Fluphenazine (oral) berbanding plasebo untuk skizofrenia
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | ||
| Methods | Allocation: random. Design: parallel. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R or RDC). Consent: written informed consent required. | |
| Interventions | 1. Oral fluphenazine: dose 15 mg/day, N = 18. | |
| Outcomes | Global state (CGI) ‐ not improved or worsened. Unable to use ‐ | |
| Notes | * Data were given only for the first 4 weeks of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random ‐ "a stratified randomization procedure...was used to assign drug treatment to balance study groups on gender, prior social function, and past duration of hospital care" (p300). No details as to randomisation methods. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Low risk | Double blind ‐ no further details. If participants experienced worsening or exacerbation of symptoms, they were removed from the study and treated on an open basis with fluphenazine. Rating scales: raters not stated to be independent of treatment. |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of participants lost to follow‐up or leaving the study early. |
| Selective reporting (reporting bias) | High risk | No scale data reported for BPRS or CGI. |
| Other bias | Unclear risk | Funding: supported in part by NIMH grants (MH‐35996 and MH‐40279). |
| Study characteristics | ||
| Methods | Allocation: random. Design: parallel. | |
| Participants | Diagnosis: chronic schizophrenia. Consent: not stated. | |
| Interventions | 1. Oral fluphenazine: dose 2‐10 mg/day. N = 18. | |
| Outcomes | Global state (using CGI): not improved or worsened; average score (CGI severity of illness*). Mental state: average score (BPRS*). Unable to use ‐ | |
| Notes | Unscheduled dose adjustments were permitted for toxicity or intolerance. *SDs imputed 'between groups' using RevMan calculator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random: "patients were assigned to treatment randomly in blocks of four" (p404) ‐ no further details. |
| Allocation concealment (selection bias) | Unclear risk | Participants were "assigned to treatment randomly in blocks of four" (p404) ‐ no further details. |
| Blinding (performance bias and detection bias) | Low risk | Double blind: "double blind design was maintained throughout the study" (p404) ‐ no further details. Identically‐appearing capsules were dispensed from a bottle labelled only with the participant name. Rating scales: raters not stated to be independent of treatment. |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up: 85% ‐ n = 2 participants left the study early, but their final measures were obtained and used in the analysis (n = 1 receiving placebo due to behavioural deterioration and n = 1 receiving fluphenazine was discharged from the hospital as markedly improved after two weeks). A further n = 11 participants, however, were dropped without final measures being obtained (placebo: n = 1 went AWOL; n = 1 on convalescent leave; n = 1 transferred to another hospital. Thioridazine group: n = 2 AWOL; n = 1 medication intolerance. Chlorpromazine: n = 1 AWOL; n = 2 refused oral medication. Fluphenazine: n = 1 administrative transfer, n = 1 AWOL). Dichotomised data presented as ITT (only n = 1 missing from placebo). LOCF for CGI and BPRS. |
| Selective reporting (reporting bias) | Unclear risk | No SDs reported for all scale data. |
| Other bias | Unclear risk | Funding: supported in part by Public Health Service Grant MH 11666 and Research Scientist Development award No. K135278 from NIMH. Medication supplied by Smith Kline and French Laboratories (chlorpromazine and placebo); Sandoz Inc (thioridazine) and ER Squibb & Sons (fluphenazine). |
| Study characteristics | ||
| Methods | Allocation: random. Design: multi‐centre, parallel. | |
| Participants | Diagnosis: schizophrenia. Consent: not stated. | |
| Interventions | 1. Oral fluphenazine: dose 1‐16 mg/day. N = 92. Additional medication: | |
| Outcomes | Leaving the study early (any reason; treatment failure; serious complication of treatment; marked early remission; incorrect diagnosis; court cases, transfer, eloped). Unable to use ‐ | |
| Notes | *44% of fluphenazine group and 5% of placebo received anti‐parkinsonian drugs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random: participants were "randomly assigned to one of four treatments on a double‐blind basis" (p247). |
| Allocation concealment (selection bias) | Unclear risk | Stratified by sex with "randomized assignment to drug treatment within each sex group" (p247). In the three out of nine hospitals participating in the study that admitted approximately equal number of White and Black participants, this was taken into account and groups were further stratified by race. |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind described, no further details. Participants received individually numbered containers of medication. A flexile dosage schedule permitted the treating physician to adjust dosage according to individuals' needs. Rating scales: raters not stated to be independent of treatment. |
| Incomplete outcome data (attrition bias) | High risk | Follow‐up: 74%. Reasons for removal of study included administrative removals (incorrect diagnoses; intercurrent medical illness; court cases, transfer, elopement, etc), treatment‐related removals (marked early remission; serious complication of treatment; treatment failure). Those lost were not included in the study report analysis. |
| Selective reporting (reporting bias) | High risk | No SDs reported for continuous data. |
| Other bias | Unclear risk | Funding: supported by NIMH grants (MH 04661, 04663, 04667, 04673, 04674, 04675, 04679, 04803). Medications provided free of charge from Sandoz Pharmaceuticals (Hanover); Squibb Institute for Medical Research (New Brunswick); Smith Kline and French Laboratories (Philiadelphia). Rating scales: raters not stated to be independent of treatment. |
