Ubat‐ubatan antipsikotik untuk ketergantungan kokain
Appendices
Appendix 1. Search strategies October 2006
In the first version of the review we identified relevant studies by searching the following sources from the earliest available date to 2006: MEDLINE (1966 toOctober 2006), EMBASE (1980 to October 2006), CINAHL (1982 to October 2006), Cochrane Drug and Alcohol Group Specialised Register (October 2006)
MEDLINE search strategy
1.exp cocaine‐related disorders/
2.((cocaine$) adj2 (abuse$ or addict$ or dependen$)).ti,ab
3.exp cocaine/ or exp crack cocaine/
4.cocaine.ti,ab
5.1 or 2 or 3 or 4
6.exp antipsychotic/
7.antipsychotic$.ti,ab
8.exp serotonin antagonists/
9.5‐HT2$.ti,ab
10.chlorpromazine.mp. or exp Chlorpromazine/
11.fluphenazine.mp. or exp Fluphenazine/
12.perphenazine.mp. or exp Perphenazine/
13.prochlorperazine.mp. or exp Prochlorperazine/
14.thioridazine.mp. or exp Thioridazine/
15.trifluoperazine.mp. or exp Trifluoperazine/
16.haloperidol.mp. or exp Haloperidol/
17.droperidol.mp. or exp Droperidol/
18.pimozide.mp. or exp Pimozide/
19.clozapine.mp. or exp Clozapine/
20.olanzapine.mp.
21.risperidone.mp. or exp Risperidone/
22.quetiapine.mp.
23.ziprasidone.mp.
24.aripiprazole.mp.
25.symbax.ti,ab.
26.tetrabenazine.mp. or exp Tetrabenazine/
27. OR 6/26
28. 5 and 27
combined with the phases 1 & 2 of the Cochrane Sensitive Search Strategy for the identification of RCTs as published in Appendix 5b2, Cochrane Handbook for Systematic Reviews of Interventions:
29.randomized controlled trial.pt.
30.randomized controlled trials/
31.controlled clinical trial.pt.
32.random allocation/
33.double blind method/
34.single blind method/
35.29/34
36.clinical trial.pt.
37.exp clinical trials/
38.(clin$ adj trial$).ab,ti.
39.((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ab,ti
40.exp PLACEBOS/
41.placebo$.ab,ti
42.random$.ab,ti
43.exp Research Design/
44.36/43
45.35 or 44
46.4 and 7 and 12
47.30 and 29
48.limit 31 to human
EMBASE search strategy
-
exp drug abuse/
-
exp Cocaine Dependence/
-
((cocaine) adj2 (abuse$ or addict$ or dependen$)).ti,ab.
-
((drug or substance) adj2 (abuse$ or addict$ or dependen$)).ti,ab.
-
1 or 2 or 3 or 4
-
exp COCAINE DERIVATIVE/ or exp COCAINE/
-
cocaine.ti,ab.
-
6 or 7
-
antipsychotic.mp.
-
serotonin agents.mp. or exp Serotonin Receptor Affecting Agent/
-
exp CHLORPROMAZINE/ or chlorpromazine.mp.
-
fluphenazine.mp. or exp FLUPHENAZINE/
-
perphenazine.mp. or exp PERPHENAZINE/
-
exp PROCHLORPERAZINE/ or prochlorperazine.mp.
-
thioridazine.mp. or exp THIORIDAZINE/
-
exp TRIFLUOPERAZINE/ or trifluoperazine.mp.
-
haloperidol.mp. or exp HALOPERIDOL/
-
exp DROPERIDOL/ or droperidol.mp.
-
pimozide.mp. or exp PIMOZIDE/
-
clozapine.mp. or exp CLOZAPINE/
-
exp OLANZAPINE/ or olanzapine.mp.
-
risperidone.mp. or exp RISPERIDONE/
-
quetiapine.mp. or exp QUETIAPINE/
-
ziprasidone.mp. or exp ZIPRASIDONE/
-
aripiprazole.mp. or exp ARIPIPRAZOLE/
-
symbax.ti,ab.
-
tetrabenazine.mp. or exp TETRABENAZINE/
-
OR 9/27
-
5 and 8 and 28
-
random$.ti,ab.
-
placebo$.ti,ab.
-
((singl$ or doubl$ or trebl$ or tripl$) adj2 (blind$ or mask$)).mp.
-
(cross‐over$ or crossover$).tw.
-
randomized controlled trial/
-
phase‐2‐clinical‐trial/
-
phase‐3‐clinical‐trial/
-
double blind procedure/
-
single blind procedure/
-
crossover procedure/
-
Latin square design/
-
exp PLACEBOS/
-
multicenter study/
-
OR 30/42
-
29 and 43
-
limit 44 to human
CINAHL search strategy
-
exp "Substance Use Disorders"/
-
(cocaine adj2 (abuse$ or dependen$))
-
TX cocaine or MH cocaine
-
TX chlorpromazine or MH chlorpromazine
-
TX fluphenazine or MH fluphenazine
-
TX perphenazine
-
MH PROCHLORPERAZINE or TX prochlorperazine
-
TX thioridazine or MH thioridazine
-
TX trifluoperazine
-
TX haloperidol or MH HALOPERIDOL
-
MH DROPERIDOL or TX droperidol
-
TX pimozide
-
TX clozapine or MH CLOZAPINE
-
TX OLANZAPINE or MH olanzapine
-
TX risperidone. or MH RISPERIDONE
-
TX quetiapine or MH QUETIAPINE
-
TX ziprasidone
-
TX aripiprazole
-
TX symbax
-
TX tetrabenazine
-
random$.tw.
-
clini$.tw.
-
trial$.tw.
-
(clin$ adj2 trial$).tw.
-
(singl$ or doubl$ or tripl$ or trebl$).mp. and (mask$ or blind$).tw.
