Intervenciones farmacológicas para el tratamiento del dolor del miembro fantasma
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Controlled clinical trial; DB followed by open phase, 3‐period, cross‐over; no wash‐out period; non‐involved doctor prepared drugs and order of administration | |
| Participants | Severe phantom pain for at least one month despite extensive pain therapy; majority of upper and lower extremity amputations of cancer aetiology; rest due to vascular and trauma; | |
| Interventions | 1) dextromethorphan 60 mg for 10 days, oral 2) dextromethorphan 90 mg for 10 days , oral 3) placebo | |
| Outcomes | Number of patients with ≥ 50% pain relief on subjective pain intensity score 0 to 100; Feeling of well‐being from 0 to 100; Sedation score from 0 to 100; Adverse events; drop‐outs/withdrawals Results: 1) & 2) all patients reported ≥ 50% pain relief; Dextromethorphan treatments with significantly better scores for feelings of well‐being at P = 0.025; Sedation significantly lower in dextromethorphan groups P = 0.01; no side effects recorded throughout drug treatment; Drop‐outs/withdrawals: None | |
| Notes | Limitations related to dosing and small sample size QS: Jadad score 3; Van Tulder score 7 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Low risk | physician not involved in study prepared batches of medications and order of administration |
| Blinding (performance bias and detection bias) | Low risk | identical capsules; outcomes assessed at the medical centre acute pain service |
| Blinding of outcome assessment (detection bias) | Unclear risk | outcomes assessed at the medical centre acute pain service but not clear if blinded |
| Incomplete outcome data (attrition bias) | Low risk | complete outcome data for all |
| Selective reporting (reporting bias) | Unclear risk | results in the DB phase reported in graphical form noting level of significance but without numerical results |
| Other bias | High risk | no wash‐out period; carry‐over effect not addressed |
| Methods | Randomised, DB, cross‐over; 6‐weeks each treatment arm; one week wash‐out period; computer‐generated randomisation | |
| Participants | Patients with phantom pain ? 6 months with pain intensity ?40 mm on 100 mm VAS scale; majority with lower limb amputations; time since amputation is 18 months; 19 participants, 15 males; Mean age, yrs (SD): 56.25 (17.5); baseline mean pain intensity (SD) : treatment group 6.1 (1.8); placebo 6.7 (1.9) | |
| Interventions | 1) gabapentin titrated in increments of 300 mg up to 2, 400 mg or maximal tolerable dose for 6 weeks; oral 2) placebo | |
| Outcomes | Change in pain intensity 100 mm VAS at end of treatment week 6 vs. baseline 1) pre 6.1 (1.8); post 2.9 (2.2) 2) pre 6.7 (1.9); post 5.1 (2.2) Mean Pain intensity difference at end of treatment week 6 (SD) 1) 3.2 (2.1) 2) 1.6 (0.7) Categorical phantom pain intensity (0 = none, 1 = mild pain, 2 = moderate pain, 3 = severe pain) end of treatment to baseline 1) pre 1.5 (0.9); post 1.45 (1) 2) pre 1.8 (0.9); post 1.6 (1.2) Median change in mood on HADS (range) 1) pre 14 (5 to 25); post 12 (4 to 23) 2) pre 15 (5 to 25); post 14 (5 to 25) Median change in function on BI (range) 1) pre 90 (70 to 105); post 85 (70 to 105) 2) pre 85 (65 to 100); post 87 (65 to 105) Median change in sleep on SIS (range) 1) pre 4 (2 to 5); post 3 (1 to 5) 2) pre 4 (2 to 5); post 4 (1 to 5) Other outcomes: number of rescue tablets Adverse events: somnolence, dizziness, headache, nausea Drop‐outs/withdrawals: 1) 2 : one did not complete and another one protocol violation 2) 3 : two did not complete and one withdrew consent Results: Significant mean pain intensity differences between two groups at 6th week in favour of gabapentin; no significant differences in rest of outcomes | |
| Notes | For lifestyle indices, sample size may be too small to rule out type 2 error; used between group analysis for comparisons | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | organized remotely (hospital pharmacist) |
| Blinding (performance bias and detection bias) | Low risk | "identical coded medication bottles containing identical tablets of gabapentin and placebo" |
| Blinding of outcome assessment (detection bias) | Unclear risk | not described who assessed and if blinded |
| Incomplete outcome data (attrition bias) | Low risk | "Data from these 19 patients were included in the results presented, using intention‐to‐treat analysis" |
| Selective reporting (reporting bias) | Low risk | it is likely that the expected outcomes were reported |
| Other bias | Low risk | carry‐over effect in cross‐over trial but probably low risk because baseline VAS pain score before placebo/gabapentin are not significantly different |
| Methods | Randomised, DB, cross‐over with 72 hours wash‐off period; Computer‐generated randomisation | |
| Participants | Patients with PLP of at least 6 months, lower extremity amputation of traumatic and vascular in aetiology; 8 participants, 6 males; mean age, yrs (SD): 70.1 (7.7); Baseline pain intensity on 0‐10 VAS, mean (SD): 7.9 (0.8) in treatment group; 7.6 (0.7) control group | |
| Interventions | 1) contralateral myofascial injection with local anaesthetic bupivacaine at 2.5 mg/ml, 1 ml, given once 2) placebo (saline) | |
| Outcomes | Pain intensity on 0 to 10 VAS, (SD) 1) pre 7.9 (0.8); post 2.6 (1.2) 2) pre 7.6 (0.7); post 6.1 (1.6) Mean difference pain intensity (SD) 1) ‐5.3 (1.4) 2) ‐1.5 (1.3) Adverse effects Drop‐outs/withdrawals Other outcomes: phantom sensation, mirror displacement in healthy limbs Results: Significant pain relief with bupivacaine contralateral myofascial injection P = 0.003. Adverse effects: no clinical signs of cardiovascular or respiratory side effects; no subjective reactions reported; no stinging sensation reported; Drop‐outs/withdrawals: None | |
| Notes | small number of patients; preliminary results QS: Jadad score 4; Van Tulder score 10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated randomisation (confirmed through email correspondence with author) |
| Allocation concealment (selection bias) | Low risk | "saline or local anaesthetic solutions prepared in a separate room by a nurse" |
| Blinding (performance bias and detection bias) | Low risk | syringes of same size; an independent physician blinded to contents of syringe performed injections" |
| Blinding of outcome assessment (detection bias) | Low risk | "The same physician who performed the basal clinical examination blinded to the treatment, visited the patients collecting number of painful muscle areas present within 1 hr of injection. The intensity of the phantom pain was evaluated before and after treatment by means of the VAS" |
