Mu‐опиоидные антагонисты при опиоид‐индуцированной дисфункции кишечника у людей с раком и людей, получающих паллиативную помощь
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, parallel, controlled, multi‐centre trial. International with sites in Australia, Czech Republic, France, Germany, Hungary, Israel, the Netherlands, Poland, and the UK | |
| Participants | Aim: to investigate whether OXN PR can improve constipation and maintain analgesia compared with OXY PR tablets, in people with cancer. Inclusion criteria: people with chronic moderate/severe cancer pain and requiring 24‐h opioid therapy Exclusion criteria: clinically unstable disease or significant cardiovascular, renal, hepatic, or psychiatric disease; clinically significant gastrointestinal disease or significant structural abnormalities of the gastrointestinal tract; cyclic chemotherapy within 2 weeks before screening visit or planned during the core trial (shown in the past to influence bowel function); radiotherapy that would influence bowel function or pain during the double‐blind phase Participants: in the intervention arm; mean age 61 years and 48/92 men. In comparison arm; mean age 64 years and 46 men and 46 women. The most common primary cancer sites were breast (19%), lung (13%), and prostate (10%). 26% had bone metastases. At the start of the trial, 183/184 (99.5%) participants had constipation‐induced or worsened by their opioid medication. A similar number were also taking laxatives. All were outpatients. | |
| Interventions | Intervention: OXN PR up to 120 mg/day, n = 92 Comparison: OXY PR up to 120 mg/day, n = 92 Duration: 4 weeks | |
| Outcomes | Primary outcomes: symptoms of constipation as measured by Bowel Function Index, efficacy for management of chronic cancer pain as measured by the Brief Pain Inventory‐Short Form Secondary outcomes: use of rescue medication, quality of life, and safety Outcomes measured: at 4 weeks | |
| Notes | Funding: Mundipharma GmbH Trial registration: NCT00513656/OXN2001 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to treatments (1:1 allocation ratio) using a pseudo‐random number generator in a computer program." |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation schedule prepared by the Clinical Supplies Department of the Sponsor or an associated company." |
| Blinding (performance bias and detection bias) | Unclear risk | Stated double‐blind, no further details provided |
| Incomplete outcome data (attrition bias) | Low risk | 133/184 completed the trial. Less than a third in each group dropped out. Similar proportion dropped out in each group |
| Selective reporting (reporting bias) | Unclear risk | No details provided |
| Sample size | Unclear risk | 50‐199 participants per treatment arm |
| Methods | Randomised, controlled, parallel, multi‐centred trial in the USA | |
| Participants | Aim: to determine the efficacy and safety of fixed‐dose subcutaneous methylnaltrexone in people with advanced illness and opioid‐induced constipation in a variety of healthcare situations (inpatient, outpatient, home, hospice, and long‐term care facilities). Inclusion criteria: participants aged > 18 years with advanced illness and a life expectancy of ≥ 1 month and opioid‐induced constipation (< 3 BM in the last week and no BM in 24 h or 48 h) and who were receiving stable doses of laxatives and opioids Exclusion criteria: people with a disease process suggestive of gastrointestinal obstruction or clinically significant active diverticular disease, fecal impaction, peritonitis, bowel surgery 10 days before dosing, or fecal ostomy, or with a bodyweight < 38 kg Participants: 118 men and 112 women. Mean age in intervention arm 65.3 years (SD 12.9) and in placebo arm 65.7 years (SD 13.0). 216/230 of white race. Primary diagnosis cancer in 66% of participants (152/230). The majority (58/78) of the other participants had pulmonary, cardiovascular, or neurological disease | |
| Interventions | Intervention: subcutaneous methylnaltrexone 8 mg (bodyweight of 38 kg to < 62 kg) or 12 mg (bodyweight > 62 kg), n = 116 Comparison: placebo, n = 114 Duration: both were administered every other day over 2 weeks | |
| Outcomes | Primary outcome: percentage of participants with RFBM within 4 h after at the most 2 of the doses in the first week of treatment Secondary outcomes: % with the first RFBM within 4 h after the first dose, number of BMs within 24 h after dosing per week Outcomes measured: over 2 weeks | |
| Notes | Funding: technical editorial and medical writing assistance from Salix Pharmaceuticals Limited Trial registration: NCT00672477 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned." No other details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly assigned." No other details |
| Blinding (performance bias and detection bias) | Unclear risk | Stated double blind, no further details provided |
| Incomplete outcome data (attrition bias) | Low risk | 27/116 in the intervention group and 20/114 in placebo were lost to follow‐up. Reason for loss were similar in both trial arms. |
| Selective reporting (reporting bias) | Unclear risk | No details |
| Sample size | Unclear risk | 50‐199 participants per treatment arm |
| Methods | Randomised, controlled, parallel trial unclear what country participants were from | |
| Participants | Aim: to evaluate the tolerability and efficacy of OXN PR doses up to oxycodone/naloxone 160 mg/80 mg compared with OXY PR formulation. Inclusion criteria: adults with cancer and non‐cancer pain requiring opioids on a stable dose of OXY PR for ≥ 4 consecutive days prior to randomisation and have a pain score of ≤ 4 with ≤ 2 doses of OXY PR analgesic rescue medication per day for either the last 3 consecutive days or 4 of the last 7 days. Constipation caused or aggravated by opioids was confirmed by the participant and the investigator and evidenced by a medical need of regular laxatives to have ≥ 3 bowel evacuations per week or by having < 3 bowel evacuations when not taking a laxative Exclusion criteria: included hypersensitivity to oxycodone, naloxone; active alcohol or drug abuse or history of opioid abuse (or both); unreported illicit drug use (including cannabis); any condition in which opioids were contraindicated or if they had diarrhoea Participants: 100 men and 143 women randomised, of which a subsample, 46, were people with cancer pain. Mean age in whole sample 57.9 years (SD 11.03) in OXN PR arm and 57.5 years (SD 12.33) in OXY PR arm. Subsample demographics on people with cancer not provided | |
| Interventions | Intervention: starting dose during the double‐blind phase dependent on the effective, stable analgesic dose established in the run‐in period, titration up to maximum daily dose of OXN PR 160 mg/80 mg was permitted after 1 week Comparison: OXY PR equivalent dosage to participants in the intervention arm Duration: up to 5 weeks | |