-
crossover.tw.
-
allocate$.tw.
-
assign$.tw.
-
(random$ adj2 (allocate$ or assign$)).tw.
-
exp Random Assignment/
-
exp Clinical Trials/
-
1 or 2 or 3
-
OR 4/20
-
OR 21/31
-
32 and 33 and 34
Cochrane Drug and Alcohol Group Specialised Register search strategy
We searched the free text "cocaine" in the field "Diagnosis" and "antipsychotic" in the field "Intervention" of the Register
Appendix 2. Cochrane Drug and Alcohol Group Specialised Register search strategy (2015)
July 15, 2015 (15 hits)
Publication Year from 2006
(cocaine*:XDI,TI AND (Antipsychotic* OR aripiprazole OR clozapine OR chlorpromazine OR droperidol OR olanzapine OR prochlorperazine OR trifluoperazine OR fluphenazine OR haloperidol OR perphenazine OR pimozide OR quetiapine OR risperidone OR tetrabenazine OR thioridazine OR ziprasidone))
Appendix 3. CENTRAL search strategy (2015)
The Cochrane Library
Issue 7, July 2015 ( 26 hits in CENTRAL; 3 hits in DARE)
-
MeSH descriptor: [Cocaine‐Related Disorders] explode all trees
-
cocaine* or crack:ti,ab,kw (Word variations have been searched)
-
#1 or #2
-
"antipsychotic":ti,ab,kw (Word variations have been searched)
-
"neuroleptic":ti,ab,kw (Word variations have been searched)
-
chlorpromazine:ti,ab,kw (Word variations have been searched)
-
"fluphenazine":ti,ab,kw (Word variations have been searched)
-
"perphenazine":ti,ab,kw (Word variations have been searched)
-
"prochlorperazine":ti,ab,kw (Word variations have been searched)
-
"thioridazine":ti,ab,kw (Word variations have been searched)
-
"trifluoperazine":ti,ab,kw (Word variations have been searched)
-
"haloperidol":ti,ab,kw (Word variations have been searched)
-
"droperidol":ti,ab,kw (Word variations have been searched)
-
"pimozide":ti,ab,kw (Word variations have been searched)
-
"clozapine":ti,ab,kw (Word variations have been searched)
-
"olanzapine":ti,ab,kw (Word variations have been searched)
-
"risperidone":ti,ab,kw (Word variations have been searched)
-
"quetiapine":ti,ab,kw (Word variations have been searched)
-
"ziprasidone":ti,ab,kw (Word variations have been searched)
-
"aripiprazole":ti,ab,kw (Word variations have been searched)
-
"tetrabenazine":ti,ab,kw (Word variations have been searched)
-
#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
-
#3 and #22 Publication Year from 2006
Appendix 4. MEDLINE search strategy (2015)
MEDLINE (via PubMed)
July 15, 2015 (71 hits)
-
Cocaine‐Related Disorders[Mesh]
-
((cocaine[tiab] OR crack[tiab]) AND (abuse*[tiab] OR dependen*[tiab] OR misuse[tiab] OR addict*[tiab]))
-
#1 or #2
-
"Antipsychotic Agents"[Mesh] OR "Antipsychotic Agents" [Pharmacological Action]
-
antipsychot*[tiab] OR anti‐psychot*[tiab] OR neuroleptic*[tiab]
-
aripiprazole
-
chlorpromazine[tiab] OR chlorpromazine[MeSH]
-
clozapine[tiab] OR clozapine[MeSH]
-
droperidol[tiab] OR droperidol[MeSH]
-
fluphenazine[tiab] OR fluphenazine[MeSH]
-
Haloperidol[tiab] OR Haloperidol[MeSH]
-
olanzapine
-
perphenazine[tiab] OR perphenazine[MeSH]
-
pimozide[tiab] OR pimozide[MeSH]
-
prochlorperazine[tiab] OR prochlorperazine[MeSH]
-
quetiapine
-
Risperidone[tiab] OR Risperidone[MeSH]
-
tetrabenazine[tiab]OR tetrabenazine[MeSH]
-
thioridazine[tiab] OR thioridazine[MeSH]
-
trifluoperazine[tiab] OR trifluoperazine[MeSH]
-
ziprasidone
-
#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
-
randomized controlled trial [pt]
-
controlled clinical trial [pt]
-
randomized [tiab]
-
placebo [tiab]
-
drug therapy [sh]
-
randomly [tiab]
-
trial [tiab]
-
groups [tiab]
-
#23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30
-
animals [mh] NOT humans [mh]
-
#31 NOT #32
-
#3 AND #22 AND #33 Filters: Publication date from 2006/10/01
Appendix 5. EMBASE search strategy (2015)
EMBASE (via embase.com)
July 15, 2015 (149 hits)