| Study characteristics | ||
| Methods | Allocation: unclear. Design: parallel. | |
| Participants | Diagnosis: chronic schizophrenia. Consent: not stated. | |
| Interventions | 1. Oral fluphenazine: dose < 14 mg/day. N = 25. | |
| Outcomes | Global state: MADRS* ‐ not improved or worsened. | |
| Notes | * Rated as either 'no change', 'clear worsening' and 'marked worsening' using the Multidimensional Rating Scale of the Veterans' Administration (Lorr 1953). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation unclear ‐ participants were "divided into three groups of 25 matched on age, chronicity and severity of illness" (p532). |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Low risk | Double blind (implied) ‐ members of the ward staff were told that powerful phenothiazine drugs were to be administered, but were unaware of which participants were receiving the active medication. All medications were identical‐looking tablets, dispensed by a medical officer who "took no part in the rating procedure" (p533). Maximum blindness preserved in evaluations claimed, as neither physicians entered the closed wards between ratings and did not observe side effects during period of treatment. A blind assessment of overall change was made at the end of the trial by the ward sister. Rating scales: raters not stated to be independent of treatment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Follow‐up: 95% ‐ n = 4 participants were lost to follow‐up by 12 weeks of treatment, only n = 2 were accounted for as having discontinued from active drugs (n = 1 from fluphenazine; n = 1 from thioproperazine). ITT used for medium‐term data. |
| Selective reporting (reporting bias) | Unclear risk | None detected. |
| Other bias | Unclear risk | Funding: all drugs and placebos were provided free of charge by May and Baker (Australia) Limited ('Majeptil') and ER Squibb and Sons (Australia) Limited ('Anatensol'). |
| Study characteristics | ||
| Methods | Allocation: randomly assigned. Design: parallel. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). Consent: not stated. | |
| Interventions | 1. Oral fluphenazine hydrochloride: dose 10 mg/day. N = 17. Additional medication ‐ Factored to: | |
| Outcomes | Relapse: defined as number of psychotic exacerbations.* Unable to use ‐ | |
| Notes | * Defined as worsening of four points or more on the sum of the BPRS clusters for thought disturbance and paranoia or increase of three or more points on either cluster. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random ‐ possible participants were stabilised on low dose fluphenazine decanoate (5 to 10 mg every 14 days) for two months and monitored every week using an idiosyncratic prodromal rating scale. Participants were randomised to either oral fluphenazine or placebo when they met criteria for a prodromal episode. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind (implied), as participants who never experienced prodromal episodes nor were assigned to either treatment were treated with 5 mg oral fluphenazine on an open label basis during exacerbations. Rating scales: raters not stated to be independent of treatment. |
| Incomplete outcome data (attrition bias) | High risk | Follow‐up: 81% ‐ n = 6 participants lost to follow‐up by two years of treatment. Only two participants accounted for as being omitted from analysis (n = 1 who dropped‐out during a prodromal episode, and n = 1 who reached the two‐year end point during a prodrome). |
| Selective reporting (reporting bias) | Unclear risk | None detected. |
| Other bias | Unclear risk | Funding: study supported by the Medical Research Service of the Department of Veterans Affairs, Washington DC (grant MH‐41573) from National Institute of Mental Health, Bethesda, Md; UCLA Mental Health Clinical Research Center for Schizophrenia (grant MH‐30911) from the National Institute of Mental Health. |
| Study characteristics | ||
| Methods | Allocation: random. Design: cross‐over (after 3 weeks). | |
| Participants | Diagnosis: chronic schizophrenia. Consent: not stated. | |
| Interventions | 1. Oral fluphenazine: dose 2.5 mg/day. N = 19. | |
| Outcomes | Relapse. Unable to use ‐ Improvement: no better or worse (data not reported by group). Lorr psychiatric rating scale (no SD, mean only). Baker and Thorpe behaviour rating scale (no SD, mean only). | |
| Notes | Participants had been receiving chlorpromazine three times a day and were "mostly stabilised" on a certain dose. They were then given doses of fluphenazine, with this drug substituted for the chlorpromazine at approx. one fortieth of the dose. A single daily dose was given of 2.5 mg (with the exception of two cases who received 20 mg) with dose increase of 2.5 mg/day (one tablet). After participants received fluphenazine for 2 months, they were rated and randomised into intervention groups, receiving dosages established in the stabilisation phase. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random ‐ participants were divided into two random groups; no further details. |
| Allocation concealment (selection bias) | Unclear risk | Only hospital pharmacist knew the composition of the groups. |
| Blinding (performance bias and detection bias) | Low risk | Double blind ‐ only hospital pharmacist knew the composition of the groups; medication was administered with matching placebo. Rating scales: raters not stated to be independent of treatment. |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of participants lost to follow‐up or leaving the study early. |
| Selective reporting (reporting bias) | High risk | Data not clearly described as to the stage of trial and relevant groups (i.e. pre‐cross‐over or post‐cross‐over). Full data not reported for continuous outcomes (missing means and SDs). |
| Other bias | Unclear risk | Funding: fluphenazine tablets were provided by ER Squibb & Sons. |