| Incomplete outcome data (attrition bias) | Low risk | all patients accounted for |
| Selective reporting (reporting bias) | Low risk | it is likely that the expected outcomes were reported |
| Other bias | Low risk | carry‐over effect in cross‐over, but probably low risk as baseline pain intensity during anaesthetic and saline not significantly different |
| Methods | Randomised, DB, cross‐over, 1 hr each treatment arm; time between infusions 48 hours; Randomisation by drawing lots by person not involved in study | |
| Participants | Patients with chronic phantom pain > 6 months duration; upper and lower extremity amputation of vascular, traumatic, cancer, chronic pain in aetiologies, mean pain intensity ≥ 3 on 10 cm VAS scale; 20 participants; 15 males; Median age, yrs (range): 57 (19.3 to 72.7); Mean baseline pain intensity on 10 cm VAS: 4.32; Duration of phantom pain, yrs: 12.41 | |
| Interventions | 1) ketamine at 0.4 mg/kg, once, one hour intravenous infusion 2) calcitonin at 200 IU once, one hour intravenous infusion 3) combination ketamine / calcitonin at 200 IE calcitonin and 0.4 mg/kg ketamine once, one hour intravenous infusion 4) placebo (saline) | |
| Outcomes | Number of patients with > 50% pain reduction on 10 cm VAS 1) 6 out of 10 2) 2 out of 20 3) 12 out of 20 4) 1 out of 19 Change in pain intensity on 10 cm VAS Adverse effects: Drop‐outs/withdrawals Other outcomes: Basal Sensory assessments Results: Ketamine alone and its combination with calcitonin but not calcitonin decreased pain intensity during and after administration of drugs compared to placebo; combination calcitonin and ketamine not superior to ketamine alone; adverse effects with 1) ketamine: severe loss of consciousness; others: light sedation and light visual hallucination/hearing impairment/impairment of position/feeling; | |
| Notes | relatively small sample size; wide range duration phantom pain; ketamine alone was given to only 10 participants QS:Jadad score 5; Van Tulder score 9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | drawing of lots |
| Allocation concealment (selection bias) | Low risk | person not involved in study randomised and prepared solutions |
| Blinding (performance bias and detection bias) | Unclear risk | "neither investigator performing experiment nor the patients were aware of the solutions infused" "In some cases, drug‐related side effects occurred which rendered blinding of physician performing the tests and patients questionable" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "In some cases, drug‐related side effects occurred which rendered blinding of physician performing the tests and patients questionable" |
| Incomplete outcome data (attrition bias) | Unclear risk | missing outcome data in one group but not related to outcome |
| Selective reporting (reporting bias) | Unclear risk | added methods (introduction of fourth session) |
| Other bias | Unclear risk | carry‐over effect |
| Methods | Randomised, cross‐over, 4‐week double blinded phase with 1 to 2 weeks wash‐out period; a long‐term open phase for responders to intervention; physician with no contact with patients randomised and kept code | |
| Participants | Patients with phantom pain, with upper and lower extremity amputations, at least 3 in 10 cm visual analog scale; 12 participants, 10 males; mean age, yrs (SD): 50.58 (14.01); Mean baseline pain intensity on 10 cm VAS (SD): 4.65 (1.06); Time since amputation, years (SD): 16.49 (14.01) | |
| Interventions | 1) Morphine sulfate titrated from 70 mg/day up to 300 mg/day / max tolerable dose for 4 weeks, oral 2) placebo | |
| Outcomes | Change in pain intensity on 0 to 10 cm VAS (SD) 1) pre 4.65 (1.06); post 3.26 (1.59) 2) pre 4.65 (1.06); post 3.99 (1.23) Number of patients with pain reduction of > 50% (10 cm VAS) 1) 5 out of 12 2) 1 out of 12 Change in mood /depression on Self‐rating Depression Score Long‐term outcomes (6 months, 12 months): only with morphine sulfate; n = 9 Other outcomes: Pain‐related self‐assessment scale; active coping and catastrophizing using West‐Haven Yale Multidimensional Pain Inventory; Brief stress scale; psychophysical thresholds, 2‐point discrimination; attentional performance with d‐2 test, magnetoenkephalography Results: significantly lower pain intensity during morphine sulphate compared to the baseline (P = 0.01) and significantly lower compared to the placebo phase (P = 0.036); Significant attentional deficits with morphine sulfate (MST); Adverse effects: tiredness, dizziness, sweating, constipation*, micturition diff, nausea, vertigo, itching, short of respiration. Side effects were moderate and higher for morphine vs. placebo. Significance noted only in constipation; drop‐outs/withdrawals: None during DB phase; mood/depression, coping & catastrophizing, stress and the other psychosocial variables with no significant relationship to pain reduction | |
| Notes | Small sample size; cortical reorganization results based on 3 participants (open phase) QS: Jadad score 4; Van Tulder score 8 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Low risk | physician with no contact with patients randomised and kept code |
| Blinding (performance bias and detection bias) | High risk | "the participants as well as treating physicians were able to identify the morphine treatment but not the placebo treatment'; blinding may have been broken |
| Blinding of outcome assessment (detection bias) | High risk | it is likely that blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | all patients accounted for in analysis of outcomes |
| Selective reporting (reporting bias) | Low risk | it is likely that the expected outcomes were reported |
| Other bias | Low risk | carry‐over effect addressed |
| Methods | Controlled clinical trial, cross‐over; 2‐hours wash‐out period; double‐blind phase then an open phase with the intervention (s‐calcitonin) for longer term assessment; drawing of lots by person not involved in study | |
| Participants | Patients with phantom pain 0 to 7 days following amputation; all except one are lower limb amputations of vascular, traumatic malignancy and infectious aetiology, at least 3 on 0 to 10 numerical analog scale; 21 participants, 12 males; Median age yrs (range): 59 (20 to 78) | |
| Interventions | 1) s‐calcitonin at 200 IU, once, 20 minutes intravenous infusion 2) saline | |
