针抽吸(needle aspiration)与切口和引流(incision and drainage)治疗扁桃体周囊肿的对比
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised trial Design: parallel‐group | |
| Participants | Number randomised: 110 Age range: 12 to 79 Gender: 89% males Setting: hospital, Taiwan Inclusion criteria: diagnosis of peritonsillar abscess Exclusion criteria: none stated Participant characteristics: mean age = 31.0 ± 15.0 years, days of symptoms prior to presentation = 4.7 ± 2.8 | |
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 55 Comparator group: incision and drainage n = 55 Use of additional interventions: antibiotics, intravenous hydration | |
| Outcomes | 1. Recurrence rate 2. Length of hospital stay 3. Pain score (Outcomes not specified as primary/secondary) | |
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Participants lost to follow‐up: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided into two groups..." Comment: specific method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not specified |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) | Low risk | All patients presumably accounted for (admitted as inpatients); no dropouts stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Incomplete methodological description (definition of recurrence/criteria for re‐intervention not described, timing of assessment for recurrence not described). |
| Methods | Allocation: randomised trial, alternation Design: parallel‐group | |
| Participants | Number randomised: 62 Age range: 15 to 35 Gender: 74% males Setting: hospital, Pakistan Inclusion criteria: not specifically stated Exclusion criteria: other associated illness Participant characteristics: mean age = 24.6 years | |
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 31 Comparator group: incision and drainage n = 31 Use of additional interventions: antibiotics (injection benzyl penicillin and metronidazole), analgesics | |
| Outcomes | 1. Recurrence rate 2. Length of hospital stay (Outcomes not specified as primary/secondary) | |
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Needle aspiration failures treated with incision and drainage Participants lost to follow‐up: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment specified |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) | Low risk | All patients presumably accounted for (admitted as inpatients); no dropouts stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Incomplete methodological description (definition of recurrence/criteria for re‐intervention not described, timing of assessment for recurrence not described). |
| Methods | Allocation: randomised trial, alternation Design: parallel‐group | |
| Participants | Number randomised: 56 Age range: 16 to 50 Gender: 71% males Setting: hospital, Pakistan Eligibility criteria: age > 15 years with peritonsillar abscess Exclusion criteria: patients with bleeding disorders, acute follicular tonsillitis Participant characteristics: mean age = 31.2 years | |
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 28 Comparator group: incision and drainage n = 28 Use of additional interventions: antibiotics (intravenous amoxicillin/clavulanate and metronidazole), povidone‐iodine (Pyodine) mouth wash, intravenous crystalloid (if necessary) | |
| Outcomes | 1. Recurrence rate 2. Symptom score 3. Length of hospital stay (Outcomes not specified as primary/secondary) | |
| Funding sources | None stated | |
| Declarations of interest | None declared | |
| Notes | Alternate basis randomisation Participants lost to follow‐up: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate basis randomisation |
| Allocation concealment (selection bias) | High risk | Alternate basis randomisation |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) | Low risk | All patients presumably accounted for (admitted as inpatients); no dropouts stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Incomplete methodological description (definition of recurrence/criteria for re‐intervention not described, timing of assessment for recurrence not described) |
| Methods | Allocation: randomised trial Design: parallel‐group | |
| Participants | Number randomised: 70 (data reported on 60) Age range: 17 to 53 Gender: 76.7% males Setting: military hospital, Pakistan Inclusion criteria: "clinical diagnosis of PTA was made on clinical features of unitonsillar erythema, swelling, odynophagia (pain on swallowing), uvular deviation towards the opposite direction and trismus", age ≥ 15 Exclusion criteria: "patients with a history of bleeding disorders or diabetes mellitus, on anticoagulant drugs or diagnosed with immunodeficiency disorders or who refused to undergo the procedure under local anesthesia"; "Patients failing to follow up were excluded from this study" Participant characteristics: mean age 32.7 | |
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 35 Comparator group: incision and drainage n = 35 Use of additional interventions: Use of additional interventions: antibiotics and analgesia ((a) co‐amoxiclav 1.2 g intravenously 8‐hourly and metronidazole 500 mg intravenously 8‐hourly for 3 days, followed by oral co‐amoxiclav 1 g twice daily and metronidazole 400 mg 3 times a day for the next 4 days, (b) paracetamol 1 g 8‐hourly orally "for fever and analgesia") | |