| Outcomes | Primary outcomes: change in mean bowel function scores, pain scores Secondary outcomes: analgesic and laxative rescue medication, complete SBMs, and quality of life (EuroQol EQ‐5D‐3L) Outcomes measured: 1, 2, 4, and 5 weeks | |
| Notes | Funding: Mundipharma GmbH Trial registration: NCT01438567 Study comprised of 3 phases: prerandomisation phase consisting of a screening period and a run‐in period, a double‐blind phase, and an extension phase. In the run‐in phase, OXY PR was titrated to analgesic effect to determine the starting dose to be used after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned." No other details |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, participant blinded, no other details on who else was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | 16/120 men and 18/123 women in whole sample dropped out per arm. Number who dropped in the subset of 46 people with cancer not reported |
| Selective reporting (reporting bias) | Low risk | Bias was unlikely as the trial listed in clinical trial registry reported same primary and secondary outcomes that were presented in the paper |
| Sample size | High risk | < 50 participants per treatment arm in subsample of people with cancer |
| Methods | Randomised, controlled, parallel, multi‐centred trial in Korea and Japan | |
| Participants | Aim: to evaluate the dose, efficacy, and safety of naldemedine for the treatment of opioid‐induced constipation in people with cancer in Japan and Korea. Inclusion criteria: adults aged ≥ 18 years with cancer pain, stable regimen of opioid for > 2 weeks, complicated with opioid‐induced constipation despite regular laxative use Exclusion criteria: constipation potentially attributable to causes other than opioid analgesics Participants: 134 men and 93 women entered trial. Mean age by trial arm: naldemedine 0.1 mg daily: 65.8 years (SD 11.5), naldemedine 0.2 mg daily: 63.4 years (SD 10.4), naldemedine 0.4 mg daily: 64.2 years (SD 10.7); placebo: 64.2 (SD 9.6). Most participants had lung cancer, other cancers included breast and colorectal. All as graded by the ECOG Performance Status were ambulatory. Care setting not stated | |
| Interventions | Intervention 1: naldemedine 0.1 mg daily, n = 56 Intervention 2: naldemedine 0.2 mg daily, n = 58 Intervention 3: naldemedine 0.4 mg daily, n = 56 Comparison: placebo, n = 57 Duration: all administered daily for 2 weeks | |
| Outcomes | Primary outcome: change from baseline in the frequency of SBM per week Secondary outcomes: SBM responder rate, change from baseline in frequency of complete SBM, change from baseline in frequency of SBM without straining, adverse events, and opiate withdrawal Outcomes measured: over 2 weeks | |
| Notes | Funding: Shionogi and Co Ltd Trial registration: JapicCTI‐111510 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Achieved "using the dynamic allocation procedure of the registration center, where the maximum intergroup difference in the participant number at each study site did not exceed two." |
| Allocation concealment (selection bias) | Unclear risk | Probably occurred as allocation provided remotely but not stated specifically |
| Blinding (performance bias and detection bias) | Low risk | All study team members and participants were blinded to treatment. |
| Incomplete outcome data (attrition bias) | Low risk | 2 participants, 1/57 in placebo group and 1/56 in naldemedine 0.1 mg were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No details |
| Sample size | Unclear risk | 50‐199 participants per treatment arm |
| Methods | Randomised, controlled, multi‐centre, parallel‐group trial in the USA | |
| Participants | Aim: to assess the efficacy and safety of subcutaneous methylnaltrexone in a population of people with advanced illness and opioid‐induced constipation, and to clarify whether there was a dose‐response relationship for the purpose of dose selection in further clinical evaluations. Inclusion criteria: advanced disease (defined as terminal or end‐stage, such as advanced metastatic cancer and AIDS but with a life expectancy of ≥ 4 weeks and stable vital signs) for which they were receiving palliative care and were receiving any opioid drug on a daily basis at a dose that had been stable for ≥ 2 weeks and were expected to remain stable for an additional ≥ 4 weeks, and despite no or conventional laxative therapy they had no BMs for 2 days and reported ongoing constipation, defined as > 2 days with no BM and a score of ≥ 3 on a 5‐point scale assessing constipation‐related distress Exclusion criteria: fever or otherwise unstable vital signs; liver function test 3 times the upper limit of normal, serum creatinine level 2 times the upper limit, or a platelet count < 50,000/mm3; new regimen or dose change of concurrent gastrointestinal motility‐altering medications during 3 weeks prior to trial enrolment; history of gastrointestinal obstruction or other condition that could compromise drug action; diagnosis of active peritoneal cancer; history of peritoneal catheter placement for chemotherapy or dialysis; known hypersensitivity to methylnaltrexone, naltrexone, or naloxone; or if any investigational drug or experimental product had been administered within the previous 30 days Participants: 15 men and 18 women. Mean age 61 years (SD 19.0) (range 20‐87 years). 79% were white people. Primary diagnoses at baseline were 28/33 cancer, 3 sickle cell disease, and 2 AIDS. 88% of participants were receiving a laxative at baseline. The mean opioid (morphine equivalent) dose at baseline was 289.9 mg/day (SD 308.0), median 180 mg/day, range 9‐1207 mg/day. Mean number of BMs per week was 1.9. Care setting not stated | |
| Interventions | Intervention 1: subcutaneous methylnaltrexone 1 mg, n = 10 Intervention 2: subcutaneous methylnaltrexone 5 mg, n = 7 Intervention 3: subcutaneous methylnaltrexone 12.5 mg, n = 10 The initial dose range of 1 mg, 5 mg, or 12.5 mg was extended by adding a 20 mg group (n = 6) during the trial while still maintaining the double‐blind. Duration: 3 doses over 1 week | |
| Outcomes | Primary outcomes: laxative response (BM) within 4 h of the initial dose. Secondary outcomes: laxation within 4 h of subsequent doses, during the 24‐h period after each dose, time to laxation, use of rescue laxatives, subjective outcomes of constipation‐associated symptoms, pain intensity, symptoms potentially due to opioid withdrawal or adverse events, and participant satisfaction Outcomes measured: up to 24 h per dose, and 30 days after last dose | |
| Notes | Funding: Progenics Pharmaceuticals Trial registration: none provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After providing consent, patients were initially randomised in a ratio of 1:1:1 to receive 1 mg, 5 mg, or 12.5 mg of methylnaltrexone." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, participant blinded, no other details on who was blinded |