| Outcomes | Change in pain intensity on 0 to 10 numeric analog scale, median 1) pre 7; post 4 2) pre 7; post 7 Number of patients with pain reduction of > 50% Long‐term (at 1 yr): number of patients with reduction of > 75% Other outcomes: number of phantom pain attacks, number of patients requiring second infusion for phantom pain recurrence Adverse events Drop‐outs/withdrawals Results: significant change in pain intensity in calcitonin phase; 19/21 with pain reduction > 50%; 8/13 surviving patients with pain reduction > 75% at 1 yr; Adverse events: headache, vertigo, nausea, vomiting, augmentation of phantom sensation, drowsiness, hot/cold flushes; Drop‐outs/withdrawals: In the long‐term follow‐up 6 months to 2 years, a total of 8 participants have succumbed | |
| Notes | QS: Jadad score 3; Van Tulder score 6 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients with PLP exceeding 3 on NAS were randomly divided into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) | Low risk | "double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | not described |
| Incomplete outcome data (attrition bias) | Unclear risk | some missing data as some patients did not have the second infusion, placebo, as their NAS did not exceed 3 |
| Selective reporting (reporting bias) | Unclear risk | numerical results for pain intensity on NAS not reported although out in graphical form and noted significance |
| Other bias | Unclear risk | carry‐over effects in cross‐over design |
| Methods | Randomised, DB, parallel; computer‐generated randomisation | |
| Participants | Patients with history of at least 12 months phantom pain of at least 4 on 11‐point numeric rating scale; upper and lower extremity amputations of traumatic aetiology in majority; 36 patients; 29 males; Median age in years (range): 62 (28 to 76) in memantine group; 61 (35 to 77) placebo; Baseline average pain intensity on 11‐point numeric rating scale (SD): 5.1 (2.13) in memantine group; 5.2 (2.02) placebo | |
| Interventions | 1) memantine at 30 mg/day, once a day, for 3 weeks, oral 2) placebo | |
| Outcomes | Change in pain intensity on 11‐point numeric rating scale, mean (SD) 1) pre 5.1 (2.1); post 3.8 (2.3) 2) pre 5.1 (2); post 3.2 (1.6) Number of participants with > 50% mean pain reduction on 11‐point numeric rating scale 1) 10/18 2) 6/18 NNT for 50% pain reduction (95%CI): 4.5 (95%CI 2.1 to 10.6) Mean change in mood / depression score on German validated depression scale (SD) 1) 22 (12) 2) 31 (18) Mean change in PDI (SD) 1) 22.1 (12) 2) 17 (7) Adverse events: vertigo, tiredness, headache, nausea, restlessness, excitation, cramps Drop‐outs/withdrawals: 1) two patients due to side effects 2) three patients due to insufficient analgesia Results: No significant difference in change in pain level in two groups; Mean pain relief was similar in both groups; no significant change in depression score; Over‐all number in severe events higher in memantine group (P < 0.05) | |
| Notes | Low powered study; Dosage too low, however, this is the limit of clinical tolerability as seen in studies QS: Jadad score 5; Van Tulder score 9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | doctor not involved in study prepared randomisation; medications prepared in hospital pharmacy |
| Blinding (performance bias and detection bias) | Low risk | "placebo and verum had same colour and size" |
| Blinding of outcome assessment (detection bias) | Unclear risk | not described |
| Incomplete outcome data (attrition bias) | Low risk | last observation carried forward |
| Selective reporting (reporting bias) | Low risk | it is likely that all expected outcomes were reported |
| Other bias | Unclear risk | baseline characteristics e.g. time since amputation, not similar; Longer time period since amputation in the memantine group but the duration of phantom pain comparable |
| Methods | Controlled clinical trial, DB, cross‐over; 3 days wash‐out period; non‐involved doctor prepared, sealed, numbered envelope for each patient containing order of drugs | |
| Participants | Patients with postamputation stump and phantom pain; upper and lower extremity amputation mostly malignancy in aetiology, rest trauma and infection, reflex dystrophy; 11 participants, 8 male; Mean age, yrs (range): 47 (32 to 78); Baseline pain intensity on 100 mm VAS: 30.2; Median duration of phantom pain, yrs (range): 4 (0.75 to 14) | |
| Interventions | 1) ketamine at 0.5 mg/kg once for 45 minutes, intravenous infusion 2) placebo (saline) | |
| Outcomes | Change in pain intensity on 0 to 100 mm VAS Adverse events Drop‐outs/withdrawals Other outcomes: McGill pain rating index, pressure pain threshold, wind‐up‐like pain, thermal stimulus response, temporal summation of heat‐induced pain, reaction time Results: All patients with significant decrease in pain intensity in ketamine group vs placebo P = 0.05; Adverse events insobriety, discomfort, elevation of mood; Drop‐outs/withdrawals: None | |
| Notes | small sample size QS: Jadad score 3; Van Tulder score 8 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Low risk | "doctor not involved in study prepared, sealed and numbered envelope for each patient containing order of ketamine and saline administration" |
| Blinding (performance bias and detection bias) | Low risk | double‐blind design; ketamine and saline same form (IV) and amount; probably done |
| Blinding of outcome assessment (detection bias) | Unclear risk | not described |
| Incomplete outcome data (attrition bias) | Low risk | all patients were analysed as to outcomes |
| Selective reporting (reporting bias) | Unclear risk | numerical results for pain intensity in VAS not reported but presented in graph as % of baseline values and noted significance |
| Other bias | Unclear risk | carry‐over effects in cross‐over design |
| Methods | Randomised, DB, parallel; randomisation by central pharmacy | |
| Participants | Patients with PLP or residual limb pain; upper and lower limb amputation of vascular, traumatic, cancer, infectious aetiologies; amputation at least 6 months, pain at least 3 months, at least 2 on 0 to 10 numerical rating scale; 39 patients, 17 males; Mean age, yrs (SD): 44.4 (9.4) in amitriptyline group; 45.3 (13.3) in control; time since amputation, yrs (SD): 11.3 (10.9) in amitriptyline; 10.6 (9.1) control; Baseline mean pain intensity on 0 to 10 NRS (SD): 3.6 (2.4) amitriptyline; 3.1 (2.6) in control | |
| Interventions | 1) amitriptyline at 10 mg/d titrate each week to max of 125 mg/d, daily for 6 weeks, oral 2) benztropine mesylate at 0.5 mg/d, daily for 6 weeks, oral | |