| Outcomes | 1. Time of resolution of odynophagia (days) 2. Fever – time to resolution (days) Above information used to define "Recovery period" as "time taken to settle both odynophagia and fever" 3. Recurrence 4. Complications | |
| Funding sources | Not specified | |
| Declarations of interest | None declared | |
| Notes | Participants lost to follow‐up: "Patients failing to follow up were excluded from this study" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a "random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | Not certain if random numbers table was open |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding; probably not done |
| Incomplete outcome data (attrition bias) | Unclear risk | 1. "Patients, who had cultured organisms resistant to [the] antibiotics [named above] were excluded from the study" 2. "Patients failing to follow up were excluded from this study" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias |
| Methods | Allocation: randomised, alternation Design: parallel‐group | |
| Participants | Number randomised: 60 Age range: "under 14" to "over 40" Gender: not specified Setting: hospital, South Africa Inclusion criteria: positive needle aspiration Exclusion criteria: negative needle aspiration Participant characteristics: mean age not provided, "all patients presented with some degree of odynophagia and drooling of saliva", 47% had trismus and 27% had pyrexia | |
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 30 Comparator group: incision and drainage n = 30 Use of additional interventions: antibiotics (penicillin), analgesics, mouthwash (unspecified) | |
| Outcomes | 1. Recurrence rate (Outcomes not specified as primary/secondary) | |
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Participants lost to follow‐up: 11 (18%) at day 1, 22 (37%) at day 7 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised on alternate basis |
| Allocation concealment (selection bias) | High risk | Randomised on alternate basis |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) | High risk | 82% of patients followed up on day 1 post‐treatment and 63% followed up on day 7 |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Allocation: randomised trial Design: parallel‐group | |
| Participants | Number randomised: 75 (only 50 patients were in groups compared in this review) Age range: 15 to 43 Gender: 33% males Setting: South Africa Inclusion criteria: not described Exclusion criteria: none stated Participant characteristics: "unilateral swelling of the tonsil and soft palate, and medial displacement of the uvula", "all patients were pyrexial" | |
| Interventions | Needle aspiration versus incision and drainage versus intravenous antibiotics alone Intervention group: intravenous antibiotics n = 25 Comparator group 1: needle aspiration n = 25 Comparator group 2: incision and drainage n = 25 Use of additional interventions: single dose of intravenous antibiotics | |
| Outcomes | 1. Distance between upper and lower incisor teeth (degree of trismus) 2. Body temperature 3. Ability to drink water 4. Microbiology cultures 5. Treatment failures (recurrence) | |
| Funding sources | None stated | |
| Declarations of interest | None declared | |
| Notes | Participants lost to follow‐up: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment specified |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition not clear, dropouts not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias |
| Methods | Allocation: randomised trial Design: parallel‐group | |
| Participants | Number randomised: 50 Age range: 22 to 43 Gender: 94% males Setting: hospital, Pakistan Inclusion criteria: "peritonsillar abscess" Exclusion criteria: none stated Participant characteristics: "all the patients were otherwise healthy and young with no immune compromising disease", no patients with previous peritonsillar abscess | |
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 25 Comparator group: incision and drainage n = 25 Use of additional interventions: antibiotics (lincomycin) | |
| Outcomes | 1. Recurrence rate 2. Length of hospital stay 3. "Period of recovery" (Outcomes not specified as primary/secondary) | |
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Participants lost to follow‐up: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment specified |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) | Low risk | All patients presumably accounted for (admitted as inpatients); no dropouts stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Incomplete methodological description (definition of recurrence/criteria for re‐intervention not described, timing of assessment for recurrence not described) |
| Methods | Allocation: randomised trial Design: parallel‐group | |
| Participants | Number randomised: 50 Age range: 18 to 51 Gender: 62% males Setting: hospital, Pakistan Inclusion criteria: "presented with peritonsillar abscess" Exclusion criteria: diabetics, less than 18 years old Participant characteristics: mean age = 32.7 years | |
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 25 Comparator group: incision and drainage n = 25 Use of additional interventions: antibiotics, chlorhexidine mouth wash | |
| Outcomes | Primary outcome: recurrence rate Secondary outcomes: none | |