| Incomplete outcome data (attrition bias) | Low risk | 22/33 completed trial. 7 discontinued "at patient request", three from the 12.5 mg arm and one each from the 1 mg and 5 mg arm and two from 20 mg arm. One in the 20mg arm discontinued because of "intolerable" adverse event |
| Selective reporting (reporting bias) | Unclear risk | No details provided |
| Sample size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, controlled, parallel‐group, multi‐centre controlled trial | |
| Participants | Aim: to assess the safety and efficacy of a single subcutaneous injection of methylnaltrexone (0.15 mg/kg or 0.3 mg/kg) versus placebo. Inclusion criteria: aged > 18 years, advanced illness (such as incurable cancer or end‐stage AIDS and life expectancy 1‐6 months) and opioid‐induced constipation. On a stable opioid regimen for the control of pain/discomfort for ≥ 3 days before randomisation, had a stable scheduled laxative regimen for ≥ 3 days prior to treatment, no clinically significant laxation within 48 h prior to the first trial drug dose, had stable vital signs, and not pregnant and using an effective method of birth control. Baseline laxative regimens taken at time of trial entry could be continued throughout the trial. Rescue laxatives, defined as laxatives administered on an as needed basis were allowed but not within 4 h before or after administration of the double‐blind dose. Exclusion criteria: previous treatment with methylnaltrexone, naltrexone, or naloxone; recent participation in any other studies involving investigational products; any disease process suggestive of gastrointestinal obstruction; any potential non‐opioid cause of bowel dysfunction; history of current peritoneal catheter for intraperitoneal administration, chemotherapy administration, or dialysis; clinically active diverticular disease; evidence of faecal impaction; surgically acute abdomen; faecal ostomy; pregnancy; or breastfeeding Participants: 84 American men and 70 American women at 17 hospice and other palliative care settings. Mean age 65.3 years (SD 14.96). Primary diagnosis cancer (125/154), cardiovascular disease (8), HIV/AIDS (1), and other (20). Apart from 8 participants, all had some level of constipation distress. 95% were using a laxative. Oral morphine equivalents, median mg/day 186.5, range 8‐12,2560 mg/day | |
| Interventions | Intervention 1: single subcutaneous injection methylnaltrexone 0.15 mg/kg, n = 47 Intervention 2: single subcutaneous injection methylnaltrexone 0.3 mg/kg, n = 55 Comparison: placebo, n = 52 Duration: 1‐week double‐blind phase, followed by 28‐day open phase | |
| Outcomes | Primary outcome: proportion of participants with rescue‐free laxation (a significant BM) within 4 h after administration of the double‐blind dose. Participants needing rescue laxative or disimpaction within 4 h of dosing were considered non‐responders. Secondary outcomes: proportion of participants with rescue‐free laxation within 24 h postdosing; improvement in GCIC scale (defined as a rating of slightly better, somewhat better, or much better); improvement in constipation distress (defined as a change by at least 1 category toward none); improvement in stool consistency; changes in baseline pain, symptoms/signs of central opioid withdrawal, and adverse events Outcomes measured: to 6 days following first dose | |
| Notes | Funding: Progenics Pharmaceuticals Trial registration: 301/NCT00401362 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned in blocks of three to the three treatment groups in a 1:1:1 ratio. Computer‐generated randomisation scheme performed by a statistician external to the sponsor." |
| Allocation concealment (selection bias) | Low risk | Quote: "computer‐generated randomisation scheme performed by a statistician external to the sponsor." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "...syringe contents were blinded to patients and staff administering injections." "each syringe had identical volume." |
| Incomplete outcome data (attrition bias) | Low risk | 152/154 completed trial (1 died and 1 was non‐compliant both in trial arm of higher dose of methylnaltrexone) Analysis on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Unclear risk | No details |
| Sample size | High risk | < 50 participants in 1 of the 2 treatment arms Although this risk was not relevant to some of our analysis. This is when we combined the trials 2 treatment groups in our exploration of the impact of mu‐opioid antagonists in comparison with placebo. |
| Methods | Randomised, controlled, single‐centre, cross‐over trial | |
| Participants | Aim: to assess in a dose‐ranging trial the use of oral naloxone in opioid‐related constipation in participants with advanced cancer Inclusion criteria: participants with advanced cancer receiving either morphine or diamorphine analgesia orally. All required laxatives prior to trial and their use was continued during the trial except for lactulose Exclusion criteria: fecal stomas or history of constipation prior to using opioid analgesia Participants: 13 men and 14 women patients in a UK hospice. Mean age 64 years, median 65 years, range 44‐88 years. 9 participants had breast cancer; 5 bronchus; 3 prostate; 2 oesophagus, and 1 each of rectum, kidney, bladder, stomach, colon, fallopian tube, malignant melanoma, and fibrosarcoma); 3 participants had liver metastases, 2 had hepatomegaly; no participant had constipation prior to using opioid analgesia | |
| Interventions | Morphine or diamorphine oral (maintenance dose) Intervention: naloxone oral every 4‐h for total daily dose of 0.5%, 1%, 2%, 5%, 10%, or 20% of total daily dose of morphine. The participants received "one level" (a lower level) of naloxone. Then after 2 participants at 0.5% to 5% had received the drug without slowing bowel transit time the dose was increased. In higher doses, the increase was following no slowing effect in 4 participants, n = 17 Comparison: placebo: chloroform water, n = 17 Duration: 2 days each treatment arm (parallel washout) | |
| Outcomes | Outcomes: small bowel transit time by lactulose/hydrogen breath test; pain by 4‐point scale (0 = no pain, 3 = severe pain) | |
| Notes | Funding: charities, Cancer Relief Macmillan Fund, and the Wolfson Foundation. Naloxone was donated by MacFarlan Smith (pharmaceutical company). Trial registration: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised but no further details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) | Unclear risk | Stated double blind but no further details provided |
| Incomplete outcome data (attrition bias) | Low risk | Data analysis of 12 participants were reported. Of the 5 not included, 1 declined. 4 were withdrawn, 2 because of diarrhoea (1 occurred while on placebo, 1 caused by the lactulose taken as part of the small bowel transit time test), 1 was withdrawn because of general deterioration, and 1 because of nausea which the trialists felt was not related to the intervention). |
