Stosowanie antybiotyków w okresie okołooperacyjnym w celu zapobiegania ostremu zapaleniu wnętrza gałki ocznej po zabiegu usunięcia zaćmy
Appendices
Appendix 1. CENTRAL search strategy
IDSearch
#1 MeSH descriptor: [Ophthalmologic Surgical Procedures] explode all trees
#2 MeSH descriptor: [Cataract] explode all trees
#3 MeSH descriptor: [Cataract Extraction] explode all trees
#4 cataract* near/3 extract* or aspirat* or operat* or remov* or surg* or excis* or implant*
#5 lens* near/3 extract* or aspirat* or operat* or remov* or surg* or excis* or implant*
#6 pha?oemulsif*
#7 lensectomy
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
#9 MeSH descriptor: [Endophthalmitis] explode all trees
#10 endophthalmitis
#11 ophthalmia
#12 #9 or #10 or #11
#13 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees
#14 antibiotic*
#15 bacteri*
#16 chloramphenicol*
#17 MeSH descriptor: [Ciprofloxacin] explode all trees
#18 ciprofloxacin*
#19 fusidic acid*
#20 gentamicin*
#21 levofloxacin*
#22 neomycin*
#23 ofloxacin*
#24 polymyxin* B
#25 cefazolin*
#26 MeSH descriptor: [Cefuroxime] explode all trees
#27 cefuroxime*
#28 moxifloxacin*
#29 norfloxacin*
#30 MeSH descriptor: [Vancomycin] explode all trees
#31 vancomycin*
#32#13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31
#33 MeSH descriptor: [Antibiotic Prophylaxis] explode all trees
#34 prophyla*
#35 prevent*
#36 #33 or #34 or #35
#37 #8 and #12 and #32
#38 #36 and #37
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt.
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. exp animals/
10. exp humans/
11. 9 not (9 and 10)
12. 8 not 11
13. exp ophthalmologic surgical procedure/
14. exp cataract/
15. exp cataract extraction/
16. ((cataract$ adj3 extract$) or aspirat$ or operat$ or remov$ or surg$ or excis$ or implant$).tw.
17. ((lens$ adj3 extract$) or aspirat$ or operat$ or remov$ or surg$ or excis$ or implant$).tw.
18. pha?oemulsif$.tw.
19. lensectomy.tw.
20. or/13‐19
21. exp endophthalmitis/
22. endophthalmitis.tw.
23. ophthalmia.tw.
24. or/21‐23
25. exp anti bacterial agents/
26. antibiotic$.tw.
27. bacteri$.tw.
28. chloramphenicol$.tw.
29. exp ciprofloxacin/
30. ciprofloxacin.tw.
31. (fusidic adj2 acid$).tw.
32. exp gentamicin/
33. gentamicin$.tw.
34. exp levofloxacin/
35. levofloxacin$.tw.
36. neomycin$.tw.
37. ofloxacin$.tw.
38. (polymyxin$ adj1 B).tw.
39. cefazolin$.tw.
40. exp cefuroxime/
41. cefuroxime$.tw.
42. moxifloxacin$.tw.
43. norfloxacin$.tw.
44. exp vancomycin/
45. vancomycin$.tw.
46. or/21‐45
47. exp antibiotic prophylaxis/
48. prophyla$.tw.
49. prevent$.tw.
50. or/47‐49
51. 20 and 24 and 46
52. 50 and 51
53. 12 and 52
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/
2. exp randomization/
3. exp double blind procedure/
4. exp single blind procedure/
5. random$.tw.
6. or/1‐5
7. (animal or animal experiment).sh.
8. human.sh.
9. 7 and 8
10. 7 not 9
11. 6 not 10
12. exp clinical trial/
13. (clin$ adj3 trial$).tw.
14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
15. exp placebo/
16. placebo$.tw.
17. random$.tw.
18. exp experimental design/
19. exp crossover procedure/
20. exp control group/
21. exp latin square design/
22. or/12‐21
23. 22 not 10
24. 23 not 11
25. exp comparative study/
26. exp evaluation/
27. exp prospective study/
28. (control$ or prospectiv$ or volunteer$).tw.
29. or/25‐28
30. 29 not 10
31. 30 not (11 or 23)
32. 11 or 24 or 31
33. exp cataract/
34. exp cataract extraction/
35. ((cataract$ adj3 extract$) or aspirat$ or operat$ or remov$ or surg$ or excis$ or implant$).tw.