'cocaine dependence'/exp OR ((cocaine OR crack) NEAR/3 (abuse* OR dependen* OR addict*)):ab,ti AND ('neuroleptic agent'/exp OR antipsychotic*:ab,ti OR 'chlorpromazine':ab,ti OR 'chlorpromazine'/exp OR 'fluphenazine'/exp OR 'fluphenazine':ab,ti OR 'perphenazine'/exp OR 'perphenazine':ab,ti OR 'prochlorperazine'/exp OR 'prochlorperazine':ab,ti OR 'thioridazine'/exp OR 'thioridazine':ab,ti OR 'trifluoperazine'/exp OR 'trifluoperazine':ab,ti OR 'haloperidol'/exp OR 'haloperidol':ab,ti OR 'droperidol'/exp OR 'droperidol':ab,ti OR 'pimozide'/exp OR 'pimozide':ab,ti OR 'clozapine'/exp OR 'clozapine':ab,ti OR 'olanzapine'/exp OR 'olanzapine':ab,ti OR 'risperidone'/exp OR 'risperidone':ab,ti OR 'quetiapine'/exp OR 'quetiapine':ab,ti OR 'ziprasidone'/exp OR 'ziprasidone':ab,ti OR 'aripiprazole'/exp OR 'aripiprazole':ab,ti) AND ('crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR 'controlled clinical trial'/exp OR 'clinical trial'/exp OR placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti) OR 'randomized controlled trial'/exp) AND [1‐10‐2006]/sd
Appendix 6. CINAHL search strategy (2015)
CINAHL (via EBSCO HOST)
July 15, 2015 (17 hits)
-
(MH "Substance Use Disorders+")
-
TX((cocaine OR crack) N3 (abuse* OR dependen* OR addict*))
-
TI cocaine OR AB cocaine OR MH cocaine
-
S1 OR S2 OR S3
-
TX (antipsychotic* OR neuroleptic* OR aripiprazole OR clozapine OR chlorpromazine OR droperidol OR olanzapine OR prochlorperazine OR trifluoperazine OR fluphenazine OR haloperidol OR perphenazine OR pimozide OR quetiapine OR risperidone OR tetrabenazine OR thioridazine OR ziprasidone)
-
S4 AND S5
-
MH "Clinical Trials+"
-
PT Clinical trial
-
TI clinic* N1 trial* or AB clinic* N1 trial*
-
TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
-
AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
-
TI randomi?ed control* trial* or AB randomi?ed control* trial*
-
MH "Random Assignment"
-
TI random* allocat* or AB random* allocat*
-
MH "Placebos"
-
TI placebo* or AB placebo*
-
MH "Quantitative Studies"
-
S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17
-
S6 AND S18 Exclude MEDLINE records
-
S6 AND S18 Publication Year from 2006
Appendix 7. Web of Science search strategy (2015)
WOS (via THOMSON REUTERS)
July 15, 2015 (94 hits)
-
TS=((cocaine OR crack) NEAR/6 (abuse* OR dependen* OR addict* OR disorder*))
-
TS=(antipsychotic* OR neuroleptic* OR aripiprazole OR clozapine OR chlorpromazine OR droperidol OR olanzapine OR prochlorperazine OR trifluoperazine OR fluphenazine OR haloperidol OR perphenazine OR pimozide OR quetiapine OR risperidone OR tetrabenazine OR thioridazine OR ziprasidone)
-
TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*)
-
#3 AND #2 AND #1Indexes=SCI‐EXPANDED, SSCI, A&HCI Timespan=2006‐2015
Appendix 8. criteria for risk of bias assessment
| Item | Judgment | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization |
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement | |
| 3. Blinding of participants and providers (performance bias) Objective outcomes | Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 4. Blinding of participants and providers (performance bias) Subjective outcomes | Low risk | Blinding of participants and providers ensured and unlikely that the blinding could have been broken; |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 5. Blinding of outcome assessor (detection bias) Objective outcomes | Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding | |
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 6.Blinding of outcome assessor (detection bias) Subjective outcomes | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding | |
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 7. Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or drop out | Low risk | No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; Missing data have been imputed using appropriate methods All randomised patients are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention to treat) |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; | |
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of drop out not reported for each group); | |
| 8 Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| High risk | Not all of the study’s pre‐specified primary outcomes have been reported; One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; The study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk |

Study flow diagram. Review update 2015.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Any antipsychotic versus placebo, Outcome 1 Dropouts.

Comparison 1 Any antipsychotic versus placebo, Outcome 2 Side effects.

Comparison 1 Any antipsychotic versus placebo, Outcome 3 Number of participants using cocaine during the treatment (as days/week by urine tests or self report).
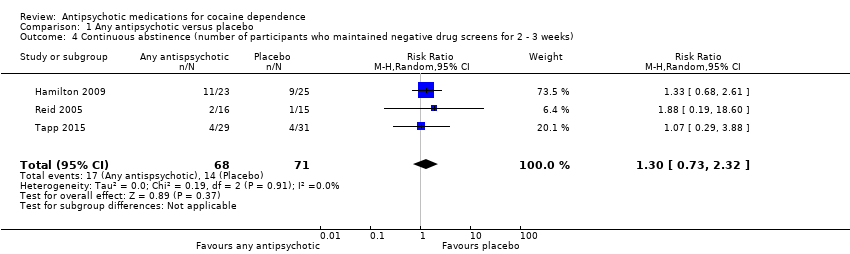
Comparison 1 Any antipsychotic versus placebo, Outcome 4 Continuous abstinence (number of participants who maintained negative drug screens for 2 ‐ 3 weeks).
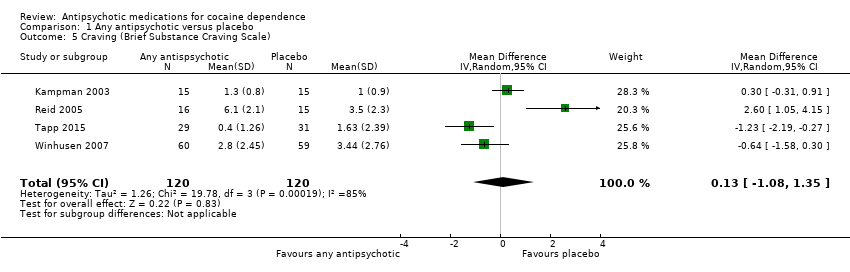
Comparison 1 Any antipsychotic versus placebo, Outcome 5 Craving (Brief Substance Craving Scale).
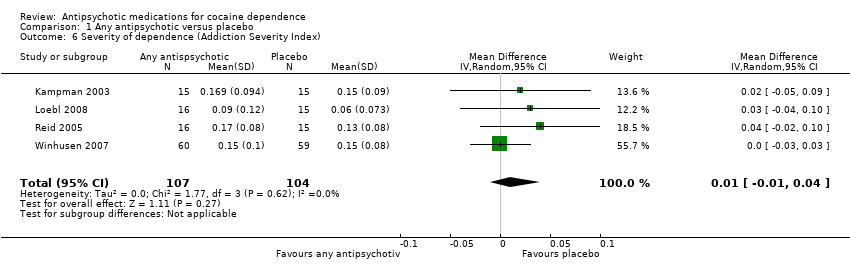
Comparison 1 Any antipsychotic versus placebo, Outcome 6 Severity of dependence (Addiction Severity Index).

Comparison 1 Any antipsychotic versus placebo, Outcome 7 Severity of dependence (Clinical Global Impression Scale).

Comparison 1 Any antipsychotic versus placebo, Outcome 8 Use of cocaine during the treatment (self‐reported as g/week).
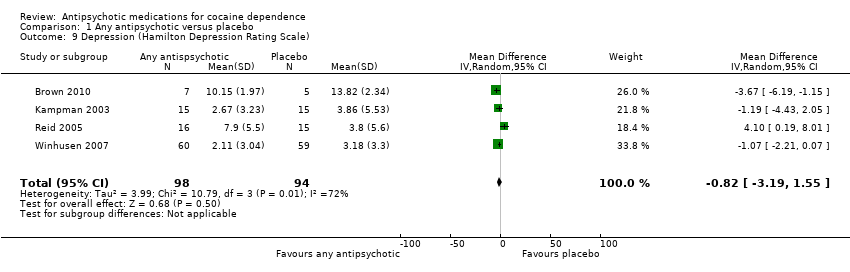
Comparison 1 Any antipsychotic versus placebo, Outcome 9 Depression (Hamilton Depression Rating Scale).

Comparison 2 Risperidone versus placebo, Outcome 1 Dropouts.

Comparison 2 Risperidone versus placebo, Outcome 2 Severity of dependence (Addiction Severity Index).
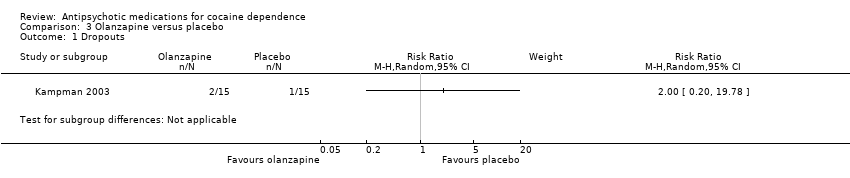
Comparison 3 Olanzapine versus placebo, Outcome 1 Dropouts.
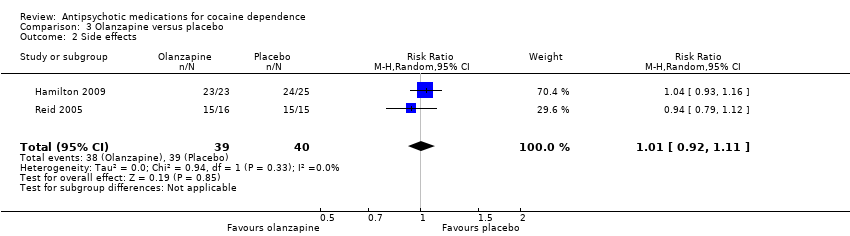
Comparison 3 Olanzapine versus placebo, Outcome 2 Side effects.

