白內障手術時期使用抗生素防止手術後急性眼內炎
Abstract
Background
Endophthalmitis is a severe inflammation of the anterior or posterior (or both) chambers of the eye that may be sterile or associated with infection. It is a potentially vision‐threatening complication of cataract surgery. Prophylactic measures for endophthalmitis are targeted against various sources of infection.
Objectives
To evaluate the effects of perioperative antibiotic prophylaxis for endophthalmitis following cataract surgery compared with no prophylaxis or other form of prophylaxis.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 12), Ovid MEDLINE, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily (January 1946 to December 2016), Embase (January 1980 to December 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to December 2016),the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We used no date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 6 December 2016. We also searched for additional studies that cited any included trials using the Science Citation Index.
Selection criteria
We included randomized controlled trials that enrolled adults undergoing cataract surgery (any method and incision type) for lens opacities due to any origin. We included trials that evaluated preoperative antibiotics, intraoperative (intracameral, subconjunctival or systemic), or postoperative antibiotic prophylaxis for acute endophthalmitis. We excluded studies that evaluated antiseptic preoperative preparations using agents such as povidone iodine or antibiotics for treating acute endophthalmitis after cataract surgery.
Data collection and analysis
Two review authors independently reviewed abstracts and full‐text articles for eligibility, assessed the risk of bias for each included study, and abstracted data.
Main results
Five studies met the inclusion criteria for this review, including 101,005 adults and 132 endophthalmitis cases. While the sample size was very large, the heterogeneity of the study designs and modes of antibiotic delivery made it impossible to conduct a formal meta‐analysis. Interventions investigated included the utility of adding vancomycin and gentamycin to the irrigating solution compared with standard balanced saline solution irrigation alone, use of intracameral cefuroxime with or without topical levofloxacin perioperatively, periocular penicillin injections and topical chloramphenicol‐sulfadimidine drops compared with topical antibiotics alone, and mode of antibiotic delivery (subconjunctival versus retrobulbar injections; fixed versus separate instillation of gatifloxacin and prednisolone). The risk of bias among studies was low to unclear due to information not being reported. We identified one ongoing study.
Two studies compared any antibiotic with no antibiotic. One study, which compared irrigation with antibiotics in balanced salt solution (BSS) versus BSS alone, was not sufficiently powered to detect differences in endophthalmitis between groups (very low‐certainty evidence). One study found reduced risk of endophthalmitis when combining intracameral cefuroxime and topical levofloxacin (risk ratio (RR) 0.14, 95% confidence interval (CI) 0.03 to 0.63; 8106 participants; high‐certainty evidence) or using intracameral cefuroxime alone (RR 0.21, CI 0.06 to 0.74; 8110 participants; high‐certainty evidence) compared with placebo, and an uncertain effect when using topical levofloxacin alone compared with placebo (RR 0.72, CI 0.32 to 1.61; 8103 participants; moderate‐certainty evidence).
Two studies found reduced risk of endophthalmitis when combining antibiotic injections during surgery and topical antibiotics compared with topical antibiotics alone (risk ratio (RR) 0.33, 95% confidence interval (CI) 0.12 to 0.92 (periocular penicillin and topical chloramphenicol‐sulfadimidine; 6618 participants; moderate‐certainty evidence); and RR 0.20, 95% CI 0.04 to 0.91 (intracameral cefuroxime and topical levofloxacin; 8101 participants; high‐certainty evidence)).
One study, which compared fixed versus separate instillation of gatifloxacin and prednisolone, was not sufficiently powered to detect differences in endophthalmitis between groups (very low‐certainty evidence). Another study found no evidence of a difference in endophthalmitis when comparing subconjunctival versus retrobulbar antibiotic injections (RR 0.85, 95% CI 0.55 to 1.32; 77,015 participants; moderate‐certainty evidence).
Two studies reported any visual acuity outcome; one study, which compared fixed versus separate instillation of gatifloxacin and prednisolone, reported only that mean visual acuity was the same for both groups at 20 days postoperation. In the other study, the difference in the proportion of eyes with final visual acuity greater than 20/40 following endophthalmitis between groups receiving intracameral cefuroxime with or without topical levofloxacin compared with no intracameral cefuroxime was uncertain (RR 0.69, 95% CI 0.22 to 2.11; 29 participants; moderate‐certainty evidence).
Only one study reported adverse events (1 of 129 eyes had pupillary membrane in front of the intraocular lens and 8 eyes showed posterior capsule opacity). No study reported outcomes related to quality of life or economic outcomes.
Authors' conclusions
Multiple measures for preventing endophthalmitis following cataract surgery have been studied. High‐certainty evidence shows that injection with cefuroxime with or without topical levofloxacin lowers the chance of endophthalmitis after surgery, and there is moderate‐certainty evidence to suggest that using antibiotic eye drops in addition to antibiotic injection probably lowers the chance of endophthalmitis compared with using injections or eye drops alone. Clinical trials with rare outcomes require very large sample sizes and are quite costly to conduct; thus, it is unlikely that many additional clinical trials will be conducted to evaluate currently available prophylaxis. Practitioners should rely on current evidence to make informed decisions regarding prophylaxis choices.
PICO
Plain language summary
白內障手術時期使用抗生素以預防眼部細菌感染
本文獻回顧的目的是什麼?
本篇考科藍文獻回顧旨於探討白內障手術時期使用抗生素是否能夠預防術後眼部細菌感染(眼內炎)。考科藍研究人員針對此問題蒐集了所有相關研究及進行分析,共搜索到五個研究。
主要結論:
白內障手術後存在發生眼內炎的微小可能性。採取手術中眼內抗生素注射的方式可以降低此感染機率(高確定性證據)。相對於單獨使用抗生素注射或抗生素點眼劑,合併使用以上兩種方式可能降低感染風險。大部分研究皆未提供不良反應的資訊。
本文獻回顧的研究內容是什麼?
眼內炎是一種不常見但可能導致失明的嚴重白內障手術併發症,起因於在接受手術當時或術後的幾天內由於細菌侵入眼內所造成。避免手術期間和手術後感染有多種方式可採用,例如:在手術時使用抗生素。可應用不同種類的抗生素,採用不同給藥方式(包括眼內注射、靜脈輸注或滴眼液)或在不同時間點使用(手術前、手術中或手術後)。
本文獻回顧的主要結果為何?考科藍研究人員共發現五個相關研究。其中有兩個研究在巴基斯坦完成,一個研究在歐洲數個國家完成,一個研究在巴西,及一個研究在土耳其完成。這些研究探討了不同的療法:其中一個研究比較四種不同療法:抗生素注射及抗生素滴眼劑合併療法,與單獨使用抗生素注射、單獨使用抗生素滴眼劑,或單獨使用安慰劑眼藥水比較;一個研究比較抗生素注射和抗生素滴眼劑合併療法,相較於單獨使用抗生素滴眼劑;一個研究比較了合併抗生素注射和類固醇,相較於單獨給予抗生素注射或類固醇;一個研究比較在眼部兩個不同位置注射抗生素;一個研究比較在手術中使用添加抗生素的無菌溶液,相較於使用未添加抗生素的無菌溶液。
文獻回顧結果顯示:
•在手術結束時採取眼內注射抗生素(cefuroxime)會降低術後發生眼內炎的機會(高確定性證據)。
•抗生素注射(cefuroxime或盤尼西林)外加抗生素滴眼劑(levofloxacin或氯黴素),相較於採用單獨抗生素注射或滴眼劑可能能降低發生眼內炎的機會(中確定性證據)。
•在白內障手術中使用的無菌灌洗液中添加抗生素是否能夠降低眼內炎發生的機會,仍屬非常不明確(非常低確定性證據)。
•單獨或合併使用抗生素和類固醇對於發生眼內炎的機會是否存在差異,仍屬非常不明確(非常低確定性證據)。
本文獻回顧資料更新日期為何?