| Outcomes | Mean change in pain intensity on 0 to 10 NRS (SD) 1) pre 3.6 (2.4); post 3.1 (2.7) 2) pre 3.1 (2.6); post 3.1 (2.9) Change in mood / depression on CES‐D (SD) 1) pre 16.2 (11.9); post 12.9 (8.5) 2) pre 16.4 (12.4); post 1.4 (13.1) Change in function on FIM (SD) 1) pre 78.4 (3.8); post 74.5 (18.8) 2) pre 78.3 (4.2); post 79.1 (3.3) Change in QOL/handicap on CHART 1) pre 417 (76); post 360 (142) 2) pre 422 (82); post 417 (75) Other outcomes: MPQ, Modified Brief Pain Inventory, satisfaction Adverse effects Drop‐outs/withdrawals Results: No significant change in pain intensity, depression scores, function, QOL; Adverse effects: dry mouth*, drowsiness, tiredness/fatigue, BOV, constipation dizziness, heartburn, poor sleep, palpitation, nausea, better sleep, urine retention, diarrhoea, tinnitus, tremor, sweating , headaches; Drop‐outs/withdrawals: two due to side effects | |
| Notes | sample represents only 18% of eligible and may have only selected those refractory to standard treatment; QS Jadad score 5; Van Tulder score 9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomised by pharmacy investigational drug service; probably appropriate random sequence generation |
| Allocation concealment (selection bias) | Low risk | random assignment and preparation of medication by hospital pharmacy |
| Blinding (performance bias and detection bias) | Low risk | "a 7 day supply of medication provided to each participant each week in identical gelatin capsules in plastic holder so that study personnel and participants were blind to medication assignment: |
| Blinding of outcome assessment (detection bias) | Low risk | "all pre and post treatments measures were administered by research assistant blinded to the subject assignment |
| Incomplete outcome data (attrition bias) | Unclear risk | not all randomised participants were included in analysis (two (10%) in amitriptyline group excluded) |
| Selective reporting (reporting bias) | Low risk | it is likely to have included the expected outcomes |
| Other bias | Low risk | similar baseline characteristics between 2 groups |
| Methods | Randomised, DB, parallel, computer‐generated randomisation by doctor not involved in study | |
| Participants | Patients with chronic PLP of at least 12 months;traumatic upper limb amputations; 16 participants, 14 males; median age 62 (35 to 71) | |
| Interventions | 1) memantine titrated up to 30 mg/day x 3 weeks 2) placebo | |
| Outcomes | Pain intensity; intracortical inhibition; intracortical facilitation Results: no significant difference in change of pain intensity in both groups; significantly increased ICI in memantine group; significantly reduced ICF in memantine group; no correlation between changes in pain intensity and neurophysiological parameters | |
| Notes | 1 participant in memantine group discontinued in study, excluded from analysis; small number of participants QS: Jadad score 5; Van Tulder 9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | doctor not involved in study did randomisation; hospital pharmacy prepared medication |
| Blinding (performance bias and detection bias) | Low risk | "the study medication was produced in hospital pharmacy using capsules of same colour and size for placebo and memantine" |
| Blinding of outcome assessment (detection bias) | Low risk | the investigator who undertook the TMS and data analysis was blinded to patients' treatment allocation and assessed pain intensity at same time |
| Incomplete outcome data (attrition bias) | Unclear risk | one patient in memantine who did not continue with drug due to adverse effects was excluded from study and not included in analysis |
| Selective reporting (reporting bias) | Low risk | likely to have included the expected outcomes |
| Other bias | Low risk | baseline characteristics between two groups similar |
| Methods | Randomised, DB, cross‐over; 6 weeks each treatment arm; wash‐out period of 5 weeks; computer‐generated randomisation | |
| Participants | Patients with PLP and residual limb pain, with upper and lower extremity amputations of vascular, traumatic, cancer, infectious aetiology; time since amputation at least 6 months; with average pain intensity of at least 3 on 0 to 10 numerical rating scale; 24 participants; 18 males; Mean age, yrs (SD): 52.1 (15.5); Baseline mean pain intensity on 0 to 10 numerical rating scale(SD): 4.38 (2.57) | |
| Interventions | 1) gabapentin titrated from 300 mg to 3,600 mg per day for 6 weeks, oral 2) placebo | |
| Outcomes | Mean change in pain intensity on 0 to 10 NRS (SD) 1) pre 4.38 (257); post 3.43 (2.45) 2) pre 4.09 (2.44); post 3.6 (2.67) Mean change in mood/depression on CES‐D (SD) 1) pre 17.5 (10.71); post 3.74 (10.17) 2) pre 18.58 (12.67); post 14.81 (9.82) Change in function on FIM Change in QOL / handicap on CHART Adverse effects Satisfaction Other outcomes: Participant rating of global improvement; modified brief pain inventory; SF MPQ sensory score; SF MPQ affective score Results: No significant difference in change in pain intensity, mood/depression scores, function, QOL, satisfaction; Adverse effects: not described but benefit of drug outweighed side effects compared with one third of placebo who reported benefit; Drop‐outs/withdrawals: Not described in paper | |
| Notes | underpowered study QS: Jadad score 4; Van Tulder score 7 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) | Low risk | "pharmacy compounded gabapentin and placebo capsules that were identical in appearance sot that study investigators and participants could not determine study assignment by the capsules" |
| Blinding of outcome assessment (detection bias) | Low risk | research study nurse contacted each participant to assess pain intensity; probably done |
| Incomplete outcome data (attrition bias) | Unclear risk | not all randomised patients were included in final analysis |
| Selective reporting (reporting bias) | Low risk | it is likely that the expected outcomes were reported |
| Other bias | Low risk | carry‐over effects addressed, within‐subject analysis |
| Methods | Randomised, DB, cross‐over, 4 weeks each treatment arm; 2 weeks wash‐out phase; randomisation by central pharmacy | |
| Participants | Patients with chronic PLP; all upper extremity amputations of traumatic aetiology; eight participants; seven males; Mean age in years (SD): 45 (12.51); Baseline pain intensity on 0 to 100 VAS endpoints (SD): 46.98 (20.38) | |
| Interventions | 1) memantine from 10 mg/day 1st week titrated to 30 mg/day 3rd‐4th week, for 4 weeks oral 2) placebo | |