| Study characteristics | ||
| Methods | Allocation: random. Design: parallel. | |
| Participants | Diagnosis: schizophrenia (remitted). Consent: informed consent obtained. | |
| Interventions | 1. Oral fluphenazine: dose 5‐20 mg/day. N = 28. Additional medication: | |
| Outcomes | Death. Unable to use ‐ | |
| Notes | * Chronic patients > 3 previous hospitalisations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random: "randomly assigned" (p44). 15% of patients referred schizophrenics were re‐diagnosed using Kraepelinian Criteria as non schizophrenic and randomised separately but treated in the same manner. |
| Allocation concealment (selection bias) | Unclear risk | "Randomly assigned" (p44) ‐ no further details. |
| Blinding (performance bias and detection bias) | Low risk | Double blind: drugs were given in a "double blind fashion" (p44). Rating scales: raters not stated to be independent of treatment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Follow‐up: 89%. Drop‐outs mainly due to moving from the geographical area or refusing further care. None of these patient's conditions had deteriorated clinically. |
| Selective reporting (reporting bias) | High risk | Not all adverse effects reported by group. No data reported for individual groups using the BPRS, CGI, PER‐C, KAS scales. |
| Other bias | Unclear risk | Funding: supported by NIMH grant MH 21337‐03. |
RDC ‐ Research Diagnostic Criteria for schizophrenia or schizoaffective disorders
DSM ‐ Diagnostic and Statistical Manual
FD ‐ Fluphenazine Decanoate
FPZ ‐ Oral fluphenazine
RatingScales:
KAS‐ Katz Adjustment Scale
Global state:
CGI ‐ Clinical Global Impression
NOSIE ‐ Nurse's Observation Scale for Inpatient Evaluation
Mental state:
BPRS ‐ Brief Psychiatric Rating Scale
IMPS ‐ Inpatient Multidimensional Psychiatric Scale
MADRS ‐ modified Montgomery‐Asberg Depression Rating Scale
PER‐C ‐ Periodic Evaluation Record‐Community
WBRS ‐ Burdock Ward Behaviour Rating Scale
Other:
CNS ‐ central nervous system
EPS ‐ extrapyramidal symptoms
ITT ‐ intention‐to‐treat
LOCF ‐ last observation carried forward
SD ‐ standard deviation
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: unclear. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: unclear. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: unclear. Participants: newly admitted patients to psychiatric wards, alcoholics, drug addicts, psychosis. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: unclear. | |
| Allocation: unclear. Participants: people with functional psychosis. | |
| Allocation: unclear. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. Participants: people with schizophrenia. Interventions: intensive behavioural skills training versus supportive group psychotherapy. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. Outcomes: no useable data ‐ results not presented for individual groups. |
IM ‐ intramuscular
SD ‐ standard deviation
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||
| 1.1 Global state: 1. Not improved or worsened Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.1  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 1: Global state: 1. Not improved or worsened | ||||||||||||||||||||||||||||||||||||||||||
| 1.1.1 short term (CGI/MDRS) | 3 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.57, 1.12] | ||||||||||||||||||||||||||||||||||||||
| 1.1.2 medium term (MDRS) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.79, 1.58] | ||||||||||||||||||||||||||||||||||||||
| 1.2 Global state: 2. Relapse Show forest plot | 3 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.07, 1.68] | ||||||||||||||||||||||||||||||||||||||
| Analysis 1.2  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 2: Global state: 2. Relapse | ||||||||||||||||||||||||||||||||||||||||||
| 1.2.1 short term | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.06, 1.03] | ||||||||||||||||||||||||||||||||||||||
| 1.2.2 long term | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.05, 3.31] | ||||||||||||||||||||||||||||||||||||||
| 1.3 Global state: 3. Percentage of time in prodrome state (skewed data) Show forest plot | 1 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.3
Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 3: Global state: 3. Percentage of time in prodrome state (skewed data) | ||||||||||||||||||||||||||||||||||||||||||
| 1.3.1 one‐year data | 1 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||
| 1.3.2 two‐year data | 1 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||
| 1.4 Global state: 4. Percentage of time in exacerbated state (skewed data) Show forest plot | 1 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.4
Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 4: Global state: 4. Percentage of time in exacerbated state (skewed data) | ||||||||||||||||||||||||||||||||||||||||||
| 1.4.1 one‐year data | 1 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||
| 1.4.2 two‐year data | 1 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||
| 1.5 Global state: 5. average score: CGI ‐ severity of illness score (high = poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.5  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 5: Global state: 5. average score: CGI ‐ severity of illness score (high = poor) | ||||||||||||||||||||||||||||||||||||||||||
| 1.5.1 short term | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐1.39, ‐0.15] | ||||||||||||||||||||||||||||||||||||||
| 1.6 Leaving the study early: 1. Non‐specific reasons Show forest plot | 5 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.49, 1.10] | ||||||||||||||||||||||||||||||||||||||
| Analysis 1.6  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 6: Leaving the study early: 1. Non‐specific reasons | ||||||||||||||||||||||||||||||||||||||||||
| 1.6.1 short term | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.43, 1.07] | ||||||||||||||||||||||||||||||||||||||