| Selective reporting (reporting bias) | Unclear risk | No details. The study does not declare a primary outcome |
| Sample size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, controlled, multi‐centre, parallel trial | |
| Participants | Aim: to assess the safety and efficacy of subcutaneous methylnaltrexone for treating opioid‐induced constipation in participants with advanced illness. Inclusion criteria: participants who had a terminal illness with a life expectancy > 1 month, were receiving stable doses of opioids for analgesia and had opioid‐induced constipation (defined as ≤ 3 laxations in the previous week or no laxation in the previous 48 h) despite having taken laxatives for ≥ 3 days. Participants could continue their baseline laxative regimen throughout the trial and take rescue laxatives as needed, though not within 4 h before or after receiving a dose of the trial drug. Exclusion criteria: participants whose constipation was not primarily caused by opioids, mechanical gastrointestinal obstruction, an indwelling peritoneal catheter, clinically active diverticular disease, fecal impaction, acute surgical abdomen, and fecal ostomy Participants: 58 men and 76 women from North America. They were from 27 nursing homes, hospice sites, or other palliative care centres in the USA and Canada (78 with cancer, 15 cardiovascular disease, 14 chronic obstructive pulmonary disease, 8 dementia, and 19 with other diseases). Median age in methylnaltrexone group 70 years (range 34‐93 years) and in the placebo group 72 years (range 39‐98 years). Opioid dose: methylnaltrexone group: mean 417 mg/day, median 150 mg/day, range 9‐4160 mg/day; placebo group: mean 339 mg/day, median 100 mg/day, range 10‐10,160 mg/day. 98% in the methylnaltrexone and 99% in placebo group were using laxatives | |
| Interventions | Intervention: subcutaneous methylnaltrexone 0.15 mg/kg bodyweight, n = 62 Comparison: placebo, n = 71 Dose every other day Duration of treatment: 2 weeks | |
| Outcomes | Primary outcome: RFBM within 4 h after first dose Secondary outcomes: laxation within 4 h after ≥ 2 of the first 4 doses. Consistency (from watery to hard) and difficulty of laxation. Adverse events were assessed using the National Cancer Institute's Common Toxicity Criteria (rated on a scale from 'none' to 'very much'). Participants were also assessed on the Modified Himmelsbach Opiate Withdrawal Scale (on 7 symptoms including yawning, lacrimation, rhinorrhoea, perspiration, tremor, piloerection, and restlessness) Outcomes measured: over 2 weeks | |
| Notes | Funding: Progenics Pharmaceuticals. Trial 302 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule, blocked according to trial centre |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The study drugs (40 mg of methylnaltrexone per millilitre or placebo) were provided in identically appearing vials." "Syringe contents were blinded to patients and staff administering injections." |
| Incomplete outcome data (attrition bias) | Low risk | 106/133 completed trial |
| Selective reporting (reporting bias) | Unclear risk | No details |
| Sample size | Unclear risk | 50‐199 participants per treatment arm |
BM: bowel movement; ECOG: Eastern Cooperative Oncology Group; CGIC: Clinical Global Impression of Change; h: hour; n: number of participants; OXN PR: oxycodone/naloxone prolonged release; OXY PR: oxycodone prolonged release; RFBM: rescue‐free bowel movements; SBM: spontaneous bowel movement; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study of people with chronic pain not palliative care or cancer | |
| Not an RCT | |
| Study of people with chronic pain not palliative care or cancer | |
| Not an RCT | |
| Study of people with chronic (low back) pain not palliative care or cancer |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT |
| Participants | Participants with opioid‐induced constipation. Participants had non‐malignant or cancer‐related pain. No breakdown provided of number with cancer and no subanalysis of effect in group with cancer |
| Interventions | Naloxegol |
| Outcomes | Spontaneous bowel movements |
| Notes | Awaiting responses from authors to clarify population details and further details for analysis |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Methylnaltrexone Bromide in the Treatment of Opioid‐Induced Constipation in Lung Cancer Patients |
| Methods | Single‐centre RCT |
| Participants | 34 participants with a life expectancy of ≥ 3 months receiving treatment for lung cancer. All participants received fentanyl |
| Interventions | Intervention: methylnaltrexone 12 mg/0.6 mL subcutaneous Comparison: placebo Duration: 4 weeks Drugs administered on alternate days |
| Outcomes | Laxation |
| Starting date | Trial completed, no published paper identified |
| Contact information | Ioannis A Dimitroulis, 6th Pulmonary Department, Sotiria Hospital for Thoracic Diseases, Athens, Greece |
| Notes |
| Trial name or title | Phase 3 Study to Evaluate the Efficacy and Safety of Naldemedine for the Treatment of Opioid‐Induced Constipation in Cancer Patients |
| Methods | RCT |
| Participants | People with cancer |
| Interventions | Intervention: naldemedine Comparison: placebo |
| Outcomes | Efficacy in improving bowel function and safety |
| Starting date | Study completed 2016, result presented in abstract only, full paper expected |
| Contact information | Toshiyuki Harada MD, PhD (harada‐[email protected]) |
| Notes | Sponsor Shionogi Limited |
| Trial name or title | A Double‐Blind, Placebo‐Controlled, Multi‐Centre Phase IIb Extension Study to Evaluate the Safety and Efficacy of Multiple Alvimopan Dosage Regimens for the Treatment of Opioid‐Induced Bowel Dysfunction in Cancer Pain Subjects |
| Methods | RCT |
| Participants | People with cancer |
| Interventions | Intervention: alvimopan Comparison: placebo |
| Outcomes | Laxation |
| Starting date | 2004 |
| Contact information | Sponsor Cubist, collaborator GlaxoSmithKline |
| Notes | ID NCT00135577 |
| Trial name or title | Trial of Alvimopan Drug for Treatment of Constipation due to Prescription Pain Medication in Cancer Patients |
| Methods | RCT |
| Participants | People with cancer |
| Interventions | Intervention: alvimopan Comparison: placebo |
| Outcomes | Not stated |
| Starting date | Start date 2003, completed 2006 |
| Contact information | Funded by Cubist |
| Notes | IDs NCT00331045 trial terminated early (with 21 participants) "as subject registration did not proceed as expected," NCT00101998 trial completed |
| Trial name or title | RCT in Symptoms of Constipation in Subjects with Non‐Malignant or Malignant Pain that Requires Around‐the‐Clock Opioid Therapy taking 50/25‐80/40 mg Twice Daily as Oxycodone/Naloxone Prolonged Release Tablets Compared to Subjects taking 50‐80 mg Twice Daily Oxycodone Prolonged Release Tablets Alone |
| Methods | RCT |