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Needle aspiration failures treated with incision and drainage Participants lost to follow‐up: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment specified |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) | Low risk | All patients presumably accounted for (admitted as inpatients); no dropouts stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Subjective definition of recurrence/criteria for re‐intervention |
| Methods | Allocation: randomised trial Design: parallel‐group | |
| Participants | Number randomised: 62 Age range: 12 to 53 Gender: "2:1 male predilection" Setting: hospital, USA Inclusion criteria: not specified Exclusion criteria: not specified Participant characteristics: median age = 24; 1 patient with bilateral abscesses | |
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 41 Comparator group: incision and drainage n = 21 Use of additional interventions: analgesics, antibiotics (penicillin V or cephalexin or erythromycin) | |
| Outcomes | 1. Recurrence rate 2. Time to resumption of normal diet (Outcomes not specified as primary/secondary) | |
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Only partial randomisation Participants lost to follow‐up: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | First 15 participants treated with needle aspiration, then patients were subsequently randomised |
| Allocation concealment (selection bias) | High risk | Randomised by hospital number |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition not clear, dropouts not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Incomplete methodological description (definition of recurrence/criteria for re‐intervention not described) |
| Methods | Allocation: randomised trial Design: parallel‐group | |
| Participants | Number randomised: 52 Age range: 13 to 60 Gender: 60% males Setting: hospital, USA Inclusion criteria: positive needle aspiration Exclusion criteria: negative needle aspiration Participant characteristics: mean age = 27 years average duration of symptoms prior to presentation = 5.3 days | |
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 24 Comparator group: incision and drainage n = 28 Use of additional interventions: antibiotics (initial dose of intramuscular penicillin G followed by intramuscular penicillin G or oral penicillin V for 10 days), erythromycin/cephalosporin/clindamycin if allergic | |
| Outcomes | 1. Recurrence rate 2. Symptom score (Outcomes not specified as primary/secondary) | |
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Authors contacted and further information/clarification obtained Participants lost to follow‐up: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | High risk | No allocation concealment performed |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not performed |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts mentioned |
| Selective reporting (reporting bias) | Low risk | All measured outcomes stated to have been reported |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Subjective definition of recurrence/criteria for re‐intervention. |
| Methods | Allocation: randomised trial Design: parallel‐group | |
| Participants | Number randomised: 62 (discrepant total = 64 in results) Age range: 8 to 57 Gender: 61.3% male Setting: hospital, Pakistan Eligibility criteria: "clinically diagnosed for peritonsillar abscess" Exclusion criteria: none stated Participant characteristics: average duration of symptoms prior to presentation = 6 days; 84% treated with antibiotics prior to presentation | |
| Interventions | Intervention group: needle aspiration n = 32 Intervention group: incision and drainage n = 32 Use of additional interventions: not stated | |
| Outcomes | 1. "Success" – not defined 2. Length of time to return to semisolid/solid food (days) 3. Proportion with "no pain by 05 days" (%) 4. Length of hospital stay (days) | |
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Participants lost to follow‐up: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment specified |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding; probably not done |
| Blinding of outcome assessment (detection bias) | High risk | No mention of blinding; probably not done |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition not clear; dropouts not mentioned; numbers in methods and results do not match |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risks of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Commentary | |
| Review paper | |
| Review paper | |
| Participants were not randomised: "All patients were divided in two groups according to surgical procedures carried out". While the study states that this was a prospective study, it seems that outcomes were assessed retrospectively. | |
| Considered a duplicate publication of Stringer 1988 | |
| Retrospective study |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | Study not available at this time. This study was inaccessible by all attempted means, including contact with the journal editor |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of recurrence Show forest plot | 10 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 3.74 [1.63, 8.59] |
| Analysis 1.1  Comparison 1 Needle aspiration versus incision and drainage, Outcome 1 Rate of recurrence. | ||||

Process for sifting search results and selecting studies for inclusion.