| Outcomes | Change in pain intensity on 0 to 100 VAS endpoints 1) pre 46.98 (20.38); post 51.51 (20.61) 2) pre 46.98 (20.38); post 49.46 (21.11) Magnetoenkephalographic recording Pain in residual limb Adverse events Drop‐outs/withdrawals Results: No significant differences in change in pain intensity in both groups; No significant differences in cortical reorganization; no significant difference in residual limb pain; adverse events: nausea, fatigue, dizziness, agitation, headaches; Drop‐outs/withdrawals: None | |
| Notes | small sample size QS: Jadad score 3; Van Tulder score 8 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the order of treatment was randomised" but not described |
| Allocation concealment (selection bias) | Low risk | "scientist not involved in study kept a record of treatment assignment" |
| Blinding (performance bias and detection bias) | Low risk | member of central pharmacy provided the blinded tablets; placebo substance of identical appearance following same scheme of dosage |
| Blinding of outcome assessment (detection bias) | Unclear risk | not described |
| Incomplete outcome data (attrition bias) | Low risk | all patients accounted for in analysis |
| Selective reporting (reporting bias) | Low risk | it is likely that the expected outcomes reported |
| Other bias | Low risk | carry‐over effects addressed |
| Methods | Randomised, DB, 40 minutes each treatment arm; cross‐over with interval of 24 hours for each infusion; block randomisation | |
| Participants | Patients with persistent postamputation pains > 6 months, lower and upper extremity amputations; 31 participants, 19 males; Mean age, yrs (SD): 54 (13); Time since amputation in months (SD): 81 (87.4) | |
| Interventions | 1) morphine at 0.2 mg/kg, once given over 40 minutes of intravenous infusion 2) lidocaine at 4 mg/kg, once given over 40 minutes of intravenous infusion 3) placebo (diphenhydramine) | |
| Outcomes | Pain intensity on computerised 0 to 100 VAS Subjective self‐reported % pain relief on 0% to 100% numeric scale, mean (SD) 1) 47.9 (38.2) 2) 25.8 (31.2) 3) 3.2 (10.1) NNT for 30% PLP pain reduction (95%CI) 1) 1.9 (95% CI 1.3 to 3.7) 2) 3.8 (95% CI:1.9 to 16.6) Other outcomes: treatment satisfaction scores on 0 to 100 numeric scale, sedation scores Adverse events Drop‐outs/ withdrawals Results: Morphine significantly decreases both stump (P < 0.01) and phantom pain (P < 0.001) vs. placebo; Lidocaine significantly decreases stump pain vs. placebo (P < 0.01) but not phantom pain (P > 0.05); Adverse events: sedation with morphine but no significant difference vs. other groups; Drop‐outs /withdrawals: 1 dropped out because of absence of pain before start of infusions | |
| Notes | Study has a power of 80%; carry‐over effects possible but baseline pain scores in both groups similar as well as short duration of action of drugs QS: Jadad score 5; Van Tulder score 9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | block randomisation |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) | Low risk | all study medications were identical in appearance; investigator administering study medication blinded from intervention; subject and research coordinator blinded |
| Blinding of outcome assessment (detection bias) | Low risk | "during the infusion, the investigator administering the study medication was blinded from the outcome assessment (pain and sedation) and the subject and research coordinators were blinded to the exact timing of study medication administration" |
| Incomplete outcome data (attrition bias) | Low risk | One dropped out from study due to absence of pain before start of infusion and was not included in analysis |
| Selective reporting (reporting bias) | Unclear risk | actual numerical VAS values for pain intensity not reported but presented in graph form and noted significance |
| Other bias | Low risk | carry‐over effects addressed and discussed; relatively short duration of action of study medications, use of good active placebo and baseline pain and sedation scores did not differ significantly between 3 days of infusion |
BI: Barthel Index; CES‐D: Center Epidemiologic Depression Scale; CHART: Craig Handicap Assessment and Reporting Technique; CI: confidence interval; DB: double blind; FIM: Functional Independence Measure; HADS: Hospital Anxiety and Depression Score; ICF: intracortical facilitation; ICI: intracortical inhibition; MPQ: McGill Pain Questionnaire; NNT: number needed to treat; NRS: numerical rating scale; NRS: numerical rating scale; PDI: Pain Disability Index; PLP: phantom limb pain; QOL: quality of life; QS: quality score; SD: standard deviation; SIS: Sleep Interference Scale; TMS, transcranial magnetic stimulation; VAS: visual analog scale; yrs: years
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Sample size of three | |
| Not reported as randomised or quasi‐randomised; no mention of treatment allocation; no description of double blinding | |
| Not established PLP/pre‐emptive therapy | |
| Not randomised; interventional | |
| Not established PLP | |
| Population composed of patients with stump pain; none had PLP | |
| Pre‐emptive/preventative therapy | |
| Case series | |
| Not established PLP but stump pain | |
| Mixed group of phantom pain and causalgias; no control | |
| Case series | |
| Pre‐emptive therapy | |
| Non‐randomised, open study | |
| Pre and post study; no control group | |
| Case report | |
| Pre‐emptive therapy | |
| Mixed diagnoses for central pain | |
| Pre‐emptive therapy | |
| Case report | |
| Case report | |
| Mixed diagnoses with no separate analyses for phantom pain | |
| No description of randomisation, allocation, double blinding, who assessed outcomes, withdrawals / drop‐outs | |
| Non‐randomised after day 3 where treatment assignment was changed based on response; not all patients were blinded; numerical results for initial responders (first 3 days) not reported | |
| Included all postamputation pains; phantom pain was not distinguished from other postamputation pains; no separate analysis for phantom pain |
PLP: phantom limb pain
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in pain intensity Show forest plot | 2 | 52 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.31, 0.79] |
| Analysis 1.1  Comparison 1 memantine vs. placebo, Outcome 1 change in pain intensity. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in pain intensity Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | ‐1.16 [‐1.94, ‐0.38] | |
| Analysis 2.1  Comparison 2 gabapentin vs. placebo, Outcome 1 change in pain intensity. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 memantine vs. placebo, Outcome 1 change in pain intensity.