| 1.6.2 medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.00 [0.25, 99.16] | ||||||||||||||||||||||||||||||||||||||
| 1.6.3 long term | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.24, 1.97] | ||||||||||||||||||||||||||||||||||||||
| 1.7 Leaving the study early: 2. Specific reason ‐ short term Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.7  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 7: Leaving the study early: 2. Specific reason ‐ short term | ||||||||||||||||||||||||||||||||||||||||||
| 1.7.1 administrative/hospital transfer | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 15.64] | ||||||||||||||||||||||||||||||||||||||
| 1.7.2 AWOL | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 15.64] | ||||||||||||||||||||||||||||||||||||||
| 1.7.3 court cases, transfer, eloped | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.65 [1.39, 81.58] | ||||||||||||||||||||||||||||||||||||||
| 1.7.4 incorrect diagnosis | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 16.78] | ||||||||||||||||||||||||||||||||||||||
| 1.7.5 marked early remission | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.20, 23.10] | ||||||||||||||||||||||||||||||||||||||
| 1.7.6 serious complication of treatment | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.71 [0.66, 208.85] | ||||||||||||||||||||||||||||||||||||||
| 1.7.7 severe extrapyramidal effects | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 70.30] | ||||||||||||||||||||||||||||||||||||||
| 1.7.8 treatment failure | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.35] | ||||||||||||||||||||||||||||||||||||||
| 1.8 Leaving the study early: 3. Marked improvement/ hospital discharge Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.8  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 8: Leaving the study early: 3. Marked improvement/ hospital discharge | ||||||||||||||||||||||||||||||||||||||||||
| 1.8.1 discharged due to marked improvement | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 69.09] | ||||||||||||||||||||||||||||||||||||||
| 1.9 Adverse effects: 1. Anticholinergic effects ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.9  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 9: Adverse effects: 1. Anticholinergic effects ‐ short term | ||||||||||||||||||||||||||||||||||||||||||
| 1.9.1 blurred vision | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.26 [0.27, 102.66] | ||||||||||||||||||||||||||||||||||||||
| 1.9.2 constipation | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.19, 4.15] | ||||||||||||||||||||||||||||||||||||||
| 1.9.3 drooling | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] | ||||||||||||||||||||||||||||||||||||||
| 1.9.4 dryness mouth or throat | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.62 [1.39, 9.42] | ||||||||||||||||||||||||||||||||||||||
| 1.9.5 gastrointestinal distress and nausea | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.30, 2.72] | ||||||||||||||||||||||||||||||||||||||
| 1.9.6 increased salivation | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.10 [1.06, 309.15] | ||||||||||||||||||||||||||||||||||||||
| 1.9.7 nasal congestion | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] | ||||||||||||||||||||||||||||||||||||||
| 1.9.8 urinary disturbance | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.20 [0.34, 30.17] | ||||||||||||||||||||||||||||||||||||||
| 1.9.9 vomiting | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.32 [0.26, 109.41] | ||||||||||||||||||||||||||||||||||||||
| 1.10 Adverse effects: 2. Cardivascular effects ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.10  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 10: Adverse effects: 2. Cardivascular effects ‐ short term | ||||||||||||||||||||||||||||||||||||||||||
| 1.10.1 dizziness, faintness, weakness | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.85, 6.49] | ||||||||||||||||||||||||||||||||||||||
| 1.10.2 hypotension | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] | ||||||||||||||||||||||||||||||||||||||
| 1.10.3 syncope | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] | ||||||||||||||||||||||||||||||||||||||
| 1.10.4 tachycardia | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] | ||||||||||||||||||||||||||||||||||||||
| 1.11 Adverse effects: 3. CNS ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.11  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 11: Adverse effects: 3. CNS ‐ short term | ||||||||||||||||||||||||||||||||||||||||||
| 1.11.1 anxiety, agitation, excitement and confusion | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.17, 6.72] | ||||||||||||||||||||||||||||||||||||||
| 1.11.2 convulsion or seizures | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.60] | ||||||||||||||||||||||||||||||||||||||
| 1.11.3 depression | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.09] | ||||||||||||||||||||||||||||||||||||||
| 1.11.4 drowsiness | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.91 [1.98, 7.71] | ||||||||||||||||||||||||||||||||||||||
| 1.11.5 headache | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.52, 2.63] | ||||||||||||||||||||||||||||||||||||||
| 1.11.6 sedation and lethargy | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.31, 3.60] | ||||||||||||||||||||||||||||||||||||||
| 1.12 Adverse effects: 4. Death ‐ long term Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.10, 55.72] | ||||||||||||||||||||||||||||||||||||||
| Analysis 1.12  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 12: Adverse effects: 4. Death ‐ long term | ||||||||||||||||||||||||||||||||||||||||||
| 1.13 Adverse effects: 5. Endocrine ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.13  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 13: Adverse effects: 5. Endocrine ‐ short term | ||||||||||||||||||||||||||||||||||||||||||