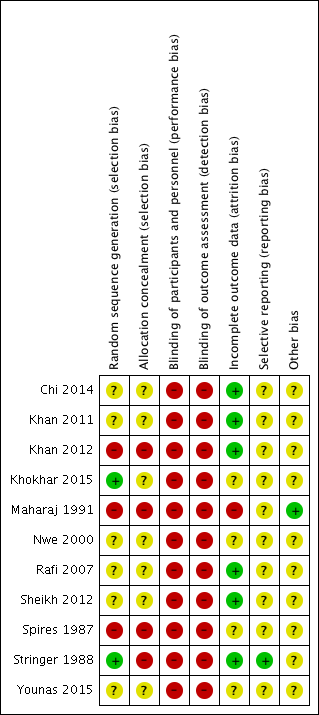
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Needle aspiration versus incision and drainage, Outcome 1 Rate of recurrence.
| Needle aspiration versus incision and drainage for the treatment of peritonsillar abscess | ||||||
| Patient or population: patients older than 8 years with peritonsillar abscess | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with incision and drainage | Risk with needle aspiration | |||||
| Primary outcome: recurrence rate | Study population | RR 3.74, 95% CI 1.63 to 8.59 | 612 (10 RCTs) | ⊕⊝⊝⊝ | — | |
| 47 per 1000 | 245 per 1000 | |||||
| Primary outcome: adverse effects/events associated with the interventions | One study reported post‐procedural bleeding in 1 patient (3.6%) in the incision and drainage group, with no adverse effects/events reported in the needle aspiration group. Two studies stated that no complications were seen in either group. | — | 226 (3 RCTs) | ⊕⊝⊝⊝ | Adverse effects/events were not mentioned as a pre‐specified outcome measure in any of the studies. | |
| Secondary outcome: time to resumption of normal diet | One study found no difference in the time to resumption of normal diet (mean 3.7 days in both groups, no confidence intervals provided). Another study found that a similar percentage of patients returned to solid food within 4 days (87%: needle aspiration, 88%: incision and drainage). | — | 124 (2 RCTs) | ⊕⊝⊝⊝ | — | |
| Secondary outcome: complications of the disease process | One study described a complication of 2 patients requiring admission to hospital for dehydration in the incision and drainage group and no complications in the needle aspiration group. One study stated that no complications were seen in either group. | — | 170 (2 RCTs) | ⊕⊝⊝⊝ | Complications of the disease process were not mentioned as a pre‐specified outcome measure in any of the studies. | |
| Secondary outcome: symptom scores | Procedural pain Study 1 Pain was less in the needle aspiration group: MD ‐0.8, 95% CI ‐1.16 to ‐0.44 (10‐point scale) Study 2 Reported less pain in the needle aspiration group Pain resolution Study 3 Pain resolution was similar between groups at 5 days post‐intervention Other symptoms Study 4 Reported comparable symptom scores between groups at presentation and 48 hours | — | Study 1 110 participants Study 2 56 participants Study 3 62 participants Study 4 52 participants | ⊕⊝⊝⊝ | — | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to serious risk of inconsistency (unexplained heterogeneity). 2Downgraded twice due to very serious risk of bias (limitations in study design). 3Adverse event (post‐procedural bleeding) was not well described. 4Incomplete data (no standard deviations or confidence intervals provided). 5Admission to hospital for rehydration is inherently subjective and depends on multiple clinical variables. 6Downgraded once due to imprecision and differences in data reporting. | ||||||
| Study ID | Definition of recurrence or criteria for re‐intervention described | Timing of assessment of recurrence |
| No | 2, 7 days (2x returned day 1) | |
| "Failure to improve symptom scale score; visual evidence of a persistent abscess" | 1, 2 days (24, 48 hours) | |
| "reaccumulation of pus" | 1, 7 days | |
| "patients in whom the trismus and pyrexia persisted 48 hours after the initial treatment" | 2 days (48 hours) | |
| No | Not stated | |
| No | Not stated | |
| No | Not stated | |
| Yes* | 0, 1, 2 days | |
| No | Not stated | |
| No | "during the course of the study", 7, 14 days | |
| N/A | N/A | |
| * "Improvement in patients was determined by examining the patient the next day after the procedure, a reduction in supra tonsillar swelling along with decrease in pain and also improvement in odynophagia were taken as criteria of improvement and termination of surgical attempts." N/A: not available | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of recurrence Show forest plot | 10 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 3.74 [1.63, 8.59] |