考科藍研究人員搜索發佈日期係至2016年12月的研究。
Authors' conclusions
Summary of findings
| Perioperative antibiotics for prevention of endophthalmitis after cataract surgery | ||||||||
| Population: participants undergoing cataract surgery Settings: eye hospital or clinic Outcome: risk of endophthalmitis after surgery | ||||||||
| Perioperative prophylaxis versus no prophylaxis | ||||||||
| Study ID | No. eyes and participants | Follow‐up | Comparison (intervention vs comparator) | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 644 eyes of 640 participants | 6 weeks | Treatment: BSS with antibiotics (vancomycin 20 mg/mL and gentamicin 8 mg/mL) | Not reported | 0/322 (0%) eyes | Not reported | 0.20 (0.01 to 4.15) | ⊕⊝⊝⊝ | |
| Control: BSS‐only irrigating infusion fluid | Not reported | 2/322 (0.62%) eyes | ||||||
| 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | 0.14 (0.03 to 0.63) | 0.10 (0.01 to 0.78) | ⊕⊕⊕⊕ | |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | 0.21 (0.06 to 0.74) | 0.20 (0.04 to 0.91) | ⊕⊕⊕⊕ | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | 0.72 (0.32 to 1.61) | 0.70 (0.27 to 1.84) | ⊕⊕⊕⊝ | |||
| Control: placebo drops | 14/4054 (0.35%) eyes | 10/4054 (0.25%) eyes | ||||||
| Comparisons of combinations of antibiotics with specific antibiotics | ||||||||
| Study ID | No. eyes and participants | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 6618 eyes of 6618 participants | 1 week | Treatment 1: combined prophylaxis (topical regimen + periocular penicillin at the time of surgery) | 5/3309 (0.15%) eyes | Not reported | 0.33 (0.12 to 0.92) | Not reported | ⊕⊕⊕⊝ | |
| Treatment 2: topical regimen alone (chloramphenicol‐sulfadimidine) | 15/3309 (0.45%) eyes | Not reported | ||||||
| 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | Treatment 1 vs treatment 2: 0.67 (0.11 to 3.99) | Treatment 1 vs treatment 2: 0.50 (0.05 to 5.52) | ⊕⊕⊕⊝ | |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | Treatment 2 vs treatment 3: 0.30 (0.08 to 1.09) | Treatment 2 vs treatment 3: 0.29 (0.06 to 1.37) | ⊕⊕⊕⊝ | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | Treatment 1 vs treatment 3: 0.20 (0.04 to 0.91) | Treatment 1 vs treatment 3: 0.14 (0.02 to 1.16) | ⊕⊕⊕⊕ | |||
| Mode of antibiotic delivery | ||||||||
| Study ID | No. eyes and patients | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 77,015 eyes of 77,015 participants | 1 week | Mode 1: Anterior sub‐Tenon injections (subconjunctival) | 38/39,752 (0.10%) eyes | Not reported | 0.85 (0.55 to 1.32) | Not reported | ⊕⊕⊕⊝ | |
| Mode 2: Posterior sub‐Tenon injections (retrobulbar) | 42/37,263 (0.11%) eyes | Not reported | ||||||
| 108 eyes of 108 participants | 3 weeks | Treatment 1: fixed combination of topical gatifloxacin 0.3% and prednisolone acetate 1% | 0/47 (0%) eyes | Not reported | 0.43 (0.02 to 10.34) | Not reported | ⊕⊝⊝⊝ | |
| Treatment 2: individual instillation of topical gatifloxacin 0.3% and prednisolone acetate 1% | 1/61 (2%) eyes | Not reported | ||||||
| GRADE Working Group grades of evidence | ||||||||
| BSS: balanced salt solution; CI: confidence interval; RR: risk ratio. *Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis. **Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR) 1 Downgraded for imprecision (‐2) as the study did not enroll a sufficient number of participants to detect differences between groups. 2 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants at the time of surgery based on the surgeon's discretion (number excluded not reported). 3 Downgraded for imprecision (‐1) as the confidence interval of the effect estimate between groups was wide. 4 Downgraded for indirectness (‐1) as the study was conducted more than 30 years ago and the techniques for cataract surgery have since changed substantially. 5 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants who did not return for follow‐up (16% of study population). | ||||||||
Background
Description of the condition
Age‐related cataract is a leading cause of reduced vision in both high‐income and low‐income countries (Friedman 2004; Resnikoff 2004). Surgery for cataract involves removal of the opaque lens and replacement with an intraocular lens (IOL). In the few cases where IOL implantation is not possible, contact lenses and glasses are valid options for the correction of the refractive error that results from being aphakic (without a lens). Endophthalmitis is a potentially vision‐threatening complication of cataract surgery. Endophthalmitis is a severe inflammation of the anterior or posterior (or both) chambers of the eye and may be sterile or associated with infection. It most commonly occurs as a complication of cataract surgery, but also may occur following other ocular procedures, trauma to the eye, metastatic systemic infections, and systemic inflammatory disorders.
Epidemiology
Reported endophthalmitis rates vary substantially, with some individual centers reporting no endophthalmitis in a several‐year period (Galvis 2014; Monica 2005), while others report rates as high as 1 in 200 or 300 surgeries (ESCRS 2007; Garcia‐Arumi 2007). One systematic review that included studies from high‐income and low‐income countries indicated a decreasing incidence of endophthalmitis following cataract surgery until the early 1990s, followed by an increase in incidence (Taban 2005a). The pooled estimate for incidence of endophthalmitis was 1.09 per 1000 surgeries from 1963 to 1999 and 2.65 per 1000 surgeries from 2000 to 2003 (Taban 2005a). In addition, an analysis of US Medicare data reported a 40% increase in the adjusted risk of endophthalmitis comparing data from 1998 to 2001 against 1994 to 1997, with annual rates ranging from 1.79 to 2.47 cases per 1000 surgeries (West 2005). An analysis of Medicare fee‐for‐service cataract surgeries reported that rates declined to 1.32 per 1000 surgeries in 2003 and 1.11 per 1000 surgeries in 2004 (Keay 2012). Rates appear to have remained relatively consistent, with two more recent Medicare analyses showing rates of 1.2 per 1000 surgeries (Coleman 2015; Du 2014). Other national‐level data have shown a decline, with Sweden's reported rate dropping from 0.48 per 1000 surgeries in 2002 through 2004 to 0.29 per 1000 surgeries for 2005 through 2010 (Friling 2013), and Iran reports an overall rate of 0.02% (Jabbarvand 2016). Furthermore, India has recently reported a rate of 0.08% among patients not receiving intracameral antibiotics and 0.02% among those receiving intracameral antibiotics (Haripriya 2016).
Presentation and diagnosis
Endophthalmitis usually presents within a few days following cataract surgery, and 80% of cases present within six weeks. Presenting features include decreased visual acuity (VA), pain, swelling and redness of the eyelids, redness of the conjunctiva, haziness of the cornea due to edema, and increased cellularity of fluid in the anterior chamber of the eye with or without hypopyon (pus). Signs of infection and inflammation of the retina and vitreous usually are observed during exam. Although endophthalmitis is a rare infection, it often results in significant long‐term morbidity, even when treated appropriately. Approximately 50% of people do not regain vision of 20/40 or better despite treatment (Gower 2015; Lalwani 2008), and often nearly one‐third have acuity worse than 20/200 following treatment (Gower 2015; Ng 2005; Sheng 2011).