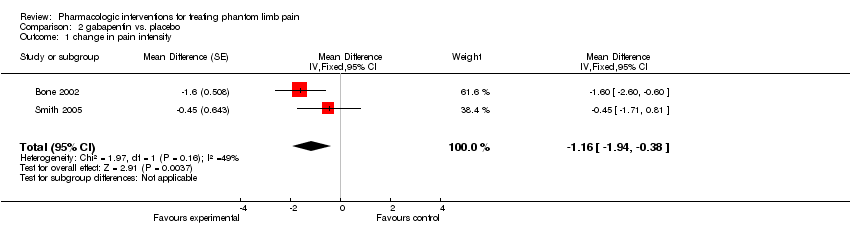
Comparison 2 gabapentin vs. placebo, Outcome 1 change in pain intensity.
| Author, Year | 1a. | 1b.
| 1c. | 2a.
| 2b. | 2c. | 3.
| Total Score |
| 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 / 5 | |
| 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 / 5 | |
| 1 | 1 | 0 | 1 | 1 | 0 | 0 | 4 / 5 | |
| 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 / 5 | |
| 1 | 0 | 0 | 1 | 1 | 0 | 1 | 4 / 5 | |
| 1 | 0 | 0 | 1 | 0 | 0 | 1 | 3 / 5 | |
| 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 / 5 | |
| 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 / 5 | |
| 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 / 5 | |
| 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 / 5 | |
| 1 | 1 | 0 | 1 | 1 | 0 | 0 | 4 / 5 | |
| 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 / 5 | |
| 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 / 5 |
| Author, Year | A | B | C | D | E | F | G | H | I | J | K | Total Score |
| 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 7 / 11 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 / 11 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 / 11 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 / 11 | |
| 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 8 / 11 | |
| 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 / 11 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 / 11 | |
| 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 8 / 11 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 9 / 11 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 9 / 11 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 7 / 11 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 8 / 11 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 9 / 11 |
| Author, yr | Number of patients | Characteristics of population | Methodology | Follow‐up | Drop‐outs/ withdrawals | Quality score |
|
|
|
|
|
|
|
| Jadad | Van Tulder |
| NMDA antagonists |
|
|
|
|
|
|
|
| Memantine |
|
|
|
|
|
|
|
| 36
| 29 males, median age 62 yrs; majority traumatic upper and lower limb amputation; at least 12 mos PLP history; at least 4 on 11pt NAS | Randomised, DB, parallel; CGR
| End of 3 wks
| Two in treatment group due to side affects; three in placebo due to insufficient analgesia | 5 | 9 | |
|
| 8
| 7 males, mean age 45 yrs; chronic PLP; traumatic upper limb amputations
| Randomised, DB, cross‐over; 2 wk wash‐out; central pharmacy randomisation | End of treatment at 30 days
| None
| 3 | 8 |
|
| 16
| 14 males; median age 62 yrs; at least 12 mos PLP; upper limb amputation | Randomised, DB, parallel, CGR
| End of treatment at 21 days | One in treatment group due to side effects | 5 | 9 |
| Dextromethorphan |
|
|
|
|
|
|
|
| 10 | 5 males, mean age 50 yrs; average of 4.8 mos PLP; severe pain at least 1 mo; majority upper, lower limb malignant amputations; others vascular and trauma | Controlled clinical trial; DB cross‐over; no wash‐out; 3‐period, DB, placebo controlled followed by open phase; non‐involved physician prepared order of administration | After 10 days of each treatment period (DB phase) | None | 3 | 7 | |
| Ketamine |
|
|
|
|
|
|
|
| 11 | 8 males; mean age 47 yrs; average of 4 yrs PLP; with postamputation and phantom pain; upper, lower limb amputation; mostly malignant others trauma, infection, reflex sympathetic dystrophy | Randomised, DB, cross‐over, 3 days wash‐out; non‐involved doctor prepared, sealed, numbered envelope for each patient containing order of drug | At end of infusion: 45 min | None | 3 | 8 | |
| 20 | 15 males, median age 57 yrs; mean pain intensity 4.32 on 10 cm VAS, average of 12 yrs PLP | Randomised, DB, cross‐over, time between infusions 48 hours; drawing of lots by person not involved in study | 30,60 min, 48 hrs after infusion | One from ketamine alone group did not get saline; two from placebo did not get heat, electrical stimulation | 5 | 9 | |
| Anticonvulsants |
|
|
|
|
|
|
|
| Gabapentin |
|
|
|
|
|
|
|
| 19 | 15 males; mean age 56 yrs; PLP ≥ 6 mo; average pain intensity ≈ 6 on VAS; majority lower limb amputations; surgical amputations | Randomised, DB, cross‐over; 1 wk wash‐out; CGR | At 6 wks | Two in treatment group, non‐completion and protocol violation; three in placebo, non‐completion; consent withdrawal | 5 | 9 | |
| 24 | 18 males; mean age 52 yrs; average pain intensity four NRS; patients with PLP and RLP; upper, lower limb amputations; vascular, traumatic, cancer, infections; at least 6 mo since amputation | Randomised, DB, cross‐over, 5 wk wash‐out; CGR | At 6 wks | Not described | 4 | 7 | |
| Antidepressants | |||||||
| Amitriptyline | |||||||
| 39 | 17 males; mean age 44 to 45 yrs; with PLP or RLP; upper, lower limb amputation; vascular, traumatic, cancer, infectious; pain at least 3 mos; pain intensity at 2 NRS | Randomised, DB, parallel Central pharmacy randomisation | At 6 wks | Two due to side effects | 5 | 9 | |
| Calcitonins | |||||||
| 21 | 12 males; median age 59 yrs; with severe PLP, 0 to 7 days after surgery; all except one are lower limb amputations; vascular, traumatic, malignant, infectious; pain intensity at least 3 NAS | Controlled clinical trial, cross‐over, 2 hr wash‐out; DB followed by open phase | Short‐term: 24 hr before and after treatment (double blind); long‐term: 6 mos, 1 to 2 yrs (open phase) | None in short‐term; total of eight patients in long‐term (open phase) | 3 | 6 | |
