Aspiración con aguja versus incisión y drenaje para el tratamiento del absceso periamigdalino
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006287.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 23 diciembre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades de oído, nariz y garganta
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Brent A Chang: literature searching, study selection, data collection, data management, 'Risk of bias' assessment, data analysis, data interpretation, drafting the protocol and review.
Andrew Thamboo: literature searching, study selection, data collection, data management, 'Risk of bias' assessment, data analysis, data interpretation, drafting the protocol and review.
Chris Diamond: protocol design, content expertise, revising the protocol and review, critical appraisal and study selection.
Martin J Burton: content expertise, data interpretation, revising the review.
Desmond A Nunez: protocol design, 'Risk of bias' assessment, content expertise, revising the protocol and review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
Declarations of interest
Brent A Chang: none to declare.
Andrew Thamboo: none to declare.
Chris Diamond: none to declare.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial sign‐off this review, which was carried out by Cochrane ENT's second Co‐ordinating Editor.
Desmond A Nunez: none to declare.
Acknowledgements
We would like to acknowledge Cochrane ENT for their helpful guidance and input.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Keir J, Almeyda R, Bowyer D and Wilbourn M were authors of the original protocol (withdrawn in 2011). New authors took over the review in 2014, publishing a new protocol (Chang 2014).
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Dec 23 | Needle aspiration versus incision and drainage for the treatment of peritonsillar abscess | Review | Brent A Chang, Andrew Thamboo, Martin J Burton, Chris Diamond, Desmond A Nunez | |
| 2014 Jul 06 | Needle aspiration versus incision and drainage for the treatment of peritonsillar abscess | Protocol | Brent A Chang, Andrew Thamboo, Chris Diamond, Desmond A Nunez | |
Differences between protocol and review
We widened the age criteria for participants in included studies based on post hoc review. A number of studies included older child (generally > 8 years old), adolescent and adult participants. Stratified data by age group (adults versus adolescents) were not available in any of the studies. Given that adolescents and older children with peritonsillar abscess are often managed similarly to adults, we thought that the review would also be applicable to this age group. Younger patients typically require a general anaesthetic for management and, therefore, have different practical considerations for intervention. We therefore made a decision to include trials with older children (age > 8), adolescents and adults.
We added the methods for the creation of a 'Summary of findings' table and GRADE assessment to the review after the protocol was published.
We also added a standard statement to clarify the role of outcomes in the review (Types of outcome measures).
Notes
The original protocol was withdrawn from Issue 11, 2011 of theCochrane Library onwards as the authors were unable to continue with the review. A new protocol by new authors was published in 2014 (Chang 2014).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Adult; Child; Humans;
PICO

Process for sifting search results and selecting studies for inclusion.
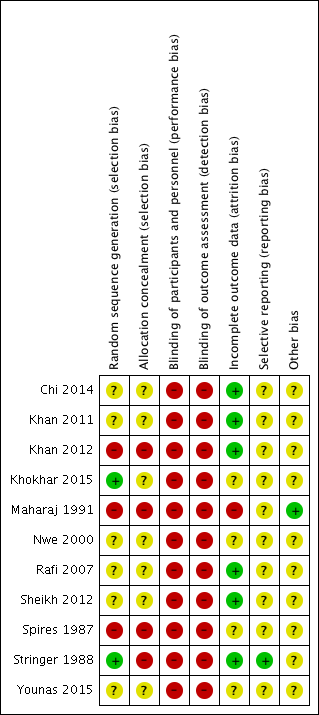
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Needle aspiration versus incision and drainage, Outcome 1 Rate of recurrence.
| Needle aspiration versus incision and drainage for the treatment of peritonsillar abscess | ||||||
| Patient or population: patients older than 8 years with peritonsillar abscess | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with incision and drainage | Risk with needle aspiration | |||||
| Primary outcome: recurrence rate | Study population | RR 3.74, 95% CI 1.63 to 8.59 | 612 (10 RCTs) | ⊕⊝⊝⊝ | — | |
| 47 per 1000 | 245 per 1000 | |||||
| Primary outcome: adverse effects/events associated with the interventions | One study reported post‐procedural bleeding in 1 patient (3.6%) in the incision and drainage group, with no adverse effects/events reported in the needle aspiration group. Two studies stated that no complications were seen in either group. | — | 226 (3 RCTs) | ⊕⊝⊝⊝ | Adverse effects/events were not mentioned as a pre‐specified outcome measure in any of the studies. | |
| Secondary outcome: time to resumption of normal diet | One study found no difference in the time to resumption of normal diet (mean 3.7 days in both groups, no confidence intervals provided). Another study found that a similar percentage of patients returned to solid food within 4 days (87%: needle aspiration, 88%: incision and drainage). | — | 124 (2 RCTs) | ⊕⊝⊝⊝ | — | |
| Secondary outcome: complications of the disease process | One study described a complication of 2 patients requiring admission to hospital for dehydration in the incision and drainage group and no complications in the needle aspiration group. One study stated that no complications were seen in either group. | — | 170 (2 RCTs) | ⊕⊝⊝⊝ | Complications of the disease process were not mentioned as a pre‐specified outcome measure in any of the studies. | |
| Secondary outcome: symptom scores | Procedural pain Study 1 Pain was less in the needle aspiration group: MD ‐0.8, 95% CI ‐1.16 to ‐0.44 (10‐point scale) Study 2 Reported less pain in the needle aspiration group Pain resolution Study 3 Pain resolution was similar between groups at 5 days post‐intervention Other symptoms Study 4 Reported comparable symptom scores between groups at presentation and 48 hours | — | Study 1 110 participants Study 2 56 participants Study 3 62 participants Study 4 52 participants | ⊕⊝⊝⊝ | — | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to serious risk of inconsistency (unexplained heterogeneity). 2Downgraded twice due to very serious risk of bias (limitations in study design). 3Adverse event (post‐procedural bleeding) was not well described. 4Incomplete data (no standard deviations or confidence intervals provided). 5Admission to hospital for rehydration is inherently subjective and depends on multiple clinical variables. 6Downgraded once due to imprecision and differences in data reporting. | ||||||
| Study ID | Definition of recurrence or criteria for re‐intervention described | Timing of assessment of recurrence |
| No | 2, 7 days (2x returned day 1) | |
| "Failure to improve symptom scale score; visual evidence of a persistent abscess" | 1, 2 days (24, 48 hours) | |
| "reaccumulation of pus" | 1, 7 days | |
| "patients in whom the trismus and pyrexia persisted 48 hours after the initial treatment" | 2 days (48 hours) | |
| No | Not stated | |
| No | Not stated | |
| No | Not stated | |
| Yes* | 0, 1, 2 days | |
| No | Not stated | |
| No | "during the course of the study", 7, 14 days | |
| N/A | N/A | |
| * "Improvement in patients was determined by examining the patient the next day after the procedure, a reduction in supra tonsillar swelling along with decrease in pain and also improvement in odynophagia were taken as criteria of improvement and termination of surgical attempts." N/A: not available | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of recurrence Show forest plot | 10 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 3.74 [1.63, 8.59] |