| Participants | People with and without cancer pain |
| Interventions | Intervention: oxycodone/naloxone Comparison: oxycodone alone |
| Outcomes | Pain and bowel function |
| Starting date | Clinical trials website reports trial complete, no publication identified |
| Contact information | Funded by Mundipharma GmbH, no contact details provided on clinical trials website |
| Notes |
| Trial name or title | To Demonstrate Equivalence in Analgesic Efficacy & Bowel Function Between Oxycodone/naloxone PR Higher Dose & Lower Dose Tablet Strengths in Subjects with Non‐cancer or Cancer Pain |
| Methods | RCT |
| Participants | People with and without cancer with pain |
| Interventions | OXN PR higher‐dose and lower‐dose tablets |
| Outcomes | Pain and bowel function |
| Starting date | 2014 |
| Contact information | Funded by Mundipharma, no contact details provided on clinical trials website |
| Notes |
| Trial name or title | Trial of Methylnaltrexone in Opioid‐Induced Constipation Patients |
| Methods | RCT |
| Participants | People with advanced illness |
| Interventions | Intervention: Subcutaneous methylnaltrexone Comparison: placebo |
| Outcomes | Laxation |
| Starting date | 2015 |
| Contact information | Shiying Yu, [email protected] |
| Notes | Sponsors: Jiangsu Chia‐tai Tianqing Pharmaceutical Co, Ltd |
| Trial name or title | Naloxegol in Cancer Opioid‐Induced Constipation |
| Methods | Randomised single‐centre trial |
| Participants | People with cancer |
| Interventions | Intervention: naloxegol Comparison: treatment as usual |
| Outcomes | Laxation, quality of life, and pain |
| Starting date | May 2016 |
| Contact information | Chelsea Hagmann, [email protected] |
| Notes | Sponsor: University of California, Collaborator: Astra Zeneca |
| Trial name or title | Tolerability, Safety, and Feasibility of Naloxegol in Patients with Cancer and OIC (Opioid Induced Constipation) |
| Methods | Randomised multi‐centre trial |
| Participants | People with cancer |
| Interventions | Intervention: naloxegol Comparison: placebo |
| Outcomes | Laxation and pain |
| Starting date | July 2016 |
| Contact information | Janet Bull, MD, [email protected] |
| Notes | Sponsor and collaborators: Hospice of Henderson County, Inc and Astra Zeneca |
| Trial name or title | Clinical Evaluation of the Efficacy of Methylnaltrexone in Resolving Constipation‐Induced by Different Opioid Subtypes Combined with Laboratory Analysis of Immunomodulatory and Antiangiogenic Effects of Methylnaltrexone |
| Methods | Multi‐centre RCT |
| Participants | People receiving palliative care with opioid‐induced constipation |
| Interventions | Intervention: methylnaltrexone Comparison: unclear |
| Outcomes | Differences in the efficacy of methylnaltrexone prescribed to resolve opioid‐induced constipation between 3 commonly used opioid subtypes: morphine sulphate, oxycodone, and fentanyl |
| Starting date | Not stated, protocol published in 2014. Trial ongoing as reported December 2015 |
| Contact information | ECW Neefjes, Department of Medical Oncology, VU University Medical Center, Cancer Center Amsterdam, The Netherlands, [email protected] |
| Notes | ID NCT01955213 |
| Trial name or title | Effect of Subcutaneous Methylnaltrexone on Patient‐Reported Outcomes in Advanced Illness Patients with Opioid‐Induced Constipation |
| Methods | RCT |
| Participants | People with advanced illness |
| Interventions | Intervention: methylnaltrexone Comparison: placebo |
| Outcomes | Participant‐reported outcomes of constipation distress, bowel movement difficulty, and Global Clinical Impression of Change |
| Starting date | Not stated, conference abstract with findings published in 2013 |
| Contact information | J Peppin. Progenics Pharmaceuticals Inc, Tarrytown, NY sponsored trial |
| Notes | Did not include results section so unclear if trial is the same as any identified in a full published paper. |
RCT: randomised controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Within 24 hours of dose Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.91, 4.04] |
| Analysis 1.1  Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 1 Within 24 hours of dose. | ||||
| 2 Within 4 hours after 4 of the 7 doses Show forest plot | 2 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.98 [4.96, 20.09] |
| Analysis 1.2  Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 2 Within 4 hours after 4 of the 7 doses. | ||||
| 3 Within 4 hours of first dose Show forest plot | 3 | 517 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.87 [2.83, 5.28] |
| Analysis 1.3  Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 3 Within 4 hours of first dose. | ||||
| 4 Within 4 hours after 1 or 2 doses of the first 4 doses Show forest plot | 2 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.89 [4.46, 10.66] |
| Analysis 1.4  Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 4 Within 4 hours after 1 or 2 doses of the first 4 doses. | ||||
| 5 Improvement in constipation distress at day 1 Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.34, 2.59] |
| Analysis 1.5  Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 5 Improvement in constipation distress at day 1. | ||||
| 6 Participant global impression of improvement in bowel status at 1 week Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.64, 3.27] |
| Analysis 1.6  Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 6 Participant global impression of improvement in bowel status at 1 week. | ||||
| 7 Clinician global impression of improvement in bowel status at 1 week Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.66, 3.38] |
| Analysis 1.7  Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 7 Clinician global impression of improvement in bowel status at 1 week. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serious adverse event Show forest plot | 2 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.93] |
| Analysis 2.1  Comparison 2 Methylnaltrexone versus placebo: serious adverse event, Outcome 1 Serious adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 3 | 518 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.94, 1.45] |
| Analysis 3.1  Comparison 3 Methylnaltrexone versus placebo: adverse event, Outcome 1 Adverse events. | ||||
| 2 Dropouts due to adverse event Show forest plot | 2 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.54, 2.76] |
| Analysis 3.2  Comparison 3 Methylnaltrexone versus placebo: adverse event, Outcome 2 Dropouts due to adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 2 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.24] |
| Analysis 4.1  Comparison 4 Oxycodone/naloxone prolonged‐release tablets versus oxycodone prolonged‐release: adverse event, Outcome 1 Adverse events. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
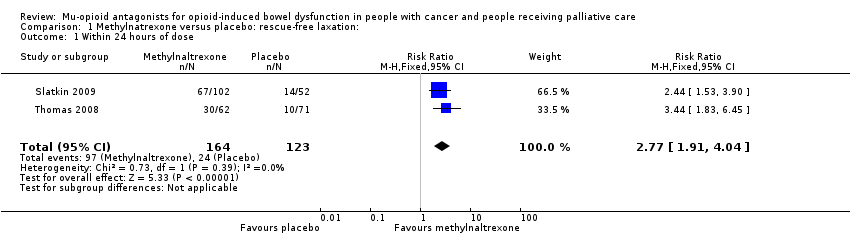
Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 1 Within 24 hours of dose.

Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 2 Within 4 hours after 4 of the 7 doses.
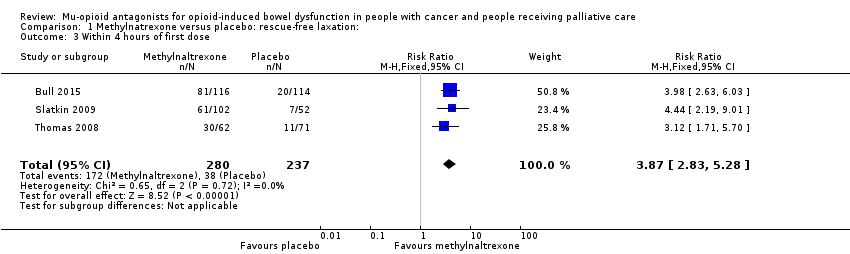
Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 3 Within 4 hours of first dose.

Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 4 Within 4 hours after 1 or 2 doses of the first 4 doses.

Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 5 Improvement in constipation distress at day 1.
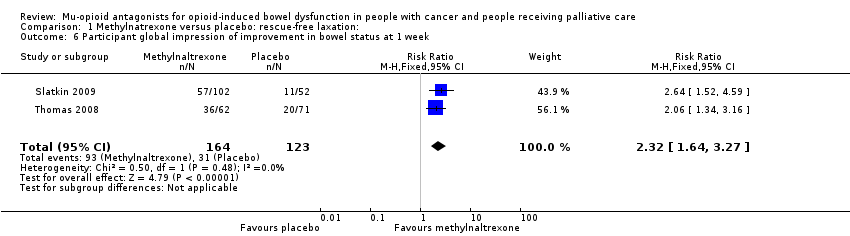
Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 6 Participant global impression of improvement in bowel status at 1 week.
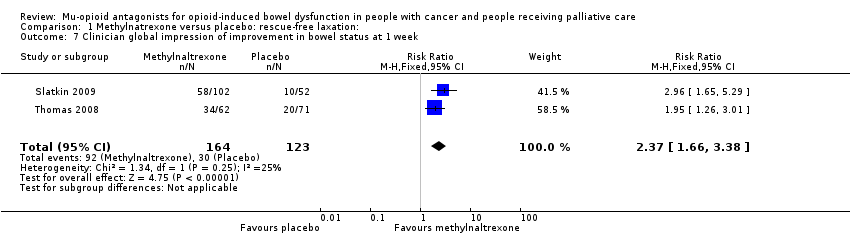
Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 7 Clinician global impression of improvement in bowel status at 1 week.

Comparison 2 Methylnaltrexone versus placebo: serious adverse event, Outcome 1 Serious adverse event.

Comparison 3 Methylnaltrexone versus placebo: adverse event, Outcome 1 Adverse events.

Comparison 3 Methylnaltrexone versus placebo: adverse event, Outcome 2 Dropouts due to adverse event.

Comparison 4 Oxycodone/naloxone prolonged‐release tablets versus oxycodone prolonged‐release: adverse event, Outcome 1 Adverse events.
| Naldemedine compared to placebo for opioid‐induced bowel dysfunction in cancer and people receiving palliative care | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Settings: cancer care Intervention: naldemedine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Naldemedine | |||||
| Laxation response within 24 hours of dose | — | — | — | — | — | Not reported |
| Laxation response between day 1 and day 14a | 375 per 1000 | 724 per 1000 | RR 1.93 (1.36 to 2.74) NNTB 2.88 (2.04 to 4.92) | 225 (1 study) | ⊕⊕⊕⊝ Moderateb | — |
| Effect on analgesia: opioid withdrawalc | — | — | 0.1 mg: MD ‐0.13 (‐0.57 to 0.31); 0.2 mg: MD ‐0.40 (‐0.87 to 0.07); 0.4 mg: MD ‐0.02 (‐0.45 to 0.41) | 225 (1 study) | ⊕⊕⊕⊝ Moderateb | — |
| Effect on analgesia: pain intensity | — | — | — | — | — | Not reported |
| Serious adverse eventsa | — | — | 5 SAEs occurred, all in naldemedine group. | 225 (1 study) | ⊕⊕⊝⊝ | — |
| Adverse eventsa | 518 per 1000 | 704 per 1000 (539 to 927) | RR 1.36 (1.04 to 1.79) | 225 (1 study) | ⊕⊕⊕⊝ Moderateb | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio; SAE: serious adverse events. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured by clinician or self‐report and in the case of adverse events using severity grades according to the Common Terminology Criteria for Adverse Events. | ||||||
| Lower‐dose naldemedine compared to higher‐dose naldemedine for opioid‐induced bowel dysfunction in cancer and people receiving palliative care | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Setting: cancer care Intervention: lower dose naldemedine 0.1 mg daily Comparison: higher dose naldemedine 0.2 mg or 0.4 mg daily | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Higher dose 0.2 mg/0.4 mg daily | Lower dose 0.1 mg daily | |||||
| Laxation response within 24 hours of dose | — | — | — | — | — | Not reported |
| Laxation response between day 1 and day 14a | 0.1 mg vs 0.2 mg: 776 per 1000 0.1 mg vs 0.4 mg: 821 per 1000 | 0.1 mg vs 0.2 mg: 564 per 1000 (430 to 739) 0.1 mg vs 0.4 mg: 564 per 1000 (433 to 733) | 0.1 mg vs 0.2 mg: RR 0.73 (0.55 to 0.95) 0.1 mg vs 0.4 mg: RR 0.69 (0.53 to 0.89) | 226 (1 study) 0.1 mg vs 0.2 mg: n = 113 0.1 mg vs 0.4 mg: n = 111 | ⊕⊕⊕⊝ Moderateb | — |
| Effect on analgesia: opioid withdrawal | — | — | — | — | — | Not reported |
| Effect on analgesia: pain intensity | — | — | — | — | — | Not reported |
| Serious adverse events | — | — | — | — | — | Not reported |
| Adverse events | — | — | — | — | — | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured by self‐report. | ||||||
| Naloxone compared with placebo for cancer and people receiving palliative care with opioid‐induced bowel dysfunction | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Settings: cancer care Intervention: naloxone Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Naloxone | |||||
| Laxation response within 24 hours of a dose | — | — | — | — | — | Not reported |
| Laxation response between day 1 and day 14 | — | — | — | — | — | Not reported |
| Effect on analgesia: opioid withdrawal | — | — | — | — | — | Not reported |
| Effect on analgesia: pain intensitya | — | — | No statistical difference in pain experienced when taking placebo or naloxone. Full data, including pre‐cross‐over results, were not provided. | 17 (1 study) | ⊕⊝⊝⊝ | — |
| Serious adverse events | — | — | — | — | — | Not reported |
| Adverse events | — | — | — | — | — | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured using 4‐point scale (0 = no pain, 3 = severe pain). | ||||||