Description of the intervention
Several factors are thought to contribute to the incidence of endophthalmitis following cataract surgery. One primary factor is the type of incision used for surgery (Lundstrom 2007; Taban 2005a; Taban 2005b). In addition, many research studies have focused on the role of antibiotics used prophylactically to target ocular surface flora. Examples of prophylactic measures include preoperative lash‐trimming and irrigation of the lacrimal drainage system with antibiotics, antiseptic preparation of the operative site using agents such as povidone iodine, and preoperative, intraoperative, and postoperative administration of antibiotics. Perioperative antibiotics may be administered through parenteral, topical, or intravitreal routes, using a variety of antibiotics. This review focuses only on perioperative antibiotic use as a prophylactic measure.
How the intervention might work
The vast majority of culture‐proven postoperative endophthalmitis cases are caused by gram‐positive bacteria, with most cases caused by Staphylococcus epidermidis and other coagulase‐negative staphylococci, flora commonly found on the ocular surface (EVSG 1995; Mollan 2007; Ng 2005; Schimel 2013). Other gram‐positive organisms and gram‐negative agents are less frequently associated with acute endophthalmitis; however, some of the less common gram‐negative organisms are associated with the worst visual outcomes. Streptococci are considered the most virulent and have the worst outcomes (Barry 2009; Gower 2015; Miller 2004; Simunovic 2012; Soriano 2006). The conjunctiva and eyelids are the most common sources of infection. The bacteria from these sites are presumably introduced into the anterior chamber through the surgical incision. Endophthalmitis occurs when the intrinsic immune defenses fail to eliminate the virulent bacterial inoculum. To assist the inborn bactericidal processes of the eye, perioperative antibiotics are used to decrease intraocular microbial contamination. Decreasing contamination can be accomplished in several ways, depending on the route of drug administration. Topical antibiotics directly penetrate the ocular surface to enter the aqueous humor of the eye (i.e. the fluid in the anterior chamber). Some antibiotics administered orally achieve intraocular concentrations via systemic delivery, while other antibiotics do not effectively penetrate the eye. Intracameral antibiotic administration is the most direct route of delivery to the site of potential infection.
Perioperative antibiotics eliminate etiologic organisms by either bacteriostatic or bactericidal mechanisms. Bacteriostatic agents arrest the growth and replication of bacteria found on the ocular surface, eyelids, or those already iatrogenically introduced into the aqueous humor. Thus, these drugs limit the spread of infection while the body's immune system eliminates the nonproliferating pathogens. Bactericidal antibiotics kill the bacteria directly, decreasing the total concentration of viable microorganisms. Bactericidal agents are more commonly used in ocular surgery as they can achieve more rapid destruction of invading bacteria. Frequently used perioperative antibiotics with bactericidal properties include fluoroquinolones, vancomycin, aminoglycosides, and cephalosporins.
Why it is important to do this review
Cataract surgery is the most common operative procedure in the aged population. While endophthalmitis is relatively rare, the frequency of the procedure makes the absolute number of cases significant enough to be a public health problem. In 2003 to 2004, nearly 1.6 million cases of cataract surgery were performed annually in the US Medicare fee‐for‐service population alone (Schein 2012), and an estimated 10 million procedures were performed worldwide annually in the 1990s (Foster 2001). Experts estimate that the annual target for cataract surgery should be above 30 million surgeries (Foster 2001). At that rate, and assuming an incidence of one case per 1000 surgeries, 30,000 cases of postcataract surgery endophthalmitis would occur annually, with about 10,000 leading to blindness in the operated eye. Visual recovery following acute postoperative endophthalmitis remains poor across different clinical settings, despite advances in treatment (Lalitha 2005; Miller 2005; Ng 2005; Sheng 2011). The extensive use of surgery to provide better vision for people with cataracts across the world calls for adoption of evidence‐based methods to prevent acute endophthalmitis. This systematic review update aims to identify the current evidence to facilitate the adoption of evidence‐based practices for prophylaxis of acute endophthalmitis after cataract surgery.
Objectives
To evaluate the effects of perioperative antibiotic prophylaxis for endophthalmitis following cataract surgery compared with no prophylaxis or other form of prophylaxis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). We employed no date or language restrictions.
Types of participants
We included trials enrolling adults undergoing cataract surgery with any procedure for lens opacities due to any origin.
Types of interventions
We included trials evaluating preoperative antibiotics, intraoperative (intracameral, subconjunctival, or systemic), or postoperative antibiotic prophylaxis for acute endophthalmitis. Comparisons of interest included:
-
any prophylaxis versus no prophylaxis;
-
preoperative versus postoperative or intraoperative prophylaxis or combinations;
-
specific antibiotics used in included trials;
-
mode of perioperative antibiotic delivery.
We excluded studies that evaluated antiseptic preoperative preparation using agents such as povidone iodine. In addition, excluded studies that evaluated antibiotics for treating acute endophthalmitis after cataract surgery.
We excluded studies with less than one week of follow‐up after surgery.
Types of outcome measures
Primary outcomes
-
Endophthalmitis: both presumed and culture‐proven endophthalmitis within six weeks after cataract surgery. Our primary analysis was based on six‐week outcomes; however, we also evaluated data from weeks one to four.
-
Visual acuity (VA) measured either as a mean logMAR score or as the number of participants with best‐corrected VA better than 20/40 and those worse than 20/200 at the different follow‐up times. Whenever multiple VA measures were available, we used acuity at six weeks after diagnosis as the primary outcome measure.
Secondary outcomes
-
Adverse effects: specific adverse effects of interest were postoperative bacterial keratitis, antibiotic resistance if documented, allergy and anaphylaxis. We also summarized other adverse effects as reported in included trials.
-
Quality of life measures.
-
Economic data
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 12), Ovid MEDLINE, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily (January 1946 to December 2016), Embase (January 1980 to December 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to December 2016),the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We used no date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 6 December 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), the ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We searched for additional studies that cited any included references using the Science Citation Index Expanded database (Web of Science).
Data collection and analysis
Selection of studies
Two review authors independently reviewed the titles and abstracts resulting from the literature searches according to the inclusion criteria. We classified abstracts as 'definitely exclude', 'unsure' or 'definitely include'. We obtained the full‐text for articles in the 'unsure' category and reassessed them for inclusion. A third review author resolved any disagreement between the two review authors. Studies excluded after full‐text review are listed in the Characteristics of excluded studies table along with the reasons for exclusion.
Data extraction and management
We developed data extraction forms to collect data from the included studies. We tested the forms using a few studies prior to extracting data for all included studies. Two review authors independently extracted study characteristics, methods, and outcomes data, and assessed risk of bias for all included studies. The two review authors compared data extraction forms and resolved discrepancies between them by discussion. One review author entered the data into Review Manager 5 (RevMan 2014), and a second review author checked the entered data for accuracy.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risk of bias according to guidelines set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and a third review author resolved any discrepancies. For each domain related to systematic biases, we made judgments of 'low risk of bias', 'unclear risk of bias', or 'high risk of bias' for each included study.
-
Selection bias: adequate sequence generation and allocation concealment. Examples of adequate sequence generation included using computerized randomization or random number lists. Methods such as centralized randomization and sequentially numbered, sealed, opaque envelopes provided adequate allocation concealment.