Comparison 3 Olanzapine versus placebo, Outcome 3 Use of cocaine during the treatment (self‐reported as days/week).
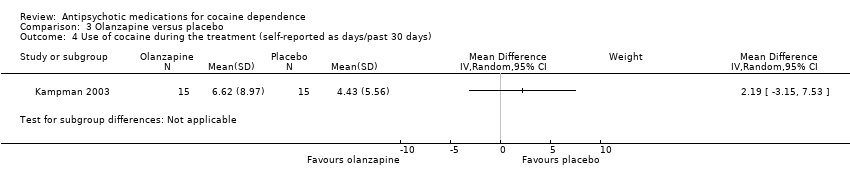
Comparison 3 Olanzapine versus placebo, Outcome 4 Use of cocaine during the treatment (self‐reported as days/past 30 days).

Comparison 3 Olanzapine versus placebo, Outcome 5 Continuous abstinence (participants who maintained negative drug screens throughout the treatment period ).

Comparison 3 Olanzapine versus placebo, Outcome 6 Craving (Brief Substance Craving Scale).
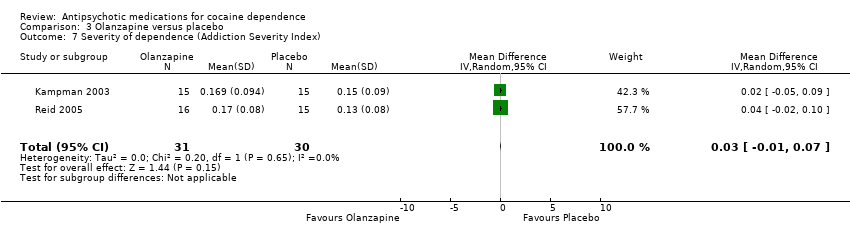
Comparison 3 Olanzapine versus placebo, Outcome 7 Severity of dependence (Addiction Severity Index).
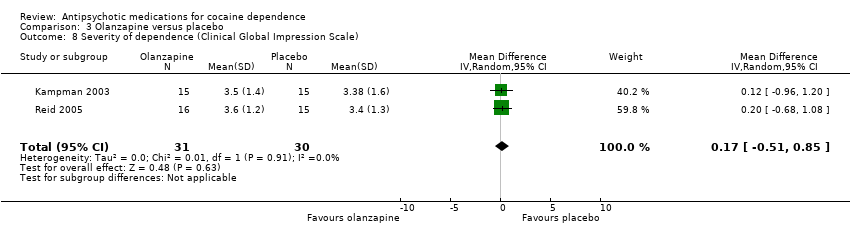
Comparison 3 Olanzapine versus placebo, Outcome 8 Severity of dependence (Clinical Global Impression Scale).

Comparison 3 Olanzapine versus placebo, Outcome 9 Amount of of cocaine use during the treatment (self‐reported as dollars spent/past 30 days).
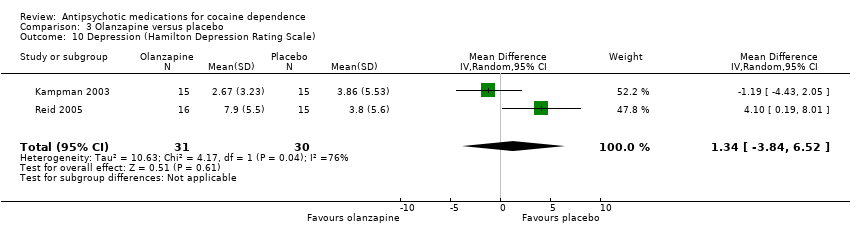
Comparison 3 Olanzapine versus placebo, Outcome 10 Depression (Hamilton Depression Rating Scale).
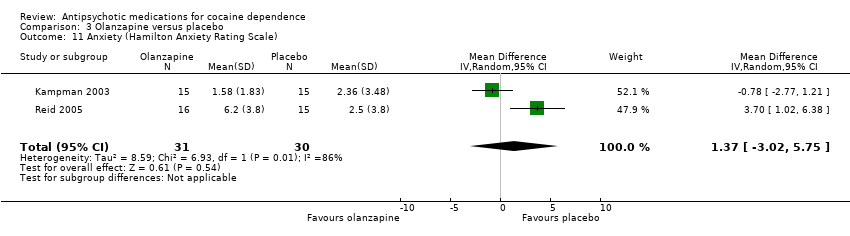
Comparison 3 Olanzapine versus placebo, Outcome 11 Anxiety (Hamilton Anxiety Rating Scale).

