ratio of IVF services to tubal surgery favours IVF, actual prevalence of tubal surgeries performed has remained static. In the United States, 3.2% of women 25 to 44 years of age with fertility problems have ever used reproductive surgery, and 3.1% have used IVF (Chandra 2014).
Potential biases in the review process
As this systematic review applied a newly designed search strategy that encompassed an extensive number of databases from conception, it is not likely that we missed relevant studies. However, we could not adequately assess publication bias by using a funnel plot owing to the scarcity of RCTs on this topic. We may have automatically excluded good quality observational studies that may adequately answer the study question owing to the nature of the protocol of this systematic review. Consideration must be given to incorporating such studies in future reviews because well‐powered RCTs continue to be few.
Agreements and disagreements with other studies or reviews
As a result of the limited nature of available data, it has repeatedly been difficult to draw reliable conclusions on the effectiveness of surgery for tubal infertility. Until data from large RCTs with adequate power become available, clinical practice must be guided on the basis of available observational studies, many of which are confounded by bias due to the traditional method of using each couple as its own internal control, hence assuming that any fertility outcome could be totally attributed to the intervention performed. Moreover, very few observational studies have incorporated direct concurrent comparison of two or more cohorts undergoing different interventions, respectively.
A previous version of this review (Pandian 2008) evaluated this topic, and a related review examined use of pelvic surgery for subfertility (Ahmad 2006). Neither these reviews nor any of the numerous non‐Cochrane systematic reviews on this topic have produced solid answers over the past three decades. Reasons for the lack of well‐designed RCTs in this area are manifold. The validity of the classification systems used to assess severity of tubal damage is questionable. Extent of tubal disease and the presence of pelvic pathology are important factors in the prognosis for success after surgical repair. The pregnancy outcome has been found to be uniformly poor after surgical treatment in patients with severe tubal disease (less than 15% pregnancy rate) (Akande 2004; Wu 1988). Selection of appropriate patients is an important determinant of outcomes after surgery and is not possible in the absence of a reliable classification system. The group of patients thought to be eligible for participation in such an RCT may, therefore, comprise a misrepresentation of the typical patient population required. Recruitment for such trials is impaired by the provision of insufficient patient information; an accepted, reliable method that can provide precise prognostic information for women with tubal damage is needed.
The advent of IVF has diminished the role of tubal surgery, and tubal infertility remains one of the major indications for IVF. Although it is expensive and invasive, IVF is the preferred choice for older women with severe tubal damage. With the reported livebirth rate per IVF cycle in most centres as high as 30% (SART 2014), and in light of uncertainties surrounding the outcomes of tubal surgery, a preference for IVF may contribute to poor recruitment for surgical RCTs. Furthermore, women with tubal damage find the spontaneous pregnancy rate unacceptably low (12‐month cumulative pregnancy rate (PR) of 2.4%); consequently, expectant management is an unattractive option for them (Evers 1998).
Funding constraints in some clinical situations mean that many women and clinicians continue to favour surgery. An additional advantage of surgery over IVF is the theoretically permanent restoration of the ability to naturally conceive for every ovulation cycle over a sustained period. This is compared with the high chance afforded by IVF over the few cycles performed and associated complications of multiple births, foetal anomalies and ovarian hyperstimulation syndrome (OHSS) (AIHW 2012; ASRM 2015; El‐Chaar 2009; HFEA 2015). Tubal surgery may be the only treatment option for couples who object to IVF for religious, moral or emotional reasons. Finally, when effective, tubal surgery leads to sustainable improvement in fertility prospects, whereas IVF (apart from frozen embryos) provides only one chance.
Specific problems have been noted with RCTs that involve surgical procedures. It is difficult to standardise the surgical procedures being tested, as procedures evolve continuously and complications decrease as surgeons gain experience. The success rate of a specific procedure depends on the experience and skill of the surgeon. It is important that all participating surgeons undergo appropriate training before the start of an RCT to reach a certain minimal level of standardisation, but this is not always possible. Blinding of participants and surgeons in surgical trials is a potential source of bias, particularly as it is not always possible to do this when one of the interventions being tested is surgical. Financial support for surgical clinical trials is limited, and this is an ongoing problem.
Despite the problems described above, serious consideration should be given to conducting RCTs to determine the effectiveness of surgery in comparison with expectant management and IVF for tubal infertility. Inclusion of women with mild and moderate tubal disease and exclusion of women with severe tubal disease may provide a reasonable way forward.
PRISMA study flow diagram.