-
Performance bias: masking of study participants and personnel. For studies in which masking was not done or not possible (e.g. surgeons administering subconjunctival versus retrobulbar injections), we considered whether the person knowing the treatment assignment could have influenced the treatment effects.
-
Detection bias: masking of outcome assessors.
-
Attrition bias: incomplete outcome data. We assessed whether follow‐up rates and reasons for losses to follow‐up were similar in the comparison groups and whether all participants were analyzed in the group to which they were randomized.
-
Reporting bias: selective outcome reporting. Studies that reported results for all study outcomes described in the methods section of the included papers were considered to have low risks of reporting bias.
-
Other sources of bias: other potential sources of bias that were considered included, but were not limited to, funding source, study design, and imbalance in baseline characteristics.
A third review author resolved any disagreement in assessments by the two review authors. In the event of missing or unclear data, we contacted the primary investigators for additional information. We allowed six weeks for a response; failing that, we used the information as available in identified reports.
Measures of treatment effect
For individual studies, we presented dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CI). We did not conduct meta‐analyses as part of this review.
Unit of analysis issues
The unit of analysis was the individual (one eye per participant) in four studies (Christy 1979; Christy 1986; Cunha 2013; ESCRS 2007). In the Sobaci 2003 study, both eyes of 4/640 (less than 1%) participants were included in the analysis; for the remaining 636 participants, only one eye was included.
Dealing with missing data
In the event of missing or unclear data, we contacted the primary investigators for additional information. We allowed six weeks for a response; failing that, we used the information as available in identified reports. We analyzed outcome data using the available data, assuming data were missing at random. We did not perform missing data statistics as the proportion of missing data was low (less than 1% of included participants) in most studies.
Assessment of heterogeneity
We assessed clinical heterogeneity using qualitative information on trial methodology, participant characteristics, interventions compared, routes of administration of prophylactic measures, duration of follow‐up, and losses to follow‐up. We performed no statistical tests for heterogeneity.
Assessment of reporting biases
Typically, funnel plots are used to examine reporting biases when 10 or more studies contribute to a given outcome. In this review with only five included studies and no meta‐analysis, funnel plots were not appropriate.
Data synthesis
Because of the small number and heterogeneity of the included studies, we described data for each study narratively.
Subgroup analysis and investigation of heterogeneity
We performed no subgroup analyses.
Sensitivity analysis
We conducted no sensitivity analyses, given the small number of included studies.
'Summary of findings' table
We prepared a 'Summary of findings' table including relative and absolute effects for the outcome of endophthalmitis for all comparisons. We assessed the certainty of evidence for all outcomes in this review using the GRADE classification system (GRADEpro 2014).
Results
Description of studies
Results of the search
Electronic literature searches as of 25 October 2012 identified 491 potentially relevant titles and abstracts for this review (Gower 2013). After duplicate independent abstract review, 12 records were assessed at the full‐text level, of which four were excluded and eight were included in the review. The eight records reported four studies. A review of references that cited the included studies and the reference lists of included studies identified one additional record that was excluded after full‐text assessment.
An updated search as of December 2016 identified 157 new records (Figure 1). The Cochrane Information Specialist removed 50 duplicate records and we screened the remaining 107 reports. We rejected 101 records after reading the abstracts and obtained the full‐text reports of six references for further assessment. We identified one new study which met the inclusion criteria (Cunha 2013), and one ongoing trial (NCT02770729). We excluded four studies (Carron 2013; Cetinkaya 2015; Li 2015; Pérez‐Canales 2015; see Characteristics of excluded studies table for details).
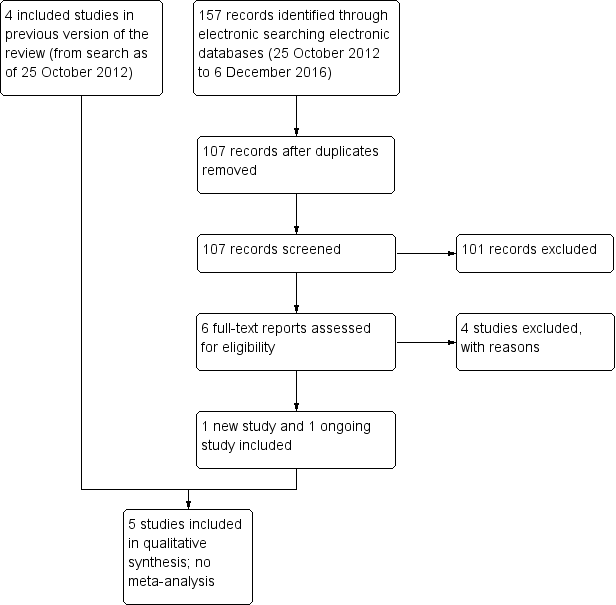
Study flow diagram.
Included studies
We include five RCTs in this review (see Characteristics of included studies table). The studies enrolled 101,005 adults undergoing cataract surgery. The five studies varied widely in the approaches and prophylactic measures examined.
The first two studies were conducted at cataract surgery camps in northern Pakistan where intracapsular cataract extraction was performed, and participants were followed postoperatively for one week (Christy 1979; Christy 1986). Endophthalmitis diagnosis was made based on clinical signs. Although intracapsular cataract extraction is rarely performed in the 21st century, and hence the relevance of these studies for contemporary consideration is reduced, the role of prophylactic antibiotics remains relevant to contemporary practice, and surgical camps remain a mainstay in many low‐income countries. Thus, these studies are described briefly.
Christy 1979 compared combined chloramphenicol‐sulfadimidine drops and periocular injection of 500,000 units of benzyl penicillin with chloramphenicol‐sulfadimidine drops alone in 6618 people/eyes. All participants were provided with a single dose of antibiotic ointment on the day prior to surgery and immediately after surgery; sulfadimidine 5% drops were instilled on subsequent days.
Christy 1986 compared subconjunctival versus retrobulbar injection of antibiotics in 77,015 people/eyes. All participants received five applications of a sulfadimidine‐chloramphenicol solution in the 20 hours before surgery. In both studies, participants were followed for one week after surgery and evaluated for endophthalmitis based on clinical signs.
The three more recent studies employed phacoemulsification.
Sobaci 2003 was conducted in Turkey and compared antibiotics (vancomycin and gentamycin) in balanced salt solution (BSS) irrigating infusion fluid with BSS‐only irrigating infusion fluid in 644 eyes of 640 participants. All were treated with ofloxacin and diclofenac sodium four times on the day prior to surgery. Povidone iodine was utilized for antisepsis at the time of surgery and a solution of ofloxacin, dexamethasone, and indomethacin was given postoperatively. Follow‐up was for six weeks postoperation. Since the incidence of endophthalmitis following cataract surgery is low (the study authors of Sobaci 2003 reported the rate of postoperative endophthalmitis at their institution was 0.109%) and because only 644 eyes were included in the study (with less than one eye expected to be affected), the study lacked sufficient power to detect valid differences between treatments.
ESCRS 2007 conducted at multiple sites throughout Europe and Turkey, implemented a two‐by‐two factorial design to evaluate intracameral cefuroxime injected at the end of surgery and topical levofloxacin given immediately preoperatively (within one hour of surgery) and up to 15 minutes following surgery in 16,603 participants. In a factorial design studying two drugs or procedures that are expected to act independently, treatment arms were allocated such that both drugs could be evaluated alone and in combination. In ESCRS 2007, the two interventions studied were intracameral cefuroxime and topical levofloxacin. One group received only intracameral cefuroxime, one group received only topical levofloxacin, one group received both intracameral cefuroxime and topical levofloxacin, and one group received neither intervention. Povidone iodine was used for antisepsis at the time of surgery and topical levofloxacin was given to all participants starting the morning after surgery. Follow‐up was for six weeks postoperation.