Comparison 3 Olanzapine versus placebo, Outcome 12 Withdrawal symptoms (Cocaine Selective Severity Assessment).
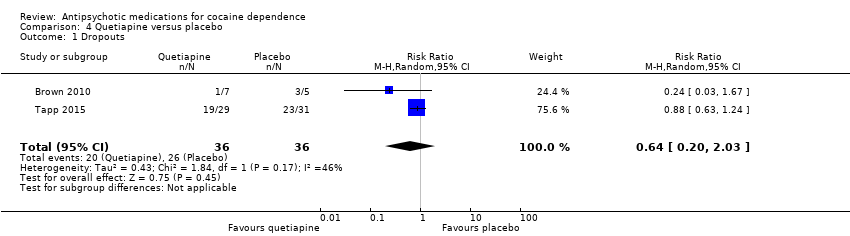
Comparison 4 Quetiapine versus placebo, Outcome 1 Dropouts.
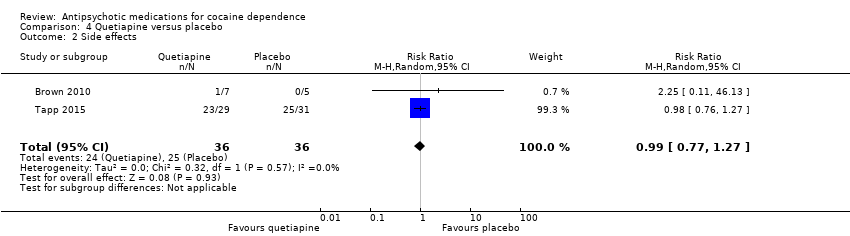
Comparison 4 Quetiapine versus placebo, Outcome 2 Side effects.

Comparison 4 Quetiapine versus placebo, Outcome 3 Use of cocaine during the treatment (self‐reported as days/week).
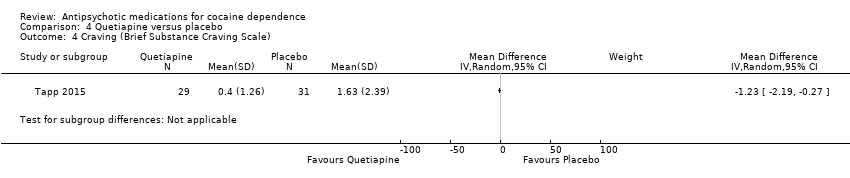
Comparison 4 Quetiapine versus placebo, Outcome 4 Craving (Brief Substance Craving Scale).

Comparison 4 Quetiapine versus placebo, Outcome 5 Amount of of cocaine use during the treatment (self‐reported as g/week).

Comparison 4 Quetiapine versus placebo, Outcome 6 Amount of of cocaine use during the treatment (self‐reported as dollars spent/week).
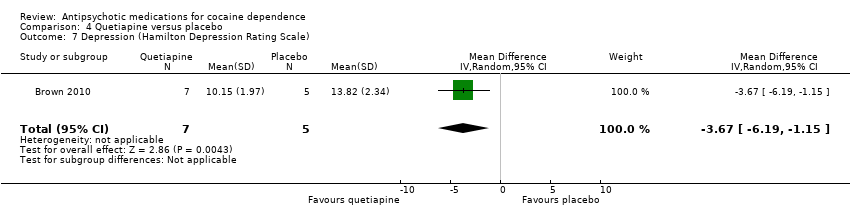
Comparison 4 Quetiapine versus placebo, Outcome 7 Depression (Hamilton Depression Rating Scale).
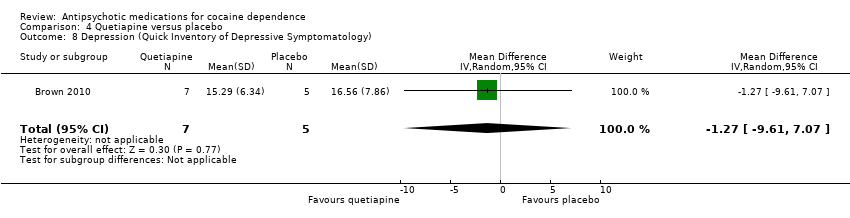
Comparison 4 Quetiapine versus placebo, Outcome 8 Depression (Quick Inventory of Depressive Symptomatology).
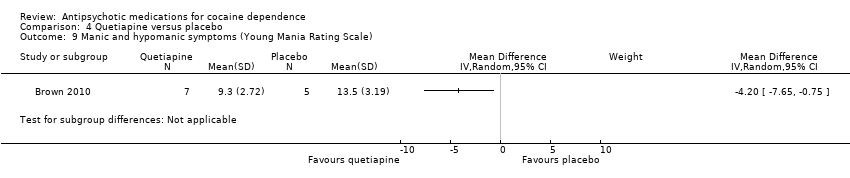
Comparison 4 Quetiapine versus placebo, Outcome 9 Manic and hypomanic symptoms (Young Mania Rating Scale).

Comparison 5 Lamotrigine versus placebo, Outcome 1 Side effects.

Comparison 6 Reserpine versus placebo, Outcome 1 Dropouts.

