Aspiracija iglom u usporedbi s incizijom s drenažom u liječenju peritonzilarnog apscesa
Appendices
Appendix 1. Search strategies
| CENTRAL | Ovid MEDLINE | EMBASE |
| #1 MeSH descriptor: [Peritonsillar Abscess] explode all trees #2 abscess* near tonsil* or abscess* near peritonsil* or abscess* near retrotonsil* or abscess* near peri‐tonsil* #3 suppurat* near tonsil* or suppurat* near peritonsil* or suppurat* near retrotonsil* or suppurat* near peri‐tonsil* #4 sepsis near tonsil* or sepsis near peritonsil* or sepsis near retrotonsil* or sepsis near peri‐tonsil* #5 septic near tonsil* or septic near peritonsil* or septic near retrotonsil* or septic near peri‐tonsil* #6 pus near tonsil* or pus near peritonsil* or pus near retrotonsil* or pus near peri‐tonsil* #7 infect* near peritonsil* or infect* near retrotonsil* or infect* near peri‐tonsil* #8 acute near peritonsil* or acute near retrotonsil* or acute near peri‐tonsil* #9 quinsy or "interval tonsil*" #10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 | 1 exp Peritonsillar Abscess/ 2 ((abscess* adj5 tonsil*) or (abscess* adj5 peritonsil*) or (abscess* adj5 retrotonsil*)).ab,ti. 3 ((suppurat* adj5 tonsil*) or (suppurat* adj5 peritonsil*) or (suppurat* adj5 retrotonsil*) or (suppurat* adj5 peri‐tonsil*)).ab,ti. 4 ((sepsis adj5 tonsil*) or (sepsis adj5 peritonsil*) or (sepsis adj5 retrotonsil*) or (sepsis adj5 peri‐tonsil*)).ab,ti. 5 ((septic adj5 tonsil*) or (septic adj5 peritonsil*) or (septic adj5 retrotonsil*) or (septic adj5 peri‐tonsil*)).ab,ti. 6 ((pus adj5 tonsil*) or (pus adj5 peritonsil*) or (pus adj5 retrotonsil*) or (pus adj5 peri‐tonsil*)).ab,ti. 7 ((infect* adj5 peritonsil*) or (infect* adj5 retrotonsil*) or (infect* adj5 peri‐tonsil*)).ab,ti. 8 ((acute adj5 peritonsil*) or (acute adj5 retrotonsil*) or (acute adj5 peri‐tonsil*)).ab,ti. 9 (quinsy or "interval tonsil*").ab,ti. 10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 | 1 exp Peritonsillar Abscess/ 2 ((abscess* adj5 tonsil*) or (abscess* adj5 peritonsil*) or (abscess* adj5 retrotonsil*)).ab,ti. 3 ((suppurat* adj5 tonsil*) or (suppurat* adj5 peritonsil*) or (suppurat* adj5 retrotonsil*) or (suppurat* adj5 peri‐tonsil*)).ab,ti. 4 ((sepsis adj5 tonsil*) or (sepsis adj5 peritonsil*) or (sepsis adj5 retrotonsil*) or (sepsis adj5 peri‐tonsil*)).ab,ti. 5 ((septic adj5 tonsil*) or (septic adj5 peritonsil*) or (septic adj5 retrotonsil*) or (septic adj5 peri‐tonsil*)).ab,ti. 6 ((pus adj5 tonsil*) or (pus adj5 peritonsil*) or (pus adj5 retrotonsil*) or (pus adj5 peri‐tonsil*)).ab,ti. 7 ((infect* adj5 peritonsil*) or (infect* adj5 retrotonsil*) or (infect* adj5 peri‐tonsil*)).ab,ti. 8 ((acute adj5 peritonsil*) or (acute adj5 retrotonsil*) or (acute adj5 peri‐tonsil*)).ab,ti. 9 (quinsy or "interval tonsil*").ab,ti. 10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 |
| CINAHL | Web of Science | ClinicalTrials.gov |
| S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S9 quinsy or "interval tonsil*" S8 acute N5 peritonsil* or acute N5 retrotonsil* or acute N5 peri‐tonsil* S7 infect* N5 peritonsil* or infect* N5 retrotonsil* or infect* N5 peri‐tonsil* S6 pus N5 tonsil* or pus N5 peritonsil* or pus N5 retrotonsil* or pus N5 peri‐tonsil* S5 TX septic N5 tonsil* or septic N5 peritonsil* or septic N5 retrotonsil* or septic N5 peri‐tonsil* S4 TX sepsis N5 tonsil* or sepsis N5 peritonsil* or sepsis N5 retrotonsil* or sepsis N5 peri‐tonsil* S3 TX suppurat* N5 tonsil* or suppurat* N5 peritonsil* or suppurat*i N5 retrotonsil* or suppurat* N5 peri‐tonsil* S2 TX abscess* N5 tonsil* or abscess* N5 peritonsil* or abscess* N5 retrotonsil* or abscess* N5 peri‐tonsil* S1 (MH "Peritonsillar Abscess") | #1 TOPIC: (abscess* near/5 tonsil* or abscess* near/5 peritonsil* or abscess* near/5 retrotonsil* or abscess* near/5 peri‐tonsil*) #2 TOPIC: (suppurat* near/5 tonsil* or suppurat* near/5 peritonsil* or suppurat* near/5 retrotonsil* or suppurat* near/5 peri‐tonsil*) #3 TOPIC: (sepsis near/5 tonsil* or sepsis near/5 peritonsil* or sepsis near/5 retrotonsil* or sepsis near/5 peri‐tonsil*) #4 TOPIC: (septic near/5 tonsil* or septic near/5 peritonsil* or septic near/5 retrotonsil* or septic near/5 peri‐tonsil*) #5 TOPIC: (pus near/5 tonsil* or pus near/5 peritonsil* or pus near/5 retrotonsil* or pus near/5 peri‐tonsil*) #6 TOPIC: (infect* near/5 peritonsil* or infect* near/5 retrotonsil* or infect* near/5 peri‐tonsil*) #7 TOPIC: (acute near/5 peritonsil* or acute near/5 retrotonsil* or acute near/5 peri‐tonsil*) #8 TOPIC: (quinsy or "interval tonsil*") #9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 | ((abscess OR sepsis OR septic OR pus OR infect OR acute) AND (tonsil* OR peritonsil* OR retrotonsil* OR per‐tonsil*)) OR quinsy OR "interval tonsil" |

Process for sifting search results and selecting studies for inclusion.