| 1.13.1 amenorrhea | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.27, 4.14] | ||||||||||||||||||||||||||||||||||||||
| 1.13.2 lactation | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.45 [0.39, 142.32] | ||||||||||||||||||||||||||||||||||||||
| 1.13.3 swelling of breasts | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.32 [0.26, 109.41] | ||||||||||||||||||||||||||||||||||||||
| 1.14 Adverse effects: 6a. Extrapyramidal effects ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.14  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 14: Adverse effects: 6a. Extrapyramidal effects ‐ short term | ||||||||||||||||||||||||||||||||||||||||||
| 1.14.1 akinesia | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] | ||||||||||||||||||||||||||||||||||||||
| 1.14.2 akathisia | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [1.23, 9.56] | ||||||||||||||||||||||||||||||||||||||
| 1.14.3 associated movements | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.37 [0.41, 133.37] | ||||||||||||||||||||||||||||||||||||||
| 1.14.4 dystonia | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.84 [0.79, 242.25] | ||||||||||||||||||||||||||||||||||||||
| 1.14.5 facial rigidity | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.03, 7.46] | ||||||||||||||||||||||||||||||||||||||
| 1.14.6 loss of associated movements | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.39 [1.95, 20.98] | ||||||||||||||||||||||||||||||||||||||
| 1.14.7 restlessness, insomnia | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.40] | ||||||||||||||||||||||||||||||||||||||
| 1.14.8 rigidity | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.54 [1.76, 7.14] | ||||||||||||||||||||||||||||||||||||||
| 1.14.9 tremor | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [1.25, 8.11] | ||||||||||||||||||||||||||||||||||||||
| 1.15 Adverse effects: 6b. Extrapyramidal effects ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.15  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 15: Adverse effects: 6b. Extrapyramidal effects ‐ medium term | ||||||||||||||||||||||||||||||||||||||||||
| 1.15.1 akathisia | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.42, 3.43] | ||||||||||||||||||||||||||||||||||||||
| 1.15.2 akinesia | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.00 [0.64, 188.95] | ||||||||||||||||||||||||||||||||||||||
| 1.15.3 dystonia | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.29] | ||||||||||||||||||||||||||||||||||||||
| 1.15.4 parkinsonism | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.50 [1.36, 22.32] | ||||||||||||||||||||||||||||||||||||||
| 1.16 Adverse effects: 7. Others ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.16  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 16: Adverse effects: 7. Others ‐ short term | ||||||||||||||||||||||||||||||||||||||||||
| 1.16.1 convulsion or seizures | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.60] | ||||||||||||||||||||||||||||||||||||||
| 1.16.2 diarrhoea | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.32 [0.26, 109.41] | ||||||||||||||||||||||||||||||||||||||
| 1.16.3 intercurrent infection | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.22, 5.14] | ||||||||||||||||||||||||||||||||||||||
| 1.16.4 rash | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.15, 3.78] | ||||||||||||||||||||||||||||||||||||||
| 1.17 Sensitivity analysis: 1. CHRONIC versus ACUTE Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.17  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 17: Sensitivity analysis: 1. CHRONIC versus ACUTE | ||||||||||||||||||||||||||||||||||||||||||
| 1.17.1 Acute: Global state ‐ not improved ‐ short term | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.42] | ||||||||||||||||||||||||||||||||||||||
| 1.17.2 Chronic: global state ‐ not improved ‐ short term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.56, 1.55] | ||||||||||||||||||||||||||||||||||||||
| 1.18 Sensitivity analysis: 2. LOW DOSES (1‐5 mg/day) versus HIGH DOSES (5mg/day>) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.18  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 18: Sensitivity analysis: 2. LOW DOSES (1‐5 mg/day) versus HIGH DOSES (5mg/day>) | ||||||||||||||||||||||||||||||||||||||||||
| 1.18.1 High dose: Global state ‐ not improved ‐ short term | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] | ||||||||||||||||||||||||||||||||||||||
| 1.18.2 Flexible dose: Global state ‐ not improved ‐ short term | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.25] | ||||||||||||||||||||||||||||||||||||||
| 1.19 Sensitivity analysis: 3. OPERATIONAL CRITERIA versus LOOSE DEFINITIONS Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.19  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 19: Sensitivity analysis: 3. OPERATIONAL CRITERIA versus LOOSE DEFINITIONS | ||||||||||||||||||||||||||||||||||||||||||
| 1.19.1 DSM‐III‐R: Global state ‐ not improved ‐ short term | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] | ||||||||||||||||||||||||||||||||||||||
| 1.19.2 Loose definition: global state ‐ not improved ‐ short term | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.25] | ||||||||||||||||||||||||||||||||||||||
| 1.20 Sensitivity analysis: 4. BEFORE 1990 versus AFTER 1990 Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||
| Analysis 1.20  Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 20: Sensitivity analysis: 4. BEFORE 1990 versus AFTER 1990 | ||||||||||||||||||||||||||||||||||||||||||
| 1.20.1 Before 1990: Global state ‐ not improved ‐ short term | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.25] | ||||||||||||||||||||||||||||||||||||||
| 1.20.2 After 1990: Global state ‐ not improved ‐ short term | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] | ||||||||||||||||||||||||||||||||||||||
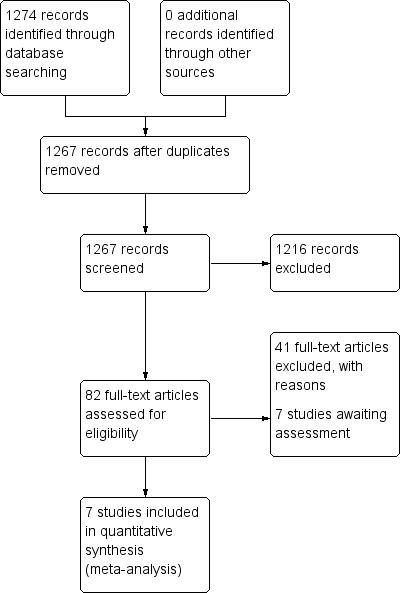
Study flow diagram: 2006 search.