In Cunha 2013, all participants underwent phacoemulsification with IOL implantation. The use of a fixed combination of gatifloxacin 0.3% and prednisolone acetate 1% (i.e. both drugs in a single bottle) was compared with the administration of gatifloxacin 0.3% alone and prednisolone acetate 1% alone. Participants instilled the drops beginning one day before the cataract surgery until 15 days postoperation. Although the study authors reported endophthalmitis as an adverse outcome, the study was not designed to assess differences in endophthalmitis rates between intervention groups. Further, with only 129 enrolled participants, the study did not have sufficient power to detect valid differences between treatment groups.
Excluded studies
We excluded nine studies overall: six were not RCTs and three did not evaluate the risk of endophthalmitis (see Characteristics of excluded studies table).
Risk of bias in included studies
Allocation
Although all the included studies were RCTs, only ESCRS 2007 reported sufficient detail to be judged as having adequate sequence generation and allocation concealment (Figure 2). ESCRS 2007 used computerized randomization for sequence generation and coded droppers to conceal treatment assignments. Cunha 2013 reported using an adequate sequence generation method, but did not report whether the allocation sequence was concealed. We judged the remaining three studies as having unclear sequence generation and allocation concealment. Christy 1986 reported that a deck of cards marked with the treatment assignments was used to randomize participants to treatment groups. The treatment administered to the participant was determined by the card that was on the top of the deck at the time of surgery; however, it was not clear whether the markings on the cards were concealed prior to surgery. Sobaci 2003 reported that participants were randomly allocated to treatment group according to the scheduled day of surgery. However, it is unclear whether the treatment assignment was stratified by the day of surgery, or whether the day's treatment assignment was revealed at the start of the operative day and alternated from day to day. Christy 1979 did not report any information regarding allocation other than that the study was randomized.
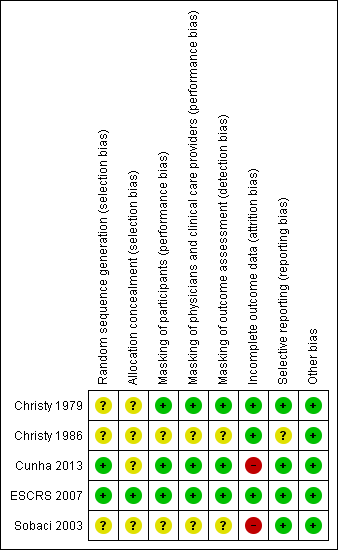
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Masking (performance bias and detection bias)
One study reported masking all study participants and personnel by distributing identical bottles with masked labels (Cunha 2013). ESCRS 2007 masked participants and clinicians by using coded droppers with either active or placebo drops. Participants and physicians were not masked for the injections since no sham injections were performed for those not receiving the intracameral cefuroxime injection. Participants and physicians who were present during the surgery were masked to the drops, and other clinical partners were masked to both drops and injections throughout the study. It was unclear whether the physicians who were present during the surgery or their clinical partners were assessing the outcomes for the study. One other study was reported to be masked, but details of who was masked and how masking was accomplished were not reported (Christy 1979). We assessed these three studies as having low risks of performance and detection bias, since masking was reported and we would not expect the diagnosis of endophthalmitis to be affected if masking was broken. The two remaining included studies did not report masking (Christy 1986; Sobaci 2003).
Incomplete outcome data
Risk of bias due to incomplete outcome data was low in three studies and high in two studies (Figure 2). In Sobaci 2003, judged as having a high risk of bias, eyes of participants for which the surgical procedure was modified according to physician discretion during surgery were excluded from the study. Reasons for modifying the protocol included administering subconjunctival antibiotics and adding a suture. The number of excluded participants was not reported. In Cunha 2013, more than 15% of participants were not included in the analyses, most due to missing the follow‐up visit. Christy 1979 and Christy 1986 reported no exclusions or losses to follow‐up; however, the study authors noted that data were limited to early postoperative infections occurring one week after surgery, since most participants lived too far away for follow‐up visits once discharged. The studies undertook no bacteriologic confirmation of infection. Although ESCRS 2007 reported following intent‐to‐treat analyses, 324 (2%) participants who were lost to follow‐up and 68 (0.4%) participants who did not undergo the planned surgery or withdrew consent (timing of withdrawal not specified) were excluded from the analyses.
Selective reporting
Risk of selective reporting bias in these studies was low. All studies employed commonly used methods for reporting endophthalmitis cases. Two studies reported results for suspected cases without bacteriological confirmation (Christy 1979; Christy 1986). Sobaci 2003 reported results for bacteriologically confirmed cases, and ESCRS 2007 reported results for all suspected cases as well as for bacteriologically confirmed cases. Cunha 2013 did not report how endophthalmitis was diagnosed.
Christy 1986 used two types of antibiotics for injections, determined by the surgeon doing the operation. The study authors reported that infection rates were similar between the two types of antibiotics and the two surgeons, but did not report infection rates for treatment groups (anterior versus posterior injections) separately by type of antibiotic.
Other potential sources of bias
We did not identify other potential sources of bias for the included studies.
Effects of interventions
The results of the five studies are described individually below. Interventions differed between them. Given the heterogeneity of study designs and modes of antibiotic delivery, we decided against conducting meta‐analyses. We describe outcome data and present a summary of postoperative endophthalmitis for all comparisons in summary of findings Table for the main comparison.
The primary outcome for four studies was postoperative endophthalmitis following cataract surgery; the fifth study investigated prophylaxis and control of inflammation following cataract surgery with endophthalmitis reported as an adverse outcome. The two earliest studies relied on clinical diagnosis of endophthalmitis (Christy 1979; Christy 1986). Sobaci 2003 reported results for bacteriologically confirmed cases only, ESCRS 2007 reported results for all suspected cases as well as the subset of bacteriologically confirmed cases, and Cunha 2013 did not report how endophthalmitis was defined.
Perioperative prophylaxis versus no prophylaxis
Irrigation with antibiotics in balanced salt solution versus balanced salt solution alone
In Sobaci 2003, at six weeks 0/322 (0%) eyes that received vancomycin and gentamycin in BSS irrigating infusion fluid had postoperative endophthalmitis compared with 2/322 (0.62%) eyes that received BSS‐only irrigating infusion fluid. The between‐group difference reflected the small number of cases that the study was not powered to detect a difference (RR 0.20, 95% CI 0.01 to 4.15). We assessed the certainty of evidence for this outcome as very low, downgrading for imprecision of the effect estimate and high risk of attrition bias in the study.
Intracameral with or without topical antibiotics versus no antibiotics
In ESCRS 2007, the risk of clinically diagnosed (presumed) postoperative endophthalmitis at six weeks was significantly reduced for eyes that received intracameral cefuroxime injections, with or without topical levofloxacin, compared with no prophylaxis (neither injection nor topical levofloxacin) (RR 0.14, 95% CI 0.03 to 0.63 with topical levofloxacin; RR 0.21, 95% CI 0.06 to 0.74 without topical drops). There were similar results when analyzing culture‐proven cases of postoperative endophthalmitis. We assessed the certainty of evidence for these outcomes as high, finding no reason to downgrade the assessment.
The effect of topical levofloxacin alone compared with no prophylaxis to reduce the risk of postoperative endophthalmitis was less certain (RR 0.72, 95% CI 0.32 to 1.61 for presumed cases; RR 0.70, 95% CI 0.27 to 1.84 for culture‐proven cases). We assessed the certainty of evidence for this outcome as moderate, downgrading for imprecision.