36. ((lens$ adj3 extract$) or aspirat$ or operat$ or remov$ or surg$ or excis$ or implant$).tw.
37. pha?oemulsif$.tw.
38. lensectomy.tw.
39. or/33‐38
40. exp endophthalmitis/
41. endophthalmitis.tw.
42. ophthalmia.tw.
43. or/40‐42
44. exp antiinfective agent/
45. antibiotic$.tw.
46. bacteri$.tw.
47. chloramphenicol$.tw.
48. ciprofloxacin.tw.
49. (fusidic adj2 acid$).tw.
50. gentamicin$.tw.
51. levofloxacin$.tw.
52. neomycin$.tw.
53. ofloxacin$.tw.
54. (polymyxin$ adj1 B).tw.
55. cefazolin$.tw.
56. cefuroxime$.tw.
57. moxifloxacin$.tw.
58. norfloxacin$.tw.
59. vancomycin$.tw.
60. or/44‐59
61. exp antibiotic prophylaxis/
62. prophyla$.tw.
63. prevent$.tw.
64. or/61‐63
65. 39 and 43 and 60
66. 64 and 65
67. 32 and 66
Appendix 4. LILACS search strategy
cataract$ or phacoemulsification or IOL and endophthalmitis
Appendix 5. ISRCTN Trials search strategy
(cataract OR phacoemulsification OR IOL) AND endophthalmitis
Appendix 6. ClinicalTrials.gov search strategy
(cataract OR phacoemulsification OR IOL) AND endophthalmitis
Appendix 7. ICTRP search strategy
cataract AND endophthalmitis
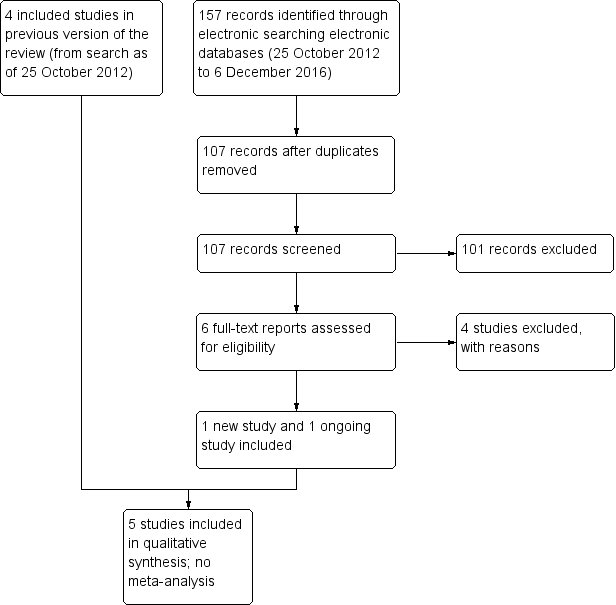
Study flow diagram.
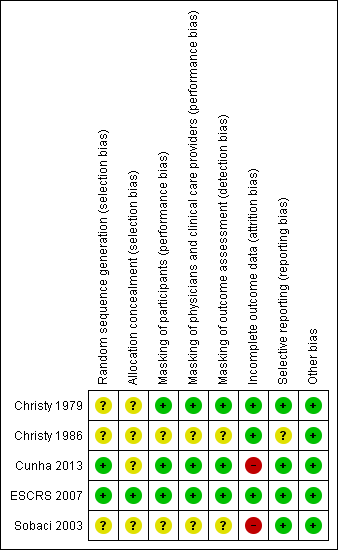
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Perioperative antibiotics for prevention of endophthalmitis after cataract surgery | ||||||||
| Population: participants undergoing cataract surgery Settings: eye hospital or clinic Outcome: risk of endophthalmitis after surgery | ||||||||
| Perioperative prophylaxis versus no prophylaxis | ||||||||
| Study ID | No. eyes and participants | Follow‐up | Comparison (intervention vs comparator) | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 644 eyes of 640 participants | 6 weeks | Treatment: BSS with antibiotics (vancomycin 20 mg/mL and gentamicin 8 mg/mL) | Not reported | 0/322 (0%) eyes | Not reported | 0.20 (0.01 to 4.15) | ⊕⊝⊝⊝ | |
| Control: BSS‐only irrigating infusion fluid | Not reported | 2/322 (0.62%) eyes | ||||||
| 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | 0.14 (0.03 to 0.63) | 0.10 (0.01 to 0.78) | ⊕⊕⊕⊕ | |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | 0.21 (0.06 to 0.74) | 0.20 (0.04 to 0.91) | ⊕⊕⊕⊕ | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | 0.72 (0.32 to 1.61) | 0.70 (0.27 to 1.84) | ⊕⊕⊕⊝ | |||
| Control: placebo drops | 14/4054 (0.35%) eyes | 10/4054 (0.25%) eyes | ||||||
| Comparisons of combinations of antibiotics with specific antibiotics | ||||||||
| Study ID | No. eyes and participants | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 6618 eyes of 6618 participants | 1 week | Treatment 1: combined prophylaxis (topical regimen + periocular penicillin at the time of surgery) | 5/3309 (0.15%) eyes | Not reported | 0.33 (0.12 to 0.92) | Not reported | ⊕⊕⊕⊝ | |
| Treatment 2: topical regimen alone (chloramphenicol‐sulfadimidine) | 15/3309 (0.45%) eyes | Not reported | ||||||