Study flow diagram: 2012 update search (no additional studies).
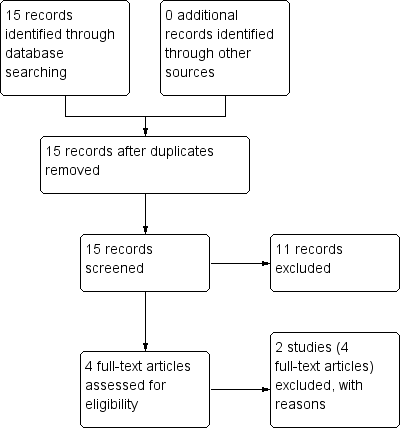
Study flow diagram: economic Cochrane Schizophrenia Group’s Health Economic Database (CSzGHED) search 23 July 2013.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 1: Global state: 1. Not improved or worsened

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 2: Global state: 2. Relapse
| Global state: 3. Percentage of time in prodrome state (skewed data) | ||||
| Study | Intervention | Mean | SD | N |
|---|---|---|---|---|
| one‐year data | ||||
| Marder 1994 | Oral fluphenazine | 10.5 | 15.90 | 17 |
| Placebo | 19.4 | 22.30 | 19 | |
| two‐year data | ||||
| Marder 1994 | Oral fluphenazine | 2.80 | 3.80 | 14 |
| Placebo | 4.90 | 5.70 | 15 | |
Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 3: Global state: 3. Percentage of time in prodrome state (skewed data)
| Global state: 4. Percentage of time in exacerbated state (skewed data) | ||||
| Study | Intervention | Mean | SD | N |
|---|---|---|---|---|
| one‐year data | ||||
| Marder 1994 | Oral fluphenazine | 11.8 | 15.00 | 17 |
| Placebo | 7.20 | 10.70 | 19 | |
| two‐year data | ||||
| Marder 1994 | Oral fluphenazine | 5.50 | 10.40 | 14 |
| Placebo | 12.9 | 13.6 | 15 | |
Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 4: Global state: 4. Percentage of time in exacerbated state (skewed data)