| Oxycodone/naloxone prolonged release tablets compared with oxycodone prolonged‐released tablets for opioid‐induced bowel dysfunction | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Settings: cancer care Intervention: oxycodone/naloxone prolonged‐release tablets Comparison: oxycodone prolonged‐released tablets | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oxycodone | Oxycodone/naloxone | |||||
| Laxation response within 24 hours of dose | — | — | — | — | — | Not reported |
| Laxation response between day 1 and day 14 | — | — | — | — | — | Not reported |
| Effect on analgesia: opioid withdrawalc | — | — | Intervention group: mean 6.64 (SD 5.97) comparison group: mean 7.29 (SD 4.59) at 7 days | 184 (1 study) | ⊕⊕⊕⊝ Moderateb | — |
| Effect on analgesia: pain intensitya | — | — | Intervention group: mean 3.50 (SD 1.88) and comparison group: mean 3.52 (SD 1.80) at 4 weeks | 184 (1 study) | ⊕⊕⊕⊝ Moderateb | Another study, Dupoiron 2017 also found outcome to be similar between trial arms, but did not provide any data. |
| Serious adverse events | 43 per 1000 | 87 per 1000 (27 to 279) | RR 2.00 (95% CI 0.62 to 6.41) | 184 (1 study) | ⊕⊕⊝⊝ Lowb,d | — |
| Adverse events | 754 per 1000 | 815 per 1000 (709 to 935) | RR 1.08 (95% CI 0.94 to 1.24) | 234 (2 studies) | ⊕⊕⊕⊝ Moderateb | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured using the Brief Pain Inventory‐Short Form. | ||||||
| Methylnaltrexone compared to placebo for opioid‐induced bowel dysfunction in cancer and people receiving palliative care | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Setting: palliative care Intervention: methylnaltrexone Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with methylnaltrexone | |||||
| Laxation response within 24 hours of dosea | 195 per 1000 | 568 per 1000 | RR 2.77 (1.91 to 4.04) | 287 | ⊕⊕⊕⊝ Moderateb | — |
| Laxation response between day 1 and day 14 (specifically within 4 hours after 4 or more of the 7 doses)a | 52 per 1000 | 517 per 1000 | RR 9.98 (4.96 to 20.09) | 305 | ⊕⊕⊕⊝ Moderate b,c | — |
| Effect on analgesia: opioid withdrawald | Study 1: day 1: MD 0.00 (‐0.46 to 0.46); day 14: MD 0.10 (‐0.63 to 0.83) Study 2: median change to day 2 = 0 in both trials arms | 236 (2 studies) | ⊕⊕⊕⊝ Moderateb | ‐ | ||
| Effect on analgesia: pain intensitye | Study 1: at 4 hours (methylnaltrexone 0.15 mg/kg: MD ‐0.76 (‐1.47 to 0.05); methylnaltrexone 0.3 mg/kg: MD ‐0.25 (‐0.91 to 0.41) Study 2: at day 1 and 14 (day 1: MD 0.20 (‐0.62 to 1.02); day 14: MD ‐0.70 (‐1.52 to 0.12) | 287 (2 studies) | ⊕⊕⊝⊝ Lowb,f | Another study, Bull 2015, found similar pain intensity experienced in trial arms, full data not provided. | ||
| Serious adverse events | 238 per 1000 | 142 per 1000 | RR 0.59 (0.38 to 0.93) | 364 | ⊕⊕⊕⊝ Moderateb | — |
| Adverse events | 700 per 1000 | 815 per 1000 | RR 1.17 (CI 0.94 to 1.45) | 518 | ⊕⊕⊝⊝ Lowb,g | Heterogeneity was substantial (74%). It was explained in sensitivity analysis by omitting the trial at a high risk of bias because of small sizes. The effect estimate was reduced. The direction of effect not changed. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured by self‐report or clinician report. dMeasured using the modified Himmelsbach Opioid Withdrawal Scale. | ||||||
| Lower dose methylnaltrexone compared to higher dose for opioid‐induced bowel dysfunction in cancer and people receiving palliative care | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Setting: palliative care Intervention 1: lower‐dose methylnaltrexone (study 1: 3 doses, 1 week, 1 mg; study 2: 1 dose, 0.15 mg/kg) Intervention 2: higher‐dose methylnaltrexone (study 1: 3 doses, 1 week, 5‐12.5 mg; study 2: 1 dose, 0.30 mg/kg) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Higher dose | Lower dose | |||||
| Laxation response within 24 hours of first dosea | Study 1: 609 per 1000 Study 2: 639 per 1000 | Study 1: 499 per 1000 (250 to 100) Study 2: 681 per 1000 (515 to 904) | Study 1: RR 0.82 (0.41 to 1.66) Study 2: RR 1.07 (0.81 to 1.42) | 135 (2 studies) Study 1: n = 33 Study 2: n = 102 | ⊕⊕⊝⊝ Lowb | Unable to combine study data as methylnaltrexone low and higher doses differed per trial |
| Laxation responsea | At 3 days: 706 per 1000 | At 3 days: 332 per 1000 | At 3 days: RR 0.47 (0.18 to 1.25) | 33 participants (1 study) | ⊕⊕⊝⊝ Lowb | Unable to combine study data as methylnaltrexone low and higher doses differed per trial |
| At 5 days: 688 per 1000 | At 5 days: 144 per 1000 | At 3 days: RR 0.21 (0.03 to 1.31) | ||||
| Effect on analgesia: opioid withdrawalc | — | — | MD ‐0.04 (‐0.73 to 0.65) | 102 participants (1 study) | ⊕⊕⊝⊝ Lowb | Another study,Portenoy 2008, also found outcome to be similar between trial arms, but did not provide any data |
| Effect on analgesia: pain intensityd | — | — | MD ‐0.51 (‐1.49 to 0.47) | 102 participants (1 study) | ⊕⊕⊝⊝ Lowb | Another study, Portenoy 2008, also found outcome to be similar between trial arms, but did not provide any data |
| Serious adverse event | — | — | — | — | Not reported | |
| Adverse event | Study 1: 1000 per 1000 Study 2: 800 per 1000 | Study 1: 1000 per 1000 (1000 to 1000) Study 2: 723 per 1000 (580 to 902) | Study 1: RR 1.00 (1.00 to 1.00) Study 2: RR 0.90 (0.73 to 1.13) | 135 (2 studies) Study 1: n = 33 Study 2: n = 102 | ⊕⊕⊝⊝ Lowb | Unable to combine study data as methylnaltrexone low and higher doses differed per trial |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured by clinician or self‐report. bDowngraded by two levels for study limitations: one for unclear risk of bias (reporting bias) and one for small sample size (high risk of bias). cMeasured using the modified Himmelsbach Opioid Withdrawal Scale. dMeasured by participant‐rated scale 0‐10. | ||||||
| Adverse event | Naldemedine (%) | Placebo (%) |
| Diarrhoea | 67 (39) | 14 (25) |
| Decreased WBC count | 9 (5) | 3 (5) |
| Abdominal pain | 6 (4) | 0 (0) |
| Vomiting | 5 (3) | 0 (0) |
| Bone marrow failure | 3 (2) | 2 (4) |
| Decreased appetite | 6 (4) | 1 (2) |
| Nasopharyngitis | 4 (2) | 1 (2) |
| Nausea | 4 (2) | 4 (7) |
| Rash | 3 (2) | 2 (4) |
| Decreased platelet count | 3 (2) | 0 (0) |
| Decreased total protein | 7 (4) | 1 (2) |
| Glucose in urine | 4 (2) | 1 (2) |
| Abnormal haematology test | 2 (1) | 0 (0) |
| Decreased RBC count | 4 (2) | 0 (0) |
| Hypertension | 2 (1) | 0 (0) |
| Increased blood alkaline phosphatase | 4 (2) | 1 (2) |
| Increased blood lactate dehydrogenase | 2 (1) | 1 (2) |
| Increased blood pressure | 2 (1) | 0 (0) |
| Increased blood urea | 4 (2) | 1 (2) |
| Increased WBC count | 1 (2) | 2 (4) |
| Protein present in urine | 5 (3) | 0 (0) |
| Upper abdominal pain | 3 (2) | 1 (2) |
| RBC: red blood cell; WBC: white blood cell. All comparisons were not statistically significant. | ||
| Methylnaltrexone vs placeboa | |
| AEs | RR 1.07, 95% CI 0.96 to 1.19 |
| AE of abdominal pain | RR 2.15, 95% CI 1.28 to 3.62 |
| AE of nausea | RR 0.87, 95% CI 0.46 to 1.65 |
| AE of vomiting | RR 0.70, 95% CI 0.33 to 1.47 |
| aomitting trial of high risk of bias. AE: adverse event; CI: confidence intervals; RR: risk ratio. | |
| Adverse event | RR (95% CI) | I²statistic on heterogeneity |
| Abdominal pain | 2.39 (1.07 to 5.34) | 65% |
| Diarrhoea | 1.02 (0.93 to 1.11) | 51% |
| Dizziness | 4.09 (0.99 to 16.83) | 0% |
| Falls | 1.02 (0.89 to 1.16) | 84% |
| Flatulence | 2.09 (1.07 to 4.08) | 0% |
| Nausea | 0.97 (0.89 to 1.06) | 63% |
| Peripheral oedema | 1.01 (0.50 to 2.03) | 0% |
| Restlessness | 0.83 (0.32 to 2.12) | 0% |
| Somnolence | 1.00 (0.93 to 1.08) | 73% |
| Vomiting | 0.99 (0.92 to 1.08) | 67% |
| CI: confidence interval; RR: risk ratio. | ||
| Adverse event | Methylnaltrexone (%) | Placebo (%) |
| Abdominal distensiona | 1 (2) | 6 (8) |
| Abdominal tendernessa | 1 (2) | 4 (6) |
| Astheniaa | 4 (6) | 4 (6) |
| Anxietyb | 5 (4.9) | 0 (0) |
| Arthralgiab | 3 (2.9) | 1 (1.9) |
| Back painc | 9 (7.8) | 3 (2.9) |
| Confusional statec | 7 (6.0) | 9 (7.9) |
| Dehydrationa | 2 (3) | 4 (6) |
| Fatigueb | 4 (3.9) | 1 (1.9) |
| Hypotensiona | 0 (0) | 4 (6) |
| Increased body temperaturea | 5 (8) | 2 (3) |
| Lethergya | 4 (6) | 4 (6) |
| Malignant‐neoplasm progressiona | 7 (11) | 9 (13) |
| Pain exacerbationb | 8 (8) | 2 (4) |
| Rhinorrhoeab | 6 (5.9) | 1 (1) |
| Sweating increasedb | 8 (7.8) | 4 (7.7) |
| Tachycardiaa | 1 (1) | 4 (6) |
| aReported in trial by Thomas 2008. bReported in trial by Slatkin 2009. cReported in trial by Bull 2015. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Within 24 hours of dose Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.91, 4.04] |
| 2 Within 4 hours after 4 of the 7 doses Show forest plot | 2 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.98 [4.96, 20.09] |
| 3 Within 4 hours of first dose Show forest plot | 3 | 517 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.87 [2.83, 5.28] |
| 4 Within 4 hours after 1 or 2 doses of the first 4 doses Show forest plot | 2 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.89 [4.46, 10.66] |
| 5 Improvement in constipation distress at day 1 Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.34, 2.59] |
| 6 Participant global impression of improvement in bowel status at 1 week Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.64, 3.27] |
| 7 Clinician global impression of improvement in bowel status at 1 week Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.66, 3.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serious adverse event Show forest plot | 2 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 3 | 518 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.94, 1.45] |
| 2 Dropouts due to adverse event Show forest plot | 2 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.54, 2.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 2 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.24] |