Comparisons of combinations of antibiotics with specific antibiotics
Chloramphenicol‐sulfadimidine drops with versus without periocular penicillin
In the Christy 1979 study of chloramphenicol‐sulfadimidine drops with or without periocular penicillin injection, 5/3309 (0.15%) eyes that received combined prophylaxis (drops and injection at the time of surgery) had postoperative endophthalmitis at one week, compared with 15/3309 (0.45%) eyes that received the topical regimen alone (RR 0.33, 95% CI 0.12 to 0.92). We assessed the certainty of evidence for this outcome as moderate, downgrading for indirectness as the study was conducted in the mid‐to‐late 1970s and the techniques for cataract surgery have since changed substantially.
Intracameral and topical antibiotics versus either antibiotic alone
In ESCRS 2007, a risk reduction was observed for eyes treated with combined intracameral cefuroxime and topical levofloxacin compared with eyes treated with topical levofloxacin alone for presumed cases of postoperative endophthalmitis (RR 0.20, 95% CI 0.04 to 0.91), but this difference was less precise for culture‐proven cases (RR 0.14, 95% CI 0.02 to 1.16). We assessed the certainty of evidence for this outcome as high, finding no reason to downgrade the assessment.
When comparing combined intracameral cefuroxime and topical levofloxacin with eyes treated with intracameral cefuroxime alone, the difference of postoperative endophthalmitis was unclear (RR 0.67, 95% CI 0.11 to 3.99 for presumed cases; RR 0.50, 95% CI 0.05 to 5.52 for proven cases). We assessed the certainty of evidence for this outcome as moderate, downgrading for imprecision.
Additionally, the head‐to‐head comparison of intracameral cefuroxime alone compared with topical levofloxacin alone suggested that intracameral cefuroxime may perform better or as good as topical levofloxacin for preventing postoperative endophthalmitis (RR 0.30, 95% CI 0.08 to 1.09 for presumed cases; RR 0.29, 95% CI 0.06 to 1.37 for proven cases). We assessed the certainty of evidence for this outcome as moderate, downgrading for imprecision.
Mode of antibiotic delivery
Subconjunctival versus retrobulbar antibiotic injection
In Christy 1986, at one week after surgery, 38/39,752 (0.10%) eyes receiving subconjunctival injection had presumed postoperative endophthalmitis, compared with 42/37,263 (0.11%) eyes receiving retrobulbar antibiotic injection. The risk of postoperative endophthalmitis was similar between groups (RR 0.85, 95% CI 0.55 to 1.32). We assessed the certainty of evidence for this outcome as moderate, downgrading for indirectness as the techniques of the cataract surgery used in the study were different compared with current cataract surgery methods.
Fixed combination versus individual instillation of topical antibiotic and corticosteroid
Cunha 2013 compared fixed combination versus individual instillation of gatifloxacin 0.3% and prednisolone acetate 1%. None of 47 eyes that received the fixed combination had postoperative endophthalmitis compared with 1/61 (2%) eyes that received the individual drops up to 20 days postoperation (RR 0.43, 95% CI 0.02 to 10.34). Due to the small number of participants and events in the study, the analysis was not powered to detect a difference between groups. We assessed the certainty of evidence for this outcome as very low, downgrading for imprecision of the effect estimate and high risk of attrition bias in the study.
Visual acuity
Only one study reported VA outcomes for postoperative endophthalmitis (ESCRS 2007). Cunha 2013 reported that mean VA was the same for both groups at baseline (0.3 logMAR) and 20 days postoperation (0.1 logMAR). No other study reported VA outcomes.
ESCRS 2007 presented outcomes in a combined manner for both intracameral cefuroxime injection groups compared to topical levofloxacin or no prophylaxis groups combined (Table 1).
| Comparisons of specific antibiotics or combinations of antibiotics | |||||||||
| Study ID | Groups | Proportion of eyes with final VA > 20/40 following endophthalmitis | RR (95% CI) | Proportion of eyes with final VA < 20/200 following endophthalmitis | RR (95% CI) | ||||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | ||
| Group 1: intracameral cefuroxime injection, with or without topical levofloxacin drops | 2/5 (40%) eyes | 1/3 (33.3%) eyes | 0.69 (0.22 to 2.11) | 0.57 (0.11 to 2.95) | 0/5 (0%) eyes | 0/3 (0%) eyes | 0.46 (0.03 to 7.48) | 0.50 (0.03 to 7.54) | |
| Group 2: no injection, with or without topical levofloxacin drops | 14/24 (58.3%) eyes | 10/17 (58.1%) eyes | 4/24 (16.7%) eyes | 4/17 (23.5%) eyes | |||||
CI: confidence interval; final VA: visual acuity at time of last follow‐up visit (range 3 weeks to 8 months); VA: visual acuity.
*Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis.
**Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR).
Proportion of eyes with final visual acuity greater than 20/40 following endophthalmitis
Among the five presumed cases of postoperative endophthalmitis who received intracameral cefuroxime injections, two (40%) had final VA better than 20/40. Among the 24 presumed cases of postoperative endophthalmitis who did not receive intracameral cefuroxime injections, 14 (58.3%) had final VA better than 20/40. The difference between antibiotic injection and no injection groups was uncertain (RR 0.69, 95% CI 0.22 to 2.11). There were similar results for culture‐proven cases between antibiotic injection (1/3 (33.3%) eyes) and no injection (10/17 (58.1%) eyes) groups (RR 0.57, 95% CI 0.11 to 2.95). We assessed the certainty of evidence for this outcome as moderate, downgrading for imprecision.
Proportion of eyes with final visual acuity less than 20/200 following endophthalmitis
Among the five presumed cases of postoperative endophthalmitis who received intracameral cefuroxime injections, none had final VA worse than 20/200. Among the 24 presumed cases of postoperative endophthalmitis who did not receive intracameral cefuroxime injections, four (16.7%) had final VA worse than 20/200. This difference between injection and no‐injection groups was very imprecise (RR 0.46, 95% CI 0.03 to 7.48). There were similar results for proven cases between the injection (0/3 (0%) eyes) and no injection (4/17 (23.5%) eyes) groups (RR 0.50, 95% CI 0.03 to 7.54). We assessed the certainty of evidence for this outcome as moderate, downgrading for imprecision.
Adverse effects
Cunha 2013 was the only study to report adverse events.
Cunha 2013 did not report information specific to postoperative bacterial keratitis, antibiotic resistance, allergy, or anaphylaxis. At 20 days postoperation, the one eye in the individual drops group with endophthalmitis had pupillary membrane in front of the IOL. Three eyes (6%) in the fixed combination group compared with five eyes (8%) in the individual drops group showed posterior capsule opacity. The study authors reported no cases of hypopyon or IOL pigmentation and no statistically significant difference between group with respect to central or incisional corneal edema.
Quality of life
No study reported outcomes related to quality of life measures.
Economic outcomes
No study reported outcomes related to economic data.
Discussion
Summary of main results
The studies included in this review were too heterogeneous for us to perform a meta‐analysis. The five included studies tested three modes of delivery for antibiotic prophylaxis measures: intraocular injection, topical drops, and antibiotics in the irrigating solution. The two studies that reported statistically significant differences among treatment arms both included antibiotic injection during surgery (one intraocular and the other periocular), and the treatment arms that included ocular injection had the lowest rates of endophthalmitis, ranging from 0.14 to 1.5 endophthalmitis cases per 1000 surgeries (Christy 1979; ESCRS 2007). Within the ESCRS 2007 study, the primary results paper combined the two intracameral injection groups for comparison against placebo and reported a 4.9‐fold increased risk of endophthalmitis when not using intracameral injection, which can be translated to an 80% decrease in endophthalmitis risk when using intracameral injection.
In this review, we calculated RRs and CIs for multiple comparisons within ESCRS 2007 using the data provided in the study reports; we compared both intracameral injection of cefuroxime and topical drops individually to placebo and to the combined regimen. Both the combined prophylaxis and the intracameral injection alone showed a reduced risk of both presumed and culture‐proven endophthalmitis compared with no prophylaxis. Comparison of the combined regimen against topical drops alone showed a reduction in risk for presumed cases, but not for culture‐proven cases only.
Sobaci 2003 compared antibiotics in the irrigating solution against BSS alone, and reported a lower endophthalmitis rate (0/322 eyes versus 2/322 eyes up to six weeks after surgery) in the treatment arm that included antibiotics (RR 0.20, 95% CI 0.01 to 4.15). If the study were adequately powered, this difference would translate to an 80% reduction in endophthalmitis. However, the overall sample size for this study was quite small (644 eyes) for a rare outcome like endophthalmitis, limiting the ability to evaluate statistical significance, as noted by the wide CIs (95% CI 0.01 to 4.15). Similarly, Cunha 2013 enrolled 129 participants, with only 108 participants analyzed at 20 days postoperation, resulting in a high degree of imprecision among outcomes.
Christy 1986 investigated the mode of delivery of antibiotics injected during surgery, and found no significant difference between subconjunctival and retrobulbar injection. The rates of endophthalmitis (1.0 to 1.1 per 1000 surgeries) in that study were comparable to endophthalmitis rates reported in the 21st century, even though surgery was performed in surgical camp settings in a developing country and that intracapsular surgery was performed. However, it is notable that this study only followed participants for one week, so some cases likely were missed.
Overall completeness and applicability of evidence
The five studies included 101,005 adults and 132 total endophthalmitis cases. While the overall sample size was quite large, the heterogeneity of the study settings, designs, and modes of antibiotic delivery made it impossible to combine the studies and make direct comparisons. Two studies were conducted in the late 1970s and early 1980s. Cataract surgery practice has changed substantially since that time, making the results of these studies less applicable today. Povidone iodine is now used routinely in most countries and is a proven measure for reducing intraocular infection (Speaker 1991). In addition, wound construction is quite different. In the 1970s and early 1980s large (180°) incisions were used routinely. Today, even in the most remote centers, much smaller incisions are employed. Small‐incision manual surgery is now the procedure of choice in the majority of surgical camp settings in low‐income countries. Despite these changes in surgical technique, Christy 1979 suggested that adding periocular penicillin injection substantially reduced the risk of endophthalmitis.
Among the five studies, the ESCRS 2007 results are most applicable to 21st century surgical practice, as it used contemporary surgical techniques and study drugs that are readily available in Europe. Its design allowed for examination of both topical and intracameral antibiotics, and included a sample size sufficient to yield statistically significant results. Thus, among the studies reviewed, it provided the firmest evidence upon which to recommend a prophylactic regimen in these settings, and suggested that intracameral antibiotic injection is useful in reducing the risk of postcataract surgery endophthalmitis. However, the choice of antibiotic remains a question for many physicians. Since the publication of ESCRS 2007, uptake of intracameral cefuroxime has varied widely. In the UK, approximately 50% of providers reported intracameral antibiotic use (Gore 2009). A retrospective analysis of billing codes in France suggested that the use of intracameral antibiotics increased from 0.60% to 80% between 2005 and 2014, likely due to the ESCRS recommendations (Creuzot‐Garcher 2016). Uptake has been more limited in the US. Results of the 2011 American Society of Cataract and Refractive Surgery (ASCRS) member survey showed less than 20% of physicians utilizing intracameral antibiotics (Leaming 2012; Vazirani 2013). By the ASCRS 2014 survey, 50% of US physicians reported use of intracameral antibiotics (Chang 2015); however, this percentage remains well below the rates in Europe, Australia and New Zealand (Behndig 2015; Meyer 2016;Schwartz 2016), and US physicians continue to express concerns about the lack of a commercially available preparation. In the US, incorporating intracameral antibiotics into standard prophylaxis practice appears to be related to surgeon volume (Chang 2008), and increased surgeon volume has been reported to be associated with reduced risk of postoperative endophthalmitis (Keay 2012). In both the US and UK, physicians not using intracameral antibiotics cite concerns regarding dilution errors and risk of contamination when compounding the drugs for doses needed for ocular injection (Gore 2009; Leaming 2012). These factors are important to consider when evaluating the applicability of the current evidence. Further discussion on this issue is provided below in the Agreements and disagreements with other studies or reviews section.
Quality of the evidence
The five studies included in this review varied substantially in the prophylaxis measures that they compared and the way data were reported. Two of these studies, Christy 1979 and Christy 1986, were conducted over 30 years ago, when standards for randomization and reporting in clinical trials were less stringent and less well‐defined. To the credit of the Christy team, they provided detailed information on both the operative procedure and the follow‐up procedures. All five studies randomized participants, but random sequence generation and allocation concealment were described fully only in ESCRS 2007. Hence, we cannot judge whether some selection bias may have occurred in the other studies.
Masking of the intervention was not complete in most of these studies. In some, the person in charge of outcome assessment was unaware of the randomization and did not actively participate in the surgery visits. These studies may be less prone to bias than those in which the examiner was present at all times and was aware of the treatment assignment.
Because of differences in the interventions used and outcomes assessed among studies, we performed no meta‐analysis. Overall, one study provided moderate‐ to high‐certainty evidence (ESCRS 2007); two studies, which were downgraded for indirectness, provided moderate‐certainty evidence (Christy 1979; Christy 1986); and two studies, which were downgraded for high risk of attrition bias and imprecision, provided very low‐certainty evidence (Cunha 2013; Sobaci 2003).
Potential biases in the review process
To minimize bias with regard to selecting studies for this review, we devised a highly sensitive search strategy to identify relevant studies from the published literature. We also searched other sources such as the reference lists of included studies, the Science Citation Index, and clinical trial registries. We imposed no date or language restrictions.
Two review authors independently performed major steps in the review process to minimize bias and errors when screening studies for inclusion, recording study characteristics, extracting quantitative data, and assessing risks of bias. The review team included clinicians, researchers, and methodologists.
Agreements and disagreements with other studies or reviews
The rare nature of endophthalmitis makes RCTs difficult to conduct, because of the very large sample sizes needed to make statistically valid comparisons. Thus, few trials have been conducted, and all that we are aware of are included here. Worldwide, numerous single‐center retrospective analyses have been conducted to examine whether changes in practice patterns resulted in reduced endophthalmitis rates. Several studies have reported reduced rates of endophthalmitis following adoption of intracameral or subconjunctival antibiotics (Beselga 2014; Garat 2009; Garcia‐Saenz 2010; Montan 2002; Myneni 2013; Packer 2011; Shorstein 2013). The antibiotic of choice has varied across studies of intracameral injections, with moxifloxacin, vancomycin, and cefuroxime all showing a reduction compared with no antibiotic injection (Packer 2011). Most notably, numerous studies have investigated the benefit of intracameral cefuroxime in light of the ESCRS study and increased availability of the antibiotic in Europe. Data from Sweden have shown extremely low rates of postoperative endophthalmitis (0.029%) in the presence of intracameral cefuroxime (Friling 2013). In Portugal, a center found that endophthalmitis decreased from 0.26% to 0.0% after introduction of the ESCRS protocol and use of intracameral cefuroxime (Beselga 2014). Several other studies also have reported declines in endophthalmitis. Across these studies, the general consensus has been that intracameral antibiotic use reduces the risk of endophthalmitis, which provides further support to the ESCRS 2007 study findings.