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 5: Global state: 5. average score: CGI ‐ severity of illness score (high = poor)

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 6: Leaving the study early: 1. Non‐specific reasons

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 7: Leaving the study early: 2. Specific reason ‐ short term

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 8: Leaving the study early: 3. Marked improvement/ hospital discharge

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 9: Adverse effects: 1. Anticholinergic effects ‐ short term

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 10: Adverse effects: 2. Cardivascular effects ‐ short term

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 11: Adverse effects: 3. CNS ‐ short term

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 12: Adverse effects: 4. Death ‐ long term

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 13: Adverse effects: 5. Endocrine ‐ short term

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 14: Adverse effects: 6a. Extrapyramidal effects ‐ short term

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 15: Adverse effects: 6b. Extrapyramidal effects ‐ medium term

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 16: Adverse effects: 7. Others ‐ short term

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 17: Sensitivity analysis: 1. CHRONIC versus ACUTE

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 18: Sensitivity analysis: 2. LOW DOSES (1‐5 mg/day) versus HIGH DOSES (5mg/day>)

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 19: Sensitivity analysis: 3. OPERATIONAL CRITERIA versus LOOSE DEFINITIONS

Comparison 1: ORAL FLUPHENAZINE versus PLACEBO, Outcome 20: Sensitivity analysis: 4. BEFORE 1990 versus AFTER 1990
| ORAL FLUPHENAZINE versus PLACEBO for Schizophrenia | ||||||
| Patient or population: patients with Schizophrenia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Control | ORAL FLUPHENAZINE versus PLACEBO | |||||
| Global state: Not improved or worsened ‐ medium term | 680 per 10001 | 762 per 1000 | RR 1.12 | 50 | ⊕⊝⊝⊝ | |
| Global state: Relapse ‐ long term | Low | RR 0.39 | 86 | ⊕⊝⊝⊝ | Note: high degree of heterogeneity between included studies. | |
| 200 per 10001,4 | 78 per 1000 | |||||
| Moderate | ||||||
| 600 per 10001,4 | 234 per 1000 | |||||
| High | ||||||
| 800 per 10001,4 | 312 per 1000 | |||||
| Adverse effects: Death ‐ long term | Low7 | RR 2.38 | 50 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate7 | ||||||
| 30 per 1000 | 71 per 1000 | |||||
| High7 | ||||||
| 90 per 1000 | 214 per 1000 | |||||
| Adverse effects: Extrapyramidal effects (akathisia) ‐ short term | Low8 | RR 3.43 | 227 | ⊕⊕⊕⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate8 | ||||||
| 100 per 1000 | 343 per 1000 | |||||
| High8 | ||||||
| 200 per 1000 | 686 per 1000 | |||||
| Adverse effects: Extrapyramidal effects (rigidity) ‐ short term | Low10 | RR 3.54 | 227 | ⊕⊕⊕⊝ | ||
| 50 per 1000 | 177 per 1000 | |||||
| Moderate10 | ||||||
| 250 per 1000 | 885 per 1000 | |||||
| High10 | ||||||
| 500 per 1000 | 1000 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Mean baseline risk presented for single study. Key: High quality ‐ no downgrading of the evidence. | ||||||
| Study | Country | Participants | Perspective | Type of Economic Evaluation | Resource Use provided | Unit Costs Provided | ICER | QALY/DALY | Net Benefit Ratio | Grading |
|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Status | Reasons for exclusion |
|---|---|---|
| Excluded | Allocation: randomised (current systematic review). | |
| Excluded | Allocation: randomised. Particiapants: schizophrenia. Interventions: fluphenazine IM. |
| Base Case | Cost per day (£) | Cost of actual relapse (£) | Cost of relapse for study population (£)** | ||
|---|---|---|---|---|---|
| Fluphenazine | Placebo | Fluphenazine | Placebo | ||
| Median length1 of stay and mean cost2 | 5,408 | 91,936 | 205,504 | 1,437 | 3,425 |
| 116 days. | |||||
| Sensitivity analyses | Cost per day (£) | Cost of actual relapse (£) | Cost of relapse for study population (£)** | ||
|---|---|---|---|---|---|
| Fluphenazine | Placebo | Fluphenazine | Placebo | ||
| Mean length of stay1 and mean cost3 | 18,049 | 306,833 | 685,862 | 4,794 | 11,431 |
| Mean length of stay1 and mean lower quartile cost4 | 15,966 | 271,422 | 606,708 | 4,241 | 10,112 |
| Mean length of stay1 and mean upper quartile cost5 | 20,078 | 341,326 | 762,964 | 5,333 | 12,716 |
| Median length of stay2 and mean lower quartile cost4 | 4,784 | 81,328 | 181,792 | 1,271 | 3,030 |
| Median length of stay2 and mean upper quartile cost5 | 6,016 | 102,272 | 228,608 | 1,598 | 3,810 |
| 153.4 days | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Global state: 1. Not improved or worsened Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 short term (CGI/MDRS) | 3 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.57, 1.12] |
| 1.1.2 medium term (MDRS) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.79, 1.58] |
| 1.2 Global state: 2. Relapse Show forest plot | 3 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.07, 1.68] |
| 1.2.1 short term | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.06, 1.03] |
| 1.2.2 long term | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.05, 3.31] |
| 1.3 Global state: 3. Percentage of time in prodrome state (skewed data) Show forest plot | 1 | Other data | No numeric data | |
| 1.3.1 one‐year data | 1 | Other data | No numeric data | |
| 1.3.2 two‐year data | 1 | Other data | No numeric data | |
| 1.4 Global state: 4. Percentage of time in exacerbated state (skewed data) Show forest plot | 1 | Other data | No numeric data | |
| 1.4.1 one‐year data | 1 | Other data | No numeric data | |
| 1.4.2 two‐year data | 1 | Other data | No numeric data | |
| 1.5 Global state: 5. average score: CGI ‐ severity of illness score (high = poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 short term | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐1.39, ‐0.15] |
| 1.6 Leaving the study early: 1. Non‐specific reasons Show forest plot | 5 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.49, 1.10] |
| 1.6.1 short term | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.43, 1.07] |
| 1.6.2 medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.00 [0.25, 99.16] |
| 1.6.3 long term | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.24, 1.97] |
| 1.7 Leaving the study early: 2. Specific reason ‐ short term Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 administrative/hospital transfer | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 15.64] |