Comparison 6 Reserpine versus placebo, Outcome 2 Craving (Brief Substance Craving Scale).
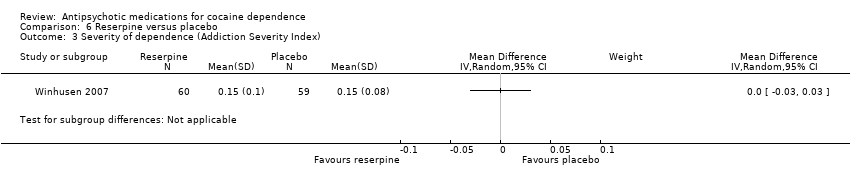
Comparison 6 Reserpine versus placebo, Outcome 3 Severity of dependence (Addiction Severity Index).

Comparison 6 Reserpine versus placebo, Outcome 4 Severity of dependence (Clinical Global Impression Scale).
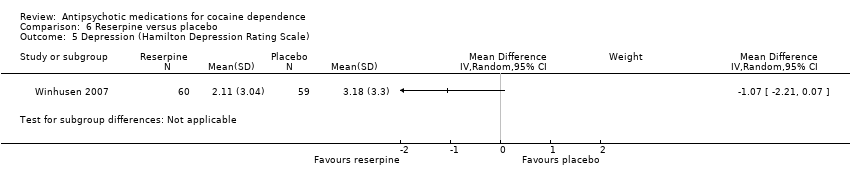
Comparison 6 Reserpine versus placebo, Outcome 5 Depression (Hamilton Depression Rating Scale).

Comparison 7 Olanzapine versus haloperidol, Outcome 1 Dropouts.

Comparison 7 Olanzapine versus haloperidol, Outcome 2 Psychopathology (Positive and Negative Syndrome Scale).
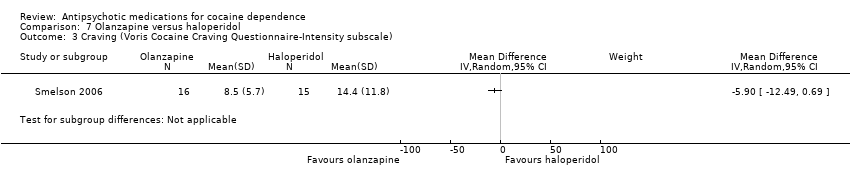
Comparison 7 Olanzapine versus haloperidol, Outcome 3 Craving (Voris Cocaine Craving Questionnaire‐Intensity subscale).
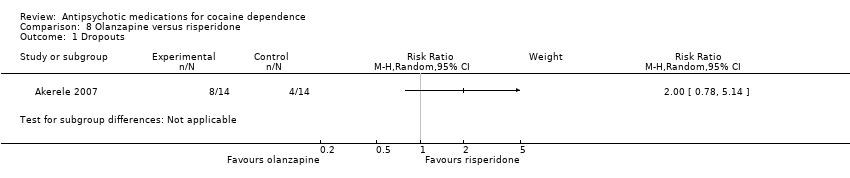
Comparison 8 Olanzapine versus risperidone, Outcome 1 Dropouts.
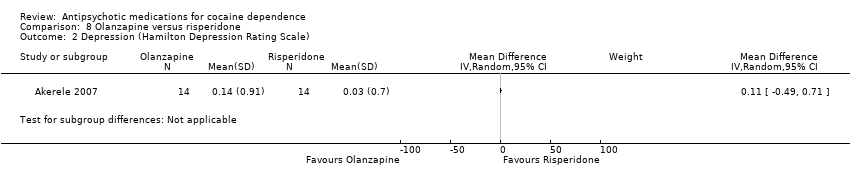
Comparison 8 Olanzapine versus risperidone, Outcome 2 Depression (Hamilton Depression Rating Scale).

Comparison 9 Aripiprazol versus ropinirol, Outcome 1 Dropouts.
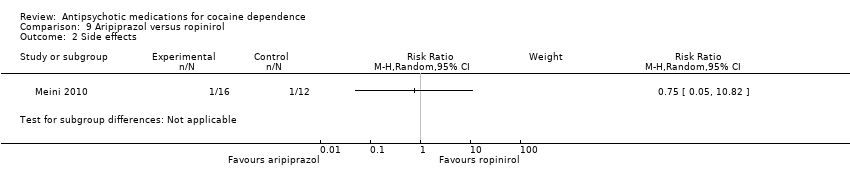
Comparison 9 Aripiprazol versus ropinirol, Outcome 2 Side effects.
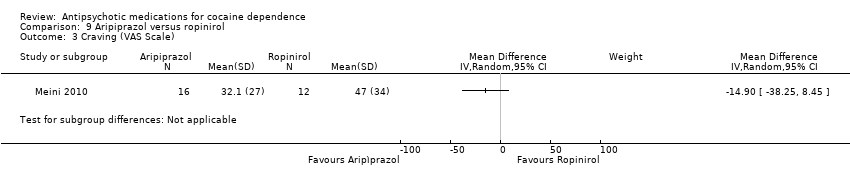
Comparison 9 Aripiprazol versus ropinirol, Outcome 3 Craving (VAS Scale).