| 20 | 15 males, median age 57 yrs; mean pain intensity 4.32 on 10 cm VAS, average of 12.41 yrs PLP | Randomised, DB, cross‐over, time between infusions 48 hours; drawing of lots by person not involved in study | 30, 60 min, 48 hrs after infusion | One from ketamine alone group did not get saline; two from placebo did not get heat, electrical stimulation | 5 | 9 | |
| Opioids | |||||||
| Morphine | |||||||
| 12 | 10 males; mean age 50 yrs; PLP; upper, lower limb amputations, pain intensity at least 3 VAS; average time since amputation 16 yrs | Randomised, cross‐over; 4‐wk DB phase, 1 to 2 wks wash‐out; long‐term phase (open) for responders; physician with no contact with patients randomised and kept code | Hourly for pain and side effect; weekly during 4 weeks of DB phase; long‐term 6, 12 mos (open) | None during double blind phase | 4 | 8 | |
| 31 | 19 males; mean age 54 yrs; patients with persistent post amputation pains 6 mos; lower, upper limb amputations; time since amputation 81 mos | Randomised, DB, cross‐over, 24 hrs wash‐out; active‐placebo controlled; block randomisation | 30 min after end of infusion | One due to absence of pain before start of infusions | 5 | 9 | |
| Anaesthetics | |||||||
| Lidocaine | |||||||
| 31 | 19 males; mean age 54 yrs; patients with persistent post amputation pain > 6 mos; lower, upper limb amputations; time since amputation 81 mos | Randomised, DB, cross‐over, 24 hrs wash‐out; active‐placebo controlled; block randomisation | 30 min after end of infusion | One due to absence of pain before start of infusions | 5 | 9 | |
| Bupivacaine | |||||||
| 8 | 6 males; mean age 70 yrs; PLP at least 6 mos; lower limb amputation; traumatic, vascular; average pain intensity ≈7 | Randomised, DB, cross‐over, 72 hrs wash‐out; CGR | 60 min after injection | none | 4 | 10 | |
| DB, double‐blind; CGR, computer‐generated randomisation; NAS, numerical analog scale; VAS, visual analog scale; NRS, numerical rating scale; PLP, phantom limb pain; RLP, residual limb pain; yr, year; yrs, years; wk, week; wks, weeks; hr, hour; hrs, hours; min, minutes; mo, month; mos, months; pt, point | |||||||
| Author, yr | Intervention | Treatment Duration | FF‐up | Outcomes | Results | Over‐all direction of efficacy | Adverse events |
| NMDA antagonist |
|
|
|
|
|
|
|
| Memantine |
|
|
|
|
|
|
|
| 1) memantine 30 mg/d; oral 2) placebo | 3 weeks | At end of 3 weeks | Pain intensity 11 pt NRS; number of participants with ≥50% pain reduction;NNT; mood;disability; adverse events | No sig diff in change in pain level, in number of participants with ≥50% pain relief; depres‐ sion scores; disability indices in 2 grps; over‐ all number severe events higher in memantine | ‐ | vertigo, tiredness, headache, nausea, restlessness, excitation, cramps | |
| 1) memantine titrated up to 30 mg/d;oral 2) placebo | 4 weeks each treatment arm | At end of 4 weeks of each arm | Pain intensity 0‐100 VAS; MEG recording; adverse events | No sig diff in change in pain intensity, cortical reorganization in both grps | ‐ | Nausea, fatigue, dizziness, agitation, headaches | |
| 1) memantine titrated up to 30 mg/d; oral 2) placebo | 3 weeks | At end of 3 weeks | Pain intensity 11 pt NRS; ICI; ICF | No sig diff in pain intensity; enhanced ICI; reduced ICF | ‐ | Not described | |
| Dextro‐ methorphan |
|
|
|
|
|
|
|
| 1) dextromethor‐ phan 120 mg/d;oral 2) dextromethor‐ phan 180 mg/d;oral 3) placebo | 10 days each treatment arm | At end of 10 days of each arm | Number of patients with ≥50% pain relief; feeling of well‐being; sedation score; adverse events | Dextromethor‐ phan grps with ≥50% pain relief; with signi‐ ficantly better feeling of well‐ being scores; with significantly lower sedation scores | + | none reported | |
| Ketamine |
|
|
|
|
|
|
|
| 1) ketamine 0.5 mg/kg once, IV infusion 2) placebo | 45 minute each treatment arm | At end of IV infusion | Pain intensity 0‐100 mm VAS;adverse events;McGill; pressure pain threshold; wind‐up like pain; thermal stimulus response; temporal summation of heat‐induced pain;reaction time | Sig dec in pain intensity; in pain‐evoked by mechanical stimulation; inc in pressure pain threshold; no alteration in temperature sensitivity in ketamine group | + | insobriety, discomfort, elevation of mood | |
| 1) ketamine 0.4 mg/kg once, IV infusion 2) calcitonin 200 IU, once, IV infusion 3) combination ketamine/ calcitonin, IV 4) placebo | 1 hour each arm | At 30, 60 mins, 48 hours after infusion | Pain intensity; number of patients with ≥50% pain reduction on 10 cm VAS; basal sensory assessment; adverse effects | Sig dec pain intensity in ketamine alone and combination vs. placebo and calcitonin; sig inc in number of responders in ketamine alone and combination vs. placebo and calcitonin; sig inc in electrical thresholds with combination treatment but no change in pressure or heat thresholds | + | loss of conscious‐ ness, light sedation, light visual hallucination, hearing impairment, position / feeling impairment | |
| Anticonvulsants |
|
|
|
|
|
|
|
| Gabapentin |
|
|
|
|
|
|
|
| 1) gabapentin titrated up to 2400 mg or max tolerable dose; oral 2) placebo | 6 weeks each arm | weekly and at end of 6 weeks | Pain intensity 100 mm VAS; pain intensity difference; depression score (HADS) function (BI); sleep (SIS); no. of rescue tabs; adverse events | Significantly greater pain intensity diff with gabapentin at end of treatment; no sig diff in depression score, function, sleep, no. of rescue tablets with the treatments | +a ‐b | somnolence, dizziness, headache, nausea | |