| 1.7.2 AWOL | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 15.64] |
| 1.7.3 court cases, transfer, eloped | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.65 [1.39, 81.58] |
| 1.7.4 incorrect diagnosis | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 16.78] |
| 1.7.5 marked early remission | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.20, 23.10] |
| 1.7.6 serious complication of treatment | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.71 [0.66, 208.85] |
| 1.7.7 severe extrapyramidal effects | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 70.30] |
| 1.7.8 treatment failure | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.35] |
| 1.8 Leaving the study early: 3. Marked improvement/ hospital discharge Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 discharged due to marked improvement | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 69.09] |
| 1.9 Adverse effects: 1. Anticholinergic effects ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 blurred vision | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.26 [0.27, 102.66] |
| 1.9.2 constipation | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.19, 4.15] |
| 1.9.3 drooling | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] |
| 1.9.4 dryness mouth or throat | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.62 [1.39, 9.42] |
| 1.9.5 gastrointestinal distress and nausea | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.30, 2.72] |
| 1.9.6 increased salivation | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.10 [1.06, 309.15] |
| 1.9.7 nasal congestion | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] |
| 1.9.8 urinary disturbance | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.20 [0.34, 30.17] |
| 1.9.9 vomiting | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.32 [0.26, 109.41] |
| 1.10 Adverse effects: 2. Cardivascular effects ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.10.1 dizziness, faintness, weakness | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.85, 6.49] |
| 1.10.2 hypotension | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] |
| 1.10.3 syncope | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] |
| 1.10.4 tachycardia | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] |
| 1.11 Adverse effects: 3. CNS ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.11.1 anxiety, agitation, excitement and confusion | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.17, 6.72] |
| 1.11.2 convulsion or seizures | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.60] |
| 1.11.3 depression | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.09] |
| 1.11.4 drowsiness | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.91 [1.98, 7.71] |
| 1.11.5 headache | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.52, 2.63] |
| 1.11.6 sedation and lethargy | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.31, 3.60] |
| 1.12 Adverse effects: 4. Death ‐ long term Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.10, 55.72] |
| 1.13 Adverse effects: 5. Endocrine ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.13.1 amenorrhea | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.27, 4.14] |
| 1.13.2 lactation | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.45 [0.39, 142.32] |
| 1.13.3 swelling of breasts | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.32 [0.26, 109.41] |
| 1.14 Adverse effects: 6a. Extrapyramidal effects ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.14.1 akinesia | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] |
| 1.14.2 akathisia | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [1.23, 9.56] |
| 1.14.3 associated movements | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.37 [0.41, 133.37] |
| 1.14.4 dystonia | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.84 [0.79, 242.25] |
| 1.14.5 facial rigidity | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.03, 7.46] |
| 1.14.6 loss of associated movements | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.39 [1.95, 20.98] |
| 1.14.7 restlessness, insomnia | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.40] |
| 1.14.8 rigidity | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.54 [1.76, 7.14] |
| 1.14.9 tremor | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [1.25, 8.11] |
| 1.15 Adverse effects: 6b. Extrapyramidal effects ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.15.1 akathisia | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.42, 3.43] |
| 1.15.2 akinesia | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.00 [0.64, 188.95] |
| 1.15.3 dystonia | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.29] |
| 1.15.4 parkinsonism | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.50 [1.36, 22.32] |
| 1.16 Adverse effects: 7. Others ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.16.1 convulsion or seizures | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.60] |
| 1.16.2 diarrhoea | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.32 [0.26, 109.41] |
| 1.16.3 intercurrent infection | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.22, 5.14] |
| 1.16.4 rash | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.15, 3.78] |
| 1.17 Sensitivity analysis: 1. CHRONIC versus ACUTE Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.17.1 Acute: Global state ‐ not improved ‐ short term | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.42] |
| 1.17.2 Chronic: global state ‐ not improved ‐ short term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.56, 1.55] |
| 1.18 Sensitivity analysis: 2. LOW DOSES (1‐5 mg/day) versus HIGH DOSES (5mg/day>) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.18.1 High dose: Global state ‐ not improved ‐ short term | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |
| 1.18.2 Flexible dose: Global state ‐ not improved ‐ short term | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.25] |
| 1.19 Sensitivity analysis: 3. OPERATIONAL CRITERIA versus LOOSE DEFINITIONS Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.19.1 DSM‐III‐R: Global state ‐ not improved ‐ short term | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |
| 1.19.2 Loose definition: global state ‐ not improved ‐ short term | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.25] |
| 1.20 Sensitivity analysis: 4. BEFORE 1990 versus AFTER 1990 Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.20.1 Before 1990: Global state ‐ not improved ‐ short term | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.25] |
| 1.20.2 After 1990: Global state ‐ not improved ‐ short term | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |