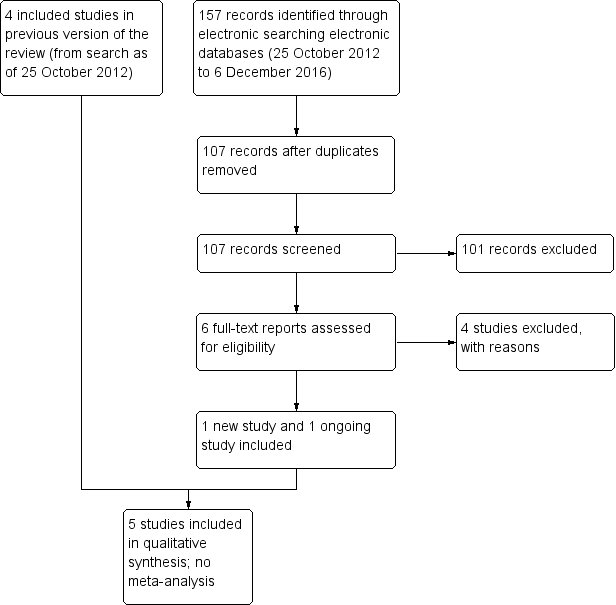
Study flow diagram.
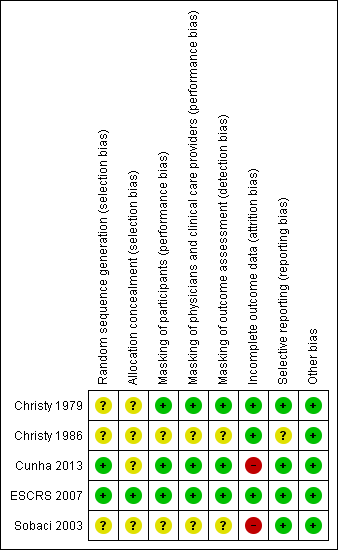
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Perioperative antibiotics for prevention of endophthalmitis after cataract surgery | ||||||||
| Population: participants undergoing cataract surgery Settings: eye hospital or clinic Outcome: risk of endophthalmitis after surgery | ||||||||
| Perioperative prophylaxis versus no prophylaxis | ||||||||
| Study ID | No. eyes and participants | Follow‐up | Comparison (intervention vs comparator) | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 644 eyes of 640 participants | 6 weeks | Treatment: BSS with antibiotics (vancomycin 20 mg/mL and gentamicin 8 mg/mL) | Not reported | 0/322 (0%) eyes | Not reported | 0.20 (0.01 to 4.15) | ⊕⊝⊝⊝ | |
| Control: BSS‐only irrigating infusion fluid | Not reported | 2/322 (0.62%) eyes | ||||||
| 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | 0.14 (0.03 to 0.63) | 0.10 (0.01 to 0.78) | ⊕⊕⊕⊕ | |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | 0.21 (0.06 to 0.74) | 0.20 (0.04 to 0.91) | ⊕⊕⊕⊕ | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | 0.72 (0.32 to 1.61) | 0.70 (0.27 to 1.84) | ⊕⊕⊕⊝ | |||
| Control: placebo drops | 14/4054 (0.35%) eyes | 10/4054 (0.25%) eyes | ||||||
| Comparisons of combinations of antibiotics with specific antibiotics | ||||||||
| Study ID | No. eyes and participants | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 6618 eyes of 6618 participants | 1 week | Treatment 1: combined prophylaxis (topical regimen + periocular penicillin at the time of surgery) | 5/3309 (0.15%) eyes | Not reported | 0.33 (0.12 to 0.92) | Not reported | ⊕⊕⊕⊝ | |
| Treatment 2: topical regimen alone (chloramphenicol‐sulfadimidine) | 15/3309 (0.45%) eyes | Not reported | ||||||
| 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | Treatment 1 vs treatment 2: 0.67 (0.11 to 3.99) | Treatment 1 vs treatment 2: 0.50 (0.05 to 5.52) | ⊕⊕⊕⊝ | |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | Treatment 2 vs treatment 3: 0.30 (0.08 to 1.09) | Treatment 2 vs treatment 3: 0.29 (0.06 to 1.37) | ⊕⊕⊕⊝ | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | Treatment 1 vs treatment 3: 0.20 (0.04 to 0.91) | Treatment 1 vs treatment 3: 0.14 (0.02 to 1.16) | ⊕⊕⊕⊕ | |||
| Mode of antibiotic delivery | ||||||||
| Study ID | No. eyes and patients | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 77,015 eyes of 77,015 participants | 1 week | Mode 1: Anterior sub‐Tenon injections (subconjunctival) | 38/39,752 (0.10%) eyes | Not reported | 0.85 (0.55 to 1.32) | Not reported | ⊕⊕⊕⊝ | |
| Mode 2: Posterior sub‐Tenon injections (retrobulbar) | 42/37,263 (0.11%) eyes | Not reported | ||||||
| 108 eyes of 108 participants | 3 weeks | Treatment 1: fixed combination of topical gatifloxacin 0.3% and prednisolone acetate 1% | 0/47 (0%) eyes | Not reported | 0.43 (0.02 to 10.34) | Not reported | ⊕⊝⊝⊝ | |
| Treatment 2: individual instillation of topical gatifloxacin 0.3% and prednisolone acetate 1% | 1/61 (2%) eyes | Not reported | ||||||
| GRADE Working Group grades of evidence | ||||||||
| BSS: balanced salt solution; CI: confidence interval; RR: risk ratio. *Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis. **Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR) 1 Downgraded for imprecision (‐2) as the study did not enroll a sufficient number of participants to detect differences between groups. 2 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants at the time of surgery based on the surgeon's discretion (number excluded not reported). 3 Downgraded for imprecision (‐1) as the confidence interval of the effect estimate between groups was wide. 4 Downgraded for indirectness (‐1) as the study was conducted more than 30 years ago and the techniques for cataract surgery have since changed substantially. 5 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants who did not return for follow‐up (16% of study population). | ||||||||
| Comparisons of specific antibiotics or combinations of antibiotics | |||||||||
| Study ID | Groups | Proportion of eyes with final VA > 20/40 following endophthalmitis | RR (95% CI) | Proportion of eyes with final VA < 20/200 following endophthalmitis | RR (95% CI) | ||||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | ||
| Group 1: intracameral cefuroxime injection, with or without topical levofloxacin drops | 2/5 (40%) eyes | 1/3 (33.3%) eyes | 0.69 (0.22 to 2.11) | 0.57 (0.11 to 2.95) | 0/5 (0%) eyes | 0/3 (0%) eyes | 0.46 (0.03 to 7.48) | 0.50 (0.03 to 7.54) | |
| Group 2: no injection, with or without topical levofloxacin drops | 14/24 (58.3%) eyes | 10/17 (58.1%) eyes | 4/24 (16.7%) eyes | 4/17 (23.5%) eyes | |||||
| CI: confidence interval; final VA: visual acuity at time of last follow‐up visit (range 3 weeks to 8 months); VA: visual acuity. *Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis. **Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR). | |||||||||