Comparison 9 Aripiprazol versus ropinirol, Outcome 4 Severity of dependence (Clinical Global Impression Scale).
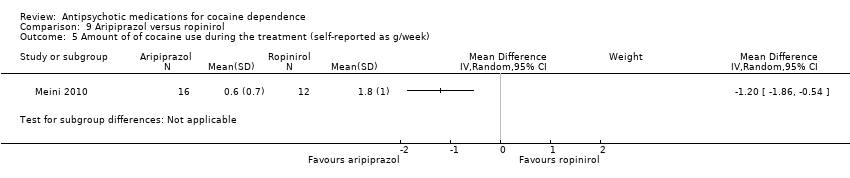
Comparison 9 Aripiprazol versus ropinirol, Outcome 5 Amount of of cocaine use during the treatment (self‐reported as g/week).
| Any antipsychotic versus placebo for cocaine dependence | ||||||
| Patient or population: people with cocaine dependence | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any antipsychotic versus placebo | |||||
| Dropouts | Study population | RR 0.75 | 397 | ⊕⊕⊕⊝ | ||
| 547 per 1000 | 411 per 1000 | |||||
| Moderate | ||||||
| 500 per 1000 | 375 per 1000 | |||||
| Side effects | Study population | RR 1.01 | 291 | ⊕⊕⊝⊝ | ||
| 497 per 1000 | 502 per 1000 | |||||
| Moderate | ||||||
| 465 per 1000 | 470 per 1000 | |||||
| Number of participants using cocaine during the treatment (as days/week by urine tests or self report) | Study population | RR 1.02 | 91 | ⊕⊕⊝⊝ | ||
| 478 per 1000 | 488 per 1000 | |||||
| Moderate | ||||||
| 596 per 1000 | 608 per 1000 | |||||
| Continuous abstinence (number of participants who maintained negative drug screens for 2 ‐ 3 weeks) | Study population | RR 1.30 | 139 | ⊕⊕⊝⊝ | ||
| 197 per 1000 | 256 per 1000 | |||||
| Moderate | ||||||
| 129 per 1000 | 168 per 1000 | |||||
| Craving (Brief Substance Craving Scale) | The mean craving (brief substance craving scale) in the control groups was | The mean craving (Brief Substance Craving Scale) in the intervention groups was | 240 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1All the studies were at unclear risk of selection bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 8 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.57, 0.97] |
| 2 Side effects Show forest plot | 6 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.93, 1.10] |
| 3 Number of participants using cocaine during the treatment (as days/week by urine tests or self report) Show forest plot | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.62] |
| 4 Continuous abstinence (number of participants who maintained negative drug screens for 2 ‐ 3 weeks) Show forest plot | 3 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.73, 2.32] |
| 5 Craving (Brief Substance Craving Scale) Show forest plot | 4 | 240 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐1.08, 1.35] |
| 6 Severity of dependence (Addiction Severity Index) Show forest plot | 4 | 211 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 7 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 3 | 180 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.38, 0.39] |
| 8 Use of cocaine during the treatment (self‐reported as g/week) Show forest plot | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.92, ‐0.16] |
| 9 Depression (Hamilton Depression Rating Scale) Show forest plot | 4 | 192 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐3.19, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 4 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.04] |
| 2 Severity of dependence (Addiction Severity Index) Show forest plot | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Side effects Show forest plot | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 3 Use of cocaine during the treatment (self‐reported as days/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Use of cocaine during the treatment (self‐reported as days/past 30 days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Continuous abstinence (participants who maintained negative drug screens throughout the treatment period ) Show forest plot | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.71, 2.61] |
| 6 Craving (Brief Substance Craving Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐0.91, 3.58] |
| 7 Severity of dependence (Addiction Severity Index) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 8 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.51, 0.85] |
| 9 Amount of of cocaine use during the treatment (self‐reported as dollars spent/past 30 days) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10 Depression (Hamilton Depression Rating Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐3.84, 6.52] |
| 11 Anxiety (Hamilton Anxiety Rating Scale) Show forest plot | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐3.02, 5.75] |
| 12 Withdrawal symptoms (Cocaine Selective Severity Assessment) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.20, 2.03] |
| 2 Side effects Show forest plot | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.77, 1.27] |
| 3 Use of cocaine during the treatment (self‐reported as days/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Craving (Brief Substance Craving Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Amount of of cocaine use during the treatment (self‐reported as g/week) Show forest plot | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.92, ‐0.16] |
| 6 Amount of of cocaine use during the treatment (self‐reported as dollars spent/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Depression (Hamilton Depression Rating Scale) Show forest plot | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐3.67 [‐6.19, ‐1.15] |
| 8 Depression (Quick Inventory of Depressive Symptomatology) Show forest plot | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐9.61, 7.07] |
| 9 Manic and hypomanic symptoms (Young Mania Rating Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Side effects Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Craving (Brief Substance Craving Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Severity of dependence (Addiction Severity Index) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Depression (Hamilton Depression Rating Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Psychopathology (Positive and Negative Syndrome Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Craving (Voris Cocaine Craving Questionnaire‐Intensity subscale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Depression (Hamilton Depression Rating Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Side effects Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Craving (VAS Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Severity of dependence (Clinical Global Impression Scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Amount of of cocaine use during the treatment (self‐reported as g/week) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |