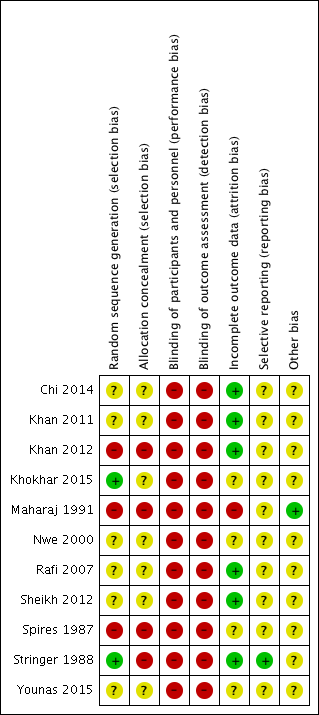
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Needle aspiration versus incision and drainage, Outcome 1 Rate of recurrence.
| Needle aspiration versus incision and drainage for the treatment of peritonsillar abscess | ||||||
| Patient or population: patients older than 8 years with peritonsillar abscess | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with incision and drainage | Risk with needle aspiration | |||||
| Primary outcome: recurrence rate | Study population | RR 3.74, 95% CI 1.63 to 8.59 | 612 (10 RCTs) | ⊕⊝⊝⊝ | — | |
| 47 per 1000 | 245 per 1000 | |||||
| Primary outcome: adverse effects/events associated with the interventions | One study reported post‐procedural bleeding in 1 patient (3.6%) in the incision and drainage group, with no adverse effects/events reported in the needle aspiration group. Two studies stated that no complications were seen in either group. | — | 226 (3 RCTs) | ⊕⊝⊝⊝ | Adverse effects/events were not mentioned as a pre‐specified outcome measure in any of the studies. | |
| Secondary outcome: time to resumption of normal diet | One study found no difference in the time to resumption of normal diet (mean 3.7 days in both groups, no confidence intervals provided). Another study found that a similar percentage of patients returned to solid food within 4 days (87%: needle aspiration, 88%: incision and drainage). | — | 124 (2 RCTs) | ⊕⊝⊝⊝ | — | |
| Secondary outcome: complications of the disease process | One study described a complication of 2 patients requiring admission to hospital for dehydration in the incision and drainage group and no complications in the needle aspiration group. One study stated that no complications were seen in either group. | — | 170 (2 RCTs) | ⊕⊝⊝⊝ | Complications of the disease process were not mentioned as a pre‐specified outcome measure in any of the studies. | |
| Secondary outcome: symptom scores | Procedural pain Study 1 Pain was less in the needle aspiration group: MD ‐0.8, 95% CI ‐1.16 to ‐0.44 (10‐point scale) Study 2 Reported less pain in the needle aspiration group Pain resolution Study 3 Pain resolution was similar between groups at 5 days post‐intervention Other symptoms Study 4 Reported comparable symptom scores between groups at presentation and 48 hours | — | Study 1 110 participants Study 2 56 participants Study 3 62 participants Study 4 52 participants | ⊕⊝⊝⊝ | — | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to serious risk of inconsistency (unexplained heterogeneity). 2Downgraded twice due to very serious risk of bias (limitations in study design). 3Adverse event (post‐procedural bleeding) was not well described. 4Incomplete data (no standard deviations or confidence intervals provided). 5Admission to hospital for rehydration is inherently subjective and depends on multiple clinical variables. 6Downgraded once due to imprecision and differences in data reporting. | ||||||
| Study ID | Definition of recurrence or criteria for re‐intervention described | Timing of assessment of recurrence |
| No | 2, 7 days (2x returned day 1) | |
| "Failure to improve symptom scale score; visual evidence of a persistent abscess" | 1, 2 days (24, 48 hours) | |
| "reaccumulation of pus" | 1, 7 days | |
| "patients in whom the trismus and pyrexia persisted 48 hours after the initial treatment" | 2 days (48 hours) | |
| No | Not stated | |
| No | Not stated | |
| No | Not stated | |
| Yes* | 0, 1, 2 days | |
| No | Not stated | |
| No | "during the course of the study", 7, 14 days | |
| N/A | N/A | |
| * "Improvement in patients was determined by examining the patient the next day after the procedure, a reduction in supra tonsillar swelling along with decrease in pain and also improvement in odynophagia were taken as criteria of improvement and termination of surgical attempts." N/A: not available | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of recurrence Show forest plot | 10 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 3.74 [1.63, 8.59] |