| 1) gabapentin titrated up to 3600 mg/d; oral 2) placebo | 6 weeks | At end of 6 wks of each arm | Pain intensity 0‐10 NRS; depression score (CES‐D); function (FIM); handicap (CHART); satisfaction; global improvement rating; pain inventory; McGill | No sig group diff on any outcomes at end of treatment | ‐c | not described | |
| Antidepressants |
|
|
|
|
|
|
|
| Amitriptyline |
|
|
|
|
|
|
|
| 1) amitriptyline 10 mg/d titrated to max of 125 mg/d; oral 2) benztropine mesylate 0.5 mg/d; oral | 6 weeks | At end of 6 weeks | Pain intensity 0‐10 NRS depression score (CES‐D); function (FIM); handicap (CHART);pain inventory; McGill; satisfaction | No sig group diff on any outcomes at end of treatment | ‐ | dry mouth (more severe), dizziness | |
| Calcitonins |
|
|
|
|
|
|
|
| 1) s‐calcitonin 200 IU, IV infusion 2) saline | 20 minute IV infusion; once | 24 hours after infusion (DB); 7‐152 days, weekly (open phase) | Pain intensity 0‐10 NAS in open phase/ long‐term; number of patients with ≥50%,75% pain relief; adverse events | Sig dec in median pain intensity with s‐ calcitonin at 24 hours after infusion; at 1 yr, 62% of patients with 75% pain reduction | + | headache, vertigo, nausea, vomiting, phantom sensation, drowsiness, hot/cold flushes | |
| 1) ketamine 0.4 mg/kg, once, IV infusion 2) calcitonin 200 IU, once, IV infusion 3) combination ketamine / calcitonin, IV 4) placebo | 1 hour each arm | At 30, 60 mins, 48 hours after infusion | Pain intensity; number of patients with ≥50% pain relief on 10 cm VAS; basal sensory assessments; adverse effects | No sig dec in pain intensity with calcitonin vs. placebo at 48 hrs; number of responders not significantly different from placebo | _ | drowsiness, nausea, facial flushing, hot/cold flushes, dizziness | |
| Opioids | |||||||
| Morphine | |||||||
| 1) Morphine sulfate titrated up to 300 mg/d or max tolerable dose; oral 2) placebo | 4 weeks each arm (DB) | End of each treat‐ ment phase of 4 weeks | Pain intensity 10 cm VAS; number of patients with 50% pain reduction; depression score; pain‐ related self‐ assessment scale; WHYMPI; BSS; psycho‐ physical thresholds; 2‐point discrimination; attentional performance; MEG | Sig pain reduction during morphine; 42% with >50% pain relief; 8% with 25‐50% pain relief during morphine; no sig change in perception and pain thresholds; significantly lower attentional performance during morphine; scores on pain experience scale, depres‐ sion score, WHYMPI, BSS with no sig relationship with pain reduction; 2 of 3 with clear cortical reorganization | + | constipation only sig adverse effect among others e.g. tiredness, dizziness, sweating, micturition difficulty, vertigo, itching, respiration | |
| 1) morphine 0.2 mg/kg, IV infusion 2) lidocaine 4 mg/kg, IV infusion 3) placebo (diphenhydra‐ mine) | 40 minutes of IV infusion | 30 minutes after end of infusion | Pain relief 0‐ 100% numeric scale; NNT for 30% pain reduction; satisfaction, sedation scores, adverse events | Sig dec in phantom and stump pain intensity during IV morphine; NNT=2; significantly higher satisfaction with morphine; no sig diff in sedation scores | + | sedation (but no sig diff with other groups) | |
| Anaesthetics | |||||||
| Lidocaine | |||||||
| 1) morphine 0.2 mg/kg, IV infusion 2) lidocaine 4 mg/kg, IV infusion 3) placebo (diphenhydra‐ mine) | 40 minutes of IV infusion | 30 minutes after end of infusion | Pain relief 0‐ 100% numeric scale; NNT for 30% pain reduction; satisfaction; sedation scores; adverse events | No sig dec in PLP vs. placebo; NNT= 3.8; significantly higher satisfaction with lidocaine vs. placebo; no sig diff in sedation scores | _ | sedation scores not significantly different from placebo | |
| Bupivacaine | |||||||
| 1) bupivacaine 2.5 mg/ml,1ml, contralateral myofascial injection 2) placebo (saline) | Injections given once | After one hour | Pain intensity 0‐10 VAS; pain intensity difference; phantom sensation; mirror displacement in healthy limbs; adverse effects | Sig pain relief with bupivacaine; reduction in phantom sensation in 6 of 8 patients | + | none | |
| yr, year; d, day; mins, minutes; NRS, numerical rating scale; NNT, number needed to treat; VAS, visual analog scale; sig, significant; dec, decrease; inc, increase; grps, groups; grp, group; max, maximum; ICI, intracortical inhibition; ICF, intracortical facilitation; IV, intravenous; DB, double‐blind; MEG, magnetoencephalography; HADS, Hospital Anxiety and Depression Scale; BI, Barthel Index; NRS, numerical rating scale; CES‐D, Center Epidemiologic Depression Scale; FIM, Functional Independence Measure; CHART, Craig Handicap Assessment and Reporting Technique; NAS, numerical analog scale; WHYMPI, West‐Haven Yale Multidimensional Inventory; BSS, Brief Stress Scale; apain intensity; bmood, sleep, function; cpain intensity, mood, function, handicap, satisfaction | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in pain intensity Show forest plot | 2 | 52 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.31, 0.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in pain intensity Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | ‐1.16 [‐1.94, ‐0.38] | |