| 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | Treatment 1 vs treatment 2: 0.67 (0.11 to 3.99) | Treatment 1 vs treatment 2: 0.50 (0.05 to 5.52) | ⊕⊕⊕⊝ | |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | Treatment 2 vs treatment 3: 0.30 (0.08 to 1.09) | Treatment 2 vs treatment 3: 0.29 (0.06 to 1.37) | ⊕⊕⊕⊝ | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | Treatment 1 vs treatment 3: 0.20 (0.04 to 0.91) | Treatment 1 vs treatment 3: 0.14 (0.02 to 1.16) | ⊕⊕⊕⊕ | |||
| Mode of antibiotic delivery | ||||||||
| Study ID | No. eyes and patients | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 77,015 eyes of 77,015 participants | 1 week | Mode 1: Anterior sub‐Tenon injections (subconjunctival) | 38/39,752 (0.10%) eyes | Not reported | 0.85 (0.55 to 1.32) | Not reported | ⊕⊕⊕⊝ | |
| Mode 2: Posterior sub‐Tenon injections (retrobulbar) | 42/37,263 (0.11%) eyes | Not reported | ||||||
| 108 eyes of 108 participants | 3 weeks | Treatment 1: fixed combination of topical gatifloxacin 0.3% and prednisolone acetate 1% | 0/47 (0%) eyes | Not reported | 0.43 (0.02 to 10.34) | Not reported | ⊕⊝⊝⊝ | |
| Treatment 2: individual instillation of topical gatifloxacin 0.3% and prednisolone acetate 1% | 1/61 (2%) eyes | Not reported | ||||||
| GRADE Working Group grades of evidence | ||||||||
| BSS: balanced salt solution; CI: confidence interval; RR: risk ratio. *Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis. **Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR) 1 Downgraded for imprecision (‐2) as the study did not enroll a sufficient number of participants to detect differences between groups. 2 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants at the time of surgery based on the surgeon's discretion (number excluded not reported). 3 Downgraded for imprecision (‐1) as the confidence interval of the effect estimate between groups was wide. 4 Downgraded for indirectness (‐1) as the study was conducted more than 30 years ago and the techniques for cataract surgery have since changed substantially. 5 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants who did not return for follow‐up (16% of study population). | ||||||||
| Comparisons of specific antibiotics or combinations of antibiotics | |||||||||
| Study ID | Groups | Proportion of eyes with final VA > 20/40 following endophthalmitis | RR (95% CI) | Proportion of eyes with final VA < 20/200 following endophthalmitis | RR (95% CI) | ||||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | ||
| Group 1: intracameral cefuroxime injection, with or without topical levofloxacin drops | 2/5 (40%) eyes | 1/3 (33.3%) eyes | 0.69 (0.22 to 2.11) | 0.57 (0.11 to 2.95) | 0/5 (0%) eyes | 0/3 (0%) eyes | 0.46 (0.03 to 7.48) | 0.50 (0.03 to 7.54) | |
| Group 2: no injection, with or without topical levofloxacin drops | 14/24 (58.3%) eyes | 10/17 (58.1%) eyes | 4/24 (16.7%) eyes | 4/17 (23.5%) eyes | |||||
| CI: confidence interval; final VA: visual acuity at time of last follow‐up visit (range 3 weeks to 8 months); VA: visual acuity. *Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis. **Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR). | |||||||||

